Advances in Targeted Delivery of Doxorubicin for Cancer Chemotherapy
Abstract
1. Introduction
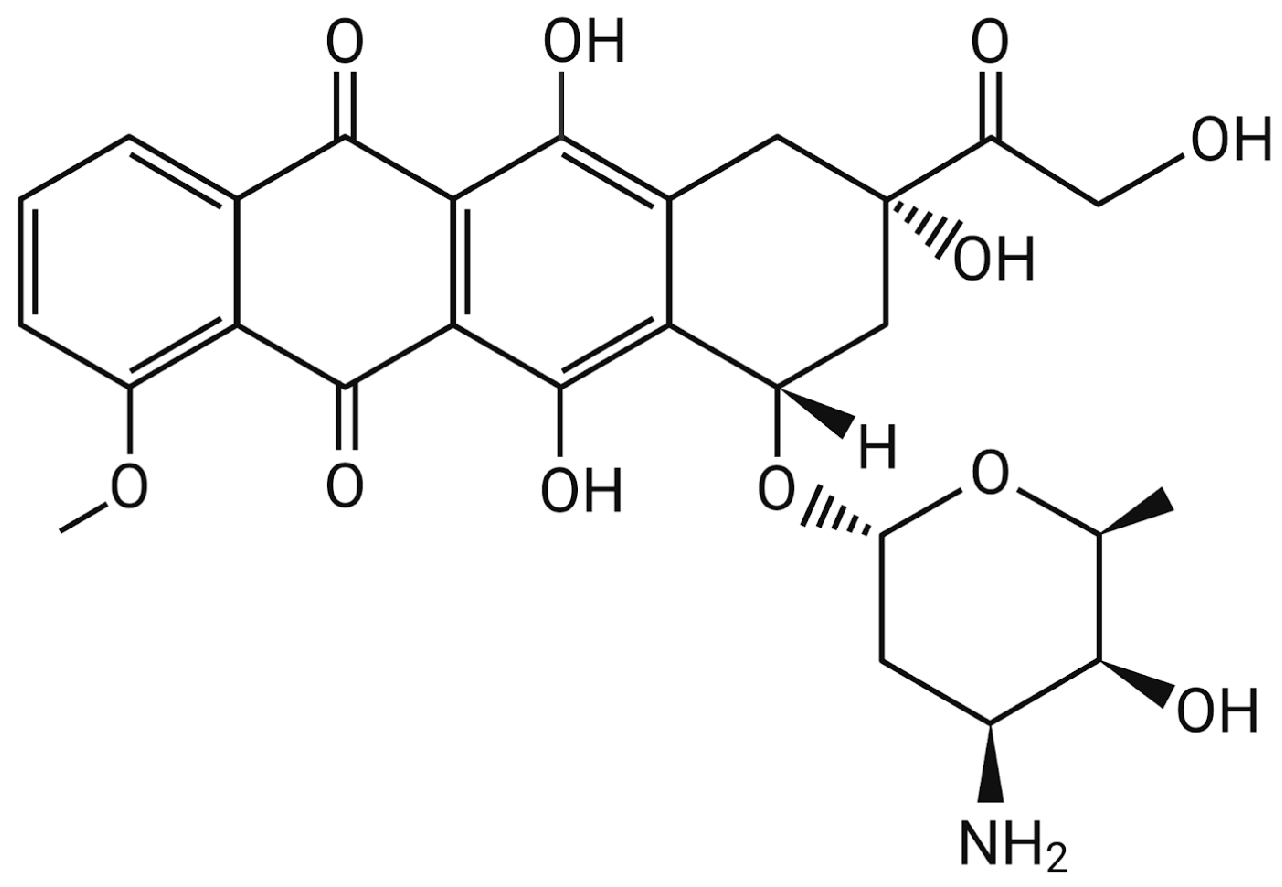
2. Doxorubicin (DOX)
2.1. Mechanism of Action
2.2. Limitations
2.2.1. Side Effects
2.2.2. Drug Resistance
3. Strategies Used in Cancer Therapy
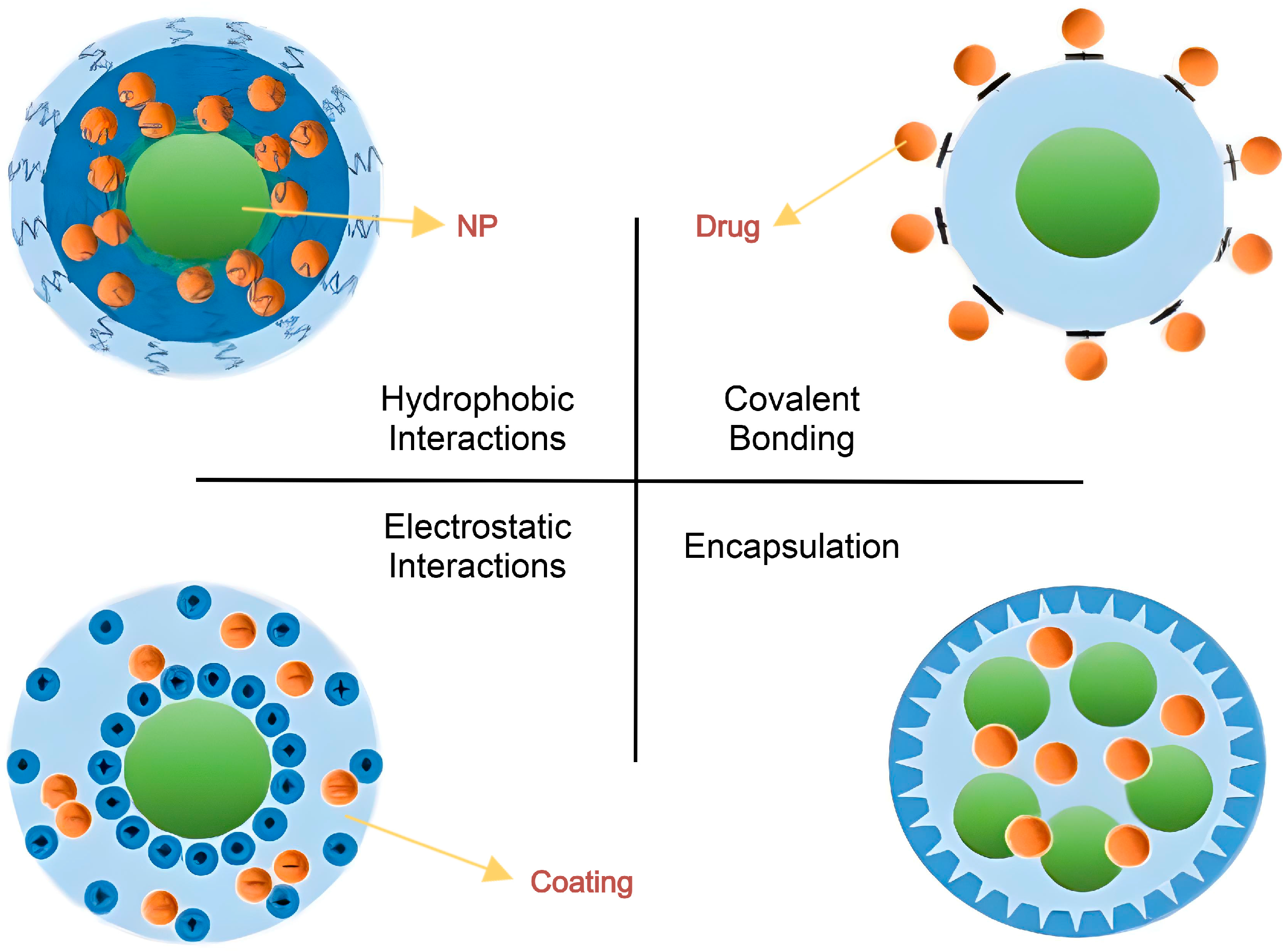
3.1. Loading Methods
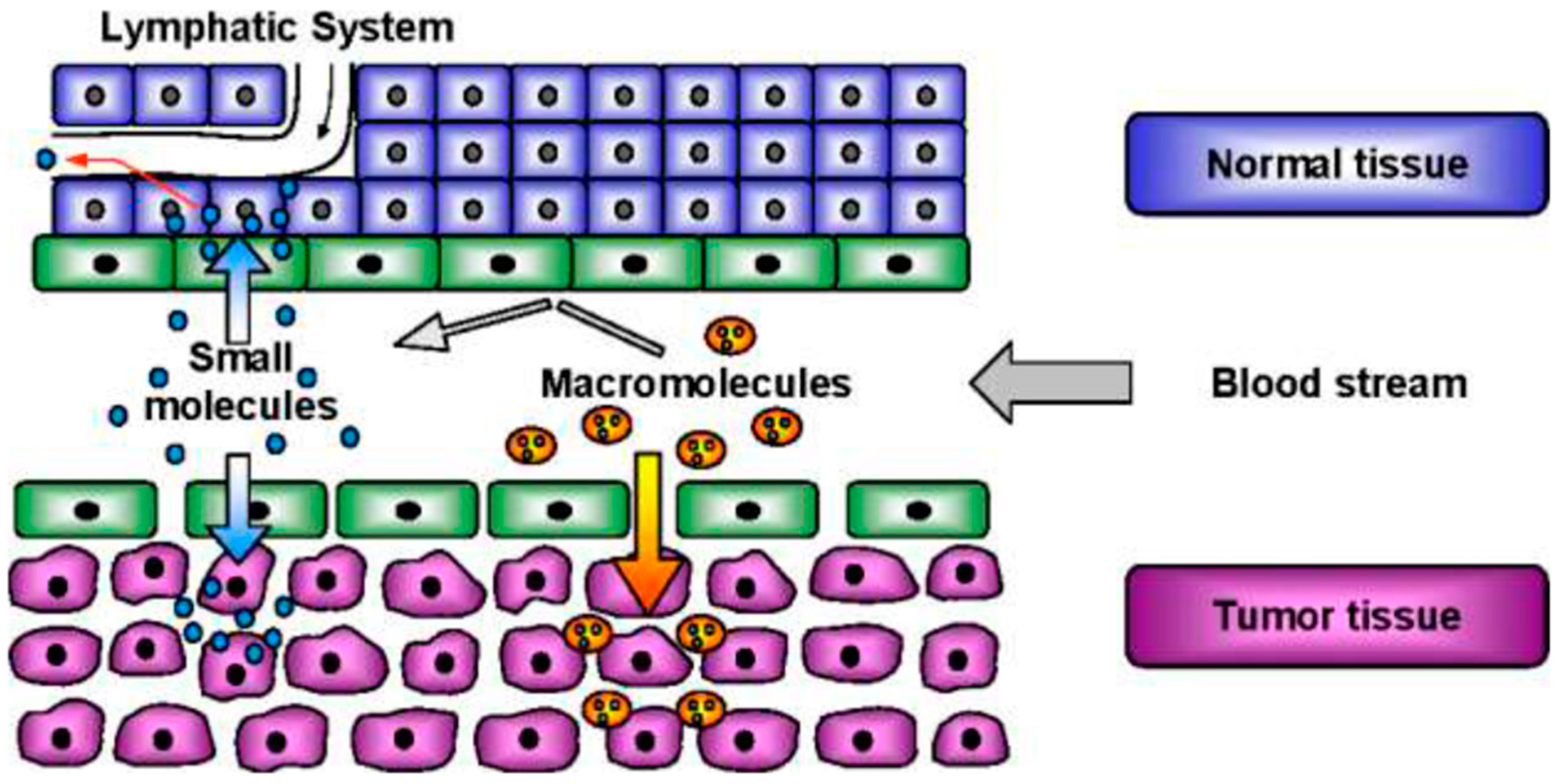
3.2. Targeting
4. Nanocarriers Used for Targeted DOX Delivery
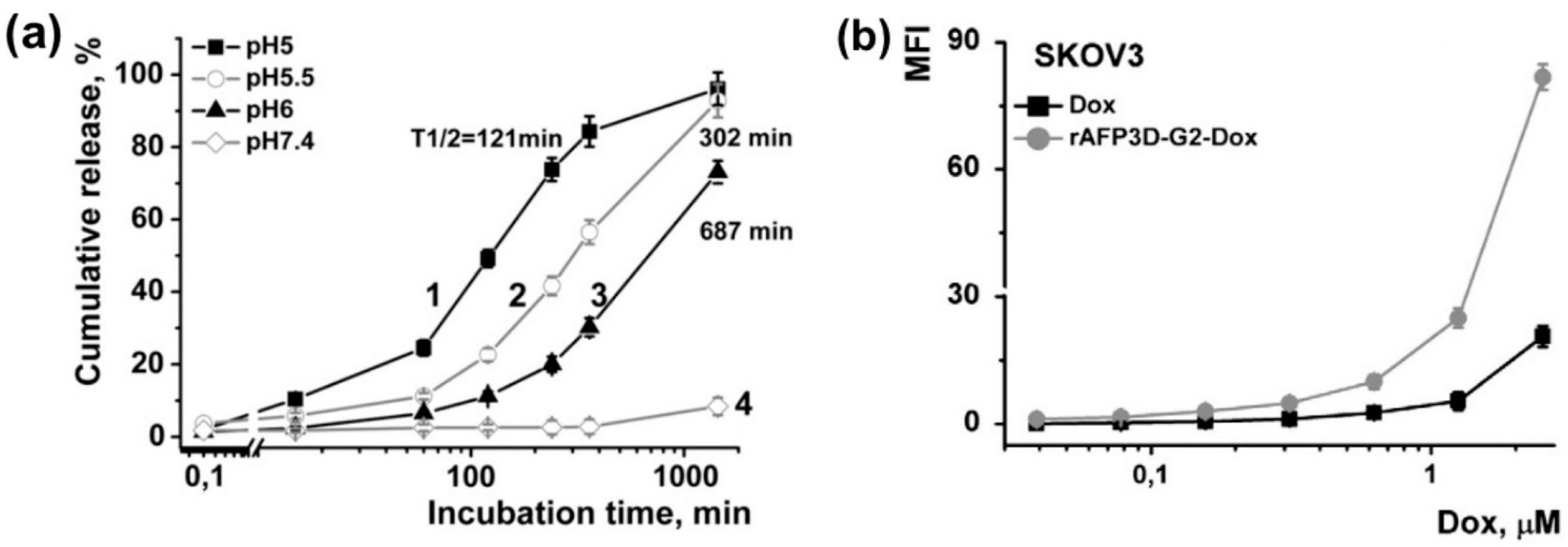
4.1. Liposomes
4.2. Micelles
4.3. Polymeric Nanoparticles
4.3.1. Nanogels
4.3.2. Dendrimer
4.4. Carbon-Based Nanomaterials
4.4.1. Nanographene Oxide (NGO)
4.4.2. Carbon Nanotubes (CNTs)
4.5. Other Advanced Nanocarriers
5. Limitations and Challenges
6. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hsu, C.-Y.; Rheima, A.M.; Kadhim, M.M.; Ahmed, N.N.; Mohammed, S.H.; Abbas, F.H.; Abed, Z.T.; Mahdi, Z.M.; Abbas, Z.S.; Hachim, S.K.; et al. An overview of nanoparticles in drug delivery: Properties and applications. S. Afr. J. Chem. Eng. 2023, 46, 233–270. [Google Scholar] [CrossRef]
- Lakkakula, J.R.; Gujarathi, P.; Pansare, P.; Tripathi, S. A comprehensive review on alginate-based delivery systems for the delivery of chemotherapeutic agent: Doxorubicin. Carbohydr. Polym. 2021, 259, 117696. [Google Scholar] [CrossRef]
- Yadav, A.; Singh, S.; Sohi, H.; Dang, S. Advances in Delivery of Chemotherapeutic Agents for Cancer Treatment. AAPS PharmSciTech 2021, 23, 25. [Google Scholar] [CrossRef] [PubMed]
- Almajidi, Y.Q.; Kadhim, M.M.; Alsaikhan, F.; Turki Jalil, A.; Hassan Sayyid, N.; Alexis Ramírez-Coronel, A.; Hassan Jawhar, Z.; Gupta, J.; Nabavi, N.; Yu, W.; et al. Doxorubicin-loaded micelles in tumor cell-specific chemotherapy. Environ. Res. 2023, 227, 115722. [Google Scholar] [CrossRef] [PubMed]
- Radu, E.R.; Semenescu, A.; Voicu, S.I. Recent Advances in Stimuli-Responsive Doxorubicin Delivery Systems for Liver Cancer Therapy. Polymers 2022, 14, 5249. [Google Scholar] [CrossRef]
- Sun, Y.; Kang, C.; Liu, F.; Zhou, Y.; Luo, L.; Qiao, H. RGD Peptide-Based Target Drug Delivery of Doxorubicin Nanomedicine. Drug Dev. Res. 2017, 78, 283–291. [Google Scholar] [CrossRef]
- Markandeywar, T.S.; Narang, R.K.; Singh, D.; Rai, V.K. Targeted Delivery of Doxorubicin as a Potential Chemotherapeutic Agent. Curr. Drug Deliv. 2023, 20, 904–918. [Google Scholar] [CrossRef]
- Sohail, M.; Sun, Z.; Li, Y.; Gu, X.; Xu, H. Research progress in strategies to improve the efficacy and safety of doxorubicin for cancer chemotherapy. Expert Rev. Anticancer Ther. 2021, 21, 1385–1398. [Google Scholar] [CrossRef]
- Vyas, M.; Simbo, D.A.; Mursalin, M.; Mishra, V.; Bashary, R.; Khatik, G.L. Drug Delivery Approaches for Doxorubicin in the Management of Cancers. Curr. Cancer Ther. Rev. 2020, 16, 320–331. [Google Scholar] [CrossRef]
- Bisht, A.; Avinash, D.; Sahu, K.K.; Patel, P.; Das Gupta, G.; Kurmi, B.D. A comprehensive review on doxorubicin: Mechanisms, toxicity, clinical trials, combination therapies and nanoformulations in breast cancer. Drug Deliv. Transl. Res. 2024, 15, 102–133. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Saebfar, H.; Gholami, M.H.; Hushmandi, K.; Zabolian, A.; Bikarannejad, P.; Hashemi, M.; Daneshi, S.; Mirzaei, S.; Sharifi, E.; et al. Doxorubicin-loaded graphene oxide nanocomposites in cancer medicine: Stimuli-responsive carriers, co-delivery and suppressing resistance. Expert Opin. Drug Deliv. 2022, 19, 355–382. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Shim, M.K.; Kim, W.J.; Choi, J.; Nam, G.-H.; Kim, J.; Kim, J.; Moon, Y.; Kim, H.Y.; Park, J.; et al. Cancer-activated doxorubicin prodrug nanoparticles induce preferential immune response with minimal doxorubicin-related toxicity. Biomaterials 2021, 272, 120791. [Google Scholar] [CrossRef]
- Wei, Q.Y.; Xu, Y.M.; Lau, A.T.Y. Recent Progress of Nanocarrier-Based Therapy for Solid Malignancies. Cancers 2020, 12, 2783. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Luo, Y.; Gan, D.; Zhang, Y.; Deng, H.; Liu, G. Advances in Doxorubicin-based nano-drug delivery system in triple negative breast cancer. Front. Bioeng. Biotechnol. 2023, 11, 1271420. [Google Scholar] [CrossRef]
- Ibrahim, M.; Abuwatfa, W.H.; Awad, N.S.; Sabouni, R.; Husseini, G.A. Encapsulation, Release, and Cytotoxicity of Doxorubicin Loaded in Liposomes, Micelles, and Metal-Organic Frameworks: A Review. Pharmaceutics 2022, 14, 254. [Google Scholar] [CrossRef]
- Zhao, N.; Woodle, M.C.; Mixson, A.J. Advances in delivery systems for doxorubicin. J. Nanomed. Nanotechnol. 2018, 9, 519. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.A. Nanocarriers-Based Products in the Market, FDA Approval, Commercialization of Nanocarriers, and Global Market. In Nano Drug Delivery for Cancer Therapy: Principles and Practices; Springer Nature: Singapore, 2024; pp. 137–148. [Google Scholar] [CrossRef]
- Kozak, A.; Lavrih, E.; Mikhaylov, G.; Turk, B.; Vasiljeva, O. Navigating the Clinical Landscape of Liposomal Therapeutics in Cancer Treatment. Pharmaceutics 2025, 17, 276. [Google Scholar] [CrossRef]
- Imantay, A.; Mashurov, N.; Zhaisanbayeva, B.A.; Mun, E.A. Doxorubicin-Conjugated Nanoparticles for Potential Use as Drug Delivery Systems. Nanomaterials 2025, 15, 133. [Google Scholar] [CrossRef]
- Barenholz, Y.C. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Doxil Label—accessdata.fda.gov. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/050718s029lbl.pdf (accessed on 19 March 2025).
- Leonard, R.C.F.; Williams, S.; Tulpule, A.; Levine, A.M.; Oliveros, S. Improving the therapeutic index of anthracycline chemotherapy: Focus on liposomal doxorubicin (Myocet™). Breast 2009, 18, 218–224. [Google Scholar] [CrossRef]
- Agency, E.M. Myocet Liposomal (Previously Myocet), European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/myocet-liposomal#overview (accessed on 19 March 2025).
- Burade, V.; Bhowmick, S.; Maiti, K.; Zalawadia, R.; Ruan, H.; Thennati, R. Lipodox® (generic doxorubicin hydrochloride liposome injection): In vivo efficacy and bioequivalence versus Caelyx® (doxorubicin hydrochloride liposome injection) in human mammary carcinoma (MX-1) xenograft and syngeneic fibrosarcoma (WEHI 164) mouse models. BMC Cancer 2017, 17, 405. [Google Scholar] [CrossRef]
- Lipodox (Doxorubicin): Side Effects, Uses, Dosage, Interactions, Warnings, RxList. Available online: https://www.rxlist.com/lipodox-drug.htm#description (accessed on 19 March 2025).
- Munster, P.; Krop, I.E.; LoRusso, P.; Ma, C.; Siegel, B.A.; Shields, A.F.; Molnár, I.; Wickham, T.J.; Reynolds, J.; Campbell, K.; et al. Safety and pharmacokinetics of MM-302, a HER2-targeted antibody—Liposomal doxorubicin conjugate, in patients with advanced HER2-positive breast cancer: A phase 1 dose-escalation study. Br. J. Cancer 2018, 119, 1086–1093. [Google Scholar] [CrossRef]
- Cagel, M.; Grotz, E.; Bernabeu, E.; Moretton, M.A.; Chiappetta, D.A. Doxorubicin: Nanotechnological overviews from bench to bedside. Drug Discov. Today 2017, 22, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002; Available online: https://www.ncbi.nlm.nih.gov/books/NBK26871/ (accessed on 19 March 2025).
- Chowdhury, N.; Chaudhry, S.; Hall, N.; Olverson, G.; Zhang, Q.-J.; Mandal, T.; Dash, S.; Kundu, A. Targeted Delivery of Doxorubicin Liposomes for Her-2+ Breast Cancer Treatment. AAPS PharmSciTech 2020, 21, 202. [Google Scholar] [CrossRef]
- Meng, F.; Zhong, Y.; Cheng, R.; Deng, C.; Zhong, Z. Ph-Sensitive Polymeric Nanoparticles for Tumor-Targeting Doxorubicin Delivery: Concept and Recent Advances. Nanomedicine 2014, 9, 487–499. [Google Scholar] [CrossRef]
- Ma, B.; Zhuang, W.; Wang, Y.; Luo, R.; Wang, Y. pH-sensitive doxorubicin-conjugated prodrug micelles with charge-conversion for cancer therapy. Acta Biomater. 2018, 70, 186–196. [Google Scholar] [CrossRef]
- Lu, J.; Zhao, W.; Huang, Y.; Liu, H.; Marquez, R.; Gibbs, R.B.; Li, J.; Venkataramanan, R.; Xu, L.; Li, S.; et al. Targeted Delivery of Doxorubicin by Folic Acid-Decorated Dual Functional Nanocarrier. Mol. Pharm. 2014, 11, 4164–4178. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, U.; Bukhari, N.; Ovais, M.; Abass, N.; Hussain, K.; Raza, A. Advances in Nano-delivery Systems for Doxorubicin: An updated insight. J. Drug Target. 2017, 26, 296–310. [Google Scholar] [CrossRef]
- Xu, X.; Wang, X.; Luo, W.; Qian, Q.; Li, Q.; Han, B.; Li, Y. Triple cell-responsive nanogels for delivery of drug into cancer cells. Colloids Surf. B Biointerfaces 2018, 163, 362–368. [Google Scholar] [CrossRef]
- Yabbarov, N.G.; Posypanova, G.A.; Vorontsov, E.A.; Obydenny, S.I.; Severin, E.S. A new system for targeted delivery of doxorubicin into tumor cells. J. Control. Release 2013, 168, 135–141. [Google Scholar] [CrossRef]
- Lim, D.J.; Sim, M.; Oh, L.; Lim, K.; Park, H. Carbon-based drug delivery carriers for cancer therapy. Arch. Pharm. Res. 2014, 37, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.C.; Guo, Z.Y.; Liu, Z.M.; Zhang, W.; Wan, M.M.; Yang, B.W. Folic acid-conjugated graphene oxide for cancer targeted chemo-photothermal therapy. J. Photochem. Photobiol. B Biol. 2013, 120, 156–162. [Google Scholar] [CrossRef]
- Wikipedia Contributors. Available online: https://en.wikipedia.org/w/index.php?title=HeLa&oldid=1252925862 (accessed on 9 January 2024).
- Wikipedia Contributors. Available online: https://en.wikipedia.org/w/index.php?title=Buckminsterfullerene&oldid=1257698806 (accessed on 15 February 2024).
- Chadar, R.; Afzal, O.; Alqahtani, S.M.; Kesharwani, P. Carbon nanotubes as an emerging nanocarrier for the delivery of doxorubicin for improved chemotherapy. Colloids Surf. B Biointerfaces 2021, 208, 112044. [Google Scholar] [CrossRef]
- Lu, Y.-J.; Wei, K.-C.; Ma, C.-C.M.; Yang, S.-Y.; Chen, J.-P. Dual targeted delivery of doxorubicin to cancer cells using folate-conjugated magnetic multi-walled carbon nanotubes. Colloids Surf. B Biointerfaces 2012, 89, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mita, A.C.; Mita, M.M.; Nawrocki, S.T.; Giles, F.J. Survivin: Key Regulator of Mitosis and Apoptosis and Novel Target for Cancer Therapeutics. Clin. Cancer Res. 2012, 14, 5000–5005. [Google Scholar] [CrossRef] [PubMed]
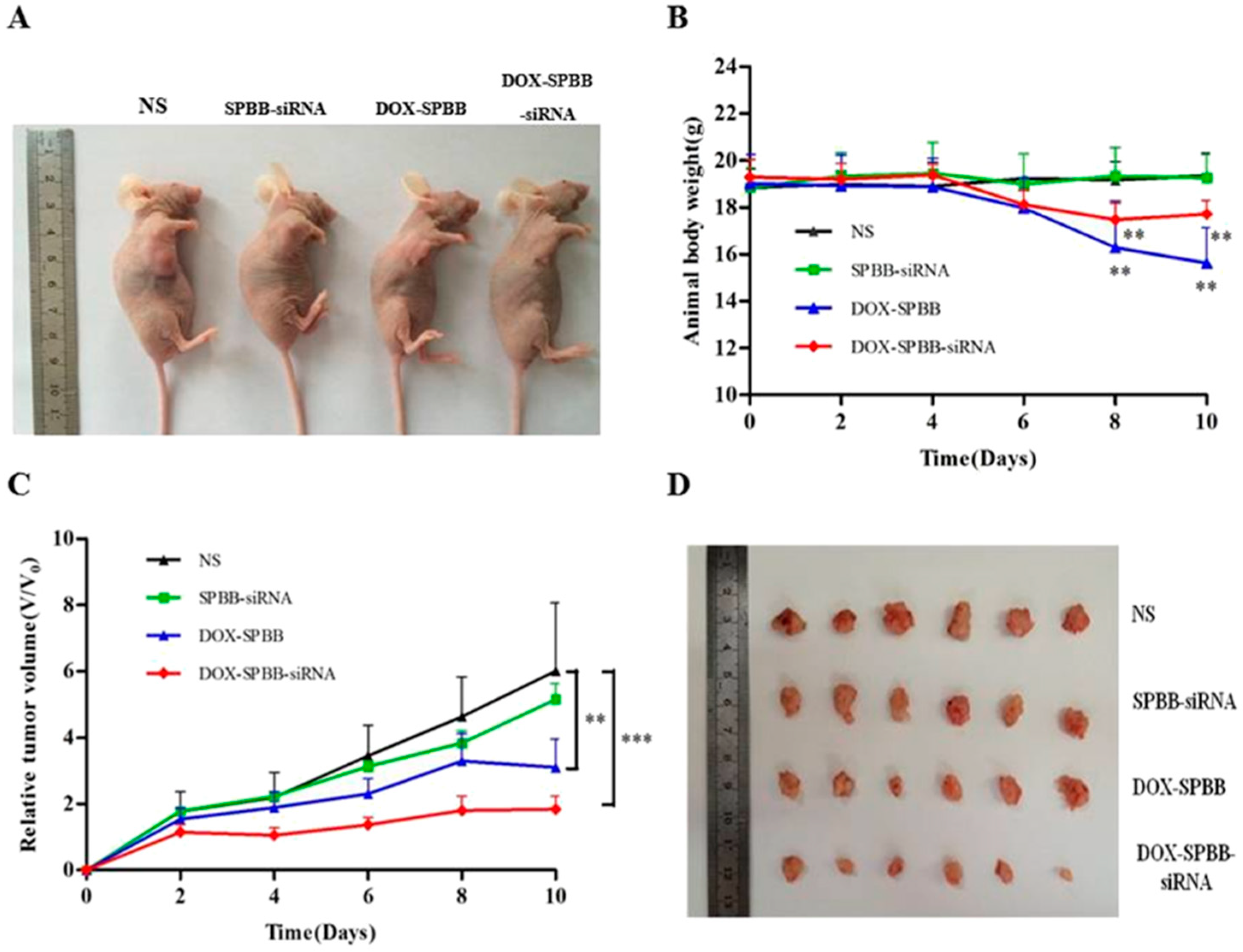
- Cao, Y.; Huang, H.-Y.; Chen, L.-Q.; Du, H.-H.; Cui, J.-H.; Zhang, L.W.; Lee, B.-J.; Cao, Q.-R. Enhanced Lysosomal Escape of pH-Responsive Polyethyleneimine—Betaine Functionalized Carbon Nanotube for the Codelivery of Survivin Small Interfering RNA and Doxorubicin. ACS Appl. Mater. Interfaces 2019, 11, 9763–9776. [Google Scholar] [CrossRef]
- Ahmadi, F.; Sodagar-Taleghani, A.; Ebrahimnejad, P.; Moghaddam, S.P.H.; Ebrahimnejad, F.; Asare-Addo, K.; Nokhodchi, A. A review on the latest developments of mesoporous silica nanoparticles as a promising platform for diagnosis and treatment of cancer. Int. J. Pharm. 2022, 625, 122099. [Google Scholar] [CrossRef]
- Gupta, Y.-D.; Mackeyev, Y.; Krishnan, S.; Bhandary, S. Mesoporous silica nanotechnology: Promising advances in augmenting cancer theranostics. Cancer Nanotechnol. 2024, 15, 9. [Google Scholar] [CrossRef]
- Moodley, T.; Singh, M. Sterically stabilised polymeric mesoporous silica nanoparticles improve doxorubicin efficiency: Tailored cancer therapy. Molecules 2020, 253, 742. Available online: https://www.mdpi.com/1420-3049/25/3/742 (accessed on 2 March 2025). [CrossRef]
- Kanniyappan, H.; Jose, J.; Chakraborty, S.; Ramasamy, M.; Muthuvijayan, V. pH-responsive drug release from positively charged mesoporous silica nanoparticles and their potential for anticancer drug delivery. J. Aust. Ceram. Soc. 2023, 59, 207–220. [Google Scholar] [CrossRef]
- Dong, J.-H.; Ma, Y.; Li, R.; Zhang, W.-T.; Zhang, M.-Q.; Meng, F.-N.; Ding, K.; Jiang, H.-T.; Gong, Y.-K. Smart MSN-drug-delivery system for tumor cell targeting and tumor microenvironment release. ACS Appl. Mater. Interfaces 2021, 13, 42522–42532. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Gong, C.; Yang, Q.; Zheng, K.; Wang, Z.; Zhang, W. Biomimetic nano-drug delivery system: An emerging platform for promoting tumor treatment. Int. J. Nanomed. 2024, 19, 571–608. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.; Lopes, D.; Motallebi, M.; Ye, M.; Xue, Y.; Vieira, A.C.F.; Singh, S.K.; Dua, K.; Veiga, F.; Sethi, G.; et al. Biomembrane-coated nanosystems as next-generation delivery systems for the treatment of gastrointestinal cancers. Bioeng. Transl. Med. 2025, e70006. [Google Scholar] [CrossRef]
- Zahiri, M.; Falsafi, M.; Lamei, K.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Targeted biomimetic hollow mesoporous organosilica nanoparticles for delivery of doxorubicin to colon adenocarcinoma: In vitro and in vivo evaluation. Microporous Mesoporous Mater. 2022, 335, 111841. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Han, S.; Kong, X.; Quan, C.; Wu, J.; Zhang, W. Cancer cell membrane camouflaged biomimetic gelatin-based nanogel for tumor inhibition. Chin. Chem. Lett. 2024, 35, 109578. [Google Scholar] [CrossRef]
- Milewska, S.; Niemirowicz-Laskowska, K.; Siemiaszko, G.; Nowicki, P.; Wilczewska, A.Z.; Car, H. Current Trends and Challenges in Pharmacoeconomic Aspects of Nanocarriers as Drug Delivery Systems for Cancer Treatment. Int. J. Nanomed. 2021, 16, 6593–6644. [Google Scholar] [CrossRef]
- Zheng, C.; Li, M.; Ding, J. Challenges and opportunities of nanomedicines in clinical translation. Bio Integr. 2021, 2, 57. Available online: https://www.scienceopen.com/hosted-document?doi=10.15212/bioi-2021-0016 (accessed on 23 March 2025). [CrossRef]
- Alshawwa, S.Z.; Kassem, A.A.; Farid, R.M.; Mostafa, S.K.; Labib, G.S. Nanocarrier drug delivery systems: Characterization, limitations, future perspectives and implementation of artificial intelligence. Pharmaceutics 2022, 14, 883. Available online: https://www.mdpi.com/1999-4923/14/4/883 (accessed on 23 March 2025). [CrossRef]

| Nanocarrier | Loading Efficiency | Advantages | Disadvantages |
|---|---|---|---|
| Liposomes | Moderate to high loading | High biocompatibility | Rapid reticuloendothelial system (RES) clearance |
| Micelles | High for hydrophobic drugs | Small size for deep tissue penetration | Potential premature drug release |
| Nanogels | Moderate loading | Excellent for stimuli-triggered release | Lower loading for very hydrophobic drugs |
| Dendrimers | Very high loading | High functionalization versatility | Complex synthesis and high cost |
| Nanographene Oxide (NGO) | Extremely high loading | Ease of surface modification for targeting | Potential toxicity and poor aqueous dispersibility |
| Carbon Nanotubes (CNTs) | High loading | Exceptional mechanical and thermal stability | Potential toxicity and poor water solubility |
| Mesoporous Silica Nanoparticles (MSNs) | Very high loading | High surface area and tunability | Possibility of long-term accumulation in organs |
| Cell Membrane-Coated Nanoparticles | Depending on the core nanoparticle | Enhanced targeting and reduced immunogenicity | More complex and costly to fabricate |
| Formulation | Status | Indications | Description | References |
|---|---|---|---|---|
| Doxil®/Caelyx® * | Approved (1995) | Ovarian cancer, AIDS-related Kaposi’s Sarcoma, Multiple Myeloma | The first FDA-approved nanodrug, pegylated liposomes, ammonium sulfate gradient | [20,21,22] |
| Myocet® | Approved (2000) | Metastatic breast cancer | Approved in Europe and Canada, non-pegylated liposomes, citrate transmembrane gradient | [23] |
| Lipodox® | Approved (2013) | Ovarian cancer, breast cancer, Kaposi’s sarcoma | Generic version of Doxil®, pegylated liposomes, ammonium sulfate gradient | [24,25] |
| ThermoDox® | Phase III | Hepatocellular carcinoma | Thermosensitive liposomes, pegylated liposomes, ammonium sulfate gradient | [18] |
| MM-302 | Phase II | HER2-positive breast cancer | PEG-modified liposomes with doxorubicin and HER2-specific antibodies | [19,26] |
| 2B3-101 | Phase II | Breast cancer metastases in the brain | Liposomal doxorubicin hydrochloride with glutathione ligands | [19] |
| SP1049C | Phase III | Adenocarcinoma in the esophagus and gastroesophageal junction | A micellar formulation using Pluronics® L61 and F127 | [19,27] |
| NK911 | Phase II | Metastatic pancreatic cancer | Another micellar formulation | [19,27] |
| FCE28068/PK1 | Phase II | Breast, colorectal, and non-small cell lung cancers | A polymer conjugate, N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer covalently bound to DOX by a peptidyl linker | [19,27] |
| FCE28068/PK2 | Phase II | Liver cancer | Builds upon PK1 by adding galactosamine, asialoglycoprotein receptors | [19,27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, W.; King, M.W. Advances in Targeted Delivery of Doxorubicin for Cancer Chemotherapy. Bioengineering 2025, 12, 430. https://doi.org/10.3390/bioengineering12040430
Xia W, King MW. Advances in Targeted Delivery of Doxorubicin for Cancer Chemotherapy. Bioengineering. 2025; 12(4):430. https://doi.org/10.3390/bioengineering12040430
Chicago/Turabian StyleXia, Wenhui, and Martin W. King. 2025. "Advances in Targeted Delivery of Doxorubicin for Cancer Chemotherapy" Bioengineering 12, no. 4: 430. https://doi.org/10.3390/bioengineering12040430
APA StyleXia, W., & King, M. W. (2025). Advances in Targeted Delivery of Doxorubicin for Cancer Chemotherapy. Bioengineering, 12(4), 430. https://doi.org/10.3390/bioengineering12040430








