Predicting Hospitalization Length in Geriatric Patients Using Artificial Intelligence and Radiomics
Abstract
1. Introduction
1.1. Overview of COVID-19 and Prognostic Challenges
1.2. Summary of Existing Research
1.3. Research Objective and Hypotheses
1.4. Structure of the Paper
2. Materials and Methods
2.1. Participants
2.2. CT Image Segmentation
- Lung parenchyma extraction: In the first phase, lung parenchyma extraction has been conducted, using Advantage Workstation 4.2 (General Electric, Milwaukee, WI, USA). Images of extracted lung parenchyma and the original acquisitions were then exported in DICOM format and saved for further analysis. In this phase, the images were reviewed by a radiologist and a technician to assess the quality of acquisition and the correct extraction of parenchyma.
- Ground-glass opacity segmentation: The extracted lung images were converted into numerical matrices using Python (ver. 3.8.10), and voxels with Hounsfield unit (HU) values between −760 and −368 in lung parenchyma images were selected to identify GGO regions.
2.3. Image Pre-Processing and Feature Extraction
- Resampled to isotropic voxels of one millimeter per size using sitkBSpline for interpolation.
- Filtered through a wavelet filter—8 decompositions, applying all combinations of high- or low-pass filters in each of the three dimensions
- Filtered through Laplacian of Gaussian filter with 5 values of sigma ([1.0, 2.0, 3.0, 4.0, 5.0]).
2.4. Feature Reduction
2.5. Machine Learning Classification
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| AUC-ROC | Area Under Receiver Operating Characteristic |
| CFS | Clinical Frailty Scale |
| CHF | Congestive Heart Failure |
| CKD | Chronic Kidney Disease |
| COPD | Chronic Obstructive Pulmonary Disease |
| CT | Computed Tomography |
| ESD | Ensemble Subspace Discriminant |
| FIO2 | Inspiratory oxygen flow |
| FN | False Negative |
| FP | False Positive |
| GGO | Ground-Glass Opacities |
| LASSO | Last Absolute Shrinkage and Selection Operator |
| LoS | Length of Hospital Stay |
| LSVM | Linear Support Vector Machine |
| ML | Machine Learning |
| MNN | Medium Neural Network |
| PO2 | Partial Oxygen Pressure |
| TN | True Negative |
| TP | True Positive |
References
- Woolf, S.H.; Chapman, D.A.; Lee, J.H. COVID-19 as the leading cause of death in the united states. JAMA 2021, 325, 123–124. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: Summary of a report of 72,314 cases from the chinese center for disease control and prevention. JAMA 2020, 323, 1239–1242. [Google Scholar]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in china, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Lai, C.-C.; Shih, T.-P.; Ko, W.-C.; Tang, H.-J.; Hsueh, P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [PubMed]
- WHO COVID-19 Dashboard. Available online: https://covid19.who.int/ (accessed on 19 September 2022).
- Menachemi, N.; Dixon, B.E.; Wools-Kaloustian, K.K.; Yiannoutsos, C.-S.T.; Halverson, P.K. How many SARS-CoV-2–infected people require hospitalization using random sample testing to better inform preparedness efforts. J. Public Health Manag. Pract. 2021, 27, 246–250. [Google Scholar]
- Center for Disease Control and Prevention. Available online: https://www.cdc.gov/nchs/nvss/vsrr/covid_weekly/index.htm (accessed on 21 September 2022).
- Lai, C.-C.; Ko, W.-C.; Lee, P.-I.; Jean, S.-S.; Hsueh, P.-R. Extra-respiratory manifestations of COVID-19. Int. J. Antimicrob. Agents 2020, 56, 106024. [Google Scholar]
- Gill, T.M. The central role of prognosis in clinical decision making. JAMA 2012, 307, 199–200. [Google Scholar]
- Ai, T.; Yang, Z.; Hou, H.; Zhan, C.; Chen, C.; Lv, W.; Tao, Q.; Sun, Z.; Xia, L. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: A report of 1014 cases. Radiology 2020, 296, E32–E40. [Google Scholar] [PubMed]
- Fang, Y.; Zhang, H.; Xie, J.; Lin, M.; Ying, L.; Pang, P.; Ji, W. Sensitivity of chest CT for COVID-19: Comparison to RT-PCR. Radiology 2020, 296, E115–E117. [Google Scholar]
- Bai, H.X.; Hsieh, B.; Xiong, Z.; Halsey, K.; Choi, J.W.; Tran, T.M.L.; Pan, I.; Shi, L.-B.; Wang, D.-C.; Mei, J.; et al. Performance of radiologists in differentiating COVID-19 from non-COVID-19 viral pneumonia at chest CT. Radiology 2020, 296, E46–E54. [Google Scholar]
- Iancu, R.I.; Zară, A.D.; Mirestean, C.C.; Iancu, D.P.T. Radiomics in COVID-19: The time for (r) evolution has came. BioMed 2022, 2, 60–68. [Google Scholar] [CrossRef]
- Kumar, V.; Gu, Y.; Basu, S.; Berglund, A.; Eschrich, S.A.; Schabath, M.B.; Forster, K.; Aerts, H.J.W.; Dekker, A.; Fenstermacher, D.; et al. Radiomics: The process and the challenges. Magn. Reson. Imaging 2012, 30, 1234–1248. [Google Scholar] [CrossRef] [PubMed]
- Yip, S.S.F.; Aerts, H.J.W. Applications and limitations of radiomics. Phys. Med. Biol. 2016, 61, R150. [Google Scholar] [CrossRef]
- Mostafaei, S.; Abdollahi, H.; Dehkordi, S.K.; Shiri, I.; Razzaghdoust, A.; Moghaddam, S.H.Z.; Saadipoor, A.; Koosha, F.; Cheraghi, S.; Mahdavi, S.R. CT imaging markers to improve radiation toxicity prediction in prostate cancer radiotherapy by stacking regression algorithm. La Radiol. Med. 2020, 125, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Nazari, M.; Shiri, I.; Hajianfar, G.; Oveisi, N.; Abdollahi, H.; Deevband, M.R.; Oveisi, M.; Zaidi, H. Noninvasive fuhrman grading of clear cell renal cell carcinoma using computed tomography radiomic features and machine learning. La Radiol. Med. 2020, 125, 754–762. [Google Scholar] [CrossRef]
- Tandel, G.S.; Balestrieri, A.; Jujaray, T.; Khanna, N.N.; Saba, L.; Suri, J.S. Multiclass magnetic resonance imaging brain tumor classification using artificial intelligence paradigm. Comput. Biol. Med. 2020, 122, 103804. [Google Scholar]
- Molina, D.; Pérez-Beteta, J.; Martínez-González, A.; Martino, J.; Velásquez, C.; Arana, E.; Pérez-García, V.M. Influence of gray level and space discretization on brain tumor heterogeneity measures obtained from magnetic resonance images. Comput. Biol. Med. 2016, 78, 49–57. [Google Scholar] [CrossRef]
- Li, L.; Wang, K.; Ma, X.; Liu, Z.; Wang, S.; Du, J.; Tian, K.; Zhou, X.; Sun, K.; Lin, Y.; et al. Radiomic analysis of multiparametric magnetic resonance imaging for differentiating skull base chordoma and chondrosarcoma. Eur. J. Radiol. 2019, 118, 81–87. [Google Scholar] [CrossRef]
- Shiri, I.; Sorouri, M.; Geramifar, P.; Nazari, M.; Abdollahi, M.; Salimi, Y.; Khosravi, B.; Askari, D.; Aghaghazvini, L.; Hajianfar, G.; et al. Machine learning-based prognostic modeling using clinical data and quantitative radiomic features from chest CT images in COVID-19 patients. Comput. Biol. Med. 2021, 132, 104304. [Google Scholar] [CrossRef]
- Fu, L.; Li, Y.; Cheng, A.; Pang, P.; Shu, Z. A novel machine learning-derived radiomic signature of the whole lung differentiates stable from progressive COVID-19 infection: A retrospective cohort study. J. Thorac. imaging 2020, 35, 361. [Google Scholar]
- Yip, S.S.F.; Klanecek, Z.; Naganawa, S.; Kim, J.; Studen, A.; Rivetti, L.; Jeraj, R. Performance and robustness of machine learning-based radiomic COVID-19 severity prediction. medRxiv 2020. [Google Scholar] [CrossRef]
- Grasselli, G.; Greco, M.; Zanella, A.; Albano, G.; Antonelli, M.; Bellani, G.; Bonanomi, E.; Cabrini, L.; Carlesso, E.; Castelli, G.; et al. Risk Factors Associated with Mortality Among Patients with COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern Med. 2020, 180, 1345–1355, Erratum in JAMA Intern Med. 2021, 181, 1021. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; The Northwell COVID-19 Research Consortium; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; et al. Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059, Erratum in JAMA 2020, 323, 2098. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062, Erratum in Lancet 2020, 395, 1038. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shu, Z.; Fang, S.; Ding, Z.; Mao, D.; Cai, R.; Chen, Y.; Pang, P.; Gong, X. Mri-based radiomics nomogram to detect primary rectal cancer with synchronous liver metastases. Sci. Rep. 2019, 9, 3374. [Google Scholar] [CrossRef]
- Wu, S.; Shen, G.; Mao, J.; Gao, B. CT radiomics in predicting egfr mutation in non-small cell lung cancer: A single institutional study. Front. Oncol. 2020, 10, 542957. [Google Scholar]
- Huang, Y.-Q.; Liang, C.-H.; He, L.; Tian, J.; Liang, C.-S.; Chen, X.; Ma, Z.-L.; Liu, Z.-Y. Development and validation of a radiomics nomogram for preoperative prediction of lymph node metastasis in colorectal cancer. J. Clin. Oncol. 2016, 34, 2157–2164. [Google Scholar]
- Abdollahi, H.; Mostafaei, S.; Cheraghi, S.; Shiri, I.; Mahdavi, S.R.; Kazemnejad, A. Cochlea CT radiomics predicts chemoradiotherapy induced sensorineural hearing loss in head and neck cancer patients: A machine learning and multi-variable modelling study. Phys. Med. 2018, 45, 192–197. [Google Scholar]
- Zhu, C.; Huang, H.; Liu, X.; Chen, H.; Jiang, H.; Liao, C.; Pang, Q.; Dang, J.; Liu, P.; Lu, H. A clinical-radiomics nomogram based on computed tomography for predicting risk of local recurrence after radiotherapy in nasopharyngeal carcinoma. Front. Oncol. 2021, 11, 637687. [Google Scholar]
- Tateishi, S.; Matsui, H.; Konishi, S. Nonlinear regression modeling via the lasso-type regularization. J. Stat. Plan. Inference 2010, 140, 1125–1134. [Google Scholar]
- Hepp, T.; Schmid, M.; Gefeller, O.; Waldmann, E.; Mayr, A. Approaches to regularized regression—A comparison between gradient boosting and the lasso. Methods Inf. Med. 2016, 55, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Krafft, S.P.; Rao, A.; Stingo, F.; Briere, T.M.; Court, L.E.; Liao, Z.; Martel, M.K. The utility of quantitative CT radiomics features for improved prediction of radiation pneumonitis. Med. Phys. 2018, 45, 5317–5324. [Google Scholar] [CrossRef]
- Bergstra, J.; Bengio, Y. Random search for hyperparameter optimization. J. Mach. Learn. Res. 2012, 13, 281–305. [Google Scholar]
- Mathur, A.; Foody, G.M. Multiclass and binary svm classification: Implications for training and classification users. IEEE Geosci. Remote Sens. Lett. 2008, 5, 241–245. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, Y.; Li, M.; Liu, Y.; Wang, F.; Weng, Q.; You, R.; Cao, D. Machine-learning-based computed tomography radiomic analysis for histologic subtype classification of thymic epithelial tumours. Eur. J. Radiol. 2020, 126, 108929. [Google Scholar] [CrossRef]
- Tian, Y.; Feng, Y. RaSE: Random Subspace Ensemble Classification. J. Mach. Learn. Res. 2021, 22, 45. [Google Scholar]
- Wang, M.; Wu, D.; Liu, C.-H.; Li, Y.; Hu, J.; Wang, W.; Jiang, W.; Zhang, Q.; Huang, Z.; Bai, L.; et al. Predicting progression to severe covid-19 using the paint score. BMC Infect. Dis. 2022, 22, 498. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, L.; Milione, S.; Fascione, M.C.; Pafundi, P.C.; Altruda, C.; Di Caterino, M.; Monaco, L.; Reginelli, A.; Perrotta, F.; Porta, G.; et al. Relevance of lung ultrasound in the diagnostic algorithm of respiratory diseases in a real-life setting: A multicentre prospective study. Respirology 2020, 25, 535–542. [Google Scholar] [CrossRef]
- Perrotta, F.; Corbi, G.; Mazzeo, G.; Boccia, M.; Aronne, L.; D’Agnano, V.; Komici, K.; Mazzarella, G.; Parrella, R.; Bianco, A. COVID-19 and the elderly: Insights into pathogenesis and clinical decision-making. Aging Clin. Exp. Res. 2020, 32, 1599–1608. [Google Scholar] [CrossRef]
- Shiri, I.; Salimi, Y.; Pakbin, M.; Hajianfar, G.; Avval, A.H.; Sanaat, A.; Mostafaei, S.; Akhavanallaf, A.; Saberi, A.; Mansouri, Z.; et al. COVID-19 prognostic modeling using CT radiomic features and machine learning algorithms: Analysis of a multi-institutional dataset of 14, 339 patients. Comput. Biol. Med. 2022, 145, 105467. [Google Scholar] [CrossRef]

| Article | Shiri et al. [21] | Fu et al. [22] | Yip et al. [23] |
|---|---|---|---|
| Aim | Develop prognostic models for survival prediction in COVID-19 patients using clinical, radiomic data from chest CT | Investigating the performance and robustness of radiomics in predicting the severity of COVID-19 | Use radiomic signatures derived from whole-lung machine learning to assess the prognosis of patients with COVID-19 |
| Study design | Retrospective | Retrospective | Retrospective |
| Patients (n) | 152 | 1110 | 64 |
| Data | Clinical data, radiological scores, radiomic features from lungs and segmented lesions on CT scan | Chest CT images and severity classifications (mild, moderate, severe) | Clinical data and lung CT scans |
| Segmentation | Whole lung and infective lesion manually segmented | Whole lung segmented through watershed algorithm | Whole lung segmented through automatic AI software |
| Feature extraction | 130 features (first order, shape, GLCM, GLRLM, GLSZM, NGTDM, GLDM) | 107 unfiltered features (first order, shape, GLCM, GLRLM, GLSZM, NGTDM, GLDM) | Shape, GLCM, RLM, GLZSM) |
| ML Models | XGBoost | Logistic regression | Support vector machine |
| Results | Combined model (lung + lesion + clinical data) showed the best prognostic performance (AUC = 0.95, accuracy = 0.88, sensitivity = 0.88, specificity = 0.89) | For mild vs severe classification, AUCvalid ≈ 0.80, moderate vs severe prediction was less accurate (AUC ≈ 0.65) | For classifying between stable and progressive infection with AUC = 0.833, sensitivity = 80.95%, specificity = 74.42% |
| Total | LoS ≤ 14 days | LoS > 14 days | p | |
|---|---|---|---|---|
| n = 168 | n = 91 | n = 77 | ||
| Female gender, n (%) | 104 (61.9%) | 64 (70.3%) | 40 (51.9%) | 0.015 |
| Age, mean ± sd | 86.5 ± 6.4 | 86.3 ± 6.9 | 86.7 ± 5.7 | 0.727 |
| CFS categories, n (%) | 0.178 | |||
| 0–3 | 30 (17.9%) | 14 (15.4%) | 16 (20.8%) | |
| 4–7 | 89 (53.0%) | 48 (52.7%) | 41 (53.2%) | |
| 8–9 | 44 (26.2%) | 24 (26.4%) | 20 (26.0%) | |
| NA | 3 (2.9%) | 5 (5.5%) | 0 (0.0%) | |
| Comorbidities | ||||
| Infarction, n (%) | 15 (8.9%) | 4 (4.4%) | 11 (14.3%) | 0.025 |
| Dementia, n (%) | 57 (33.9%) | 32 (35.2%) | 25 (32.5%) | 0.713 |
| CKD, n (%) | 39 (23.2%) | 21 (23.1%) | 18 (23.4%) | 0.963 |
| Hypertension, n (%) | 111 (66.1%) | 53 (58.2%) | 58 (75.3%) | 0.020 |
| Stroke, n (%) | 20 (11.9%) | 7 (7.7%) | 13 (16.9%) | 0.067 |
| COPD, n (%) | 21 (12.5%) | 13 (14.3%) | 8 (10.4%) | 0.447 |
| Atrial fibrillation, n (%) | 49 (29.2%) | 25 (27.5%) | 24 (31.2%) | 0.599 |
| Cancer, n (%) | 37 (22%) | 16 (17.6%) | 21 (27.3%) | 0.131 |
| CHF, n (%) | 41 (24.4%) | 20 (22%) | 21 (27.3%) | 0.426 |
| Diabetes, n (%) | 39 (23.2%) | 20 (22%) | 19 (24.7%) | 0.680 |
| Treatments | ||||
| Number of drugs, mean ± sd | 2.7 ± 2.0 | 2.0 ± 1.4 | 3.4 ± 2.3 | <0.001 |
| Oxygen therapy, n (%) | 119 (70.8%) | 62 (68.1%) | 57 (74.0%) | 0.028 |
| Symptoms | ||||
| Cough, n (%) | 38 (22.6%) | 17 (18.7%) | 21 (27.3%) | 0.235 |
| Dyspnea, n (%) | 91 (54.2%) | 54 (59.3%) | 37 (48.1%) | 0.070 |
| Diarrhea, n (%) | 14 (8.3%) | 6 (6.6%) | 8 (10.4%) | 0.420 |
| Nausea, n (%) | 4 (2.4%) | 2 (2.2%) | 2 (2.6%) | 0.899 |
| Vomit, n (%) | 12 (7.1%) | 7 (7.7%) | 5 (6.5%) | 0.707 |
| Conjunctivitis, n (%) | 2 (1.2%) | 1 (1.1%) | 1 (1.3%) | 0.929 |
| Ageusia, n (%) | 1 (0.6%) | 0 (0%) | 1 (1.3%) | 0.286 |
| Anosmia, n (%) | 1(0.6%) | 0(0%) | 1(1.3%) | 0.286 |
| Emogas Analysis | ||||
| PO2, mean ± sd | 64.3 ± 13.9 | 63.8 ± 11.2 | 64.9 ± 16.4 | 0.621 |
| FIO2, mean ± sd | 36.7 ± 16.4 | 34.4 ± 15.5 | 39.1 ± 17.1 | 0.094 |
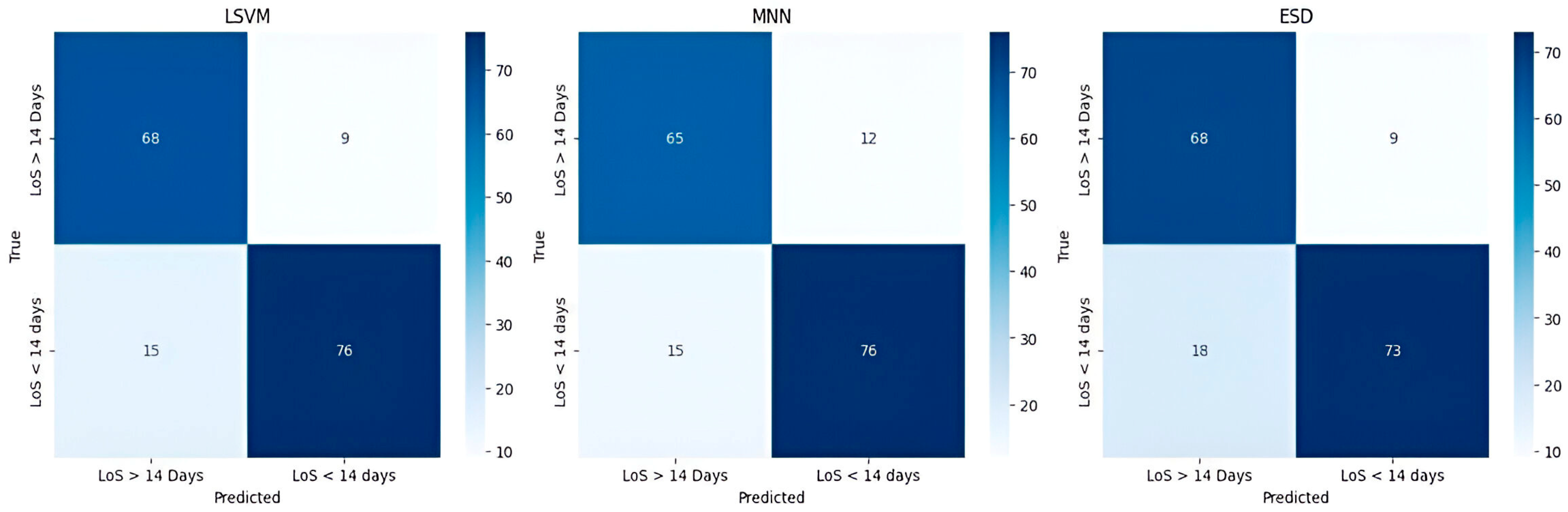
| LSVM Train/Test | MNN Train/Test | ESD Train/Test | |
|---|---|---|---|
| Accuracy (%) | 85.4/86.0 | 80.3/84.0 | 79.5/84.0 |
| Precision (%) | 82.2/84.6 | 79.3/84.0 | 75.4/81.5 |
| Sensitivity (%) | 89.5/88.0 | 80.7/84.0 | 86.0/88.0 |
| Specificity (%) | 81.784.0 | 80.0/84.0 | 73.3/80.1 |
| F1-score (%) | 85.7/86.2 | 80.0/84.0 | 80.3/84.6 |
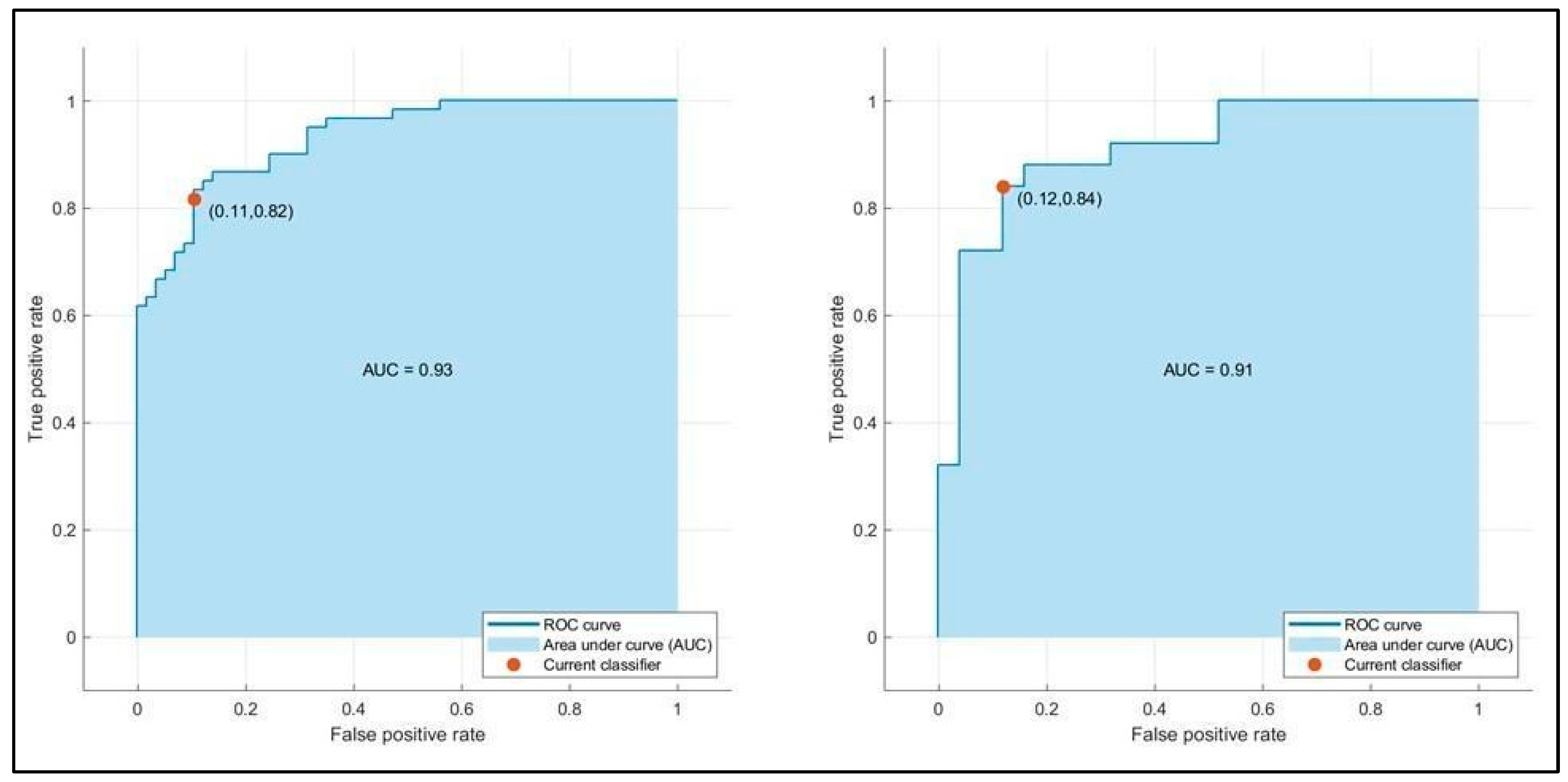
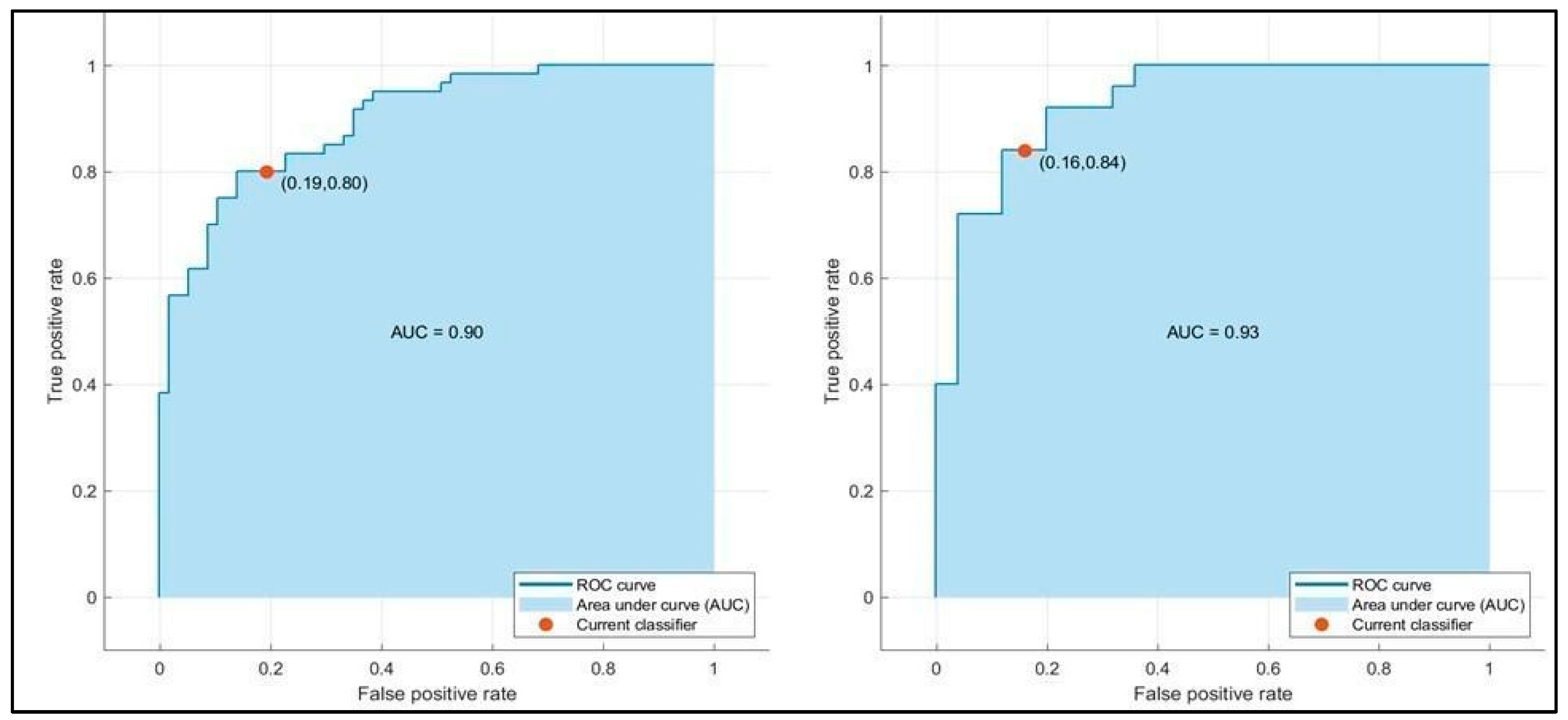
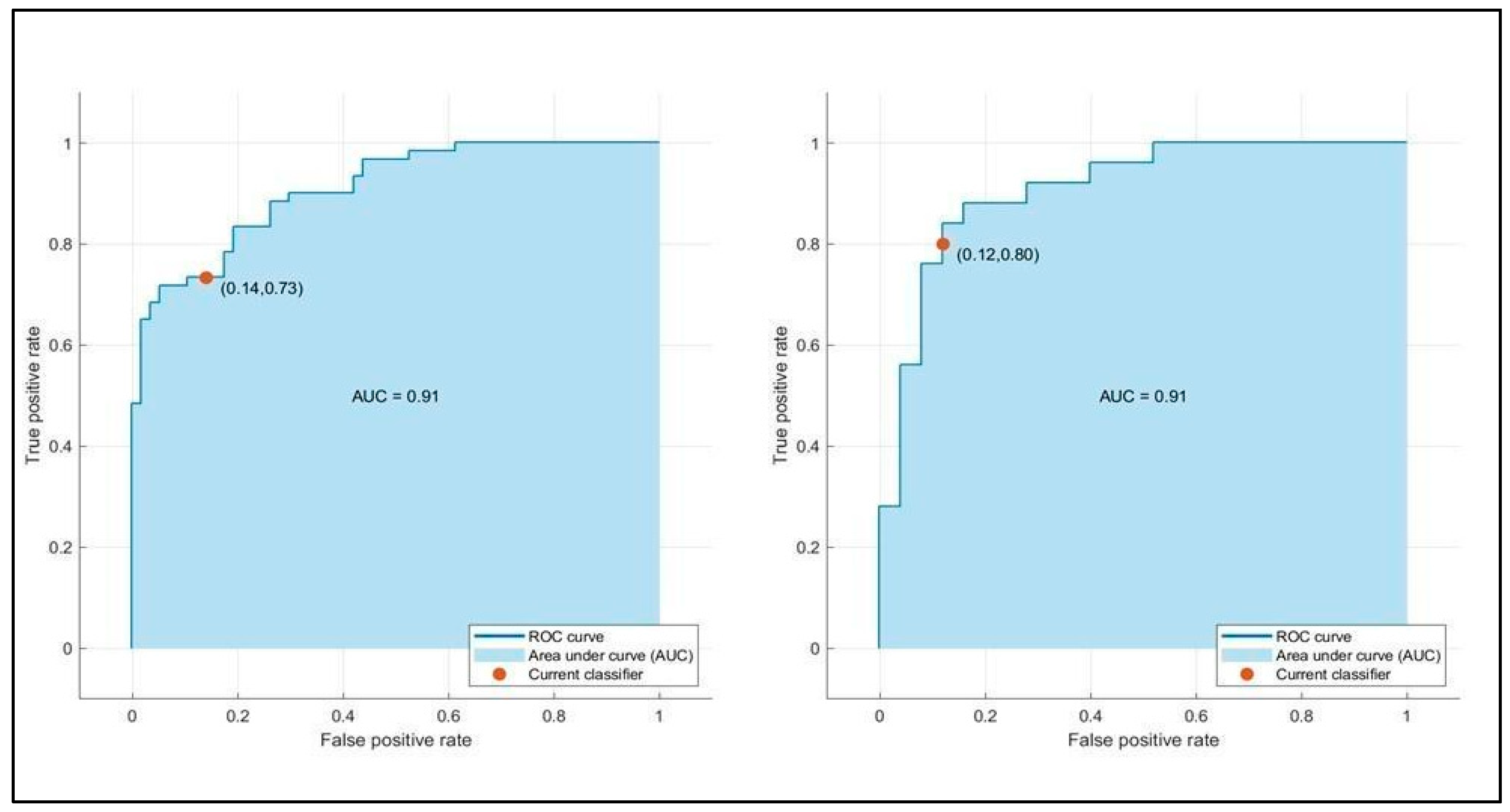
| AUC | 0.91/0.93 | 0.90/0.93 | 0.91/0.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fantechi, L.; Barbarossa, F.; Cecchini, S.; Zoppi, L.; Amabili, G.; Di Rosa, M.; Paci, E.; Fornarelli, D.; Bonfigli, A.R.; Lattanzio, F.; et al. Predicting Hospitalization Length in Geriatric Patients Using Artificial Intelligence and Radiomics. Bioengineering 2025, 12, 368. https://doi.org/10.3390/bioengineering12040368
Fantechi L, Barbarossa F, Cecchini S, Zoppi L, Amabili G, Di Rosa M, Paci E, Fornarelli D, Bonfigli AR, Lattanzio F, et al. Predicting Hospitalization Length in Geriatric Patients Using Artificial Intelligence and Radiomics. Bioengineering. 2025; 12(4):368. https://doi.org/10.3390/bioengineering12040368
Chicago/Turabian StyleFantechi, Lorenzo, Federico Barbarossa, Sara Cecchini, Lorenzo Zoppi, Giulio Amabili, Mirko Di Rosa, Enrico Paci, Daniela Fornarelli, Anna Rita Bonfigli, Fabrizia Lattanzio, and et al. 2025. "Predicting Hospitalization Length in Geriatric Patients Using Artificial Intelligence and Radiomics" Bioengineering 12, no. 4: 368. https://doi.org/10.3390/bioengineering12040368
APA StyleFantechi, L., Barbarossa, F., Cecchini, S., Zoppi, L., Amabili, G., Di Rosa, M., Paci, E., Fornarelli, D., Bonfigli, A. R., Lattanzio, F., Maranesi, E., & Bevilacqua, R. (2025). Predicting Hospitalization Length in Geriatric Patients Using Artificial Intelligence and Radiomics. Bioengineering, 12(4), 368. https://doi.org/10.3390/bioengineering12040368









