Bioengineered Approaches for Esophageal Regeneration: Advancing Esophageal Cancer Therapy
Abstract
1. Introduction
2. Clinical Overview of Esophageal Cancer
2.1. Comprehensive Overview of Esophageal Cancer
2.2. Prevalence
2.3. Key Risk Factors for Esophageal Cancer
3. Clinical Strategies and Limitations
3.1. Current Treatment Strategies
3.2. Surgical Approaches & Limitations
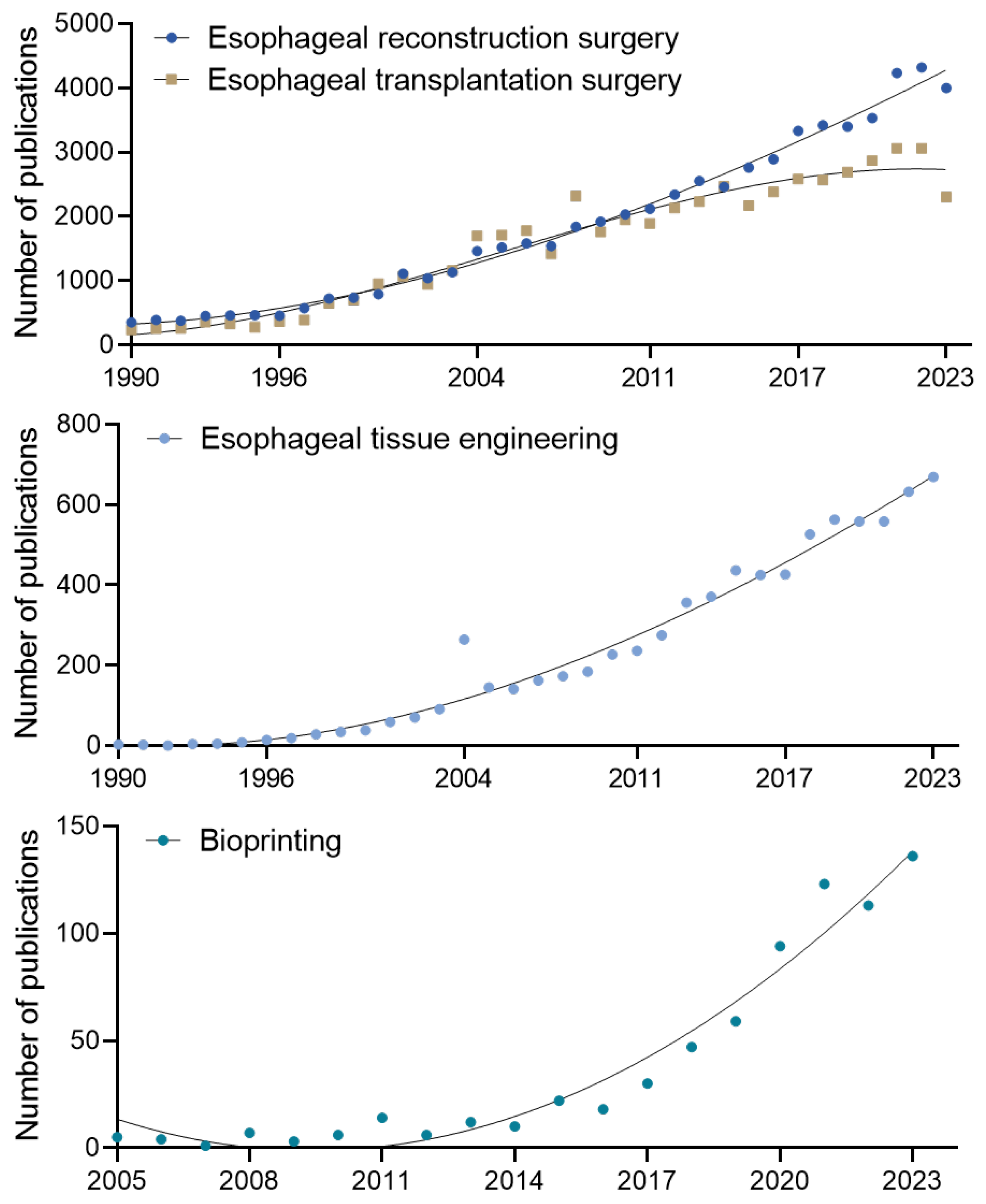
3.3. Research Trends and Emerging Needs
4. Recent Advances in Esophageal Tissue Engineering
4.1. 3D Biofabrication
4.1.1. Patch-Type Structure
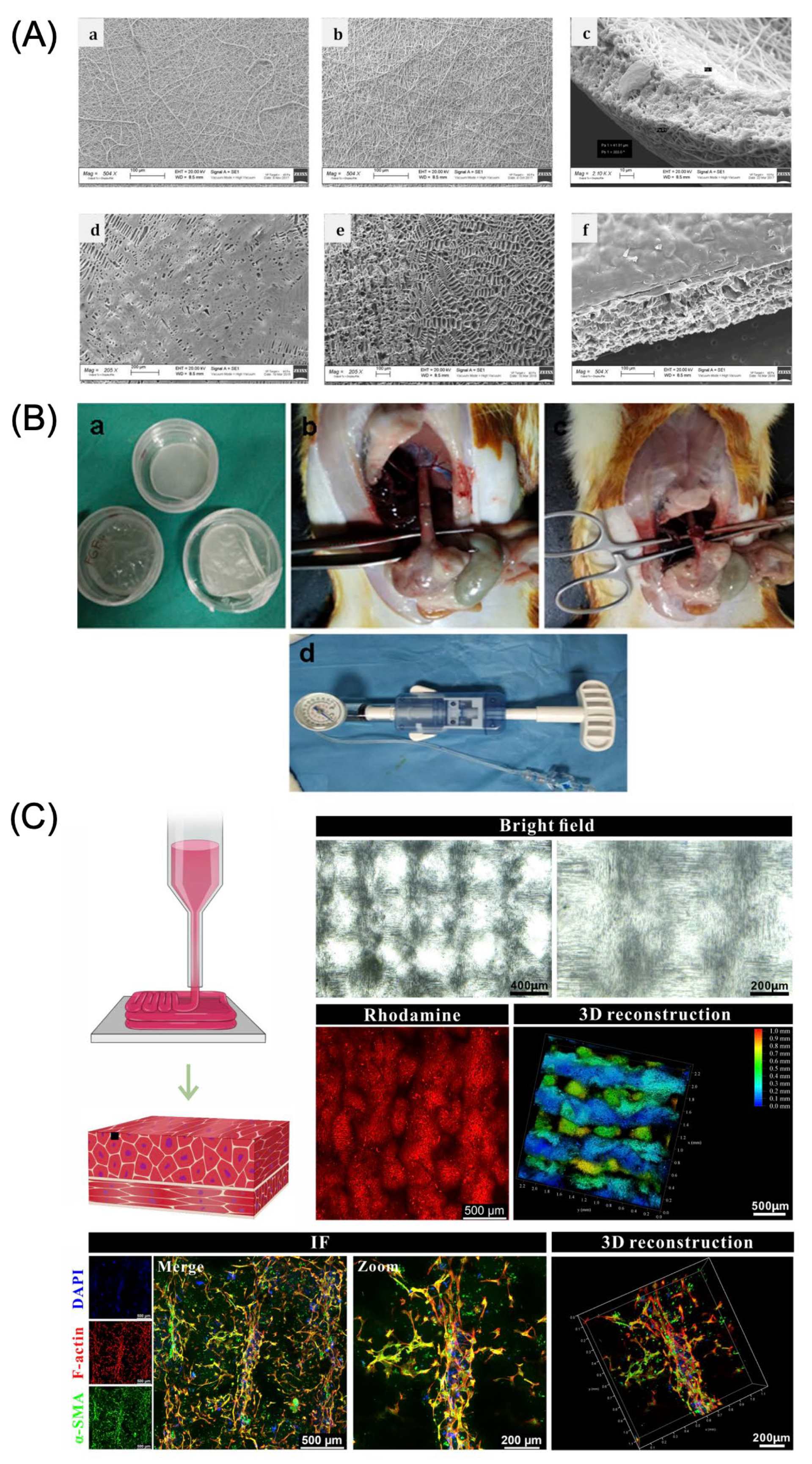
4.1.2. Tubular-Type Structure
5. Animal Models in Esophageal Tissue Engineering Research
5.1. Overview of Animal Models for Esophageal Tissue Engineering
5.1.1. Rat
5.1.2. Rabbit
5.1.3. Pig
5.2. Application of Three-Dimensional Biofabrication in Animal Models for Esophageal Regeneration
6. Challenges and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Sivarao, D.V.; Goyal, R.K. Functional anatomy and physiology of the upper esophageal sphincter. Am. J. Med. 2000, 108, 27–37. [Google Scholar] [CrossRef]
- Brinster, C.J.; Singhal, S.; Lee, L.; Marshall, M.B.; Kaiser, L.R.; Kucharczuk, J.C. Evolving options in the management of esophageal perforation. Ann. Thorac. Surg. 2004, 77, 1475–1483. [Google Scholar] [CrossRef]
- Kovesi, T.; Rubin, S. Long-term Complications of Congenital Esophageal Atresia and/or Tracheoesophageal Fistula. Chest 2004, 126, 915–925. [Google Scholar] [CrossRef]
- Napier, K.J.; Scheerer, M.; Misra, S. Esophageal cancer: A Review of epidemiology, pathogenesis, staging workup and treatment modalities. World J. Gastrointest. Oncol. 2014, 6, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, M.; Roshandel, G.; McCormack, V.; Malekzadeh, R. Current Status and Future Prospects for Esophageal Cancer. Cancers 2023, 15, 765. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.G.; Zhang, W.W.; He, Z.Y.; Sun, J.Y.; Chen, Y.X.; Guo, L. Sites of metastasis and overall survival in esophageal cancer: A population-based study. Cancer Manag. Res. 2017, 9, 781–788. [Google Scholar] [CrossRef]
- Cense, H.A.; Visser, M.R.; van Sandick, J.W.; de Boer, A.G.; Lamme, B.; Obertop, H.; van Lanschot, J.J. Quality of life after colon interposition by necessity for esophageal cancer replacement. J. Surg. Oncol. 2004, 88, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Nam, H.; Jang, J.; Lee, S.J. 3D Bioprinting Strategies for the Regeneration of Functional Tubular Tissues and Organs. Bioengineering 2020, 7, 32. [Google Scholar] [CrossRef]
- Xu, R.; Fang, X.; Wu, S.; Wang, Y.; Zhong, Y.; Hou, R.; Zhang, L.; Shao, L.; Pang, Q.; Zhang, J.; et al. Development and Prospect of Esophageal Tissue Engineering. Front. Bioeng. Biotechnol. 2022, 10, 2022. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, M.; Kim, E.; Lin, S.; Liu, K.; Lan, X.; Que, J. Development and stem cells of the esophagus. Semin. Cell Dev. Biol. 2017, 66, 25–35. [Google Scholar] [CrossRef]
- Kantarcioglu, M.; Caliskan, B.; Demirci, H.; Karacalioglu, O.; Kekilli, M.; Polat, Z.; Gunal, A.; Akinci, M.; Uysal, C.; Eksert, S.; et al. The Efficacy of Mesenchymal Stem Cell Transplantation in Caustic Esophagus Injury: An Experimental Study. Stem Cells Int. 2014, 2014, 939674. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Choi, J.W.; Park, J.-K.; Song, E.H.; Park, S.A.; Kim, Y.S.; Shin, Y.S.; Kim, C.-H. Tissue-engineered artificial oesophagus patch using three-dimensionally printed polycaprolactone with mesenchymal stem cells: A preliminary report. Interact. Cardiovasc. Thorac. Surg. 2016, 22, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Jiang, X.; Li, H.; Guo, L.; Jiang, W.; Lu, S.-H. miR-203 Inhibits the Proliferation and Self-Renewal of Esophageal Cancer Stem-Like Cells by Suppressing Stem Renewal Factor Bmi-1. Stem Cells Dev. 2013, 23, 576–585. [Google Scholar] [CrossRef]
- Zhang, Y. Epidemiology of esophageal cancer. World J. Gastroenterol. 2013, 19, 5598–5606. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef]
- Liu, K.S.; Raza, S.A.; El-Serag, H.B.; Thrift, A.P. Trends in Esophageal Adenocarcinoma and Esophageal Squamous Cell Carcinoma Incidence in the United States from 1992 to 2019. Cancers 2022, 14, 6049. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Hernandez Gonzalo, D.; Lai, J.; Liu, X. Histopathology of Barrett’s Esophagus and Early-Stage Esophageal Adenocarcinoma: An Updated Review. Gastrointest. Disord. 2018, 1, 147–163. [Google Scholar] [CrossRef]
- Tarazi, M.; Chidambaram, S.; Markar, S.R. Risk Factors of Esophageal Squamous Cell Carcinoma beyond Alcohol and Smoking. Cancers 2021, 13, 1009. [Google Scholar] [CrossRef]
- Sijben, J.; Huibertse, L.J.; Rainey, L.; Broeders, M.J.M.; Peters, Y.; Siersema, P.D. Oesophageal cancer awareness and anticipated time to help-seeking: Results from a population-based survey. Br. J. Cancer 2024, 130, 1795–1802. [Google Scholar] [CrossRef]
- Meves, V.; Behrens, A.; Pohl, J. Diagnostics and Early Diagnosis of Esophageal Cancer. Viszeralmedizin 2015, 31, 315–318. [Google Scholar] [CrossRef]
- Leong, C.K.; Foo, A.Z.X.; Goh, K.J.; Hsu, A.A.L.; Ho, A.R.; Ng, M.C.H.; Anantham, D.; Lee, P. Airway interventions for tracheobronchial involvement in esophageal carcinoma: A retrospective cohort outcome study and algorithmic approach. J. Thorac. Dis. 2022, 14, 2565–2578. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.A.; Li, F.; Zhu, J.; Chen, X.; Ren, W.; Gao, B. Multidisciplinary nutritional management improves nutritional and hospitalized outcomes of patients with esophageal cancer undergoing chemoradiotherapy: A randomized control trial. Medicine 2023, 102, e33335. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.T.; Li, Q.; Hao, L.; Ni, Y.J.; Luan, W.Y.; Yang, Z.; Chen, X.D.; Zhang, T.T.; Miao, Y.D.; Zhang, F. Esophageal cancer screening, early detection and treatment: Current insights and future directions. World J. Gastrointest. Oncol. 2024, 16, 1180–1191. [Google Scholar] [CrossRef]
- Yang, C.S.; Chen, X.; Tu, S. Etiology and Prevention of Esophageal Cancer. Gastrointest. Tumors 2016, 3, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Uhlenhopp, D.J.; Then, E.O.; Sunkara, T.; Gaduputi, V. Epidemiology of esophageal cancer: Update in global trends, etiology and risk factors. Clin. J. Gastroenterol. 2020, 13, 1010–1021. [Google Scholar] [CrossRef]
- Marabotto, E.; Pellegatta, G.; Sheijani, A.D.; Ziola, S.; Zentilin, P.; De Marzo, M.G.; Giannini, E.G.; Ghisa, M.; Barberio, B.; Scarpa, M.; et al. Prevention Strategies for Esophageal Cancer-An Expert Review. Cancers 2021, 13, 2183. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Toda, S.; Hatta, W.; Asanuma, K.; Asano, N.; Ono, Y.; Abe, H.; Ogata, Y.; Saito, M.; Kanno, T.; Jin, X.; et al. Decreased Expression of NRF2 Target Genes after Alcohol Exposure in the Background Esophageal Mucosa of Patients with Esophageal Squamous Cell Carcinoma. Tohoku J. Exp. Med. 2022, 258, 195–206. [Google Scholar] [CrossRef]
- Kumagai, N.; Wakai, T.; Akazawa, K.; Ling, Y.; Wang, S.; Shan, B.; Okuhara, Y.; Hatakeyama, Y.; Kataoka, H. Heavy alcohol intake is a risk factor for esophageal squamous cell carcinoma among middle-aged men: A case-control and simulation study. Mol. Clin. Oncol. 2013, 1, 811–816. [Google Scholar] [CrossRef]
- Prabhu, A.; Obi, K.O.; Rubenstein, J.H. The Synergistic Effects of Alcohol and Tobacco Consumption on the Risk of Esophageal Squamous Cell Carcinoma: A Meta-Analysis. Off. J. Am. Coll. Gastroenterol. ACG 2014, 109, 822–827. [Google Scholar] [CrossRef]
- Du, X.; Hidayat, K.; Shi, B.M. Abdominal obesity and gastroesophageal cancer risk: Systematic review and meta-analysis of prospective studies. Biosci. Rep. 2017, 37, BSR20160474. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, A.N.; Murad, M.H.; Buttar, N.S.; El-Serag, H.B.; Katzka, D.A.; Iyer, P.G. Central adiposity is associated with increased risk of esophageal inflammation, metaplasia, and adenocarcinoma: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2013, 11, 1399–1412.e7. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, A.K.; Katzka, D.A.; Iyer, P.G. Endoscopic Screening for Barrett’s Esophagus and Esophageal Adenocarcinoma: Rationale, Candidates, and Challenges. Gastrointest. Endosc. Clin. 2021, 31, 27–41. [Google Scholar] [CrossRef]
- Elliott, J.A.; Donlon, N.E.; Beddy, P.; Donohoe, C.L.; Doyle, S.L.; King, S.; Ravi, N.; Reynolds, J.V. Visceral obesity with and without metabolic syndrome: Incidence and clinical impact in esophageal adenocarcinoma treated with curative intent. Dis. Esophagus 2022, 35, doab094. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, X.; Gao, R.; Wang, Y.; Wei, T.; Zang, Z.; Zhu, L.; Li, Q.; Zhang, Y.; Liu, F. Role of diet in the risks of esophageal adenocarcinoma and squamous cell carcinoma: An updated umbrella review. Eur. J. Nutr. 2024, 63, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Jakszyn, P.; Gonzalez, C.A. Nitrosamine and related food intake and gastric and oesophageal cancer risk: A systematic review of the epidemiological evidence. World J. Gastroenterol. 2006, 12, 4296–4303. [Google Scholar] [CrossRef]
- Yao, L.; Zhong, X.; Huang, G.; Ma, Q.; Xu, L.; Xiao, H.; Guo, X. Investigation on the Potential Correlation Between TP53 and Esophageal Cancer. Front. Cell Dev. Biol. 2021, 9, 730337. [Google Scholar] [CrossRef]
- Zhou, C.; Li, J.; Li, Q. CDKN2A methylation in esophageal cancer: A meta-analysis. Oncotarget 2017, 8, 50071–50083. [Google Scholar] [CrossRef]
- Farha, N.; Lyu, R.; Liska, D.; Bhatt, A.; Macaron, C.; Burke, C.A. Prevalence and risk factors of barrett’s esophagus in lynch syndrome. Fam. Cancer 2023, 22, 55–60. [Google Scholar] [CrossRef]
- Guo, Y.M.; Wang, Q.; Liu, Y.Z.; Chen, H.M.; Qi, Z.; Guo, Q.H. Genetic polymorphisms in cytochrome P4502E1, alcohol and aldehyde dehydrogenases and the risk of esophageal squamous cell carcinoma in Gansu Chinese males. World J. Gastroenterol. 2008, 14, 1444–1449. [Google Scholar] [CrossRef]
- Dulak, A.M.; Stojanov, P.; Peng, S.; Lawrence, M.S.; Fox, C.; Stewart, C.; Bandla, S.; Imamura, Y.; Schumacher, S.E.; Shefler, E.; et al. Exome and whole-genome sequencing of esophageal adenocarcinoma identifies recurrent driver events and mutational complexity. Nat. Genet. 2013, 45, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Fatehi Hassanabad, A.; Chehade, R.; Breadner, D.; Raphael, J. Esophageal carcinoma: Towards targeted therapies. Cell. Oncol. 2020, 43, 195–209. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C. AJCC Cancer Staging Manual; Springer: Berlin/Heidelberg, Germany, 2017; Volume 1024. [Google Scholar]
- McLaren, P.J.; Dolan, J.P. Esophagectomy as a treatment consideration for early-stage esophageal cancer and high-grade dysplasia. J. Laparoendosc. Adv. Surg. Tech. 2016, 26, 757–762. [Google Scholar]
- Gao, G.; Lee, J.H.; Jang, J.; Lee, D.H.; Kong, J.S.; Kim, B.S.; Choi, Y.J.; Jang, W.B.; Hong, Y.J.; Kwon, S.M. Tissue engineered bio-blood-vessels constructed using a tissue-specific bioink and 3D coaxial cell printing technique: A novel therapy for ischemic disease. Adv. Funct. Mater. 2017, 27, 1700798. [Google Scholar]
- Briel, J.W.; Tamhankar, A.P.; Hagen, J.A.; DeMeester, S.R.; Johansson, J.; Choustoulakis, E.; Peters, J.H.; Bremner, C.G.; DeMeester, T.R. Prevalence and risk factors for ischemia, leak, and stricture of esophageal anastomosis: Gastric pull-up versus colon interposition. J. Am. Coll. Surg. 2004, 198, 536–541. [Google Scholar] [PubMed]
- German, J.C.; Waterston, D.J. Colon interposition for the replacement of the esophagus in children. J. Pediatr. Surg. 1976, 11, 227–234. [Google Scholar]
- Malik, S.; Sharma, G.; Sanaka, M.R.; Thota, P.N. Role of endoscopic therapy in early esophageal cancer. World J. Gastroenterol. 2018, 24, 3965. [Google Scholar] [PubMed]
- Wander, P.; Tokar, J.L. Endoscopic management of early esophageal cancer: A literature review. Ann. Esophagus 2023, 6, 16. [Google Scholar]
- American Cancer Society. Treating Esophageal Cancer; American Cancer Society: Atlanta, GA, USA, 2025. [Google Scholar]
- van Hagen, P.; Hulshof, M.; Van Lanschot, J.; Steyerberg, E.; Henegouwen, M.V.B.; Wijnhoven, B.; Richel, D.; Nieuwenhuijzen, G.; Hospers, G.; Bonenkamp, J. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar]
- Watanabe, M.; Otake, R.; Kozuki, R.; Toihata, T.; Takahashi, K.; Okamura, A.; Imamura, Y. Recent progress in multidisciplinary treatment for patients with esophageal cancer. Surg. Today 2020, 50, 12–20. [Google Scholar]
- Kutup, A.; Nentwich, M.F.; Bollschweiler, E.; Bogoevski, D.; Izbicki, J.R.; Hölscher, A.H. What should be the gold standard for the surgical component in the treatment of locally advanced esophageal cancer: Transthoracic versus transhiatal esophagectomy. Ann. Surg. 2014, 260, 1016–1022. [Google Scholar] [PubMed]
- Biere, S.S.; van Berge Henegouwen, M.I.; Maas, K.W.; Bonavina, L.; Rosman, C.; Garcia, J.R.; Gisbertz, S.S.; Klinkenbijl, J.H.; Hollmann, M.W.; de Lange, E.S. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: A multicentre, open-label, randomised controlled trial. Lancet 2012, 379, 1887–1892. [Google Scholar] [PubMed]
- Bothereau, H.; Munoz-Bongrand, N.; Lambert, B.; Montemagno, S.; Cattan, P.; Sarfati, E. Esophageal reconstruction after caustic injury: Is there still a place for right coloplasty? Am. J. Surg. 2007, 193, 660–664. [Google Scholar]
- D’Journo, X.B.; Thomas, P.A. Current management of esophageal cancer. J. Thorac. Dis. 2014, 6, S253. [Google Scholar] [PubMed]
- Luketich, J.D.; Pennathur, A.; Awais, O.; Levy, R.M.; Keeley, S.; Shende, M.; Christie, N.A.; Weksler, B.; Landreneau, R.J.; Abbas, G. Outcomes after minimally invasive esophagectomy: Review of over 1000 patients. Ann. Surg. 2012, 256, 95–103. [Google Scholar]
- Kim, B.S.; Lee, J.-S.; Gao, G.; Cho, D.-W. Direct 3D cell-printing of human skin with functional transwell system. Biofabrication 2017, 9, 025034. [Google Scholar]
- Jang, J.; Park, H.-J.; Kim, S.-W.; Kim, H.; Park, J.Y.; Na, S.J.; Kim, H.J.; Park, M.N.; Choi, S.H.; Park, S.H. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials 2017, 112, 264–274. [Google Scholar] [PubMed]
- Jeong, H.-J.; Nam, H.; Kim, J.-S.; Cho, S.; Park, H.-H.; Cho, Y.-S.; Jeon, H.; Jang, J.; Lee, S.-J. Dragging 3D printing technique controls pore sizes of tissue engineered blood vessels to induce spontaneous cellular assembly. Bioact. Mater. 2024, 31, 590–602. [Google Scholar]
- Nam, H.; Choi, Y.-m.; Cho, S.; Gao, G.; Kim, D.; Kim, J.; Choi, H.; Lee, S.-H.; Jang, J. Modular assembly of bioprinted perfusable blood vessel and tracheal epithelium for studying inflammatory respiratory diseases. Biofabrication 2022, 15, 014101. [Google Scholar]
- Nam, H.; Choi, Y.-m.; Jang, J. Vascularized lower respiratory-physiology-on-a-chip. Appl. Sci. 2020, 10, 900. [Google Scholar] [CrossRef]
- Heo, J.; Nam, H.; Hwang, D.; Cho, S.J.; Jung, S.-Y.; Cho, D.-W.; Shim, J.-H.; Lim, G. Enhanced cellular distribution and infiltration in a wet electrospun three-dimensional fibrous scaffold using eccentric rotation-based hydrodynamic conditions. Sens. Actuators B Chem. 2016, 226, 357–363. [Google Scholar]
- Farhat, W.; Chatelain, F.; Marret, A.; Faivre, L.; Arakelian, L.; Cattan, P.; Fuchs, A. Trends in 3D bioprinting for esophageal tissue repair and reconstruction. Biomaterials 2021, 267, 120465. [Google Scholar]
- Yang, P.; Ju, Y.; Hu, Y.; Xie, X.; Fang, B.; Lei, L. Emerging 3D bioprinting applications in plastic surgery. Biomater. Res. 2023, 27, 1. [Google Scholar]
- Ramesh, S.; Harrysson, O.L.; Rao, P.K.; Tamayol, A.; Cormier, D.R.; Zhang, Y.; Rivero, I.V. Extrusion bioprinting: Recent progress, challenges, and future opportunities. Bioprinting 2021, 21, e00116. [Google Scholar]
- Ng, W.L.; Shkolnikov, V. Jetting-based bioprinting: Process, dispense physics, and applications. Bio-Des. Manuf. 2024, 7, 771–799. [Google Scholar]
- Bonetti, L.; Scalet, G. 4D fabrication of shape-changing systems for tissue engineering: State of the art and perspectives. Prog. Addit. Manuf. 2025, 10, 1913–1943. [Google Scholar] [CrossRef]
- Han, H.; Park, Y.; Choi, Y.m.; Yong, U.; Kang, B.; Shin, W.; Min, S.; Kim, H.J.; Jang, J. A bioprinted tubular intestine model using a colon-specific extracellular matrix bioink. Adv. Healthc. Mater. 2022, 11, 2101768. [Google Scholar]
- Kang, B.; Park, Y.; Hwang, D.G.; Kim, D.; Yong, U.; Lim, K.S.; Jang, J. Facile bioprinting process for fabricating size-controllable functional microtissues using light-activated decellularized extracellular matrix-based bioinks. Adv. Mater. Technol. 2022, 7, 2100947. [Google Scholar]
- Yong, U.; Lee, S.; Jung, S.; Jang, J. Interdisciplinary approaches to advanced cardiovascular tissue engineering: ECM-based biomaterials, 3D bioprinting, and its assessment. Prog. Biomed. Eng. 2020, 2, 042003. [Google Scholar]
- Jang, J.; Park, J.Y.; Gao, G.; Cho, D.-W. Biomaterials-based 3D cell printing for next-generation therapeutics and diagnostics. Biomaterials 2018, 156, 88–106. [Google Scholar]
- Hou, L.; Jin, J.; Lv, J.; Chen, L.; Zhu, Y.; Liu, X. Constitution and in vivo test of micro-porous tubular scaffold for esophageal tissue engineering. J. Biomater. Appl. 2015, 30, 568–578. [Google Scholar]
- Adel, I.M.; ElMeligy, M.F.; Elkasabgy, N.A. Conventional and recent trends of scaffolds fabrication: A superior mode for tissue engineering. Pharmaceutics 2022, 14, 306. [Google Scholar] [CrossRef] [PubMed]
- Nieponice, A.; Ciotola, F.F.; Nachman, F.; Jobe, B.A.; Hoppo, T.; Londono, R.; Badylak, S.; Badaloni, A.E. Patch esophagoplasty: Esophageal reconstruction using biologic scaffolds. Ann. Thorac. Surg. 2014, 97, 283–288. [Google Scholar]
- Pisani, S.; Croce, S.; Chiesa, E.; Dorati, R.; Lenta, E.; Genta, I.; Bruni, G.; Mauramati, S.; Benazzo, A.; Cobianchi, L. Tissue engineered esophageal patch by mesenchymal stromal cells: Optimization of electrospun patch engineering. Int. J. Mol. Sci. 2020, 21, 1764. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kim, I.G.; Wu, Y.; Cho, H.; Shin, J.W.; Park, S.A.; Chung, E.J. Experimental investigation of esophageal reconstruction with electrospun polyurethane nanofiber and 3D printing polycaprolactone scaffolds using a rat model. Head Neck 2021, 43, 833–848. [Google Scholar]
- Cesur, O.; Tanir, T.E.; Celepli, P.; Ozarslan, F.; Hucumenoglu, S.; Karaibrahimoglu, A.; Hasirci, N. Enhancing esophageal repair with bioactive bilayer mesh containing FGF. Sci. Rep. 2021, 11, 19203. [Google Scholar]
- Qin, J.; Zhao, J.; Wu, Y.; Li, L.; Li, D.; Deng, H.; Liu, J.; Zhang, L. Chitosan/collagen layer-by-layer deposition for improving the esophageal regeneration ability of nanofibrous mats. Carbohydr. Polym. 2022, 286, 119269. [Google Scholar]
- Yeo, M.; Yoon, J.W.; Park, G.T.; Shin, S.C.; Song, Y.C.; Cheon, Y.I.; Lee, B.J.; Kim, G.H.; Kim, J.H. Esophageal wound healing by aligned smooth muscle cell-laden nanofibrous patch. Mater. Today Bio 2023, 19, 100564. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, Y.; Gu, Z.; Ni, R.; Feng, P.; Hu, Z.; Song, L.; Shen, X.; Gu, C.; Li, J.; et al. Engineered muscle from micro-channeled PEG scaffold with magnetic Fe(3)O(4) fixation towards accelerating esophageal muscle repair. Mater. Today Bio 2023, 23, 100853. [Google Scholar] [CrossRef]
- Luo, Y.; Hu, Z.; Ni, R.; Xu, R.; Zhao, J.; Feng, P.; Zhu, T.; Chen, Y.; Yao, J.; Yao, Y.; et al. Fabrication of 3D Biomimetic Smooth Muscle Using Magnetic Induction and Bioprinting for Tissue Regeneration. Biomater. Res. 2024, 28, 0076. [Google Scholar] [CrossRef]
- Gundogdu, G.; Morhardt, D.; Cristofaro, V.; Algarrahi, K.; Yang, X.; Costa, K.; Alegria, C.G.; Sullivan, M.P.; Mauney, J.R. Evaluation of Bilayer Silk Fibroin Grafts for Tubular Esophagoplasty in a Porcine Defect Model. Tissue Eng. Part A 2021, 27, 103–116. [Google Scholar] [CrossRef]
- Farhat, W.; Ayollo, D.; Arakelian, L.; Thierry, B.; Mazari-Arrighi, E.; Caputo, V.; Faivre, L.; Cattan, P.; Larghero, J.; Chatelain, F.; et al. Biofabrication of an Esophageal Tissue Construct from a Polymer Blend Using 3D Extrusion-Based Printing. Adv. Eng. Mater. 2022, 24. [Google Scholar] [CrossRef]
- Nam, H.; Jeong, H.-J.; Jo, Y.; Lee, J.Y.; Ha, D.-H.; Kim, J.H.; Chung, J.H.; Cho, Y.-S.; Cho, D.-W.; Lee, S.-J.; et al. Multi-layered Free-form 3D Cell-printed Tubular Construct with Decellularized Inner and Outer Esophageal Tissue-derived Bioinks. Sci. Rep. 2020, 10, 7255. [Google Scholar] [CrossRef]
- Sarrafian, T.L.; Brazzell, J.L.; Barron, M.; Aho, J.; Blanco, E.; Powell, C.; Johnson, J.; Wigle, D.A. Serial evaluation of segmental esophageal reconstruction using a polyurethane scaffold in a pig model. J. Thorac. Dis. 2022, 14, 1830–1839. [Google Scholar] [CrossRef] [PubMed]
- Yeleswarapu, S.; Chameettachal, S.; Pati, F. Integrated 3D Printing-Based Framework—A Strategy to Fabricate Tubular Structures with Mechanocompromised Hydrogels. ACS Appl. Bio Mater. 2021, 4, 6982–6992. [Google Scholar] [CrossRef]
- Qiu, S.; Liang, L.; Zou, P.; Chen, Q. Decellularized small intestine submucosa/polylactic-co-glycolic acid composite scaffold for potential application in hypopharyngeal and cervical esophageal tissue repair. Regen. Biomater. 2021, 8, rbaa061. [Google Scholar] [CrossRef]
- Pisani, S.; Croce, S.; Mauramati, S.; Marmonti, M.; Cobianchi, L.; Herman, I.; Dorati, R.; Avanzini, M.A.; Genta, I.; Benazzo, M.; et al. Engineered Full Thickness Electrospun Scaffold for Esophageal Tissue Regeneration: From In Vitro to In Vivo Approach. Pharmaceutics 2022, 14, 252. [Google Scholar] [CrossRef]
- Piedrahita, J.A.; Williams, J.K. Animal Models in Tissue Engineering. Part I. Tissue Eng. Part C Methods 2017, 23, 641–642. [Google Scholar] [CrossRef] [PubMed]
- Piedrahita, J.A.; Williams, J.K. * Animal Models in Tissue Engineering. Part II. Tissue Eng. Part C Methods 2017, 23, 827–828. [Google Scholar] [CrossRef]
- Kapoor, H.; Lohani, K.R.; Lee, T.H.; Agrawal, D.K.; Mittal, S.K. Animal Models of Barrett’s Esophagus and Esophageal Adenocarcinoma-Past, Present, and Future. Clin. Transl. Sci. 2015, 8, 841–847. [Google Scholar] [CrossRef]
- Macke, R.A.; Nason, K.S.; Mukaisho, K.-i.; Hattori, T.; Fujimura, T.; Sasaki, S.; Oyama, K.; Miyashita, T.; Ohta, T.; Miwa, K.; et al. Barrett’s esophagus and animal models. Ann. N. Y. Acad. Sci. 2011, 1232, 392–400. [Google Scholar] [CrossRef]
- Nair, D.V.; Reddy, A.G. Laboratory animal models for esophageal cancer. Vet. World 2016, 9, 1229–1232. [Google Scholar] [CrossRef] [PubMed]
- Bryda, E.C. The Mighty Mouse: The impact of rodents on advances in biomedical research. Mo. Med. 2013, 110, 207–211. [Google Scholar] [PubMed]
- Kim, H.B.; Jo, Y.; Woo, S.H.; Han, S.Y.; Lee, S.H.; Chang, Y.-T.; Park, J.Y.; Jang, J.; Han, H.H. The Effect of 3-Dimensional–Printed Sequential Dual Drug–Releasing Patch on the Capsule Formation Around the Silicone Implant in a Rat Model. Aesthetic Surg. J. 2024, 44, NP411–NP420. [Google Scholar] [CrossRef]
- Sun, K.-H.; Liu, Z.; Liu, C.; Yu, T.; Shang, T.; Huang, C.; Zhou, M.; Liu, C.; Ran, F.; Li, Y.; et al. Evaluation of in vitro and in vivo biocompatibility of a myo-inositol hexakisphosphate gelated polyaniline hydrogel in a rat model. Sci. Rep. 2016, 6, 23931. [Google Scholar] [CrossRef]
- Su, Y.; Chen, X.; Klein, M.; Fang, M.; Wang, S.; Yang, C.S.; Goyal, R.K. Phenotype of columnar-lined esophagus in rats with esophagogastroduodenal anastomosis: Similarity to human Barrett’s esophagus. Lab. Investig. 2004, 84, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Diemer, P.; Markoew, S.; Le, D.Q.S.; Qvist, N. Poly-ε-caprolactone mesh as a scaffold for in vivo tissue engineering in rabbit esophagus. Dis. Esophagus 2015, 28, 240–245. [Google Scholar] [CrossRef]
- Hannon, E.; Pellegrini, M.; Scottoni, F.; Durkin, N.; Shibuya, S.; Lutman, R.; Proctor, T.J.; Hutchinson, J.C.; Arthurs, O.J.; Phylactopoulos, D.-E.; et al. Lessons learned from pre-clinical testing of xenogeneic decellularized esophagi in a rabbit model. iScience 2022, 25, 105174. [Google Scholar] [CrossRef]
- Luc, G.; Charles, G.; Gronnier, C.; Cabau, M.; Kalisky, C.; Meulle, M.; Bareille, R.; Roques, S.; Couraud, L.; Rannou, J.; et al. Decellularized and matured esophageal scaffold for circumferential esophagus replacement: Proof of concept in a pig model. Biomaterials 2018, 175, 1–18. [Google Scholar] [CrossRef]
- Perry, Y.; Epperly, M.W.; Fernando, H.C.; Klein, E.; Finkelstein, S.; Greenberger, J.S.; Luketich, J.D. Photodynamic therapy induced esophageal stricture—An animal model: From mouse to pig. J. Surg. Res. 2005, 123, 67–74. [Google Scholar] [CrossRef]
- Diaz del Consuelo, I.; Pizzolato, G.-P.; Falson, F.; Guy, R.H.; Jacques, Y. Evaluation of pig esophageal mucosa as a permeability barrier model for buccal tissue. J. Pharm. Sci. 2005, 94, 2777–2788. [Google Scholar] [CrossRef] [PubMed]
- Lubin, J.H.; Cook, M.B.; Pandeya, N.; Vaughan, T.L.; Abnet, C.C.; Giffen, C.; Webb, P.M.; Murray, L.J.; Casson, A.G.; Risch, H.A.; et al. The importance of exposure rate on odds ratios by cigarette smoking and alcohol consumption for esophageal adenocarcinoma and squamous cell carcinoma in the Barrett’s Esophagus and Esophageal Adenocarcinoma Consortium. Cancer Epidemiol. 2012, 36, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Belsky, K.; Smiell, J. Navigating the Regulatory Pathways and Requirements for Tissue-Engineered Products in the Treatment of Burns in the United States. J. Burn Care Res. 2020, 42, 774–784. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Guidance for the Content of Premarket Notifications for Esophageal and Tracheal Prostheses; Food and Drug Administration (FDA): Rockville, MD, USA, 1998.
- Model, L.; Wiesel, O. A narrative review of esophageal tissue engineering and replacement: Where are we? Ann. Transl. Med. 2021, 9, 910. [Google Scholar] [CrossRef]
- Poghosyan, T.; Catry, J.; Luong-Nguyen, M.; Bruneval, P.; Domet, T.; Arakelian, L.; Sfeir, R.; Michaud, L.; Vanneaux, V.; Gottrand, F.; et al. Esophageal tissue engineering: Current status and perspectives. J. Visc. Surg. 2016, 153, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Kalogeropoulou, M.; Díaz-Payno, P.J.; Mirzaali, M.J.; van Osch, G.J.V.M.; Fratila-Apachitei, L.E.; Zadpoor, A.A. 4D printed shape-shifting biomaterials for tissue engineering and regenerative medicine applications. Biofabrication 2024, 16, 022002. [Google Scholar] [CrossRef]

| Category | Criteria | |
|---|---|---|
| Primary Tumor (T) | TX | Primary tumors cannot be assessed |
| T0 | No evidence of a primary tumor | |
| Tis | High-grade dysplasia (carcinoma in situ) | |
| T1 | Tumor invades the lamina propria, muscularis mucosae, or submucosa | |
| T1a | Tumor invades the lamina propria or muscularis mucosae | |
| T1b | Tumor invades the submucosa | |
| T2 | Tumor invades the muscularis propria | |
| T3 | Tumor invades the adventitia | |
| T4 | Tumor invades adjacent structures | |
| T4a | Resectable tumor invading the pleura, pericardium, or diaphragm | |
| T4b | Unresectable tumors invading critical adjacent structures, such as the aorta, vertebral body, or trachea | |
| Regional Lymph Nodes (N) | NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis | |
| N1 | Metastasis in 1–2 regional lymph nodes | |
| N2 | Metastasis in 3–6 regional lymph nodes | |
| N3 | Metastasis in 7 or more regional lymph nodes | |
| Distant Metastasis (M) | M0 | No distant metastasis |
| M1 | Distant metastasis present | |
| Bioprinting | Electrospinning | Mold-Based Fabrication | |
|---|---|---|---|
| Description | A layer-by-layer additive manufacturing technique that precisely deposits cell-laden bioinks to construct three-dimensional tissue-like structures. | A fabrication technique that uses a high-voltage electric field to draw polymer solutions into nanofibers, forming scaffolds that mimic the extracellular matrix (ECM). | A traditional technique that involves casting biomaterials or cell-laden hydrogels into pre-designed molds to form tubular or complex tissue structures. |
| Strengths of 3D Biofabrication in Esophageal Tissue Engineering |
|
|
|
| Limitations of 3D Biofabrication in Esophageal Tissue Engineering |
|
|
|
| Advantages for esophageal scaffold |
|
|
|
| Disadvantages for esophageal scaffold |
|
|
|
| Animal Model | Defect Shape | Defect Size | Scaffold Type | Fabrication Techniques (Materials) | Experiment Period | Functional Outcomes | Ref. |
|---|---|---|---|---|---|---|---|
| Rat | Wedge | 1.5 × 2 mm2 | Patch | Electrospinning (PU) 3D printing (PCL) | 4 weeks | PU nanofiber exhibits a tendency to increase re-epithelialization PCL scaffold shows a tendency for more muscle regeneration | [5] |
| Patch (sheet) | Electrospinning (nylon6/silk fibroin) | 4 weeks | LBL-structured nanofibrous mats display good hydrophilicity, facilitating cell adhesion and proliferation The antibacterial capacity of the mats against E. coli and S. aureus is >90% | [18] | |||
| Half-circle | 0.5 × 0.5 cm2 | Patch | Solvent casting (PCL) Crosslinking (gelatin, FGF) | 4 weeks | A bilayer polymeric mesh containing FGF significantly enhances bioactivity, promoting epithelial regeneration and collagen accumulation | [17] | |
| Circle | Ø 2 mm | Patch | 3D printing (PCL) Cell electrospinning (MSCs, SMCs, alginate, PEO) | 2 weeks | CE-SMC patch significantly enhances vascularization, leading to the formation of abundant new blood vessels The CE SMC patch increases the expression of SM22α and vimentin, indicating higher esophageal muscle regeneration | [23] | |
| Linear | 4 mm | Patch | UV molding (PEGDA) Nanoparticle alignment (Fe3O4) + sputtering (Au) | 5 days | Fe3O4 micro-/nano-stripes, used as alignment inducers within a microchannel-patterned scaffold, promote esophageal muscle tissue regeneration and muscle repair | [24] | |
| Rabbit | Circumferential | 1.6 cm | Tubular | Decellularization (pig esophagus) | 16 days | A vascularized muscle flap successfully promotes decellularized scaffold anastomoses and neovascularization However, long-term survival remains limited owing to the fragility of the animal model, requiring further testing in larger models | [31] |
| Square | ~3 × 5 mm2 | Patch | 3D bioprinting (GelMA, SFMA, Fe3O4, BMSC) | 9 days | Hydrogel scaffold supports cell growth and differentiation, aligning BMSCs into SMCs to create a transplantable biomimetic muscle construct It effectively restores smooth muscle structure by enhancing SMC alignment and ECM remodeling | [25] | |
| Pig | Circumferential | 4 cm | Cylindrical patch | Decellularization (pig esophagus) QMR | 3 months | A QMR-treated scaffold maintains its structural integrity while forming an interconnected network, enhancing cell adhesion and integration BM-MSC-seeded scaffolds enhance esophageal muscle regeneration and reduce inflammation | [32] |
| 5, 10 cm | Tubular | Electrospinning (PU) | 13 months | Although complete esophageal layer formation is not achieved, esophageal healing is observed with stent use A structurally intact tube with patency and no leakage shows significant clinical potential, even if it does not fully replicate the native esophagus | [29] | ||
| 2.5 cm | Tubular | Electrospinning (PLA, PCL) | 7 days | A PLA-PCL electrospun scaffold provides effective support for promoting esophageal regeneration, as confirmed by preliminary in vitro and in vivo studies | [104] | ||
| 2 cm | Tubular | Solvent-casting/salt-leaching (silk fibroin) | 3 months | Acellular tubular BLSF implants promote esophageal tissue regeneration, including innervated, vascularized epithelial, and muscular components These grafts show minimal immune reactions and preserve implantation site integrity while, in some cases, enabling oral food consumption | [26] * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-S.; Nam, H.; Kim, E.C.; Jeong, H.-J.; Lee, S.-J. Bioengineered Approaches for Esophageal Regeneration: Advancing Esophageal Cancer Therapy. Bioengineering 2025, 12, 479. https://doi.org/10.3390/bioengineering12050479
Kim J-S, Nam H, Kim EC, Jeong H-J, Lee S-J. Bioengineered Approaches for Esophageal Regeneration: Advancing Esophageal Cancer Therapy. Bioengineering. 2025; 12(5):479. https://doi.org/10.3390/bioengineering12050479
Chicago/Turabian StyleKim, Jae-Seok, Hyoryung Nam, Eun Chae Kim, Hun-Jin Jeong, and Seung-Jae Lee. 2025. "Bioengineered Approaches for Esophageal Regeneration: Advancing Esophageal Cancer Therapy" Bioengineering 12, no. 5: 479. https://doi.org/10.3390/bioengineering12050479
APA StyleKim, J.-S., Nam, H., Kim, E. C., Jeong, H.-J., & Lee, S.-J. (2025). Bioengineered Approaches for Esophageal Regeneration: Advancing Esophageal Cancer Therapy. Bioengineering, 12(5), 479. https://doi.org/10.3390/bioengineering12050479






