Susceptibility-Weighted Imaging (SWI): Technical Aspects and Applications in Brain MRI for Neurodegenerative Disorders
Abstract
1. Introduction
2. Materials and Methods
3. Technical Fundamentals of SWI
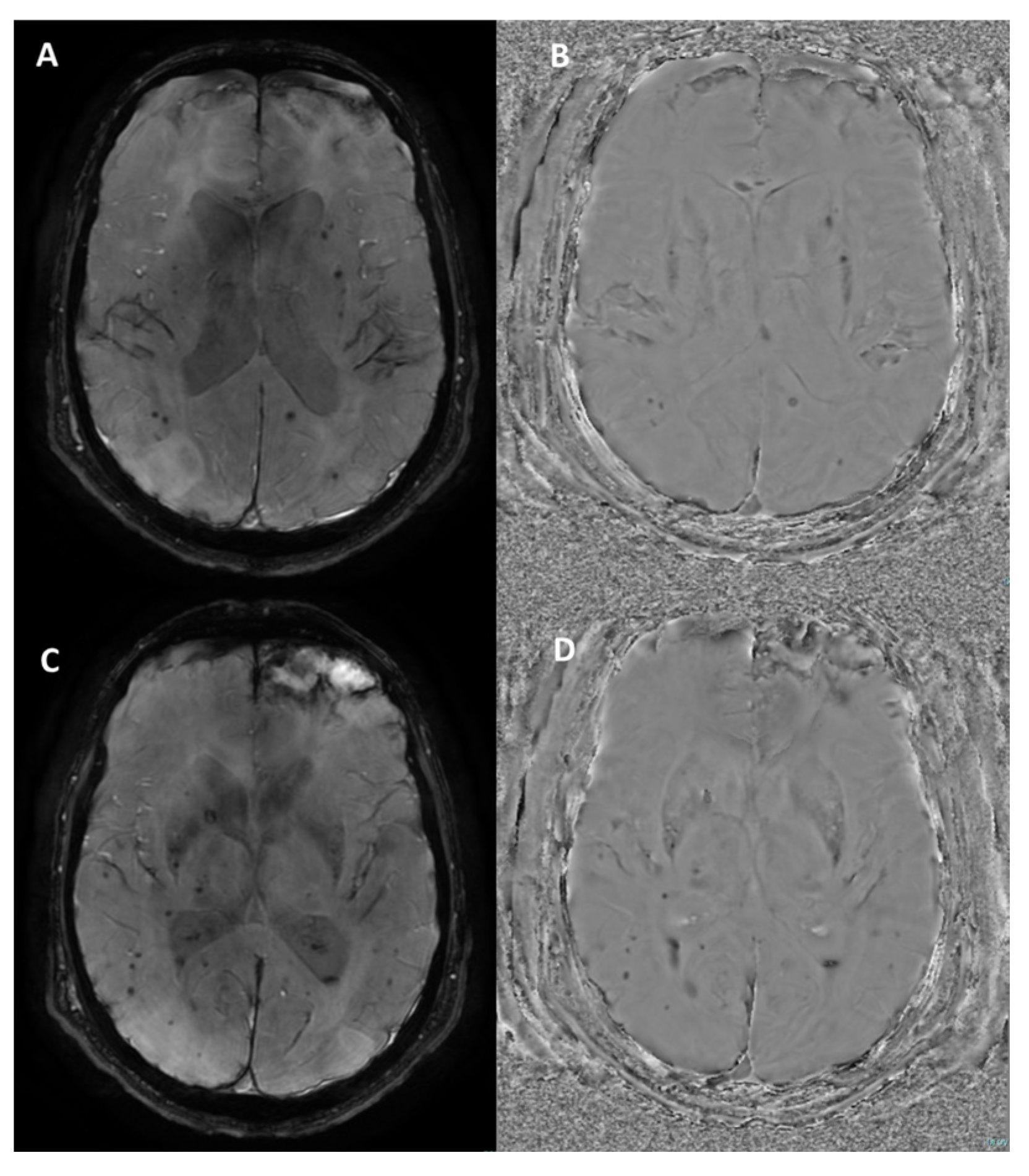
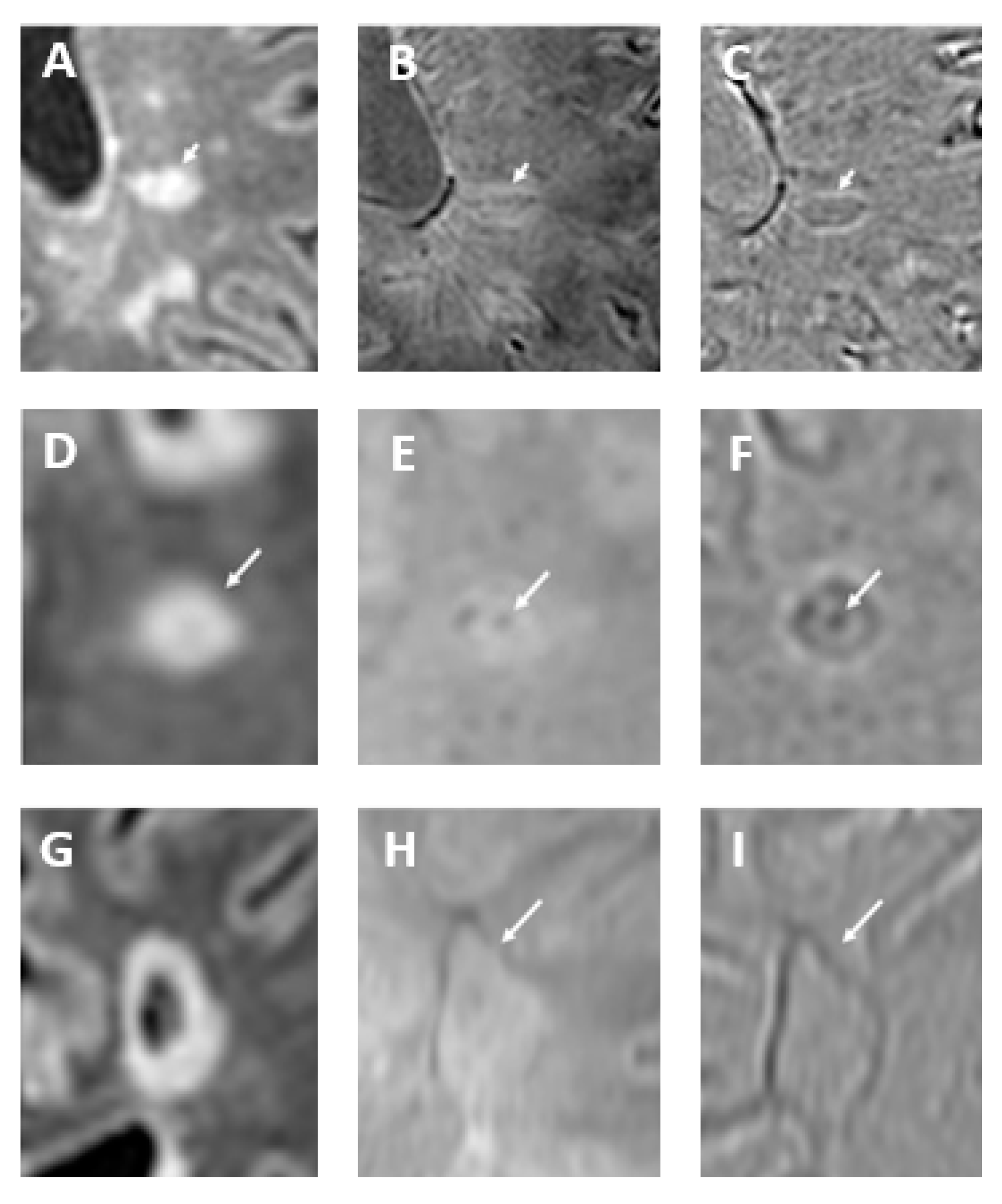
How Can Calcifications and Blood Be Differentiated on an SWI Phase Map?
4. SWI Applications in Neurodegenerative Disorders
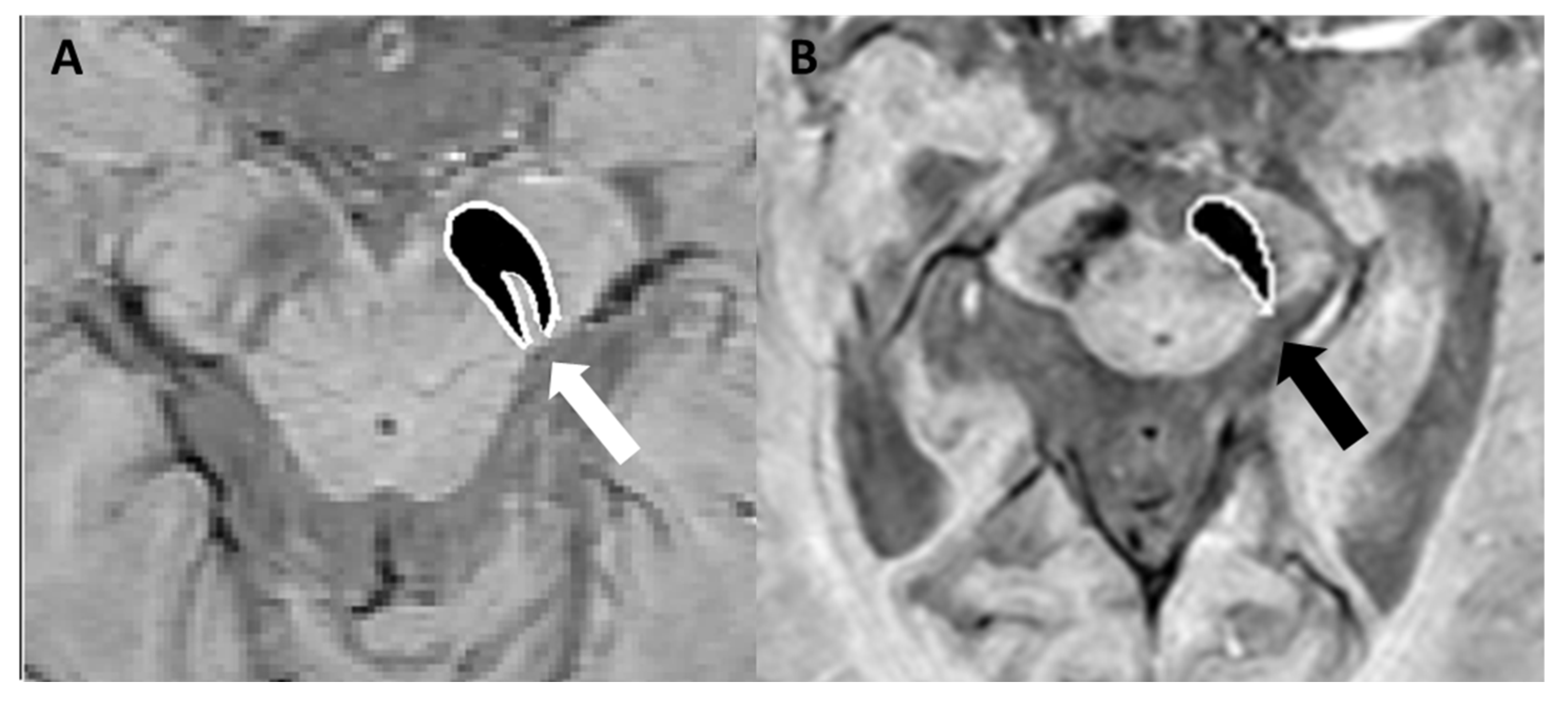
4.1. Parkinson’s Disease, Lewy Body Dementia, and Atypical Parkinsonian Syndromes
4.2. Multiple Sclerosis
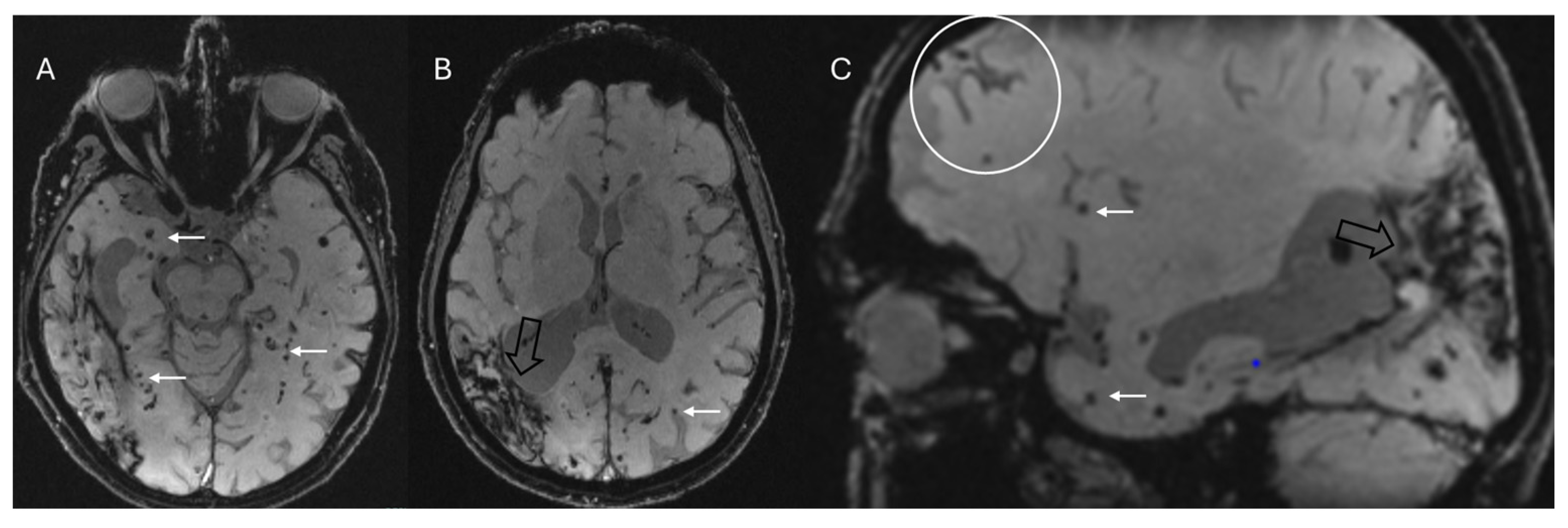
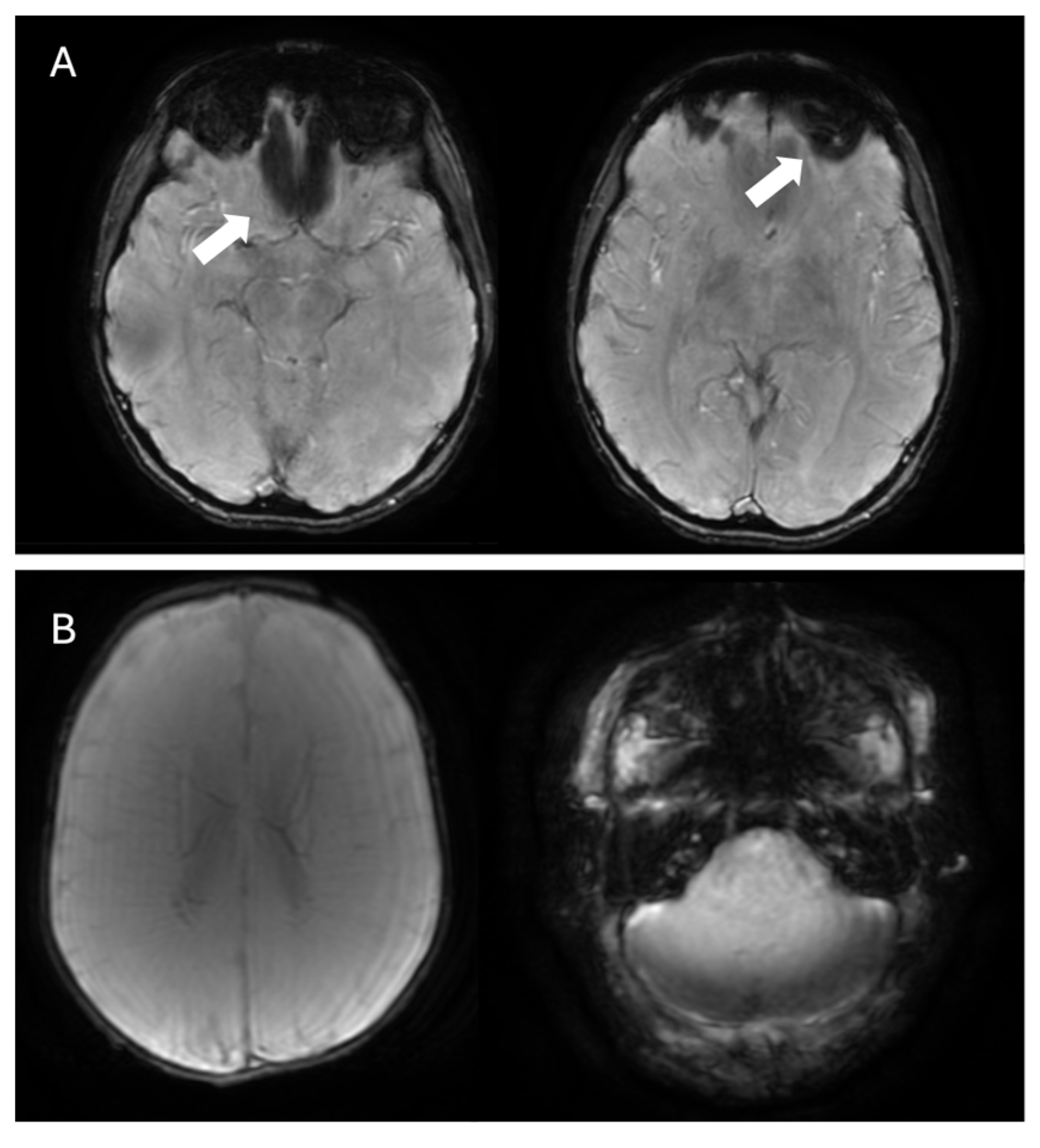
4.3. Cerebral Amyloid Angiopathy
4.4. Other Conditions
- -
- Amyotrophic lateral sclerosis is a progressive neurodegenerative disease affecting upper and lower motor neuron function. While traditional MRI sequences struggle to reliably visualize axonal degeneration in the corticospinal tracts of amyotrophic lateral sclerosis patients, SWI has shown promise in revealing abnormalities in the motor cortex. Specifically, a low signal intensity region, termed the “motor band sign”, is observed more frequently in younger patients and may reflect iron deposition associated with upper motor neuron involvement [69]. The high contrast observed in the motor cortex via QSM may serve as a valuable and sensitive tool for both the diagnosis and prognosis of amyotrophic lateral sclerosis [2].
- -
- Hereditary ataxias are characterized by the slow, progressive degeneration of the cerebellum and its pathways, resulting in motor incoordination and balance impairments. These impairments present as limb ataxia, gait and stance ataxia, dysarthria, and oculomotor signs. SWI imaging reveals atrophy of the cerebellar nuclei in spinocerebellar ataxia 6, Friedreich’s ataxia, and spinocerebellar ataxia 3 [70]. In patients with oculomotor apraxia, a key diagnostic indicator is the absence of the normal hypointensity in the dentate nucleus on 3T SWI and FLAIR scans, exhibiting both high sensitivity and specificity [71].
- -
- Huntington’s disease, an autosomal dominant neurodegenerative disorder, involves the progressive loss of GABAergic neurons in the basal ganglia, notably the caudate and putamen (dorsal striatum). This neuronal loss leads to chorea, subcortical cognitive impairment, behavioral changes, and depression, typically beginning in midlife. Iron deposition within the basal ganglia (mainly globus pallidus) can sometimes manifest as decreased T2 signal and blooming on SWI [72].
- -
- Neurodegeneration with brain iron accumulation refers to a diverse and progressive group of disorders characterized by excessive iron deposition in the brain, particularly within the basal ganglia, and the majority of these conditions have a genetic origin [73]. The “eye of the tiger” sign in the globus pallidus is the most recognized imaging feature, typically associated with pantothenate kinase-associated neurodegeneration (formerly known as Hallervorden–Spatz disease) [74]. However, it has been demonstrated that this sign is not pathognomonic, particularly in adult patients [75]. Caution is therefore needed to avoid misinterpreting this finding, as some authors have even reported that it may appear as a normal finding on 3T MRI scanners [76]. Furthermore, QSM shows promise in clearly visualizing age-atypical iron accumulation in the globus pallidus due to its high contrast capabilities [2,77].
- -
- Chronic traumatic encephalopathy is characterized by perivascular accumulations of hyperphosphorylated tau in neurons and cellular processes, especially at the depths of the sulci. This neurodegenerative disease is associated with repeated head injuries, frequently encountered in contact sports [78]. Repetitive head impacts and traumatic brain injury can lead to microbleeds, which may have implications for the pathogenesis of chronic traumatic encephalopathy. Microhemorrhages associated with diffuse axonal injury frequently occur at the gray–white matter junction, in the corpus callosum, and within the brainstem. SWI can accurately identify these hemorrhagic foci, some of which may exhibit a linear configuration [79]. However, microbleeds have been observed infrequently in studies examining retired professional athletes from contact sports [80]. SWI has been shown to be more accurate and sensitive in detecting microbleeds associated with diffuse axonal injury compared to T2*-GRE sequences. Nevertheless, these findings have low specificity for chronic traumatic encephalopathy and may also be present in other traumatic brain injuries [79].
5. Pitfalls and Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| BSI | blood-sensitive imaging |
| CAA | cerebral amyloid angiopathy |
| CVS | central vein sign |
| FLAIR | fluid-attenuated inversion recovery |
| FSBB | flow-sensitive black blood |
| GRE | gradient recalled echo |
| MinIP | minimum intensity projection |
| MRI | magnetic resonance imaging |
| MS | multiple sclerosis |
| MSA | multiple system atrophy |
| PD | Parkinson’s disease |
| PSP | progressive supranuclear palsy |
| QSM | quantitative susceptibility mapping |
| QSMART | QSM artifact reduction technique |
| SWAN | susceptibility-weighted angiography |
| SWI | susceptibility-weighted imaging |
| SWI-p | susceptibility-weighted imaging-phase |
| TE | echo time |
| TR | repetition time |
References
- Haller, S.; Haacke, E.M.; Thurnher, M.M.; Barkhof, F. Susceptibility-weighted Imaging: Technical Essentials and Clinical Neurologic Applications. Radiology 2021, 299, 3–26. [Google Scholar] [CrossRef]
- Harada, T.; Kudo, K.; Fujima, N.; Yoshikawa, M.; Ikebe, Y.; Sato, R.; Shirai, T.; Bito, Y.; Uwano, I.; Miyata, M. Quantitative Susceptibility Mapping: Basic Methods and Clinical Applications. RadioGraphics 2022, 42, 1161–1176. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Buch, S.; Chen, Y.; Choi, H.; Dai, Y.; Habib, C.; Hu, J.; Jung, J.; Luo, Y.; Utriainen, D.; et al. Susceptibility Weighted Imaging: Current Status and Future Directions. NMR Biomed. 2017, 30, e3552. [Google Scholar] [CrossRef] [PubMed]
- Parillo, M.; Quattrocchi, C.; Pilato, F.; Di Lazzaro, V.; Zobel, B.B. Whole-body computed tomography as first-line imaging procedure to exclude cancer in patients with neurological suspicion of paraneoplastic syndromes: Shall clinical practice adhere to recommendations? Radiography 2023, 29, 8–13. [Google Scholar] [CrossRef]
- Parillo, M.; Vaccarino, F.; Quattrocchi, C.C. Imaging findings in a case of leptomeningeal myelomatosis, a rare but critical central nervous system complication of multiple myeloma. Neuroradiol. J. 2023, 36, 616–620. [Google Scholar] [CrossRef]
- Parillo, M.; Vertulli, D.; Mallio, C.A.; Quattrocchi, C.C. Imaging findings in carcinomatous encephalitis secondary to malignant melanoma. Egypt. J. Neurol. Psychiatry Neurosurg. 2023, 59, 76. [Google Scholar] [CrossRef]
- Parillo, M.; Vertulli, D.; Vaccarino, F.; Mallio, C.A.; Zobel, B.B.; Quattrocchi, C.C. The sensitivity of MIPs of 3D contrast-enhanced VIBE T1-weighted imaging for the detection of small brain metastases (≤5 mm) on 1.5 tesla MRI. Neuroradiol. J. 2024, 37, 744–750. [Google Scholar] [CrossRef]
- Béjot, Y.; Yaffe, K. Ageing Population: A Neurological Challenge. Neuroepidemiology 2019, 52, 76–77. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- SWI. Questions and Answers in MRI. Available online: https://mriquestions.com/making-an-sw-image.html (accessed on 20 February 2025).
- Haacke, E.M.; Makki, M.; Ge, Y.; Maheshwari, M.; Sehgal, V.; Hu, J.; Selvan, M.; Wu, Z.; Latif, Z.; Xuan, Y.; et al. Characterizing iron deposition in multiple sclerosis lesions using susceptibility weighted imaging. J. Magn. Reson. Imaging 2009, 29, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Arrighini, G.P.; Maestro, M.; Moccia, R. Magnetic Properties of Polyatomic Molecules. I. Magnetic Susceptibility of H2O, NH3, CH4, H2O2. J. Chem. Phys. 1968, 49, 882–889. [Google Scholar] [CrossRef]
- Schenck, J.F. Health and Physiological Effects of Human Exposure to Whole-Body Four-Tesla Magnetic Fields during MRI. Ann. N. Y. Acad. Sci. 1992, 649, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Duyn, J.H.; Schenck, J. Contributions to magnetic susceptibility of brain tissue. NMR Biomed. 2016, 30. [Google Scholar] [CrossRef]
- Deistung, A.; Schäfer, A.; Schweser, F.; Biedermann, U.; Turner, R.; Reichenbach, J.R. Toward in vivo histology: A comparison of quantitative susceptibility mapping (QSM) with magnitude-, phase-, and R2⁎-imaging at ultra-high magnetic field strength. NeuroImage 2013, 65, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Yablonskiy, D.A.; Haacke, E.M. Theory of NMR signal behavior in magnetically inhomogeneous tissues: The static dephasing regime. Magn. Reson. Med. 1994, 32, 749–763. [Google Scholar] [CrossRef]
- Sedlacik, J.; Rauscher, A.; Reichenbach, J.R. Quantification of modulated blood oxygenation levels in single cerebral veins by investigating their MR signal decay. Z. Med. Phys. 2009, 19, 48–57. [Google Scholar] [CrossRef]
- Rimkus, C.d.M.; Otsuka, F.S.; Nunes, D.M.; Chaim, K.T.; Otaduy, M.C.G. Central Vein Sign and Paramagnetic Rim Lesions: Susceptibility Changes in Brain Tissues and Their Implications for the Study of Multiple Sclerosis Pathology. Diagnostics 2024, 14, 1362. [Google Scholar] [CrossRef]
- Halefoglu, A.M.; Yousem, D.M. Susceptibility weighted imaging: Clinical applications and future directions. World J. Radiol. 2018, 10, 30–45. [Google Scholar] [CrossRef]
- Chavhan, G.B.; Babyn, P.S.; Thomas, B.; Shroff, M.M.; Haacke, E.M. Principles, Techniques, and Applications of T2*-based MR Imaging and Its Special Applications. RadioGraphics 2009, 29, 1433–1449. [Google Scholar] [CrossRef]
- Haacke, E.M.; Xu, Y.; Cheng, Y.N.; Reichenbach, J.R. Susceptibility weighted imaging (SWI). Magn. Reson. Med. 2004, 52, 612–618. [Google Scholar] [CrossRef]
- Haacke, E.M.; Mittal, S.; Wu, Z.; Neelavalli, J.; Cheng, Y.-C. Susceptibility-Weighted Imaging: Technical Aspects and Clinical Applications, Part 1. Am. J. Neuroradiol. 2009, 30, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Sotoudeh, H.; Sarrami, A.H.; Wang, J.; Saadatpour, Z.; Razaei, A.; Gaddamanugu, S.; Choudhary, G.; Shafaat, O.; Singhal, A. Susceptibility-Weighted Imaging in Neurodegenerative Disorders: A Review. J. Neuroimaging 2021, 31, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Haacke, E.M.; Cheng, N.Y.C.; House, M.J.; Liu, Q.; Neelavalli, J.; Ogg, R.J.; Khan, A.; Ayaz, M.; Kirsch, W.; Obenaus, A. Imaging iron stores in the brain using magnetic resonance imaging. Magn. Reson. Imaging 2005, 23, 1–25. [Google Scholar] [CrossRef]
- Smith, E.E. Cerebral amyloid angiopathy as a cause of neurodegeneration. J. Neurochem. 2018, 144, 651–658. [Google Scholar] [CrossRef]
- Wilson, D.M.; Cookson, M.R.; Bosch, L.V.D.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of neurodegenerative diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef]
- Sparacia, G.; Agnello, F.; La Tona, G.; Iaia, A.; Midiri, F.; Sparacia, B. Assessment of cerebral microbleeds by susceptibility-weighted imaging in Alzheimer’s disease patients: A neuroimaging biomarker of the disease. Neuroradiol. J. 2017, 30, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Hirtz, D.; Thurman, D.J.; Gwinn-Hardy, K.; Mohamed, M.; Chaudhuri, A.R.; Zalutsky, R. How common are the “common” neurologic disorders? Neurology 2007, 68, 326–337. [Google Scholar] [CrossRef]
- Damier, P.; Hirsch, E.C.; Agid, Y.; Graybiel, A.M. The substantia nigra of the human brain. Brain 1999, 122 Pt 8, 1421–1436. [Google Scholar] [CrossRef]
- Schwarz, S.T.; Afzal, M.; Morgan, P.S.; Bajaj, N.; Gowland, P.A.; Auer, D.P. The ‘Swallow Tail’ Appearance of the Healthy Nigrosome—A New Accurate Test of Parkinson’s Disease: A Case-Control and Retrospective Cross-Sectional MRI Study at 3T. PLoS ONE 2014, 9, e93814. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; He, N.; Huang, P.; Li, Y.; Tang, R.; Sethi, S.K.; Ghassaban, K.; Yerramsetty, K.K.; Palutla, V.K.; Chen, S.; et al. Imaging the Nigrosome 1 in the substantia nigra using susceptibility weighted imaging and quantitative susceptibility mapping: An application to Parkinson’s disease. NeuroImage Clin. 2020, 25, 102103. [Google Scholar] [CrossRef]
- Schmidt, M.A.; Engelhorn, T.; Marxreiter, F.; Winkler, J.; Lang, S.; Kloska, S.; Goelitz, P.; Doerfler, A. Ultra high-field SWI of the substantia nigra at 7T: Reliability and consistency of the swallow-tail sign. BMC Neurol. 2017, 17, 194. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Zhou, P.-Y.; Li, G.; Zhang, G.-B.; Wang, P.-Q.; Liu, J.-Z.; Xu, F.; Yang, F.; Wu, X.-X. Visualization of nigrosomes-1 in 3T MR susceptibility weighted imaging and its absence in diagnosing Parkinson’s disease. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4603–4609. [Google Scholar] [PubMed]
- Mahlknecht, P.; Krismer, F.; Poewe, W.; Seppi, K. Meta-analysis of dorsolateral nigral hyperintensity on magnetic resonance imaging as a marker for Parkinson’s disease. Mov. Disord. 2017, 32, 619–623. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, J.; He, N.; Li, Y.; Wen, Y.; Xu, H.; Tang, R.; Jin, Z.; Haacke, E.M.; Yan, F.; et al. Radiomic Features of the Nigrosome-1 Region of the Substantia Nigra: Using Quantitative Susceptibility Mapping to Assist the Diagnosis of Idiopathic Parkinson’s Disease. Front. Aging Neurosci. 2019, 11, 167. [Google Scholar] [CrossRef]
- Shams, S.; Fällmar, D.; Schwarz, S.; Wahlund, L.-O.; van Westen, D.; Hansson, O.; Larsson, E.-M.; Haller, S. MRI of the Swallow Tail Sign: A Useful Marker in the Diagnosis of Lewy Body Dementia? Am. J. Neuroradiol. 2017, 38, 1737–1741. [Google Scholar] [CrossRef]
- Lee, T.-W.; Chen, C.-Y.; Chen, K.; Tso, C.-W.; Lin, H.-H.; Lai, Y.-L.L.; Hsu, F.-T.; Chung, H.-W.; Liu, H.-S. Evaluation of the Swallow-Tail Sign and Correlations of Neuromelanin Signal with Susceptibility and Relaxations. Tomography 2021, 7, 107–119. [Google Scholar] [CrossRef]
- Harding, A.J.; Broe, G.A.; Halliday, G.M. Visual hallucinations in Lewy body disease relate to Lewy bodies in the temporal lobe. Brain 2002, 125, 391–403. [Google Scholar] [CrossRef]
- Meijer, F.J.; Steens, S.C.; van Rumund, A.; Walsum, A.-M.v.C.v.; Küsters, B.; Esselink, R.A.; Verbeek, M.M.; Bloem, B.R.; Goraj, B. Nigrosome-1 on Susceptibility Weighted Imaging to Differentiate Parkinson’s Disease from Atypical Parkinsonism: An In Vivo and Ex Vivo Pilot Study. Pol. J. Radiol. 2016, 81, 363–369. [Google Scholar] [CrossRef]
- Meijer, F.; van Rumund, A.; Fasen, B.; Titulaer, I.; Aerts, M.; Esselink, R.; Bloem, B.; Verbeek, M.; Goraj, B. Susceptibility-Weighted Imaging Improves the Diagnostic Accuracy of 3T Brain MRI in the Work-Up of Parkinsonism. Am. J. Neuroradiol. 2015, 36, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Saini, J.; Kesavadas, C.; Sarma, P.S.; Kishore, A. Utility of susceptibility-weighted MRI in differentiating Parkinson’s disease and atypical parkinsonism. Neuroradiology 2010, 52, 1087–1094. [Google Scholar] [CrossRef]
- Kim, E.Y.; Sung, Y.H.; Shin, H.-G.; Noh, Y.; Nam, Y.; Lee, J. Diagnosis of Early-Stage Idiopathic Parkinson’s Disease Using High-Resolution Quantitative Susceptibility Mapping Combined with Histogram Analysis in the Substantia Nigra at 3 T. J. Clin. Neurol. 2018, 14, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, H.; Granberg, T.; Westman, E.; Svenningsson, P. Quantitative susceptibility mapping differentiates between parkinsonian disorders. Park. Relat. Disord. 2017, 44, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Ohtsuka, C.; Yoshioka, K.; Kameda, H.; Yokosawa, S.; Sato, R.; Terayama, Y.; Sasaki, M. Differential diagnosis of parkinsonism by a combined use of diffusion kurtosis imaging and quantitative susceptibility mapping. Neuroradiology 2017, 59, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Bu, S.; Pang, H.; Li, X.; Zhao, M.; Wang, J.; Liu, Y.; Yu, H. Multi-parametric radiomics of conventional T1 weighted and susceptibility-weighted imaging for differential diagnosis of idiopathic Parkinson’s disease and multiple system atrophy. BMC Med. Imaging 2023, 23, 204. [Google Scholar] [CrossRef]
- Ren, Q.; Wang, Y.; Leng, S.; Nan, X.; Zhang, B.; Shuai, X.; Zhang, J.; Xia, X.; Li, Y.; Ge, Y.; et al. Substantia Nigra Radiomics Feature Extraction of Parkinson’s Disease Based on Magnitude Images of Susceptibility-Weighted Imaging. Front. Neurosci. 2021, 15, 646617. [Google Scholar] [CrossRef] [PubMed]
- Reich, D.S.; Lucchinetti, C.F.; Calabresi, P.A. Multiple Sclerosis. N. Engl. J. Med. 2018, 378, 169–180. [Google Scholar] [CrossRef]
- Parillo, M.; Vaccarino, F.; Di Gennaro, G.; Kumar, S.; Van Goethem, J.; Zobel, B.B.; Quattrocchi, C.C.; Parizel, P.M.; Mallio, C.A. Overview of the Current Knowledge and Conventional MRI Characteristics of Peri- and Para-Vascular Spaces. Brain Sci. 2024, 14, 138. [Google Scholar] [CrossRef] [PubMed]
- McDonald, W.I.; Compston, A.; Edan, G.; Goodkin, D.; Hartung, H.; Lublin, F.D.; McFarland, H.F.; Paty, D.W.; Polman, C.H.; Reingold, S.C.; et al. Recommended diagnostic criteria for multiple sclerosis: Guidelines from the international panel on the diagnosis of multiple sclerosis. Ann. Neurol. 2001, 50, 121–127. [Google Scholar] [CrossRef]
- Brownlee, W.J.; Swanton, J.K.; Altmann, D.R.; Ciccarelli, O.; Miller, D.H. Earlier and more frequent diagnosis of multiple sclerosis using the McDonald criteria. J. Neurol. Neurosurg. Psychiatry 2015, 86, 584–585. [Google Scholar] [CrossRef] [PubMed]
- Runia, T.F.; Jafari, N.; Hintzen, R.Q. Application of the 2010 revised criteria for the diagnosis of multiple sclerosis to patients with clinically isolated syndromes. Eur. J. Neurol. 2013, 20, 1510–1516. [Google Scholar] [CrossRef]
- Solomon, A.J.; Bourdette, D.N.; Cross, A.H.; Applebee, A.; Skidd, P.M.; Howard, D.B.; Spain, R.I.; Cameron, M.H.; Kim, E.; Mass, M.K.; et al. The contemporary spectrum of multiple sclerosis misdiagnosis: A multicenter study. Neurology 2016, 87, 1393–1399. [Google Scholar] [CrossRef]
- Aliaga, E.S.; Barkhof, F. MRI mimics of multiple sclerosis. Handb. Clin. Neurol. 2014, 122, 291–316. [Google Scholar] [CrossRef] [PubMed]
- Hammond, K.E.; Metcalf, M.; Carvajal, L.; Okuda, D.T.; Srinivasan, R.; Vigneron, D.; Nelson, S.J.; Pelletier, D. Quantitative in vivo magnetic resonance imaging of multiple sclerosis at 7 Tesla with sensitivity to iron. Ann. Neurol. 2008, 64, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, H. Multiple Sclerosis Pathology: Evolution of Pathogenetic Concepts. Brain Pathol. 2005, 15, 217–222. [Google Scholar] [CrossRef]
- Tallantyre, E.; Dixon, J.; Donaldson, I.; Owens, T.; Morgan, P.; Morris, P.; Evangelou, N. Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology 2011, 76, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Suh, C.H.; Kim, S.J.; Jung, S.C.; Choi, C.G.; Kim, H.S. The “Central Vein Sign” on T2*-weighted Images as a Diagnostic Tool in Multiple Sclerosis: A Systematic Review and Meta-analysis using Individual Patient Data. Sci. Rep. 2019, 9, 18188. [Google Scholar] [CrossRef]
- Maggi, P.; Sati, P.; Nair, G.; Cortese, I.C.M.; Jacobson, S.; Smith, B.R.; Nath, A.; Ohayon, J.; van Pesch, V.; Perrotta, G.; et al. Paramagnetic Rim Lesions are Specific to Multiple Sclerosis: An International Multicenter 3T MRI Study. Ann. Neurol. 2020, 88, 1034–1042. [Google Scholar] [CrossRef]
- La Rosa, F.; Wynen, M.; Al-Louzi, O.; Beck, E.S.; Huelnhagen, T.; Maggi, P.; Thiran, J.-P.; Kober, T.; Shinohara, R.T.; Sati, P.; et al. Cortical lesions, central vein sign, and paramagnetic rim lesions in multiple sclerosis: Emerging machine learning techniques and future avenues. NeuroImage Clin. 2022, 36, 103205. [Google Scholar] [CrossRef]
- Meaton, I.; Altokhis, A.; Allen, C.M.; A Clarke, M.; Sinnecker, T.; Meier, D.; Enzinger, C.; Calabrese, M.; De Stefano, N.; Pitiot, A.; et al. Paramagnetic rims are a promising diagnostic imaging biomarker in multiple sclerosis. Mult. Scler. J. 2022, 28, 2212–2220. [Google Scholar] [CrossRef]
- Chawla, S.; Kister, I.; Wuerfel, J.; Brisset, J.C.; Liu, S.; Sinnecker, T.; Dusek, P.; Haacke, E.M.; Paul, F.; Ge, Y. Iron and Non-Iron-Related Characteristics of Multiple Sclerosis and Neuromyelitis Optica Lesions at 7T MRI. Am. J. Neuroradiol. 2016, 37, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Knipe, H.; Ravindran, R. McDonald Diagnostic Criteria for Multiple Sclerosis. Reference Article, Radiopaedia.org. Available online: https://radiopaedia.org/articles/mcdonald-diagnostic-criteria-for-multiple-sclerosis-4?lang=us (accessed on 20 February 2025).
- Mesker, D.J.; Poels, M.M.F.; Ikram, M.A.; Vernooij, M.W.; Hofman, A.; Vrooman, H.A.; van der Lugt, A.; Breteler, M.M.B. Lobar Distribution of Cerebral Microbleeds. Arch. Neurol. 2011, 68, 656–659. [Google Scholar] [CrossRef] [PubMed]
- Rubin, A.; Waszczuk, Ł.; Trybek, G.; Kapetanakis, S.; Bladowska, J. Application of susceptibility weighted imaging (SWI) in diagnostic imaging of brain pathologies—A practical approach. Clin. Neurol. Neurosurg. 2022, 221, 107368. [Google Scholar] [CrossRef]
- Sharma, R.; Dearaugo, S.; Infeld, B.; O’Sullivan, R.; Gerraty, R.P. Cerebral amyloid angiopathy: Review of clinico-radiological features and mimics. J. Med. Imaging Radiat. Oncol. 2018, 62, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Linn, J.; Halpin, A.; Demaerel, P.; Ruhland, J.; Giese, A.; Dichgans, M.; van Buchem, M.; Bruckmann, H.; Greenberg, S. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology 2010, 74, 1346–1350. [Google Scholar] [CrossRef] [PubMed]
- Lopes, P.A.; Raposo, N.; Charidimou, A.; Zotin, M.Z.; Gurol, M.E.; Greenberg, S.; Viswanathan, A. SWI versus GRE-T2*: Assessing cortical superficial siderosis in advanced cerebral amyloid angiopathy. Rev. Neurol. 2024, 180, 532–538. [Google Scholar] [CrossRef]
- Charidimou, A.; Boulouis, G.; Frosch, M.P.; Baron, J.-C.; Pasi, M.; Albucher, J.F.; Banerjee, G.; Barbato, C.; Bonneville, F.; Brandner, S.; et al. The Boston criteria version 2.0 for cerebral amyloid angiopathy: A multicentre, retrospective, MRI–neuropathology diagnostic accuracy study. Lancet Neurol. 2022, 21, 714–725. [Google Scholar] [CrossRef]
- Roeben, B.; Wilke, C.; Bender, B.; Ziemann, U.; Synofzik, M. The motor band sign in ALS: Presentations and frequencies in a consecutive series of ALS patients. J. Neurol. Sci. 2019, 406, 116440. [Google Scholar] [CrossRef]
- Stefanescu, M.R.; Dohnalek, M.; Maderwald, S.; Thürling, M.; Minnerop, M.; Beck, A.; Schlamann, M.; Diedrichsen, J.; Ladd, M.E.; Timmann, D. Structural and functional MRI abnormalities of cerebellar cortex and nuclei in SCA3, SCA6 and Friedreich’s ataxia. Brain 2015, 138, 1182–1197. [Google Scholar] [CrossRef]
- Ronsin, S.; Hannoun, S.; Thobois, S.; Petiot, P.; Vighetto, A.; Cotton, F.; Tilikete, C. A new MRI marker of ataxia with oculomotor apraxia. Eur. J. Radiol. 2019, 110, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Macerollo, A.; Perry, R.; Stamelou, M.; Batla, A.; Mazumder, A.A.; Adams, M.E.; Bhatia, K.P. Susceptibility-weighted imaging changes suggesting brain iron accumulation in Huntington’s disease: An epiphenomenon which causes diagnostic difficulty. Eur. J. Neurol. 2014, 21, e16–e17. [Google Scholar] [CrossRef]
- Kruer, M.; Boddaert, N.; Schneider, S.; Houlden, H.; Bhatia, K.; Gregory, A.; Anderson, J.; Rooney, W.; Hogarth, P.; Hayflick, S. Neuroimaging Features of Neurodegeneration with Brain Iron Accumulation. Am. J. Neuroradiol. 2012, 33, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Guillerman, R.P. The Eye-of-the-Tiger Sign. Radiology 2000, 217, 895–896. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Lin, C. Eye-of-the-Tiger sign is not Pathognomonic of Pantothenate Kinase-Associated Neurodegeneration in Adult Cases. Brain Behav. 2011, 1, 55–56. [Google Scholar] [CrossRef] [PubMed]
- Sethi, K.D.; Mehta, S.H.; Morgan, J.C. Defining the Eye-of-the-Tiger Sign. JAMA Neurol. 2015, 72, 606. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Sato, N.; Ishiyama, A.; Shigemoto, Y.; Suzuki, F.; Fujii, H.; Maikusa, N.; Matsuda, H.; Nishioka, K.; Hattori, N.; et al. Serial MRI alterations of pediatric patients with beta-propeller protein associated neurodegeneration (BPAN). J. Neuroradiol. 2021, 48, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Alosco, M.L.; Culhane, J.; Mez, J. Neuroimaging Biomarkers of Chronic Traumatic Encephalopathy: Targets for the Academic Memory Disorders Clinic. Neurotherapeutics 2021, 18, 772–791. [Google Scholar] [CrossRef]
- Shetty, T.; Raince, A.; Manning, E.; Tsiouris, A.J. Imaging in Chronic Traumatic Encephalopathy and Traumatic Brain Injury. Sports Health Multidiscip. Approach 2016, 8, 26–36. [Google Scholar] [CrossRef]
- Gerges, J.A.; Chalhoub, I.; Atallah, C.; Khoury, R. Biomarkers of chronic traumatic encephalopathy: A state-of-the art review. Biomark. Neuropsychiatry 2023, 8, 100066. [Google Scholar] [CrossRef]
- Wu, Z.; Mittal, S.; Kish, K.; Yu, Y.; Hu, J.; Haacke, E.M. Identification of calcification with MRI using susceptibility-weighted imaging: A case study. J. Magn. Reson. Imaging 2009, 29, 177–182. [Google Scholar] [CrossRef]
- Yaghmaie, N.; Syeda, W.T.; Wu, C.; Zhang, Y.; Zhang, T.D.; Burrows, E.L.; Brodtmann, A.; Moffat, B.A.; Wright, D.K.; Glarin, R.; et al. QSMART: Quantitative susceptibility mapping artifact reduction technique. NeuroImage 2021, 231, 117701. [Google Scholar] [CrossRef]
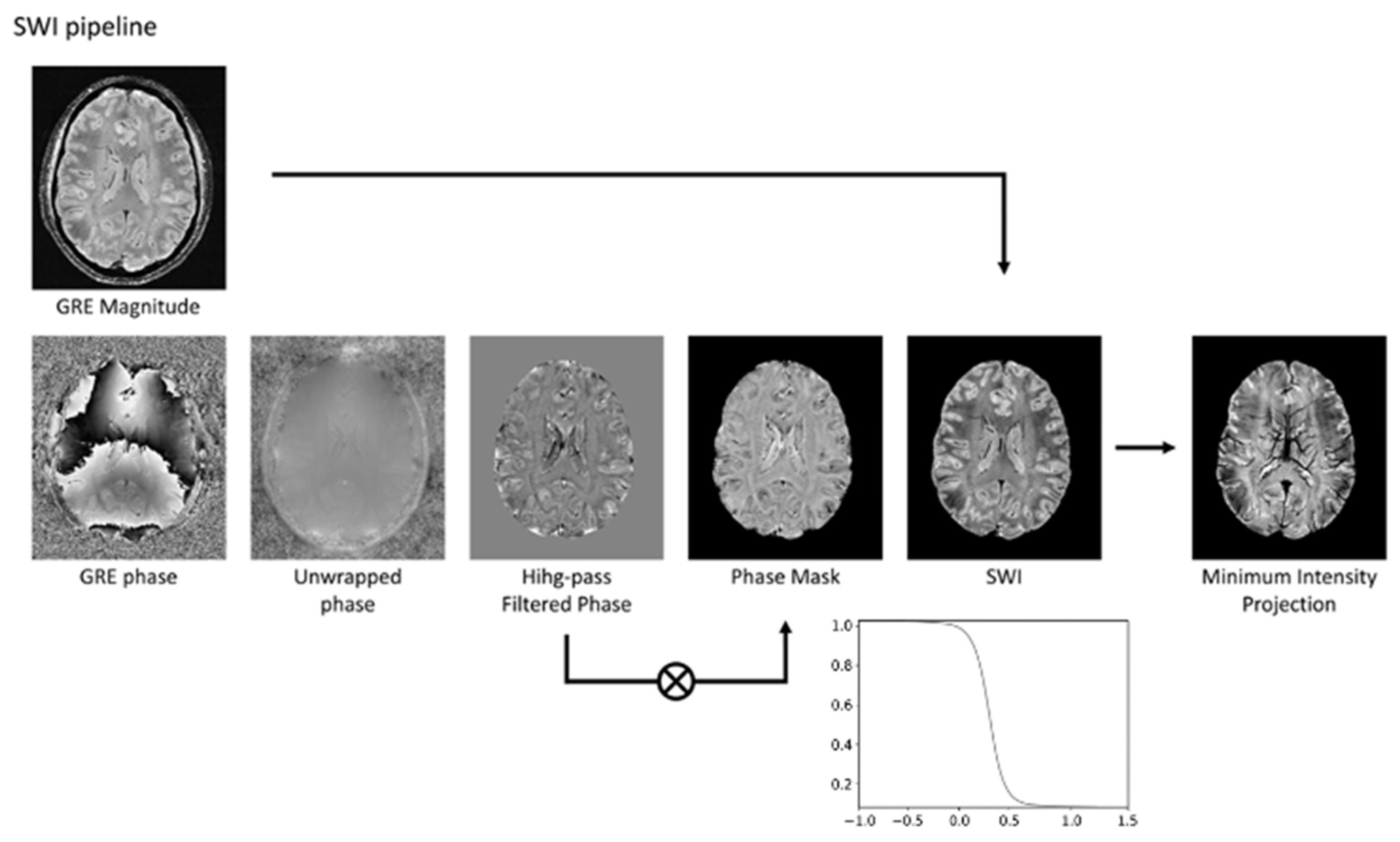
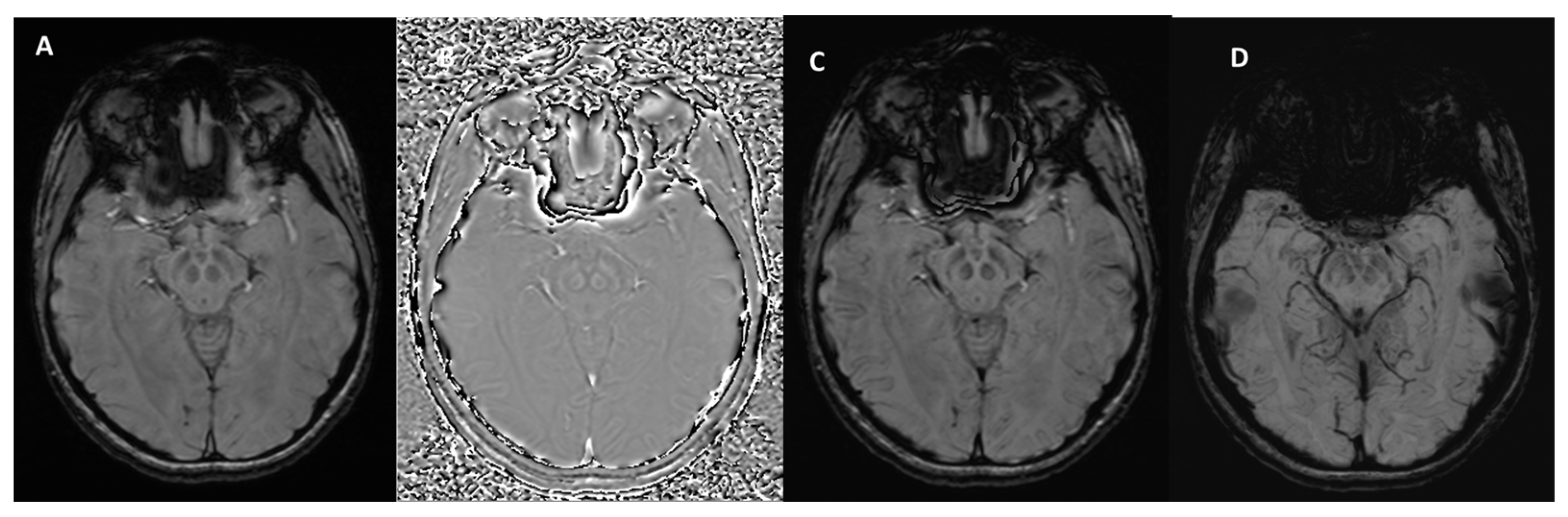
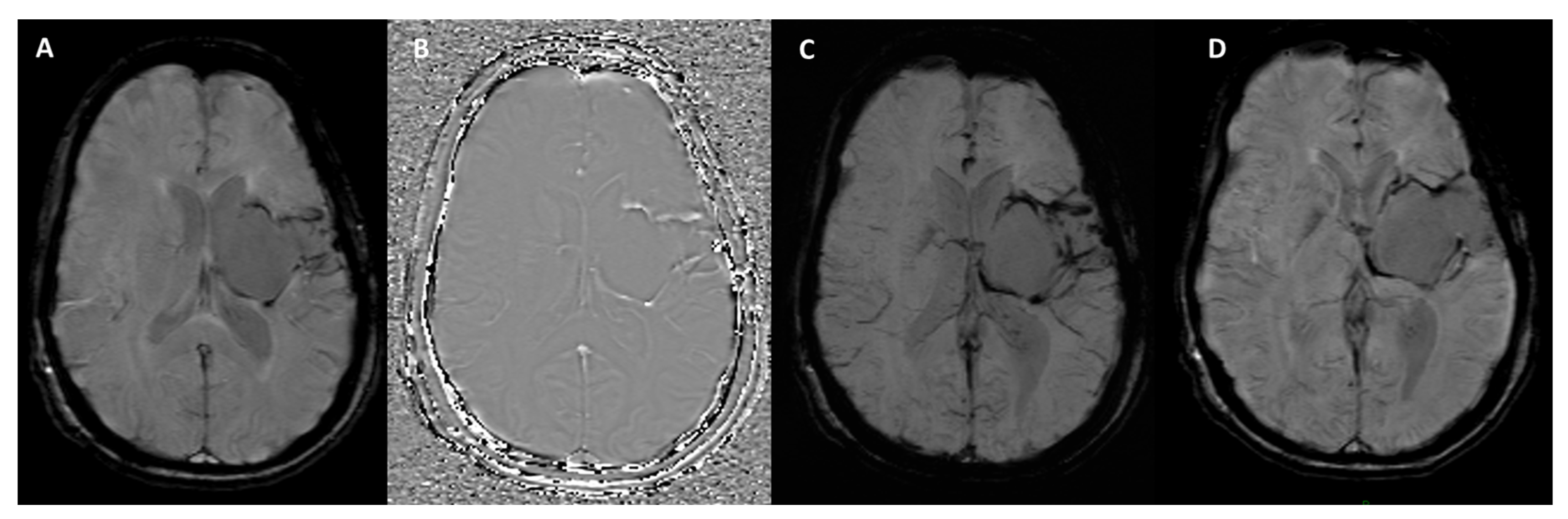
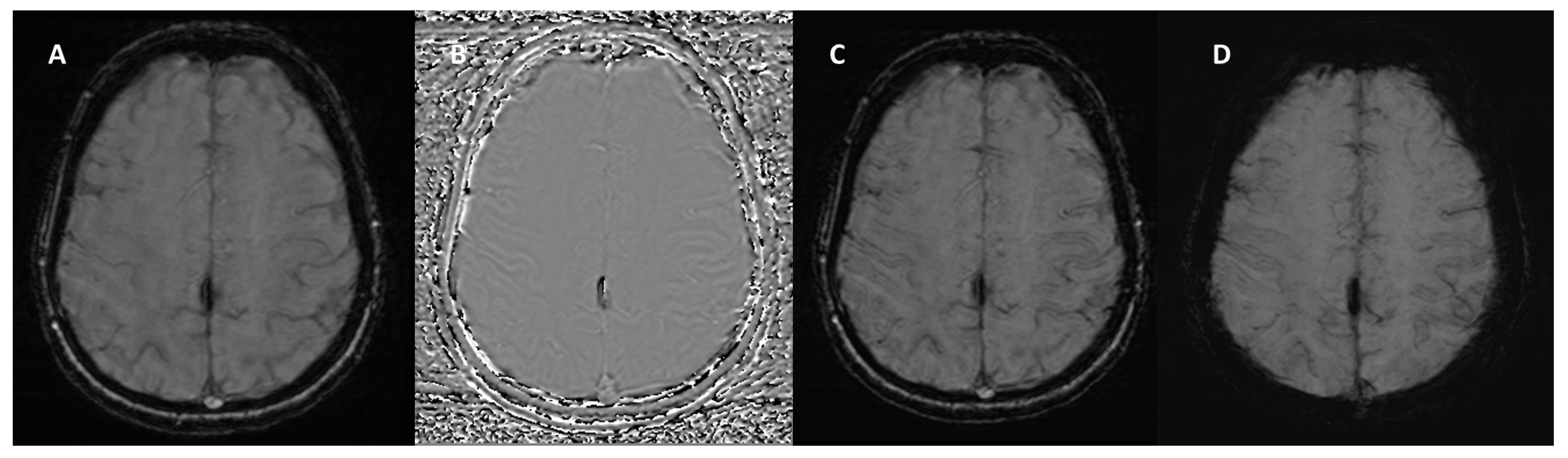
| Neurodegenerative Diseases | SWI Findings | Description |
|---|---|---|
| Parkinson’s disease, Lewy body dementia, and atypical parkinsonian syndromes | Absent swallow-tail sign | Loss of the normal bright signal in the posterior third of the substantia nigra (nigrosome-1) |
| Iron deposition | MSA-P: hypointensity in the putamen PSP: hypointesity in basal ganglia, red nucleus, substantia nigra pars reticulata, and cerebellar dentate nucleus | |
| Multiple sclerosis | Central vein sign | Punctate or linear hypointensity at the center of a hyperintense lesion in at least 2 of 3 orthogonal planes (>2 mm) |
| Paramagnetic rim lesions | Hypointense rim surrounding an internal lesion that is isointense to adjacent normal white matter | |
| Cerebral amyloid angiopathy | Cortical or cortico–subcortical microbleeds | Small (2–10 mm), multiple (≥2), round or ovoid, and uniformly hypointense, primarily located in the frontal and parietal lobes (usually sparing the basal ganglia, assisting in the differential diagnosis with hypertensive microangiopathy) |
| Convexity subarachnoid hemorrhage/cortical superficial siderosis | Curvilinear regions of signal drop-out localized to one or more sulci | |
| Amyotrophic lateral sclerosis | Motor band sign | Curvilinear bands of reduced signal in the gray matter of the primary motor cortex |
| Hereditary ataxias | Abnormal dentate nuclei | Atrophy in spinocerebellar ataxia 6, Friedreich’s ataxia, and spinocerebellar ataxia 3; Decreased iron concentration in oculomotor apraxia |
| Huntington’s disease | Iron deposition | Hypointesity in the basal ganglia (mainly globus pallidus) |
| Neurodegeneration with brain iron accumulation | Eye of the tiger sign | Symmetric bilateral abnormal low signal in the globus pallidus with central high signal |
| Chronic traumatic encephalopathy | Diffuse axonal injury (microbleeds) | Punctate or linear hypointensity at the gray–white matter junction, in the corpus callosum or the brainstem |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaccarino, F.; Quattrocchi, C.C.; Parillo, M. Susceptibility-Weighted Imaging (SWI): Technical Aspects and Applications in Brain MRI for Neurodegenerative Disorders. Bioengineering 2025, 12, 473. https://doi.org/10.3390/bioengineering12050473
Vaccarino F, Quattrocchi CC, Parillo M. Susceptibility-Weighted Imaging (SWI): Technical Aspects and Applications in Brain MRI for Neurodegenerative Disorders. Bioengineering. 2025; 12(5):473. https://doi.org/10.3390/bioengineering12050473
Chicago/Turabian StyleVaccarino, Federica, Carlo Cosimo Quattrocchi, and Marco Parillo. 2025. "Susceptibility-Weighted Imaging (SWI): Technical Aspects and Applications in Brain MRI for Neurodegenerative Disorders" Bioengineering 12, no. 5: 473. https://doi.org/10.3390/bioengineering12050473
APA StyleVaccarino, F., Quattrocchi, C. C., & Parillo, M. (2025). Susceptibility-Weighted Imaging (SWI): Technical Aspects and Applications in Brain MRI for Neurodegenerative Disorders. Bioengineering, 12(5), 473. https://doi.org/10.3390/bioengineering12050473








