Advanced Artificial Intelligence Technologies Transforming Contemporary Pharmaceutical Research
Abstract
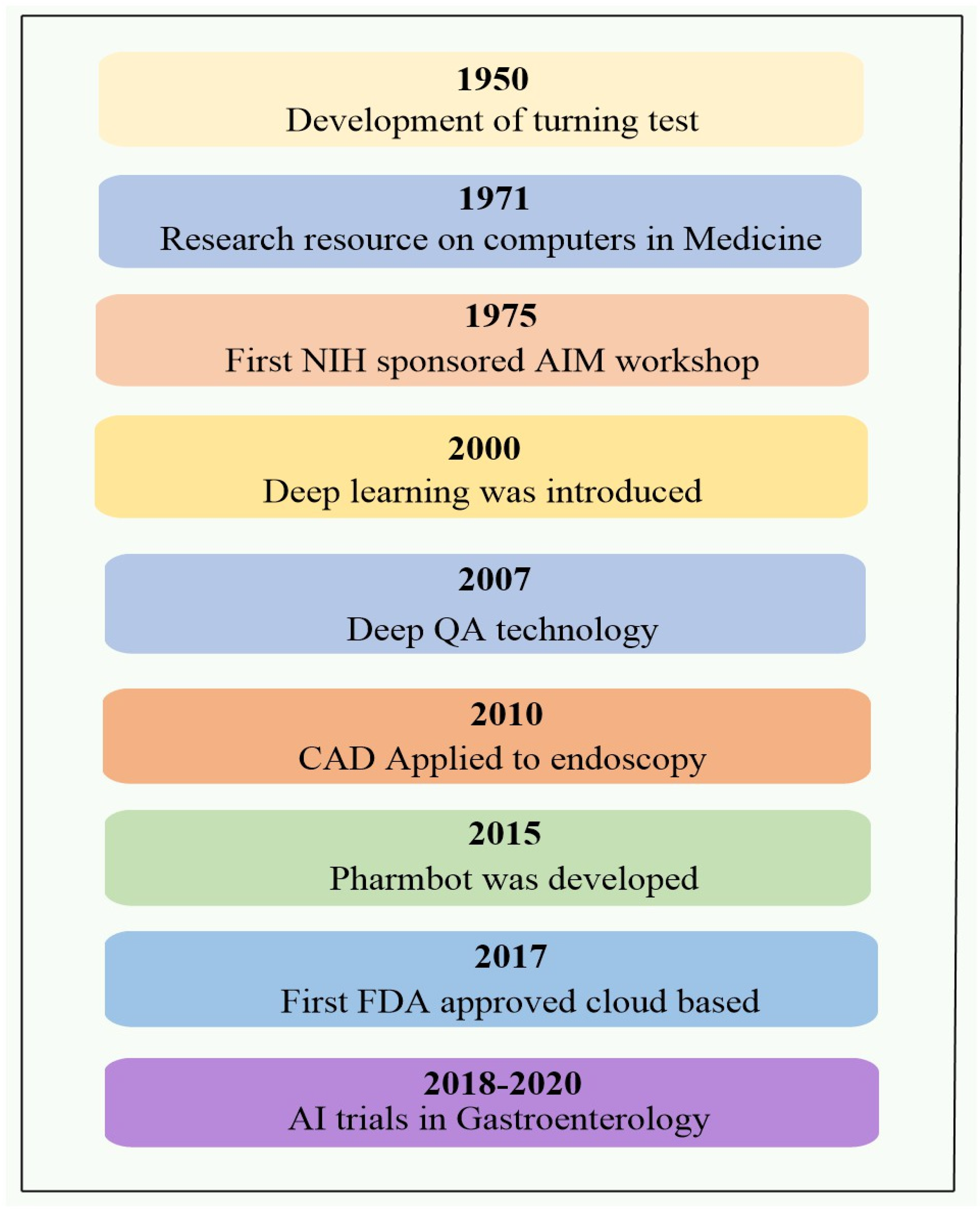
1. Introduction
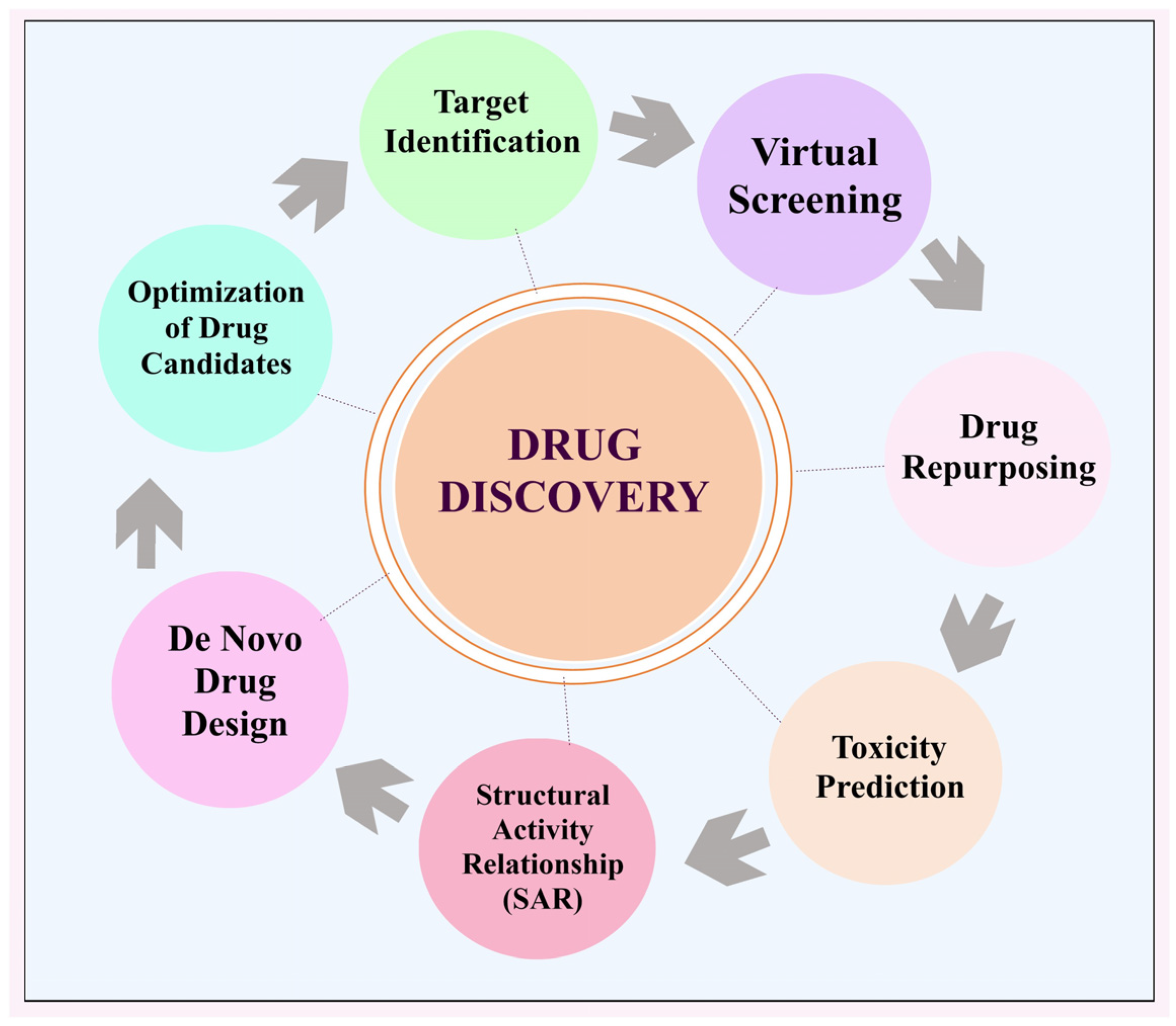
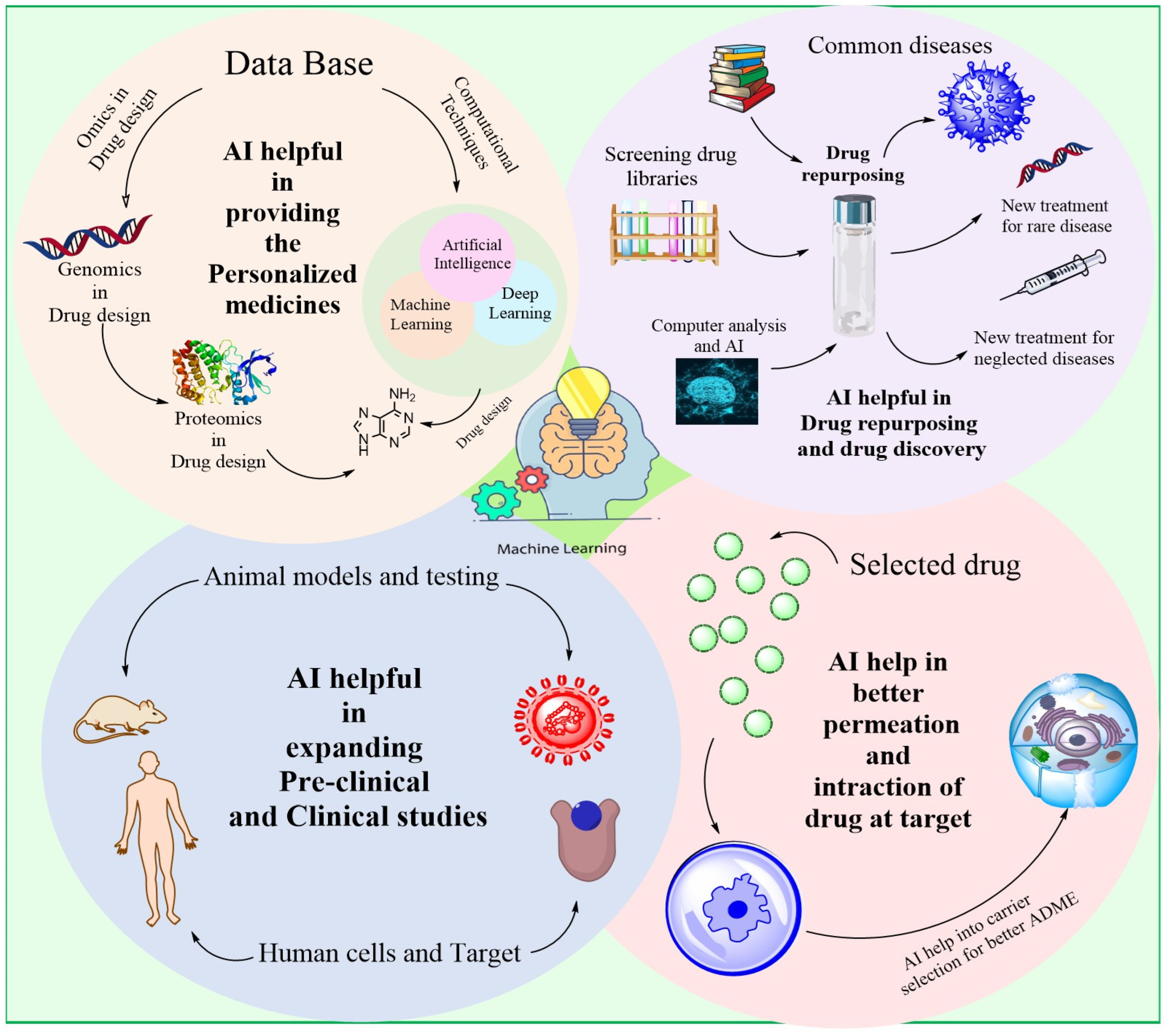
2. AI for Drug Discovery
2.1. Recognising Objectives
2.2. Screening via Simulation
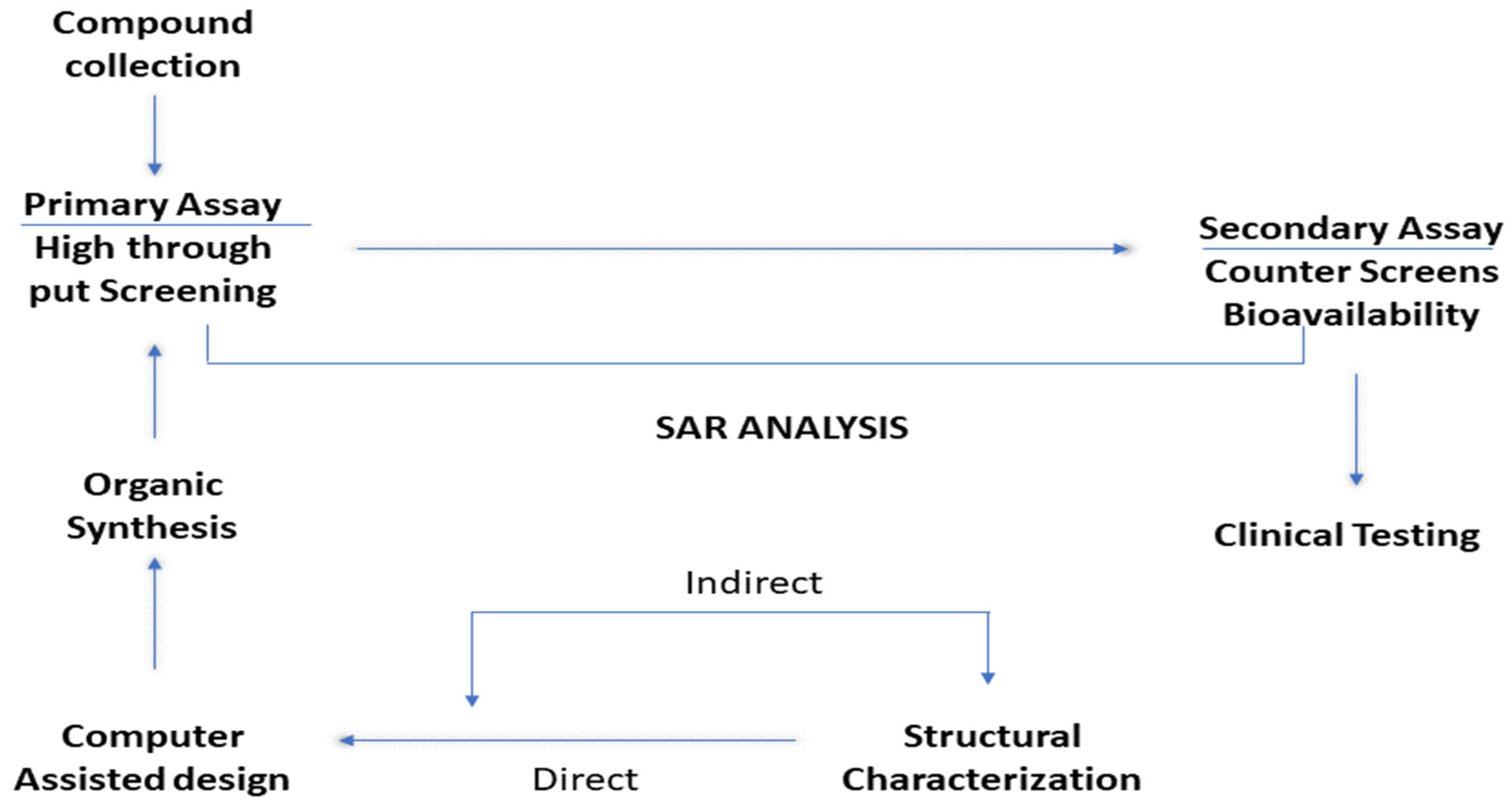
2.3. Relationship Between Structure and Activity (SAR)
2.4. Novel Drug Formulation
2.5. Improvement of Potential Medicines
2.6. Repurposing Pharmaceuticals
2.7. Predicting Toxic Effects
2.8. Artificial Intelligence Methods for Pharmaceutical Research
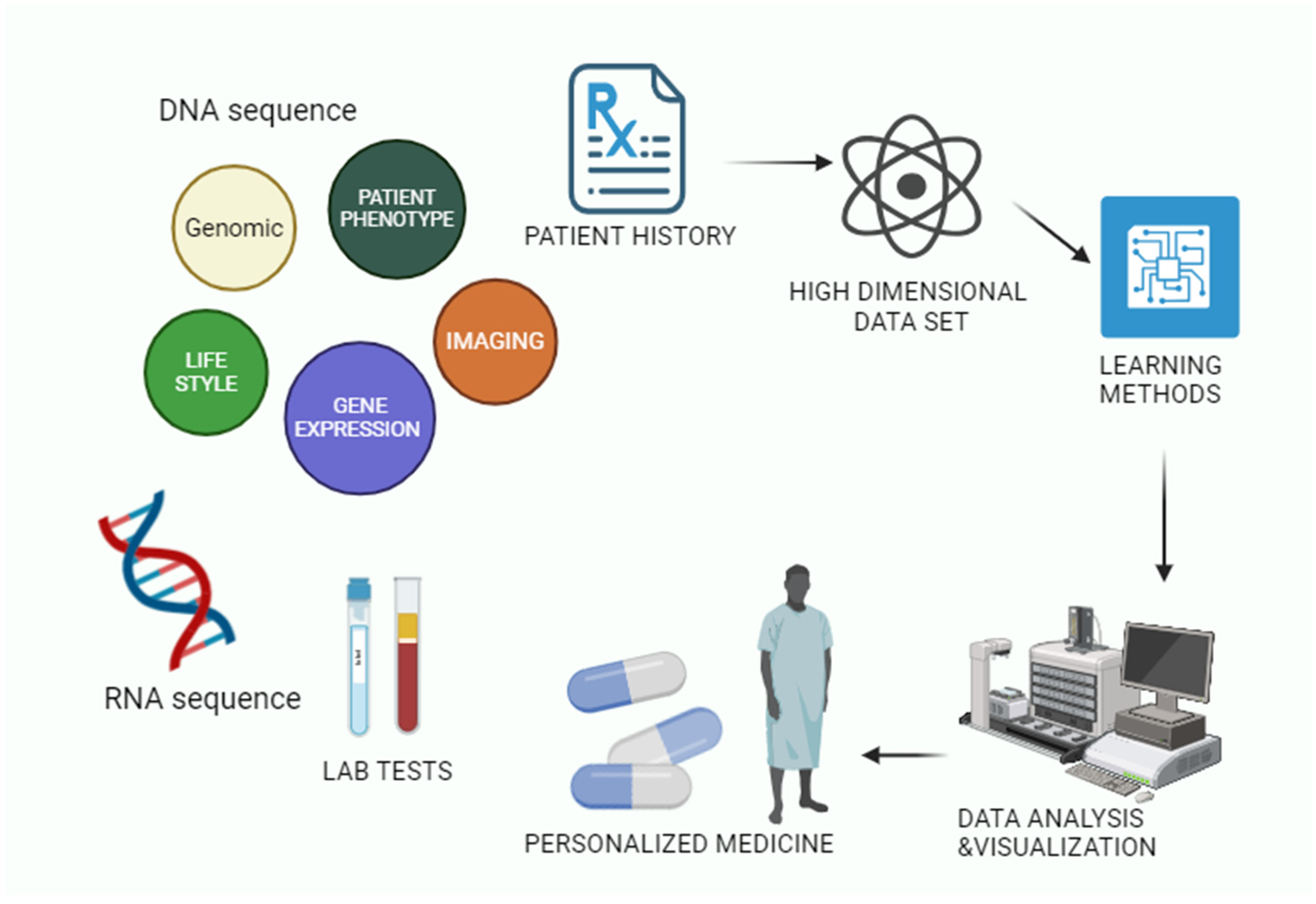
3. Artificial Intelligence in Individualised Computerised Therapy
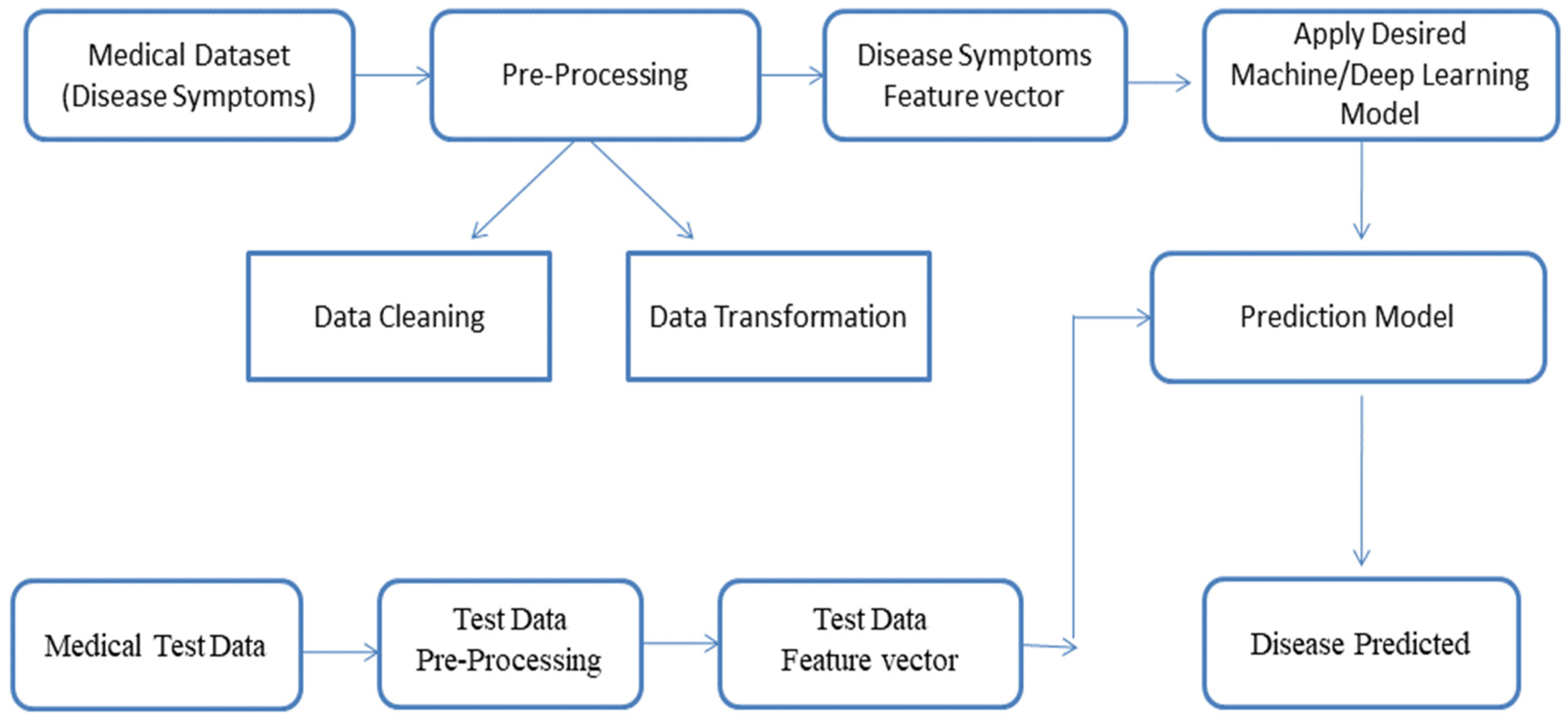
3.1. AI-Based Illness Prediction Modelling Framework
3.2. Diagnostic Imaging in Medicine
3.3. Optimization of Pharmaceutical Products
4. AI-Based Drug Delivery System
4.1. Microemulsions and Emulsions
4.2. Tablets
4.3. Beads, Microparticles, and Nanoparticles (Multiparticulates)
4.4. Application of AI Tools in the Design of Dosage Forms
5. Ai-Powered Resources for the Creation of Biologics
6. AI for Epidemic and Pandemic Forecasting
7. Intelligent Systems for Pharmacokinetics and Pharmacodynamics
7.1. Parameters for Drug Release and Absorption Prediction
7.2. Estimation of Metabolism and Excretion Indices
8. Hospital Pharmacy AI
9. Applications of AI to Polypharmacology
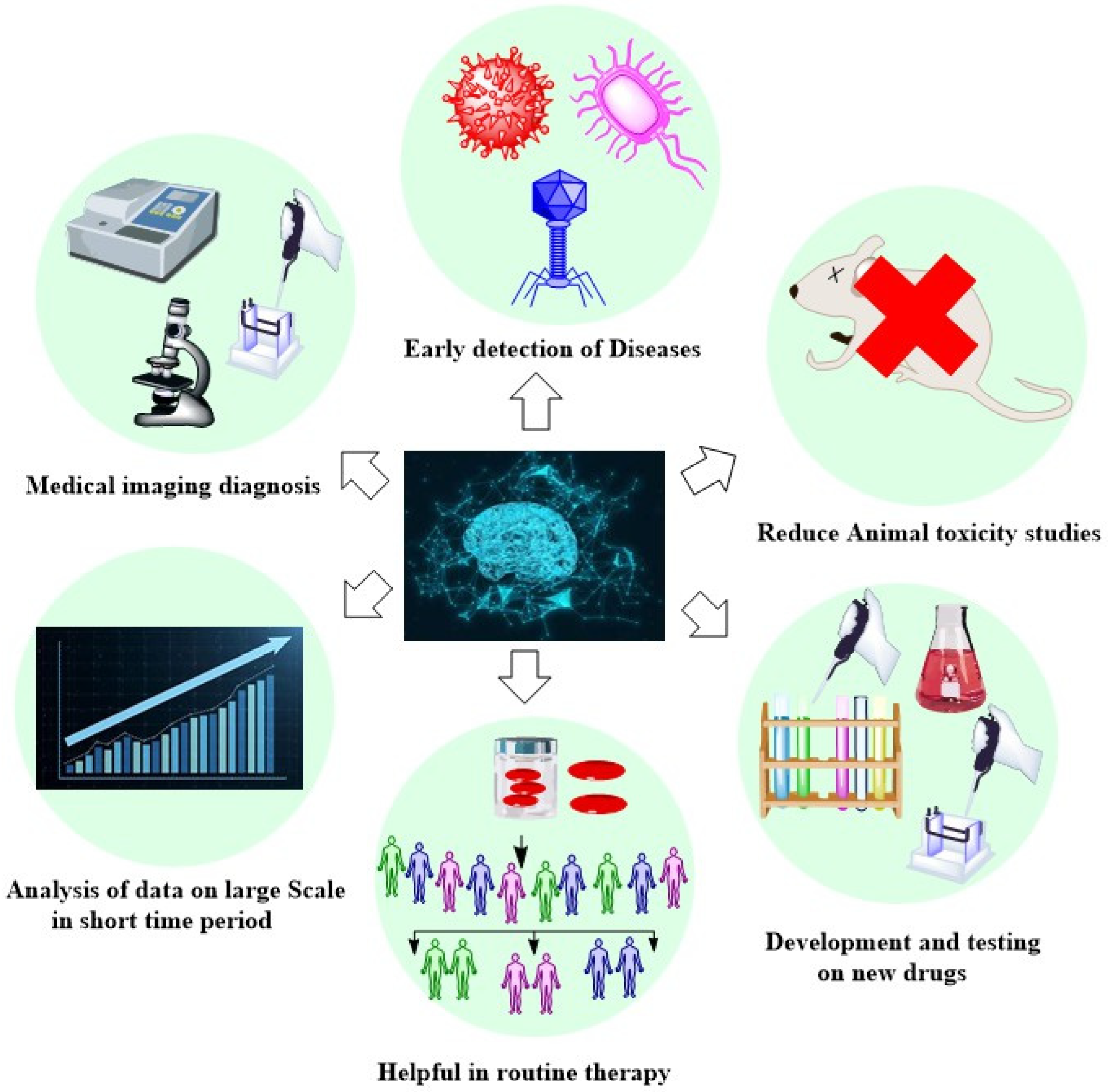
10. Benefits of Artificial Intelligence
11. Drawbacks Associated with AI Technology
12. The Constraints of AI Tools
13. The Significance of Explainability in Deep Learning Models, Particularly in the Medical Field
13.1. The Necessity of Regulation
13.2. The Necessity of Innovation
13.3. The Necessity of Advancement
14. Countering Adversarial Attacks and AI Evasion in Cybersecurity
14.1. Adversarial Attack
14.2. Current Mitigation Techniques
15. Innovational Models That Cater to the Needs of Individuals
16. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| FDA | Food and Drug Administration |
| GAN | Generative Adversarial Networks |
| RNN | Recurrent Neural Networks |
| CNN | Convolutional Neural Networks |
| LSTM | Long Short-Term Memory Networks |
| GNN | Graph Neural Networks |
| RL | Reinforcement Learning |
| DQN | Deep Q-Networks |
| SAR | Structure-Activity Relationship |
| TF | Target Fishing Technology |
| ANN | Artificial Neural Networks |
| FES | Fuzzy Expert Systems |
| CT | Computed Tomography |
| AMM | Anonymized Mobility Maps |
| VDPV | Vaccine-Derived Poliovirus |
| SVM | Support Vector Machine |
| MPNN | Multilayer Perceptron Neural Network Classifier |
| CMC | Composite Monte Carlo |
| CF | Corrective Feedback |
| PNN | Polynomial Neural Network |
| DNN | Deep Neural Networks |
References
- Dastha, J.F. Application of artificial intelligence to pharmacy and medicine. Hospital 1992, 27, 312–315, 319–322. [Google Scholar]
- Duch, W.; Swaminathan, K.; Meller, J. Artificial intelligence approaches for rational drug design and discovery. Curr. Pharm. Des. 2007, 13, 1497–1508. [Google Scholar]
- Scannell, J.W.; Blanckley, A.; Boldon, H.; Warrington, B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat. Rev. Drug Discov. 2012, 11, 191200. [Google Scholar]
- Munos, B. Lessons from 60 years of pharmaceutical innovation. Nat. Rev. Drug Discov. 2009, 8, 95968. [Google Scholar]
- Mak, K.K.; Pichika, M.R. Artificial intelligence in drug development: Present status and future prospects. Drug Discov. Today 2019, 24, 773–780. [Google Scholar] [PubMed]
- Times of India Pharmaceutical Supply Chain Management: Crises and Innovations. Available online: https://timesofindia.indiatimes.com/blogs/voices/pharmaceutical-supply-chain-management-crises-and-innovations/ (accessed on 15 May 2023).
- Sharma, R.; Shishodia, A.; Gunasekaran, A.; Min, H.; Munim, Z.H. The Role of Artificial Intelligence in Supply Chain Management: Mapping the Territory. Int. J. Prod. Res. 2022, 60, 7527–7550. [Google Scholar]
- Sousa, T.; Correia, J.; Pereira, V.; Rocha, M. Generative Deep Learning for Targeted Compound Design. J. Chem. Inf. Model. 2021, 61, 5343–5361. [Google Scholar] [PubMed]
- Rajalingham, R.; Piccato, A.; Jazayeri, M. Recurrent Neural Networks with Explicit Representation of Dynamic Latent Variables Can Mimic Behavioral Patterns in a Physical Inference Task. Nat. Commun. 2022, 13, 5865. [Google Scholar]
- Nag, S.; Baidya, A.T.K.; Mandal, A.; Mathew, A.T.; Das, B.; Devi, B.; Kumar, R. Deep Learning Tools for Advancing Drug Discovery and Development. 3 Biotech 2022, 12, 110. [Google Scholar]
- Liu, X.; Liu, C.; Huang, R.; Zhu, H.; Liu, Q.; Mitra, S.; Wang, Y. Long Short-Term Memory Recurrent Neural Network for Pharmacokinetic-Pharmacodynamic Modeling. Int. J. Clin. Pharmacol. Ther. 2021, 59, 138–146. [Google Scholar]
- Turchin, A.; Masharsky, S.; Zitnik, M. Comparison of BERT Implementations for Natural Language Processing of Narrative Medical Documents. Inform. Med. Unlocked 2023, 36, 101139. [Google Scholar]
- Huo, L.; Tang, Y. Multi-Objective Deep Reinforcement Learning for Personalized Dose Optimization Based on Multi-Indicator Experience Replay. Appl. Sci. 2022, 13, 325. [Google Scholar] [CrossRef]
- Olivier, A.; Shields, M.D.; Graham-Brady, L. Bayesian Neural Networks for Uncertainty Quantification in Data-Driven Materials Modeling. Comput. Methods Appl. Mech. Eng. 2021, 386, 114079. [Google Scholar]
- Magris, M.; Iosifidis, A. Bayesian Learning for Neural Networks: An Algorithmic Survey. Artif. Intell. Rev. 2023, 56, 11773–11823. [Google Scholar]
- Pham, T.-H.; Qiu, Y.; Zeng, J.; Xie, L.; Zhang, P. A Deep Learning Framework for High-Throughput Mechanism-Driven Phenotype Compound Screening and Its Application to COVID-19 Drug Repurposing. Nat. Mach. Intell. 2021, 3, 247–257. [Google Scholar]
- Meyers, J.; Fabian, B.; Brown, N. De Novo Molecular Design and Generative Models. Drug Discov. Today 2021, 26, 2707–2715. [Google Scholar]
- Khadela, A.; Popat, S.; Ajabiya, J.; Valu, D.; Savale, S.; Chavda, V.P. AI, ML and Other Bioinformatics Tools for Preclinical and Clinical Development of Drug Products. In Bioinformatics Tools for Pharmaceutical Drug Product Development; Wiley: Hoboken, NJ, USA, 2023; pp. 255–284. ISBN 978-1-119-86572-8. [Google Scholar]
- Koutroumpa, N.-M.; Papavasileiou, K.D.; Papadiamantis, A.G.; Melagraki, G.; Afantitis, A. A Systematic Review of Deep Learning Methodologies Used in the Drug Discovery Process with Emphasis on In Vivo Validation. Int. J. Mol. Sci. 2023, 24, 6573. [Google Scholar] [CrossRef]
- Rang, M.; Li, B.; Chen, H. Application of Message Passing Neural Networks for Molecular Property Prediction. Curr. Opin. Struct. Biol. 2023, 81, 102616. [Google Scholar]
- Reiser, P.; Neubert, M.; Eberhard, A.; Torresi, L.; Zhou, C.; Shao, C.; Metni, H.; van Hoesel, C.; Schopmans, H.; Sommer, T.; et al. Graph Neural Networks for Materials Science and Chemistry. Commun. Mater. 2022, 3, 93. [Google Scholar]
- Jenkins, J.L.; Bender, A.; Davies, J.W. In Silico Target Fishing: Predicting Biological Targets from Chemical Structure. Drug Discov. Today Technol. 2006, 3, 413–421. [Google Scholar]
- Afzal, A.M.; Mussa, H.Y.; Turner, R.E.; Bender, A.; Glen, R.C. A Multi-Label Approach to Target Prediction Taking Ligand Promiscuity into Account. J. Cheminform. 2015, 7, 24. [Google Scholar] [PubMed]
- Wang, L.; Xie, X.-Q. Computational Target Fishing: What Should Chemogenomics Researchers Expect for the Future of in Silico Drug Design and Discovery? Future Med. Chem. 2014, 6, 247–249. [Google Scholar] [PubMed]
- CIorio, F.; Bosotti, R.; Scacheri, E.; Belcastro, V.; Mithbaokar, P.; Ferriero, R.; Murino, L.; Tagliaferri, R.; Brunetti-Pierri, N.; Isacchi, A.; et al. Discovery of Drug Mode of Action and Drug Repositioning from Transcriptional Responses. Proc. Natl. Acad. Sci. USA 2010, 107, 14621–14626. [Google Scholar]
- Begam, B.F.; Kumar, J.S. A Study on Cheminformatics and Its Applications on Modern Drug Discovery. Procedia Eng. 2012, 38, 1264–1275. [Google Scholar]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug Repurposing: Progress, Challenges and Recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar]
- Nettles, J.H.; Jenkins, J.L.; Bender, A.; Deng, Z.; Davies, J.W.; Glick, M. Bridging Chemical and Biological Space: “Target Fishing” Using 2D and 3D Molecular Descriptors. J. Med. Chem. 2006, 49, 6802–6810. [Google Scholar]
- Galati, S.; Di Stefano, M.; Martinelli, E.; Poli, G.; Tuccinardi, T. Recent Advances in In Silico Target Fishing. Molecules 2021, 26, 5124. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, X.; Wang, Y.; Yu, Y.; Huang, N.; Li, G.; Li, X.; Wu, J.C.; Yang, S. Artificial intelligence in drug development. Nat. Med. 2025, 1–15. [Google Scholar]
- Hassanzadeh, P.; Atyabi, F.; Dinarvand, R. The significance of artificial intelligence in drug delivery system design. Adv. Drug Deliv. Rev. 2019, 151, 169–190. [Google Scholar]
- Lakdawala, A.S.; Okafo, G.; Baldoni, J.; Palovich, M.; Sikosek, T.; Sahni, V. Adapting drug discovery to artificial intelligence. Drug Target Rev. 2018, 50–52. [Google Scholar]
- Reymond, J.L.; Deursen, R.V.; Blum, L.C.; Ruddigkeit, L. Chemical space as a source for new drugs. Med. Chem. Commun. 2010, 1, 30–38. [Google Scholar]
- Ferrero, E.; Dunham, I.; Sanseau, P. In silico prediction of novel therapeutic targets using gene-disease association data. J. Transl. Med. 2017, 15, 182. [Google Scholar] [PubMed]
- Katsila, T.; Spyroulias, G.A.; Patrinos, G.P.; Matsoukas, M.T. Computational approaches in target identification and drug discovery. Comput. Struct. Biotechnol. J. 2016, 14, 177–184. [Google Scholar] [PubMed]
- Yuan, Y.; Pei, J.; Lai, L. Lig Builder 2: A practical de novo drug design approach. J. Chem. Inf. Model. 2011, 51, 1083–1091. [Google Scholar]
- Fleming, N. How artificial intelligence is changing drug discovery. Nature 2018, 557, S55–S57. [Google Scholar]
- Miha, S.; Jiménez, J.; Sabbadin, D.; De Fabritiis, G. Shape-based generative modeling for de novo drug design. J. Chem. Inf. Model. 2019, 59, 1205–1214. [Google Scholar]
- Merk, D.; Friedrich, L.; Grisoni, F.; Schneider, G. De novo Design of Bioactive Small Molecules by Artificial Intelligence Daniel. Mol. Inform. 2018, 37, 201700153. [Google Scholar]
- Blaschke, T.; Olivecrona, M.; Engkvist, O.; Bajorath, J.; Chen, H. Application of Generative Autoencoder in de nov Molecular Design. Mol. Inform. 2018, 37, 201700123. [Google Scholar]
- Gupta, A.; Müller, A.T.; Huisman, B.J.; Fuchs, J.A.; Schneider, P.; Schneider, G. Generative Recurrent Networks for de nov Drug Design. Mol. Inform. 2018, 37, 201700111. [Google Scholar]
- Zhang, X.; Wu, F.; Yang, N.; Zhan, X.; Liao, J.; Mai, S.; Huang, Z. In silico methods for identification of potential therapeutic targets. Interdiscip. Sci. Comput. Life Sci. 2022, 1–26. [Google Scholar]
- Muller, A.T.; Hiss, J.A.; Schneider, G. Recurrent Neural Network Model for Constructive Peptide Design. J. Chem. Inf. Model. 2018, 58, 472–479. [Google Scholar]
- Kadurin, A.; Nikolenko, S.; Khrabrov, K.; Aliper, A.; Zhavoronkov, A. druGAN: An advanced generative adversarial autoencoder model for de novo generation of new molecules with desired molecular properties in silico. Mol. Pharm. 2017, 14, 3098–3104. [Google Scholar]
- Ramesh, A.N.; Kambhampati, C.; Monson, J.R.; Drew, P.J. Artificial intelligence in medicine. Ann. R. Coll. Surg. Engl. 2004, 86, 334. [Google Scholar] [PubMed]
- Albu, A.; Ungureanu, L. Artificial neural network in medicine. Telemed. J. e-Health 2012, 18, 446–453. [Google Scholar]
- Hopfield, J.J. Artificial neural networks. IEEE Circuits Syst. Mag. 1988, 4, 3–10. [Google Scholar]
- Minaee, S.; Kafieh, R.; Sonka, M.; Yazdani, S.; Soufi, G. Deep-COVID: Predicting COVID-19 from chest X-ray images using deep transfer learning. Med. Image Anal. 2020, 65, 101794. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.; Singla, R. Federated learning systems for healthcare: Perspective and recent progress. In Federated Learning Systems; Studies in Computational, Intelligence; Rehman, M.H., Gaber, M.M., Eds.; Springer: Cham, Switzerland, 2021; Volume 965. [Google Scholar]
- Ali, M.; Tengnah, J.; Sooklall, R. A predictive model for hypertension diagnosis using machine learning techniques. In Telemedicine Technologies; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar] [CrossRef]
- Jo, T.; Nho, K.; Saykin, A.J. Deep learning in Alzheimer’s disease: Diagnostic classification and prognostic prediction using neuroimaging data. Front. Aging Neurosci. 2019, 11, 220. [Google Scholar] [CrossRef]
- Chen, P.; Gadepalli, K.; MacDonald, R.; Liu, Y.; Dean, J. An augmented reality microscope with real time artificial intelligence integration for cancer diagnosis. Nat. Med. 2019, 25, 1453–1457. [Google Scholar] [CrossRef]
- Chen, J.; Remulla, D.; Nguyen, J.; Aastha, D.; Liu, Y.; Dasgupta, P. Current status of artificial intelligence applications in urology and their potential to influence clinical practice. BJU Int. 2019, 124, 567–577. [Google Scholar] [CrossRef]
- Nasser, I.; Naser, S. Predicting tumor category using artificial neural network. Int. J. Acad. Health Med. Res. 2019, 3, 1–7. [Google Scholar]
- Sarao, V.; Veritti, D.; Paolo, L. Automated diabetic retinopathy detection with two different retinal imaging devices using artificial intelligence. Graefe’s Arch. Clin. Exp. Opthamol. 2020, 258, 2647–2654. [Google Scholar] [CrossRef]
- Keenan, T.; Clemons, T.; Domalpally, A.; Elman, M.; Havilio, M.; Agron, E.; Chew, E.; Benyamini, G. Retinal specialist versus artificial intelligence detection of retinal fluid from OCT: Age-related eye disease study 2: 10 year follow on study. Ophthalmology 2020, 128, 100–109. [Google Scholar] [CrossRef]
- Rajalakshmi, R.; Subashini, R.; Anjana, R.; Mohan, V. Automated diabetic retinopathy detection in smartphone-based fundus photography using artificial intelligence. Eye 2018, 32, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Bibault, J.; Xing, L. Screening for chronic obstructive pulmonary disease with artificial intelligence. Lancet Digit. Health 2020, 2, e216–e217. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sha, M.; Zhao, X.; Ma, J.; Ni, H.; Gao, W.; Ming, D. Automated detection of pathologic white matter alterations in Alzheimer’s disease using combined diffusivity and kurtosis method. Psychiatry Res. Neuroimaging 2017, 264, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Sanap, G.; Shenoy, S.; Kalyane, D.; Kalia, K.; Tekade, R.K. Artificial intelligence in drug discovery and development. Drug Discov. Today 2021, 26, 80–93. [Google Scholar] [CrossRef]
- Miles, J.C.; Walker, A.J. The potential application of artificial intelligence in transport. In IEE Proceedings-Intelligent Transport Systems; IET: London, UK, 2006; Volume 153, pp. 183–198. [Google Scholar]
- Yang, Y.; Siau, K. Qualitative research on marketing and sales in the artificial intelligence age. In Proceedings of the 13th Annual Conference of the Midwest United States Association for Information Systems (MWAIS), St. Louis, MO, USA, 17–18 May 2018. 2018 proceedings. [Google Scholar]
- Yildirim, O.; Gottwald, M.; Schüler, P.; Michel, M.C. Opportunities and challenges for drug development: Public–private partnerships, adaptive designs and big data. Front. Pharmacol. 2016, 7, 461. [Google Scholar] [CrossRef]
- Shah, N.; Patel, N.; Patel, K.R. A sequential review on intelligent drug delivery system. J. Pharm. Sci. Biosci. Res. 2013, 3, 158–162. [Google Scholar]
- Medarevic, D.P.; Kleinebudde, P.; Djuris, J.; Djuric, Z.; Ibric, S. Combined application of mixture experimental design and artificial neural networks in the solid dispersion development. Drug Dev. Ind. Pharm. 2016, 42, 389–402. [Google Scholar] [CrossRef]
- Barmpalexis, P.; Koutsidis, I.; Karavas, E.; Louk, D. Development of PVP/PEG mixtures as appropriate carriers for the preparation of drug solid dispersions by melt mixing technique and optimization of dissolution using artificial neural networks. Eur. J. Pharm. Biopharm. 2013, 85, 1219–1231. [Google Scholar] [CrossRef]
- Kumar, K.J.; Panpalia, G.M.; Priyadarshini, S. Application of artificial neural networks in optimizing the fatty alcohol concentration in the formulation of an O/W emulsion. Acta Pharm. 2011, 61, 249–256. [Google Scholar] [PubMed]
- Podlogar, F.; Šibanc, R.; Gašperlin, M. Evolutionary artificial neural networks as tools for predicting the internal structure of microemulsions. J. Pharm. Pharmaceut. Sci. 2008, 11, 67–76. [Google Scholar]
- Agatonovic-Kustrin, S.; Glass, B.D.; Wisch, M.H.; Alany, R.G. Prediction of a stable microemulsion formulation for the oral delivery of a combination of antitubercular drugs using ANN methodology. Pharm. Res. 2003, 20, 1760–1765. [Google Scholar] [PubMed]
- Petrovic, J.; Ibric, S.; Betz, G.; Duric, Z. Optimization of matrix tablets controlled drug release using Elman dynamic neural networks and decision trees. Int. J. Pharm. 2012, 428, 57–67. [Google Scholar] [PubMed]
- Mandal, U.; Gowda, V.; Ghosh, A.; Bose, A.; Bhaumik, U.; Chatterjee, B. Optimization of metformin HCl 500 mg sustained release matrix tablets using artificial neural network (ANN) based on multilayer perceptrons (MLP) model. Chem. Pharm. Bull. 2008, 56, 150–155. [Google Scholar]
- Barmpalexis, P.; Kanaze, F.I.; Kachrimanis, K.; Georgarakis, E. Artificial neural networks in the optimization of a nimodipine controlled release tablet formulation. Eur. J. Pharm. Biopharm. 2010, 74, 316–323. [Google Scholar]
- Zhang, Z.H.; Wang, Y.; Wu, W.F.; Zhao, X.; Sun, X.C.; Wang, H.Q. Development of glipizide push-pull osmotic pump controlled release tablets by using expert system and artificial neural network. Yao Xue Xue Bao 2012, 47, 1687–1695. [Google Scholar] [PubMed]
- Patel, A.; Mehta, T.; Patel, M.; Patel, K.; Patel, N. Design porosity osmotic tablet for delivering low and pH-dependent soluble drug using an artificial neural network. Curr. Drug Deliv. 2012, 9, 459–467. [Google Scholar] [CrossRef]
- Vaithiyalingam, S.; Khan, M.A. Optimization and characterization of controlled release multi-particulate beads formulated with customized cellulose acetate butyrate dispersion. Int. J. Pharm. 2002, 234, 179–193. [Google Scholar]
- Sankalia, M.G.; Mashru, R.C.; Sankalia, J.M.; Sutariya, V.B. Papain entrapment in alginate beads for stability improvement and site-specific delivery: Physicochemical characterization and factorial optimization using neural network modeling. AAPS Pharm. Sci. Tech. 2005, 6, E209–E222. [Google Scholar]
- Zhang, A.Y.; Fan, T.Y. Optimization of calcium alginate floating microspheres loading aspirin by artificial neural networks and response surface methodology. Beijing Da Xue Xue Bao Yi Xue Ban 2010, 42, 197–201. [Google Scholar] [PubMed]
- Labouta, H.I.; El-khordagui, L.K.; Molokhia, A.M.; Ghaly, G.M. Multivariate modelling of encapsulation and release of an ionizable drug from polymer microspheres. J. Pharm. Sci. 2009, 98, 4603–4615. [Google Scholar]
- Chavda, V.P. Nanotherapeutics and Nanobiotechnology. In Applications of Targeted Nano Drugs and Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–13. [Google Scholar]
- Colombo, S. Applications of Artificial Intelligence in Drug Delivery and Pharmaceutical Development. In Artificial Intelligence in Healthcare; Elsevier: Amsterdam, The Netherlands, 2020; pp. 85–116. [Google Scholar]
- Das, P.J.; Preuss, C.; Mazumder, B. Artificial Neural Network as Helping Tool for Drug Formulation and Drug Administration Strategies. In Artificial Neural Network for Drug Design, Delivery and Disposition; Elsevier: Amsterdam, The Netherlands, 2016; pp. 263–276. [Google Scholar]
- Bhhatarai, B.; Walters, W.P.; Hop, C.E.C.A.; Lanza, G.; Ekins, S. Opportunities and Challenges Using Artificial Intelligence in ADME/Tox. Nat. Mater. 2019, 18, 418–422. [Google Scholar] [PubMed]
- Siepmann, J.; Siepmann, F. Modeling of Diffusion Controlled Drug Delivery. J. Control. Release 2012, 161, 351–362. [Google Scholar] [PubMed]
- Yang, S.-Y.; Huang, Q.; Li, L.-L.; Ma, C.-Y.; Zhang, H.; Bai, R.; Teng, Q.-Z.; Xiang, M.-L.; Wei, Y.-Q. An Integrated Scheme for Feature Selection and Parameter Setting in the Support Vector Machine Modeling and Its Application to the Prediction of Pharmacokinetic Properties of Drugs. Artif. Intell. Med. 2009, 46, 155–163. [Google Scholar]
- Yu, L.X.; Ellison, C.D.; Hussain, A.S. Predicting Human Oral Bioavailability Using in Silico Models. In Applications of Pharmacokinetic Principles in Drug Development; Springer: Boston, MA, USA, 2004; pp. 53–74. [Google Scholar]
- Menden, M.P.; Iorio, F.; Garnett, M.; McDermott, U.; Benes, C.H.; Ballester, P.J.; Saez-Rodriguez, J. Machine Learning Prediction of Cancer Cell Sensitivity to Drugs Based on Genomic and Chemical Properties. PLoS ONE 2013, 8, e61318. [Google Scholar]
- Magill, E.; Demartis, S.; Gavini, E.; Dian Permana, A.; Raj Singh Thakur, R.; Faris Adrianto, M.; Waite, D.; Glover, K.; Picco, C.J.; Korelidou, A.; et al. Solid Implantable Devices for Sustained Drug Delivery. Adv. Drug Deliv. Rev. 2023, 199, 114950. [Google Scholar]
- Wang, N.; Zhang, Y.; Wang, W.; Ye, Z.; Chen, H.; Hu, G.; Ouyang, D. How Can Machine Learning and Multiscale Modeling Benefit Ocular Drug Development? Adv. Drug Deliv. Rev. 2023, 196, 114772. [Google Scholar]
- Vora, L.K.; Moffatt, K.; Tekko, I.A.; Paredes, A.J.; Volpe-Zanutto, F.; Mishra, D.; Peng, K.; Raj Singh Thakur, R.; Donnelly, R.F. Microneedle Array Systems for Long-Acting Drug Delivery. Eur. J. Pharm. Biopharm. 2021, 159, 44–76. [Google Scholar] [CrossRef]
- Wu, Y.; Vora, L.K.; Mishra, D.; Adrianto, M.F.; Gade, S.; Paredes, A.J.; Donnelly, R.F.; Singh, T.R.R. Nanosuspension-Loaded Dissolving Bilayer Microneedles for Hydrophobic Drug Delivery to the Posterior Segment of the Eye. Biomater. Adv. 2022, 137, 212767. [Google Scholar] [CrossRef]
- Bagde, A.; Dev, S.; Madhavi, K.; Sriram, L.; Spencer, S.D.; Kalvala, A.; Nathani, A.; Salau, O.; Mosley-Kellum, K.; Dalvaigari, H.; et al. Biphasic Burst and Sustained Transdermal Delivery in Vivo Using an AI-Optimized 3D-Printed MN Patch. Int. J. Pharm. 2023, 636, 122647. [Google Scholar]
- Chavda, V.P.; Vora, L.K.; Pandya, A.K.; Patravale, V.B. Intranasal Vaccines for SARS-CoV-2: From Challenges to Potential in COVID-19 Management. Drug Discov. Today 2021, 26, 2619–2636. [Google Scholar] [PubMed]
- Chavda, V.P.; Pandya, R.; Apostolopoulos, V. DNA Vaccines for SARS-CoV-2: Toward Third-Generation Vaccination Era. Expert Rev. Vaccines 2021, 20, 1549–1560. [Google Scholar] [PubMed]
- Chavda, V.P.; Prajapati, R.; Lathigara, D.; Nagar, B.; Kukadiya, J.; Redwan, E.M.; Uversky, V.N.; Kher, M.N.; Patel, R. Therapeutic Monoclonal Antibodies for COVID-19 Management: An Update. Expert Opin. Biol. Ther. 2022, 22, 763–780. [Google Scholar]
- Chavda, V.P.; Soni, S.; Vora, L.K.; Soni, S.; Khadela, A.; Ajabiya, J. MRNA-Based Vaccines and Therapeutics for COVID-19 and Future Pandemics. Vaccines 2022, 10, 2150. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Dawre, S.; Pandya, A.; Vora, L.K.; Modh, D.H.; Shah, V.; Dave, D.J.; Patravale, V. Lyotropic Liquid Crystals for Parenteral Drug Delivery. J. Control. Release 2022, 349, 533–549. [Google Scholar] [CrossRef]
- Chavda, V.P.; Pandya, A.; Kumar, L.; Raval, N.; Vora, L.K.; Pulakkat, S.; Patravale, V.; Salwa, D.Y.; Tang, B.Z. Exosome Nanovesicles: A Potential Carrier for Therapeutic Delivery. Nano Today 2023, 49, 101771. [Google Scholar]
- Huang, Z.; Chavda, V.P.; Bezbaruah, R.; Uversky, V.N.; Palagati, S.; Patel, A.B.; Chen, Z.-S. An Ayurgenomics Approach: Prakriti-Based Drug Discovery and Development for Personalized Care. Front. Pharmacol. 2022, 13, 866827. [Google Scholar] [CrossRef]
- Chavda, V.P.; Balar, P.C.; Teli, D.; Davidson, M.; Bojarska, J.; Apostolopoulos, V. Antibody–Biopolymer Conjugates in Oncology: A Review. Molecules 2023, 28, 2605. [Google Scholar] [CrossRef]
- Chavda, V.P.; Bezbaruah, R.; Dolia, S.; Shah, N.; Verma, S.; Savale, S.; Ray, S. Convalescent Plasma (Hyperimmune Immunoglobulin) for COVID-19 Management: An Update. Process Biochem. 2023, 127, 66–81. [Google Scholar] [CrossRef]
- Kabra, R.; Singh, S. Evolutionary Artificial Intelligence Based Peptide Discoveries for Effective COVID-19 Therapeutics. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2021, 1867, 165978. [Google Scholar]
- Akbar, R.; Bashour, H.; Rawat, P.; Robert, P.A.; Smorodina, E.; Cotet, T.-S.; Flem-Karlsen, K.; Frank, R.; Mehta, B.B.; Vu, M.H.; et al. Progress and Challenges for the Machine Learning-Based Design of Fit-for-Purpose Monoclonal Antibodies. MAbs 2022, 14, 2008790. [Google Scholar] [PubMed]
- Sharma, A.; Virmani, T.; Pathak, V.; Sharma, A.; Pathak, K.; Kumar, G.; Pathak, D. Artificial Intelligence-Based Data-Driven Strategy to Accelerate Research, Development, and Clinical Trials of COVID Vaccine. BioMed Res. Int. 2022, 2022, 7205241. [Google Scholar]
- Dhakal, A.; McKay, C.; Tanner, J.J.; Cheng, J. Artificial Intelligence in the Prediction of Protein–Ligand Interactions: Recent Advances and Future Directions. Brief. Bioinform. 2022, 23, bbab476. [Google Scholar]
- Vishnoi, S.; Matre, H.; Garg, P.; Pandey, S.K. Artificial Intelligence and Machine Learning for Protein Toxicity Prediction Using Proteomics Data. Chem. Biol. Drug Des. 2020, 96, 902–920. [Google Scholar]
- Huang, Z.; Chavda, V.P.; Bezbaruah, R.; Dhamne, H.; Yang, D.-H.; Zhao, H.-B. CAR T-Cell Therapy for the Management of Mantle Cell Lymphoma. Mol. Cancer 2023, 22, 67. [Google Scholar]
- Madhav, N.; Oppenheim, B.; Gallivan, M.; Mulembakani, P.; Rubin, E.; Wolfe, N. Pandemics: Risks, Impacts, and Mitigation. In Disease Control Priorities: Improving Health and Reducing Poverty, 3rd ed.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2017; Volume 9, pp. 315–345. [Google Scholar]
- Steele, L.; Orefuwa, E.; Bino, S.; Singer, S.R.; Lutwama, J.; Dickmann, P. Earlier Outbreak Detection—A Generic Model and Novel Methodology to Guide Earlier Detection Supported by Data from Low- and Mid-Income Countries. Front. Public Health 2020, 8, 452. [Google Scholar]
- Gopinath, N. Artificial intelligence: Potential tool to subside SARS-CoV-2 pandemic. Process Biochem. 2021, 110, 94–99. [Google Scholar]
- Burke, R.M.; Shah, M.P.; Wikswo, M.E.; Barclay, L.; Kambhampati, A.; Marsh, Z.; Cannon, J.L.; Parashar, U.D.; Vinjé, J.; Hall, A.J. The Norovirus Epidemiologic Triad: Predictors of Severe Outcomes in US Norovirus Outbreaks, 2009–2016. J. Infect. Dis. 2018, 219, 1364–1372. [Google Scholar]
- Carlson, C.J.; Dougherty, E.; Boots, M.; Getz, W.; Ryan, S.J. Consensus and conflict among ecological forecasts of Zika virus out-breaks in the United States. Sci. Rep. 2018, 8, 4921. [Google Scholar]
- Kleiven, E.F.; Henden, J.-A.; Ims, R.A.; Yoccoz, N.G. Seasonal difference in temporal transferability of an ecological model: Near-term predictions of lemming outbreak abundances. Sci. Rep. 2018, 8, 15252. [Google Scholar]
- OECD. Artificial Intelligence in Society; OECD: Paris, France, 2019; Volume 134. [Google Scholar]
- Su, K.; Xu, L.; Li, G.; Ruan, X.; Li, X.; Deng, P.; Li, X.; Li, Q.; Chen, X.; Xiong, Y.; et al. Forecasting influenza activity using self-adaptive AI model and multi-source data in Chongqing, China. Ebiomedicine 2019, 47, 284–292. [Google Scholar]
- Cheng, H.-Y.; Wu, Y.-C.; Lin, M.-H.; Liu, Y.-L.; Tsai, Y.-Y.; Wu, J.-H.; Pan, K.-H.; Ke, C.-J.; Chen, C.-M.; Liu, D.-P.; et al. Applying Machine Learning Models with An Ensemble Approach for Accurate Real-Time Influenza Forecasting in Taiwan: Development and Validation Study. J. Med. Internet Res. 2020, 22, e15394. [Google Scholar] [PubMed]
- Khan, M.A.; Abidi, W.U.H.; Al Ghamdi, M.A.; Almotiri, S.H.; Saqib, S.; Alyas, T.; Khan, K.M.; Mahmood, N. Forecast the influenza pandemic using machine learning. Comput. Mater. Contin. 2021, 66, 331–340. [Google Scholar]
- Venkatramanan, S.; Sadilek, A.; Fadikar, A.; Barrett, C.L.; Biggerstaff, M.; Chen, J.; Dotiwalla, X.; Eastham, P.; Gipson, B.; Higdon, D.; et al. Forecasting Influenza Activity Using Machine-Learned Mobility Map. Nat. Commun. 2021, 12, 726. [Google Scholar]
- Soni, U.; Gupta, N.; Sakshi. An Artificial Intelligence Approach for Forecasting Ebola Disease. J. Phys. Conf. Ser. 2021, 1950, 012038. [Google Scholar]
- Zhang, P.; Chen, B.; Ma, L.; Li, Z.; Song, Z.; Duan, W.; Qiu, X. The Large Scale Machine Learning in an Artificial Society: Prediction of the Ebola Outbreak in Beijing. Comput. Intell. Neurosci. 2015, 2015, 531650. [Google Scholar]
- Akhtar, M.; Kraemer, M.U.G.; Gardner, L.M. A dynamic neural network model for predicting risk of Zika in real time. BMC Med. 2019, 17, 171. [Google Scholar]
- Cui, P.; Wang, S. Application of Microfluidic Chip Technology in Pharmaceutical Analysis: A Review. J. Pharm. Anal. 2019, 9, 238–247. [Google Scholar]
- Tuntland, T.; Ethell, B.; Kosaka, T.; Blasco, F.; Zang, R.X.; Jain, M.; Gould, T.; Hoffmaster, K. Implementation of Pharmacokinetic and Pharmacodynamic Strategies in Early Research Phases of Drug Discovery and Development at Novartis Institute of Biomedical Research. Front. Pharmacol. 2014, 5, 174. [Google Scholar]
- Mager, D.E.; Woo, S.; Jusko, W.J. Scaling Pharmacodynamics from In Vitro and Preclinical Animal Studies to Humans. Drug Metab. Pharmacokinet. 2009, 24, 16–24. [Google Scholar] [CrossRef]
- Alsultan, A.; Alghamdi, W.A.; Alghamdi, J.; Alharbi, A.F.; Aljutayli, A.; Albassam, A.; Almazroo, O.; Alqahtani, S. Clinical Pharmacology Applications in Clinical Drug Development and Clinical Care: A Focus on Saudi Arabia. Saudi Pharm. J. 2020, 28, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Keutzer, L.; You, H.; Farnoud, A.; Nyberg, J.; Wicha, S.G.; Maher-Edwards, G.; Vlasakakis, G.; Moghaddam, G.K.; Svensson, E.M.; Menden, M.P.; et al. Machine Learning and Pharmacometrics for Prediction of Pharmacokinetic Data: Differences, Similarities and Challenges Illustrated with Rifampicin. Pharmaceutics 2022, 14, 1530. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Ertas, Y.N.; Walhekar, V.; Modh, D.; Doshi, A.; Shah, N.; Anand, K.; Chhabria, M. Advanced Computational Methodologies Used in the Discovery of New Natural Anticancer Compounds. Front. Pharmacol. 2021, 12, 702611. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedula, A.; Calad-Thomson, S.; Liu, C.; Sale, M.; Gattu, N.; Goyal, N. Artificial Intelligence and Pharmacometrics: Time to Embrace, Capitalize, and Advance? CPT Pharmacomet. Syst. Pharmacol. 2019, 8, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Shah, M. Artificial Intelligence and Machine Learning in Drug Discovery and Development. Intell. Med. 2022, 2, 134–140. [Google Scholar] [CrossRef]
- Obrezanova, O. Artificial Intelligence for Compound Pharmacokinetics Prediction. Curr. Opin. Struct. Biol. 2023, 79, 102546. [Google Scholar] [CrossRef]
- Mhatre, S.; Shukla, S.; Chavda, V.P.; Gandikota, L.; Patravale, V. AI and ML for Development of Cell and Gene Therapy for Personalized Treatment. In Bioinformatics Tools for Pharmaceutical Drug Product Development; Wiley: Hoboken, NJ, USA, 2023; pp. 371–400. ISBN 978-1-119-86572-8. [Google Scholar]
- Chou, W.-C.; Lin, Z. Machine Learning and Artificial Intelligence in Physiologically Based Pharmacokinetic Modeling. Toxicol. Sci. 2023, 191, 1–14. [Google Scholar] [CrossRef]
- Van Tran, T.T.; Tayara, H.; Chong, K.T. Artificial Intelligence in Drug Metabolism and Excretion Prediction: Recent Advances, Challenges, and Future Perspectives. Pharmaceutics 2023, 15, 1260. [Google Scholar] [CrossRef]
- Li, Y.; Meng, Q.; Yang, M.; Liu, D.; Hou, X.; Tang, L.; Wang, X.; Lyu, Y.; Chen, X.; Liu, K.; et al. Current Trends in Drug Metabolism and Pharmacokinetics. Acta Pharm. Sin. B 2019, 9, 1113–1144. [Google Scholar] [CrossRef]
- Parikh, P.K.; Savjani, J.K.; Gajjar, A.K.; Chhabria, M.T. Bioinformatics and Cheminformatics Tools in Early Drug Discovery. In Bioinformatics Tools for Pharmaceutical Drug Product Development; Wiley: Hoboken, NJ, USA, 2023; pp. 147–181. ISBN 978-1-119-86572-8. [Google Scholar]
- Alsmadi, M.M.; Idkaidek, N. The Analysis of Pethidine Pharmacokinetics in Newborn Saliva, Plasma, and Brain Extracellular Fluid After Prenatal Intrauterine Exposure from Pregnant Mothers Receiving Intramuscular Dose Using PBPK Modeling. Eur. J. Drug Metab. Pharmacokinet. 2023, 48, 281–300. [Google Scholar] [PubMed]
- Zhang, Z.; Tang, W. Drug Metabolism in Drug Discovery and Development. Acta Pharm. Sin. B 2018, 8, 721–732. [Google Scholar]
- Khan, S.R.; Al Rijjal, D.; Piro, A.; Wheeler, M.B. Integration of AI and Traditional Medicine in Drug Discovery. Drug Discov. Today 2021, 26, 982–992. [Google Scholar] [CrossRef]
- Selvaraj, C.; Chandra, I.; Singh, S.K. Artificial Intelligence and Machine Learning Approaches for Drug Design: Challenges and Opportunities for the Pharmaceutical Industries. Mol. Divers. 2022, 26, 1893–1913. [Google Scholar] [PubMed]
- Nuhn, L. Artificial Intelligence Assists Nanoparticles to Enter Solid Tumours. Nat. Nanotechnol. 2023, 18, 550–551. [Google Scholar] [PubMed]
- Zhou, H.; Hartford, A.; Tsai, K. A Bayesian Approach for PK/PD Modeling with PD Data Below Limit of Quantification. J. Biopharm. Stat. 2012, 22, 1220–1243. [Google Scholar] [CrossRef]
- Dansirikul, C.; Morris, R.G.; Tett, S.E.; Duffull, S.B. A Bayesian Approach for Population Pharmacokinetic Modelling of Sirolimus. Br. J. Clin. Pharmacol. 2006, 62, 420–434. [Google Scholar]
- You, W.; Widmer, N.; De Micheli, G. Example-Based Support Vector Machine for Drug Concentration Analysis. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; IEEE: New York, NY, USA, 2011; pp. 153–157. [Google Scholar]
- You, W.; Simalatsar, A.; Widmer, N.; Micheli, G. De Personalized Drug Administrations Using Support Vector Machine. BioNanoScience 2013, 3, 378–393. [Google Scholar]
- Camps-Valls, G.; Soria-Olivas, E.; Perez-Ruixo, J.J.; Artes-Rodriguez, A.; Jimenez-Torres, N.V. Therapeutic Drug Monitoring of Kidney Transplant Recipients Using Profiled Support Vector Machines. IEEE Trans. Syst. Man Cybern. Part C Appl. Rev. 2007, 37, 359–372. [Google Scholar]
- Farhana, N.A.; Afendi, F.M.; Fitrianto, A.; Wijaya, S.H. Classification Modeling of Support Vector Machine (SVM) and Random Forest in Predicting Pharmacodynamics Interactions. J. Phys. Conf. Ser. 2021, 1863, 012067. [Google Scholar]
- Woillard, J.-B.; Labriffe, M.; Prémaud, A.; Marquet, P. Estimation of Drug Exposure by Machine Learning Based on Simulations from Published Pharmacokinetic Models: The Example of Tacrolimus. Pharmacol. Res. 2021, 167, 105578. [Google Scholar]
- Woillard, J.; Labriffe, M.; Debord, J.; Marquet, P. Tacrolimus Exposure Prediction Using Machine Learning. Clin. Pharmacol. Ther. 2021, 110, 361–369. [Google Scholar]
- Ng, C.; Xiao, Y.; Putnam, W.; Lum, B.; Tropsha, A. Quantitative Structure–pharmacokinetic Parameters Relationships (QSPKR) Analysis of Antimicrobial Agents in Humans Using Simulated Annealing K-nearest-neighbor and Partial Least-square Analysis Methods. J. Pharm. Sci. 2004, 93, 2535–2544. [Google Scholar] [PubMed]
- Peng, J.; Li, J.; Shang, X. A Learning-Based Method for Drug-Target Interaction Prediction Based on Feature Representation Learning and Deep Neural Network. BMC Bioinform. 2020, 21, 394. [Google Scholar]
- Wang, Y.-B.; You, Z.-H.; Yang, S.; Yi, H.-C.; Chen, Z.-H.; Zheng, K. A Deep Learning-Based Method for Drug-Target Interaction Prediction Based on Long Short-Term Memory Neural Network. BMC Med. Inform. Decis. Mak. 2020, 20, 49. [Google Scholar]
- Fouchécourt, M.-O.; Béliveau, M.; Krishnan, K. Quantitative structure–pharmacokinetic relationship modelling. Sci. Total Environ. 2001, 274, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Jiang, Y.; Zhi, H. Artificial intelligence in healthcare: Past, present and future. Stroke Vasc. Neurol. 2017, 2, 230–243. [Google Scholar] [PubMed]
- Manikiran, S.S.; Prasanthi, N.L. Artificial Intelligence: Milestones and Role in Pharma and Healthcare Sector. Pharma Times 2019, 51, 10–11. [Google Scholar]
- Singh, A.; Sengupta, S.; Lakshminarayanan, V. Explainable deep learning models in medical image analysis. J. Imaging 2020, 6, 52. [Google Scholar] [CrossRef]
- Gulum, M.A.; Trombley, C.M.; Kantardzic, M. A review of explainable deep learning cancer detection models in medical imaging. Appl. Sci. 2021, 11, 4573. [Google Scholar] [CrossRef]
- Dhar, T.; Dey, N.; Borra, S.; Sherratt, R.S. Challenges of deep learning in medical image analysis—Improving explainability and trust. IEEE Trans. Technol. Soc. 2023, 4, 68–75. [Google Scholar] [CrossRef]
- Jin, D.; Sergeeva, E.; Weng, W.H.; Chauhan, G.; Szolovits, P. Explainable deep learning in healthcare: A methodological survey from an attribution view. WIREs Mech. Dis. 2022, 14, e1548. [Google Scholar] [CrossRef]
- Lisboa, P.J.; Saralajew, S.; Vellido, A.; Fernández-Domenech, R.; Villmann, T. The coming of age of interpretable and explainable machine learning models. Neurocomputing 2023, 535, 25–39. [Google Scholar] [CrossRef]
- Wei, Q.; Chen, H.; Kaeberlein, M.; Lee, S.-I. ExplaiNAble BioLogical Age (ENABL Age): An artificial intelligence framework for interpretable biological age. Lancet Healthy Longev. 2023, 4, e711–e723. [Google Scholar]
- Hassija, V.; Chamola, V.; Mahapatra, A.; Singal, A.; Goel, D.; Huang, K.; Scardapane, S.; Spinelli, I.; Mahmud, M.; Hussain, A. Interpreting black-box models: A review on explainable artificial intelligence. Cogn. Comput. 2024, 16, 45–74. [Google Scholar] [CrossRef]
- Ras, G.; van Gerven, M.; Haselager, P. Explanation Methods in Deep Learning: Users, Values, Concerns and Challenges; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Vorobeva, A.A.; Matuzko, M.A.; Sivkov, D.I.; Safiullin, R.I.; Menshchikov, A.A. A new method for countering evasion adversarial attacks on information systems based on artificial intelligence. J. Sci. Tech. Inf. Technol. Mech. Opt. 2024, 24, 256–266. [Google Scholar] [CrossRef]
- Malatji, M.; Tolah, A. Artificial intelligence (AI) cybersecurity dimensions: A comprehensive framework for understanding adversarial and offensive AI. AI Ethics 2024, 1–28. [Google Scholar] [CrossRef]
- Papernot, N.; McDaniel, P.; Wu, X.; Jha, S.; Swami, A. Distillation as a defense to adversarial perturbations against deep neural networks. In Proceedings of the 2016 IEEE Symposium on Security and Privacy (SP), San Jose, CA, USA, 22–26 May 2016; pp. 582–597. [Google Scholar]
- Liang, Y.; Samavi, R. Advanced defensive distillation with ensemble voting and noisy logits. Appl. Intell. 2023, 53, 3069–3094. [Google Scholar] [CrossRef]
- Chakraborty, U.; Roy, S.; Kumar, S. Rise of Generative AI and ChatGPT: Understand how Generative AI and ChatGPT Are Transforming and Reshaping the Business World (English Edition); BPB Publications: Noida, India, 2023. [Google Scholar]
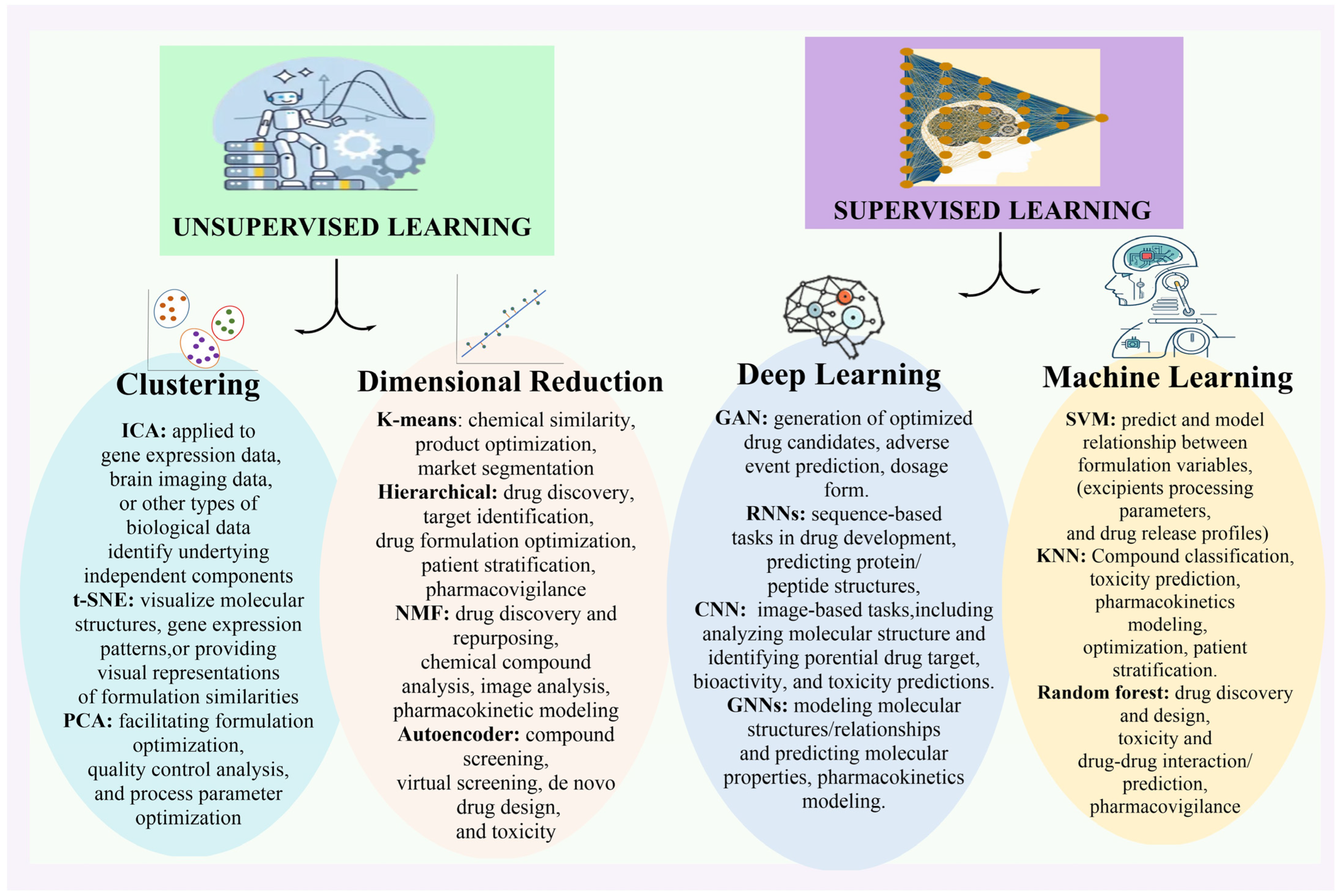
| Sr No. | Softwires Powered by AI | Application | References |
|---|---|---|---|
| 1. | GAN (Generative Adversarial Networks) | 1. Drug research and innovation makes extensive use of GANs for the generation of new chemical compounds and the optimisation of their attributes. 2. To generate structurally varied as well as functionally optimised potential drugs. 3. GANs use a generator network to produce new compounds and an evaluation network to assess its quality. | [9] |
| 2. | RNN (Recurrent Neural Networks) | 1. In the field of pharmaceutical research, RNN is frequently used for pattern-based activities like protein coding, dna data analysis, and protein configuration estimation. 2. It is able to learn features and then implementation into new patterns, capturing sequential relationships in the process. | [10] |
| 3. | CNN (Convolutional Neural Networks) | 1. Molecular structural analysis and the identification of possible therapeutic targets are two examples of based on images activities, where CNNs excel. 2. To help with medication development and receptor recognition, they may derive useful information through molecular pictures. | [11] |
| 4. | LSTM (Long Short-Term Memory Networks) | 1. When it comes to modelling and forecasting temporal relationships, LSTMs, a form of RNN, particularly emerge. 2. ADME Studies as well as pharmacodynamics of any drug have made quite easy to make drug profile by the use of this software. | [12] |
| 5. | Graph Neural Networks (GNNs) | 1. Drug discovery activities like the structure of molecules are well-suited to GNNs. 2. Their capabilities include molecular graph modelling, property prediction, simulated testing, and completely novel pharmaceutical development assistance. | [13] |
| 6. | RL (Reinforcement Learning) | 1. Drug dosage procedures and individualised treatment programmes have both benefited from the application of RL approaches. 2. RL techniques enhance medical results by learning from environmental interactions and making sequential judgements which assist in optimisation of dose. | [14] |
| 7. | DQN (Deep Q-Networks) | 1. By optimising drug development procedures through chemical activity prediction, DQNs—a hybrid of profound neural networks and reinforcement learning—have been employed. 2. Recommending promising subjects for additional testing. | [15,16] |
| 8. | Bayesian Models | 1. In order to quantify uncertainty and make decisions, the pharmaceutical industry uses Bayesian models like Gaussian processes and Bayesian networks. 2. Scientists can use them to optimise designs for experiments, conduct risk assessments, and generate probabilistic guesses. | [17,18] |
| 9. | Autoencoders | 1. The method for developing drugs makes use of autoencoders, which are autonomous neural models, to reduce multiplicity and retrieve features. 2. In addition to aiding in simulated analysis and combinatorial screening, they are able to capture crucial molecular properties. | [19,20] |
| 10. | Transformer Models | 1. One area where transformer models have found use is in the field of pharmaceutical natural language processing. One such model is BERT, which stands for Bidirectional Encoder Representations from Transformers. 2. Researchers can make better decisions about medication development with the help of their ability to retrieve useful information through data from clinical trials, data for patent, and also literature. | [21,22] |
| Drug Discovery AI Tools | Information | Respective Websites |
|---|---|---|
| Chemputer | Improved structure for documenting the synthesis of chemicals | https://zenodo.org/record/1481731 |
| ODDT | For application in the fields of molecular modelling & chemo informatics | https://github.com/oddt/oddt |
| ORGANIC | Molecules with certain properties can be synthesised using this instrument. | https://github.com/aspuru-guzik-group/ORGANIC |
| DeepChem | An artificial intelligence instrument to make drug discovery estimations that is built on Python | https://github.com/deepchem/deepchem |
| DeepNeuralNet-QSAR | Hypotheses concerning the action of molecules | https://github.com/Merck/DeepNeuralNet-QSAR |
| Neural Graph Fingerprints | Prediction for properties of newly discovered compounds | https://github.com/HIPS/neural-fingerprint |
| Hit Dexter | Predicting which compounds may react to biological experiments using artificial intelligence algorithms | http://hitdexter2.zbh.uni-hamburg.de |
| DeepTox | Assessment of the potential for toxicity along with biological compatibility | www.bioinf.jku.at/research/DeepTox |
| PotentialNet | An artificial graph convolutional artificial intelligent model for ligand binding. | https://pubs.acs.org/doi/full/10.1021/acscentsci.8b00507 |
| REINVENT | Regenerative neural network-based molecular design | https://github.com/MarcusOlivecrona/REINVENT |
| DeltaVina | A scoring function for rescoring protein–ligand binding affinity | https://github.com/chengwang88/deltavina |
| AlphaFold | Forecasting the three-dimensional structure of proteins | https://deepmind.com/blog/alphafold |
| S.N | Imaging Type for Medical Purposes | Detailed Description |
|---|---|---|
| 1. | X-rays (radiographic imaging) | Uses X-rays and other forms of ionising electromagnetic energy to produce images of things. |
| 2. | Infrared imaging | Builds low-quality moving projection images of the physical internal structures in real time by repeatedly exposing them to X-rays at a reduced dose rate. |
| 3. | Coronary angiography | Applied for the diagnosis of aortic aneurysms, stenosis, blockages, new vascular formation, and stent and catheter implantation. |
| 4. | DEXA | The osteoporosis test uses Dual X-ray Absorptiometry, which is also called bone densitometry. |
| 5. | CT Scan (Computed tomography) | Uses a computer and a lot of ionising radiation to create pictures of both solid and delicate tissues. |
| 6. | MRI (Magnetic resonance imaging) | Imaging of the body made possible by a powerful magnet and radiofrequency waves; used in medical diagnostics. |
| 7. | Ultrasound imaging | Makes use of broadband, sound waves with high frequencies reflected off of tissues to generate three-dimensional pictures. |
| 8. | Bone scan | A method of monitoring bone repair that makes use of a radioactive substance. |
| 9. | Electron microscopy | A high-resolution microscope that can magnify minute details. |
| 10. | Radiation therapy (Nuclear Medicine) | Includes the use of nuclear characteristics for both medical evaluation and therapy. |
| 11. | Magnetic resonance angiography scans | Draws blood vessel structures in the body with remarkable clarity. |
| Computer Programmes (Software) | Target | Benefits | Drawbacks | PK/PD/Both | Reference |
|---|---|---|---|---|---|
| WinBUGS/Bayesian | In order to deal with information that is not yet quantifiable | Previous research can be utilised for model-fitting purposes without any additional processing. | Excessive processing time—Impossible negative results in specific PK/PD models | Both | [140] |
| Bayesian + PKBUGS + WinBUGS + version 1.3 | Investigation of sirolimus concentration data using pharmacokinetic models | Possible covariate association identification; Simple integration of historical data with present-day data | Datasets are few and often lack useful information. | PK | [141] |
| Least Squares Support Vector Machine | Analysis of drug concentrations using patient profiles | A unique model is created for each individual patient—When compared to the PK modelling method, SVM-based approaches provide more accurate predictions of drug concentrations | Sample outliers significantly impact the model, reducing its accuracy. | PK | [142] |
| Random Sample Consensus (RANSAC) and Support Vector Machine with Drug Administration Decision Support System (DADSS) | Concentration, optimal dose, and dose interval prediction | Improved adaptability and structural adjustability; algorithmic predictability affected by dataset noise | Algorithm predictability is affected by dataset noise. | PK | [143] |
| Model Dependent Support Vector Machine/Profile SVM | Therapeutic medication tracking in patients undergoing kidney transplantation | an economical and crucial dosage Benefit nonlinear models | Extensive datasets—Time-consuming | PK | [144] |
| An SVM combined with a random forest model | How drugs interact with one another pharmacodynamically according to SES, CS, and TPC (Target Protein Connectedness) | The accuracy of the PDI predictions was 89.93% and the AUC value was 79.96%. | More extensive data processing and filtering is necessary. | PD | [145] |
| Gradient Boosting Machines, Random Forest, Linear Regressions (LASSO), and XGBoost | Forecasting the area under the curve (AUC) and plasma concentration-time series (0–24 h following multiple doses of rifampicin | Analyses that save time Covariate selections are made easier with this strategy. | Possible results that are not applicable to clinical practice | PK | [146] |
| XGBoost | Estimation of drug AUC of tacrolimus or mycophenolate mofetil (MMF) | PK datasets from renal, liver, and heart transplant patients were accurately predicted | Not possible to calculate the probability of target attainment and accurate dosing | PK | [147,148] |
| Simulated Annealing k-Nearest-Neighbor (SA-kNN)/Partial Least-Square (PLS)/Multiple Linear Regression (MLR)/Sybyl version 6.7 | Prediction of pharmacokinetic parameters of antimicrobial agents based on molecular structure | Cost-effective—Requires less sample size | Requires multiple model generation methods—Interpretation of individual descriptors is almost impossible | Both | [149] |
| Drug Target Interaction Convolutional Neural Network (DTICNN) | Identification of drug-target interactions and prediction of potential drug molecules | Cost-effective—Time-saving | Large datasets are required | PD | [150] |
| Deep Long Short-Term Memory (DeepLSTM) | Computational methods to validate drug-target interactions | Based on Position Specific Scoring Matrix (PSSM) and Legendre Moment (LM) | Large datasets are required | PD | [151] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, P.; Chaudhary, B.; Arya, P.; Chauhan, R.; Devi, S.; Parejiya, P.B.; Gupta, M.M. Advanced Artificial Intelligence Technologies Transforming Contemporary Pharmaceutical Research. Bioengineering 2025, 12, 363. https://doi.org/10.3390/bioengineering12040363
Kumar P, Chaudhary B, Arya P, Chauhan R, Devi S, Parejiya PB, Gupta MM. Advanced Artificial Intelligence Technologies Transforming Contemporary Pharmaceutical Research. Bioengineering. 2025; 12(4):363. https://doi.org/10.3390/bioengineering12040363
Chicago/Turabian StyleKumar, Parveen, Benu Chaudhary, Preeti Arya, Rupali Chauhan, Sushma Devi, Punit B. Parejiya, and Madan Mohan Gupta. 2025. "Advanced Artificial Intelligence Technologies Transforming Contemporary Pharmaceutical Research" Bioengineering 12, no. 4: 363. https://doi.org/10.3390/bioengineering12040363
APA StyleKumar, P., Chaudhary, B., Arya, P., Chauhan, R., Devi, S., Parejiya, P. B., & Gupta, M. M. (2025). Advanced Artificial Intelligence Technologies Transforming Contemporary Pharmaceutical Research. Bioengineering, 12(4), 363. https://doi.org/10.3390/bioengineering12040363








