Epidemiology and Risk Prediction Model of Multidrug-Resistant Organism Infections After Liver Transplant Recipients: A Single-Center Cohort Study
Abstract
1. Introduction
2. Material and Methods
2.1. Ethics Approval
2.2. Study Design
2.3. Related Definitions
2.4. Data Collection and Immunosuppressive Regimen
2.5. Statistical Analysis and Modeling
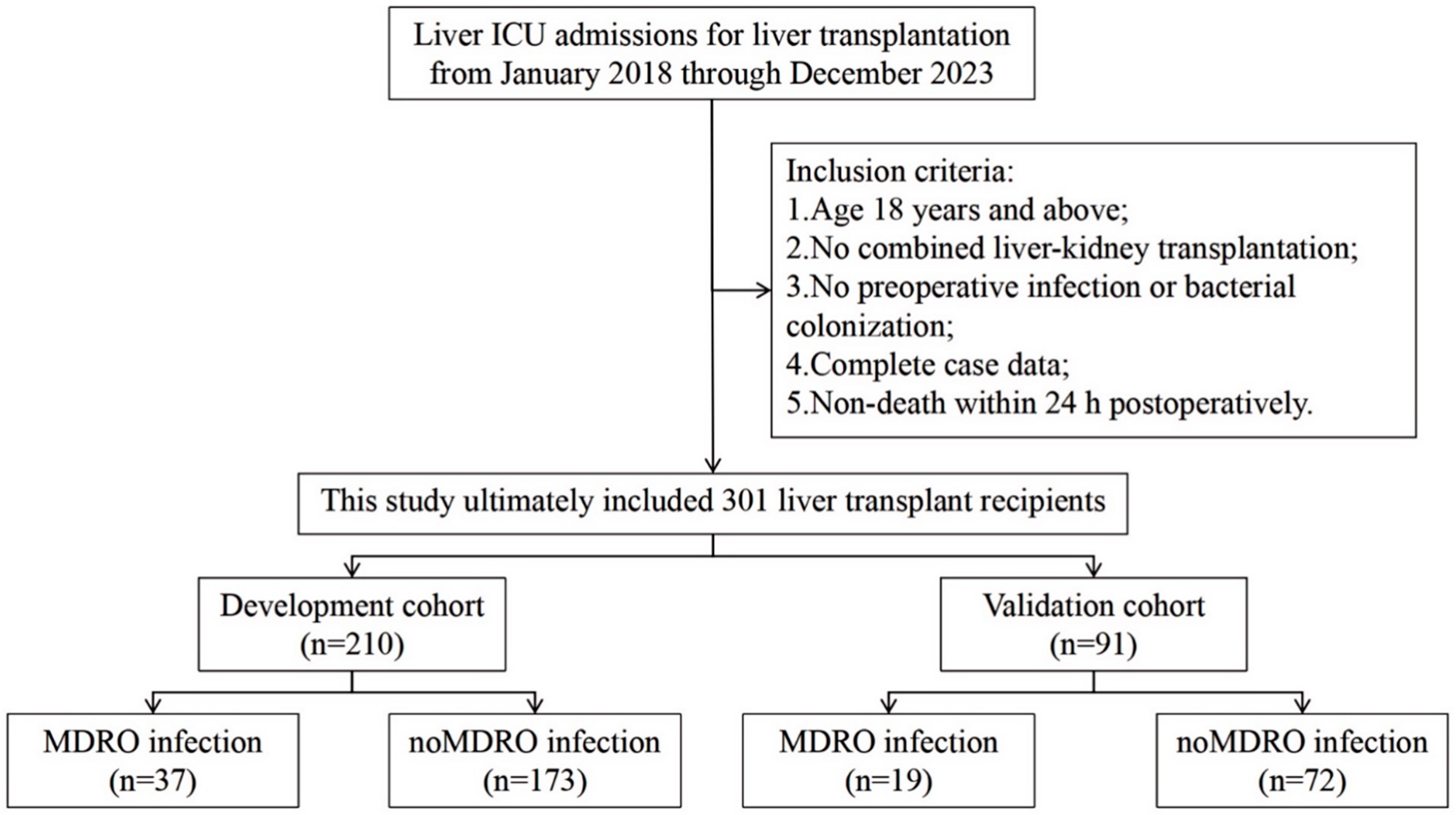
3. Results
3.1. Characteristics of the Study Cohort
3.2. Epidemiology and Origin of Pathogenic Bacteria
3.3. Impact of MDRO Infections on Postoperative Outcomes
3.4. Multivariate Logistic Regression Model for Predictors of Post-LT MDRO Infection
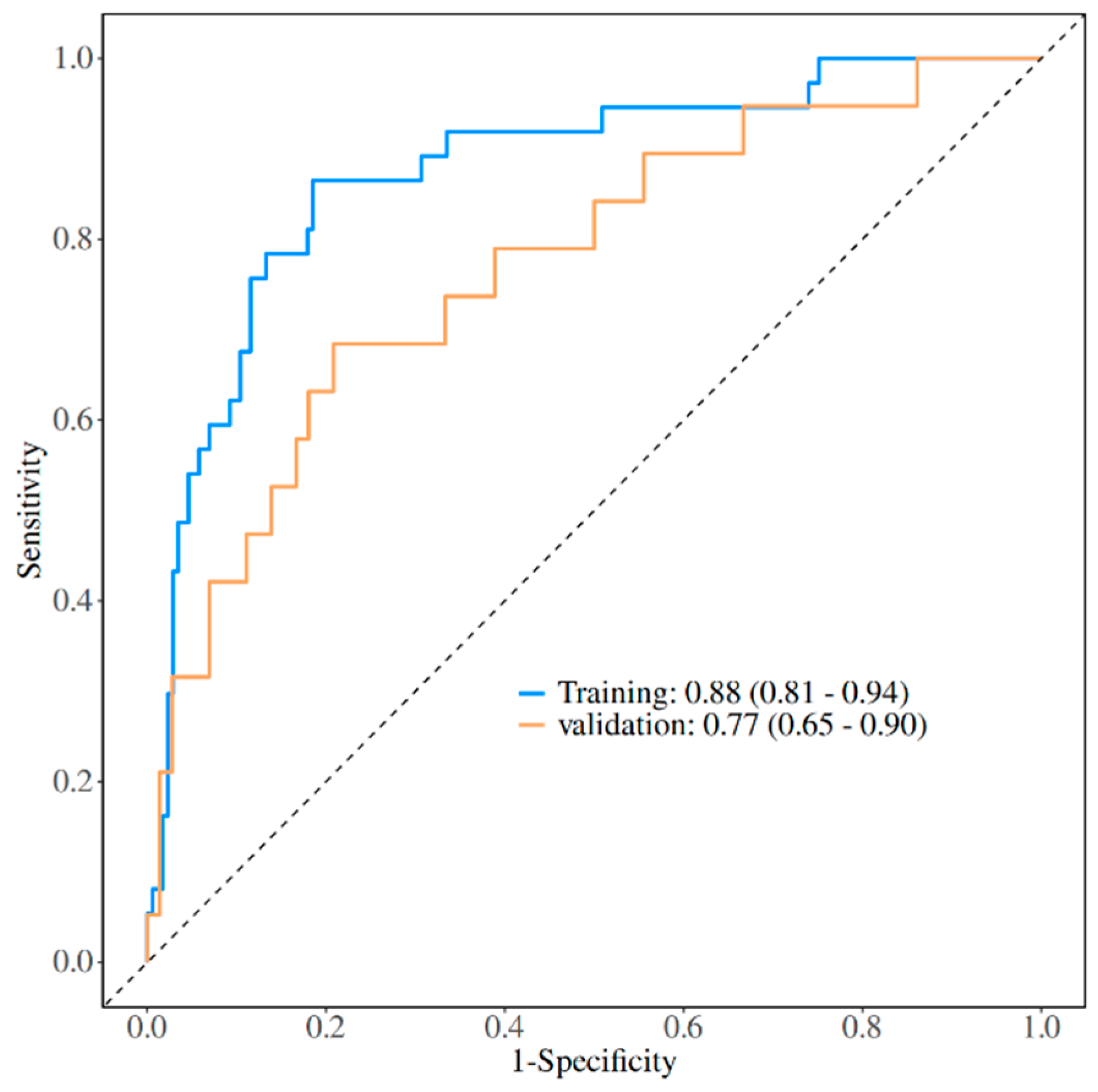
3.5. Development of a Nomogram for the Prediction of Post-LT MDRO Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Oriol, I.; Sabé, N.; Simonetti, A.F.; Lladó, L.; Manonelles, A.; González, J.; Tubau, F.; Carratalà, J. Changing trends in the aetiology, treatment and outcomes of bloodstream infection occurring in the first year after solid organ transplantation: A single-centre prospective cohort study. Transplant. Int. 2017, 30, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Viehman, J.A.; Clancy, C.J.; Clarke, L.; Shields, R.K.; Silveira, F.P.; Kwak, E.J.; Vergidis, P.; Hughes, C.; Humar, A.; Nguyen, M.H. Surgical Site Infections After Liver Transplantation: Emergence of Multidrug-Resistant Bacteria and Implications for Prophylaxis and Treatment Strategies. Transplantation 2016, 100, 2107–2114. [Google Scholar] [CrossRef] [PubMed]
- van Duin, D.; van Delden, C. Multidrug-resistant gram-negative bacteria infections in solid organ transplantation. Am. J. Transplant. 2013, 13 (Suppl. S4), 31–41. [Google Scholar] [CrossRef]
- Winston, D.J.; Emmanouilides, C.; Busuttil, R.W. Infections in liver transplant recipients. Clin. Infect. Dis. 1995, 21, 1077–1089, quiz 1090-1071. [Google Scholar] [CrossRef]
- Martin-Mateos, R.; Martínez-Arenas, L.; Carvalho-Gomes, Á.; Aceituno, L.; Cadahía, V.; Salcedo, M.; Arias, A.; Lorente, S.; Odriozola, A.; Zamora, J.; et al. Multidrug-resistant bacterial infections after liver transplantation: Prevalence, impact, and risk factors. J. Hepatol. 2024, 80, 904–912. [Google Scholar] [CrossRef]
- Neofytos, D.; Stampf, S.; Hoessly, L.D.; D’Asaro, M.; Tang, G.N.; Boggian, K.; Hirzel, C.; Khanna, N.; Manuel, O.; Mueller, N.J.; et al. Bacteremia During the First Year After Solid Organ Transplantation: An Epidemiological Update. Open Forum Infect. Dis. 2023, 10, ofad247. [Google Scholar] [CrossRef]
- Singh, N.; Gayowski, T.; Rihs, J.D.; Wagener, M.M.; Marino, I.R. Evolving trends in multiple-antibiotic-resistant bacteria in liver transplant recipients: A longitudinal study of antimicrobial susceptibility patterns. Liver Transplant. 2001, 7, 22–26. [Google Scholar] [CrossRef]
- Lanini, S.; Costa, A.N.; Puro, V.; Procaccio, F.; Grossi, P.A.; Vespasiano, F.; Ricci, A.; Vesconi, S.; Ison, M.G.; Carmeli, Y.; et al. Incidence of carbapenem-resistant gram negatives in Italian transplant recipients: A nationwide surveillance study. PLoS ONE 2015, 10, e0123706. [Google Scholar] [CrossRef] [PubMed]
- Freire, M.P.; Pierrotti, L.C.; Oshiro, I.C.; Bonazzi, P.R.; Oliveira, L.M.; Machado, A.S.; Van Der Heijden, I.M.; Rossi, F.; Costa, S.F.; D’Albuquerque, L.A.; et al. Carbapenem-resistant Acinetobacter baumannii acquired before liver transplantation: Impact on recipient outcomes. Liver Transplant. 2016, 22, 615–626. [Google Scholar] [CrossRef]
- Coskun, K. Risk factors and outcomes of carbapenem-resistant Klebsiella pneumoniae infections in liver transplant recipients. Liver Transplant. 2016, 22, 130. [Google Scholar] [CrossRef]
- Chen, C.; Guan, Q.; Li, D.; Sheng, B.; Zhang, Z.; Hu, Y. Clinical characteristics and risk factor analysis of recipients with multidrug-resistant bacterial bloodstream infections after liver transplantation: A single-centre retrospective study. J. Pharm. Policy Pract. 2024, 17, 2390072. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, W.; Kang, M.; Wu, S.; Liu, Y.; Liao, Q.; Xiao, Y.; Ma, Y.; Xie, Y. Bacterial and Fungal Infections After Liver Transplantation: Microbial Epidemiology, Risk Factors for Infection and Death with Infection. Ann. Transplant. 2020, 25, e921591. [Google Scholar] [CrossRef] [PubMed]
- Taimur, S.; Pouch, S.M.; Zubizarreta, N.; Mazumdar, M.; Rana, M.; Patel, G.; Freire, M.P.; Pellett Madan, R.; Kwak, E.J.; Blumberg, E.; et al. Impact of pre-transplant carbapenem-resistant Enterobacterales colonization and/or infection on solid organ transplant outcomes. Clin. Transplant. 2021, 35, e14239. [Google Scholar] [CrossRef] [PubMed]
- Mouloudi, E.; Massa, E.; Papadopoulos, S.; Iosifidis, E.; Roilides, I.; Theodoridou, T.; Piperidou, M.; Orphanou, A.; Passakiotou, M.; Imvrios, G.; et al. Bloodstream infections caused by carbapenemase-producing Klebsiella pneumoniae among intensive care unit patients after orthotopic liver transplantation: Risk factors for infection and impact of resistance on outcomes. Transplant. Proc. 2014, 46, 3216–3218. [Google Scholar] [CrossRef]
- Rasmussen, D.B.; Møller, D.L.; Knudsen, A.D.; Rostved, A.A.; Knudsen, J.D.; Rasmussen, A.; Nielsen, S.D. Enterococcal Infections the First Year after Liver Transplantation-A Prospective Cohort Study. Microorganisms 2021, 9, 1740. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.; Li, R.D.; Ai, J.W.; Zhu, Y.M.; Zhou, X.; Qian, Y.Y.; Chen, X.C.; Wang, X.Y.; Zhang, H.C.; Li, Y.; et al. Infection within 2 weeks before liver transplantation closely related to prognosis of posttransplant infection: A single-center retrospective observational study in China. Hepatobiliary Pancreat. Dis. Int. 2020, 19, 358–364. [Google Scholar] [CrossRef]
- Kaido, T.; Mori, A.; Ogura, Y.; Ogawa, K.; Hata, K.; Yoshizawa, A.; Yagi, S.; Uemoto, S. Pre- and perioperative factors affecting infection after living donor liver transplantation. Nutrition 2012, 28, 1104–1108. [Google Scholar] [CrossRef]
- El-Badrawy, M.K.; Ali, R.E.; Yassen, A.; AbouElela, M.A.; Elmorsey, R.A. Early-Onset Pneumonia After Liver Transplant: Microbial Causes, Risk Factors, and Outcomes, Mansoura University, Egypt, Experience. Exp. Clin. Transplant. 2017, 15, 547–553. [Google Scholar] [CrossRef]
- Dolci, G.; Burastero, G.J.; Paglia, F.; Cervo, A.; Meschiari, M.; Guaraldi, G.; Chester, J.; Mussini, C.; Franceschini, E. Epidemiology and Prevention of Early Infections by Multi-Drug-Resistant Organisms in Adults Undergoing Liver Transplant: A Narrative Review. Microorganisms 2023, 11, 1606. [Google Scholar] [CrossRef]
- Fishman, J.A.; Greenwald, M.A.; Grossi, P.A. Transmission of infection with human allografts: Essential considerations in donor screening. Clin. Infect. Dis. 2012, 55, 720–727. [Google Scholar] [CrossRef]
- Bartoletti, M.; Giannella, M.; Tedeschi, S.; Viale, P. Multidrug-Resistant Bacterial Infections in Solid Organ Transplant Candidates and Recipients. Infect. Dis. Clin. N. Am. 2018, 32, 551–580. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.; Fang, J.; Wu, S.; Huang, J.; Lu, C.; Mao, S.; Lu, C. Analysis and Prediction of Risk Factors for Early Infection After Liver Transplantation. Chin. J. Transplant. 2022, 16, 216–223. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Wang, D.; Su, W.; Yang, X. Nomogram Prediction for Postoperative Mortality of Orthotopic Liver Transplantation. Exp. Clin. Transplant. 2022, 20, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Giannella, M.; Freire, M.; Rinaldi, M.; Abdala, E.; Rubin, A.; Mularoni, A.; Gruttadauria, S.; Grossi, P.; Shbaklo, N.; Tandoi, F.; et al. Development of a Risk Prediction Model for Carbapenem-resistant Enterobacteriaceae Infection After Liver Transplantation: A Multinational Cohort Study. Clin. Infect. Dis. 2021, 73, e955–e966. [Google Scholar] [CrossRef]
- Shen, G.; Zhang, L.; Fan, W.; Lv, H.; Wang, F.; Ye, Q.; Lin, M.; Yu, X.; Cai, H.; Wu, X. Establishment of a risk prediction model for multidrug-resistant bacteria in deceased organ donors: A retrospective cohort study in China. Front. Cell Infect. Microbiol. 2023, 13, 1181630. [Google Scholar] [CrossRef]
- Guo, D.; Wang, H.; Lai, X.; Li, J.; Xie, D.; Zhen, L.; Jiang, C.; Li, M.; Liu, X. Development and validation of a nomogram for predicting acute kidney injury after orthotopic liver transplantation. Ren. Fail. 2021, 43, 1588–1600. [Google Scholar] [CrossRef]
- Chen, W.; Wu, S.; Gong, L.; Guo, Y.; Wei, L.; Jin, H.; Zhou, Y.; Li, C.; Lu, C.; Xu, L. Exploring the risk factors of early sepsis after liver transplantation: Development of a novel predictive model. Front. Med. 2023, 10, 1274961. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Wu, X.; Ren, J. Chinese Guidelines for the Diagnosis and Treatment of Intra-abdominal Infections (2019 Edition). Chin. J. Pract. Surg. 2020, 40, 1–16. [Google Scholar] [CrossRef]
- Li, G.; Shi, B.; Ju, C.; Sun, L. Standardized Diagnostic and Therapeutic Techniques for Postoperative Infections in Solid Organ Transplantation (2019 Edition)—General Discussion and Bacterial Pneumonia. Organ. Transplant. 2019, 10, 343–351. [Google Scholar]
- van Delden, C.; Stampf, S.; Hirsch, H.H.; Manuel, O.; Meylan, P.; Cusini, A.; Hirzel, C.; Khanna, N.; Weisser, M.; Garzoni, C.; et al. Burden and Timeline of Infectious Diseases in the First Year After Solid Organ Transplantation in the Swiss Transplant Cohort Study. Clin. Infect. Dis. 2020, 71, e159–e169. [Google Scholar] [CrossRef] [PubMed]
- Baganate, F.; Beal, E.W.; Tumin, D.; Azoulay, D.; Mumtaz, K.; Black, S.M.; Washburn, K.; Pawlik, T.M. Early mortality after liver transplantation: Defining the course and the cause. Surgery 2018, 164, 694–704. [Google Scholar] [CrossRef]
- Pérez-Cameo, C.; Oriol, I.; Lung, M.; Lladó, L.; Dopazo, C.; Nuvials, X.; Los-Arcos, I.; Sabé, N.; Castells, L.; Len, O. Impact of Prophylactic Norfloxacin in Multidrug Resistant Bacterial Infections in the Early Liver Posttransplant Period. Exp. Clin. Transplant. 2023, 21, 236–244. [Google Scholar] [CrossRef]
- Tezcan, H.; Altunsoy, A.; Turan Gökçe, D.; Gökcan, H.; Arı, D.; Aydın, O.; Bostancı, E.B.; Akdoğan Kayhan, M. Multidrug-Resistant Infections After Liver Transplantation, Etiology and Risk Factors: A Single-Center Experience. Exp. Clin. Transplant. 2023, 21, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Taddei, R.; Riccardi, N.; Tiseo, G.; Galfo, V.; Biancofiore, G. Early Intra-Abdominal Bacterial Infections after Orthotopic Liver Transplantation: A Narrative Review for Clinicians. Antibiotics 2023, 12, 1316. [Google Scholar] [CrossRef]
- Safdar, N.; Said, A.; Lucey, M.R.; Knechtle, S.J.; D’Alessandro, A.; Musat, A.; Pirsch, J.; McDermott, J.; Kalayoglu, M.; Maki, D.G. Infected bilomas in liver transplant recipients: Clinical features, optimal management, and risk factors for mortality. Clin. Infect. Dis. 2004, 39, 517–525. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reid, G.E.; Grim, S.A.; Sankary, H.; Benedetti, E.; Oberholzer, J.; Clark, N.M. Early intra-abdominal infections associated with orthotopic liver transplantation. Transplantation 2009, 87, 1706–1711. [Google Scholar] [CrossRef]
- Li, Z.J.; Wang, K.W.; Liu, B.; Zang, F.; Zhang, Y.; Zhang, W.H.; Zhou, S.M.; Zhang, Y.X. The Distribution and Source of MRDOs Infection: A Retrospective Study in 8 ICUs, 2013–2019. Infect. Drug Resist. 2021, 14, 4983–4991. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhang, L.; Feng, C.; Lu, S.; Li, X.; Liu, T.; Wang, W.; Di, J. Targeted surveillance analysis of nosocomial infections in the intensive care unit over the past 13 years. Chin. J. Infect. Control 2023, 22, 1282–1290. [Google Scholar] [CrossRef]
- Smithson, A.; Ramos, J.; Niño, E.; Culla, A.; Pertierra, U.; Friscia, M.; Bastida, M.T. Characteristics of febrile urinary tract infections in older male adults. BMC Geriatr. 2019, 19, 334. [Google Scholar] [CrossRef]
- Wang, S.; Wang, P.; Lyu, T.; Yang, J.; Kong, L.; Xu, G.; Song, J.; Xie, K.; Zhao, D.; Que, W.; et al. Multicenter expert consensus on the diagnosis and treatment of multidrug-resistant bacterial infections after liver transplantation. Chin. J. General Surg. 2025, 32, 1–8. [Google Scholar]
- Shi, S.H.; Kong, H.S.; Xu, J.; Zhang, W.J.; Jia, C.K.; Wang, W.L.; Shen, Y.; Zhang, M.; Zheng, S.S. Multidrug resistant gram-negative bacilli as predominant bacteremic pathogens in liver transplant recipients. Transplant. Infect. Dis. 2009, 11, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Barchiesi, F.; Montalti, R.; Castelli, P.; Nicolini, D.; Staffolani, S.; Mocchegiani, F.; Fiorentini, A.; Manso, E.; Vivarelli, M. Carbapenem-Resistant Klebsiella pneumoniae influences the outcome of early infections in liver transplant recipients. BMC Infect. Dis. 2016, 16, 538. [Google Scholar] [CrossRef]
- Freire, M.P.; Pouch, S.; Manesh, A.; Giannella, M. Burden and Management of Multi-Drug Resistant Organism Infections in Solid Organ Transplant Recipients Across the World: A Narrative Review. Transplant. Int. 2024, 37, 12469. [Google Scholar] [CrossRef]
- Bodro, M.; Sabé, N.; Tubau, F.; Lladó, L.; Baliellas, C.; Roca, J.; Cruzado, J.M.; Carratalà, J. Risk factors and outcomes of bacteremia caused by drug-resistant ESKAPE pathogens in solid-organ transplant recipients. Transplantation 2013, 96, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.J.; Dennis, A.S.M.; Fariha, Z.; Pai Mangalore, R.; Macesic, N. Multidrug-resistant organism bloodstream infections in solid organ transplant recipients and impact on mortality: A systematic review. JAC Antimicrob. Resist. 2024, 6, dlae152. [Google Scholar] [CrossRef]
- Xu, X.; Ding, H.; Li, W.; Jia, J.; Wei, L.; Duan, Z.; Linghu, E.; Zhuang, H. Diagnostic and Therapeutic Guidelines for Cirrhotic Ascites and Related Complications. J. Clin. Hepatol. Gastroenterol. 2017, 33, 1847–1863. [Google Scholar]
- Planas, R.; Montoliu, S.; Ballesté, B.; Rivera, M.; Miquel, M.; Masnou, H.; Galeras, J.A.; Giménez, M.D.; Santos, J.; Cirera, I.; et al. Natural history of patients hospitalized for management of cirrhotic ascites. Clin. Gastroenterol. Hepatol. 2006, 4, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.I.; Kim, Y.J.; Jun, Y.H.; Wie, S.H.; Kim, Y.R.; Choi, J.Y.; Yoon, S.K.; Moon, I.S.; Kim, D.G.; Lee, M.D.; et al. Epidemiology and risk factors for bacteremia in 144 consecutive living-donor liver transplant recipients. Yonsei Med. J. 2009, 50, 112–121. [Google Scholar] [CrossRef]
- Habib, S.; Berk, B.; Chang, C.C.; Demetris, A.J.; Fontes, P.; Dvorchik, I.; Eghtesad, B.; Marcos, A.; Shakil, A.O. MELD and prediction of post-liver transplantation survival. Liver Transplant. 2006, 12, 440–447. [Google Scholar] [CrossRef]
- Lai, M.; Wang, X.; Yao, Q.; Liu, H.; Xu, Y.; He, L.; Li, G. The predictive value of the first postoperative MELD score and its derived scores on early survival rate after liver transplantation in patients with liver failure. Organ. Transplant. 2022, 13, 489–494. [Google Scholar] [CrossRef]
- Ferstl, P.G.; Filmann, N.; Heilgenthal, E.M.; Schnitzbauer, A.A.; Bechstein, W.O.; Kempf, V.A.J.; Villinger, D.; Schultze, T.G.; Hogardt, M.; Stephan, C.; et al. Colonization with multidrug-resistant organisms is associated with in increased mortality in liver transplant candidates. PLoS ONE 2021, 16, e0245091. [Google Scholar] [CrossRef]
- Li, S.; Lu, J.; Song, F.; Xu, J. New strategies and technologies beyond the MELD score applied in liver transplantation. Liver 2022, 27, 739–741. [Google Scholar] [CrossRef]
- Ai, C.; Zhang, A.; Zhang, A.; Ji, A.; Li, A.; Mao, A. Clinical Analysis of Perioperative Human Serum Albumin Application in Liver Transplant Patients. Clin. Pharmacol. Ther. J. 2020, 18, 21–24. [Google Scholar] [CrossRef]
- Singh, N.; Paterson, D.L.; Gayowski, T.; Wagener, M.M.; Marino, I.R. Predicting bacteremia and bacteremic mortality in liver transplant recipients. Liver Transplant. 2000, 6, 54–61. [Google Scholar] [CrossRef]
- Lee, I.K.; Chang, P.H.; Yeh, C.H.; Li, W.F.; Yin, S.M.; Lin, Y.C.; Tzeng, W.J.; Chen, C.L.; Lin, C.C.; Wang, C.C. Risk factors and crucial prognostic indicators of mortality in liver transplant recipients with bloodstream infections: A comprehensives study of 1049 consecutive liver transplants over an 11-year period. J. Microbiol. Immunol. Infect. 2024, 57, 771–781. [Google Scholar] [CrossRef]
- Ertmer, C.; Kampmeier, T.G.; Volkert, T.; Wolters, H.; Rehberg, S.; Morelli, A.; Schmidt, H.; Lange, M.; Boschin, M.; Van Aken, H.; et al. Impact of human albumin infusion on organ function in orthotopic liver transplantation—A retrospective matched-pair analysis. Clin. Transplant. 2015, 29, 67–75. [Google Scholar] [CrossRef]
- Tufoni, M.; Baldassarre, M.; Zaccherini, G.; Antognoli, A.; Caraceni, P. Hemodynamic and Systemic Effects of Albumin in Patients with Advanced Liver Disease. Curr. Hepatol. Rep. 2020, 19, 147–158. [Google Scholar] [CrossRef]
- Wu, N.; Liu, T.; Tian, M.; Liu, C.; Ma, S.; Cao, H.; Bian, H.; Wang, L.; Feng, Y.; Qi, J. Albumin, an interesting and functionally diverse protein, varies from ‘native’ to ‘effective’ (Review). Mol. Med. Rep. 2024, 29, 24. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, R.; Mateu, X.; Maseda, E.; Yébenes, J.C.; Aldecoa, C.; De Haro, C.; Ruiz-Rodriguez, J.C.; Garnacho-Montero, J. Non-oncotic properties of albumin. A multidisciplinary vision about the implications for critically ill patients. Expert. Rev. Clin. Pharmacol. 2018, 11, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.E.; D’Agata, E.M.; Paterson, D.L.; Clarke, L.; Qureshi, Z.A.; Potoski, B.A.; Peleg, A.Y. Pseudomonas aeruginosa bacteremia over a 10-year period: Multidrug resistance and outcomes in transplant recipients. Transplant. Infect. Dis. 2009, 11, 227–234. [Google Scholar] [CrossRef]
- Huang, X.; Li, G.; Yi, L.; Li, M.; Wang, J. Analysis of the colonization status and risk factors of multidrug-resistant bacteria in the intensive care unit. Chin. J. Crit. Care Med. 2015, 8, 667–671. [Google Scholar] [CrossRef]
- Zhong, L.; Men, T.Y.; Li, H.; Peng, Z.H.; Gu, Y.; Ding, X.; Xing, T.H.; Fan, J.W. Multidrug-resistant gram-negative bacterial infections after liver transplantation—Spectrum and risk factors. J. Infect. 2012, 64, 299–310. [Google Scholar] [CrossRef]
- Min, E.K.; Yim, S.H.; Choi, M.C.; Lee, J.G.; Joo, D.J.; Kim, M.S.; Kim, D.G. Incidence, mortality, and risk factors associated with carbapenem-resistant Acinetobacter baumannii bacteremia within 30 days after liver transplantation. Clin. Transplant. 2023, 37, e14956. [Google Scholar] [CrossRef]
- Ao, H.; Song, H.; Li, J. A Nomogram for Predicting the Effectiveness of Consultations on Multi-Drug Resistant Infections: An Exploration for Clinical Pharmacy Services. Infect. Drug Resist. 2024, 17, 3439–3450. [Google Scholar] [CrossRef]
- Guo, L.; Qin, H.; Zhang, S.; Zhang, Y.; Lian, H. Construction and Validation of a Nomogram Model for the Risk of Postoperative Multidrug-Resistant Bacterial Infections in Patients with Bone Trauma. Chin. J. Infect. Control. 2022, 21, 584–591. [Google Scholar] [CrossRef]
- He, J.; Qu, T. Research Progress on Predictive Models for Multidrug-Resistant Bacteria Infection Risk in Intensive Care Units. Chin. J. Clin. Infect. Dis. 2023, 16, 384–390. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, J.; Wang, X.; Deng, H.; Wang, L.; Zhu, L.; Shao, M.; Yang, N.; Ma, Y.; Xu, Y. Interpretation and Enlightenment of the External Validation Guide for Clinical Risk Prediction Models (2024 Edition). J. Nurs. Sci. 2024, 39, 52–56. [Google Scholar] [CrossRef]
- Duncan, S.; Annunziato, R.A.; Dunphy, C.; LaPointe Rudow, D.; Shneider, B.L.; Shemesh, E. A systematic review of immunosuppressant adherence interventions in transplant recipients: Decoding the streetlight effect. Pediatr Transplant. 2018, 22, e13086. [Google Scholar] [CrossRef]
- Mellon, L.; Doyle, F.; Hickey, A.; Ward, K.D.; de Freitas, D.G.; McCormick, P.A.; O’Connell, O.; Conlon, P. Interventions for increasing immunosuppressant medication adherence in solid organ transplant recipients. Cochrane Database Syst. Rev. 2022, 9, Cd012854. [Google Scholar] [CrossRef]


| Characteristic | Total | Training Cohort | Validtion Cohort |
|---|---|---|---|
| (n = 301) | (n = 210) | (n = 91) | |
| Age (years) | 53.0 (46.0, 60.0) | 53.0 (46.0, 59.8) | 52.0 (46.0, 60.5) |
| Sex | |||
| Male | 244 (81.1%) | 79 (86.8%) | 165 (78.6%) |
| Female | 57 (18.9%) | 12 (13.2%) | 45 (21.4%) |
| BMI (kg/m2) | 22.0 (20.0, 24.0) | 22.0 (20.0, 24.0) | 22.0 (21.0, 24.0) |
| Primary disease for LT | |||
| Hepatocellular carcinoma | 130 (43.2%) | 91 (43.3%) | 39 (42.9%) |
| Hepatitis B virus cirrhosis | 79 (26.3%) | 56 (26.7%) | 23 (25.3%) |
| Alcoholic liver cirrhosis | 29 (9.6%) | 18 (8.6%) | 11 (12.1%) |
| Acute liver failure | 18 (6%) | 13 (6.2%) | 5 (5.5%) |
| Primary biliary cirrhosis | 13 (4.3%) | 12 (5.7%) | 1 (1.1%) |
| Autoimmune liver disease | 10 (3.3%) | 6 (2.9%) | 4 (4.4%) |
| Cholangiocarcinoma | 8 (2.7%) | 4 (1.9%) | 4 (4.4%) |
| Hepatitis C virus cirrhosis | 5 (1.7%) | 4 (1.9%) | 1 (1.1%) |
| Others | 9 (3%) | 6 (2.9%) | 3 (3.3%) |
| Comorbidities | |||
| Diabetes | 49 (16.3%) | 37 (17.6%) | 12 (13.2%) |
| Hypertension | 36 (12%) | 23 (11%) | 13 (14.3%) |
| Hypertension and diabetes | 15 (5%) | 9 (4.3%) | 6 (6.6%) |
| Operation | |||
| Classic orthotopic liver transplantation | 251 (83.4%) | 178 (84.8%) | 73 (80.2%) |
| Piggyback liver transplantation | 46 (15.3%) | 29 (13.8%) | 17 (18.7%) |
| Split liver transplantation | 4 (1.3%) | 3 (1.4%) | 1 (1.1%) |
| Ascites | 143 (47.5%) | 96 (45.7%) | 47 (51.7%) |
| Biliary intestinal anastomosis | 33 (11%) | 19 (9.1%) | 14 (15.4%) |
| MELD score ≥25 | 34 (11.3%) | 23 (11%) | 11 (12.1%) |
| Child-Pugh score C | 32 (10.6%) | 23 (11%) | 9 (9.9%) |
| Hepatic encephalopathy | 28 (9.3%) | 18 (8.6%) | 10 (11%) |
| Antibacterial drug use within 30 days before LT | 25 (8.3%) | 19 (9.1%) | 6 (6.6%) |
| Preoperative ICU stay history | 27 (9%) | 20 (9.5%) | 7 (7.7%) |
| Surgical time (h) | 8.0 (7.0, 9.0) | 8.0 (7.0, 9.0) | 8.0 (8.0, 10.0) |
| Intraoperative blood loss (ml) | 400.0 (200.0, 600.0) | 400.0 (200.0, 600.0) | 400.0 (200.0, 600.0) |
| Total bilirubin (μmol/L) | 137.0 (100.0, 210.0) | 132.0 (99.3, 212.3) | 139.0 (100.0, 208.5) |
| Albumin (g/L) | 35.0 (32.0, 37.0) | 35.0 (32.0, 37.0) | 35.0 (31.0, 37.0) |
| MDRO | 56 (18.6%) | 37 (17.6%) | 19 (20.9%) |
| WBC (*109/L) | 5.8 (4.6, 8.6) | 5.9 (4.6, 8.6) | 5.6 (4.5, 8.2%) |
| Transplantation or open laparotomy again | 12 (4%) | 10 (4.8%) | 2 (2.2%) |
| CRRT | 12 (4%) | 7 (3.3%) | 5 (5.5%) |
| Biliary complications | 20 (6.6%) | 14 (6.7%) | 6 (6.6%) |
| Intestinal complications | 17 (5.7%) | 15 (7.1%) | 2 (2.2%) |
| Ventilator support time (h) | 8.0 (6.0, 10.0) | 8.0 (6.0, 10.0) | 8.0 (6.0, 10.0) |
| Length of ICU stay days | 6.0 (4.0, 8.0) | 5.5 (4.0, 8.0) | 6.0 (4.0, 8.0) |
| Acute rejection reaction | 48 (16%) | 38 (18.1%) | 10 (11%) |
| Total length of hospital stay (d) | 16.0 (12.0, 22.0) | 16.0 (12.0, 23.0) | 16.0 (11.0, 20.5) |
| 28 d all-cause mortality | 4 (1.3%) | 3 (1.4%) | 1 (1.1%) |
| 90 d all-cause mortality | 9 (3%) | 8 (3.8%) | 1 (1.1%) |
| Variables | Total | No MDRO | MDRO | p-Value |
|---|---|---|---|---|
| (n = 210) | (n = 173) | (n = 37) | ||
| Age (years) | 53.0 (46.0, 59.8) | 53.0 (46.0, 59.0) | 56.0 (42.0, 62.0) | 0.720 |
| Sex | 0.975 | |||
| Male | 165 (78.6%) | 136 (78.6%) | 29 (78.4%) | |
| Female | 45 (21.4%) | 37 (21.4%) | 8 (21.6%) | |
| BMI (kg/m2) | 22.0 (20.0, 24.0) | 22.0 (20.0, 24.0) | 22.0 (21.0, 24.0) | 0.337 |
| Primary disease for LT | ||||
| Hepatocellular carcinoma | 91 (43.3%) | 76 (43.9%) | 15 (40.5%) | 0.129 |
| Hepatitis B virus cirrhosis | 56 (26.7%) | 50 (28.9%) | 6 (16.2%) | |
| Alcoholic liver cirrhosis | 18 (8.6%) | 14 (8.1%) | 4 (10.8%) | |
| Acute liver failure | 13 (6.2%) | 8 (4.6%) | 5 (13.5%) | |
| Primary biliary cirrhosis | 12 (5.7%) | 11 (6.4%) | 1 (2.7%) | |
| Autoimmune liver disease | 6 (2.9%) | 4 (2.3%) | 2 (5.4%) | |
| Cholangiocarcinoma | 4 (1.9%) | 2 (1.2%) | 2 (5.4%) | |
| Hepatitis C virus cirrhosis | 4 (1.9%) | 3 (1.7%) | 1 (2.7%) | |
| Others | 6 (2.9%) | 5 (2.9%) | 1 (2.7%) | |
| Comorbidities | 0.166 | |||
| Diabetes | 37 (17.6%) | 27 (15.6%) | 10 (27%) | |
| Hypertension | 23 (11%) | 20 (11.6%) | 3 (8.1%) | |
| Hypertension and diabetes | 9 (4.3%) | 6 (3.5%) | 3 (8.1%) | |
| Operation | 0.049 | |||
| Classic orthotopic liver transplantation | 178 (84.8%) | 150 (86.7%) | 28 (75.7%) | |
| Piggyback liver transplantation | 29 (13.8%) | 22 (12.7%) | 7 (18.9%) | |
| Split liver transplantation | 3 (1.4%) | 1 (0.6%) | 2 (5.4%) | |
| Ascites | 96 (45.7%) | 68 (39.3%) | 28 (75.7%) | <0.001 |
| Biliary intestinal anastomosis | 19 (9.1%) | 13 (7.5%) | 6 (16.2%) | 0.174 |
| MELD score ≥25 | 23 (11%) | 16 (9.3%) | 7 (18.9%) | 0.156 |
| Child-Pugh score C | 23 (11%) | 15 (8.7%) | 8 (21.6%) | 0.046 |
| Hepatic encephalopathy | 18 (8.6%) | 14 (8.1%) | 4 (10.8%) | 0.832 |
| Antibacterial drug use within 30 days before LT | 19 (9.1%) | 13 (7.5%) | 6 (16.2%) | 0.174 |
| Preoperative ICU stay history | 20 (9.5%) | 12 (6.9%) | 8 (21.6%) | 0.014 |
| Surgical time (h) | 8.0 (7.0, 9.0) | 8.0 (7.0, 9.0) | 9.0 (8.0, 12.0) | <0.001 |
| Intraoperative blood loss (ml) | 400.0 (200.0, 600.0) | 400.0 (200.0, 500.0) | 600.0 (300.0, 800.0) | <0.001 |
| Total bilirubin (μmol/L) | 132.0 (99.3, 212.3) | 116.0 (95.0, 200.0) | 217.0 (187.0, 379.0) | <0.001 |
| Albumin (g/L) | 35.0 (32.0, 37.0) | 35.0 (32.0, 38.0) | 33.0 (31.0, 35.0) | 0.002 |
| WBC (*109/L) | 5.9 (4.6, 8.6) | 5.9 (4.6, 8.6) | 5.9 (5.0, 8.5) | 0.639 |
| Transplantation or open laparotomy again | 10 (4.8%) | 6 (3.5%) | 4 (10.8%) | 0.139 |
| CRRT | 7 (3.3%) | 5 (2.9%) | 2 (5.4%) | 0.788 |
| Biliary complications | 14 (6.7%) | 8 (4.6%) | 6 (16.2%) | 0.028 |
| Intestinal complications | 15 (7.1%) | 11 (6.4%) | 4 (10.8%) | 0.547 |
| Ventilator support time (h) | 8.0 (6.0, 10.0) | 7.0 (6.0, 9.0) | 12.0 (6.0, 23.0) | 0.001 |
| Length of ICU stay days | 5.5 (4.0, 8.0) | 5.0 (4.0, 7.0) | 8.0 (6.0, 15.0) | <0.001 |
| Acute rejection reaction | 38 (18.1%) | 17 (9.8%) | 21 (56.8%) | <0.001 |
| Total length of hospital stay (d) | 16.0 (12.0, 23.0) | 15.0 (12.0, 20.0) | 38.0 (21.0, 43.0) | <0.001 |
| 28 d all-cause mortality | 3 (1.4%) | 2 (1.2%) | 1 (2.7%) | 0.443 |
| 90 d all-cause mortality | 8 (3.8%) | 2 (1.2%) | 6 (16.2%) | <0.001 |
| Pathogen | The Number of Pathogen Detections (56) | Percentage (%) |
|---|---|---|
| Drug-resistant gram-negative bacteria | 49 | 0.875 |
| Klebsiella pneumoniae CRE | 26 | 0.464 |
| Acinetobacter baumannii CRAB | 15 | 0.268 |
| Escherichia coli ESBLs | 4 | 0.071 |
| Enterobacter cloacae CRE | 2 | 0.036 |
| Pseudomonas aeruginosa CRPA | 2 | 0.036 |
| Drug-resistant gram-positive bacteria | 7 | 0.125 |
| Staphylococcus aureus MRSA | 3 | 0.054 |
| Staphylococcus epidermidis MRS | 1 | 0.018 |
| Staphylococcus caprae MRS | 1 | 0.018 |
| Staphylococcus hominis MRS | 1 | 0.018 |
| Enterococcus faecium VRE | 1 | 0.018 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| β | OR (95%CI) | p-Value | β | OR (95%CI) | p-Value | |
| Ascites | 1.57 | 4.80 (2.14~10.81) | <0.001 | 1.25 | 3.48 (1.33~9.14) | 0.011 |
| Child-Pugh score C | 1.07 | 2.91 (1.13~7.48) | 0.027 | |||
| Preoperative ICU stay history | 1.31 | 3.70 (1.39~9.84) | 0.009 | |||
| Total bilirubin (μmol/L) | 0.01 | 1.01 (1.01~1.01) | <0.001 | 0.01 | 1.01 (1.01~1.01) | <0.001 |
| Albumin (g/L) | −0.17 | 0.84 (0.76~0.93) | <0.001 | −0.17 | 0.85 (0.75~0.96) | 0.010 |
| Biliary complications | 1.38 | 3.99 (1.29~12.31) | 0.016 | |||
| Length of ICU stay days | 0.14 | 1.15 (1.07~1.23) | <0.001 | 0.09 | 1.09 (1.01~1.17) | 0.019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Li, D.; Zhou, Z.; Guan, Q.; Sheng, B.; Hu, Y.; Zhang, Z. Epidemiology and Risk Prediction Model of Multidrug-Resistant Organism Infections After Liver Transplant Recipients: A Single-Center Cohort Study. Bioengineering 2025, 12, 417. https://doi.org/10.3390/bioengineering12040417
Chen C, Li D, Zhou Z, Guan Q, Sheng B, Hu Y, Zhang Z. Epidemiology and Risk Prediction Model of Multidrug-Resistant Organism Infections After Liver Transplant Recipients: A Single-Center Cohort Study. Bioengineering. 2025; 12(4):417. https://doi.org/10.3390/bioengineering12040417
Chicago/Turabian StyleChen, Chuanlin, Desheng Li, Zhengdon Zhou, Qinghua Guan, Bo Sheng, Yongfang Hu, and Zhenyu Zhang. 2025. "Epidemiology and Risk Prediction Model of Multidrug-Resistant Organism Infections After Liver Transplant Recipients: A Single-Center Cohort Study" Bioengineering 12, no. 4: 417. https://doi.org/10.3390/bioengineering12040417
APA StyleChen, C., Li, D., Zhou, Z., Guan, Q., Sheng, B., Hu, Y., & Zhang, Z. (2025). Epidemiology and Risk Prediction Model of Multidrug-Resistant Organism Infections After Liver Transplant Recipients: A Single-Center Cohort Study. Bioengineering, 12(4), 417. https://doi.org/10.3390/bioengineering12040417








