A Review on Bioengineering the Bovine Mammary Gland: The Role of the Extracellular Matrix and Reconstruction Prospects
Abstract
1. Introduction
2. Macro and Microscopic Characterization of the Bovine Mammary Gland
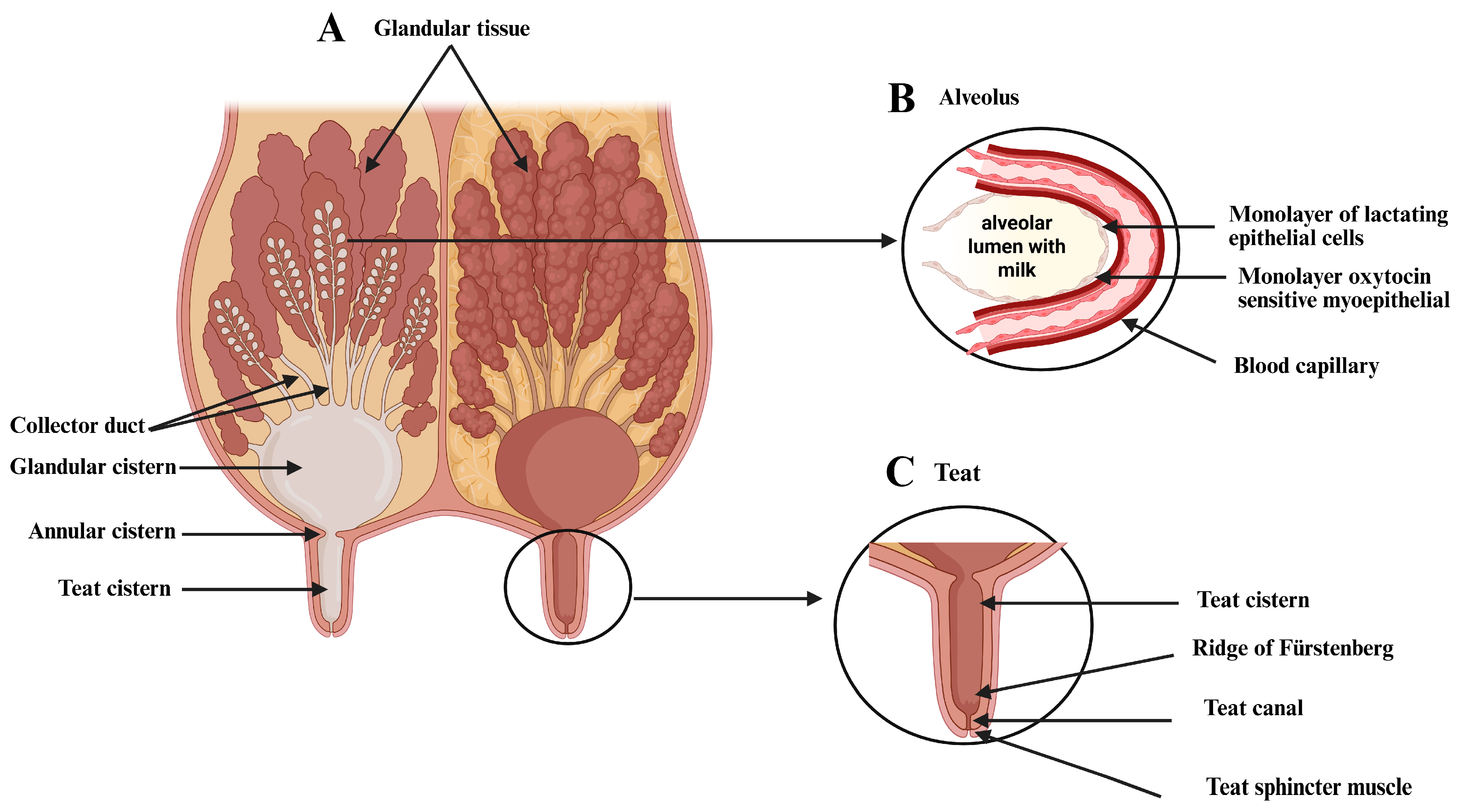
2.1. The Anatomy of the Bovine Mammary Gland
2.2. Histological Characterization
2.3. The Extracellular Matrix of the Mammary Gland
2.4. Comparative Analysis of the Extracellular Matrix of the Mammary Gland Between Cattle and Small Domestic Ruminants
2.5. The Role of the Extracellular Matrix in the Immune Response During Mastitis
2.6. Therapeutic Strategies for Bovine Mastitis
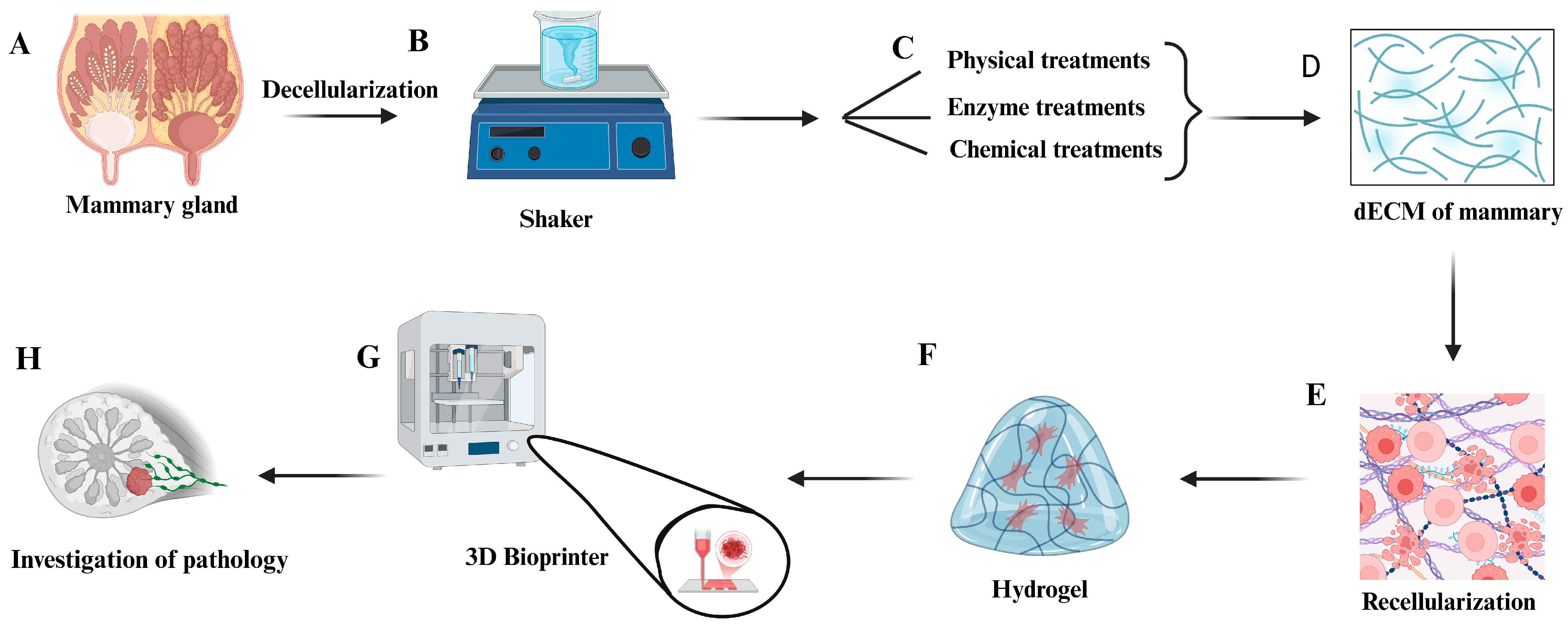
3. Decellularization Method for Obtaining Extracellular Matrix
3.1. Analysis of Decellularization Efficiency
3.2. Ideal Properties of ECM
4. Recellularization Method
4.1. Cell Types Used for the Recellularization of Parenchymal Organs
4.2. Support Cells
5. Decellularization and Recellularization of the Mammary Gland
3D Printing Is Used for the Recellularization Process
6. Limitations of Extracellular Matrix Recellularization
7. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional | TN | Tenascin |
| CHAPS | 3Cholamidopropyl dimethylammonio-1propanesulfonate | DAPI | 4,6-diamidino-2-phenylindole |
| CS | Chondroitin sulfate | FGFR2 | Fibroblast growth factor receptor-2 |
| dECM | Decellularized extracellular matrix | IL-6 | Interleukin-6 |
| DS | Dermatan sulfate | H&E | Hematoxylin and eosin |
| DAMPs | Damage-associated molecular patterns | pH | Hydrogen ion potential |
| ECM | Extracellular matrix | DNA | Deoxyribonucleic acid |
| EDTA | Ethylenediaminetetraacetic acid | SGBTR | Scaffold-guided breast tissue regeneration |
| EGTA | Ethylene glycol-bis(β)-aminoethyl ether | iPSCs | Induced pluripotent stem cells |
| FN | Fibronectin | VEGF | Vascular endothelial growth factor |
| FnBPs | Fibronectin-binding proteins | pO2 | Partial pressure of oxygen |
| GAGs | Glycosaminoglycans | TGF-β | Transforming growth factor-beta |
| LN | Laminin | MSCs | Mesenchymal stem cells |
| LN-111 | Laminin-111 | EGFR | Epidermal growth factor receptor |
| LN-332 | Laminin-332 | pCO2 | Partial pressure of carbon dioxide |
| MMPs | Metalloproteinases | LOX | Lysyl oxidases |
| MW | Molecular weight | ESCs | Embryonic stem cells |
| SDS | Sodium dodecyl sulfate |
References
- Jaswal, S.; Jena, M.K.; Anand, V.; Jaswal, A.; Kancharla, S.; Kolli, P.; Mandadapu, G.; Kumar, S.; Mohanty, A.K. Critical Review on Physiological and Molecular Features during Bovine Mammary Gland Development: Recent Advances. Cells 2022, 11, 3325. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Jeong, D.K. Stem Cell Research: A Novel Boulevard towards Improved Bovine Mastitis Management. Int. J. Biol. Sci. 2013, 9, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Wang, J.; Wang, Y.; Wang, C.; Liu, X.; Han, Z.; Fu, Y.; Yang, Z. Effects of Neutrophil Extracellular Traps on Bovine Mammary Epithelial Cells in Vitro. Front. Immunol. 2019, 10, 1003. [Google Scholar] [CrossRef]
- Rifatbegović, M.; Nicholas, R.A.J.; Mutevelić, T.; Hadžiomerović, M.; Maksimović, Z. Pathogens Associated with Bovine Mastitis: The Experience of Bosnia and Herzegovina. Vet. Sci. 2024, 11, 63. [Google Scholar] [CrossRef]
- Barbacena Rosa dos Santos, W.; Coelho de Oliveira, N.; de Lima Vieira, M.; Corrêa Ribeiro, J.; Santos Cezário, A.; Maria Bianchini Oliveira, E.; Sousa Camargos, A.; Neves Pereira Valente, T. Mastite Bovina: Uma Revisão. Colloq. Agrar. 2017, 13, 301–314. [Google Scholar] [CrossRef]
- Cobirka, M.; Tancin, V.; Slama, P. Epidemiology and Classification of Mastitis. Animals 2020, 10, 2212. [Google Scholar] [CrossRef]
- Gelgie, A.E.; Korsa, M.G.; Kerro Dego, O. Mycoplasma Bovis Mastitis. Curr. Res. Microb. Sci. 2022, 3, 100123. [Google Scholar] [CrossRef]
- Mehmood, S.; Ashraf, M.; Khan, M.T.S. Antimicrobial Resistance and Virulence Determinants of E. Coli in Bovine Clinical Mastitis in Dairy Farms. Cont. Vet. J. 2023, 3, 54–59. [Google Scholar]
- Suzuki, K.; Kaneko, F.; Matsushita, A.; Hata, E. Outbreaks of Bovine Mastitis Caused by Specific Mycoplasma Bovis Strains Recurring at Multi-Year Intervals. J. Vet. Diagn. Investig. 2024, 36, 457–462. [Google Scholar] [CrossRef]
- Rai, A.K.; Nayak, A.; Jogi, J.; Gupta, V.; Singh, R.V. Prevalence of Clinical and Subclinical Mastitis in Dairy Cows and Buffaloes of Jabalpur District of Madhya Pradesh. Pharma Innov. J. 2022, 11, 4771–4773. [Google Scholar]
- Hiitiö, H.; Vakkamäki, J.; Simojoki, H.; Autio, T.; Junnila, J.; Pelkonen, S.; Pyörälä, S. Prevalence of Subclinical Mastitis in Finnish Dairy Cows: Changes during Recent Decades and Impact of Cow and Herd Factors. Acta Vet. Scand. 2017, 59, 22. [Google Scholar] [CrossRef] [PubMed]
- McDougall, S.; Castle, R. Cow-Level Risk Factors for Clinical Mastitis in the Dry Period in Cows Treated with an Internal Teat Sealant Alone at the End of Lactation. N. Z. Vet. J. 2021, 69, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, M.E.; Ledesma, M.M.; Lombarte Serrat, A.; Vay, C.; Sordelli, D.O.; Giacomodonato, M.N.; Buzzola, F.R. Growth Conditions Affect Biofilms of Staphylococcus Aureus Producing Mastitis: Contribution of MALDI-TOF-MS to Strain Characterization. Curr. Res. Microb. Sci. 2021, 2, 100073. [Google Scholar] [CrossRef]
- Da Fonseca, M.E. Mastite Bovina: Revisão. Pubvet 2021, 15, 162. [Google Scholar] [CrossRef]
- Morales-Ubaldo, A.L.; Rivero-Perez, N.; Valladares-Carranza, B.; Velázquez-Ordoñez, V.; Delgadillo-Ruiz, L.; Zaragoza-Bastida, A. Bovine Mastitis, a Worldwide Impact Disease: Prevalence, Antimicrobial Resistance, and Viable Alternative Approaches. Vet. Anim. Sci. 2023, 21, 100306. [Google Scholar] [CrossRef]
- Anwar, M.A.; Aziz, S.; Ashfaq, K.; Aqib, A.I.; Shoaib, M.; Naseer, M.A.; Alvi, M.A.; Muzammil, I.; Bhutta, Z.A.; Sattar, H.; et al. Trends in Frequency, Potential Risks and Antibiogram of E. Coli Isolated from Semi-Intensive Dairy Systems. Pak. Vet. J. 2022, 42, 167–172. [Google Scholar]
- Acosta, A.C.; da Silva, L.B.G.; Medeiros, E.S.; Pinheiro-Júnior, J.W.; Mota, R.A. Mastitis in Ruminants in Brazil. Pesqui. Vet. Bras. 2016, 36, 565–573. [Google Scholar] [CrossRef]
- Khasapane, N.G.; Byaruhanga, C.; Thekisoe, O.; Nkhebenyane, S.J.; Khumalo, Z.T.H. Prevalence of Subclinical Mastitis, Its Associated Bacterial Isolates and Risk Factors among Cattle in Africa: A Systematic Review and Meta-Analysis. BMC Vet. Res. 2023, 19, 123. [Google Scholar] [CrossRef]
- Finot, L.; Chanat, E.; Dessauge, F. Mammary Gland 3D Cell Culture Systems in Farm Animals. Vet. Res. 2021, 52, 78. [Google Scholar] [CrossRef]
- Conci, C.; Bennati, L.; Bregoli, C.; Buccino, F.; Danielli, F.; Gallan, M.; Gjini, E.; Raimondi, M.T. Tissue Engineering and Regenerative Medicine Strategies for the Female Breast. J. Tissue Eng. Regen. Med. 2020, 14, 369–387. [Google Scholar] [CrossRef]
- Balakrishnan-nair, D.K.; Nair, N.D.; Venugopal, S.K.; Das, V.N.; George, S.; Abraham, M.J.; Eassow, S.; Alison, M.R.; Sainulabdeen, A.; Anilkumar, T.V. An Immunopathological Evaluation of the Porcine Cholecyst Matrix as a Muscle Repair Graft in a Male Rat Abdominal Wall Defect Model. Toxicol. Pathol. 2018, 46, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, M.d.C. Descelularização Pancreática Visando à Recelularização Como Alternativa Terapêutica Para o Diabetes Mellitus Tipo I. Ph.D. Thesis, University of São Paulo, São Paulo, Brazil, 2019; p. 182. [Google Scholar]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The Extracellular Matrix at a Glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Mandal, S.S.; Bauri, S.; Maiti, P. 3D Bioprinting and Its Innovative Approach for Biomedical Applications. MedComm 2022, 4, e194. [Google Scholar] [CrossRef]
- FAO Mozambique. FAO Emergency and Resilience; Food and Agriculture Organization of the United Nations: Rome, Italy, 2025. [Google Scholar]
- Gil, M.; Rudy, M.; Duma-Kocan, P.; Stanisławczyk, R.; Krajewska, A.; Dziki, D.; Hassoon, W.H. Sustainability of Alternatives to Animal Protein Sources, a Comprehensive Review. Sustainability 2024, 16, 7701. [Google Scholar] [CrossRef]
- Cruz, S.O.; Júnior, V.R.; Correia, B.R.; Barreto, L.M.G. Saúde Da Glândula Mamária de Vacas Em Lactação: Revisão de Literatura. Ens. E Ciência Ciências Biológicas Agrárias E Da Saúde 2022, 26, 262–269. [Google Scholar] [CrossRef]
- Hovey, R.C.; Trott, J.F.; Vonderhaar, B.K. Establishing a Framework for the Functional Mammary Gland: From Endocrinology to Morphology. J. Mammary Gland Biol. Neoplasia 2002, 7, 17–38. [Google Scholar] [CrossRef]
- Barreto, R.S.N.; Carvalho, H.J.C.; Matias, G.S.S.; Silva, M.G.K.C.; Ribeiro, R.R.; Campanelli, T.B.; Rigoglio, N.N.; Carreira, A.C.O.; Miglino, M.A. The Extracellular Matrix Protein Pattern in the Canine Neoplastic Mammary Gland. Tissue Cell 2023, 82, 102050. [Google Scholar] [CrossRef]
- Ribeiro, E.A.; Araujo, R.S.; Nunes, G.O.; de Ávila Filho, S.H.; Borges, P.A.C. Uso da Ultrassonografia na Glândula Mamária de Ruminantes. Enciclopédia Biosf. 2021, 18, 530–543. [Google Scholar] [CrossRef]
- Klein, B.G. Cunningham Tratado de Fisiologia Veterinária, 6th ed.; GEN Guanabara Koogan: Rio de Janeiro, Brazil, 2021. [Google Scholar]
- Sumbal, J.; Budkova, Z.; Traustadóttir, G.Á.; Koledova, Z. Mammary Organoids and 3D Cell Cultures: Old Dogs with New Tricks. J. Mammary Gland Biol. Neoplasia 2020, 25, 273–288. [Google Scholar] [CrossRef]
- Araujo, G.D.; de Souza, K.B.; de Oliveira, L.A.d.S.; Ribeiro, R.P. Aspectos Morfológicos e Fisiológicos de Glândulas Mamárias de Fêmeas Bovinas—Revisão de Literatura. Pubvet 2015, 6, 36. [Google Scholar] [CrossRef]
- Gopalakrishnan, K.; Teitelbaum, S.L.; Wetmur, J.; Manservisi, F.; Falcioni, L.; Panzacchi, S.; Gnudi, F.; Belpoggi, F.; Chen, J. Histology and Transcriptome Profiles of the Mammary Gland across Critical Windows of Development in Sprague Dawley Rats. J. Mammary Gland Biol. Neoplasia 2018, 23, 149–163. [Google Scholar] [CrossRef]
- Janjanam, J.; Singh, S.; Jena, M.K.; Varshney, N.; Kola, S.; Kumar, S.; Kaushik, J.K.; Grover, S.; Dang, A.K.; Mukesh, M.; et al. Comparative 2D-DIGE Proteomic Analysis of Bovine Mammary Epithelial Cells during Lactation Reveals Protein Signatures for Lactation Persistency and Milk Yield. PLoS ONE 2014, 9, e102515. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, R.; Han, C.; Huang, L. Extracellular Matrix Grafts: From Preparation to Application (Review). Int. J. Mol. Med. 2021, 47, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Karousou, E.; D’Angelo, M.L.; Kouvidi, K.; Vigetti, D.; Viola, M.; Nikitovic, D.; De Luca, G.; Passi, A. Collagen VI and Hyaluronan: The Common Role in Breast Cancer. BioMed Res. Int. 2014, 2014, 606458. [Google Scholar] [CrossRef] [PubMed]
- Varallo, G.R.; Jardim-Perassi, B.V.; Alexandre, P.A.; Fukumasu, H.; Zuccari, D.A.P.C. Global Gene Expression Profile in Canine Mammary Carcinomas. Vet. J. 2019, 254, 105393. [Google Scholar] [CrossRef]
- Stoian, A.; Adil, A.; Biniazan, F.; Haykal, S. Two Decades of Advances and Limitations in Organ Recellularization. Curr. Issues Mol. Biol. 2024, 46, 9179–9214. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Liu, G.; Li, S.; Yuan, H.; Yun, Z.; Zhang, W.; Zhang, S.; Dai, Y.; Ma, Y. Decellularized Breast Matrix as Bioactive Microenvironment for in Vitro Three-Dimensional Cancer Culture. J. Cell. Physiol. 2019, 234, 3425–3435. [Google Scholar] [CrossRef]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of Tissues and Organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef]
- Llacua, L.A.; Faas, M.M.; de Vos, P. Extracellular Matrix Molecules and Their Potential Contribution to the Function of Transplanted Pancreatic Islets. Diabetologia 2018, 61, 1261–1272. [Google Scholar] [CrossRef]
- Tamayo-Angorrilla, M.; López de Andrés, J.; Jiménez, G.; Marchal, J.A. The Biomimetic Extracellular Matrix: A Therapeutic Tool for Breast Cancer Research. Transl. Res. 2022, 247, 117–136. [Google Scholar] [CrossRef]
- Maller, O.; Martinson, H.; Schedin, P. Extracellular Matrix Composition Reveals Complex and Dynamic Stromal-Epithelial Interactions in the Mammary Gland. J. Mammary Gland Biol. Neoplasia 2010, 15, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Biniazan, F.; Stoian, A.; Haykal, S. Adipose-Derived Stem Cells: Angiogenetic Potential and Utility in Tissue Engineering. Int. J. Mol. Sci. 2024, 25, 2356. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular Matrix Structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Marettová, E.; Maretta, M. The Distribution of Collagen and Elastic Fibres in the Lactating Bovine Mammary Gland. Folia Vet. 2019, 63, 60–65. [Google Scholar] [CrossRef]
- Park, J.; Schwarzbauer, J.E. Mammary Epithelial Cell Interactions with Fibronectin Stimulate Epithelial-Mesenchymal Transition. Oncogene 2014, 33, 1649–1657. [Google Scholar] [CrossRef]
- Katsumi, A.; Orr, A.W.; Tzima, E.; Schwartz, M.A. Integrins in Mechanotransduction. J. Biol. Chem. 2004, 279, 12001–12004. [Google Scholar] [CrossRef]
- Aumailley, M.; Bruckner-Tuderman, L.; Carter, W.G.; Deutzmann, R.; Edgar, D.; Ekblom, P.; Engel, J.; Engvall, E.; Hohenester, E.; Jones, J.C.R.; et al. A Simplified Laminin Nomenclature. Matrix Biol. 2005, 24, 326–332. [Google Scholar] [CrossRef]
- O’Brien, J.; Lyons, T.; Monks, J.; Lucia, M.S.; Wilson, R.S.; Hines, L.; Man, Y.G.; Borges, V.; Schedin, P. Alternatively Activated Macrophages and Collagen Remodeling Characterize the Postpartum Involuting Mammary Gland across Species. Am. J. Pathol. 2010, 176, 1241–1255. [Google Scholar] [CrossRef]
- Oldberg, A.; Antonsson, P.; Hedbom, E.; Heinegard, D. Structure and Function of Extracellular Matrix Proteoglycans. Biochem. Soc. Trans. 1990, 18, 789–792. [Google Scholar] [CrossRef]
- Somuncu, Ö.S. Decellularization Concept in Regenerative Medicine. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2019; Volume 1212, pp. 71–85. ISBN 9783030328221. [Google Scholar]
- Biswas, S.K.; Banerjee, S.; Baker, G.W.; Kuo, C.Y.; Chowdhury, I. The Mammary Gland: Basic Structure and Molecular Signaling during Development. Int. J. Mol. Sci. 2022, 23, 3883. [Google Scholar] [CrossRef]
- Lérias, J.R.; Hernández-Castellano, L.E.; Suárez-Trujillo, A.; Castro, N.; Pourlis, A.; Almeida, A.M. The Mammary Gland in Small Ruminants: Major Morphological and Functional Events Underlying Milk Production—A Review. J. Dairy Res. 2014, 81, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Paramasivan, S.; Ramesh, G.; Ushakumary, S.; Sivagnanam, S. Micromorphology of Myoepithelial Cells in the Mammary Glands of Madras Red Sheep During Various Physiological Status. Shanlax Int. J. Vet. Sci. 2014, 1. [Google Scholar]
- Senthilkumar, S.; Kannan, T.A.; Ramesh, G.; Sumanthi, D. Histoarchitectural Changes in the Stromal Tissue of Udder of Small Ruminants. SSRN Electron. J. 2020, 31, 164–165. [Google Scholar] [CrossRef]
- Mahdi, A.; Atyia, A. Anatomical, Histological and Radiological Study of the Mammary Gland of Small Ruminants. Basrah J. Vet. Res. 2009, 8, 10–22. [Google Scholar] [CrossRef]
- Berry, S.D.K.; Howard, R.D.; Akers, R.M. Mammary Localization and Abundance of Laminin, Fibronectin, and Collagen IV Proteins in Prepubertal Heifers. J. Dairy Sci. 2003, 86, 2864–2874. [Google Scholar] [CrossRef]
- Kumar, V. Age Related Histomorphological and Histochemical Studies on Mammary Gland of Goat. Master’s Thesis, Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana, India, 2022. [Google Scholar]
- Lepucki, A.; Orlińska, K.; Mielczarek-Palacz, A.; Kabut, J.; Olczyk, P.; Komosińska-Vassev, K. The Role of Extracellular Matrix Proteins in Breast Cancer. J. Clin. Med. 2022, 11, 1250. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Xiong, G.; Trinkle, C.; Xu, R. Integrated Extracellular Matrix Signaling in Mammary Gland Development and Breast Cancer Progression. Histol. Histopathol. 2014, 29, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K.; Dandara, C. The Extracellular Matrix: Its Composition, Function, Remodeling, and Role in Tumorigenesis. Biomimetics 2023, 8, 146. [Google Scholar] [CrossRef]
- Li, H.; Zheng, H.; Li, L.; Shen, X.; Zang, W.; Sun, Y. The Effects of Matrix Metalloproteinase-9 on Dairy Goat Mastitis and Cell Survival of Goat Mammary Epithelial Cells. PLoS ONE 2016, 11, e0160989. [Google Scholar] [CrossRef]
- Yang, B. Lipoteichoic Acid Disrupts Mammary Epithelial Barrier Integrity by Altering Expression of Occludin and Zonula Occluden (ZO)-1. Kafkas Univ. Vet. Fak. Derg. 2023, 29, 423–428. [Google Scholar] [CrossRef]
- Yang, J.; Bahcecioglu, G.; Zorlutuna, P. The Extracellular Matrix and Vesicles Modulate the Breast Tumor Microenvironment. Bioengineering 2020, 7, 124. [Google Scholar] [CrossRef]
- Cheng, J.; Zhou, M.; Nobrega, D.B.; Barkema, H.W.; Xu, S.; Li, M.; Kastelic, J.P.; Shi, Y.; Han, B.; Gao, J. Genetic Diversity and Molecular Epidemiology of Outbreaks of Klebsiella Pneumoniae Mastitis on Two Large Chinese Dairy Farms. J. Dairy Sci. 2021, 104, 762–775. [Google Scholar] [CrossRef]
- Abegewi, U.A.; Esemu, S.N.; Ndip, R.N.; Ndip, L.M. Prevalence and Risk Factors of Coliform-Associated Mastitis and Antibiotic Resistance of Coliforms from Lactating Dairy Cows in North West Cameroon. PLoS ONE 2022, 17, e0268247. [Google Scholar] [CrossRef]
- Han, G.; Zhang, B.; Luo, Z.; Lu, B.; Luo, Z.; Zhang, J.; Wang, Y.; Luo, Y.; Yang, Z.; Shen, L.; et al. Molecular Typing and Prevalence of Antibiotic Resistance and Virulence Genes in Streptococcus Agalactiae Isolated from Chinese Dairy Cows with Clinical Mastitis. PLoS ONE 2022, 17, e0268262. [Google Scholar] [CrossRef] [PubMed]
- Kovačević, Z.; Kladar, N.; Čabarkapa, I.; Radinović, M.; Maletić, M.; Erdeljan, M.; Božin, B. New Perspective of Origanum Vulgare, L. and Satureja Montana, L. Essential Oils as Bovine Mastitis Treatment Alternatives. Antibiotics 2021, 10, 1460. [Google Scholar] [CrossRef]
- Saeed, S.I.; Vivian, L.; Zalati, C.W.S.C.W.; Sani, N.I.M.; Aklilu, E.; Mohamad, M.; Noor, A.A.M.; Muthoosamy, K.; Kamaruzzaman, N.F. Antimicrobial Activities of Graphene Oxide against Biofilm and Intracellular Staphylococcus Aureus Isolated from Bovine Mastitis. BMC Vet. Res. 2023, 19, 10. [Google Scholar] [CrossRef]
- Kour, S.; Sharma, N.; Balaji, N.; Kumar, P.; Soodan, J.S.; dos Santos, M.V.; Son, Y.O. Advances in Diagnostic Approaches and Therapeutic Management in Bovine Mastitis. Vet. Sci. 2023, 10, 449. [Google Scholar] [CrossRef] [PubMed]
- McInnes, A.D.; Moser, M.A.J.; Chen, X. Preparation and Use of Decellularized Extracellular Matrix for Tissue Engineering. J. Funct. Biomater. 2022, 13, 240. [Google Scholar] [CrossRef] [PubMed]
- Kasturi, M.; Vasanthan, K.S. Effect of Decellularization Using Sodium Dodecyl Sulfate on Glycosaminoglycans Content in the Liver. Regen. Med. 2023, 18, 527–530. [Google Scholar] [CrossRef]
- Scarritt, M.E.; Pashos, N.C.; Bunnell, B.A. A Review of Cellularization Strategies for Tissue Engineering of Whole Organs. Front. Bioeng. Biotechnol. 2015, 3, 43. [Google Scholar] [CrossRef]
- Mendibil, U.; Ruiz-Hernandez, R.; Retegi-Carrion, S.; Garcia-Urquia, N.; Olalde-Graells, B.; Abarrategi, A. Tissue-Specific Decellularization Methods: Rationale and Strategies to Achieve Regenerative Compounds. Int. J. Mol. Sci. 2020, 21, 5447. [Google Scholar] [CrossRef] [PubMed]
- Neishabouri, A.; Soltani Khaboushan, A.; Daghigh, F.; Kajbafzadeh, A.M.; Majidi Zolbin, M. Decellularization in Tissue Engineering and Regenerative Medicine: Evaluation, Modification, and Application Methods. Front. Bioeng. Biotechnol. 2022, 10, 805299. [Google Scholar] [CrossRef] [PubMed]
- Mota, C.; Camarero-Espinosa, S.; Baker, M.B.; Wieringa, P.; Moroni, L. Bioprinting: From Tissue and Organ Development to in Vitro Models. Chem. Rev. 2020, 120, 11032. [Google Scholar] [CrossRef]
- Aeberhard, P.A.; Grognuz, A.; Peneveyre, C.; McCallin, S.; Hirt-Burri, N.; Antons, J.; Pioletti, D.; Raffoul, W.; Applegate, L.A. Efficient Decellularization of Equine Tendon with Preserved Biomechanical Properties and Cytocompatibility for Human Tendon Surgery Indications. Artif. Organs 2020, 44, E161–E171. [Google Scholar] [CrossRef]
- Burk, J.; Erbe, I.; Berner, D.; Kacza, J.; Kasper, C.; Pfeiffer, B.; Winter, K.; Brehm, W. Freeze-Thaw Cycles Enhance Decellularization of Large Tendons. Tissue Eng.-Part C Methods 2014, 20, 276–284. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Hong, H.; Hu, R.; Liu, J.; Liu, C. Decellularized Extracellular Matrix Scaffolds: Recent Trends and Emerging Strategies in Tissue Engineering. Bioact. Mater. 2022, 10, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Hussein, K.H.; Ahmadzada, B.; Correa, J.C.; Sultan, A.; Wilken, S.; Amiot, B.; Nyberg, S.L. Liver Tissue Engineering Using Decellularized Scaffolds: Current Progress, Challenges, and Opportunities. Bioact. Mater. 2024, 40, 280–305. [Google Scholar] [CrossRef]
- Brouki Milan, P.; Masoumi, F.; Biazar, E.; Zare Jalise, S.; Mehrabi, A. Exploiting the Potential of Decellularized Extracellular Matrix (ECM) in Tissue Engineering: A Review Study. Macromol. Biosci. 2024, 25, e2400322. [Google Scholar] [CrossRef]
- Allu, I.; Sahi, A.K.; Koppadi, M.; Gundu, S.; Sionkowska, A. Decellularization Techniques for Tissue Engineering: Towards Replicating Native Extracellular Matrix Architecture in Liver Regeneration. J. Funct. Biomater. 2023, 14, 518. [Google Scholar] [CrossRef]
- White, L.J.; Taylor, A.J.; Faulk, D.M.; Keane, T.J.; Saldin, L.T.; Reing, J.E.; Swinehart, I.T.; Turner, N.J.; Ratner, B.D.; Badylak, S.F. The Impact of Detergents on the Tissue Decellularization Process: A ToF-SIMS Study. Acta Biomater. 2016, 50, 207. [Google Scholar] [CrossRef]
- Elder, B.D.; Eleswarapu, S.V.; Athanasiou, K.A. Extraction Techniques for the Decellularization of Tissue Engineered Articular Cartilage Constructs. Biomaterials 2009, 30, 3749. [Google Scholar] [CrossRef]
- Jalili, A.; Shojaei-Ghahrizjani, F.; Tabatabaiefar, M.A.; Rahmati, S. Decellularized Skin Pretreatment by Monophosphoryl Lipid A and Lactobacillus Casei Supernatant Accelerate Skin Recellularization. Mol. Biol. Rep. 2024, 51, 675. [Google Scholar] [CrossRef] [PubMed]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An Overview of Tissue and Whole Organ Decellularization Processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef]
- Hudson, T.W.; Zawko, S.; Deister, C.; Lundy, S.; Hu, C.Y.; Lee, K.; Schmidt, C.E. Optimized Acellular Nerve Graft Is Immunologically Tolerated and Supports Regeneration. Tissue Eng. 2004, 10, 1641–1651. [Google Scholar] [CrossRef] [PubMed]
- Funamoto, S.; Nam, K.; Kimura, T.; Murakoshi, A.; Hashimoto, Y.; Niwaya, K.; Kitamura, S.; Fujisato, T.; Kishida, A. The Use of High-Hydrostatic Pressure Treatment to Decellularize Blood Vessels. Biomaterials 2010, 31, 3590–3595. [Google Scholar] [CrossRef]
- Morawski, M.; Krasnodębski, M.; Rochoń, J.; Kubiszewski, H.; Marzęcki, M.; Topyła, D.; Murat, K.; Staszewski, M.; Szczytko, J.; Maleszewski, M.; et al. Decellularized Liver Matrices for Expanding the Donor Pool—An Evaluation of Existing Protocols and Future Trends. Biomolecules 2025, 15, 98. [Google Scholar] [CrossRef] [PubMed]
- Eldeeb, A.E.; Salah, S.; Elkasabgy, N.A. Biomaterials for Tissue Engineering Applications and Current Updates in the Field: A Comprehensive Review. AAPS PharmSciTech 2022, 23, 267. [Google Scholar] [CrossRef]
- Brown, M.; Li, J.; Moraes, C.; Tabrizian, M.; Li-Jessen, N.Y.K. Decellularized Extracellular Matrix: New Promising and Challenging Biomaterials for Regenerative Medicine. Biomaterials 2022, 289, 121786. [Google Scholar] [CrossRef]
- Golebiowska, A.A.; Intravaia, J.T.; Sathe, V.M.; Kumbar, S.G.; Nukavarapu, S.P. Decellularized Extracellular Matrix Biomaterials for Regenerative Therapies: Advances, Challenges and Clinical Prospects. Bioact. Mater. 2024, 32, 98–123. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, B. Biodegradation of Silk Biomaterials. Int. J. Mol. Sci. 2009, 10, 1514–1524. [Google Scholar] [CrossRef]
- Noro, J.; Vilaça-Faria, H.; Reis, R.L.; Pirraco, R.P. Extracellular Matrix-Derived Materials for Tissue Engineering and Regenerative Medicine: A Journey from Isolation to Characterization and Application. Bioact. Mater. 2024, 34, 494–519. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the Extracellular Matrix in Development and Disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Yue, B. Biology of the Extracellular Matrix: An Overview. J. Glaucoma 2014, 23, S20–S23. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Xing, Y.; Li, J.; Deng, C.; Li, Y.; Ren, X.; Zhang, D. Rebuilding the Vascular Network: In Vivo and in Vitro Approaches. Front. Cell Dev. Biol. 2021, 9, 639299. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; Saleh, T.; Xu, M. Recellularization of Native Tissue Derived Acellular Scaffolds with Mesenchymal Stem Cells. Cells 2021, 10, 1787. [Google Scholar] [CrossRef]
- Robertson, M.J.; Soibam, B.; O’Leary, J.G.; Sampaio, L.C.; Taylor, D.A. Recellularization of Rat Liver: An in Vitro Model for Assessing Human Drug Metabolism and Liver Biology. PLoS ONE 2018, 13, e0191892. [Google Scholar] [CrossRef]
- Hillebrandt, K.H.; Everwien, H.; Haep, N.; Keshi, E.; Pratschke, J.; Sauer, I.M. Strategies Based on Organ Decellularization and Recellularization. Transpl. Int. 2019, 32, 571–585. [Google Scholar] [CrossRef]
- Garreta, E.; Oria, R.; Tarantino, C.; Pla-Roca, M.; Prado, P.; Fernández-Avilés, F.; Campistol, J.M.; Samitier, J.; Montserrat, N. Tissue Engineering by Decellularization and 3D Bioprinting. Mater. Today 2017, 20, 166–178. [Google Scholar] [CrossRef]
- Badylak, S.F.; Taylor, D.; Uygun, K. Whole-Organ Tissue Engineering: Decellularization and Recellularization of Three-Dimensional Matrix Scaffolds. Annu. Rev. Biomed. Eng. 2011, 13, 27–53. [Google Scholar] [CrossRef]
- Rozen, W.M.; Pesce, M.; Uygun, B.E.; Plock, J.A.; Haykal, S.; Adil, A.; Xu, M. Recellularization of Bioengineered Scaffolds for Vascular Composite Allotransplantation. Front. Surg. 2022, 9, 843677. [Google Scholar] [CrossRef]
- Inman, J.L.; Robertson, C.; Mott, J.D.; Bissell, M.J. Mammary Gland Development: Cell Fate Specification, Stem Cells and the Microenvironment. Development 2015, 142, 1028–1042. [Google Scholar] [CrossRef] [PubMed]
- Bruno, R.D.; Fleming, J.M.; George, A.L.; Boulanger, C.A.; Schedin, P.; Smith, G.H. Mammary Extracellular Matrix Directs Differentiation of Testicular and Embryonic Stem Cells to Form Functional Mammary Glands in Vivo. Sci. Rep. 2017, 7, 40196. [Google Scholar] [CrossRef]
- Ogiso, S.; Yasuchika, K.; Fukumitsu, K.; Ishii, T.; Kojima, H.; Miyauchi, Y.; Yamaoka, R.; Komori, J.; Katayama, H.; Kawai, T.; et al. Efficient Recellularisation of Decellularised Whole-Liver Grafts Using Biliary Tree and Foetal Hepatocytes. Sci. Rep. 2016, 6, 35887. [Google Scholar] [CrossRef]
- Beane, O.S.; Fonseca, V.C.; Cooper, L.L.; Koren, G.; Darling, E.M. Impact of Aging on the Regenerative Properties of Bone Marrow-, Muscle-, and Adipose-Derived Mesenchymal Stem/Stromal Cells. PLoS ONE 2014, 9, e115963. [Google Scholar] [CrossRef] [PubMed]
- de Haan, M.J.A.; Witjas, F.M.R.; Engelse, M.A.; Rabelink, T.J. Have We Hit a Wall with Whole Kidney Decellularization and Recellularization: A Review. Curr. Opin. Biomed. Eng. 2021, 20, 100335. [Google Scholar] [CrossRef]
- Jiang, W.C.; Cheng, Y.H.; Yen, M.H.; Chang, Y.; Yang, V.W.; Lee, O.K. Cryo-Chemical Decellularization of the Whole Liver for Mesenchymal Stem Cells-Based Functional Hepatic Tissue Engineering. Biomaterials 2014, 35, 3607–3617. [Google Scholar] [CrossRef] [PubMed]
- Mendez, J.J.; Ghaedi, M.; Steinbacher, D.; Niklason, L.E. Epithelial Cell Differentiation of Human Mesenchymal Stromal Cells in Decellularized Lung Scaffolds. Tissue Eng.-Part A 2014, 20, 1735–1746. [Google Scholar] [CrossRef]
- Wagner, D.E.; Bonenfant, N.R.; Parsons, C.S.; Sokocevic, D.; Brooks, E.M.; Borg, Z.D.; Lathrop, M.J.; Wallis, J.D.; Daly, A.B.; Lam, Y.W.; et al. Comparative Decellularization and Recellularization of Normal versus Emphysematous Human Lungs. Biomaterials 2014, 35, 3281–3297. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Zhang, S.; Peng, G.; Liu, T.; Li, Y.; Xiang, D.; Wassler, M.J.; Shelat, H.S.; Geng, Y. Rotating Microgravity-Bioreactor Cultivation Enhances the Hepatic Differentiation of Mouse Embryonic Stem Cells on Biodegradable Polymer Scaffolds. Tissue Eng. Part A 2012, 18, 2376–2385. [Google Scholar] [CrossRef]
- Hutchings, G.; Janowicz, K.; Moncrieff, L.; Dompe, C.; Strauss, E.; Kocherova, I.; Nawrocki, M.J.; Kruszyna, Ł.; Wasiatycz, G.; Antosik, P.; et al. The Proliferation and Differentiation of Adipose-Derived Stem Cells in Neovascularization and Angiogenesis. Int. J. Mol. Sci. 2020, 21, 3790. [Google Scholar] [CrossRef]
- Bourget, J.M.; Gauvin, R.; Larouche, D.; Lavoie, A.; Labbé, R.; Auger, F.A.; Germain, L. Human Fibroblast-Derived ECM as a Scaffold for Vascular Tissue Engineering. Biomaterials 2012, 33, 9205–9213. [Google Scholar] [CrossRef] [PubMed]
- White, E.S. Lung Extracellular Matrix and Fibroblast Function. Ann. Am. Thorac. Soc. 2015, 12 (Suppl. S1), S30–S33. [Google Scholar] [CrossRef]
- Tracy, L.E.; Minasian, R.A.; Caterson, E.J. Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef]
- Wang, F.; Cai, X.; Shen, Y.; Meng, L. Cell–Scaffold Interactions in Tissue Engineering for Oral and Craniofacial Reconstruction. Bioact. Mater. 2022, 23, 16. [Google Scholar] [CrossRef] [PubMed]
- Palladino, F.; Marcelino, P.R.F.; Schlogl, A.E.; José, Á.H.M.; Rodrigues, R.d.C.L.B.; Fabrino, D.L.; Santos, I.J.B.; Rosa, C.A. Bioreactors: Applications and Innovations for a Sustainable and Healthy Future—A Critical Review. Appl. Sci. 2024, 14, 9346. [Google Scholar] [CrossRef]
- Moffat, D.; Ye, K.; Jin, S. Decellularization for the Retention of Tissue Niches. J. Tissue Eng. 2022, 13, 20417314221101150. [Google Scholar] [CrossRef]
- Rauner, G. Using Organoids to Tap Mammary Gland Diversity for Novel Insight. J. Mammary Gland Biol. Neoplasia 2024, 29, 7. [Google Scholar] [CrossRef]
- Bhattacharya, T.; Kumari, M.; Kaur, K.; Kaity, S.; Arumugam, S.; Ravichandiran, V.; Roy, S. Decellularized Extracellular Matrix-Based Bioengineered 3D Breast Cancer Scaffolds for Personalized Therapy and Drug Screening. J. Mater. Chem. B 2024, 12, 8843–8867. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, X.; Dowbaj, A.M.; Sljukic, A.; Bratlie, K.; Lin, L.; Fong, E.L.S.; Balachander, G.M.; Chen, Z.; Soragni, A.; et al. Organoids. Nat. Rev. Methods Primers 2022, 2, 94. [Google Scholar] [CrossRef]
- Avagliano, A.; Fiume, G.; Ruocco, M.R.; Martucci, N.; Vecchio, E.; Insabato, L.; Russo, D.; Accurso, A.; Masone, S.; Montagnani, S.; et al. Influence of Fibroblasts on Mammary Gland Development, Breast Cancer Microenvironment Remodeling, and Cancer Cell Dissemination. Cancers 2020, 12, 1697. [Google Scholar] [CrossRef]
- Ballesteros, A.C.V.; Puello, H.R.S.; Lopez-Garcia, J.A.; Bernal-Ballen, A.; Mosquera, D.L.N.; Forero, D.M.M.; Charry, J.S.S.; Bejarano, Y.A.N. Bovine Decellularized Amniotic Membrane: Extracellular Matrix as Scaffold for Mammalian Skin. Polymers 2020, 12, 590. [Google Scholar] [CrossRef] [PubMed]
- Mollica, P.A.; Booth-Creech, E.N.; Reid, J.A.; Zamponi, M.; Sullivan, S.M.; Palmer, X.L.; Sachs, P.C.; Bruno, R.D. 3D Bioprinted Mammary Organoids and Tumoroids in Human Mammary Derived ECM Hydrogels. Acta Biomater. 2019, 95, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Klyshnikov, K.Y.; Rezvova, M.A.; Belikov, N.V.; Glushkova, T.V.; Ovcharenko, E.A. Enhancing Decellularized Vascular Scaffolds with PVDF and PCL Reinforcement: A Fused Deposition Modeling Approach. Front. Cardiovasc. Med. 2023, 10, 1257812. [Google Scholar] [CrossRef]
- Fercana, G.; Bowser, D.; Portilla, M.; Langan, E.M.; Carsten, C.G.; Cull, D.L.; Sierad, L.N.; Simionescu, D.T. Platform Technologies for Decellularization, Tunic-Specific Cell Seeding, and In Vitro Conditioning of Extended Length, Small Diameter Vascular Grafts. Tissue Eng. Part C Methods 2014, 20, 1016. [Google Scholar] [CrossRef] [PubMed]
- Kasravi, M.; Ahmadi, A.; Babajani, A.; Mazloomnejad, R.; Hatamnejad, M.R.; Shariatzadeh, S.; Bahrami, S.; Niknejad, H. Immunogenicity of Decellularized Extracellular Matrix Scaffolds: A Bottleneck in Tissue Engineering and Regenerative Medicine. Biomater. Res. 2023, 27, 10. [Google Scholar] [CrossRef]
- Parihar, A.; Pandita, V.; Kumar, A.; Parihar, D.S.; Puranik, N.; Bajpai, T.; Khan, R. 3D Printing: Advancement in Biogenerative Engineering to Combat Shortage of Organs and Bioapplicable Materials. Regen. Eng. Transl. Med. 2021, 8, 173–199. [Google Scholar] [CrossRef]
- Bernardo, M.P.; Paschoalin, R.T.; Dos Santos, D.M.; Bilatto, S.; Farinas, C.S.; Correa, D.S.; Oliveira, O.N.; Mattoso, L.H.C. Processing and Application of Polymeric Biomaterials: Recent Advances and Perspectives. Quim. Nova 2021, 44, 1311–1327. [Google Scholar] [CrossRef]
- Noroozi, R.; Arif, Z.U.; Taghvaei, H.; Khalid, M.Y.; Sahbafar, H.; Hadi, A.; Sadeghianmaryan, A.; Chen, X. 3D and 4D Bioprinting Technologies: A Game Changer for the Biomedical Sector? Ann. Biomed. Eng. 2023, 51, 1683–1712. [Google Scholar] [CrossRef]
- Amukarimi, S.; Rezvani, Z.; Eghtesadi, N.; Mozafari, M. Corrigendum to “Smart Biomaterials: From 3D Printing to 4D Bioprinting” [Methods 205 (2022) 191–199, (S1046202322001608), (10.1016/j.Ymeth.2022.07.006)]. Methods 2022, 208, 27. [Google Scholar] [CrossRef]
- Do, A.V.; Khorsand, B.; Geary, S.M.; Salem, A.K. 3D Printing of Scaffolds for Tissue Regeneration Applications. Adv. Healthc. Mater. 2015, 4, 1742. [Google Scholar] [CrossRef]
- Saini, G.; Segaran, N.; Mayer, J.L.; Saini, A.; Albadawi, H.; Oklu, R. Applications of 3D Bioprinting in Tissue Engineering and Regenerative Medicine. J. Clin. Med. 2021, 10, 4966. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Yang, M.; Wang, L.; Li, W.; Liu, M.; Jin, Y.; Wang, Y.; Yang, R.; Wang, Y.; Zhang, K.; et al. Hydrogels for 3D Bioprinting in Tissue Engineering and Regenerative Medicine: Current Progress and Challenges. Int. J. Bioprinting 2023, 9, 759. [Google Scholar] [CrossRef]
- Gómez, S.; Vlad, M.D.; López, J.; Fernández, E. Design and Properties of 3D Scaffolds for Bone Tissue Engineering. Acta Biomater. 2016, 42, 341–350. [Google Scholar] [CrossRef]
- Chung, J.J.; Im, H.; Kim, S.H.; Park, J.W.; Jung, Y. Toward Biomimetic Scaffolds for Tissue Engineering: 3D Printing Techniques in Regenerative Medicine. Front. Bioeng. Biotechnol. 2020, 8, 586406. [Google Scholar] [CrossRef]
- Wang, P.; Sun, Y.; Shi, X.; Shen, H.; Ning, H.; Liu, H. 3D Printing of Tissue Engineering Scaffolds: A Focus on Vascular Regeneration. Bio-Des. Manuf. 2021, 4, 344–378. [Google Scholar] [CrossRef]
- Kim, H.Y.; Sinha, I.; Sears, K.E.; Kuperwasser, C.; Rauner, G. Expanding the Evo-Devo Toolkit: Generation of 3D Mammary Tissue from Diverse Mammals | Development | The Company of Biologists. Stem Cell Regen. 2024, 151, 2021–2034. [Google Scholar]
- Koskinen, L.M.; Nieminen, L.; Arjonen, A.; Guzmán, C.; Peurla, M.; Peuhu, E. Spatial Engineering of Mammary Epithelial Cell Cultures with 3D Bioprinting Reveals Growth Control by Branch Point Proximity. J. Mammary Gland Biol. Neoplasia 2024, 29, 5. [Google Scholar] [CrossRef] [PubMed]
- Reid, J.A.; Mollica, P.M.; Bruno, R.D.; Sachs, P.C. Consistent and Reproducible Cultures of Large-Scale 3D Mammary Epithelial Structures Using an Accessible Bioprinting Platform. Breast Cancer Res. 2018, 20, 122. [Google Scholar] [CrossRef]
- Blanco-Fernandez, B.; Rey-Vinolas, S.; Baǧcl, G.; Rubi-Sans, G.; Otero, J.; Navajas, D.; Perez-Amodio, S.; Engel, E. Bioprinting Decellularized Breast Tissue for the Development of Three-Dimensional Breast Cancer Models. ACS Appl. Mater. Interfaces 2022, 14, 29467–29482. [Google Scholar] [CrossRef]
- Huniadi, M.; Nosálová, N.; Almášiová, V.; Horňáková, Ľ.; Valenčáková, A.; Hudáková, N.; Cizkova, D. Three-Dimensional Cultivation a Valuable Tool for Modelling Canine Mammary Gland Tumour Behaviour In Vitro. Cells 2024, 13, 695. [Google Scholar] [CrossRef]
- Huang, G.; Li, F.; Zhao, X.; Ma, Y.; Li, Y.; Lin, M.; Jin, G.; Lu, T.J.; Genin, G.M.; Xu, F. Functional and Biomimetic Materials for Engineering of the Three-Dimensional Cell Microenvironment. Chem. Rev. 2017, 117, 12764–12850. [Google Scholar] [CrossRef] [PubMed]
- Micha, R. Tendon Functional Extracellular Matrix, H.R.C. Physiol. Behav. 2017, 176, 100–106. [Google Scholar]
- Wolf, K.; te Lindert, M.; Krause, M.; Alexander, S.; te Riet, J.; Willis, A.L.; Hoffman, R.M.; Figdor, C.G.; Weiss, S.J.; Friedl, P. Physical Limits of Cell Migration: Control by ECM Space and Nuclear Deformation and Tuning by Proteolysis and Traction Force. J. Cell Biol. 2013, 201, 1069–1084. [Google Scholar] [CrossRef]
- Chan, B.P.; Leong, K.W. Scaffolding in Tissue Engineering: General Approaches and Tissue-Specific Considerations. Eur. Spine J. 2008, 17, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Doyle, A.D.; Nazari, S.S.; Yamada, K.M. Cell-Extracellular Matrix Dynamics. Phys. Biol. 2022, 19, 021002. [Google Scholar] [CrossRef]
- Polak, J.M.; Bishop, A.E. Stem Cells and Tissue Engineering: Past, Present, and Future. Ann. N. Y. Acad. Sci. 2006, 1068, 352–366. [Google Scholar] [CrossRef]
- Liu, W.Y.; Lin, S.G.; Zhuo, R.Y.; Xie, Y.Y.; Pan, W.; Lin, X.F.; Shen, F.X. Xenogeneic Decellularized Scaffold: A Novel Platform for Ovary Regeneration. Tissue Eng.-Part C Methods 2017, 23, 61–71. [Google Scholar] [CrossRef]
- Muiznieks, L.D.; Keeley, F.W. Molecular Assembly and Mechanical Properties of the Extracellular Matrix: A Fibrous Protein Perspective. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2013, 1832, 866–875. [Google Scholar] [CrossRef]
| Mammary Gland Structure | Components | Description | Applications | Reference |
|---|---|---|---|---|
| Basal lamina | Collagen IV | It is a network-forming class. It is the main component of the basal lamina of the mammary gland. It is a heterotrimer and composed of six possible α chains. It is also a significant basal lamina component and is considered its primary scaffold protein. | It supports the basal lamina structure during embryogenesis and in mammary epithelial cells. | [44] |
| Nidogens | Mesodermally derived fibroblasts synthesize sulfated glycoproteins (150 kDa). | They produce Laminins (LN, Ln, Lm, or Lam), which are stabilizing components of the basal lamina. They allow for the connection between LN-111 and collagen IV. | [44] | |
| Intra and interlobular stroma | Collagen I | Fibrillar collagen I is the main protein of the stroma of the mammary gland that supports the formation of the mammary duct. | They form bundles of varying thickness and length, associated with other macromolecules of the ECM, which determines their architectural structure. This structure allows the mammary epithelium to be supported during pregnancy and lactation, providing the elastic capacity that enables the tissue to return to its original shape after stretching. | [44,46,47] |
| Collagen III | This forms structures with characteristics of fibrillar collagen I and is a homotrimer consisting of a single α chain. | |||
| Collagen V | This forms structures with characteristics of fibrillar collagen I. It is a heterotrimer composed of three different α chains. | |||
| FN | This is a dimeric glycoprotein (~500 kDa) that mediates cell adhesion, migration, proliferation, and branching morphogenesis. | It is a precursor of the fibril that interacts with other components of the ECM and organizes the interstitial matrix, allowing for the attachment of breast tissue cells. | [44,48,49] | |
| TN | Glycoproteins have five members: TN-C, TN-R, TN-W, TN-X, and TN-Y. In the mammary gland, TN-C is transiently expressed in the dense stroma surrounding the budding epithelium during embryogenesis. | TN-X maintains tissue elasticity during lactation. | [44,50] | |
| Sparc | It is a small glycoprotein of 32 kDa. | It is upregulated in mammary gland development during the transition from lactation to postpartum involution, and increased collagen and FN levels correlate with this. | [44,51] | |
| Laminins (LN, Ln, Lm, or Lam) | The primary protein membrane comprises three polypeptide chains: α, β, and γ. In the mammary gland, LN-111 and LN-332 are abundant at the level of the basement membrane. | They are responsible for acinar formation and induction of contact with epithelial cells. They stimulate milk secretion. | [50] | |
| Decoration | This is a decorin core protein (~38 kDa) linked to a single chain of CS or DS. | It controls the spatial alignment of collagen fibers in the stroma. It is crucial for the proper organization of fibrillar collagen. | [44,52] | |
| Biglycan | It comprises a 38 kDa core protein covalently linked to two GAG chains (chondroitin sulfate and/or dermatan sulfate) with an overall MW of 150–240 kDa. | It plays a role in inducing the elastic properties of the gland during periods of expansion. | [44,53] | |
| Fibrous connective tissue | Elastic fibers | They have components such as elastin, fibulins, and proteoglycans associated with microfibrils, forming elastic fibers. | It provides structural support and elasticity to various tissues during lactation. | [44] |
| Characteristic | Bovines | Small Ruminants (Sheep/Goats) | References |
|---|---|---|---|
| Predominant collagen type | Type I collagen is more abundant, contributing to greater tissue rigidity. | Higher proportion of type III collagen, favoring elasticity. | [55,56,57] |
| FN distribution | More abundant in the parenchyma than in the mammary fat pad. | Similar, but with less quantitative detail in studies. | [56,58,59] |
| LN distribution | Present in the parenchymal stroma, associated with epithelial organization. | Similar distribution, with analogous structural function. | [56,59] |
| Elastic fibers | Not specifically emphasized. | Significant presence, associated with tissue elasticity. | [57,59,60] |
| GAG composition | Lower relative proportion of hydrating GAGs. | A higher presence of chondroitin and heparan sulfate contributes to hydration. | [57,58] |
| ECM density | High fibrillar density and greater mechanical resistance. | Lower density and a more flexible matrix. | [1,57] |
| MMP activity | Moderate activity and slower remodeling. | High activity, facilitating tissue regeneration. | [44,60] |
| Methods of Decellularization | Advantages | Disadvantages | Reference | |
|---|---|---|---|---|
| Physical | Flash freezing | This technique is considered safe because it does not produce residual chemicals and has minimal impact on tissue structure and biochemical composition after decellularization. | Damage or rupture of the ECM due to extremely low temperatures. | [73,79,80,81] |
| Mechanical force | Cell rupture followed by washing to remove cellular material. | The application of pressure compromises the integrity of the ECM. | [75,80] | |
| Mechanical agitation | Increases exposure to chemical reagents for cell removal. | Damage to the ECM in cases of agitation or excessive sonication. | [73,75] | |
| Sonication | Facilitates the penetration of chemical detergents and accelerates the removal of cellular debris. | Potential damage to cell membranes and the ECM due to cavitation. | [82] | |
| Chemical | Ionic detergents (SDS) | SDS solubilizes cytoplasmic and nuclear membranes, inducing cell lysis. In the ECM, SDS removes residual cytoplasmic and cellular proteins. | SDS concentrations exceeding 10 μg/mg dry weight can induce cytotoxicity due to the difficulty of removing SDS from decellularized tissues. As it forms strong hydrophobic bonds with ECM proteins, it may disrupt the native tissue structure, deplete GAGs, and damage collagen and other structural proteins. | [73,83] |
| Nonionic detergents | Triton X-100 disrupts lipid–lipid and lipid–protein hydrophobic interactions while preserving protein–protein interactions. | Its efficiency varies by tissue type, yielding mixed results regarding ECM integrity and may deplete GAGs. | [82,83] | |
| Alkaline and acidic agents | These agents solubilize cytoplasmic components and destroy nucleic acids. | They also result in a significant loss of GAGs from the ECM. | [83,84] | |
| Zwitterionic detergents | Compounds such as CHAPS exhibit properties of both ionic and nonionic detergents, facilitating cell removal and ECM disruption similar to Triton X-100. | It may damage ECM proteins depending on the tissue and concentration used. | [81,85] | |
| Hypotonic/hypertonic solutions | Induces cell lysis by osmotic shock. | Ineffective in removing residual cell contents. | [83,86] | |
| EDTA/EGTA | Disrupts cell–ECM adhesions by chelating divalent metal ions. | It is often used with enzymatic methods, such as trypsin digestion, and has a limited impact when used alone. | [82,87] | |
| Enzymatic | Trypsin | Removes specific cell proteins, facilitating decellularization. | Prolonged exposure can disrupt the structure of the ECM, removing essential components such as LN, FN, elastin, and GAGs. | [81,88] |
| Endonucleases | Enzymes catalyze the hydrolysis of internal bonds in ribonucleotide and deoxyribonucleotide chains. | They can complicate the removal of intact cells. | [87,88,89] | |
| Exonucleases | These enzymes catalyze the hydrolysis of terminal bonds in ribonucleotide and deoxyribonucleotide chains. | Limited impact when used alone. | [81,90] | |
| Cellular Type | Definition | Advantages | Disadvantages | Applications | Reference |
|---|---|---|---|---|---|
| Fetal and Adult Cells | Fetal cells: maintain phenotypic markers and spatial organization when cultured on biological scaffolds. Adult cells: include renal/alveolar epithelial cells and fibroblasts, often obtained via biopsy. | Fetal cells: show promising functional capabilities in lung, liver, and kidney scaffolds. Adult cells: ease of acquisition via biopsy. | Fetal cells are unsuitable for clinical applications; adult cells have low proliferative capacity and limited scalability for organ repopulation. | Fetal cells recellularize scaffolds, such as rat lung, liver, and kidney; adult cells are used for kidney and lung recellularization, but are limited by low proliferation. | [108,109] |
| ESCs | Pluripotent stem cells can expand in vitro and differentiate into multiple lineages. | High proliferative capacity; ability to differentiate into multiple lineages; influence cell differentiation in organ matrices. | Ethical concerns regarding the source; potential for teratoma formation (tumorigenicity); risk of uncontrolled differentiation. | Widely used in tissue engineering studies for the recellularization of organ scaffolds. | [75,109,110] |
| MSCs | Multipotent stem cells isolated from bone marrow or adipose tissue can differentiate into various cell types and support tissue repair. | Robust proliferation in culture; differentiation into multiple lineages; secretion of cytokines and chemokines for tissue repair; provision of stromal support. | Differentiation may be inconsistent across different scaffolds. Require precise culture conditions for lineage-specific differentiation. | Hepatic dECM: accelerates differentiation into hepatocytes; cardiac dECM: differentiates into cardiomyocytes under stimulation; pulmonary dECM: differentiates into epithelial lineages. | [110,111,112,113,114,115] |
| iPSCs | Stem cells are generated by reprogramming somatic cells to express pluripotency genes, mimicking ESCs. | Adhere to and proliferate on dECM; express alveolar markers in pulmonary scaffolds; and have pluripotent characteristics similar to ESCs. | Lower adhesion in specific scaffolds (e.g., cardiac dECM); risk of genetic instability; labor-intensive reprogramming process. | Pulmonary dECM: promotes adhesion and proliferation. Cardiac dECM: shows lower adhesion than MSCs, requiring further research to optimize use. | [110,115] |
| Species Studied | Cell Type Recellularized | Results | Reference |
|---|---|---|---|
| Bovine | Sheep skin cells | The new material was a biological scaffold for in vitro skin cell culture. | [126] |
| Rat and Human | Normal mammary and breast cancer cells | The study described a novel mammary-specific culture protocol that combines a self-gelling hydrogel comprised solely of an ECM from decellularized rat or human breast tissue with a 3D bioprinting platform. | [127] |
| Bovine | No cells were used | A series of in vitro tests demonstrated the consistency and potential of this approach for decellularized xenogenic scaffolds, a concept that had not been explored before. | [128] |
| Bovine | Endothelial cells | Potential grafts for the treatment of acute ischemia were developed. | [129] |
| Human | Adipose stem cells | The study illustrated the potential of regenerative medicine in terms of mammary gland reconstruction to restore breast physiology and morphology damaged by mastectomy. | [26] |
| Minipig | No cells were used | SGBTR regenerates soft tissue by implanting additively manufactured bioresorbable scaffolds filled with autologous fat grafts. | [130] |
| Species Studied | Main Results | Reference |
|---|---|---|
| Multiple eutherian mammals and a marsupial (gray short-tailed opossum) | Successfully created next-generation 3D mammary gland organoids from eight eutherian mammals and the first branched organoid of a marsupial mammary gland, providing a model for studying mammary gland evolution and development. | [141] |
| Human mammary epithelial cells | Developed a 3D bioprinting protocol for normal and cancerous mammary epithelial cells into a branched Y shape, facilitating the study of cell positioning in regulating proliferation and invasion. | [94,142] |
| Mouse mammary epithelial cells | Detailed the use of a 3D bioprinting platform to control the formation of organoids through the “self-assembly” of mammary epithelial cells, enabling consistent and reproducible cultures of large-scale 3D mammary epithelial tissues. | [143] |
| Porcine breast tissue | Developed a method for decellularizing and delipidating porcine breast tissue compatible with hydrogel formation, advancing the development of tissue-engineered breast models. | [94,144] |
| Canine mammary gland tumors | Utilized 3D culture methods to model canine mammary gland tumors, providing insights into tumor biology and potential therapeutic approaches. | [145] |
| Various organoids | Discussed organoid bioprinting approaches that control the 3D arrangement of organoids, contributing to the development of functional tissue for regenerative medicine. | [138,146] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chissico Júnior, F.; Santos da Silva, T.; Vieira Meirelles, F.; Monzani, P.S.; Fornari Laurindo, L.; Maria Barbalho, S.; Miglino, M.A. A Review on Bioengineering the Bovine Mammary Gland: The Role of the Extracellular Matrix and Reconstruction Prospects. Bioengineering 2025, 12, 501. https://doi.org/10.3390/bioengineering12050501
Chissico Júnior F, Santos da Silva T, Vieira Meirelles F, Monzani PS, Fornari Laurindo L, Maria Barbalho S, Miglino MA. A Review on Bioengineering the Bovine Mammary Gland: The Role of the Extracellular Matrix and Reconstruction Prospects. Bioengineering. 2025; 12(5):501. https://doi.org/10.3390/bioengineering12050501
Chicago/Turabian StyleChissico Júnior, Fernando, Thamires Santos da Silva, Flávio Vieira Meirelles, Paulo Sérgio Monzani, Lucas Fornari Laurindo, Sandra Maria Barbalho, and Maria Angélica Miglino. 2025. "A Review on Bioengineering the Bovine Mammary Gland: The Role of the Extracellular Matrix and Reconstruction Prospects" Bioengineering 12, no. 5: 501. https://doi.org/10.3390/bioengineering12050501
APA StyleChissico Júnior, F., Santos da Silva, T., Vieira Meirelles, F., Monzani, P. S., Fornari Laurindo, L., Maria Barbalho, S., & Miglino, M. A. (2025). A Review on Bioengineering the Bovine Mammary Gland: The Role of the Extracellular Matrix and Reconstruction Prospects. Bioengineering, 12(5), 501. https://doi.org/10.3390/bioengineering12050501








