Bacteriophages as Targeted Therapeutic Vehicles: Challenges and Opportunities
Abstract
1. Introduction
2. Advantages and Disadvantages of Bacteriophage-Based Therapeutics and Regulatory Issues
2.1. Advantages of Phage Therapy
2.2. Disadvantages and Challenges of Phage Therapy
2.3. Regulatory Issues Associated with Phage Therapy
3. Bacteriophage-Based Therapeutic Strategies: Phage Display Technology, Biopanning, and Applications Using Phage Display Technology
3.1. Phage Display Technology
3.2. Production of Monoclonal Antibodies (mAbs) Using Phage Display Technology
3.3. Biopanning Strategy
3.4. Phage Cocktails
3.5. Phage Encapsulation
3.6. Stability of Phages and Phage Encapsulation Using Liposomes and Polymeric Microparticles to Enhance Phage Stability and Efficacy
3.7. Phage Engineering Using CRISPR-Cas9 Technology
4. Phage Applications in Medicine
4.1. Bacterial Infections Therapy
| Infection Type | Pathogens/Infections | Findings | Models Used | Ref. |
|---|---|---|---|---|
| Skin | Pyogenic infections (E. coli, Proteus, S. aureus, Pseudomonas, Klebsiella) | 86% full recovery, 14% improvement | Human patients | [105] |
| S. aureus, E. coli, Streptococcus, Proteus, P. aeruginosa in ulcers | 70% healing, 23% bacterial reduction | Human patients | [106] | |
| P. aeruginosa, Enterococcus, Staphylococcus in diabetic ulcers | Infection alleviated, no MRSA infection | - | [107] | |
| K. pneumoniae in burn wounds | More effective than gentamycin and silver nitrate | Mouse models | [108] | |
| A. baumannii | Smaller, cleaner wounds | Balb/c mice | [109] | |
| S. aureus in eczema and acne vulgaris | Reduced symptoms, no harm to commensals | Human patients | [110] | |
| Oral | A. actinomycetemcomitans in periodontitis | 99% bacterial killing | In vitro studies | [111] |
| S. sobrinus, S. mutans dental biofilms | Reduced biofilm severity, decreased caries | Sprague Dawley rats | [112] | |
| Endodontic infection (E. faecalis) | Degraded biofilm | Ex vivo models | [113] | |
| Gastrointestinal | C. difficile | Symptom resolution, stool normalization | Human studies | [114] |
| Diarrhea (EPEC) | Infection controlled | Balb/c mice | [115] | |
| Respiratory | K. pneumoniae | Reduced inflammation, bacterial burden | Swiss Webster mice | [116] |
| Chronic P. aeruginosa infection | 70% bacteria cleared | Mouse models | [117] | |
| Urinary Tract | K. pneumoniae UTI | Cured by phage-antibiotic combo | Human patients | [118] |
| Eye | P. aeruginosa keratitis | Preserved corneal integrity, reduced bacterial load | Murine models | [119] |
| Ear | Chronic P. aeruginosa otitis | Reduced bacterial counts, no adverse effects | Human patients | [120,121] |
| Nasal | Chronic rhinosinusitis (S. aureus) | 20% favorable outcomes | Human patients | [122] |
| Sepsis/Bacteremia | E. coli | 95–100% survival rates | Murine models | [123] |
| Liver | Cytolytic E. faecalis | Lytic phages attenuated ethanol-elicited liver disease | Humanized mouse models | [124] |
| Orthopedic | Multidrug-resistant E. coli, E. faecalis (VRE), S. aureus (MRSA) in osteoarticular infections | Controlled infections associated with implants | Human patients | [125] |
4.2. Phage Therapy Against Human Viral Diseases
4.3. Phage Therapy in the Treatment of Veterinary Diseases
4.4. Phage Technology in Neurotherapy
4.5. Phages and Cancer Therapy
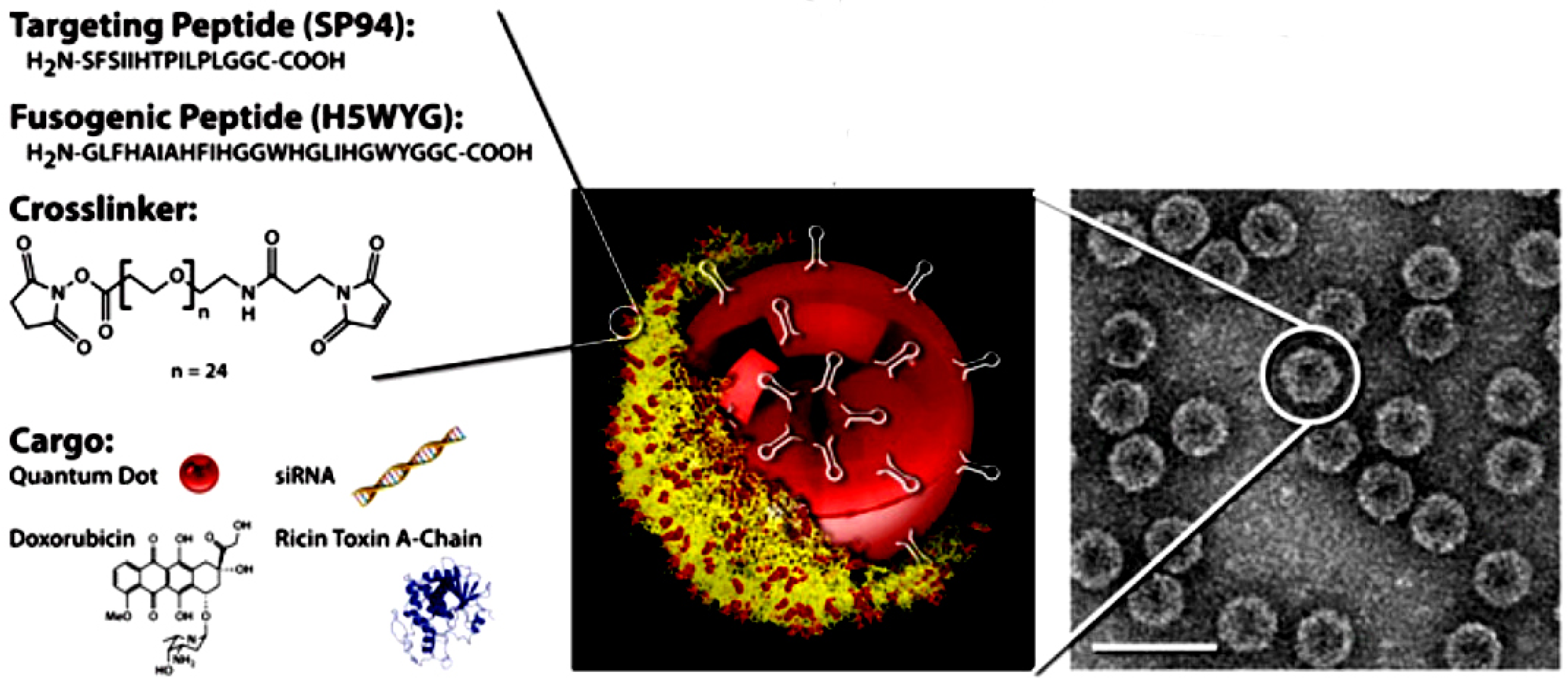
4.5.1. Phages in Targeted Drug Delivery
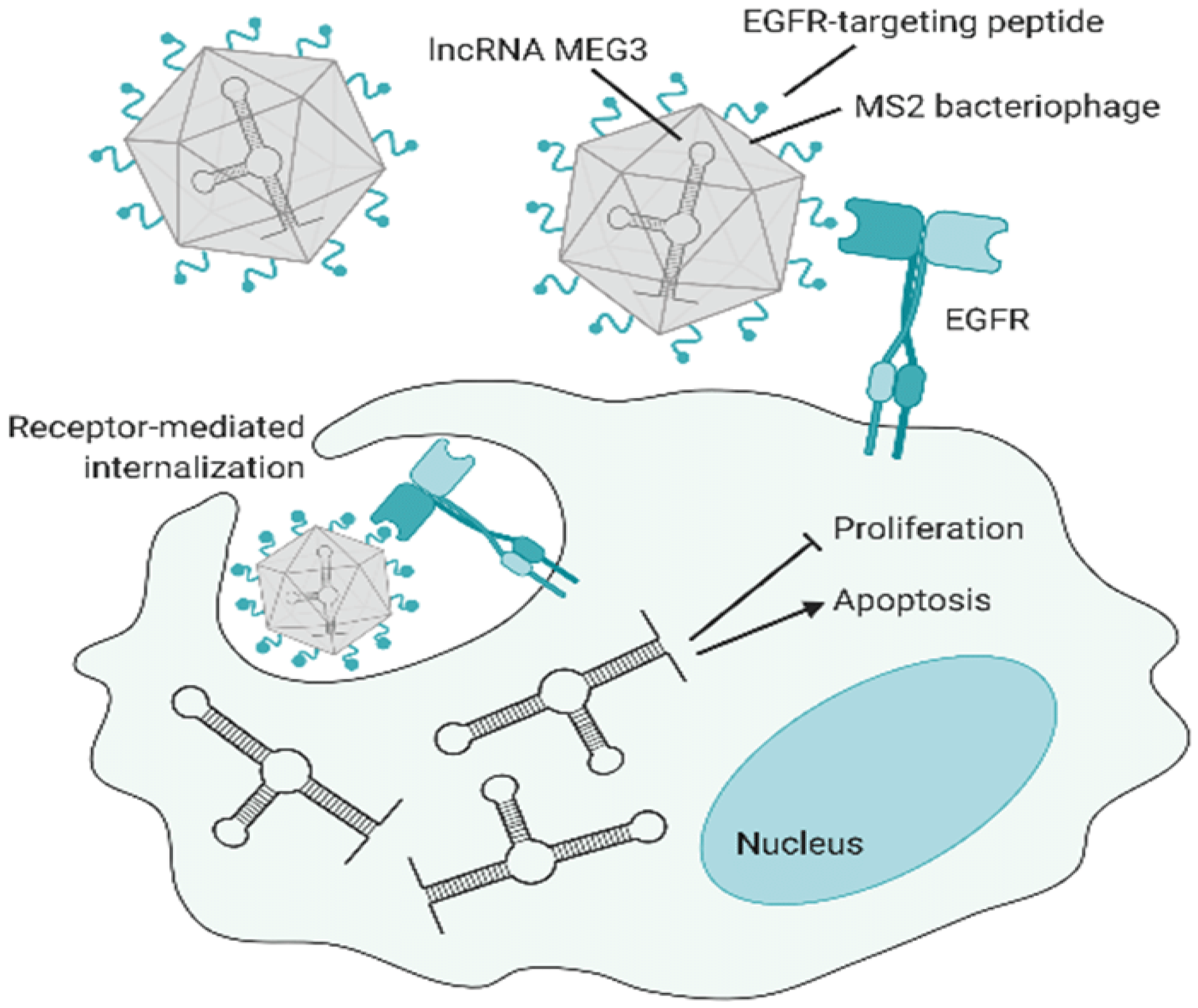
4.5.2. Phages in Targeted Gene Therapy
4.5.3. Phages in PTT and PDT
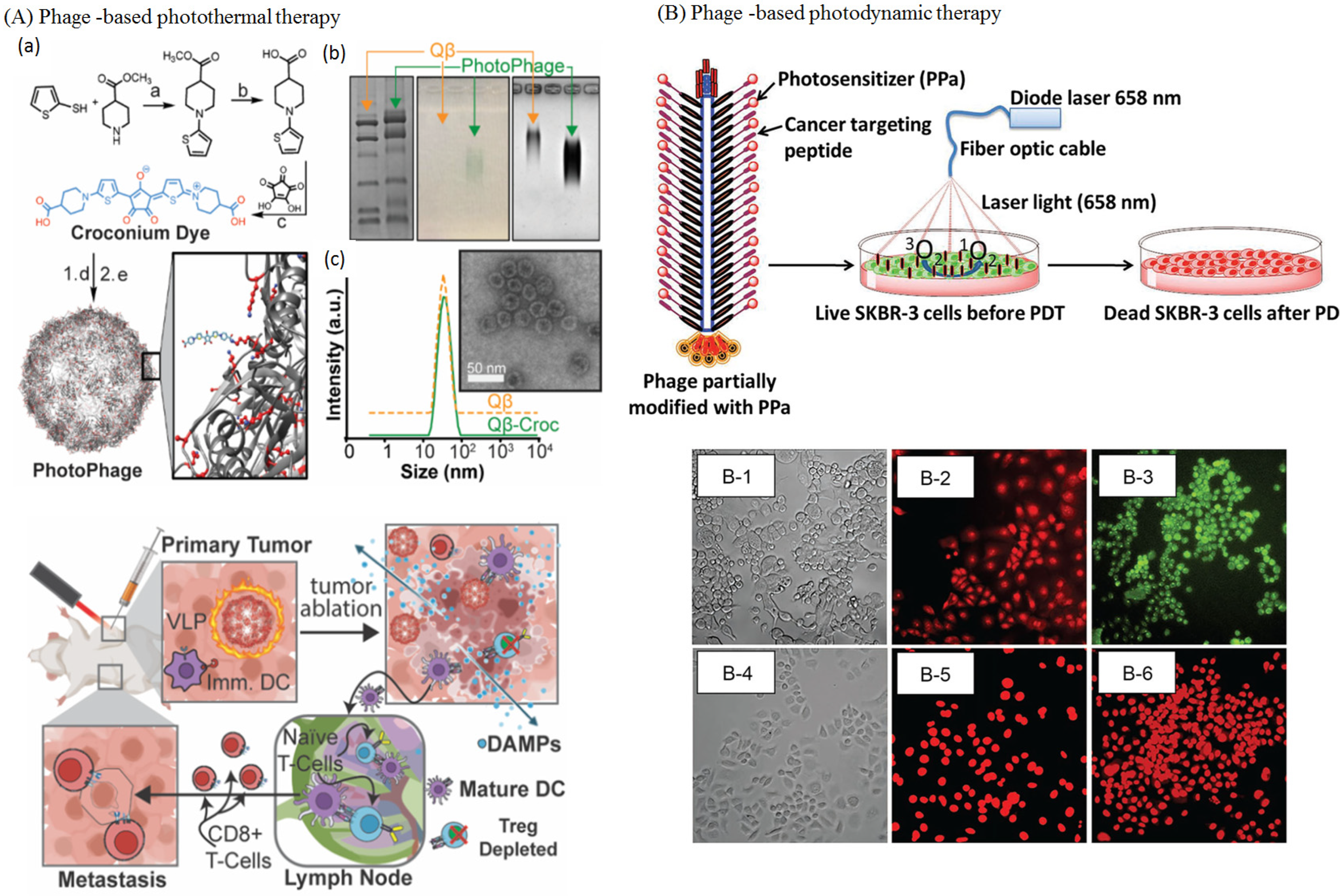
4.5.4. Phages in Cancer Immunotherapy
4.5.5. Phages in Combination Therapy
4.5.6. Phages as Bioimaging Agents
4.5.7. Phages in Theragnostics
5. Recent Developments in Phage Engineering and Therapy
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wommack, K.E.; Colwell, R.R. Virioplankton: Viruses in aquatic ecosystems. Microbiol. Mol. Biol. Rev. 2000, 64, 69–114. [Google Scholar] [CrossRef] [PubMed]
- Bertozzi Silva, J.; Storms, Z.; Sauvageau, D. Host receptors for bacteriophage adsorption. FEMS Microbiol. Lett. 2016, 363, fnw002. [Google Scholar] [CrossRef] [PubMed]
- Doss, J.; Culbertson, K.; Hahn, D.; Camacho, J.; Barekzi, N. A review of phage therapy against bacterial pathogens of aquatic and terrestrial organisms. Viruses 2017, 9, 50. [Google Scholar] [CrossRef]
- Cui, L.; Watanabe, S.; Miyanaga, K.; Kiga, K.; Sasahara, T.; Aiba, Y.; Tan, X.-E.; Veeranarayanan, S.; Thitiananpakorn, K.; Nguyen, H.M. A comprehensive review on phage therapy and phage-based drug development. Antibiotics 2024, 13, 870. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, Y.; Xu, Y.; Chen, Y.; Zhang, W.; Liu, T.; Chen, G.; Wang, K. Phage-based delivery systems: Engineering, applications, and challenges in nanomedicines. J. Nanobiotechnol. 2024, 22, 365. [Google Scholar] [CrossRef]
- Akanda, Z.Z.; Taha, M.; Abdelbary, H. Current review—The rise of bacteriophage as a unique therapeutic platform in treating peri-prosthetic joint infections. J. Orthop. Res. 2018, 36, 1051–1060. [Google Scholar] [CrossRef]
- Smith, H.W.; Huggins, M. Successful treatment of experimental Escherichia coli infections in mice using phage: Its general superiority over antibiotics. Microbiology 1982, 128, 307–318. [Google Scholar] [CrossRef]
- Abedon, S.T.; Kuhl, S.J.; Blasdel, B.G.; Kutter, E.M. Phage treatment of human infections. Bacteriophage 2011, 1, 66–85. [Google Scholar] [CrossRef]
- Romero-Calle, D.; Guimarães Benevides, R.; Góes-Neto, A.; Billington, C. Bacteriophages as alternatives to antibiotics in clinical care. Antibiotics 2019, 8, 138. [Google Scholar] [CrossRef]
- El-Shibiny, A.; El-Sahhar, S. Bacteriophages: The possible solution to treat infections caused by pathogenic bacteria. Can. J. Microbiol. 2017, 63, 865–879. [Google Scholar] [CrossRef]
- Weber-Dąbrowska, B.; Jończyk-Matysiak, E.; Żaczek, M.; Łobocka, M.; Łusiak-Szelachowska, M.; Górski, A. Bacteriophage procurement for therapeutic purposes. Front. Microbiol. 2016, 7, 1177. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, K. Phage therapy: What factors shape phage pharmacokinetics and bioavailability? Systematic and critical review. Med. Res. Rev. 2019, 39, 2000–2025. [Google Scholar] [CrossRef] [PubMed]
- Olawade, D.B.; Fapohunda, O.; Egbon, E.; Ebiesuwa, O.A.; Usman, S.O.; Faronbi, A.O.; Fidelis, S.C. Phage therapy: A targeted approach to overcoming antibiotic resistance. Microb. Pathog. 2024, 197, 107088. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, H.; Gan, D.; Wang, M.; Deng, H.; Yang, Q.E. Antibacterial effect of phage cocktails and phage-antibiotic synergy against pathogenic Klebsiella pneumoniae. Msystems 2024, 9, e00607-24. [Google Scholar] [CrossRef]
- Gibb, B.P.; Hadjiargyrou, M. Bacteriophage therapy for bone and joint infections: An instructional review. Bone Jt. J. 2021, 103, 234–244. [Google Scholar] [CrossRef]
- Skurnik, M.; Pajunen, M.; Kiljunen, S. Biotechnological challenges of phage therapy. Biotechnol. Lett. 2007, 29, 995–1003. [Google Scholar] [CrossRef]
- Krylov, V. Phagotherapy in terms of bacteriophage genetics: Hopes, perspectives, safety, limitations. Genetika 2001, 37, 869–887. [Google Scholar]
- Abedon, S.T.; Thomas-Abedon, C. Phage therapy pharmacology. Curr. Pharm. Biotechnol. 2010, 11, 28–47. [Google Scholar] [CrossRef]
- Kutateladze, M.; Adamia, R. Bacteriophages as potential new therapeutics to replace or supplement antibiotics. Trends Biotechnol. 2010, 28, 591–595. [Google Scholar] [CrossRef]
- Goodridge, L.D. Designing phage therapeutics. Curr. Pharm. Biotechnol. 2010, 11, 15–27. [Google Scholar] [CrossRef]
- Loc-Carrillo, C.; Abedon, S.T. Pros and cons of phage therapy. Bacteriophage 2011, 1, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Kronheim, S.; Daniel-Ivad, M.; Duan, Z.; Hwang, S.; Wong, A.I.; Mantel, I.; Nodwell, J.R.; Maxwell, K.L. A chemical defence against phage infection. Nature 2018, 564, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Krylov, V.; Shaburova, O.; Pleteneva, E.; Bourkaltseva, M.; Krylov, S.; Kaplan, A.; Chesnokova, E.; Kulakov, L.; Magill, D.; Polygach, O. Modular approach to select bacteriophages targeting Pseudomonas aeruginosa for their application to children suffering with cystic fibrosis. Front. Microbiol. 2016, 7, 1631. [Google Scholar] [CrossRef]
- Anomaly, J. The future of phage: Ethical challenges of using phage therapy to treat bacterial infections. Public Health Ethics 2020, 13, 82–88. [Google Scholar] [CrossRef]
- Zalewska-Piątek, B. Phage therapy—Challenges, opportunities and future prospects. Pharmaceuticals 2023, 16, 1638. [Google Scholar] [CrossRef]
- Gabiatti, N.; Yu, P.; Mathieu, J.; Lu, G.W.; Wang, X.; Zhang, H.; Soares, H.M.; Alvarez, P.J. Bacterial endospores as phage genome carriers and protective shells. Appl. Environ. Microbiol. 2018, 84, e01186-01118. [Google Scholar] [CrossRef]
- Van Belleghem, J.D.; Dąbrowska, K.; Vaneechoutte, M.; Barr, J.J. Phage interaction with the mammalian immune system. In Phage Therapy: A Practical Approach; Springer: Cham, Switzerland, 2019; pp. 91–122. [Google Scholar]
- Pelfrene, E.; Willebrand, E.; Cavaleiro Sanches, A.; Sebris, Z.; Cavaleri, M. Bacteriophage therapy: A regulatory perspective. J. Antimicrob. Chemother. 2016, 71, 2071–2074. [Google Scholar] [CrossRef]
- Khan Mirzaei, M.; Nilsson, A.S. Isolation of phages for phage therapy: A comparison of spot tests and efficiency of plating analyses for determination of host range and efficacy. PLoS ONE 2015, 10, e0118557. [Google Scholar] [CrossRef]
- Cui, Z.; Guo, X.; Feng, T.; Li, L. Exploring the whole standard operating procedure for phage therapy in clinical practice. J. Transl. Med. 2019, 17, 373. [Google Scholar] [CrossRef]
- Jones, J.D.; Trippett, C.; Suleman, M.; Clokie, M.R.; Clark, J.R. The future of clinical phage therapy in the United Kingdom. Viruses 2023, 15, 721. [Google Scholar] [CrossRef]
- Onsea, J.; Uyttebroek, S.; Chen, B.; Wagemans, J.; Lood, C.; Van Gerven, L.; Spriet, I.; Devolder, D.; Debaveye, Y.; Depypere, M. Bacteriophage therapy for difficult-to-treat infections: The implementation of a multidisciplinary phage task force (the PHAGEFORCE study protocol). Viruses 2021, 13, 1543. [Google Scholar] [CrossRef]
- Khatami, A.; Foley, D.A.; Warner, M.S.; Barnes, E.H.; Peleg, A.Y.; Li, J.; Stick, S.; Burke, N.; Lin, R.C.; Warning, J. Standardised treatment and monitoring protocol to assess safety and tolerability of bacteriophage therapy for adult and paediatric patients (STAMP study): Protocol for an open-label, single-arm trial. BMJ Open 2022, 12, e065401. [Google Scholar] [CrossRef] [PubMed]
- Strathdee, S.A.; Hatfull, G.F.; Mutalik, V.K.; Schooley, R.T. Phage therapy: From biological mechanisms to future directions. Cell 2023, 186, 17–31. [Google Scholar] [CrossRef]
- Verbeken, G.; Pirnay, J.-P.; De Vos, D.; Jennes, S.; Zizi, M.; Lavigne, R.; Casteels, M.; Huys, I. Optimizing the European regulatory framework for sustainable bacteriophage therapy in human medicine. Arch. Immunol. Ther. Exp. 2012, 60, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.P. Filamentous fusion phage: Novel expression vectors that display cloned antigens on the virion surface. Science 1985, 228, 1315–1317. [Google Scholar] [CrossRef]
- Smith, G.P.; Petrenko, V.A. Phage display. Chem. Rev. 1997, 97, 391–410. [Google Scholar] [CrossRef]
- Xu, P.; Ghosh, S.; Gul, A.R.; Bhamore, J.R.; Park, J.P.; Park, T.J. Screening of specific binding peptides using phage-display techniques and their biosensing applications. TrAC Trends Anal. Chem. 2021, 137, 116229. [Google Scholar] [CrossRef]
- Nixon, A.E.; Sexton, D.J.; Ladner, R.C. Drugs derived from phage display: From candidate identification to clinical practice. mAbs 2014, 6, 73–85. [Google Scholar] [CrossRef]
- Winter, G.; Griffiths, A.D.; Hawkins, R.E.; Hoogenboom, H.R. Making antibodies by phage display technology. Annu. Rev. Immunol. 1994, 12, 433–455. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Gao, H.; Qing, G. Phage display derived peptides for alzheimer’s disease therapy and diagnosis. Theranostics 2022, 12, 2041. [Google Scholar] [CrossRef]
- Anand, T.; Virmani, N.; Bera, B.C.; Vaid, R.K.; Vashisth, M.; Bardajatya, P.; Kumar, A.; Tripathi, B.N. Phage display technique as a tool for diagnosis and antibody selection for coronaviruses. Curr. Microbiol. 2021, 78, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Love, K.R.; Swoboda, J.G.; Noren, C.J.; Walker, S. Enabling glycosyltransferase evolution: A facile substrate-attachment strategy for phage-display enzyme evolution. ChemBioChem 2006, 7, 753–756. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Chung, W.J.; McFarland, S.; Lee, S.W. Assembly of bacteriophage into functional materials. Chem. Rec. 2013, 13, 43–59. [Google Scholar] [CrossRef]
- Zhou, X.; Cao, P.; Zhu, Y.; Lu, W.; Gu, N.; Mao, C. Phage-mediated counting by the naked eye of miRNA molecules at attomolar concentrations in a Petri dish. Nat. Mater. 2015, 14, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Gray, B.P.; Brown, K.C. Combinatorial peptide libraries: Mining for cell-binding peptides. Chem. Rev. 2014, 114, 1020–1081. [Google Scholar] [CrossRef]
- Piggott, A.M.; Karuso, P. Identifying the cellular targets of natural products using T7 phage display. Nat. Prod. Rep. 2016, 33, 626–636. [Google Scholar] [CrossRef]
- Yang, M.; Sunderland, K.; Mao, C. Virus-derived peptides for clinical applications. Chem. Rev. 2017, 117, 10377–10402. [Google Scholar] [CrossRef]
- Hussein, A.H.; Davis, E.M.; Halperin, S.A.; Lee, S.F. Construction and characterization of single-chain variable fragment antibodies directed against the Bordetella pertussis surface adhesins filamentous hemagglutinin and pertactin. Infect. Immun. 2007, 75, 5476–5482. [Google Scholar] [CrossRef]
- Braganza, A.; Wallace, K.; Pell, L.; Parrish, C.R.; Siegel, D.L.; Mason, N.J. Generation and validation of canine single chain variable fragment phage display libraries. Vet. Immunol. Immunopathol. 2011, 139, 27–40. [Google Scholar] [CrossRef]
- Dabiri, M.; Tehrani, M.; Rafiei, A.; Valadan, R. Production and functional analysis of a phage displayed scFv recombinant antibody targeting EGFR/HER2 dimerization domain. Protein Expr. Purif. 2025, 228, 106649. [Google Scholar] [CrossRef]
- Zhang, Y. Evolution of phage display libraries for therapeutic antibody discovery. Mabs 2023, 15, 2213793. [Google Scholar] [CrossRef] [PubMed]
- Galán, A.; Comor, L.; Horvatić, A.; Kuleš, J.; Guillemin, N.; Mrljak, V.; Bhide, M. Library-based display technologies: Where do we stand? Mol. Biosyst. 2016, 12, 2342–2358. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-Y.; Lin, C.-T.; Kuo, S.-Y.; Chang, D.-K.; Wu, H.-C. Peptide-mediated targeting to tumor blood vessels of lung cancer for drug delivery. Cancer Res. 2007, 67, 10958–10965. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Feng, L.; Fan, L.; Zha, Y.; Guo, L.; Zhang, Q.; Chen, J.; Pang, Z.; Wang, Y.; Jiang, X. Targeting the brain with PEG–PLGA nanoparticles modified with phage-displayed peptides. Biomaterials 2011, 32, 4943–4950. [Google Scholar] [CrossRef]
- Peabody, J.; Core, S.B.; Ronsard, L.; Lingwood, D.; Peabody, D.S.; Chackerian, B. An Approach for Antigen-Agnostic Identification of Virus-Like Particle-Displayed Epitopes that Engage Specific Antibody V Gene Regions. In Therapeutic Proteins: Methods and Protocols; Springer: Nature, Switzerland, 2023; pp. 55–74. [Google Scholar]
- Frietze, K.M.; Pascale, J.M.; Moreno, B.; Chackerian, B.; Peabody, D.S. Pathogen-specific deep sequence-coupled biopanning: A method for surveying human antibody responses. PLoS ONE 2017, 12, e0171511. [Google Scholar] [CrossRef]
- Chen, L.; Yuan, S.; Liu, Q.; Mai, G.; Yang, J.; Deng, D.; Zhang, B.; Liu, C.; Ma, Y. In vitro design and evaluation of phage cocktails against Aeromonas salmonicida. Front. Microbiol. 2018, 9, 1476. [Google Scholar] [CrossRef]
- Yoo, S.; Lee, K.-M.; Kim, N.; Vu, T.N.; Abadie, R.; Yong, D. Designing phage cocktails to combat the emergence of bacteriophage-resistant mutants in multidrug-resistant Klebsiella pneumoniae. Microbiol. Spectr. 2024, 12, e01258-23. [Google Scholar] [CrossRef]
- Schmerer, M.; Molineux, I.J.; Bull, J.J. Synergy as a rationale for phage therapy using phage cocktails. PeerJ 2014, 2, e590. [Google Scholar] [CrossRef]
- Ly-Chatain, M.H. The factors affecting effectiveness of treatment in phages therapy. Front. Microbiol. 2014, 5, 51. [Google Scholar] [CrossRef]
- Abubakar, S.; Hauwa-Suleiman, B.; Ali Abbagana, B.; Alhaji-Mustafa, I.; Abbas-Musa, I. Novel uses of bacteriophages in the treatment of human infections and antibiotic resistance. Am. J. Biosci 2016, 4, 34. [Google Scholar] [CrossRef][Green Version]
- Gou, Z.; Yao, P.; Xiong, L.; Wang, X.; Yuan, Q.; Sun, F.; Cheng, Y.; Xia, P. Potential of a phage cocktail in the treatment of multidrug-resistant Klebsiella pneumoniae pulmonary infection in mice. BMC Microbiol. 2025, 25, 151. [Google Scholar] [CrossRef] [PubMed]
- Emencheta, S.C.; Onugwu, A.L.; Kalu, C.F.; Ezinkwo, P.N.; Eze, O.C.; Vila, M.M.D.C.; Balcão, V.M.; Attama, A.A.; Onuigbo, E.B. Bacteriophages as nanocarriers for targeted drug delivery and enhanced therapeutic effects. Mater. Adv. 2024, 5, 986–1016. [Google Scholar] [CrossRef]
- Peng, H.; Chen, I.A.; Qimron, U. Engineering Phages to Fight Multidrug-Resistant Bacteria. Chem. Rev. 2024, 16, 933–971. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Mirshekari, H.; Moosavi Basri, S.M.; Bahrami, S.; Moghoofei, M.; Hamblin, M.R. Bacteriophages and phage-inspired nanocarriers for targeted delivery of therapeutic cargos. Adv. Drug Deliv. Rev. 2016, 106, 45–62. [Google Scholar] [CrossRef]
- Yacoby, I.; Bar, H.; Benhar, I. Targeted drug-carrying bacteriophages as antibacterial nanomedicines. Antimicrob. Agents Chemother. 2007, 51, 2156–2163. [Google Scholar] [CrossRef]
- Crooke, S.N.; Schimer, J.; Raji, I.; Wu, B.; Oyelere, A.K.; Finn, M.G. Lung Tissue Delivery of Virus-Like Particles Mediated by Macrolide Antibiotics. Mol. Pharm. 2019, 16, 2947–2955. [Google Scholar] [CrossRef]
- Knezevic, P.; Obreht, D.; Curcin, S.; Petrusic, M.; Aleksic, V.; Kostanjsek, R.; Petrovic, O. Phages of Pseudomonas aeruginosa: Response to environmental factors and in vitro ability to inhibit bacterial growth and biofilm formation. J. Appl. Microbiol. 2011, 111, 245–254. [Google Scholar] [CrossRef]
- Malik, D.J.; Sokolov, I.J.; Vinner, G.K.; Mancuso, F.; Cinquerrui, S.; Vladisavljevic, G.T.; Clokie, M.R.J.; Garton, N.J.; Stapley, A.G.F.; Kirpichnikova, A. Formulation, stabilisation and encapsulation of bacteriophage for phage therapy. Adv. Colloid Interface Sci. 2017, 249, 100–133. [Google Scholar] [CrossRef]
- Briers, Y.; Miroshnikov, K.; Chertkov, O.; Nekrasov, A.; Mesyanzhinov, V.; Volckaert, G.; Lavigne, R. The structural peptidoglycan hydrolase gp181 of bacteriophage phiKZ. Biochem. Biophys. Res. Commun. 2008, 374, 747–751. [Google Scholar] [CrossRef]
- Colom, J.; Cano-Sarabia, M.; Otero, J.; Cortés, P.; Maspoch, D.; Llagostera, M. Liposome-Encapsulated Bacteriophages for Enhanced Oral Phage Therapy against Salmonella spp. Appl. Environ. Microbiol. 2015, 81, 4841–4849. [Google Scholar] [CrossRef]
- Cinquerrui, S.; Mancuso, F.; Vladisavljević, G.T.; Bakker, S.E.; Malik, D.J. Nanoencapsulation of Bacteriophages in Liposomes Prepared Using Microfluidic Hydrodynamic Flow Focusing. Front. Microbiol. 2018, 9, 2172. [Google Scholar] [CrossRef] [PubMed]
- Otero, J.; García-Rodríguez, A.; Cano-Sarabia, M.; Maspoch, D.; Marcos, R.; Cortés, P.; Llagostera, M. Biodistribution of Liposome-Encapsulated Bacteriophages and Their Transcytosis During Oral Phage Therapy. Front. Microbiol. 2019, 10, 689. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Yuan, L.; Lin, L. Novel chitosan film embedded with liposome-encapsulated phage for biocontrol of Escherichia coli O157:H7 in beef. Carbohydr. Polym. 2017, 177, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Chuang, J.-J.; Huang, Y.-Y.; Lo, S.-H.; Hsu, T.-F.; Huang, W.-Y.; Huang, S.-L.; Lin, Y.-S. Effects of pH on the Shape of Alginate Particles and Its Release Behavior. Int. J. Polym. Sci. 2017, 2017, 3902704. [Google Scholar] [CrossRef]
- Wall, S.K.; Zhang, J.; Rostagno, M.H.; Ebner, P.D. Phage therapy to reduce preprocessing Salmonella infections in market-weight swine. Appl Environ. Microbiol. 2010, 76, 48–53. [Google Scholar] [CrossRef]
- Saez, A.C.; Zhang, J.; Rostagno, M.H.; Ebner, P.D. Direct feeding of microencapsulated bacteriophages to reduce Salmonella colonization in pigs. Foodborne Pathog. Dis. 2011, 8, 1269–1274. [Google Scholar] [CrossRef]
- Jamaledin, R.; Sartorius, R.; Di Natale, C.; Vecchione, R.; De Berardinis, P.; Netti, P.A. Recombinant Filamentous Bacteriophages Encapsulated in Biodegradable Polymeric Microparticles for Stimulation of Innate and Adaptive Immune Responses. Microorganisms 2020, 8, 650. [Google Scholar] [CrossRef]
- Martel, B.; Moineau, S. CRISPR-Cas: An efficient tool for genome engineering of virulent bacteriophages. Nucleic Acids Res. 2014, 42, 9504–9513. [Google Scholar] [CrossRef]
- Mitsunaka, S.; Yamazaki, K.; Pramono, A.K.; Ikeuchi, M.; Kitao, T.; Ohara, N.; Kubori, T.; Nagai, H.; Ando, H. Synthetic engineering and biological containment of bacteriophages. Proc. Natl. Acad. Sci. USA 2022, 119, e2206739119. [Google Scholar] [CrossRef]
- Wang, C.; Xia, Q.; Zhang, Q.; Qu, Y.; Su, S.; Cheng, J.K.; Hughes, N.W.; Cong, L. CRISPR-Cas12a System With Synergistic Phage Recombination Proteins for Multiplex Precision Editing in Human Cells. Front. Cell Dev. Biol. 2022, 9, 719705. [Google Scholar] [CrossRef]
- Cheng, L.; Deng, Z.; Tao, H.; Song, W.; Xing, B.; Liu, W.; Kong, L.; Yuan, S.; Ma, Y.; Wu, Y. Harnessing stepping-stone hosts to engineer, select, and reboot synthetic bacteriophages in one pot. Cell Rep. Methods 2022, 2, 100217. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Batra, H.; Dong, J.; Chen, C.; Rao, V.B.; Tao, P. Genetic engineering of bacteriophages against infectious diseases. Front. Microbiol. 2019, 10, 954. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Li, Y.; Yang, M.; Mao, C. T7 phage as an emerging nanobiomaterial with genetically tunable target specificity. Adv. Sci. 2022, 9, 2103645. [Google Scholar] [CrossRef] [PubMed]
- Duong, M.M.; Carmody, C.M.; Ma, Q.; Peters, J.E.; Nugen, S.R. Optimization of T4 phage engineering via CRISPR/Cas9. Sci. Rep. 2020, 10, 18229. [Google Scholar] [CrossRef]
- Krishnamurthy, M.; Moore, R.T.; Rajamani, S.; Panchal, R.G. Bacterial genome engineering and synthetic biology: Combating pathogens. BMC Microbiol. 2016, 16, 258. [Google Scholar] [CrossRef]
- Shen, J.; Zhou, J.; Chen, G.-Q.; Xiu, Z.-L. Efficient genome engineering of a virulent Klebsiella bacteriophage using CRISPR-Cas9. J. Virol. 2018, 92, e00534-18. [Google Scholar] [CrossRef]
- Hoshiga, F.; Yoshizaki, K.; Takao, N.; Miyanaga, K.; Tanji, Y. Modification of T2 phage infectivity toward Escherichia coli O157: H7 via using CRISPR/Cas9. FEMS Microbiol. Lett. 2019, 366, fnz041. [Google Scholar] [CrossRef]
- Ali, Y.; Inusa, I.; Sanghvi, G.; Mandaliya, V.; Bishoyi, A.K. The current status of phage therapy and its advancement towards establishing standard antimicrobials for combating multi drug-resistant bacterial pathogens. Microb. Pathog. 2023, 181, 106199. [Google Scholar] [CrossRef]
- Dedrick, R.M.; Guerrero-Bustamante, C.A.; Garlena, R.A.; Russell, D.A.; Ford, K.; Harris, K.; Gilmour, K.C.; Soothill, J.; Jacobs-Sera, D.; Schooley, R.T. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat. Med. 2019, 25, 730–733. [Google Scholar] [CrossRef]
- Kiga, K.; Tan, X.-E.; Ibarra-Chávez, R.; Watanabe, S.; Aiba, Y.; Sato’o, Y.; Li, F.-Y.; Sasahara, T.; Cui, B.; Kawauchi, M. Development of CRISPR-Cas13a-based antimicrobials capable of sequence-specific killing of target bacteria. Nat. Commun. 2020, 11, 2934. [Google Scholar] [CrossRef]
- Li, F.-Y.; Tan, X.-E.; Shimamori, Y.; Kiga, K.; Veeranarayanan, S.; Watanabe, S.; Nishikawa, Y.; Aiba, Y.; Sato’o, Y.; Miyanaga, K. Phagemid-based capsid system for CRISPR-Cas13a antimicrobials targeting methicillin-resistant Staphylococcus aureus. Commun. Biol. 2024, 7, 1129. [Google Scholar] [CrossRef] [PubMed]
- Shimamori, Y.; Tan, X.-E.; Li, F.-Y.; Nishikawa, Y.; Watanabe, S.; Sasahara, T.; Miyanaga, K.; Aiba, Y.; Veeranarayanan, S.; Thitiananpakorn, K. Efficient synthesis of CRISPR-Cas13a-antimicrobial capsids against MRSA facilitated by silent mutation incorporation. Sci. Rep. 2024, 14, 16225. [Google Scholar] [CrossRef] [PubMed]
- Kilcher, S.; Studer, P.; Muessner, C.; Klumpp, J.; Loessner, M.J. Cross-genus rebooting of custom-made, synthetic bacteriophage genomes in L-form bacteria. Proc. Natl. Acad. Sci. USA 2018, 115, 567–572. [Google Scholar] [CrossRef]
- Pirnay, J.-P.; Djebara, S.; Steurs, G.; Griselain, J.; Cochez, C.; De Soir, S.; Glonti, T.; Spiessens, A.; Vanden Berghe, E.; Green, S. Personalized bacteriophage therapy outcomes for 100 consecutive cases: A multicentre, multinational, retrospective observational study. Nat. Microbiol. 2024, 9, 1434–1453. [Google Scholar] [CrossRef]
- Kebriaei, R.; Lehman, S.M.; Shah, R.M.; Stamper, K.C.; Kunz Coyne, A.J.; Holger, D.; El Ghali, A.; Rybak, M.J. Optimization of phage-antibiotic combinations against Staphylococcus aureus biofilms. Microbiol. Spectr. 2023, 11, e04918-22. [Google Scholar] [CrossRef]
- Khalifa, L.; Shlezinger, M.; Beyth, S.; Houri-Haddad, Y.; Coppenhagen-Glazer, S.; Beyth, N.; Hazan, R. Phage therapy against Enterococcus faecalis in dental root canals. J. Oral Microbiol. 2016, 8, 32157. [Google Scholar] [CrossRef]
- El-Telbany, M.; El-Didamony, G.; Askora, A.; Ariny, E.; Abdallah, D.; Connerton, I.F.; El-Shibiny, A. Bacteriophages to control multi-drug resistant Enterococcus faecalis infection of dental root canals. Microorganisms 2021, 9, 517. [Google Scholar] [CrossRef]
- El-Telbany, M.; Lin, C.-Y.; Abdelaziz, M.N.; Maung, A.T.; El-Shibiny, A.; Mohammadi, T.N.; Zayda, M.; Wang, C.; Zar Chi Lwin, S.; Zhao, J. Potential application of phage vB_EfKS5 to control Enterococcus faecalis and its biofilm in food. AMB Express 2023, 13, 130. [Google Scholar] [CrossRef]
- Chung, K.M.; Liau, X.L.; Tang, S.S. Bacteriophages and their host range in multidrug-resistant bacterial disease treatment. Pharmaceuticals 2023, 16, 1467. [Google Scholar] [CrossRef]
- Jault, P.; Leclerc, T.; Jennes, S.; Pirnay, J.P.; Que, Y.-A.; Resch, G.; Rousseau, A.F.; Ravat, F.; Carsin, H.; Le Floch, R.; et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): A randomised, controlled, double-blind phase 1/2 trial. Lancet Infect. Dis. 2019, 19, 35–45. [Google Scholar] [CrossRef]
- Pasivkina, M.A.; Anurova, M.N.; Kiseleva, I.A.; Andreeva, A.A.; Vorobev, A.M.; Mizaeva, T.E.; Mekhtiev, E.R.; Zubkova, E.S.; Alieva, K.M.; Kuzmin, A.R.; et al. Isolation and Characterization of Salmonella Bacteriophages as Potential Agents for Phage Therapy of Antibiotic-Resistant Intestinal Infections. Bull. Exp. Biol. Med. 2024, 177, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Sabzali, S.; Bouzari, M. Isolation, identification and some characteristics of two lytic bacteriophages against Salmonella enterica serovar Paratyphi B and S. enterica serovar Typhimurium from various food sources. FEMS Microbiol. Lett. 2021, 368, fnab037. [Google Scholar] [CrossRef] [PubMed]
- Weber-Dąbrowska, B.; Mulczyk, M.; Górski, A. Bacteriophage therapy of bacterial infections: An update of our institute’s experience. Arch. Immunol. Ther. Exp. 2000, 48, 547–551. [Google Scholar]
- Markoishvili, K.; Tsitlanadze, G.; Katsarava, R.; Glenn, J.; Morris, M., Jr.; Sulakvelidze, A. A novel sustained-release matrix based on biodegradable poly (ester amide) s and impregnated with bacteriophages and an antibiotic shows promise in management of infected venous stasis ulcers and other poorly healing wounds. Int. J. Dermatol. 2002, 41, 453–458. [Google Scholar] [CrossRef]
- Morozova, V.V.; Kozlova, Y.N.; Ganichev, D.A.; Tikunova, N.V. Bacteriophage treatment of infected diabetic foot ulcers. In Bacteriophage Therapy: From Lab to Clinical Practice; Springer: Cham, Switzerland, 2017; pp. 151–158. [Google Scholar]
- Kumari, S.; Harjai, K.; Chhibber, S. Bacteriophage versus antimicrobial agents for the treatment of murine burn wound infection caused by Klebsiella pneumoniae B5055. J. Med. Microbiol. 2011, 60, 205–210. [Google Scholar] [CrossRef]
- Yin, S.; Huang, G.; Zhang, Y.; Jiang, B.; Yang, Z.; Dong, Z.; You, B.; Yuan, Z.; Hu, F.; Zhao, Y. Phage Abp1 rescues human cells and mice from infection by pan-drug resistant Acinetobacter baumannii. Cell. Physiol. Biochem. 2018, 44, 2337–2345. [Google Scholar] [CrossRef]
- Totté, J.E.; van Doorn, M.B.; Pasmans, S.G. Successful treatment of chronic Staphylococcus aureus-related dermatoses with the topical endolysin Staphefekt SA. 100: A report of 3 cases. Case Rep. Dermatol. 2017, 9, 19–25. [Google Scholar] [CrossRef]
- Castillo-Ruiz, M.; Vinés, E.D.; Montt, C.; Fernández, J.; Delgado, J.M.; Hormazábal, J.C.; Bittner, M. Isolation of a novel Aggregatibacter actinomycetemcomitans serotype b bacteriophage capable of lysing bacteria within a biofilm. Appl. Environ. Microbiol. 2011, 77, 3157–3159. [Google Scholar] [CrossRef]
- Xu, J.; Yang, H.; Bi, Y.; Li, W.; Wei, H.; Li, Y. Activity of the chimeric lysin ClyR against common Gram-positive oral microbes and its anticaries efficacy in rat models. Viruses 2018, 10, 380. [Google Scholar] [CrossRef]
- Li, W.; Yang, H.; Gong, Y.; Wang, S.; Li, Y.; Wei, H. Effects of a chimeric lysin against planktonic and sessile Enterococcus faecalis hint at potential application in endodontic therapy. Viruses 2018, 10, 290. [Google Scholar] [CrossRef]
- Ott, S.J.; Waetzig, G.H.; Rehman, A.; Moltzau-Anderson, J.; Bharti, R.; Grasis, J.A.; Cassidy, L.; Tholey, A.; Fickenscher, H.; Seegert, D. Efficacy of sterile fecal filtrate transfer for treating patients with Clostridium difficile infection. Gastroenterology 2017, 152, 799–811.e7. [Google Scholar] [CrossRef] [PubMed]
- Vahedi, A.; Soltan Dallal, M.M.; Douraghi, M.; Nikkhahi, F.; Rajabi, Z.; Yousefi, M.; Mousavi, M. Isolation and identification of specific bacteriophage against enteropathogenic Escherichia coli (EPEC) and in vitro and in vivo characterization of bacteriophage. FEMS Microbiol. Lett. 2018, 365, fny136. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Wang, X.; Wang, L.; Li, Z.; Che, J.; Wang, L.; Li, X.; Cao, Z.; Zhang, J.; Jin, L. Evaluation of the efficacy of a bacteriophage in the treatment of pneumonia induced by multidrug resistance Klebsiella pneumoniae in mice. BioMed Res. Int. 2015, 2015, 752930. [Google Scholar] [CrossRef] [PubMed]
- Waters, E.M.; Neill, D.R.; Kaman, B.; Sahota, J.S.; Clokie, M.R.; Winstanley, C.; Kadioglu, A. Phage therapy is highly effective against chronic lung infections with Pseudomonas aeruginosa. Thorax 2017, 72, 666–667. [Google Scholar] [CrossRef]
- Bao, J.; Wu, N.; Zeng, Y.; Chen, L.; Li, L.; Yang, L.; Zhang, Y.; Guo, M.; Li, L.; Li, J. Non-active antibiotic and bacteriophage synergism to successfully treat recurrent urinary tract infection caused by extensively drug-resistant Klebsiella pneumoniae. Emerg. Microbes Infect. 2020, 9, 771–774. [Google Scholar] [CrossRef]
- Fukuda, K.; Ishida, W.; Uchiyama, J.; Rashel, M.; Kato, S.-I.; Morita, T.; Muraoka, A.; Sumi, T.; Matsuzaki, S.; Daibata, M. Pseudomonas aeruginosa keratitis in mice: Effects of topical bacteriophage KPP12 administration. PLoS ONE 2012, 7, e47742. [Google Scholar] [CrossRef]
- Wright, A.; Hawkins, C.; Änggård, E.; Harper, D. A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clin. Otolaryngol. 2009, 34, 349–357. [Google Scholar] [CrossRef]
- Hawkins, C.; Harper, D.; Burch, D.; Änggård, E.; Soothill, J. Topical treatment of Pseudomonas aeruginosa otitis of dogs with a bacteriophage mixture: A before/after clinical trial. Vet. Microbiol. 2010, 146, 309–313. [Google Scholar] [CrossRef]
- Ooi, M.L.; Drilling, A.J.; Morales, S.; Fong, S.; Moraitis, S.; Macias-Valle, L.; Vreugde, S.; Psaltis, A.J.; Wormald, P.-J. Safety and tolerability of bacteriophage therapy for chronic rhinosinusitis due to Staphylococcus aureus. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 723–729. [Google Scholar] [CrossRef]
- Schneider, G.; Szentes, N.; Horváth, M.; Dorn, Á.; Cox, A.; Nagy, G.; Doffkay, Z.; Maróti, G.; Rákhely, G.; Kovács, T. Kinetics of targeted phage rescue in a mouse model of systemic Escherichia coli K1. BioMed Res. Int. 2018, 2018, 7569645. [Google Scholar] [CrossRef]
- Duan, Y.; Llorente, C.; Lang, S.; Brandl, K.; Chu, H.; Jiang, L.; White, R.C.; Clarke, T.H.; Nguyen, K.; Torralba, M. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature 2019, 575, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Barros, J.; Melo, L.D.; Poeta, P.; Igrejas, G.; Ferraz, M.P.; Azeredo, J.; Monteiro, F.J. Lytic bacteriophages against multidrug-resistant Staphylococcus aureus, Enterococcus faecalis and Escherichia coli isolates from orthopaedic implant-associated infections. Int. J. Antimicrob. Agents 2019, 54, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Racenis, K.; Lacis, J.; Rezevska, D.; Mukane, L.; Vilde, A.; Putnins, I.; Djebara, S.; Merabishvili, M.; Pirnay, J.-P.; Kalnina, M. Successful bacteriophage-antibiotic combination therapy against multidrug-resistant Pseudomonas aeruginosa left ventricular assist device driveline infection. Viruses 2023, 15, 1210. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, J.; Nakamura, K.; Ishiguro, Y.; Iwano, H. Using phage to drive selections toward restoring antibiotic sensitivity in Pseudomonas aeruginosa via chromosomal deletions. Front. Microbiol. 2024, 15, 1401234. [Google Scholar] [CrossRef]
- Altamirano, F.L.G.; Kostoulias, X.; Subedi, D.; Korneev, D.; Peleg, A.Y.; Barr, J.J. Phage-antibiotic combination is a superior treatment against Acinetobacter baumannii in a preclinical study. EBioMedicine 2022, 80, 104045. [Google Scholar]
- Sharun, K.; Dhama, K.; Tiwari, R.; Gugjoo, M.B.; Iqbal Yatoo, M.; Patel, S.K.; Pathak, M.; Karthik, K.; Khurana, S.K.; Singh, R. Advances in therapeutic and managemental approaches of bovine mastitis: A comprehensive review. Vet. Q. 2021, 41, 107–136. [Google Scholar] [CrossRef]
- Haq, I.U.; Chaudhry, W.N.; Akhtar, M.N.; Andleeb, S.; Qadri, I. Bacteriophages and their implications on future biotechnology: A review. Virol. J. 2012, 9, 9. [Google Scholar] [CrossRef]
- Tiwari, R.; Dhama, K.; Kumar, A.; Rahal, A.; Kapoor, S. Bacteriophage therapy for safeguarding animal and human health: A review. Pak. J. Biol. Sci. 2014, 17, 301–315. [Google Scholar] [CrossRef]
- Carson, L.; Gorman, S.P.; Gilmore, B.F. The use of lytic bacteriophages in the prevention and eradication of biofilms of Proteus mirabilis and Escherichia coli. FEMS Immunol. Med. Microbiol. 2010, 59, 447–455. [Google Scholar] [CrossRef]
- Verbanic, S.; Deacon, J.M.; Chen, I.A. The chronic wound phageome: Phage diversity and associations with wounds and healing outcomes. Microbiol. Spectr. 2022, 10, e0277721. [Google Scholar] [CrossRef]
- Akturk, E.; Melo, L.D.; Oliveira, H.; Crabbé, A.; Coenye, T.; Azeredo, J. Combining phages and antibiotic to enhance antibiofilm efficacy against an in vitro dual species wound biofilm. Biofilm 2023, 6, 100147. [Google Scholar] [CrossRef] [PubMed]
- Fiscarelli, E.V.; Rossitto, M.; Rosati, P.; Essa, N.; Crocetta, V.; Di Giulio, A.; Lupetti, V.; Di Bonaventura, G.; Pompilio, A. In vitro newly isolated environmental phage activity against biofilms preformed by Pseudomonas aeruginosa from patients with cystic fibrosis. Microorganisms 2021, 9, 478. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Chen, H.; Zhang, M.; Zhao, Y.; Jiang, Y.; Liu, X.; Huang, W.; Ma, Y. Clinical experience of personalized phage therapy against carbapenem-resistant Acinetobacter baumannii lung infection in a patient with chronic obstructive pulmonary disease. Front. Cell. Infect. Microbiol. 2021, 11, 631585. [Google Scholar] [CrossRef] [PubMed]
- Keary, R.; Sanz-Gaitero, M.; J van Raaij, M.; Mahony, J.; Fenton, M.; McAuliffe, O.; Hill, C.; Paul Ross, R.; Coffey, A. Characterization of a bacteriophage-derived murein peptidase for elimination of antibiotic-resistant Staphylococcus aureus. Curr. Protein Pept. Sci. 2016, 17, 183–190. [Google Scholar] [CrossRef]
- Mirzaei, A.; Wagemans, J.; Nasr Esfahani, B.; Lavigne, R.; Moghim, S. A phage cocktail to control surface colonization by proteus mirabilis in catheter-associated urinary tract infections. Microbiol. Spectr. 2022, 10, e0209222. [Google Scholar] [CrossRef]
- Kowalski, J.; Górska, R.; Cieślik, M.; Górski, A.; Jończyk-Matysiak, E. What are the potential benefits of using bacteriophages in periodontal therapy? Antibiotics 2022, 11, 446. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, Z.; Lin, H.; Tian, Y.; Zhang, P.; Chen, H.; Wang, Y.; Shen, Y. The feasibility of phage therapy for periodontitis. Future Microbiol. 2021, 16, 649–656. [Google Scholar] [CrossRef]
- Schmalstig, A.A.; Wiggins, A.; Badillo, D.; Wetzel, K.S.; Hatfull, G.F.; Braunstein, M. Bacteriophage infection and killing of intracellular Mycobacterium abscessus. mbio 2024, 15, e0292423. [Google Scholar] [CrossRef]
- Garcia, P.; Martinez, B.; Obeso, J.; Rodriguez, A. Bacteriophages and their application in food safety. Lett. Appl. Microbiol. 2008, 47, 479–485. [Google Scholar] [CrossRef]
- Kropinski, A.M.; Sulakvelidze, A.; Konczy, P.; Poppe, C. Salmonella phages and prophages—Genomics and practical aspects. Methods Mol. Biol. 2007, 394, 133–175. [Google Scholar]
- Chan, B.K.; Abedon, S.T.; Loc-Carrillo, C. Phage cocktails and the future of phage therapy. Future Microbiol. 2013, 8, 769–783. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Liu, Q.; Lian, T.; Shao, L. The ΦCPG1 chlamydiaphage can infect Chlamydia trachomatis and significantly reduce its infectivity. Virus Res. 2019, 267, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pan, O.C.-C.; Miller, S.; Patel, R.; Mukhopadhyay, S.; Sarullo, G.; Go, G.; Galli, J.; Hessels, J.; Schlingmann-Molina, B.; Ndashimye, E. Discovery of Antibodies Against Endemic Coronaviruses with NGS-Based Human Fab Phage Display Platform. Antibodies 2025, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Bahadir, A.O.; Balcioglu, B.K.; Uzyol, K.S.; Hatipoglu, I.; Sogut, I.; Basalp, A.; Erdag, B. Phage displayed HBV core antigen with immunogenic activity. Appl. Biochem. Biotechnol. 2011, 165, 1437–1447. [Google Scholar] [CrossRef]
- González-Mora, A.; Hernández-Pérez, J.; Iqbal, H.M.; Rito-Palomares, M.; Benavides, J. Bacteriophage-based vaccines: A potent approach for antigen delivery. Vaccines 2020, 8, 504. [Google Scholar] [CrossRef]
- Palma, M. Aspects of phage-based vaccines for protein and epitope immunization. Vaccines 2023, 11, 436. [Google Scholar] [CrossRef]
- Li, W.; Schäfer, A.; Kulkarni, S.S.; Liu, X.; Martinez, D.R.; Chen, C.; Sun, Z.; Leist, S.R.; Drelich, A.; Zhang, L. High potency of a bivalent human VH domain in SARS-CoV-2 animal models. Cell 2020, 183, 429–441.e16. [Google Scholar] [CrossRef]
- Sathaliyawala, T.; Rao, M.; Maclean, D.M.; Birx, D.L.; Alving, C.R.; Rao, V.B. Assembly of human immunodeficiency virus (HIV) antigens on bacteriophage T4: A novel in vitro approach to construct multicomponent HIV vaccines. J. Virol. 2006, 80, 7688–7698. [Google Scholar] [CrossRef]
- Zulkarneev, E.R.; Laishevtsevtsev, A.I.; Kiseleva, I.A.; Efimova, O.G.; Mizaeva, T.E.; Pasivkina, M.A.; Zubkova, E.S.; Aleshkin, A.V.; Karaulov, A.V. Assessment of the Safety of Anti-Salmonella Disinfectant for Veterinary Use Based on a Cocktail of Bacteriophages. Bull. Exp. Biol. Med. 2024, 177, 482–487. [Google Scholar] [CrossRef]
- Fenton, M.; Keary, R.; McAuliffe, O.; Ross, R.P.; O′ Mahony, J.; Coffey, A. Bacteriophage-derived peptidase CHAPk eliminates and prevents staphylococcal biofilms. Int. J. Microbiol. 2013, 2013, 625341. [Google Scholar] [CrossRef]
- Silva, E.C.; Oliveira, T.J.; Moreli, F.C.; Harada, L.K.; Vila, M.; Balcão, V.M. Newly isolated lytic bacteriophages for Staphylococcus intermedius, structurally and functionally stabilized in a hydroxyethylcellulose gel containing choline geranate: Potential for transdermal permeation in veterinary phage therapy. Res. Vet. Sci. 2021, 135, 42–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ji, Y.; Su, J.; Xue, Y.; Xi, H.; Wang, Z.; Bi, L.; Zhao, R.; Zhang, H.; Yang, L.; et al. Therapeutic Efficacy of Phage P(IZ) SAE-01E2 against Abortion Caused by Salmonella enterica Serovar Abortusequi in Mice. Appl Environ. Microbiol. 2020, 86, e01366-20. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, T.; Iwano, H.; Higuchi, H.; Yokota, H.; Usui, M.; Iwasaki, T.; Tamura, Y. Bacteriophage can lyse antibiotic-resistant Pseudomonas aeruginosa isolated from canine diseases. J. Vet. Med. Sci. 2016, 78, 1035–1038. [Google Scholar] [CrossRef]
- Santos, T.M.; Ledbetter, E.C.; Caixeta, L.S.; Bicalho, M.L.; Bicalho, R.C. Isolation and characterization of two bacteriophages with strong in vitro antimicrobial activity against Pseudomonas aeruginosa isolated from dogs with ocular infections. Am. J. Vet. Res. 2011, 72, 1079–1086. [Google Scholar] [CrossRef]
- Freitag, T.; Squires, R.A.; Schmid, J. Naturally occurring bacteriophages lyse a large proportion of canine and feline uropathogenic Escherichia coli isolates in vitro. Res. Vet. Sci. 2008, 85, 1–7. [Google Scholar] [CrossRef]
- Alomari, M.M.M.; Dec, M.; Nowaczek, A.; Puchalski, A.; Wernicki, A.; Kowalski, C.; Urban-Chmiel, R. Therapeutic and Prophylactic Effect of the Experimental Bacteriophage Treatment to Control Diarrhea Caused by E. coli in Newborn Calves. ACS Infect. Dis. 2021, 7, 2093–2101. [Google Scholar] [CrossRef]
- Thanki, A.M.; Mignard, G.; Atterbury, R.J.; Barrow, P.; Millard, A.D.; Clokie, M.R.J. Prophylactic Delivery of a Bacteriophage Cocktail in Feed Significantly Reduces Salmonella Colonization in Pigs. Microbiol. Spectr. 2022, 10, e0042222. [Google Scholar] [CrossRef]
- Ji, W.; Huang, Q.; Sun, L.; Wang, H.; Yan, Y.; Sun, J. A novel endolysin disrupts Streptococcus suis with high efficiency. FEMS Microbiol. Lett. 2015, 362, fnv205. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, C.; Wang, H.; Yan, Y.X.; Sun, J. A novel prophage lysin Ply5218 with extended lytic activity and stability against Streptococcus suis infection. FEMS Microbiol. Lett. 2016, 363, fnw186. [Google Scholar] [CrossRef][Green Version]
- Wang, Z.; Ma, J.; Wang, J.; Yang, D.; Kong, L.; Fu, Q.; Cheng, Y.; Wang, H.; Yan, Y.; Sun, J. Application of the Phage Lysin Ply5218 in the Treatment of Streptococcus suis Infection in Piglets. Viruses 2019, 11, 715. [Google Scholar] [CrossRef]
- El-Shibiny, A.; Scott, A.; Timms, A.; Metawea, Y.; Connerton, P.; Connerton, I. Application of a group II Campylobacter bacteriophage to reduce strains of Campylobacter jejuni and Campylobacter coli colonizing broiler chickens. J. Food Prot. 2009, 72, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, M.; Trotereau, A.; Culot, A.; Moodley, A.; Atterbury, R.; Wagemans, J.; Lavigne, R.; Velge, P.; Schouler, C. Isolation and Characterization of a Novel Phage Collection against Avian-Pathogenic Escherichia coli. Microbiol. Spectr. 2023, 11, e0429622. [Google Scholar] [CrossRef] [PubMed]
- Canter, R.G.; Penney, J.; Tsai, L.-H. The road to restoring neural circuits for the treatment of Alzheimer’s disease. Nature 2016, 539, 187–196. [Google Scholar] [CrossRef]
- De Strooper, B.; Karran, E. The cellular phase of Alzheimer’s disease. Cell 2016, 164, 603–615. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, X.-L.; Yang, Q.-G.; Xu, W.-H.; Wang, F.; Chen, Y.-P.; Chen, G.-H. A peptide that binds specifically to the β-amyloid of Alzheimer’s disease: Selection and assessment of anti-β-amyloid neurotoxic effects. PLoS ONE 2011, 6, e27649. [Google Scholar] [CrossRef]
- Van Groen, T.; Wiesehan, K.; Funke, S.A.; Kadish, I.; Nagel-Steger, L.; Willbold, D. Reduction of Alzheimer’s Disease Amyloid Plaque Load in Transgenic Mice by D3, ad-Enantiomeric Peptide Identified by Mirror Image Phage Display. ChemMedChem Chem. Enabling Drug Discov. 2008, 3, 1848–1852. [Google Scholar]
- Funke, S.A.; van Groen, T.; Kadish, I.; Bartnik, D.; Nagel-Steger, L.; Brener, O.; Sehl, T.; Batra-Safferling, R.; Moriscot, C.; Schoehn, G. Oral treatment with the D-enantiomeric peptide D3 improves the pathology and behavior of Alzheimer’s disease transgenic mice. ACS Chem. Neurosci. 2010, 1, 639–648. [Google Scholar] [CrossRef]
- van Groen, T.; Kadish, I.; Funke, S.A.; Bartnik, D.; Willbold, D. Treatment with D3 removes amyloid deposits, reduces inflammation, and improves cognition in aged AβPP/PS1 double transgenic mice. J. Alzheimer’s Dis. 2013, 34, 609–620. [Google Scholar] [CrossRef]
- Jiang, N.; Leithold, L.H.; Post, J.; Ziehm, T.; Mauler, J.; Gremer, L.; Cremer, M.; Schartmann, E.; Shah, N.J.; Kutzsche, J. Preclinical pharmacokinetic studies of the tritium labelled D-enantiomeric peptide D3 developed for the treatment of Alzheimer s disease. PLoS ONE 2015, 10, e0128553. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Ito, S.; Masuda, T.; Couraud, P.-O.; Ohtsuki, S. Novel cyclic peptides facilitating transcellular blood-brain barrier transport of macromolecules in vitro and in vivo. J. Control. Release 2020, 321, 744–755. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Li, Y.; Zhong, M.; Zhao, P.; Guo, C.; Xu, H.; Wang, T.; Gao, H. Brain targeting and Aβ binding bifunctional nanoparticles inhibit amyloid protein aggregation in APP/PS1 transgenic mice. ACS Chem. Neurosci. 2021, 12, 2110–2121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wan, X.; Zheng, X.; Shao, X.; Liu, Q.; Zhang, Q.; Qian, Y. Dual-functional nanoparticles targeting amyloid plaques in the brains of Alzheimer’s disease mice. Biomaterials 2014, 35, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Janelidze, S.; Stomrud, E.; Smith, R.; Palmqvist, S.; Mattsson, N.; Airey, D.C.; Proctor, N.K.; Chai, X.; Shcherbinin, S.; Sims, J.R. Cerebrospinal fluid p-tau217 performs better than p-tau181 as a biomarker of Alzheimer’s disease. Nat. Commun. 2020, 11, 1683. [Google Scholar] [CrossRef] [PubMed]
- Janelidze, S.; Mattsson, N.; Palmqvist, S.; Smith, R.; Beach, T.G.; Serrano, G.E.; Chai, X.; Proctor, N.K.; Eichenlaub, U.; Zetterberg, H. Plasma P-tau181 in Alzheimer’s disease: Relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat. Med. 2020, 26, 379–386. [Google Scholar] [CrossRef]
- Carmody, C.M.; Goddard, J.M.; Nugen, S.R. Bacteriophage capsid modification by genetic and chemical methods. Bioconjug. Chem. 2021, 32, 466–481. [Google Scholar] [CrossRef]
- Veeranarayanan, S.; Azam, A.H.; Kiga, K.; Watanabe, S.; Cui, L. Bacteriophages as solid tumor theragnostic agents. Int. J. Mol. Sci. 2021, 23, 402. [Google Scholar] [CrossRef]
- Marino, S.M.; Gladyshev, V.N. Cysteine function governs its conservation and degeneration and restricts its utilization on protein surfaces. J. Mol. Biol. 2010, 404, 902–916. [Google Scholar] [CrossRef]
- Liyanagedera, S.B.; Williams, J.; Wheatley, J.P.; Biketova, A.Y.; Hasan, M.; Sagona, A.P.; Purdy, K.J.; Puxty, R.J.; Feher, T.; Kulkarni, V. SpyPhage: A cell-free TXTL platform for rapid engineering of targeted phage therapies. ACS Synth. Biol. 2022, 11, 3330–3342. [Google Scholar] [CrossRef]
- Sanmukh, S.G.; Felisbino, S.L. Bacteriophages in cancer biology and therapies. Clin. Oncol. 2017, 2, 1295. [Google Scholar]
- Abbineni, G.; Modali, S.; Safiejko-Mroczka, B.; Petrenko, V.A.; Mao, C. Evolutionary selection of new breast cancer cell-targeting peptides and phages with the cell-targeting peptides fully displayed on the major coat and their effects on actin dynamics during cell internalization. Mol. Pharm. 2010, 7, 1629–1642. [Google Scholar] [CrossRef]
- Sunderland, K.S.; Yang, M.; Mao, C. Phage-enabled nanomedicine: From probes to therapeutics in precision medicine. Angew. Chem. Int. Ed. 2017, 56, 1964–1992. [Google Scholar] [CrossRef] [PubMed]
- Sanmukh, S.G.; Dos Santos, N.J.; Barquilha, C.N.; De Carvalho, M.; Dos Reis, P.P.; Delella, F.K.; Carvalho, H.F.; Latek, D.; Fehér, T.; Felisbino, S.L. Bacterial RNA virus MS2 exposure increases the expression of cancer progression genes in the LNCaP prostate cancer cell line. Oncol. Lett. 2023, 25, 86. [Google Scholar] [CrossRef]
- Gibb, B.; Hyman, P.; Schneider, C.L. The many applications of engineered bacteriophages—An overview. Pharmaceuticals 2021, 14, 634. [Google Scholar] [CrossRef]
- Manivannan, A.C.; Dhandapani, R.; Velmurugan, P.; Thangavelu, S.; Paramasivam, R.; Ragunathan, L.; Saravanan, M. Phage in cancer treatment–Biology of therapeutic phage and screening of tumor targeting peptide. Expert Opin. Drug Deliv. 2022, 19, 873–882. [Google Scholar] [CrossRef]
- Choi, D.S.; Jin, H.-E.; Yoo, S.Y.; Lee, S.-W. Cyclic RGD peptide incorporation on phage major coat proteins for improved internalization by HeLa cells. Bioconjug. Chem. 2014, 25, 216–223. [Google Scholar] [CrossRef]
- Moradi, M.; Ghaleh, H.E.G.; Bolandian, M.; Dorostkar, R. New role of bacteriophages in medical oncology. Biotechnol. Appl. Biochem. 2023, 70, 2017–2024. [Google Scholar] [CrossRef]
- Du, B.; Han, H.; Wang, Z.; Kuang, L.; Wang, L.; Yu, L.; Wu, M.; Zhou, Z.; Qian, M. Targeted drug delivery to hepatocarcinoma in vivo by phage-displayed specific binding peptide. Mol. Cancer Res. 2010, 8, 135–144. [Google Scholar] [CrossRef]
- Bar, H.; Yacoby, I.; Benhar, I. Killing cancer cells by targeted drug-carrying phage nanomedicines. BMC Biotechnol. 2008, 8, 37. [Google Scholar] [CrossRef]
- Turrini, E.; Ulfo, L.; Costantini, P.E.; Saporetti, R.; Di Giosia, M.; Nigro, M.; Petrosino, A.; Pappagallo, L.; Kaltenbrunner, A.; Cantelli, A.; et al. Molecular engineering of a spheroid-penetrating phage nanovector for photodynamic treatment of colon cancer cells. Cell. Mol. Life Sci. 2024, 81, 144. [Google Scholar] [CrossRef]
- Sittiju, P.; Wudtiwai, B.; Chongchai, A.; Hajitou, A.; Kongtawelert, P.; Pothacharoen, P.; Suwan, K. Bacteriophage-based particles carrying the TNF-related apoptosis-inducing ligand (TRAIL) gene for targeted delivery in hepatocellular carcinoma. Nanoscale 2024, 16, 6603–6617. [Google Scholar] [CrossRef]
- Chongchai, A.; Bentayebi, K.; Chu, G.; Yan, W.; Waramit, S.; Phitak, T.; Kongtawelert, P.; Pothacharoen, P.; Suwan, K.; Hajitou, A. Targeted treatment of chondrosarcoma with a bacteriophage-based particle delivering a secreted tumor necrosis factor-related apoptosis-inducing ligand. Mol. Ther. Oncol. 2024, 32, 200805. [Google Scholar] [CrossRef] [PubMed]
- Shoae-Hassani, A.; Keyhanvar, P.; Seifalian, A.M.; Mortazavi-Tabatabaei, S.A.; Ghaderi, N.; Issazadeh, K.; Amirmozafari, N.; Verdi, J. λ Phage nanobioparticle expressing apoptin efficiently suppress human breast carcinoma tumor growth in vivo. PLoS ONE 2013, 8, e79907. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-R.; Zhou, Y.; Ye, B.-C. Tumor-Targeted Delivery of PD-1-Displaying Bacteriophages by Escherichia coli for Adjuvant Treatment of Colorectal Cancer. ACS Synth. Biol. 2025, 14, 407–419. [Google Scholar] [CrossRef]
- Catala, A.; Dzieciatkowska, M.; Wang, G.; Gutierrez-Hartmann, A.; Simberg, D.; Hansen, K.C.; D’Alessandro, A.; Catalano, C.E. Targeted intracellular delivery of trastuzumab using designer phage lambda nanoparticles alters cellular programs in human breast cancer cells. ACS Nano 2021, 15, 11789–11805. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lei, L.; Yan, J.; Wang, X.; Li, L.; Liu, Q.; Wang, Y.; Chen, T.; Shao, J.; Yu, L.; et al. Bifunctional Phage Particles Augment CD40 Activation and Enhance Lymph Node-Targeted Delivery of Personalized Neoantigen Vaccines. ACS Nano 2025, 19, 6955–6976. [Google Scholar] [CrossRef]
- Yue, H.; Li, Y.; Yang, T.; Wang, Y.; Bao, Q.; Xu, Y.; Liu, X.; Miao, Y.; Yang, M.; Mao, C. Filamentous phages as tumour-targeting immunotherapeutic bionanofibres. Nat. Nanotechnol. 2025, 20, 167–176. [Google Scholar] [CrossRef]
- Saw, P.E.; Song, E.-W. Phage display screening of therapeutic peptide for cancer targeting and therapy. Protein Cell 2019, 10, 787–807. [Google Scholar] [CrossRef]
- Lei, L.; Yan, J.; Xin, K.; Li, L.; Sun, Q.; Wang, Y.; Chen, T.; Wu, S.; Shao, J.; Liu, B.; et al. Engineered Bacteriophage-Based In Situ Vaccine Remodels a Tumor Microenvironment and Elicits Potent Antitumor Immunity. ACS Nano 2024, 18, 12194–12209. [Google Scholar] [CrossRef]
- Azizi, M.; Shahgolzari, M.; Fathi-Karkan, S.; Ghasemi, M.; Samadian, H. Multifunctional plant virus nanoparticles: An emerging strategy for therapy of cancer. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 15, e1872. [Google Scholar] [CrossRef]
- Phumyen, A.; Jantasorn, S.; Jumnainsong, A.; Leelayuwat, C. Doxorubicin-conjugated bacteriophages carrying anti-MHC class I chain-related A for targeted cancer therapy in vitro. OncoTargets Ther. 2014, 7, 2183–2195. [Google Scholar]
- Ashley, C.E.; Carnes, E.C.; Phillips, G.K.; Durfee, P.N.; Buley, M.D.; Lino, C.A.; Padilla, D.P.; Phillips, B.; Carter, M.B.; Willman, C.L. Cell-specific delivery of diverse cargos by bacteriophage MS2 virus-like particles. ACS Nano 2011, 5, 5729–5745. [Google Scholar] [CrossRef] [PubMed]
- Mozar, F.S.; Meivita, M.P.; Go, S.X.; Li, L.; Bajalovic, N.; Loke, D.K. Ultra-efficient MCF-7 cell ablation and chemotherapy-integrated electrothermal therapy with DOX–WS2–PEG–M13 nanostructures. Discov. Mater. 2024, 4, 5. [Google Scholar] [CrossRef]
- Suthiwangcharoen, N.; Li, T.; Li, K.; Thompson, P.; You, S.; Wang, Q. M13 bacteriophage-polymer nanoassemblies as drug delivery vehicles. Nano Res. 2011, 4, 483–493. [Google Scholar] [CrossRef]
- Kim, H.; Choi, H.; Bae, Y.; Kang, S. Development of target-tunable P22 VLP-based delivery nanoplatforms using bacterial superglue. Biotechnol. Bioeng. 2019, 116, 2843–2851. [Google Scholar] [CrossRef]
- Botstein, D.; Waddell, C.H.; King, J. Mechanism of head assembly and DNA encapsulation in Salmonella phage P22: I. Genes, proteins, structures and DNA maturation. J. Mol. Biol. 1973, 80, 669–695. [Google Scholar] [CrossRef]
- Kim, K.R.; Lee, A.S.; Kim, S.M.; Heo, H.R.; Kim, C.S. Virus-like nanoparticles as a theranostic platform for cancer. Front. Bioeng. Biotechnol. 2023, 10, 1106767. [Google Scholar] [CrossRef]
- Kolesanova, E.; Melnikova, M.; Bolshakova, T.; Rybalkina, E.Y.; Sivov, I. Bacteriophage MS2 as a tool for targeted delivery in solid tumor chemotherapy. Acta Nat. 2019, 11, 98–101. [Google Scholar] [CrossRef]
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRx 2005, 2, 3–14. [Google Scholar] [CrossRef]
- Anand, P.; O’Neil, A.; Lin, E.; Douglas, T.; Holford, M. Tailored delivery of analgesic ziconotide across a blood brain barrier model using viral nanocontainers. Sci. Rep. 2015, 5, 12497. [Google Scholar] [CrossRef]
- Apawu, A.K.; Curley, S.M.; Dixon, A.R.; Hali, M.; Sinan, M.; Braun, R.D.; Castracane, J.; Cacace, A.T.; Bergkvist, M.; Holt, A.G. MRI compatible MS2 nanoparticles designed to cross the blood–brain-barrier: Providing a path towards tinnitus treatment. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1999–2008. [Google Scholar] [CrossRef]
- Tsedev, U.; Lin, C.-W.; Hess, G.T.; Sarkaria, J.N.; Lam, F.C.; Belcher, A.M. Phage particles of controlled length and genome for in vivo targeted glioblastoma imaging and therapeutic delivery. ACS Nano 2022, 16, 11676–11691. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Kohli, A.G.; Moser, F.; Endy, D.; Belcher, A.M. Refactored M13 bacteriophage as a platform for tumor cell imaging and drug delivery. ACS Synth. Biol. 2012, 1, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Tuyen Ho, M.; Barrett, A.; Wang, Y.; Hu, Q. Bioinspired and Biomimetic Gene Delivery Systems. ACS Appl. Bio Mater. 2023, 7, 4914–4922. [Google Scholar] [CrossRef]
- Larocca, D.; Witte, A.; Johnson, W.; Pierce, G.F.; Baird, A. Targeting bacteriophage to mammalian cell surface receptors for gene delivery. Hum. Gene Ther. 1998, 9, 2393–2399. [Google Scholar] [CrossRef]
- Lankes, H.; Zanghi, C.; Santos, K.; Capella, C.; Duke, C.; Dewhurst, S. In vivo gene delivery and expression by bacteriophage lambda vectors. J. Appl. Microbiol. 2007, 102, 1337–1349. [Google Scholar] [CrossRef]
- Bedi, D.; Gillespie, J.W.; Petrenko, V.A., Jr.; Ebner, A.; Leitner, M.; Hinterdorfer, P.; Petrenko, V.A. Targeted delivery of siRNA into breast cancer cells via phage fusion proteins. Mol. Pharm. 2013, 10, 551–559. [Google Scholar] [CrossRef]
- Yata, T.; Lee, E.L.; Suwan, K.; Syed, N.; Asavarut, P.; Hajitou, A. Modulation of extracellular matrix in cancer is associated with enhanced tumor cell targeting by bacteriophage vectors. Mol. Cancer 2015, 14, 110. [Google Scholar] [CrossRef]
- Kao, C.-Y.; Pan, Y.-C.; Hsiao, Y.-H.; Lim, S.-K.; Cheng, T.-W.; Huang, S.-W.; Wu, S.M.-Y.; Sun, C.-P.; Tao, M.-H.; Mou, K.Y. Improvement of Gene Delivery by Minimal Bacteriophage Particles. ACS Nano 2023, 17, 14532–14544. [Google Scholar] [CrossRef]
- Hajitou, A.; Trepel, M.; Lilley, C.E.; Soghomonyan, S.; Alauddin, M.M.; Marini, F.C.; Restel, B.H.; Ozawa, M.G.; Moya, C.A.; Rangel, R. A hybrid vector for ligand-directed tumor targeting and molecular imaging. Cell 2006, 125, 385–398. [Google Scholar] [CrossRef]
- Przystal, J.M.; Waramit, S.; Pranjol, M.Z.I.; Yan, W.; Chu, G.; Chongchai, A.; Samarth, G.; Olaciregui, N.G.; Tabatabai, G.; Carcaboso, A.M. Efficacy of systemic temozolomide-activated phage-targeted gene therapy in human glioblastoma. EMBO Mol. Med. 2019, 11, e8492. [Google Scholar] [CrossRef]
- Qazi, S.; Miettinen, H.M.; Wilkinson, R.A.; McCoy, K.; Douglas, T.; Wiedenheft, B. Programmed self-assembly of an active P22-Cas9 nanocarrier system. Mol. Pharm. 2016, 13, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Haque, F.; Hu, H.; Guo, P. Bacteriophage RNA Leading the Way in RNA Nanotechnology for Targeted Cancer Therapy. In RNA Nanotechnology and Therapeutics; CRC Press: Boca Raton, FL, USA, 2022; pp. 473–483. [Google Scholar]
- Wang, G.; Jia, T.; Xu, X.; Chang, L.; Zhang, R.; Fu, Y.; Li, Y.; Yang, X.; Zhang, K.; Lin, G. Novel miR-122 delivery system based on MS2 virus like particle surface displaying cell-penetrating peptide TAT for hepatocellular carcinoma. Oncotarget 2016, 7, 59402. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, M.; Alizadeh, A.A.; Mivehroud, M.H.; Dastmalchi, S. Construction of a bacteriophage-derived vector with potential applications in targeted drug delivery and cell imaging. Biotechnol. Lett. 2024, 46, 147–159. [Google Scholar] [CrossRef]
- Foglizzo, V.; Marchiò, S. Bacteriophages as therapeutic and diagnostic vehicles in cancer. Pharmaceuticals 2021, 14, 161. [Google Scholar] [CrossRef]
- Gallo, J.; Villasante, A. Recent Advances in Biomimetic Nanocarrier-Based Photothermal Therapy for Cancer Treatment. Int. J. Mol. Sci. 2023, 24, 15484. [Google Scholar] [CrossRef] [PubMed]
- Gandra, N.; Abbineni, G.; Qu, X.; Huai, Y.; Wang, L.; Mao, C. Bacteriophage bionanowire as a carrier for both cancer-targeting peptides and photosensitizers and its use in selective cancer cell killing by photodynamic therapy. Small 2013, 9, 215–221. [Google Scholar] [CrossRef]
- Qu, X.; Qiu, P.; Zhu, Y.; Yang, M.; Mao, C. Guiding nanomaterials to tumors for breast cancer precision medicine: From tumor-targeting small-molecule discovery to targeted nanodrug delivery. NPG Asia Mater. 2017, 9, e452. [Google Scholar] [CrossRef]
- Shahrivarkevishahi, A.; Luzuriaga, M.A.; Herbert, F.C.; Tumac, A.C.; Brohlin, O.R.; Wijesundara, Y.H.; Adlooru, A.V.; Benjamin, C.; Lee, H.; Parsamian, P. PhotothermalPhage: A virus-based photothermal therapeutic agent. J. Am. Chem. Soc. 2021, 143, 16428–16438. [Google Scholar] [CrossRef]
- Cao, B.; Xu, H.; Yang, M.; Mao, C. Virus-based cancer therapeutics for targeted photodynamic therapy. Methods Mol. Biol. 2018, 1776, 643–652. [Google Scholar]
- Stephanopoulos, N.; Tong, G.J.; Hsiao, S.C.; Francis, M.B. Dual-surface modified virus capsids for targeted delivery of photodynamic agents to cancer cells. ACS Nano 2010, 4, 6014–6020. [Google Scholar] [CrossRef]
- Hou, X.-L.; Xie, X.-T.; Tan, L.-F.; Zhang, F.; Fan, J.-X.; Chen, W.; Hu, Y.-G.; Zhao, Y.-D.; Liu, B.; Xu, Q.-R. T4 phage display technology for enhanced photodynamic therapy of breast cancer. ACS Mater. Lett. 2023, 5, 2270–2281. [Google Scholar] [CrossRef]
- Ulfo, L.; Cantelli, A.; Petrosino, A.; Costantini, P.E.; Nigro, M.; Starinieri, F.; Turrini, E.; Zadran, S.K.; Zuccheri, G.; Saporetti, R. Orthogonal nanoarchitectonics of M13 phage for receptor targeted anticancer photodynamic therapy. Nanoscale 2022, 14, 632–641. [Google Scholar] [CrossRef]
- Bortot, B.; Apollonio, M.; Baj, G.; Andolfi, L.; Zupin, L.; Crovella, S.; di Giosia, M.; Cantelli, A.; Saporetti, R.; Ulfo, L. Advanced photodynamic therapy with an engineered M13 phage targeting EGFR: Mitochondrial localization and autophagy induction in ovarian cancer cell lines. Free Radic. Biol. Med. 2022, 179, 242–251. [Google Scholar] [CrossRef]
- Hou, X.-L.; Tan, L.-F.; Xie, X.-T.; Zhang, B.; Wang, Q.; Cheng, K.; Fan, J.-X.; Liu, T.-C.; Liu, B. Peroxisome-inspired T4 phage hybrid enzyme nanoreactors for photodynamic therapy of breast cancer. Chem. Eng. J. 2025, 505, 159138. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, Q.; Miao, Y.; Lyu, Y.; Xu, Y.; Yang, M.; Mao, C. Tumor-Homing Phage Nanofibers for Nanozyme-Enhanced Targeted Breast Cancer Therapy. Adv. Mater. 2025, 37, e2403756. [Google Scholar] [CrossRef]
- Sioud, M.; Zhang, Q. Precision Killing of M2 Macrophages with Phage-Displayed Peptide-Photosensitizer Conjugates. Cancers 2023, 15, 2009. [Google Scholar] [CrossRef]
- Xu, H.; Cao, B.; Li, Y.; Mao, C. Phage nanofibers in nanomedicine: Biopanning for early diagnosis, targeted therapy, and proteomics analysis. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1623. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Kawai, T.; Ikegawa, M.; Ori, D.; Akira, S. Decoding Toll-like receptors: Recent insights and perspectives in innate immunity. Immunity 2024, 57, 649–673. [Google Scholar] [CrossRef]
- Popescu, M.; Van Belleghem, J.D.; Khosravi, A.; Bollyky, P.L. Bacteriophages and the immune system. Annu. Rev. Virol. 2021, 8, 415–435. [Google Scholar] [CrossRef]
- Hashiguchi, S.; Yamaguchi, Y.; Takeuchi, O.; Akira, S.; Sugimura, K. Immunological basis of M13 phage vaccine: Regulation under MyD88 and TLR9 signaling. Biochem. Biophys. Res. Commun. 2010, 402, 19–22. [Google Scholar] [CrossRef]
- Paludan, S.R.; Bowie, A.G. Immune sensing of DNA. Immunity 2013, 38, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed]
- Champagne-Jorgensen, K.; Luong, T.; Darby, T.; Roach, D.R. Immunogenicity of bacteriophages. Trends Microbiol. 2023, 31, 1058–1071. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lamolinara, A.; Conti, L.; Giangrossi, M.; Cui, L.; Morelli, M.B.; Amantini, C.; Falconi, M.; Bartolacci, C.; Andreani, C. HER2-Displaying M13 Bacteriophages induce Therapeutic Immunity against Breast Cancer. Cancers 2022, 14, 4054. [Google Scholar] [CrossRef]
- Gajewski, T.F.; Schreiber, H.; Fu, Y.-X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013, 14, 1014–1022. [Google Scholar] [CrossRef]
- Dor-On, E.; Solomon, B. Targeting glioblastoma via intranasal administration of Ff bacteriophages. Front. Microbiol. 2015, 6, 530. [Google Scholar] [CrossRef]
- Sweere, J.M.; Van Belleghem, J.D.; Ishak, H.; Bach, M.S.; Popescu, M.; Sunkari, V.; Kaber, G.; Manasherob, R.; Suh, G.A.; Cao, X. Bacteriophage trigger antiviral immunity and prevent clearance of bacterial infection. Science 2019, 363, eaat9691. [Google Scholar] [CrossRef]
- Ghambashidze, K.; Chikhladze, R.; Saladze, T.; Hoopes, P.; Shubitidze, F.E. coli Phagelysate: A Primer to Enhance Nanoparticles and Drug Deliveries in Tumor. Cancers 2023, 15, 2315. [Google Scholar] [CrossRef]
- Garg, P. Filamentous bacteriophage: A prospective platform for targeting drugs in phage-mediated cancer therapy. J. Cancer Res. Ther. 2019, 15, S1–S10. [Google Scholar] [CrossRef]
- Gaubin, M.; Fanutti, C.; Mishal, Z.; Durrbach, A.; De Berardinis, P.; Sartorius, R.; Del Pozzo, G.; Guardiola, J.; Perham, R.N.; Piatier-Tonneau, D. Processing of filamentous bacteriophage virions in antigen-presenting cells targets both HLA class I and class II peptide loading compartments. DNA Cell Biol. 2003, 22, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Goracci, M.; Pignochino, Y.; Marchiò, S. Phage display-based nanotechnology applications in cancer immunotherapy. Molecules 2020, 25, 843. [Google Scholar] [CrossRef] [PubMed]
- Barati, N.; Razazan, A.; Nicastro, J.; Slavcev, R.; Arab, A.; Mosaffa, F.; Nikpoor, A.R.; Badiee, A.; Jaafari, M.R.; Behravan, J. Immunogenicity and antitumor activity of the superlytic λF7 phage nanoparticles displaying a HER2/neu-derived peptide AE37 in a tumor model of BALB/c mice. Cancer Lett. 2018, 424, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Pouyanfard, S.; Bamdad, T.; Hashemi, H.; Bandehpour, M.; Kazemi, B. Induction of protective anti-CTL epitope responses against HER-2-positive breast cancer based on multivalent T7 phage nanoparticles. PLoS ONE 2012, 7, e49539. [Google Scholar] [CrossRef]
- Bartolacci, C.; Andreani, C.; Curcio, C.; Occhipinti, S.; Massaccesi, L.; Giovarelli, M.; Galeazzi, R.; Iezzi, M.; Tilio, M.; Gambini, V. Phage-based anti-HER2 vaccination can circumvent immune tolerance against breast cancer. Cancer Immunol. Res. 2018, 6, 1486–1498. [Google Scholar] [CrossRef]
- Razazan, A.; Nicastro, J.; Slavcev, R.; Barati, N.; Arab, A.; Mosaffa, F.; Jaafari, M.R.; Behravan, J. Lambda bacteriophage nanoparticles displaying GP2, a HER2/neu derived peptide, induce prophylactic and therapeutic activities against TUBO tumor model in mice. Sci. Rep. 2019, 9, 2221. [Google Scholar] [CrossRef]
- Jung, E.; Chung, Y.H.; Steinmetz, N.F. TLR Agonists Delivered by Plant Virus and Bacteriophage Nanoparticles for Cancer Immunotherapy. Bioconjug. Chem. 2023, 34, 1596–1605. [Google Scholar] [CrossRef]
- Storni, T.; Ruedl, C.; Schwarz, K.; Schwendener, R.A.; Renner, W.A.; Bachmann, M.F. Nonmethylated CG motifs packaged into virus-like particles induce protective cytotoxic T cell responses in the absence of systemic side effects. J. Immunol. 2004, 172, 1777–1785. [Google Scholar] [CrossRef]
- Lemke-Miltner, C.D.; Blackwell, S.E.; Yin, C.; Krug, A.E.; Morris, A.J.; Krieg, A.M.; Weiner, G.J. Antibody Opsonization of a TLR9 Agonist–Containing Virus-like Particle Enhances In Situ Immunization. J. Immunol. 2020, 204, 1386–1394. [Google Scholar] [CrossRef]
- Wang, H.Y.; Chang, Y.-C.; Hu, C.-W.; Kao, C.-Y.; Yu, Y.-A.; Lim, S.-K.; Mou, K.Y. Development of a novel cytokine vehicle using filamentous phage display for colorectal cancer treatment. ACS Synth. Biol. 2021, 10, 2087–2095. [Google Scholar] [CrossRef]
- Dong, X.; Pan, P.; Zhang, Q.; Ye, J.-J.; Zhang, X.-Z. Engineered living bacteriophage-enabled self-adjuvanting hydrogel for remodeling tumor microenvironment and cancer therapy. Nano Lett. 2023, 23, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Neal, R.; Tharmanathan, P.; France, B.; Din, N.; Cotton, S.; Fallon-Ferguson, J.; Hamilton, W.; Hendry, A.; Hendry, M.; Lewis, R. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br. J. Cancer 2015, 112, S92–S107. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Jung, H.; Shin, H.-H.; Cho, G.; Cho, H.; Kang, S. Implementation of p22 viral capsids as intravascular magnetic resonance T 1 contrast conjugates via site-selective attachment of Gd (III)-chelating agents. Biomacromolecules 2013, 14, 2332–2339. [Google Scholar] [CrossRef] [PubMed]
- Carrico, Z.M.; Farkas, M.E.; Zhou, Y.; Hsiao, S.C.; Marks, J.D.; Chokhawala, H.; Clark, D.S.; Francis, M.B. N-Terminal labeling of filamentous phage to create cancer marker imaging agents. ACS Nano 2012, 6, 6675–6680. [Google Scholar] [CrossRef]
- Robertson, K.L.; Soto, C.M.; Archer, M.J.; Odoemene, O.; Liu, J.L. Engineered T4 viral nanoparticles for cellular imaging and flow cytometry. Bioconjug. Chem. 2011, 22, 595–604. [Google Scholar] [CrossRef]
- Aanei, I.L.; ElSohly, A.M.; Farkas, M.E.; Netirojjanakul, C.; Regan, M.; Taylor Murphy, S.; O’Neil, J.P.; Seo, Y.; Francis, M.B. Biodistribution of antibody-MS2 viral capsid conjugates in breast cancer models. Mol. Pharm. 2016, 13, 3764–3772. [Google Scholar] [CrossRef]
- Asar, M.; Newton-Northup, J.; Deutscher, S.; Soendergaard, M. Ovarian cancer targeting phage for in vivo near-infrared optical imaging. Diagnostics 2019, 9, 183. [Google Scholar] [CrossRef]
- El-Sayed, A.; Bernhard, W.; Barreto, K.; Gonzalez, C.; Hill, W.; Pastushok, L.; Fonge, H.; Geyer, C.R. Evaluation of antibody fragment properties for near-infrared fluorescence imaging of HER3-positive cancer xenografts. Theranostics 2018, 8, 4856. [Google Scholar] [CrossRef]
- Lee, K.J.; Lee, J.H.; Chung, H.K.; Ju, E.J.; Song, S.Y.; Jeong, S.-Y.; Choi, E.K. Application of peptide displaying phage as a novel diagnostic probe for human lung adenocarcinoma. Amino Acids 2016, 48, 1079–1086. [Google Scholar] [CrossRef]
- Chung, W.-J.; Lee, D.-Y.; Yoo, S.Y. Chemical modulation of M13 bacteriophage and its functional opportunities for nanomedicine. Int. J. Nanomed. 2014, 9, 5825–5836. [Google Scholar]
- Yadav, D.; Sankaranarayanan, S.; Thanekar, A.; Rengan, A. Bioinspired gold coated phage nanosomes for anti-microbial and anti-cancer theranostics. Mater. Today Nano 2023, 23, 100348. [Google Scholar] [CrossRef]
- Eghbalpoor, F.; Gorji, M.; Alavigeh, M.Z.; Moghadam, M.T. Genetically engineered phages and engineered phage-derived enzymes to destroy biofilms of antibiotics resistance bacteria. Heliyon 2024, 10, e35666. [Google Scholar] [CrossRef] [PubMed]
- Hampton, J.T.; Liu, W.R. Diversification of Phage-Displayed Peptide Libraries with Noncanonical Amino Acid Mutagenesis and Chemical Modification. Chem. Rev. 2024, 124, 6051–6077. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-H.C.; Guo, X.S.; Zhang, H.E.; Dubey, G.K.; Geng, Z.Z.; Fierke, C.A.; Xu, S.; Hampton, J.T.; Liu, W.R. Leveraging a Phage-Encoded Noncanonical Amino Acid: A Novel Pathway to Potent and Selective Epigenetic Reader Protein Inhibitors. ACS Cent. Sci. 2024, 10, 782–792. [Google Scholar] [CrossRef]
- Sankaranarayanan, S.A.; Yadav, D.N.; Yadav, S.; Srivastava, A.; Pramatha, S.R.; Kotagiri, V.R.; Joshi, H.; Rengan, A.K. Tailoring Phage Nanosomes for Enhanced Theranostic Properties of Near Infrared Dyes. Langmuir 2024, 40, 16743–16756. [Google Scholar] [CrossRef]
- Gencay, Y.E.; Jasinskytė, D.; Robert, C.; Semsey, S.; Martínez, V.; Petersen, A.Ø.; Brunner, K.; de Santiago Torio, A.; Salazar, A.; Turcu, I.C. Engineered phage with antibacterial CRISPR–Cas selectively reduce E. coli burden in mice. Nat. Biotechnol. 2024, 42, 265–274. [Google Scholar] [CrossRef]
- Hou, X.L.; Zhang, B.; Cheng, K.; Zhang, F.; Xie, X.T.; Chen, W.; Tan, L.F.; Fan, J.X.; Liu, B.; Xu, Q.R. Engineering Phage Nanocarriers Integrated with Bio-Intelligent Plasmids for Personalized and Tunable Enzyme Delivery to Enhance Chemodynamic Therapy. Adv. Sci. 2024, 11, e2308349. [Google Scholar] [CrossRef]
- Kalarical Janardhanan, S.; Narayan, S.; Abbineni, G.; Hayhurst, A.; Mao, C. Architectonics of phage-liposome nanowebs as optimized photosensitizer vehicles for photodynamic cancer therapy. Mol Cancer Ther. 2010, 9, 2524–2535. [Google Scholar] [CrossRef]
- Podlacha, M.; Grabowski, Ł.; Kosznik-Kawśnicka, K.; Zdrojewska, K.; Stasiłojć, M.; Węgrzyn, G.; Węgrzyn, A. Interactions of Bacteriophages with Animal and Human Organisms—Safety Issues in the Light of Phage Therapy. Int. J. Mol. Sci. 2021, 22, 8937. [Google Scholar] [CrossRef]






| Engineered Phage | Findings | In Vitro/In Vivo Models | Reference |
|---|---|---|---|
| HBcAg core antigen was fused to the M13 phage pIII | Immunization with these recombinant phages showed potent immunogenicity | Mice models | [147] |
| HSV-1 glycoprotein D expression cassette was inserted into M13 phage genome | Recombinant phage upon expression functioned as a potent vaccine with high capability to induce cell mediated immunity and neutralizing antiviral antibodies | Mice models | [148] |
| SARS-CoV-2 spike (S) protein and the CAKSMGDIVC peptide were respectively displayed on the phage M13 pVIII and pIII | Elicited robust specific and systemic immune reactions with no adverse effects | Mice models | [149] |
| SARS-CoV-2 pneumonia | Infection successfully neutralized by phage therapy at a low dosage of 2 mg/kg in mice while in the hamster model, phage administration proved highly therapeutic and prophylactic | Mouse and hamster models | [150] |
| HIV antigens displayed on the T4 surface as many copies | Robust and broadly neutralizing antibodies and cell-mediated T-cell responses were elicited to HIV antigens in the absence of any external adjuvants | Mice models | [151] |
| Engineered Phage | Findings | In Vitro/In Vivo Models | Reference |
|---|---|---|---|
| Phage cocktail to treat mastitis induced by S. aureus | Treatment with phage cocktail led to the highest intramammary phage titer when compared to other cohorts and possessed efficiency comparable to that induced by the antibiotic, ceftiofur sodium | Murine models | [129] |
| The novel peptidase derived from phages, CHAPK against Staphylococci involved in the formation of biofilms | Acted as an efficient biocidal agent enabling rapid disruption of bacterial biofilms suggesting that it can be incorporated in the teat-dip solution to preclude S. aureus colonization upon the udder skin surface of bovines | In vitro studies | [153] |
| Phage particles against S. intermedius causing pyoderma skin infections and collected from wounds and inner hearing channel of animals (dogs and horses) | Cutaneous permeation of phage particles conveyed in a hydroxyethylcellulose (HEC) gel and integrating ionic liquid that acted as a permeation enhancer; the ionic liquid highly enhanced transdermal permeation of the bacteriophage particles, with associated high potential of the HEC gel formulation in the antimicrobial treatment of animal skin infections | In vitro assays | [154] |
| Phage PIZ SAE-01E2 against Salmonella enterica subsp. enterica serovar Abortusequi infections causing abortion in mares and donkeys | Prophylactic and therapeutic effects observed wherein a single intraperitoneal injection of PIZ SAE-01E2 before or after bacterial challenge provided effective protection against abortions in all pregnant mice | Pregnant murine model of abortion | [155] |
| Phages P2S2 and P5U5 against multidrug-resistant pathogenic strains of P. aeruginosa sourced from canine skin diseases wherein P. aeruginosa is responsible for otitis externa in dogs, in addition to wound infection, chronic deep pyoderma, and ocular infections including ulcerative keratitis | Potent lytic activity against a wide range of P. aeruginosa strains obtained from canine ocular infections (80–100% lysis,); preparation containing both phages showed a notable inhibition of bacterial growth at all the MOIs tested | In vitro studies | [156,157] |
| Bacteriophages against uropathogenic multidrug-resistant E. coli strains in dogs and cats | Over 90% of the ten bacteriophages isolated were capable of lysing about 50% of the target E. coli sourced from feline and canine feces upon singular testing, and over 90% were able to lyse the target when administered as a cocktail | In vitro studies | [158] |
| A cocktail of φ26, φ27, and φ29 bacteriophages against Shiga-toxin-expressing Escherichia coli responsible for causing neonatal diarrhea | Suppositories containing a cocktail of the three E. coli lytic phages and Lactobacillus spp. (a probiotic bacterium) showed both prophylactic and therapeutic effects without impacting endogenous microflora | In vivo testing in calves | [159] |
| A cocktail of lytic bacteriophages SPFM14 and SPFM10 against S. typhimurium challenge in pig gastrointestinal tracts | Upon prophylactic oral administration in feeds, demonstrated a significant decrease in the colonization of target bacteria | In vivo studies in pigs | [160] |
| Two new lysins sourced from lysogenic phages (phi5218 and phi7917) targeting Streptococcus suis multiple serotypes | Efficient lytic activity and therapeutic potential | In vitro and in vivo studies in mouse and piglets | [161,162,163] |
| Virulent phage CP220 administered to broiler chicken infected with Campylobacter coli and Campylobacter jejuni | Notably lower target bacteria count within the intestines | In vivo studies in broiler chicken | [164] |
| A cocktail of 8 bacteriophages against avian pathogenic E. coli challenge | 90% protection from death in comparison to control eggs, which showed 100% mortality | In ovo inoculation into embryonated eggs | [165] |
| Therapies | Phage Type | Strategy | Therapeutic Agent | Cancer | Reference |
|---|---|---|---|---|---|
| Chemo-therapy | Phage A54 | conjugation | doxorubicin (DOX) | hepatocarcinoma cells | [190] |
| Phage fUSE5-ZZ | conjugation | hygromycin and doxoru-bicin | breast carcinoma cell lines | [191] | |
| Photodynamic therapy (PDT) | M13 phage | conjugation | Rose Bengal (RB) | colorectal cancer (CC) | [192] |
| Gene therapy | M13 | Display | TRAIL gene | Hepatocellular carcinoma (HCC), | [193] |
| M13 | Display | TRAIL gene | Chondrosarcoma (CS) | [194] | |
| Lamda (λ ZAP-CMV) | Display | Apoptin | breast carcinoma cell lines | [195] | |
| Immuno-therapy | M13 | Display | Programmed death ligand 1 (PD-L1) | colorectal cancer | [196] |
| lambda | Display | Trastuzumab | Breast cancer | [197] | |
| M13 | Display | anti-CD40 designed ankyrin repeat protein (DARPin) | Breast cancer | [198] | |
| fd | Display and biopan-ning | PD-L1-binding peptide (HH) and a melanoma-targeting peptide (IP) | Melanoma | [199] | |
| M13 | biopanning | proapoptotic peptide, D(KLAKLAK) | breast cancer | [200] | |
| Combination therapy | M13 | Display | CD40+ 10-hydroxycamptothecin (HCPT), +PD-1 blockade | [201] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkataraman, S.; Shahgolzari, M.; Yavari, A.; Hefferon, K. Bacteriophages as Targeted Therapeutic Vehicles: Challenges and Opportunities. Bioengineering 2025, 12, 469. https://doi.org/10.3390/bioengineering12050469
Venkataraman S, Shahgolzari M, Yavari A, Hefferon K. Bacteriophages as Targeted Therapeutic Vehicles: Challenges and Opportunities. Bioengineering. 2025; 12(5):469. https://doi.org/10.3390/bioengineering12050469
Chicago/Turabian StyleVenkataraman, Srividhya, Mehdi Shahgolzari, Afagh Yavari, and Kathleen Hefferon. 2025. "Bacteriophages as Targeted Therapeutic Vehicles: Challenges and Opportunities" Bioengineering 12, no. 5: 469. https://doi.org/10.3390/bioengineering12050469
APA StyleVenkataraman, S., Shahgolzari, M., Yavari, A., & Hefferon, K. (2025). Bacteriophages as Targeted Therapeutic Vehicles: Challenges and Opportunities. Bioengineering, 12(5), 469. https://doi.org/10.3390/bioengineering12050469








