The Preservation and Reuse of Lenticules Extracted via Small Incision Lenticule Extraction (SMILE): A Narrative Review
Abstract
1. Introduction
2. Storage and Preservation Methods for Corneal Stromal Lenticules
2.1. Short-Term Storage
2.1.1. Dehydrating Agents
2.1.2. Optisol GS and Other Preservation Solutions
2.2. Long-Term Storage
2.2.1. Cryopreservation
2.2.2. Hydrogel Nutrient Capsules
2.2.3. Silicone Oil
2.3. Preservation Challenges and Future Directions
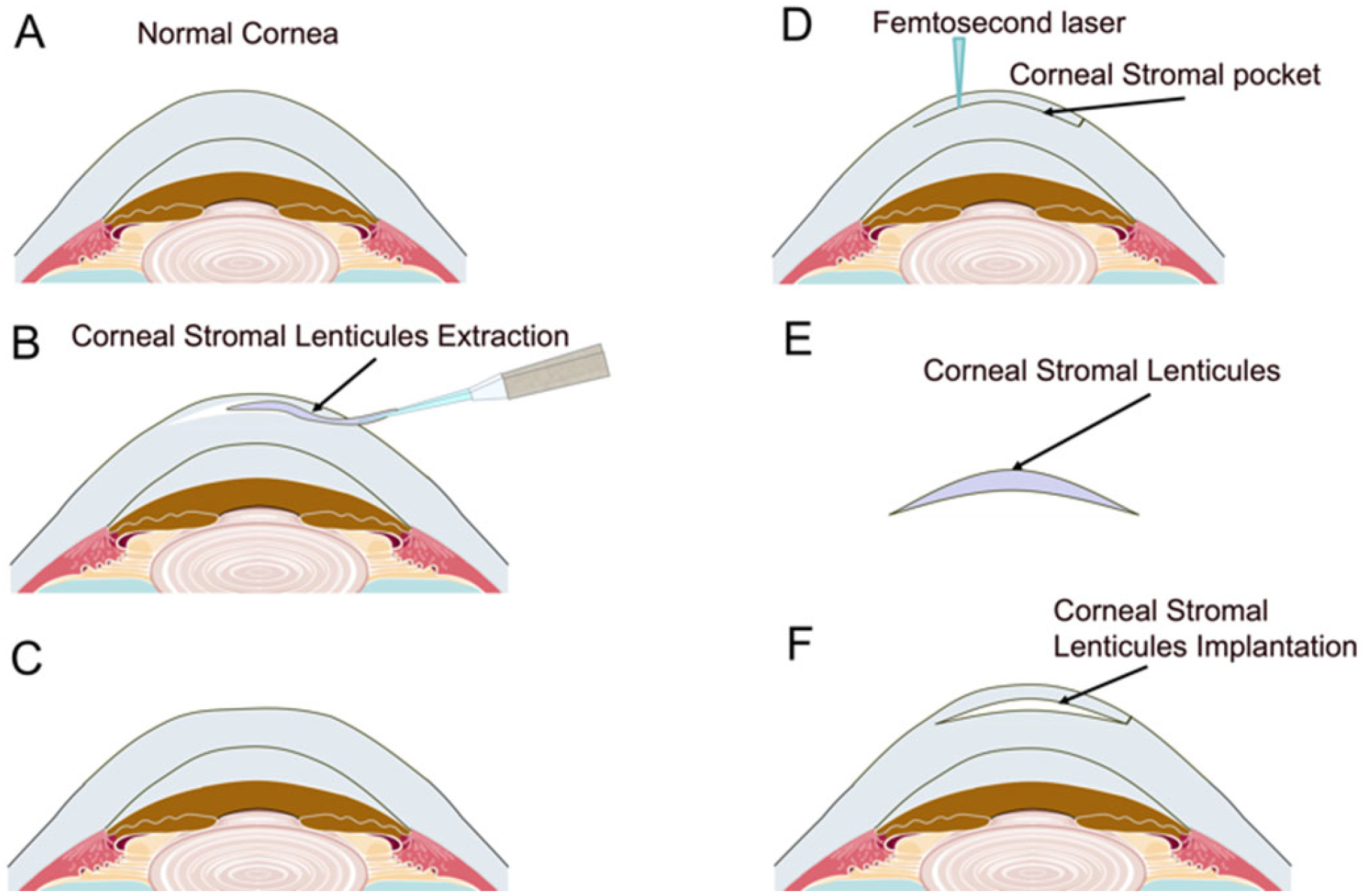
3. Re-Utilization of Corneal Stromal Lenticules
3.1. Lenticule Intrastromal Keratoplasty (LIKE)
3.1.1. Hyperopia Correction
3.1.2. Presbyopia Treatment
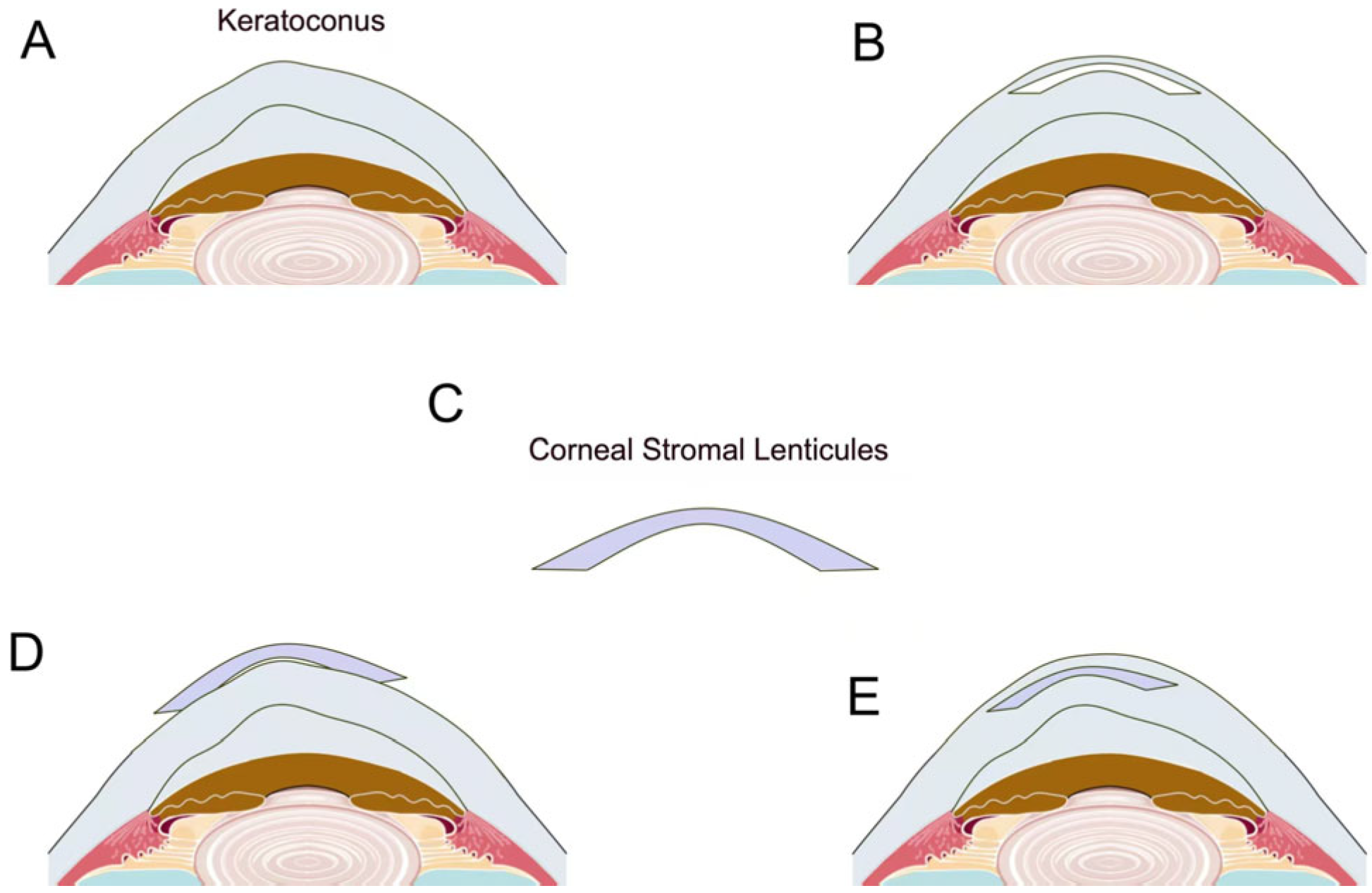
3.1.3. Keratoconus and Corneal Ectasia
3.1.4. Post-LASIK Ectasia
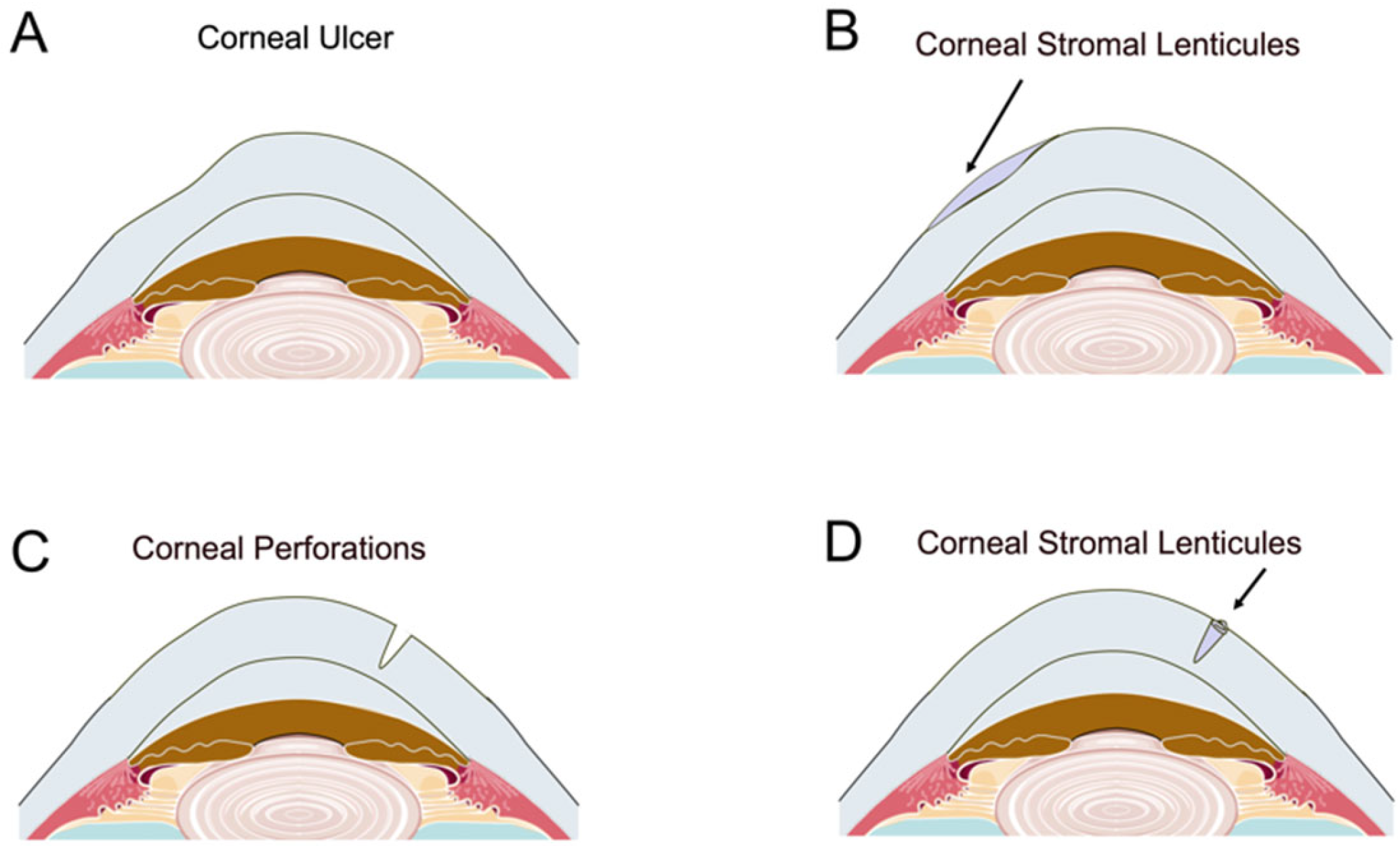
3.2. Corneal Patch Grafting
3.2.1. Corneal Ulcer and Perforation
3.2.2. Corneal Dystrophies and Epikeratophakia
3.2.3. Applications in Other Ocular Conditions
3.2.4. Glaucoma Drainage Implantation Surgery
3.2.5. Regenerative Corneal Engineering
3.2.6. Ocular Drug Delivery System
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Byrd, L.B.; Gurnani, B.; Martin, N. Corneal Ulcer; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Dua, H.S.; Faraj, L.A.; Said, D.G.; Gray, T.; Lowe, J. Human corneal anatomy redefined: A novel pre-descemet’s layer (dua’s layer). Ophthalmology 2013, 120, 1778–1785. [Google Scholar] [PubMed]
- Akter, F. Principles of Tissue Engineering. In Tissue Engineering Made Easy; Academic Press: Cambridge, MA, USA, 2016; pp. 3–16. [Google Scholar]
- Yusoff, N.Z.B.M.; Riau, A.K.; Yam, G.H.F.; Halim, N.S.H.B.; Mehta, J.S. Isolation and Propagation of Human Corneal Stromal Keratocytes for Tissue Engineering and Cell Therapy. Cells 2022, 11, 178. [Google Scholar] [CrossRef] [PubMed]
- Pascolini, D.; Mariotti, S.P. Global estimates of visual impairment: 2010. Br. J. Ophthalmol. 2011, 96, 614–618. [Google Scholar]
- Tan, D.T.H.; Dart, J.K.G.; Holland, E.J.; Kinoshita, S. Corneal transplantation. Lancet 2012, 379, 1749–1761. [Google Scholar]
- Shah, R.; Shah, S.; Sengupta, S. Results of small incision lenticule extraction: All-in-one femtosecond laser refractive surgery. J. Cataract Refract. Surg. 2011, 37, 127–137. [Google Scholar]
- Moshirfar, M.; McCaughey, M.V.; Reinstein, D.Z.; Shah, R.; Santiago-Caban, L.; Fenzl, C.R. Small-incision lenticule extraction. J. Cataract Refract. Surg. 2015, 41, 652–665. [Google Scholar]
- Reinstein, D.Z.; Archer, T.J.; Gobbe, M. Small incision lenticule extraction (SMILE) history, fundamentals of a new refractive surgery technique and clinical outcomes. Eye Vis. 2014, 1, 3. [Google Scholar]
- Mohamed-Noriega, K.; Toh, K.P.; Poh, R.; Balehosur, D.; Riau, A.; Htoon, H.M.; Peh, G.S.L.; Chaurasia, S.S.; Tan, D.T.; Mehta, J.S. Cornea lenticule viability and structural integrity after refractive lenticule extraction (ReLEx) and cryopreservation. Mol. Vis. 2011, 17, 3437–3449. [Google Scholar]
- Liu, Y.-C.; Williams, G.P.; George, B.L.; Soh, Y.Q.; Seah, X.Y.; Peh, G.S.L.; Yam, G.H.F.; Mehta, J.S. Corneal lenticule storage before reimplantation. Mol. Vis. 2017, 23, 753–764. [Google Scholar]
- Xia, F.; Zhao, J.; Fu, D.; Xu, Y.; Yao, P.; Li, M.; Aruma, A.; Zhou, X. Optical transmittance and ultrastructure of SMILE-derived lenticules subjected to three different preservative methods. Exp. Eye Res. 2020, 201, 108357. [Google Scholar]
- Feilmeier, M.R.; Tabin, G.C.; Williams, L.; Oliva, M. The use of glycerol-preserved corneas in the developing world. Middle East Afr. J. Ophthalmol. 2010, 17, 38–43. [Google Scholar] [PubMed]
- Li, Q.; Wang, H.; Dai, Z.; Cao, Y.; Jin, C. Preparation and Biomechanical Properties of an Acellular Porcine Corneal Stroma. Cornea 2017, 36, 1343–1351. [Google Scholar] [PubMed]
- Halberstadt, M.; Athmann, S.; Winter, R.; Hagenah, M. Impact of transportation on short-term preserved corneas preserved in Optisol-GS, Likorol, Likorol-DX, and MK-medium. Cornea 2000, 19, 788–791. [Google Scholar]
- Varssano, D.; Russ, V.; Linhart, Y.; Lazar, M. Air transportation of corneal tissue: Experience with local compared to transatlantic donor corneas. Cornea 2005, 24, 674–677. [Google Scholar]
- Liang, G.; Wang, L.; Pan, Z.; Zhang, F. Comparison of the Different Preservative Methods for Refractive Lenticules Following SMILE. Curr. Eye Res. 2019, 44, 832–839. [Google Scholar]
- Gao, Y.; Zhou, Q.; Qu, M.; Yang, L.; Wang, Y.; Shi, W. In vitro culture of human fetal corneal endothelial cells. Graefes Arch. Clin. Exp. Ophthalmol. 2011, 249, 663–669. [Google Scholar]
- Maathuis, M.H.J.; Leuvenink, H.G.D.; Ploeg, R.J. Perspectives in organ preservation. Transplantation 2007, 83, 1289–1298. [Google Scholar]
- Yang, J.; Pan, C.; Sui, X.; Cai, N.; Zhang, J.; Zhu, Y.; Zhang, L. The hypothermic preservation of mammalian cells with assembling extracellular-matrix-mimetic microparticles. J. Mater. Chem. B 2017, 5, 1535–1541. [Google Scholar]
- Ganesh, S.; Brar, S.; Rao, P.A. Cryopreservation of extracted corneal lenticules after small incision lenticule extraction for potential use in human subjects. Cornea 2014, 33, 1355–1362. [Google Scholar]
- Oh, J.Y.; Kim, M.K.; Lee, H.J.; Ko, J.H.; Wee, W.R.; Lee, J.H. Comparative observation of freeze-thaw-induced damage in pig, rabbit, and human corneal stroma. Vet. Ophthalmol. 2009, 12, 50–56. [Google Scholar]
- Neronov, A.; Mazgalova, J.; Cholakova, M.; Dimitrova, M.; Deligiozova, I.; Kovatcheva, S.; Nikolova, E. Integrity of endothelium in cryopreserved human cornea. Cryo Lett. 2005, 26, 131–136. [Google Scholar]
- Trias, E.; Gallon, P.; Ferrari, S.; Piteira, A.R.; Tabera, J.; Casaroli-Marano, R.P.; Parekh, M.; Ruzza, A.; Franch, A.; Ponzin, D. Banking of corneal stromal lenticules: A risk-analysis assessment with the EuroGTP II interactive tool. Cell Tissue Bank. 2020, 21, 189–204. [Google Scholar] [PubMed]
- Zhang, Z.; Sun, B.; Xia, F.; Yu, Y.; Shen, Y.; Yao, P.; Wang, X.; Zhou, X.; Zhao, J. Study on the biological properties of SMILE-derived corneal stromal lenticules after long-term cryopreservation in nutrient capsules. Exp. Eye Res. 2024, 239, 109756. [Google Scholar] [PubMed]
- Zhao, J.; Zhang, Z.; Xia, F. Nutrient capsules maintain tear film homeostasis for human corneal lenticule transplantation. Chem. Eng. J. 2022, 450, 138078. [Google Scholar]
- Perez, V.L.; Saeed, A.M.; Tan, Y.; Urbieta, M.; Cruz-Guilloty, F. The eye: A window to the soul of the immune system. J. Autoimmun. 2013, 45, 7–14. [Google Scholar]
- Ditzen, K.; Huschka, H.; Pieger, S. Laser in situ keratomileusis for hyperopia. J. Cataract Refract. Surg. 1998, 24, 42–47. [Google Scholar]
- Varley, G.A.; Huang, D.; Rapuano, C.J.; Schallhorn, S.; Boxer, W.B.S.; Sugar, A. LASIK for hyperopia, hyperopic astigmatism, and mixed astigmatism: A report by the American Academy of Ophthalmology. Ophthalmology 2004, 111, 1604–1617. [Google Scholar]
- Pradhan, K.R.; Reinstein, D.Z.; Carp, G.I.; Archer, T.J.; Gobbe, M.; Gurung, R. Femtosecond laser-assisted keyhole endokeratophakia: Correction of hyperopia by implantation of an allogeneic lenticule obtained by SMILE from a myopic donor. J. Refract. Surg. 2013, 29, 777–782. [Google Scholar]
- Studer, H.P.; Pradhan, K.R.; Reinstein, D.Z.; Businaro, E.; Archer, T.J.; Gobbe, M.; Roberts, C.J. Biomechanical Modeling of Femtosecond Laser Keyhole Endokeratophakia Surgery. J. Refract. Surg. 2015, 31, 480–486. [Google Scholar]
- Zhang, J.; Zhou, Y. Small incision lenticule extraction (SMILE) combined with allogeneic intrastromal lenticule inlay for hyperopia with astigmatism. PLoS ONE 2021, 16, e0257667. [Google Scholar]
- Wu, J.; Xiong, L.; Wang, Z.; Reinstein, D.Z.; Vida, R.S.; Archer, T.J. Correction of moderate to high hyperopia with implantation of an allogeneic refractive lenticule. J. Refract. Surg. 2020, 36, 772–779. [Google Scholar] [PubMed]
- Moshirfar, M.; Hopping, G.C.; Somani, A.N.; Vaidyanathan, U.; Liu, H.; Barnes, J.R.; Linn, S.; Ronquillo, Y.C.; Hoopes, P.C. Human allograft refractive lenticular implantation for high hyperopiccorrection. J. Cataract Refract. Surg. 2020, 46, 305–311. [Google Scholar] [PubMed]
- Sun, L.; Yao, P.; Li, M.; Shen, Y.; Zhao, J.; Zhou, X. The Safety and Predictability of Implanting Autologous Lenticule Obtained by SMILE for Hyperopia. J. Refract. Surg. 2015, 31, 374–379. [Google Scholar] [PubMed]
- Li, M.; Li, M.; Sun, L.; Han, T.; Ding, L.; Xiang, J.; Zhou, X. In vivo confocal microscopic investigation of the cornea after autologous implantation of lenticules obtained through small incision lenticule extraction for treatment of hyperopia. Clin. Exp. Optom. 2018, 101, 38–45. [Google Scholar]
- Fu, L.; Hollick, E.J. Artificial Cornea Transplantation; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Göker, S.; Er, H.; Kahvecioglu, C. Laser in situ keratomileusis to correct hyperopia from +4.25 to +8.00 diopters. J. Refract. Surg. 1998, 14, 26–30. [Google Scholar]
- Pesudovs, K. Wavefront aberration outcomes of LASIK for high myopia and hyperopia. J. Refract. Surg. 2005, 21, S508–S512. [Google Scholar]
- Soosan, J.; Kumar, D.A.; Agarwal, A.; Agarwal, A.; Aravind, R.; Saijimol, A.I. Preliminary Evidence of Successful Near Vision Enhancement with a New Technique: PrEsbyopic Allogenic Refractive Lenticule (PEARL) Corneal Inlay Using a SMILE Lenticule. J. Refract. Surg. 2017, 33, 224–229. [Google Scholar]
- Konstantopoulos, A.; Mehta, J.S. Surgical compensation of presbyopia with corneal inlays. Expert. Rev. Med. Devices 2015, 12, 341–352. [Google Scholar]
- Liu, Y.C.; Teo, E.P.W.; Ang, H.P.; Seah, X.Y.; Lwin, N.C.; Yam, G.H.F.; Mehta, J.S. Biological corneal inlay for presbyopia derived from small incision lenticule extraction (SMILE). Sci. Rep. 2018, 8, 1831. [Google Scholar]
- Arlt, E.; Krall, E.; Moussa, S.; Grabner, G.; Dexl, A. Implantable inlay devices for presbyopia: The evidence to date. Clin. Ophthalmol. 2015, 9, 129–137. [Google Scholar]
- Wolffsohn, J.S.; Davies, L.N. Presbyopia: Effectiveness of correction strategies. Prog. Retin. Eye Res. 2019, 68, 124–143. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom, R.L.; Macrae, S.M.; Pepose, J.S.; Hoopes, P.C.S. Corneal inlays for presbyopia correction. Curr. Opin. Ophthalmol. 2013, 24, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Mulet, M.E.; Alio, J.L.; Knorz, M.C. Hydrogel intracorneal inlays for the correction of hyperopia: Outcomes and complications after 5 years of follow-up. Ophthalmology 2009, 116, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Alió, J.L.; Mulet, M.E.; Zapata, L.F.; Vidal, M.T.; De Rojas, V.; Javaloy, J. Intracorneal inlay complicated by intrastromal epithelial opacification. Arch. Ophthalmol. 2004, 122, 1441–1446. [Google Scholar] [CrossRef][Green Version]
- Arnalich-Montiel, F.; Alió Del Barrio, J.L.; Alió, J.L. Corneal surgery in keratoconus: Which type, which technique, which outcomes? Eye Vis. 2016, 3, 2. [Google Scholar] [CrossRef]
- Krachmer, J.H.; Feder, R.S.; Belin, M.W. Keratoconus and related noninflammatory corneal thinning disorders. Surv. Ophthalmol. 1984, 28, 293–322. [Google Scholar] [CrossRef]
- Parker, J.S.; van Dijk, K.; Melles, G.R.J. Treatment options for advanced keratoconus: A review. Surv. Ophthalmol. 2015, 60, 459–480. [Google Scholar] [CrossRef]
- Henein, C.; Nanavaty, M.A. Systematic review comparing penetrating keratoplasty and deep anterior lamellar keratoplasty for management of keratoconus. Cont. Lens Anterior Eye 2017, 40, 3–14. [Google Scholar] [CrossRef]
- Gordon, M.O.; Steger-May, K.; Szczotka-Flynn, L.; Riley, C.; Joslin, C.E.; Weissman, B.A.; Fink, B.A.; Edrington, T.B.; Olafsson, H.E.; Zadnik, K. Baseline factors predictive of incident penetrating keratoplasty in keratoconus. Am. J. Ophthalmol. 2006, 142, 923–930. [Google Scholar] [CrossRef]
- Liu, Y.; He, Y.; Deng, Y.; Wang, L. Lenticule addition keratoplasty for the treatment of keratoconus: A systematic review and critical considerations. Indian J. Ophthalmol. 2024, 72, 167–175. [Google Scholar] [CrossRef]
- Sekundo, W.; Reinstein, D.Z.; Blum, M. Improved lenticule shape for hyperopic femtosecond lenticule extraction (ReLEx FLEx): A pilot study. Lasers Med. Sci. 2016, 31, 659–664. [Google Scholar] [PubMed]
- Pradhan, K.R.; Reinstein, D.Z.; Carp, G.I.; Archer, T.J.; Dhungana, P. Small Incision Lenticule Extraction (SMILE) for Hyperopia: 12-Month Refractive and Visual Outcomes. J. Refract. Surg. 2019, 35, 442–450. [Google Scholar] [PubMed]
- Mastropasqua, L.; Nubile, M.; Salgari, N.; Mastropasqua, R. Femtosecond Laser-Assisted Stromal Lenticule Addition Keratoplasty for the Treatment of Advanced Keratoconus: A Preliminary Study. J. Refract. Surg. 2018, 34, 36–44. [Google Scholar]
- Mastropasqua, L.; Nubile, M. Corneal thickening and central flattening induced by femtosecond laser hyperopic-shaped intrastromal lenticule implantation. Int. Ophthalmol. 2017, 37, 893–904. [Google Scholar]
- Ganesh, S.; Brar, S. Femtosecond Intrastromal Lenticular Implantation Combined with Accelerated Collagen Cross-Linking for the Treatment of Keratoconus—Initial Clinical Result in 6 Eyes. Cornea 2015, 34, 1331–1339. [Google Scholar]
- Jadidi, K.; Mosavi, S.A. Keratoconus treatment using femtosecond-assisted intrastromal corneal graft (FAISCG) surgery: A case series. Int. Med. Case Rep. J. 2018, 11, 9–15. [Google Scholar]
- Pedrotti, E.; Cozzini, T.; Fasolo, A.; Bonacci, E.; Bonetto, J.; Merz, T.; Talli, P.; Marchini, G. Small-incision lenticule addition in ex vivo model of ectatic human corneas. Int. Ophthalmol. 2019, 39, 2575–2581. [Google Scholar]
- Mastropasqua, L.; Salgari, N.; D’Ugo, E.; Lanzini, M.; Alió, D.B.J.L.; Alió, J.L.; Cochener, B.; Nubile, M. In Vivo Confocal Microscopy of Stromal Lenticule Addition Keratoplasty for Advanced Keratoconus. J. Refract. Surg. 2020, 36, 544–550. [Google Scholar]
- Jin, H.; He, M.; Liu, H.; Zhong, X.; Wu, J.; Liu, L.; Ding, H.; Zhang, C.; Zhong, X. Small-Incision Femtosecond Laser-Assisted Intracorneal Concave Lenticule Implantation in Patients with Keratoconus. Cornea 2019, 38, 446–453. [Google Scholar]
- Doroodgar, F.; Jabbarvand, M.; Niazi, S.; Karimian, F.; Niazi, F.; Sanginabadi, A.; Ghoreishi, M.; Alinia, C.; Hashemi, H.; Alió, J.L. Customized Stromal Lenticule Implantation for Keratoconus. J. Refract. Surg. 2020, 36, 786–794. [Google Scholar]
- Riau, A.K.; Htoon, H.M.; Alió, D.B.J.L.; Nubile, M.; El, Z.M.; Mastropasqua, L.; Alió, J.L.; Mehta, J.S. Femtosecond laser-assisted stromal keratophakia for keratoconus: A systemic review and meta-analysis. Int. Ophthalmol. 2021, 41, 1965–1979. [Google Scholar] [PubMed]
- Fan, Y.; Hong, Y.; Bao, H.; Huang, Y.; Zhang, P.; Zhu, D.; Jiang, Q.; Zuo, Y.; Swain, M.; Elsheikh, A.; et al. Biomechanical and histological changes associated with riboflavin ultraviolet-A-induced CXL with different irradiances in young human corneal stroma. Comput. Biol. Med. 2024, 178, 108607. [Google Scholar]
- Tan, B.U.; Purcell, T.L.; Torres, L.F.; Schanzlin, D.J. New surgical approaches to the management of keratoconus and post-LASIK ectasia. Trans. Am. Ophthalmol. Soc. 2006, 104, 212–220. [Google Scholar]
- Jiang, Y.; Li, Y.; Yang, S.; Lu, T.C. Tuck-in Lamellar keratoplasty with an lenticule obtained by small incision lenticule extraction for treatment of Post-LASIK Ectasia. Sci. Rep. 2017, 7, 17806. [Google Scholar]
- Li, M.; Zhao, F.; Li, M.; Knorz, M.C.; Zhou, X. Treatment of Corneal Ectasia by Implantation of an Allogenic Corneal Lenticule. J. Refract. Surg. 2018, 34, 347–350. [Google Scholar]
- Li, M.; Wei, R.; Yang, W.; Shang, J.; Fu, D.; Xia, F.; Choi, J.; Zhou, X. Femtosecond Laser-Assisted Allogenic Lenticule Implantation for Corneal Ectasia After LASIK: A 3-Year In Vivo Confocal Microscopic Investigation. J. Refract. Surg. 2020, 36, 714–722. [Google Scholar] [CrossRef]
- Deshmukh, R.; Stevenson, L.J.; Vajpayee, R. Management of corneal perforations: An update. Indian J. Ophthalmol. 2020, 68, 7–14. [Google Scholar]
- Pant, O.P.; Hao, J.L.; Zhou, D.D.; Pant, M.; Lu, C.W. Tectonic keratoplasty using small incision lenticule extraction-extracted intrastromal lenticule for corneal lesions. J. Int. Med. Res. 2020, 48, 300060519897668. [Google Scholar]
- Jhanji, V.; Young, A.L.; Mehta, J.S.; Sharma, N.; Agarwal, T.; Vajpayee, R.B. Management of corneal perforation. Surv. Ophthalmol. 2011, 56, 522–538. [Google Scholar]
- Yokogawa, H.; Kobayashi, A.; Yamazaki, N.; Masaki, T.; Sugiyama, K. Surgical therapies for corneal perforations: 10 years of cases in a tertiary referral hospital. Clin. Ophthalmol. 2014, 8, 2165–2170. [Google Scholar]
- Jiang, Y.; Li, Y.; Liu, X.W.; Xu, J. A Novel Tectonic Keratoplasty with Femtosecond Laser Intrastromal Lenticule for Corneal Ulcer and Perforation. Chin. Med. J. 2016, 129, 1817–1821. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, V.; Ganesh, S.; Brar, S.; Pandey, R. Application of the SMILE-Derived Glued Lenticule Patch Graft in Microperforations and Partial-Thickness Corneal Defects. Cornea 2016, 35, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Abd Elaziz, M.S.; Zaky, A.G.; El SaebaySarhan, A.R. Stromal lenticule transplantation for management of corneal perforations; One year results. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 1179–1184. [Google Scholar] [PubMed]
- Zhao, J.; Sun, L.; Shen, Y.; Tian, M.; Yao, P.; Zhou, X. Using Donor Lenticules Obtained Through SMILE for an Epikeratophakia Technique Combined with Phototherapeutic Keratectomy. J. Refract. Surg. 2016, 32, 840–845. [Google Scholar]
- Wan, Q.; Tang, J.; Han, Y.; Ye, H. Surgical treatment of corneal dermoid by using intrastromal lenticule obtained from small-incision lenticule extraction. Int. Ophthalmol. 2020, 40, 43–49. [Google Scholar]
- Jacob, S.; Narasimhan, S.; Agarwal, A.; Agarwal, A.; Ai, S. Combined interface tattooing and fibrin glue-assisted sutureless corneal resurfacing with donor lenticule obtained from small-incision lenticule extraction for limbal dermoid. J. Cataract Refract. Surg. 2017, 43, 1371–1375. [Google Scholar]
- Li, Z.; Cheng, Z.; Jia, Z.; Tang, Y. Treatment of Corneal Dermoid with Fibrin Glue Boned Multi-Layer Lenticules from Small Incision Lenticules Extraction Surgery: A Preliminary Study of Five Patients. Curr. Eye Res. 2024, 4, 1–16. [Google Scholar]
- Mutlu, S.N.; Evereklioglu, C.; Najafi, J.; Arda, H. Transparent intrastromal corneal lenticule obtained from SMILE surgery as a free graft for the treatment of primary pterygium: A pilot study. Am. J. Ophthalmol. Case Rep. 2023, 32, 101897. [Google Scholar]
- Pant, O.P.; Hao, J.L.; Zhou, D.D.; Wang, F.; Lu, C.W. A novel case using femtosecond laser-acquired lenticule for recurrent pterygium: Case report and literature review. J. Int. Med. Res. 2018, 46, 2474–2480. [Google Scholar]
- Jacob, S.; Dhawan, P.; Tsatsos, M.; Agarwal, A.; Narasimhan, S.; Kumar, A. Fibrin Glue-Assisted Closure of Macroperforation in Predescemetic Deep Anterior Lamellar Keratoplasty with a Donor Obtained from Small Incision Lenticule Extraction. Cornea 2019, 38, 775–779. [Google Scholar]
- Hu, S.S.; Ding, H.; Meng, X.Y.; Ouyang, B.W.; Yang, Z.D.; Chen, X.D.; Zhong, X.W. Treatment of superficial corneal opacities with corneal stromal lenticule obtained through SMILE surgery. Int. J. Ophthalmol. 2024, 17, 2221–2228. [Google Scholar] [CrossRef] [PubMed]
- Nagi, K.S.; Cumba, R.J.; Bell, N.P.; Blieden, L.S.; Chuang, A.Z.; Mankiewicz, K.A.; Nguyen, D.; Feldman, R.M. Short-Term Outcomes of KeraSys Patch Graft for Glaucoma Drainage Devices: A Case Series. J. Ophthalmol. 2013, 2013, 784709. [Google Scholar] [PubMed]
- Spierer, O.; Waisbourd, M.; Golan, Y.; Newman, H.; Rachmiel, R. Partial thickness corneal tissue as a patch graft material for prevention of glaucoma drainage device exposure. BMC Ophthalmol. 2016, 16, 20. [Google Scholar]
- Wang, Y.; Liu, J.; Huang, W.; Xu, Y.; Cheng, M.; Shen, Z. The best thickness of cornea graft from SMILE surgery as patch graft in glaucoma drainage implant surgery. Medicine 2021, 100, 25828. [Google Scholar]
- Wang, Y.; Li, X.; Huang, W.; Liu, J.; Xu, Y.; Chen, M.; Wang, Q. Partial thickness cornea tissue from small incision lenticule extraction: A novel patch graft in glaucoma drainage implant surgery. Medicine 2019, 98, 14500. [Google Scholar]
- Yin, Y.; Lu, Y.; Xiang, A.; Fu, Y.; Zhao, Y.; Li, Y.; Hu, T.; Du, K.; Hu, S.; Fu, Q.; et al. Comparison of the optical quality after SMILE and FS-LASIK for high myopia by OQAS and iTrace analyzer: A one-year retrospective study. BMC Ophthalmol. 2021, 21, 292. [Google Scholar] [CrossRef]
- García-Gareta, E.; Abduldaiem, Y.; Sawadkar, P.; Kyriakidis, C.; Lali, F.; Greco, K.V. Decellularised scaffolds: Just a framework? Current knowledge and future directions. J. Tissue Eng. 2020, 11, 2041731420942903. [Google Scholar]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef]
- Wilson, S.L.; Sidney, L.E.; Dunphy, S.E.; Rose, J.B.; Hopkinson, A. Keeping an eye on decellularized corneas: A review of methods, characterization and applications. J. Funct. Biomater. 2013, 4, 114–161. [Google Scholar] [CrossRef]
- Chen, Y.; Yin, Y.W.; Zhao, Y.; Wu, X.Y.; Young, K.; Song, W.T.; Xia, X.B.; Wen, D. Differentiation of human embryonic stem cells derived mesenchymal stem cells into corneal epithelial cells after being seeded on decellularized SMILE-derived lenticules. Int. J. Ophthalmol. 2019, 12, 717–724. [Google Scholar]
- Hong, H.; Huh, M.I.; Park, S.M.; Lee, K.P.; Kim, H.K.; Kim, D.S. Decellularized corneal lenticule embedded compressed collagen: Toward a suturable collagenous construct for limbal reconstruction. Biofabrication 2018, 10, 045001. [Google Scholar]
- Surovtseva, M.A.; Krasner, K.Y.; Kim, I.I.; Surovtsev, N.V.; Chepeleva, E.V.; Bondarenko, N.A.; Lykov, A.P.; Bgatova, N.P.; Alshevskaya, A.A.; Trunov, A.N.; et al. Reversed Corneal Fibroblasts Therapy Restores Transparency of Mouse Cornea After Injury. Int. J. Mol. Sci. 2024, 25, 7053. [Google Scholar] [CrossRef] [PubMed]
- Mastropasqua, L.; Nubile, M.; Acerra, G.; Detta, N.; Pelusi, L.; Lanzini, M.; Mattioli, S.; Santalucia, M.; Pietrangelo, L.; Allegretti, M.; et al. Bioengineered Human Stromal Lenticule for Recombinant Human Nerve Growth Factor Release: A Potential Biocompatible Ocular Drug Delivery System. Front. Bioeng. Biotechnol. 2022, 10, 887414. [Google Scholar]
- Hori, J.; Niederkorn, J.Y. Immunogenicity and immune privilege of corneal allografts. Chem. Immunol. Allergy 2007, 92, 290–299. [Google Scholar]
- Li, J.; Yu, Z.; Han, M.; Zeng, Q.; Zhang, Y.; Wei, S.; Wu, L.; Du, J.; Li, J.; Gao, J.; et al. Biochemical component analysis of human myopic corneal stroma using the Raman spectrum. Int. Ophthalmol. 2024, 44, 153. [Google Scholar]
- Oruz, O.; Şaker, D.; Şimşek, F.; Eroğlu, M.; Polat, S. Histochemical and ultrastructural evaluation of myopic corneal lenticules based on refractive error. Clin. Exp. Ophthalmol. 2024, 52, 704–712. [Google Scholar]
- Wu, W.; Song, Y.; Sun, M.; Li, Y.; Xu, Y.; Xu, M.; Yang, Y.; Li, S.; Zhang, F. Corneal metabolic biomarkers for moderate and high myopia in human. Exp. Eye Res. 2023, 237, 109689. [Google Scholar]
- Zeng, X.; Qi, P.; Li, M.; Cheng, Z.; Lin, L.; Liu, W.; Wang, Y.; Zhang, N. Nonlinear optical properties of human cornea measured by spectral domain Z-scan method. Opt. Express 2021, 29, 38870–38878. [Google Scholar]
- Rodriguez-Pozo, J.A.; Ramos-Lopez, J.F.; Gonzalez-Gallardo, M.C.; Campos, F.; Sanchez-Porras, D.; Oyonarte, S.; Oruezabal, R.I.; Campos, A.; Martin-Piedra, M.A.; Alaminos, M. Evaluation of myopic cornea lenticules. A histochemical and clinical correlation. Exp. Eye Res. 2020, 196, r108066. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Li, J.; Wu, Z.; Li, Y.; Wu, G.; Wei, S. The Preservation and Reuse of Lenticules Extracted via Small Incision Lenticule Extraction (SMILE): A Narrative Review. Bioengineering 2025, 12, 380. https://doi.org/10.3390/bioengineering12040380
Zhang Y, Li J, Wu Z, Li Y, Wu G, Wei S. The Preservation and Reuse of Lenticules Extracted via Small Incision Lenticule Extraction (SMILE): A Narrative Review. Bioengineering. 2025; 12(4):380. https://doi.org/10.3390/bioengineering12040380
Chicago/Turabian StyleZhang, Yaohua, Jing Li, Zhiqing Wu, Yong Li, Guoxi Wu, and Shengsheng Wei. 2025. "The Preservation and Reuse of Lenticules Extracted via Small Incision Lenticule Extraction (SMILE): A Narrative Review" Bioengineering 12, no. 4: 380. https://doi.org/10.3390/bioengineering12040380
APA StyleZhang, Y., Li, J., Wu, Z., Li, Y., Wu, G., & Wei, S. (2025). The Preservation and Reuse of Lenticules Extracted via Small Incision Lenticule Extraction (SMILE): A Narrative Review. Bioengineering, 12(4), 380. https://doi.org/10.3390/bioengineering12040380






