Modelling and Simulation of the Interactions Between the Cardiovascular System and the Combined Use of VA ECMO and IABP: Comparison Between Peripheral and Central Configurations
Abstract
1. Introduction
2. Materials and Methods
- Minimum, maximum and mean values of left atrial pressure (LAP); aortic pressure (AoP) and pulmonary arterial pressure (PAP).
- Pulmonary capillary wedge pressure (PCWP), which is an indirect estimate of left atrial pressure and therefore left ventricular filling pressure. This gives information about the severity of left ventricular failure, pulmonary hypertension and blood volume status.
- Total flow (native plus assistance flow).
- Coronary blood flow (CBF), which is the ratio between coronary perfusion pressure and coronary vascular resistance. Coronary perfusion pressure is defined as the difference between aortic diastolic pressure and left ventricular end-diastolic pressure.
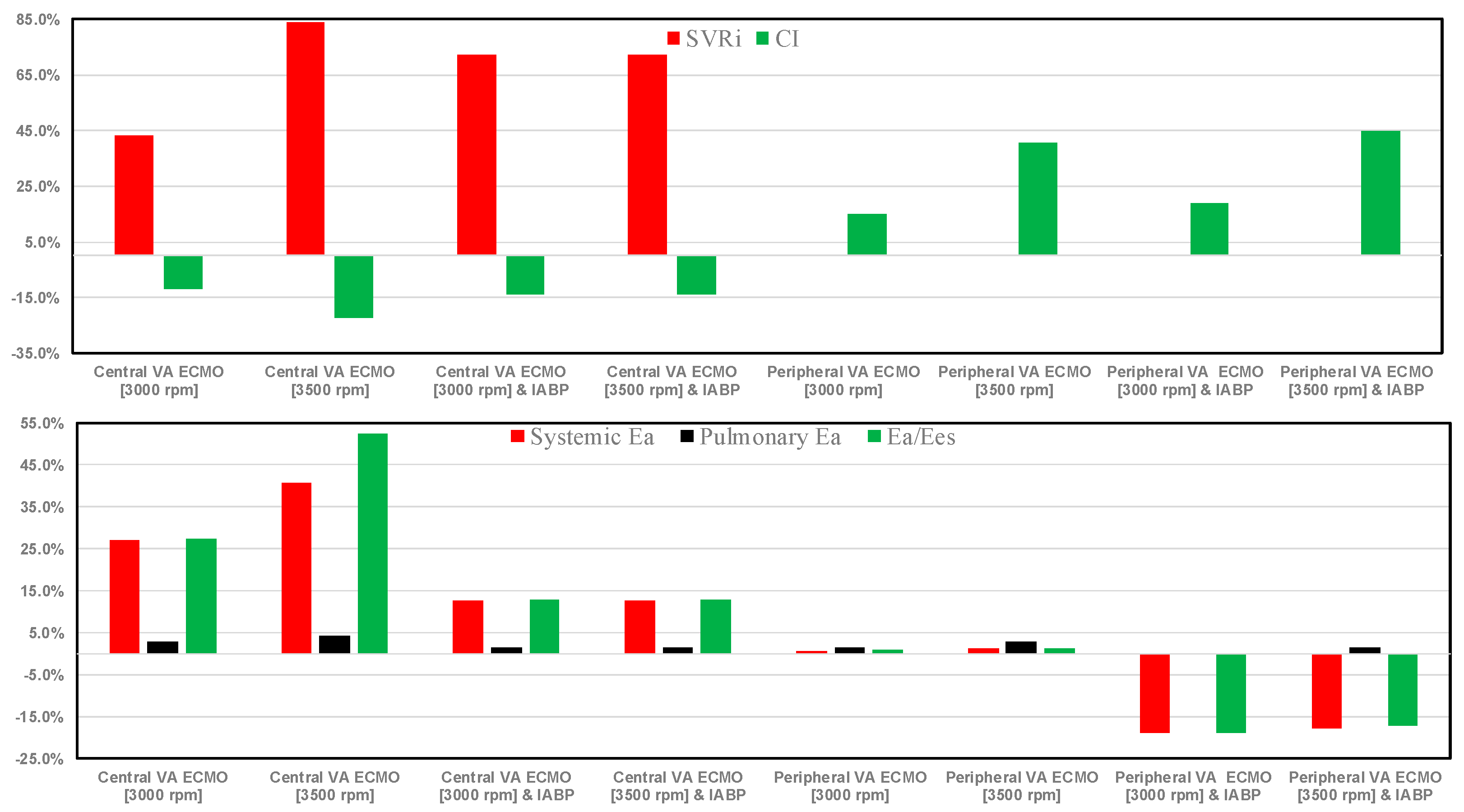
- Cardiac index (CI), which is the ratio between cardiac output and body surface area.
- Left ventricular end systolic (diastolic) volume LVESV (LVEDV).
- Right ventricular end systolic (diastolic) volume RVESV (RVEDV).
- The systemic vascular resistance index (SVRi), which is the ratio between systemic vascular resistance and body surface area. Systemic vascular resistance is the ratio between pressure difference (mean arterial pressure minus central venous pressure) and cardiac output.
- ✓ Left (right) atrial pressure-volume loop area LA-PVLA (RA-PVLA);
- ✓ Left (right) ventricular external work LVEW (RVEW);
- ✓ Left (right) ventricular pressure-volume area LVPVA (RVPVA).
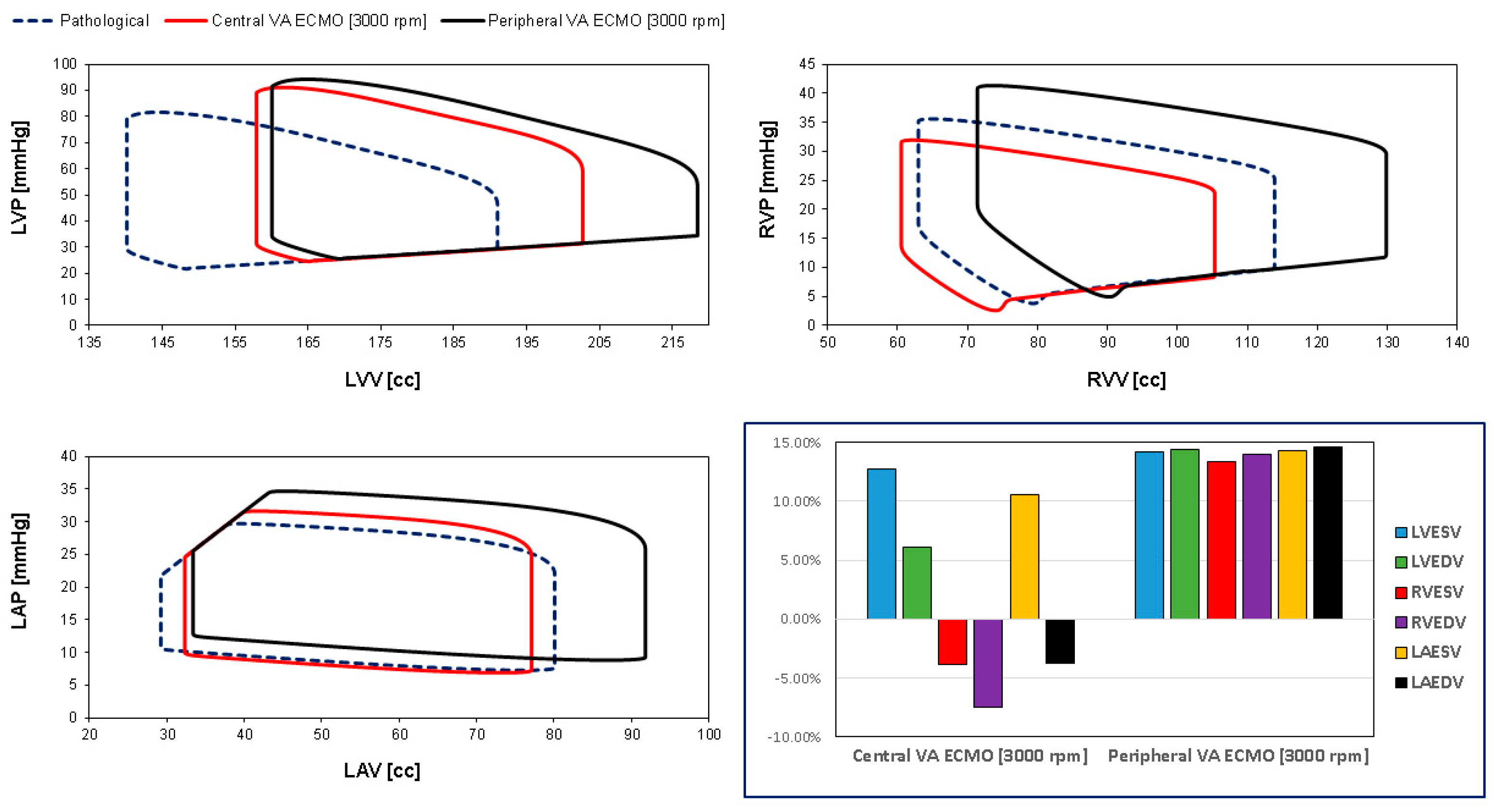
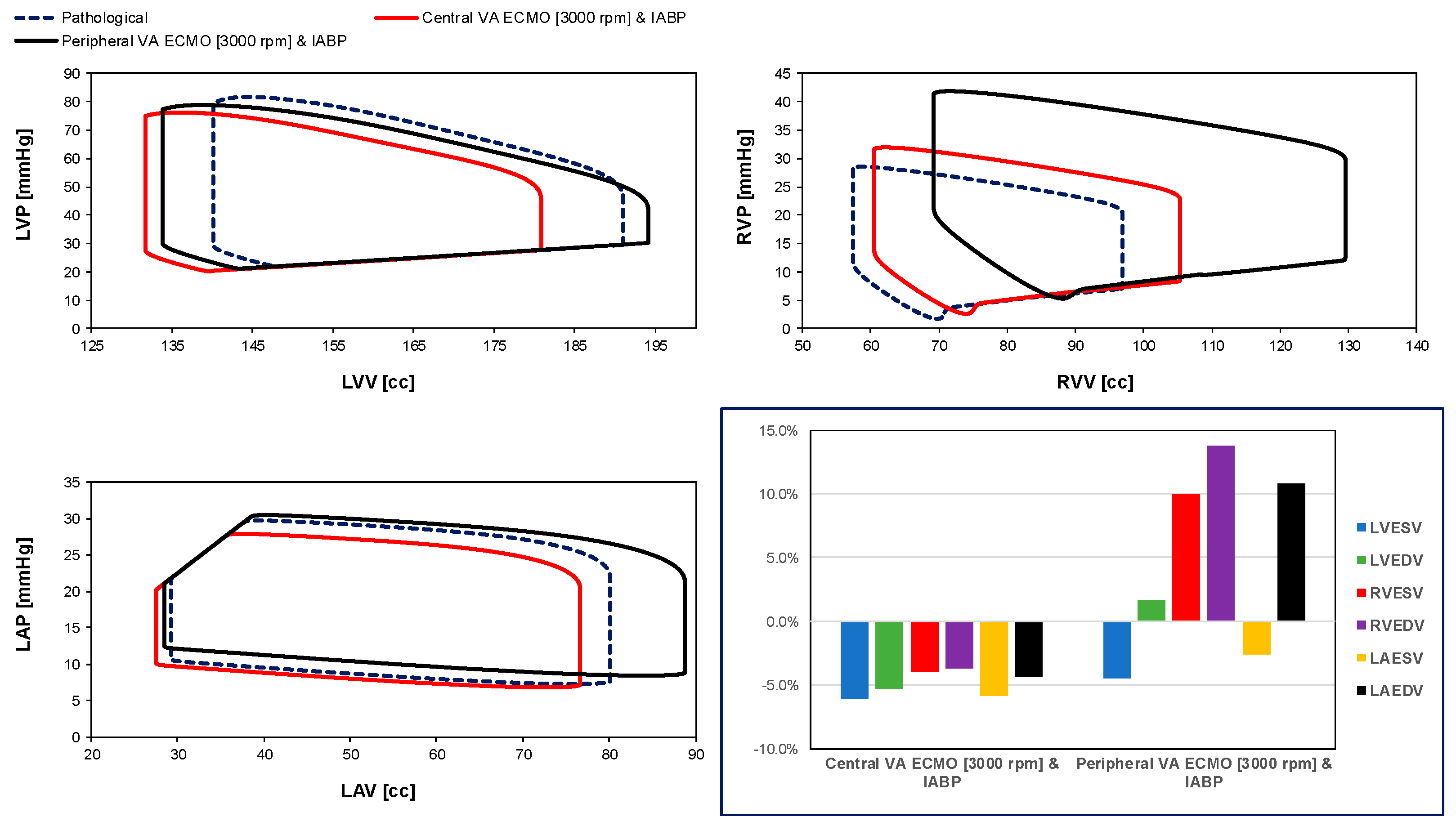
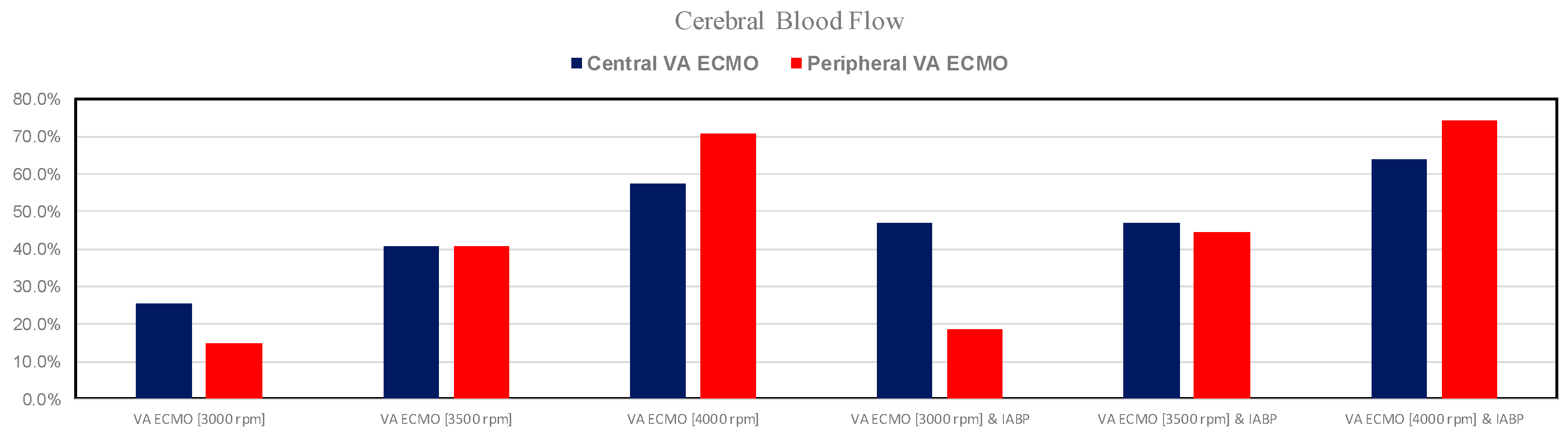
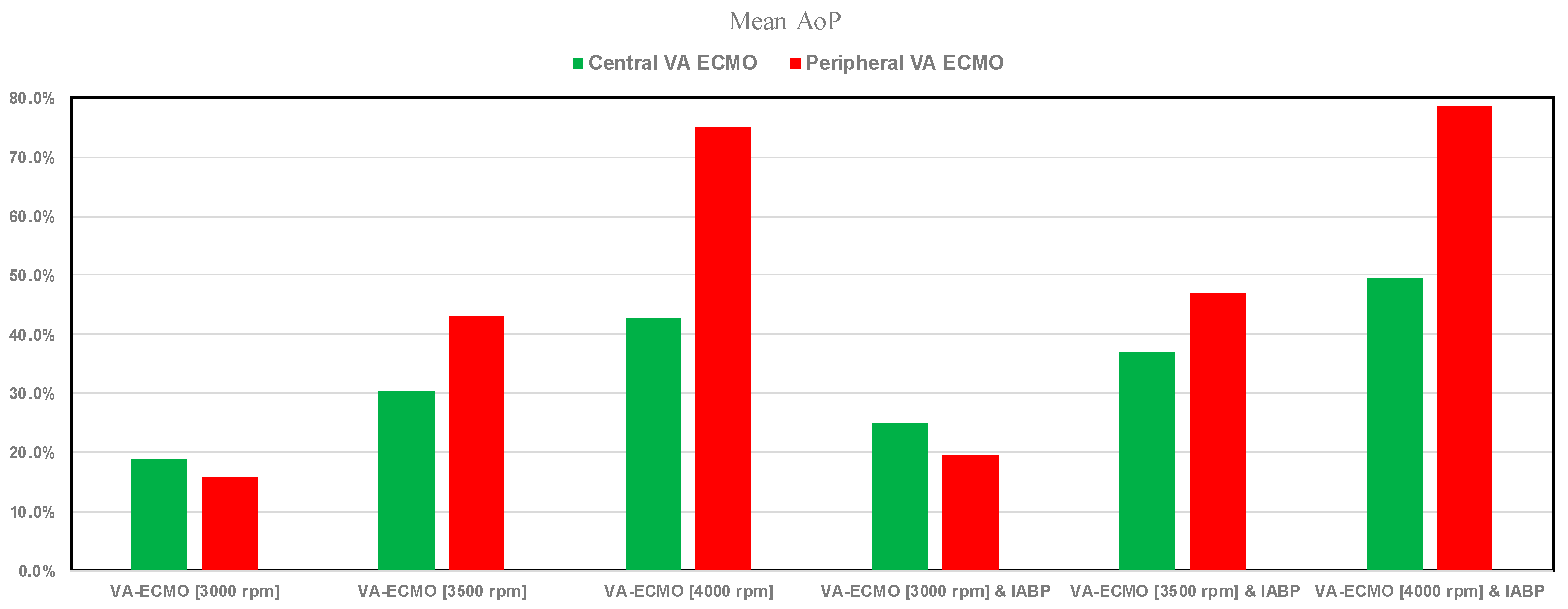
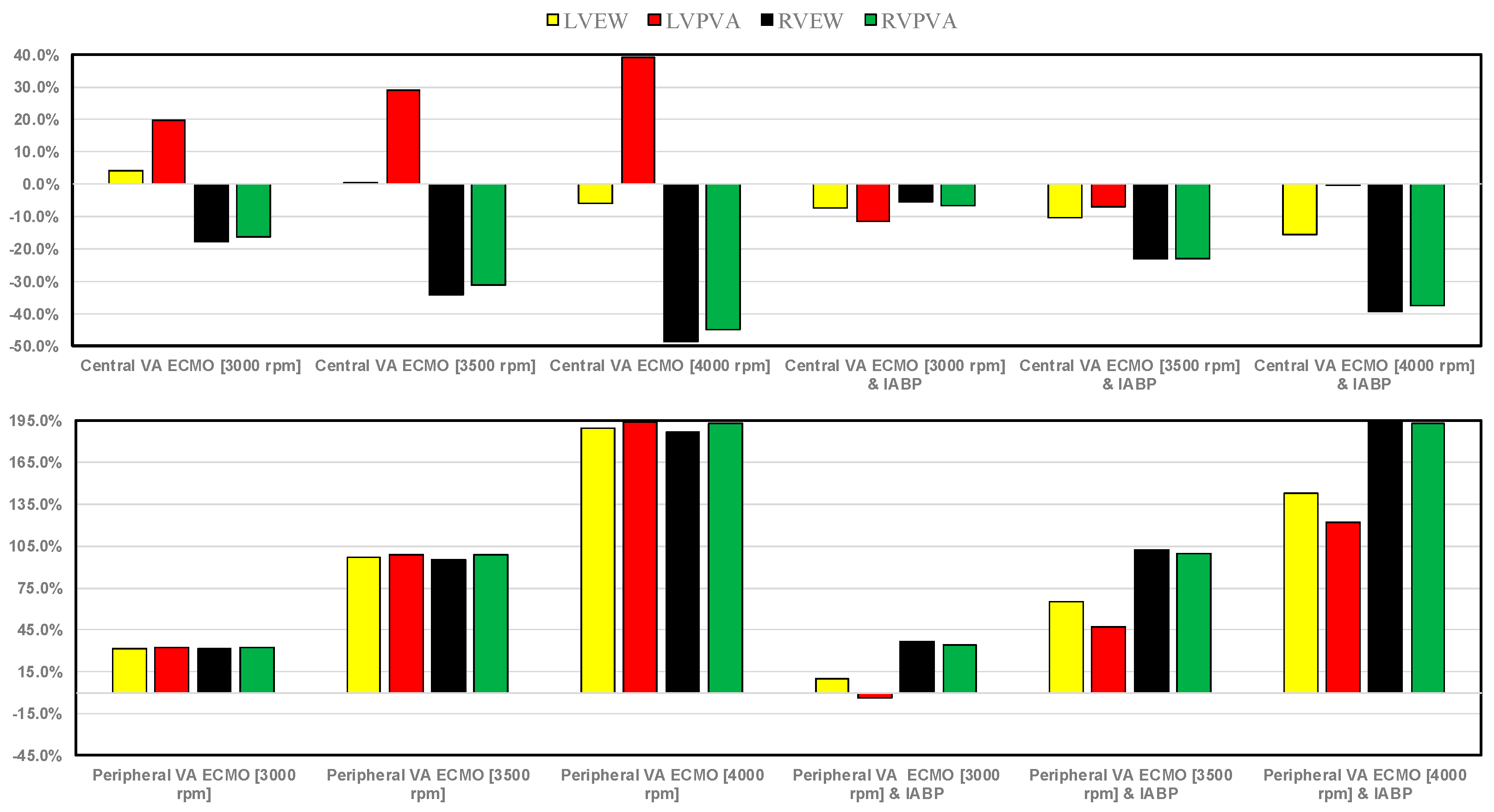
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meani, P.; Gelsomino, S.; Natour, E.; Johnson, D.M.; Rocca, H.-P.B.L.; Pappalardo, F.; Bidar, E.; Makhoul, M.; Raffa, G.; Heuts, S. Modalities and Effects of Left Ventricle Unloading on Extracorporeal Life Support: A Review of the Current Literature. Eur. J. Heart Fail. 2017, 19, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, R.; Meani, P.; Raffa, G.M.; Kowalewski, M. Extracorporeal Membrane Oxygenation and Left Ventricular Unloading: What Is the Evidence? JTCVS Tech. 2022, 13, 101. [Google Scholar] [CrossRef]
- Nishi, T.; Ishii, M.; Tsujita, K.; Okamoto, H.; Koto, S.; Nakai, M.; Sumita, Y.; Iwanaga, Y.; Kobayashi, Y.; Hirata, K. Outcomes of VA-ECMO plus IABP versus VA-ECMO Alone for Treatment of Acute Myocardial Infarction Complicated by Cardiogenic Shock: Insight from the JROADDPC Registry. J. Am. Coll. Cardiol. 2022, 79, 954. [Google Scholar] [CrossRef]
- Li, Y.; Yan, S.; Gao, S.; Liu, M.; Lou, S.; Liu, G.; Ji, B.; Gao, B. Effect of an Intra-Aortic Balloon Pump with Venoarterial Extracorporeal Membrane Oxygenation on Mortality of Patients with Cardiogenic Shock: A Systematic Review and Meta-Analysis. Eur. J. Cardiothorac. Surg. 2019, 55, 395–404. [Google Scholar] [CrossRef]
- Vallabhajosyula, S.; O’Horo, J.C.; Antharam, P.; Ananthaneni, S.; Vallabhajosyula, S.; Stulak, J.M.; Eleid, M.F.; Dunlay, S.M.; Gersh, B.J.; Rihal, C.S. Concomitant Intra-Aortic Balloon Pump Use in Cardiogenic Shock Requiring Veno-Arterial Extracorporeal Membrane Oxygenation: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Interv. 2018, 11, e006930. [Google Scholar] [CrossRef]
- Björnsdóttir, B.; Biancari, F.; Dalén, M.; Dell’Aquila, A.M.; Jónsson, K.; Fiore, A.; Mariscalco, G.; El-Dean, Z.; Gatti, G.; Zipfel, S. Postcardiotomy Venoarterial Extracorporeal Membrane Oxygenation with and without Intra-Aortic Balloon Pump. J. Cardiothorac. Vasc. Anesth. 2022, 36, 2876–2883. [Google Scholar] [CrossRef] [PubMed]
- Hasde, A.I.; Sarıcaoğlu, M.C.; Dikmen Yaman, N.; Baran, Ç.; Özçınar, E.; Çakıcı, M.; İnan, M.B.; Akar, A.R. Comparison of Left Ventricular Unloading Strategies on Venoarterial Extracorporeal Life Support. Interact. Cardiovasc. Thorac. Surg. 2021, 32, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-Y.; Liao, C.-W.; Wang, C.-H.; Chi, N.-H.; Yu, H.-Y.; Chou, N.-K.; Hwang, J.-J.; Lin, J.-L.; Chiang, F.-T.; Chen, Y.-S. Effects of Additional Intra-Aortic Balloon Counter-Pulsation Therapy to Cardiogenic Shock Patients Supported by Extra-Corporeal Membranous Oxygenation. Sci. Rep. 2016, 6, 23838. [Google Scholar] [CrossRef]
- Wang, D.; Chao, V.; Yap, K.H.; Tan, T.E. Does Concurrent Use of Intra-Aortic Balloon Pumps Improve Survival in Patients with Cardiogenic Shock Requiring Venoarterial Extracorporeal Membrane Oxygenation? Interact. Cardiovasc. Thorac. Surg. 2020, 30, 312–315. [Google Scholar] [CrossRef]
- Tepper, S.; Garcia, M.B.; Fischer, I.; Ahmed, A.; Khan, A.; Balsara, K.R.; Masood, M.F.; Itoh, A. Clinical Outcomes and Reduced Pulmonary Artery Pressure With Intra-Aortic Balloon Pump During Central Extracorporeal Life Support. ASAIO J. 2019, 65, 173–179. [Google Scholar] [CrossRef]
- Baran, C.; Ozcinar, E.; Kayan, A.; Dikmen, N.; Baran, C.S.; Inan, M.B. Comparison of ECMO, IABP and ECMO+ IABP in the Postoperative Period in Patients with Postcardiotomy Shock. J. Cardiovasc. Dev. Dis. 2024, 11, 283. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Hou, J.; Tang, H.; Hu, S. Concurrent Initiation of Intra-Aortic Balloon Pumping with Extracorporeal Membrane Oxygenation Reduced in-Hospital Mortality in Postcardiotomy Cardiogenic Shock. Ann. Intensive Care 2019, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kida, H.; Sotomi, Y.; Hikoso, S.; Nakatani, D.; Mizuno, H.; Suna, S.; Okada, K.; Kitamura, T.; Komukai, S.; Dohi, T. Prognostic Significance of Intra-Aortic Balloon Pumping Support in Patients with Acute Myocardial Infarction and Veno-Arterial Extracorporeal Membrane Oxygenation Therapy. J. Cardiol. 2022, 79, 179–185. [Google Scholar] [CrossRef]
- Loforte, A.; Murana, G.; Cefarelli, M.; Jafrancesco, G.; Sabatino, M.; Martin Suarez, S.; Pilato, E.; Pacini, D.; Grigioni, F.; Bartolomeo, R.D. Role of Intra-aortic Balloon Pump and Extracorporeal Membrane Oxygenation in Early Graft Failure after Cardiac Transplantation. Artif. Organs 2016, 40, E136–E145. [Google Scholar] [CrossRef] [PubMed]
- Nuding, S.; Werdan, K. IABP plus ECMO—Is One and One More than Two? J. Thorac. Dis. 2017, 9, 961. [Google Scholar] [CrossRef] [PubMed]
- Zeng, P.; Yang, C.; Chen, J.; Fan, Z.; Cai, W.; Huang, Y.; Xiang, Z.; Yang, J.; Zhang, J.; Yang, J. Comparison of the Efficacy of ECMO with or without IABP in Patients with Cardiogenic Shock: A Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 917610. [Google Scholar] [CrossRef]
- Bréchot, N.; Demondion, P.; Santi, F.; Lebreton, G.; Pham, T.; Dalakidis, A.; Gambotti, L.; Luyt, C.-E.; Schmidt, M.; Hekimian, G. Intra-Aortic Balloon Pump Protects against Hydrostatic Pulmonary Oedema during Peripheral Venoarterial-Extracorporeal Membrane Oxygenation. Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 62–69. [Google Scholar] [CrossRef]
- Raffa, G.M.; Kowalewski, M.; Brodie, D.; Ogino, M.; Whitman, G.; Meani, P.; Pilato, M.; Arcadipane, A.; Delnoij, T.; Natour, E. Meta-Analysis of Peripheral or Central Extracorporeal Membrane Oxygenation in Postcardiotomy and Non-Postcardiotomy Shock. Ann. Thorac. Surg. 2019, 107, 311–321. [Google Scholar] [CrossRef]
- Xu, B.; Li, C.; Cai, T.; Cui, Y.; Du, Z.; Fan, Q.; Guo, D.; Jiang, C.; Xing, Z.; Xin, M. Intra-aortic Balloon Pump Impacts the Regional Haemodynamics of Patients with Cardiogenic Shock Treated with Femoro-femoral Veno-arterial Extracorporeal Membrane Oxygenation. ESC Heart Fail. 2022, 9, 2610–2617. [Google Scholar] [CrossRef]
- Tay, C.K.; Yoo, K.H.; Cho, Y.H. Intraaortic Balloon Pulsation in Peripheral Venoarterial Extracorporeal Membrane Oxygenation: More Is Not Always Better. Crit. Care Med. 2016, 44, e1251. [Google Scholar] [CrossRef]
- Yang, F.; Jia, Z.; Xing, J.; Wang, Z.; Liu, Y.; Hao, X.; Jiang, C.; Wang, H.; Jia, M.; Hou, X. Effects of Intra-Aortic Balloon Pump on Cerebral Blood Flow during Peripheral Venoarterial Extracorporeal Membrane Oxygenation Support. J. Transl. Med. 2014, 12, 106. [Google Scholar] [CrossRef] [PubMed]
- Bělohlávek, J.; Mlček, M.; Huptych, M.; Svoboda, T.; Havránek, Š.; Ošt’ádal, P.; Bouček, T.; Kovárník, T.; Mlejnský, F.; Mrázek, V. Coronary versus Carotid Blood Flow and Coronary Perfusion Pressure in a Pig Model of Prolonged Cardiac Arrest Treated by Different Modes of Venoarterial ECMO and Intraaortic Balloon Counterpulsation. Crit. Care 2012, 16, R50. [Google Scholar] [CrossRef]
- Jung, C.; Lauten, A.; Roediger, C.; Fritzenwanger, M.; Schumm, J.; Figulla, H.R.; Ferrari, M. In Vivo Evaluation of Tissue Microflow under Combined Therapy with Extracorporeal Life Support and Intra-Aortic Balloon Counterpulsation. Anaesth. Intensive Care 2009, 37, 833–835. [Google Scholar] [CrossRef] [PubMed]
- Petroni, T.; Harrois, A.; Amour, J.; Lebreton, G.; Brechot, N.; Tanaka, S.; Luyt, C.-E.; Trouillet, J.-L.; Chastre, J.; Leprince, P. Intra-Aortic Balloon Pump Effects on Macrocirculation and Microcirculation in Cardiogenic Shock Patients Supported by Venoarterial Extracorporeal Membrane Oxygenation. Crit. Care Med. 2014, 42, 2075–2082. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, I.; Liakopoulos, O.; Elskamp, M.; Maier-Trauth, J.; Gerfer, S.; Mühlbauer, T.; Slottosch, I.; Kuhn, E.; Sabashnikov, A.; Rademann, P. Concomitant Intra-Aortic Balloon Pumping Significantly Reduces Left Ventricular Pressure during Central Veno-Arterial Extracorporeal Membrane Oxygenation—Results from a Large Animal Model. Life 2022, 12, 1859. [Google Scholar] [CrossRef]
- Farag, J.; Stephens, A.F.; Chong, W.J.; Gregory, S.D.; Marasco, S.F. Intra-Aortic Balloon Pump Use with Extra Corporeal Membrane Oxygenation—A Mock Circulation Loop Study. ASAIO J. 2022, 68, 669–675. [Google Scholar] [CrossRef]
- Gerfer, S.; Djordjevic, I.; Maier, J.; Movahed, A.; Elskamp, M.; Kuhn, E.; Liakopoulos, O.; Wahlers, T.; Deppe, A.C. Endothelial and Hemodynamic Function in a Large Animal Model in Relation to Different Extracorporeal Membrane Oxygenation Cannulation Strategies and Intra-Aortic Balloon Pumping. J. Clin. Med. 2023, 12, 4038. [Google Scholar] [CrossRef]
- Gu, K.; Zhang, Y.; Gao, B.; Chang, Y.; Zeng, Y. Hemodynamic Differences between Central ECMO and Peripheral ECMO: A Primary CFD Study. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 717. [Google Scholar] [CrossRef]
- Gu, K.; Zhang, Z.; Gao, B.; Chang, Y.; Wan, F. Hemodynamic Effects of Perfusion Level of Peripheral ECMO on Cardiovascular System. Biomed. Eng. Online 2018, 17, 59. [Google Scholar] [CrossRef]
- Gu, K.; Gao, S.; Zhang, Z.; Ji, B.; Chang, Y. Hemodynamic Effect of Pulsatile on Blood Flow Distribution with VA ECMO: A Numerical Study. Bioengineering 2022, 9, 487. [Google Scholar] [CrossRef]
- Reymond, P.; Bendjelid, K.; Giraud, R.; Richard, G.; Murith, N.; Cikirikcioglu, M.; Huber, C. To Balloon or Not to Balloon? The Effects of an Intra-Aortic Balloon-Pump on Coronary Artery Flow during Extracorporeal Circulation Simulating Normal and Low Cardiac Output Syndromes. J. Clin. Med. 2021, 10, 5333. [Google Scholar] [CrossRef] [PubMed]
- De Lazzari, B.; Badagliacca, R.; Filomena, D.; Papa, S.; Vizza, C.D.; Capoccia, M.; De Lazzari, C. CARDIOSIM©: The First Italian Software Platform for Simulation of the Cardiovascular System and Mechanical Circulatory and Ventilatory Support. Bioengineering 2022, 9, 383. [Google Scholar] [CrossRef]
- De Lazzari, C.; Staltieri, D. CARDIOSIM© Website. 2021. Available online: https://Cardiosim.Dsb.Cnr.It (accessed on 29 May 2022).
- De Lazzari, C. Interaction between the Septum and the Left (Right) Ventricular Free Wall in Order to Evaluate the Effects on Coronary Blood Flow: Numerical Simulation. Comput. Methods Biomech. Biomed. Engin. 2012, 15, 1359–1368. [Google Scholar] [CrossRef]
- Capoccia, M.; Marconi, S.; Singh, S.A.; Pisanelli, D.M.; De Lazzari, C. Simulation as a Preoperative Planning Approach in Advanced Heart Failure Patients. A Retrospective Clinical Analysis. Biomed. Eng. Online 2018, 17, 52. [Google Scholar] [CrossRef]
- De Lazzari, B.; Iacovoni, A.; Mottaghy, K.; Capoccia, M.; Badagliacca, R.; Vizza, C.D.; De Lazzari, C. ECMO Assistance during Mechanical Ventilation: Effects Induced on Energetic and Haemodynamic Variables. Comput. Methods Programs Biomed. 2021, 202, 106003, Erratum in Comput. Methods Programs Biomed. 2021, 212, 106478. [Google Scholar] [CrossRef]
- De Lazzari, C.; De Lazzari, C. A Pumping Heart Coupled to Lumped Description of the Cardiovascular Compartments (CARDIOSIM©). In Modelling Cardiovascular System and Mechanical Circulatory Support; CNR: Roma, Italy, 2007; pp. 65–67. [Google Scholar]
- Capoccia, M.; Marconi, S.; De Lazzari, C. Decision-Making in Advanced Heart Failure Patients Requiring LVAD Insertion: Can Preoperative Simulation Become the Way Forward? A Case Study. J. Biomed. Eng. Inform. 2018, 4, 8–20. [Google Scholar]
- De Lazzari, B.; Capoccia, M.; Badagliacca, R.; Bozkurt, S.; De Lazzari, C. IABP versus Impella Support in Cardiogenic Shock: “In Silico” Study. J. Cardiovasc. Dev. Dis. 2023, 10, 140. [Google Scholar] [CrossRef]
- Vahdatpour, C.; Collins, D.; Goldberg, S. Cardiogenic Shock. J. Am. Heart Assoc. 2019, 8, e011991. [Google Scholar] [CrossRef] [PubMed]
- Alkhunaizi, F.A.; Smith, N.; Brusca, S.B.; Furfaro, D. The Management of Cardiogenic Shock From Diagnosis to Devices. A Narrat. Review. CHEST Crit. Care 2024, 2, 100071. [Google Scholar] [CrossRef]
- Sinha, S.S.; Morrow, D.A.; Kapur, N.K.; Kataria, R.; Roswell, R.O. 2025 Concise Clinical Guidance: An ACC Expert Consensus Statement on the Evaluation and Management of Cardiogenic Shock: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2025, 85, 1618–1641. [Google Scholar] [CrossRef]
- Pankaj, J. Acute Haemodynamic Effects of Venoarterial Extracorporeal Membrane Oxygenation on Left Ventricular Mechano-energetics: The Ramp and Clamp Study. Interv. Cardiol. Rev. 2021, 16 (Suppl. S2), 1–22. [Google Scholar] [CrossRef]
- Brewer, J.M.; Capoccia, M.; Maybauer, D.M.; Lorusso, R.; Swol, J.; Maybauer, M.O. The ProtekDuo dual-lumen cannula for temporary acute mechanical circulatory support in right heart failure: A systematic review. Perfusion 2023, 38 (Suppl. S1), 59–67. [Google Scholar] [CrossRef] [PubMed]
- Maybauer, M.O.; Capoccia, M.; Maybauer, D.M.; Lorusso, R.; Swol, J.; Brewer, J.M. The ProtekDuo in ECMO configuration for ARDS secondary to COVID-19: A systematic review. Int. J. Artif. Organs 2023, 46, 93–98. [Google Scholar] [CrossRef]
- Capoccia, M.; Brewer, J.M.; Rackauskas, M.; Becker, T.K.; Maybauer, D.M.; Stukov, Y.; Lorusso, R.; Maybauer, M.O. Outcome of Veno-Pulmonary Extracorporeal Life Support in Lung Transplantation Using ProtekDuo Cannula: A Systematic Review and Description of Configurations. J. Clin. Med. 2024, 13, 4111. [Google Scholar] [CrossRef]
- Sauren, L.D.; Reesink, K.D.; Selder, J.L.; Beghi, C.; Van Der Veen, F.H.; Maessen, J.G. The Acute Effect of Intra-aortic Balloon Counterpulsation during Extracorporeal Life Support: An Experimental Study. Artif. Organs 2007, 31, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Baumann, S.L. The Limitations of Evidence-Based Practice. Nurs. Sci. Q. 2010, 23, 226–230. [Google Scholar] [CrossRef]
- Doshi, D.; Burkhoff, D. Cardiovascular Simulation of Heart Failure Pathophysiology and Therapeutics. J. Card. Fail. 2016, 22, 303–311. [Google Scholar] [CrossRef]
- Saltarocchi, S. Simulation in Advanced Heart Failure with a View to Selection and Optimisation of Device Therapy. Int. J. Eng. Sci. Res. Tech. Thesis 2018. [Google Scholar] [CrossRef]
- De Lazzari, C.; Neglia, D.; Ferrari, G.; Bernini, F.; Micalizzi, M.; L’Abbate, A.; Trivella, M.G. Computer Simulation of Coronary Flow Waveforms during Caval Occlusion. Methods Inf. Med. 2009, 48, 113–122. [Google Scholar]
- De Lazzari, C.; Darowski, M.; Wolski, P.; Ferrari, G.; Tosti, G.; Pisanelli, D.M. In Vivo and Simulation Study of Artificial Ventilation Effects on Energetic Variables in Cardiosurgical Patients. Methods Inf. Med. 2005, 44, 98–105. [Google Scholar]
- Hester, R.L.; Iliescu, R.; Summers, R.; Coleman, T.G. Systems biology and integrative physiological modelling. J. Physiol. 2011, 589, 1053–1060. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Lazzari, B.; Capoccia, M.; Badagliacca, R.; Maybauer, M.O.; De Lazzari, C. Modelling and Simulation of the Interactions Between the Cardiovascular System and the Combined Use of VA ECMO and IABP: Comparison Between Peripheral and Central Configurations. Bioengineering 2025, 12, 540. https://doi.org/10.3390/bioengineering12050540
De Lazzari B, Capoccia M, Badagliacca R, Maybauer MO, De Lazzari C. Modelling and Simulation of the Interactions Between the Cardiovascular System and the Combined Use of VA ECMO and IABP: Comparison Between Peripheral and Central Configurations. Bioengineering. 2025; 12(5):540. https://doi.org/10.3390/bioengineering12050540
Chicago/Turabian StyleDe Lazzari, Beatrice, Massimo Capoccia, Roberto Badagliacca, Marc O. Maybauer, and Claudio De Lazzari. 2025. "Modelling and Simulation of the Interactions Between the Cardiovascular System and the Combined Use of VA ECMO and IABP: Comparison Between Peripheral and Central Configurations" Bioengineering 12, no. 5: 540. https://doi.org/10.3390/bioengineering12050540
APA StyleDe Lazzari, B., Capoccia, M., Badagliacca, R., Maybauer, M. O., & De Lazzari, C. (2025). Modelling and Simulation of the Interactions Between the Cardiovascular System and the Combined Use of VA ECMO and IABP: Comparison Between Peripheral and Central Configurations. Bioengineering, 12(5), 540. https://doi.org/10.3390/bioengineering12050540





