Emerging Image-Guided Navigation Techniques for Cardiovascular Interventions: A Scoping Review
Abstract
1. Introduction
1.1. Review Methodology
1.1.1. Search Strategy and Databases
1.1.2. Inclusion and Exclusion Criteria
- Were published in English in peer-reviewed journals or leading international conferences;
- Focused on imaging techniques, navigation systems, or image-guided interventions specifically applied to cardiovascular procedures;
- Demonstrated technical novelty, clinical validation, or translational relevance, such as evaluations of performance, workflow integration, or outcome improvements;
- Included clinical, preclinical, simulation-based, or computational studies with clearly defined methodologies.
- Editorials, commentaries, abstracts, or opinion pieces without supporting data;
- Studies limited to non-cardiac applications or purely diagnostic modalities without interventional relevance;
- Studies focusing primarily on the development or evaluation of contrast media are excluded, as this review is centered on imaging techniques rather than contrast agent innovation.
1.1.3. Screening and Selection
2. Advancements in Imaging Techniques for Cardiac Intervention
2.1. Fluoroscopy and X-Ray Imaging Techniques
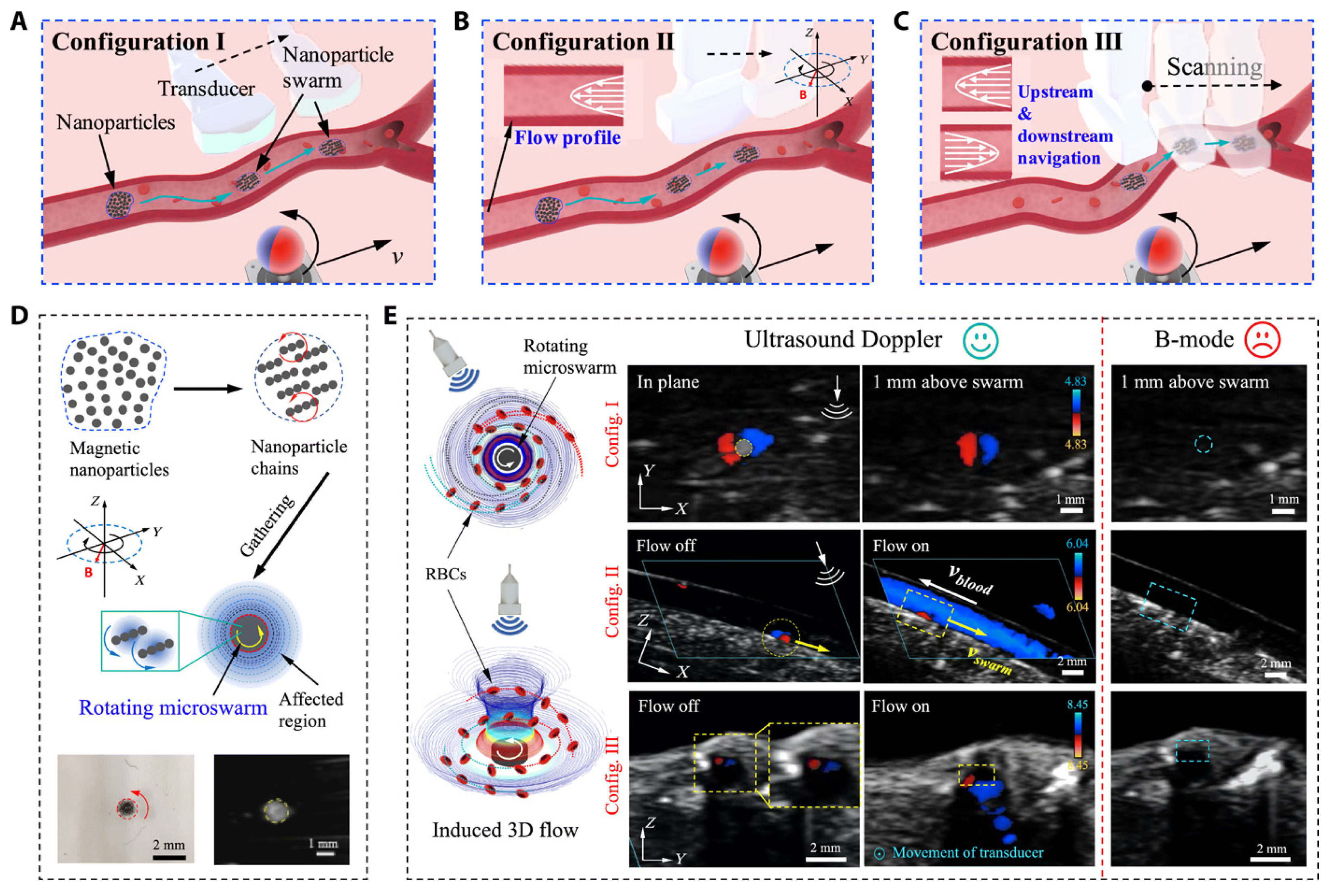
2.2. Ultrasound-Based Navigation and Control
2.3. MRI-Based Navigation and Control
2.4. Optical Coherence Tomography (OCT) Technique
3. Emerging Imaging Techniques for Next-Generation Cardiac Interventions
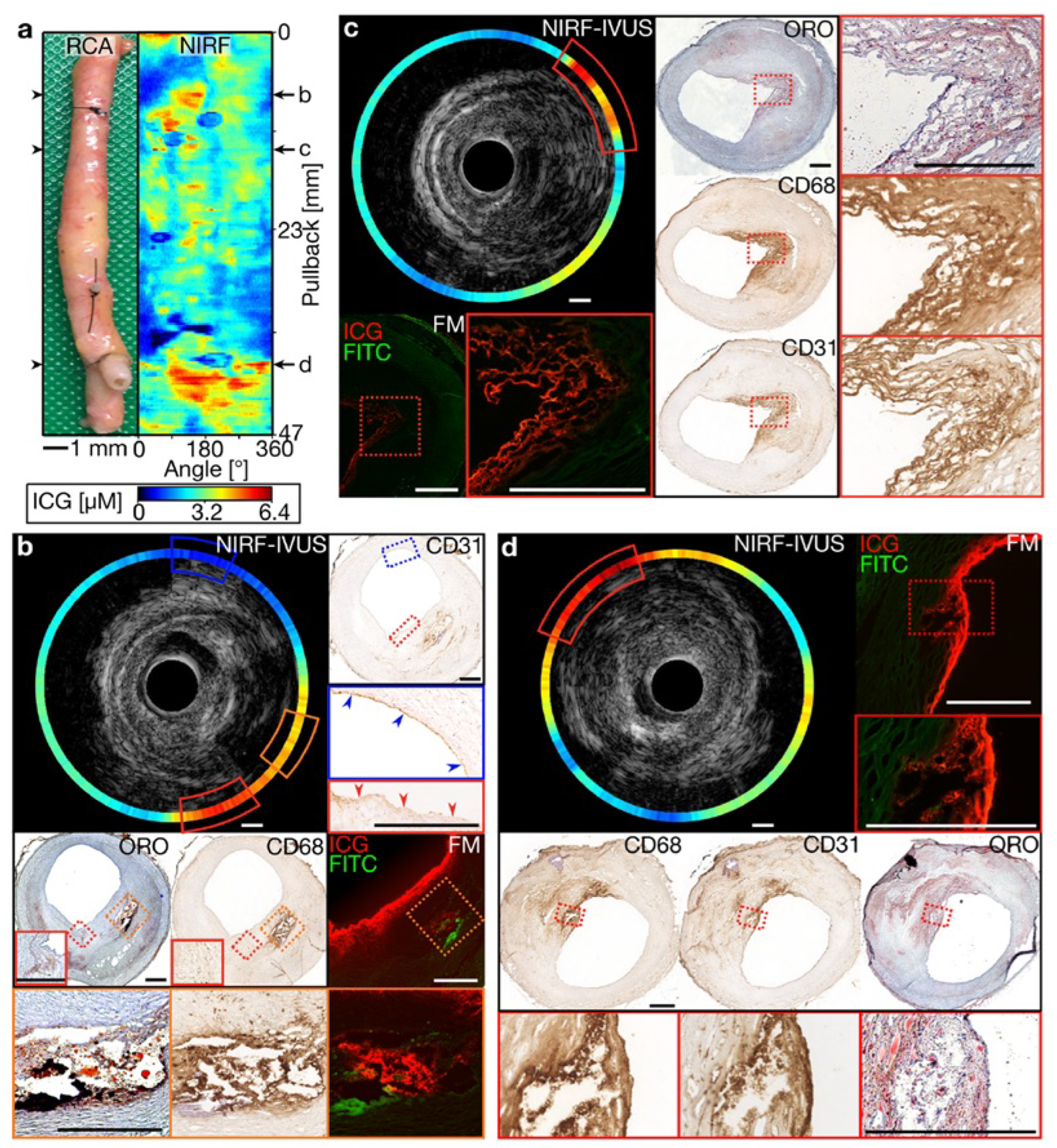
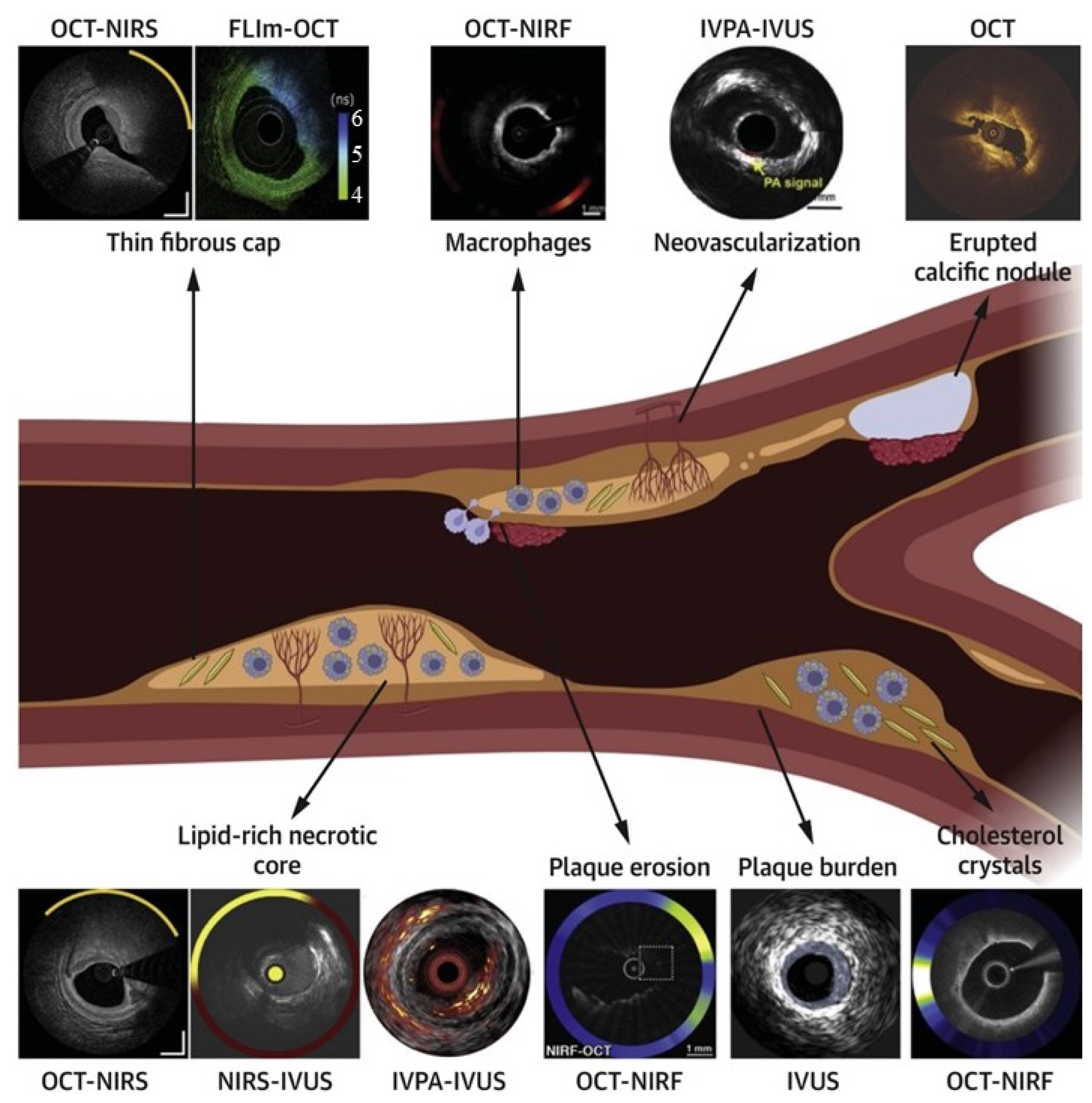
3.1. Near-Infrared Fluorescence (NIRF) and Near-Infrared Spectroscopy (NIRS) in Intravascular Imaging
3.2. Nuclear Imaging
3.3. Multimodalities Imaging Techniques
3.4. Alternative Navigation Methods
4. AI-Assisted Image-Guided Navigation
4.1. AI-Enhanced Image Processing and Interpretation
4.2. AI-Powered Procedural Guidance and Automation
4.3. Implementation Challenges and Clinical Validation
5. Challenges and Limitations
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ullah, A.; Kumar, M.; Sayyar, M.; Sapna, F.; John, C.; Memon, S.; Qureshi, K.; Agbo, E.C.; Ariri, H.I.; Chukwu, E.J.; et al. Revolutionizing cardiac care: A comprehensive narrative review of cardiac rehabilitation and the evolution of cardiovascular medicine. Cureus 2023, 15, e46469. [Google Scholar] [CrossRef]
- Vento, V.; Kuntz, S.; Lejay, A.; Chakfe, N. Evolutionary trends and innovations in cardiovascular intervention. Front. Med. Technol. 2024, 6, 1384008. [Google Scholar] [CrossRef] [PubMed]
- Rogers, T.; Campbell-Washburn, A.E.; Ramasawmy, R.; Yildirim, D.K.; Bruce, C.G.; Grant, L.P.; Stine, A.M.; Kolandaivelu, A.; Herzka, D.A.; Ratnayaka, K.; et al. Interventional cardiovascular magnetic resonance: State-of-the-art. J. Cardiovasc. Magn. Reson. 2023, 25, 48. [Google Scholar] [CrossRef]
- Sachdeva, R.; Armstrong, A.K.; Arnaout, R.; Grosse-Wortmann, L.; Han, B.K.; Mertens, L.; Moore, R.A.; Olivieri, L.J.; Parthiban, A.; Powell, A.J. Novel techniques in imaging congenital heart disease: JACC scientific statement. J. Am. Coll. Cardiol. 2024, 83, 63–81. [Google Scholar] [CrossRef]
- Sermesant, M.; Delingette, H.; Cochet, H.; Jaïs, P.; Ayache, N. Applications of artificial intelligence in cardiovascular imaging. Nat. Rev. Cardiol. 2021, 18, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Wehbe, R.M.; Katsaggelos, A.K.; Hammond, K.J.; Hong, H.; Ahmad, F.S.; Ouyang, D.; Shah, S.J.; McCarthy, P.M.; Thomas, J.D. Deep learning for cardiovascular imaging: A review. JAMA Cardiol. 2023, 8, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Alvandi, M.; Javid, R.N.; Shaghaghi, Z.; Farzipour, S.; Nosrati, S. An in-depth analysis of the adverse effects of ionizing radiation exposure on cardiac catheterization staffs. Curr. Radiopharm. 2024, 17, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhou, D.; Ye, L.; Housden, R.J.; Fazili, A.; Rhode, K.S. A tensor-based catheter and wire detection and tracking framework and its clinical applications. IEEE Trans. Biomed. Eng. 2021, 69, 635–644. [Google Scholar] [CrossRef]
- Zhou, J.J.; Quadri, A.; Sewani, A.; Alawneh, Y.; Gilliland-Rocque, R.; Magnin, C.; Dueck, A.; Wright, G.A.; Tavallaei, M.A. The CathPilot: A novel approach for accurate interventional device steering and tracking. IEEE/ASME Trans. Mechatron. 2022, 27, 5812–5823. [Google Scholar] [CrossRef]
- Chang, P.L.; Rolls, A.; De Praetere, H.; Vander Poorten, E.; Riga, C.V.; Bicknell, C.D.; Stoyanov, D. Robust catheter and guidewire tracking using b-spline tube model and pixel-wise posteriors. IEEE Robot. Autom. Lett. 2016, 1, 303–308. [Google Scholar] [CrossRef]
- Roshanfar, M.; Fekri, P.; Dargahi, J. A deep learning model for tip force estimation on steerable catheters via learning-from-simulation. In Proceedings of the Hamlyn Symposium on Medical Robotics, London, UK, 26–29 June 2023. [Google Scholar]
- Fekri, P.; Khodashenas, H.; Lachapelle, K.; Cecere, R.; Zadeh, M.; Dargahi, J. Y-net: A deep convolutional architecture for 3d estimation of contact forces in intracardiac catheters. IEEE Robot. Autom. Lett. 2022, 7, 3592–3599. [Google Scholar] [CrossRef]
- Fekri, P.; Zadeh, M.; Dargahi, J. H-Net: A Multitask Architecture for Simultaneous 3D Force Estimation and Stereo Semantic Segmentation in Intracardiac Catheters. IEEE Robot. Autom. Lett. 2024, 10, 844–851. [Google Scholar] [CrossRef]
- Agricola, E.; Meucci, F.; Ancona, F.; Sanz, A.P.; Zamorano, J.L. Echocardiographic guidance in transcatheter structural cardiac interventions. Euro Interv. 2022, 17, 1205. [Google Scholar] [CrossRef]
- Wang, S.; Singh, D.; Lau, D.; Reddy, K.; Althoefer, K.; Rhode, K.; Housden, R.J. Probe tracking and its application in automatic acquisition using a trans-esophageal ultrasound robot. In Proceedings of the Computer-Assisted and Robotic Endoscopy: Third International Workshop, CARE 2016, Held in Conjunction with MICCAI 2016, Athens, Greece, 17 October 2016; Revised Selected Papers 3. Springer: Berlin/Heidelberg, Germany, 2017; pp. 14–23. [Google Scholar]
- Yang, Z.; Yang, L.; Zhang, M.; Wang, Q.; Yu, S.C.H.; Zhang, L. Magnetic control of a steerable guidewire under ultrasound guidance using mobile electromagnets. IEEE Robot. Autom. Lett. 2021, 6, 1280–1287. [Google Scholar] [CrossRef]
- Wang, Q.; Chan, K.F.; Schweizer, K.; Du, X.; Jin, D.; Yu, S.C.H.; Nelson, B.J.; Zhang, L. Ultrasound Doppler-guided real-time navigation of a magnetic microswarm for active endovascular delivery. Sci. Adv. 2021, 7, eabe5914. [Google Scholar] [CrossRef] [PubMed]
- Padhan, J.; Tsekos, N.; Al-Ansari, A.; Abinahed, J.; Deng, Z.; Navkar, N.V. Dynamic Guidance Virtual Fixtures for Guiding Robotic Interventions: Intraoperative MRI-guided Transapical Cardiac Intervention Paradigm. In Proceedings of the 2022 IEEE 22nd International Conference on Bioinformatics and Bioengineering (BIBE), Taichung, Taiwan, 7–9 November 2022; IEEE: Piscataway, NY, USA, 2022; pp. 265–270. [Google Scholar]
- Velasco Forte, M.N.; Roujol, S.; Ruijsink, B.; Valverde, I.; Duong, P.; Byrne, N.; Krueger, S.; Weiss, S.; Arar, Y.; Reddy, S.R.V.; et al. MRI for guided right and left heart cardiac catheterization: A prospective study in congenital heart disease. J. Magn. Reson. Imaging 2021, 53, 1446–1457. [Google Scholar] [CrossRef]
- Dong, Z.; Wang, X.; Fang, G.; He, Z.; Ho, J.D.L.; Cheung, C.L.; Tang, W.L.; Xie, X.; Liang, L.; Chang, H.C.; et al. Shape tracking and feedback control of cardiac catheter using MRI-guided robotic platform—Validation with pulmonary vein isolation simulator in MRI. IEEE Trans. Robot. 2022, 38, 2781–2798. [Google Scholar] [CrossRef]
- Kensicher, T.; Leclerc, J.; Biediger, D.; Shah, D.J.; Seimenis, I.; Becker, A.T.; Tsekos, N.V. Towards MRI-guided and actuated tetherless milli-robots: Preoperative planning and modeling of control. In Proceedings of the 2017 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Vancouver, Canada, 24–28 September 2017; IEEE: Piscataway, NY, USA, 2017; pp. 6440–6447. [Google Scholar]
- Joshi, N.V.; Vesey, A.T.; Williams, M.C.; Shah, A.S.; Calvert, P.A.; Craighead, F.H.; Yeoh, S.E.; Wallace, W.; Salter, D.; Fletcher, A.M.; et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: A prospective clinical trial. Lancet 2014, 383, 705–713. [Google Scholar] [CrossRef]
- Yeniaras, E.; Lamaury, J.; Navkar, N.V.; Shah, D.J.; Chin, K.; Deng, Z.; Tsekos, N.V. Magnetic resonance based control of a robotic manipulator for interventions in the beating heart. In Proceedings of the 2011 IEEE International Conference on Robotics and Automation, Shanghai, China, 9–13 May 2011; IEEE: Piscataway, NY, USA, 2011; pp. 6270–6275. [Google Scholar]
- Kurogi, K.; Ishii, M.; Yamamoto, N.; Yamanaga, K.; Tsujita, K. Optical coherence tomography-guided percutaneous coronary intervention: A review of current clinical applications. Cardiovasc. Interv. Ther. 2021, 36, 169–177. [Google Scholar] [CrossRef]
- Amabile, N.; Range, G.; Souteyrand, G.; Godin, M.; Boussaada, M.; Meneveau, N.; Cayla, G.; Casassus, F.; Lefevre, T.; Hakim, R.; et al. Optical coherence tomography to guide percutaneous coronary intervention of the left main coronary artery: The LEMON study: OCT guidance in LM PCI. Euro Interv. 2021, 17, e124. [Google Scholar]
- Yonetsu, T.; Jang, I.K. Cardiac optical coherence tomography: History, current status, and perspective. JACC Asia 2024, 4, 89–107. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.Y.; Ahn, J.M.; Yun, S.C.; Hur, S.H.; Cho, Y.K.; Lee, C.H.; Hong, S.J.; Lim, S.; Kim, S.W.; Won, H.; et al. Guiding intervention for complex coronary lesions by optical coherence tomography or intravascular ultrasound. J. Am. Coll. Cardiol. 2024, 83, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.A.; Rathod, K.S.; Koganti, S.; Hamshere, S.; Astroulakis, Z.; Lim, P.; Sirker, A.; O’Mahony, C.; Jain, A.K.; Knight, C.J.; et al. Angiography alone versus angiography plus optical coherence tomography to guide percutaneous coronary intervention: Outcomes from the pan-London PCI cohort. JACC Cardiovasc. Interv. 2018, 11, 1313–1321. [Google Scholar] [CrossRef]
- Chamié, D.; Costa Jr, J.R.; Damiani, L.P.; Siqueira, D.; Braga, S.; Costa, R.; Seligman, H.; Brito, F.; Barreto, G.; Staico, R.; et al. Optical coherence tomography versus intravascular ultrasound and angiography to guide percutaneous coronary interventions: The iSIGHT randomized trial. Circ. Cardiovasc. Interv. 2021, 14, e009452. [Google Scholar] [CrossRef] [PubMed]
- IJsselmuiden, A.; Zwaan, E.; Oemrawsingh, R.; Bom, M.; Dankers, F.; de Boer, M.J.; Camaro, C.; van Geuns, R.; Daemen, J.; Van Der Heijden, D.J.; et al. Appropriate use criteria for optical coherence tomography guidance in percutaneous coronary interventions: Recommendations of the working group of interventional cardiology of the Netherlands Society of Cardiology. Neth. Heart J. 2018, 26, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.M.; Piao, Z.; Albaghdadi, M.S.; Coughlin, P.A.; Rudd, J.H.; Tearney, G.J.; Jaffer, F.A. Intravascular fluorescence molecular imaging of atherosclerosis. In Atherosclerosis: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2022; pp. 853–872. [Google Scholar]
- Hogue, C.W.; Levine, A.; Hudson, A.; Lewis, C. Clinical applications of near-infrared spectroscopy monitoring in cardiovascular surgery. Anesthesiology 2021, 134, 784. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, W.; Zhou, C.; Li, M.; Wang, X.; Zhang, W.; Liu, Z.; Wu, L.; James, T.D.; Li, P.; et al. Precision navigation of hepatic ischemia–reperfusion injury guided by lysosomal viscosity-activatable NIR-II fluorescence. J. Am. Chem. Soc. 2022, 144, 13586–13599. [Google Scholar] [CrossRef]
- Rauschendorfer, P.; Lenz, T.; Nicol, P.; Wild, L.; Beele, A.; Sabic, E.; Klosterman, G.; Laugwitz, K.L.; Jaffer, F.A.; Gorpas, D.; et al. Intravascular ICG-enhanced NIRF-IVUS imaging to assess progressive atherosclerotic lesions in excised human coronary arteries. npj Cardiovasc. Health 2024, 1, 14. [Google Scholar] [CrossRef]
- Bao, K.; Tully, M.; Cardenas, K.; Wang, H.; Srinivas, S.; Rho, J.; Jeon, O.H.; Dinh, J.; Yokomizo, S.; McDonnell, R.; et al. Ultralow Background Near-Infrared Fluorophores with Dual-Channel Intraoperative Imaging Capability. Adv. Healthc. Mater. 2023, 12, 2203134. [Google Scholar] [CrossRef]
- Chakravarty, R. Development of Radionuclide Generators for Biomedical Applications. Ph.D. Thesis, Homi Bhabha National Institute, Mumbai, India, 2011. [Google Scholar]
- Ford, E.C.; Herman, J.; Yorke, E.; Wahl, R.L. 18F-FDG PET/CT for image-guided and intensity-modulated radiotherapy. J. Nucl. Med. 2009, 50, 1655–1665. [Google Scholar] [CrossRef]
- Bokhari, S.; Castaño, A.; Pozniakoff, T.; Deslisle, S.; Latif, F.; Maurer, M.S. 99mTc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ. Cardiovasc. Imaging 2013, 6, 195–201. [Google Scholar] [CrossRef]
- Youssef, G.; Leung, E.; Mylonas, I.; Nery, P.; Williams, K.; Wisenberg, G.; Gulenchyn, K.Y.; Dekemp, R.A.; DaSilva, J.; Birnie, D.; et al. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: A systematic review and metaanalysis including the Ontario experience. J. Nucl. Med. 2012, 53, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Dweck, M.R.; Jones, C.; Joshi, N.V.; Fletcher, A.M.; Richardson, H.; White, A.; Marsden, M.; Pessotto, R.; Clark, J.C.; Wallace, W.A.; et al. Assessment of valvular calcification and inflammation by positron emission tomography in patients with aortic stenosis. Circulation 2012, 125, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Cartlidge, T.R.; Doris, M.K.; Sellers, S.L.; Pawade, T.A.; White, A.C.; Pessotto, R.; Kwiecinski, J.; Fletcher, A.; Alcaide, C.; Lucatelli, C.; et al. Detection and prediction of bioprosthetic aortic valve degeneration. J. Am. Coll. Cardiol. 2019, 73, 1107–1119. [Google Scholar] [CrossRef]
- Thorn, S.L.; Shuman, J.A.; Stacy, M.R.; Purcell, B.P.; Doviak, H.; Burdick, J.A.; Spinale, F.G.; Sinusas, A.J. Matrix metalloproteinase-targeted SPECT/CT imaging for evaluation of therapeutic hydrogels for the early modulation of post-infarct myocardial remodeling. J. Cardiovasc. Transl. Res. 2023, 16, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Tufaro, V.; Jaffer, F.A.; Serruys, P.W.; Onuma, Y.; van der Steen, A.F.; Stone, G.W.; Muller, J.E.; Marcu, L.; Van Soest, G.; Courtney, B.K.; et al. Emerging hybrid intracoronary imaging technologies and their applications in clinical practice and research. Cardiovasc. Interv. 2024, 17, 1963–1979. [Google Scholar] [CrossRef]
- Noori, M.; Hougaard, M.; Maehara, A.; Trøan, J.; Hansen, K.; Ellert, J.; Veien, K.; Hansen, H.; Junker, A.; Lassen, J.; et al. TCT-53 Near-Infrared Spectroscopy and Intravascular Ultrasound-Guided vs Angiography-Guided Percutaneous Coronary Intervention in Patients With Acute Myocardial Infarction: The NIRVUS Trial. J. Am. Coll. Cardiol. 2024, 84, B174. [Google Scholar] [CrossRef]
- Schneider, V.S.; Böhm, F.; Blum, K.; Riedel, M.; Abdelwahed, Y.S.; Klotsche, J.; Steiner, J.K.; Heuberger, A.; Skurk, C.; Mochmann, H.C.; et al. Impact of real-time angiographic co-registered optical coherence tomography on percutaneous coronary intervention: The OPTICO-integration II trial. Clin. Res. Cardiol. 2021, 110, 249–257. [Google Scholar] [CrossRef]
- Lim, S.; Cha, J.J.; Joo, H.J.; Park, J.H.; Yu, C.W.; Ahn, T.H.; Lim, D.S.; Hong, S.J. The Role of Lipid Core Burden Index Measured by Near-Infrared Spectroscopy in Predicting Slow TIMI Flow After Coronary Intervention. Circulation 2021, 144, A12190. [Google Scholar] [CrossRef]
- Sheth, T.N.; Pinilla-Echeverri, N.; Mehta, S.R.; Courtney, B.K. First-in-human images of coronary atherosclerosis and coronary stents using a novel hybrid intravascular ultrasound and optical coherence tomographic catheter. JACC Cardiovasc. Interv. 2018, 11, 2427–2430. [Google Scholar] [CrossRef]
- Li, J. Development of an Ultrafast Integrated IVUS-OCT System and Catheter for In Vivo Applications. Ph.D. Thesis, UC Irvine, Irvine, CA, USA, 2015. [Google Scholar]
- Muller, J.; Madder, R. OCT-NIRS imaging for detection of coronary plaque structure and vulnerability. Front. Cardiovasc. Med. 2020, 7, 90. [Google Scholar] [CrossRef]
- Kassab, M.; Thrapp, A.; Gardecki, J.A.; Ahsen, O.O.; Kawamura, Y.; Mauskapf, A.; Spicer, G.; Gavgiotaki, E.; Kumar, A.; Modi, M.; et al. LUM015, A Translatable Molecular Imaging Agent, Enables OCT-NIRF Imaging of Inflammatory Protease Activity in Preclinical and Human Atherosclerosis. J. Am. Coll. Cardiol. 2022, 79, 1762. [Google Scholar] [CrossRef]
- Cochennec, F.; Riga, C.; Hamady, M.; Cheshire, N.; Bicknell, C. Improved catheter navigation with 3D electromagnetic guidance. J. Endovasc. Ther. 2013, 20, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.J.; Torabinia, M.; Dhrif, H.; Caprio, A.; Liu, J.; Wong, S.C.; Mosadegh, B. Development of a hybrid training simulator for structural heart disease interventions. Adv. Intell. Syst. 2020, 2, 2000109. [Google Scholar] [CrossRef]
- Finnesgard, E.J.; Simons, J.P.; Marecki, H.; Ofori, I.; Kölbel, T.; Schurink, G.W.H.; van Herwaarden, J.A.; Schanzer, A. Fiber Optic RealShape technology in endovascular surgery. Semin. Vasc. Surg. 2021, 34, 241–246. [Google Scholar] [CrossRef]
- van Herwaarden, J.A.; Jansen, M.M.; Vonken, E.j.P.; Bloemert-Tuin, T.; Bullens, R.W.; de Borst, G.J.; Hazenberg, C.E. First in human clinical feasibility study of endovascular navigation with Fiber Optic RealShape (FORS) technology. Eur. J. Vasc. Endovasc. Surg. 2021, 61, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Sibbald, M.; Mitchell, H.R.; Buccola, J.; Pinilla-Echeverri, N. Impact of Artificial Intelligence-Enhanced Optical Coherence Tomography Software on Percutaneous Coronary Intervention Decisions. J. Soc. Cardiovasc. Angiogr. Interv. 2025, 4, 102438. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y. Improving the diagnosis and treatment of congenital heart disease through the combination of three-dimensional echocardiography and image guided surgery. BMC Med. Imaging 2024, 24, 61. [Google Scholar] [CrossRef]
- Gandhi, S.; Mosleh, W.; Shen, J.; Chow, C.M. Automation, machine learning, and artificial intelligence in echocardiography: A brave new world. Echocardiography 2018, 35, 1402–1418. [Google Scholar] [CrossRef]
- Kweon, J.; Kim, K.; Lee, C.; Kwon, H.; Park, J.; Song, K.; Kim, Y.I.; Park, J.; Back, I.; Roh, J.H.; et al. Deep reinforcement learning for guidewire navigation in coronary artery phantom. IEEE Access 2021, 9, 166409–166422. [Google Scholar] [CrossRef]
- Ma, Y.; Gogin, N.; Cathier, P.; Housden, R.J.; Gijsbers, G.; Cooklin, M.; O’Neill, M.; Gill, J.; Rinaldi, C.A.; Razavi, R.; et al. Real-time x-ray fluoroscopy-based catheter detection and tracking for cardiac electrophysiology interventions. Med. Phys. 2013, 40, 071902. [Google Scholar] [CrossRef]
- Unberath, M.; Gao, C.; Hu, Y.; Judish, M.; Taylor, R.H.; Armand, M.; Grupp, R. The impact of machine learning on 2d/3d registration for image-guided interventions: A systematic review and perspective. Front. Robot. AI 2021, 8, 716007. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, A.; Xu, Y.; Xiong, H.; Meng, M.Q.H. Rl-tee: Autonomous probe guidance for transesophageal echocardiography based on attention-augmented deep reinforcement learning. IEEE Trans. Autom. Sci. Eng. 2023, 21, 1526–1538. [Google Scholar] [CrossRef]
- Kienzlen, A.; Jaensch, F.; Verl, A.; Cheng, L. Concept for a reinforcement learning approach to navigate catheters through blood vessels. In Proceedings of the 2022 28th International Conference on Mechatronics and Machine Vision in Practice (M2VIP), Nanjing, China, 16–18 November 2022; IEEE: Piscataway, NY, USA, 2022; pp. 1–4. [Google Scholar]
- Omisore, O.M.; Akinyemi, T.; Duan, W.; Du, W.; Wang, L. A novel sample-efficient deep reinforcement learning with episodic policy transfer for PID-based control in cardiac catheterization robots. arXiv 2021, arXiv:2110.14941. [Google Scholar]
- Bian, G.; Lipowicz, M.; Kruger, G.H. Self-learning of inverse kinematics for feedforward control of intracardiac robotic ablation catheters. In Proceedings of the 2015 Pattern Recognition Association of South Africa and Robotics and Mechatronics International Conference (PRASA-RobMech), Port Elizabeth, South Africa, 26–27 November 2015; IEEE: Piscataway, NY, USA, 2015; pp. 72–77. [Google Scholar]
- Liu, W.; Tian, T.; Xu, W.; Liang, B.; Lu, Q.; Pan, X.; Zhao, W.; Yang, H.; Su, R. Image-Guided Autonomous Guidewire Navigation in Robot-Assisted Endovascular Interventions using Reinforcement Learning. arXiv 2024, arXiv:2403.05748. [Google Scholar]
- Li, K.; Xu, Y.; Wang, J.; Ni, D.; Liu, L.; Meng, M.Q.H. Image-guided navigation of a robotic ultrasound probe for autonomous spinal sonography using a shadow-aware dual-agent framework. IEEE Trans. Med. Robot. Bionics 2021, 4, 130–144. [Google Scholar] [CrossRef]
- Annabestani, M.; Sriram, S.; Caprio, A.; Janghorbani, S.; Wong, S.C.; Sigaras, A.; Mosadegh, B. High-fidelity pose estimation for real-time extended reality (XR) visualization for cardiac catheterization. Sci. Rep. 2024, 14, 26962. [Google Scholar] [CrossRef]
- Sharafuddin, M.J.; Marjan, A.E. Current status of carbon dioxide angiography. J. Vasc. Surg. 2017, 66, 618–637. [Google Scholar] [CrossRef]
- Ali, M.; Noureldin, M.; Kashef, O.E.; Zaghlol, H. Safety and effectiveness of carbon dioxide contrast medium in infra-inguinal endovascular interventions for patients with chronic threatening lower limb ischemia and renal impairment: A multicentric trial. J. Endovasc. Ther. 2024, 31, 772–783. [Google Scholar] [CrossRef]
- Roshanfar, M.; Salimi, M.; Kaboodrangi, A.H.; Jang, S.J.; Sinusas, A.J.; Wong, S.C.; Mosadegh, B. Advanced Robotics for the Next-Generation of Cardiac Interventions. Micromachines 2025, 16, 363. [Google Scholar] [CrossRef]
- Salimi, M.; Roshanfar, M.; Tabatabaei, N.; Mosadegh, B. Machine Learning-Assisted Short-Wave InfraRed (SWIR) Techniques for Biomedical Applications: Towards Personalized Medicine. J. Pers. Med. 2023, 14, 33. [Google Scholar] [CrossRef] [PubMed]
- Pevnick, J.M.; Birkeland, K.; Zimmer, R.; Elad, Y.; Kedan, I. Wearable technology for cardiology: An update and framework for the future. Trends Cardiovasc. Med. 2018, 28, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Dagher, L.; Shi, H.; Zhao, Y.; Marrouche, N.F. Wearables in cardiology: Here to stay. Heart Rhythm. 2020, 17, 889–895. [Google Scholar] [CrossRef]
- Cheung, C.C.; Krahn, A.D.; Andrade, J.G. The emerging role of wearable technologies in detection of arrhythmia. Can. J. Cardiol. 2018, 34, 1083–1087. [Google Scholar] [CrossRef] [PubMed]
- Jan, A.; Nimmi, K.; Wajid, M.A. Revolutionizing Healthcare with IoT in Cardiology. In Wellness Management Powered by AI Technologies; Scrivener: Beverly, MA, USA, 2025; pp. 231–273. [Google Scholar]
- Yashudas, A.; Gupta, D.; Prashant, G.; Dua, A.; AlQahtani, D.; Reddy, A.S.K. Deep-cardio: Recommendation system for cardiovascular disease prediction using iot network. IEEE Sens. J. 2024, 24, 14539–14547. [Google Scholar] [CrossRef]
- Roshanfar, M.; Jang, S.J.; Sinusas, A.; Wong, S.C.; Mosadegh, B. Addressing Peri-Device Leaks in Next-Generation Transcatheter Left Atrial Appendage Occluders: An Open Question. Surgeries 2025, 6, 15. [Google Scholar] [CrossRef]

| Modality | Parameters | Explanation | |
|---|---|---|---|
| X-ray modalities | Fluoroscopy | Resolution Penetration Depth Depth Estimation Field of View Enhanced Vision Safety and Hazards | Moderate to high resolution depending on the system, ranging from 1- to 2-line pairs per mm. Excellent penetration through soft tissues, limited by bone density. Limited depth perception, as it primarily provides a 2D real-time image. Broad field of view, suitable for dynamic visualization during interventions. Contrast agents enhance visibility, aiding in detailed visualization of vessels and cardiac structures. Exposure to ionizing radiation, requiring careful management to minimize risks. |
| Angiography | Resolution Penetration Depth Depth Estimation Field of View Enhanced Vision Safety and Hazards | Similar to fluoroscopy, offering moderate to high resolution. Excellent penetration through soft tissues and vessels. Limited depth perception, primarily providing 2D images. Well suited for visualizing blood vessels and assessing patency. Utilizes contrast agents to enhance visibility and assess vascular structures. Involves exposure to ionizing radiation, and there may be risks associated with contrast agents. | |
| CT Angiography | Resolution Penetration Depth Depth Estimation Field of View Enhanced Vision Safety and Hazards | High spatial resolution, typically around 0.5 to 1 mm. Limited penetration through bone, excellent visualization of soft tissues. 3D imaging provides detailed depth perception. Comprehensive field of view, capturing detailed anatomy for pre-procedural planning. Iodine-based contrast agents enhance vascular visibility, allowing for detailed assessment of arteries. Involves exposure to ionizing radiation, though advancements aim to minimize radiation dose. | |
| Ultrasound | TTE | Resolution Penetration Depth Depth Estimation Field of View Enhanced Vision Safety and Hazards | Variable, but can achieve high resolution, typically ranging from 1 to 2 mm. Limited penetration through bone, excellent for cardiac imaging. 2D imaging with limited depth perception. Well suited for assessing cardiac structures and functions. Real-time imaging provides dynamic visualization without ionizing radiation. Non-ionizing radiation, considered safe with no known harmful effects. |
| TEE | Resolution Penetration Depth Depth Estimation Field of View Enhanced Vision Safety and Hazards | High resolution, often better than transthoracic echocardiography. Excellent penetration due to close proximity of the probe. 3D imaging enhances depth perception. Detailed visualization of cardiac structures and adjacent areas. Provides clearer images, particularly beneficial for guiding interventions near the heart. Generally considered safe, though it involves inserting a probe into the esophagus. | |
| IVUS | Resolution Penetration Depth Depth Estimation Field of View Enhanced Vision Safety and Hazards | High resolution, typically around 100 to 200 µm. Limited to blood vessels, provides detailed imaging within. 2D cross-sectional imaging, allowing precise assessment of vessel walls. Focused imaging within blood vessels. Direct visualization of vessel walls aids in guiding stent placement. Generally considered safe, though it involves catheterization. | |
| MRI | MRI Imaging | Resolution Penetration Depth Depth Estimation Field of View Enhanced Vision Safety and Hazards | High spatial resolution, typically around 1 to 3 mm. Excellent penetration through tissues, limited by bone. 3D imaging provides detailed depth perception. Comprehensive imaging of cardiac structures. Offers excellent soft tissue contrast without ionizing radiation. Non-ionizing radiation, generally considered safe, but contraindicated in certain conditions. |
| MR Angiography | Resolution Penetration Depth Depth Estimation Field of View Enhanced Vision Safety and Hazards | High spatial resolution, typically around 1 to 2 mm. Excellent for vascular imaging. 3D imaging provides detailed depth perception. Excellent for comprehensive vascular assessments. Contrast-enhanced imaging enhances visibility of blood vessels. Non-ionizing radiation, generally considered safe, but contraindicated in certain conditions. | |
| Optical Imaging | OCT | Resolution Penetration Depth Depth Estimation Field of View Enhanced Vision Safety and Hazards | Very high resolution, typically around 10 to 20 µm. Limited to a few millimeters, providing microscopic imaging. 2D cross-sectional imaging with detailed depth perception at a microscopic level. Narrow field of view but offers microscopic details of coronary arteries. Utilizes near-infrared light for exceptional resolution, providing detailed views of vessel walls. Generally considered safe, non-invasive, and does not involve ionizing radiation. |
| NIRS | Resolution Penetration Depth Depth Estimation Field of View Enhanced Vision Safety and Hazards | Moderate to high resolution, typically around 1 to 2 mm. Limited to a few millimeters, suitable for assessing arterial plaque composition. Provides information about tissue composition within the imaged depth. Specific to the area of interest, focusing on lipid content within arterial plaques. Near-infrared light to assess lipid content in plaques, aiding in decision-making during interventions. Generally considered safe, non-invasive, and does not involve ionizing radiation. |
| Imaging Modality | Spatial Resolution | Temporal Resolution | Radiation Exposure | Real-Time Guidance | Clinical Applications and Outcomes |
|---|---|---|---|---|---|
| Fluoroscopy | 200–300 μm | Excellent (30 fps) | High (5–15 mSv per procedure) | Excellent | Advantages: Wide field of view, excellent device visibility Limitations: Poor soft tissue contrast, radiation exposure Outcomes: Standard of care for catheter navigation; serves as reference for emerging techniques |
| CT Angiography | 350–500 μm | Limited (75–250 ms) | Moderate to high (3–15 mSv) | Limited, primarily pre-procedural | Advantages: 3D volumetric data, excellent calcification assessment Limitations: Limited intra-procedural use, significant radiation Outcomes: Reduced complications in structural interventions |
| Transthoracic Echocardiography | 0.5–1.5 mm | Excellent (>30 fps) | None | Good | Advantages: No radiation, real-time functional assessment Limitations: Operator dependent, limited windows Outcomes: Improved guidance for structural interventions with a reduction in paravalvular leak |
| Transesophageal Echocardiography | 0.5–1 mm | Excellent (>30 fps) | None | Excellent | Advantages: Superior image quality, real-time 3D capabilities Limitations: Semi-invasive, requires sedation Outcomes: Reduction in procedural complications for structural interventions |
| Intravascular Ultrasound | 70–150 μm | Good (20–30 fps) | None | Good | Advantages: Full vessel cross-section, excellent media- adventitia visualization Limitations: Limited plaque characterization, requires vessel access Outcomes: Reduction in MACE |
| Optical Coherence Tomography | 10–20 μm | Good (15–25 fps) | None | Good | Advantages: Highest resolution, superior stent assessment Limitations: Limited penetration, requires blood clearance Outcomes: Reduction in-stent thrombosis |
| MRI | 1–2 mm | Moderate (10–50 ms frame rate) | None | Limited by acquisition time | Advantages: No radiation, excellent soft tissue contrast Limitations: Limited device visualization, slow acquisition Outcomes: Improved procedural success in congenital interventions |
| Near-Infrared Spectroscopy | 1–2 mm | Goode | None | Moderate | Advantages: Lipid core detection, identifies vulnerable plaques Limitations: No structural information alone, limited to lipid detection Outcomes: Prediction of periprocedural MI |
| Near-Infrared Fluorescence | 0.5–1 mm | Moderate | None | Moderate | Advantages: Molecular imaging, detects inflammatory activity Limitations: Limited clinical validation, requires specific probes Outcomes: Emerging evidence for plaque inflammation assessment |
| NIRS-IVUS | IVUS: 70–150 μm NIRS: 1–2 mm | Good (20 fps) | None | Good | Advantages: Combined structural and molecular imaging Limitations: Moderate resolution, higher cost than single modality Outcomes: Reduction in MACE compared to angiography-guided PCI |
| OCT-NIRS | OCT: 10–20 μm NIRS: 1–2 mm | Good (15–20 fps) | None | Good | Advantages: Highest resolution structural imaging with lipid characterization Limitations: Limited penetration, requires blood clearance Outcomes: Reduced edge dissections |
| OCT-NIRF | OCT: 10–20 μm NIRF: 0.5–1 mm | Good | None | Moderate | Advantages: Combined structural and inflammation assessment Limitations: Limited clinical validation, specialty probes required Outcomes: Emerging evidence for inflammation-directed intervention |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roshanfar, M.; Salimi, M.; Jang, S.-J.; Sinusas, A.J.; Kim, J.; Mosadegh, B. Emerging Image-Guided Navigation Techniques for Cardiovascular Interventions: A Scoping Review. Bioengineering 2025, 12, 488. https://doi.org/10.3390/bioengineering12050488
Roshanfar M, Salimi M, Jang S-J, Sinusas AJ, Kim J, Mosadegh B. Emerging Image-Guided Navigation Techniques for Cardiovascular Interventions: A Scoping Review. Bioengineering. 2025; 12(5):488. https://doi.org/10.3390/bioengineering12050488
Chicago/Turabian StyleRoshanfar, Majid, Mohammadhossein Salimi, Sun-Joo Jang, Albert J. Sinusas, Jiwon Kim, and Bobak Mosadegh. 2025. "Emerging Image-Guided Navigation Techniques for Cardiovascular Interventions: A Scoping Review" Bioengineering 12, no. 5: 488. https://doi.org/10.3390/bioengineering12050488
APA StyleRoshanfar, M., Salimi, M., Jang, S.-J., Sinusas, A. J., Kim, J., & Mosadegh, B. (2025). Emerging Image-Guided Navigation Techniques for Cardiovascular Interventions: A Scoping Review. Bioengineering, 12(5), 488. https://doi.org/10.3390/bioengineering12050488










