Current and Near-Future Technologies to Quantify Nanoparticle Therapeutic Loading Efficiency and Surface Coating Efficiency with Targeted Moieties
Abstract
1. Introduction
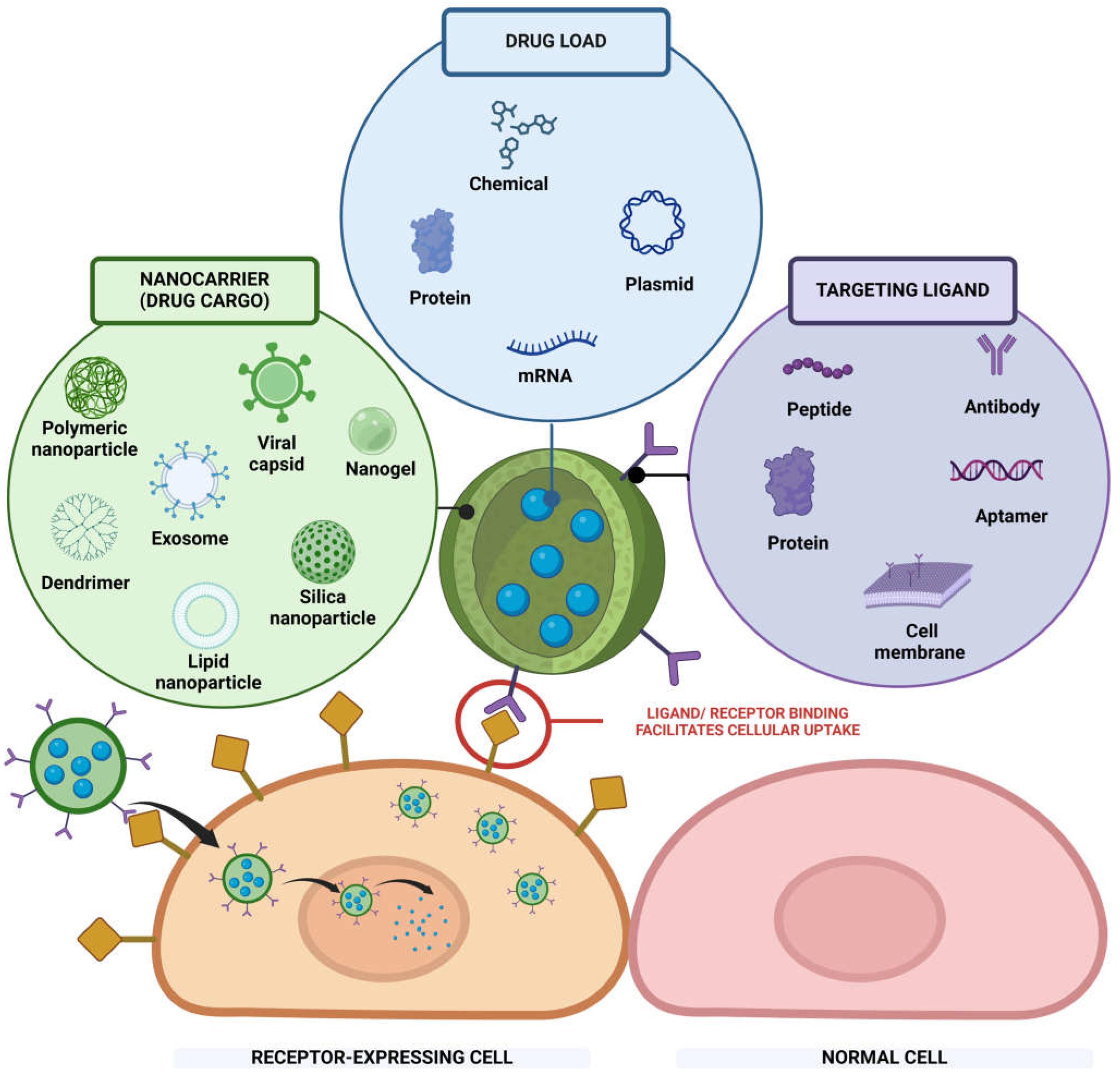
2. Nanoparticles for Active Targeting Delivery
2.1. Components of Nanoparticles for Active Targeting Delivery
2.2. Current Barriers in Translating Targeted Nanoparticle Delivery to the Clinic
3. Methods to Quantify Sizes of Nanoparticles
3.1. Bulk Detection
3.2. Single-Nanoparticle Detection
4. Methods to Quantify the Drug Loading Efficacies of Nanoparticles
4.1. Bulk Detection of Payloads in NPs
| Payload | Detection Methods | Detection Range | Advantages | Disadvantages | Application Examples |
| Protein based | UV Absorbance 280 nm | 20–3000 µg [126] | Simple, highly specific, sample can be used after measurement | Applicable to proteins with tryptophan and tyrosine; requires non-sequence-specific absorbance calibration and accounting for nucleic acid background noise. | Nerolidol-Loaded Chitosan–Alginate Nanoparticles [130] |
| UV Absorbance 205 nm | 1–100 µg [126] | More sensitive and displays less protein-to-protein variability than 280 nm [131] | Background of solvent can interfere with the reading. | N/A | |
| Coomassie Blue (Bradford Assay) | 1–50 µg [126] | Simple | Contaminate from surfactant. | Platelet-rich-plasma-loaded chitosan nanoparticles (wound healing) [132], tumor-antigen-loaded PLGA NPs (cancer vaccine) [133] | |
| Lowry (Alkaline Copper Reduction Assays) | 5–100 µg [126] | Highly sensitive | Long preparation and complex procedure, contaminated by reduction reaction. | BSA-Loaded PLGA–Chitosan Composite Nanoparticles [134] | |
| Bicinchoninic Acid (BCA) | 0.2–50 µg [126] | Simpler, highly sensitive | Thiol, phospholipid, and ammonium sulfate interference. | PLGA-containing anti-CTLA4 (endometriosis) [135] PLGA-R837@Cat nanoparticles (tumors) [136] | |
| Mass-spectrometry (MS) | Flexible, specific, multiple targets simultaneously, precision and accuracy, requiring minimal material for analysis [137] | High equipment costs; stable electricity, ventilation, high-purity gases, and skilled staff are required. Compounds must be volatile enough to transfer from liquid to mobile carrier gas for detector elution [137]. | Docetaxel-loaded PLGA NPs (cancer) [138] | ||
| Nucleic acids | UV Absorbance 260 nm | NanoDrop 1/1: 0.2 *–27,500 ng/µL Nanodrop 3300: 0.05–2000 picograms/μL (Thermofisher, Waltham, Massachusetts, USA) | Quick and easy | Cannot differentiate between RNA and DNA, limited sensitivity at low concentrations. | DNA-loaded PLA-PEG-PLA NPs (SS-Nanodrop) [139] DNA/PLL NPs (Binding Kinetics) [140] |
| Fluorescence-based assays: Picogreen assay (DNA) Ribogreen assay (RNA) | 1 ng/mL to 1000 ng/mL (Picogreen assay) 1 ng/mL to 1 µg/mL Ribogreen assay—RNA) [141] iQuant RNA BR Assay Kit (RNA): 20–1000 ng RNA | Higher sensitivity and specificity [129] | Susceptibility to compounds such as salts and chemical reagents [129]. | cDNA PLGA NPs-Notch SIgnaling [142] IL-29 cDNA-immunology (HCV, cancer) [143] RNAi PEI-PLGA for gene delivery [144] Various types of RNA-loaded LNPs [141] Reactive oxygen species (ROS) mRNA LNPs [145] mRNA LNPs for prenatal treatment of congenital disorders [146] mRNA-encoded cystic fibrosis transmembrane conductance regulator (CFTR)-loaded LNPs for pulmonary delivery [147] LNPs incorporating hydroxycholesterols to enhance mRNA delivery to T cells [148] | |
| Liquid chromatography–mass spectrometry (LC-MS) | used for measuring mRNA | Sample does not require mRNA extraction, detergents, or enzyme | Expensive, limited sample throughput. | mRNA loaded in lipid nanoparticles (LNPs) [149] | |
| Other pharmaceutical compounds | High-performance liquid chromatography (HPLC) | 190–800 nm | High sensitivity, specificity, rapidity, accuracy, precision, and ease of automation | Expensive, complicated to troubleshoot, and time-consuming. | DOX-loaded liposomes (cancer) [150], Isoniazid- and Rifampicin-Loaded Bovine Serum Albumin Nanoparticles (tuberculosis) [151], melatonin-loaded human serum albumin NPs (neurodegenerative eye diseases) [152] |
| UV/VIS | variable | Easy to use, fast and efficient analysis, inexpensive, non-destructive, minimal processing | Lower sensitivity and selectivity; light scattering and multiple absorbing species may interfere with accuracy. | Itraconazole-loaded chitosan-silver nanoparticles [153] necrosulfonamide-loaded mesoporous nanoparticles (inflammation) [154] Cisplatin-loaded Glutathione-responsive biodegradable polyurethane nanoparticles (cancer): UV–Vis spectrophotometer at 703 nm [155] Curcumin-loaded self-assembled WPI@SLG core–shell nanoparticles (antioxidant activity in gastrointestinal conditions): absorbance at the wavelength of 426 nm [156] Ciprofloxacin-loaded PEG–PLGA NPs (regenerative endodontic treatment): absorbance 275 nm [157] ATP-loaded albumin nanoparticles (cancer): UV/Vis spectrophotometer at 257 nm [158] | |
| Optical Density (OD) | Variable | Easy to use, inexpensive | Low sensitivity. | Ponatinib- and dasatinib-loaded exosomes (cancer): The optical density (OD) value for ponatinib and dasatinib was recorded at 285 and 233 nm [159] |
4.2. Single-Nanoparticle Therapeutic Load Detection
5. Methods to Quantify the Number of Targeted Ligands per Nanoparticle
5.1. Bulk Detection of the Average Number of Ligands per Nanoparticle
5.2. Single-Nanoparticle Ligand Density Detection
6. Summary and Future Outlook
Funding
Acknowledgments
Conflicts of Interest
References
- Ross, K.A.; Brenza, T.M.; Binnebose, A.M.; Phanse, Y.; Kanthasamy, A.G.; Gendelman, H.E.; Salem, A.K.; Bartholomay, L.C.; Bellaire, B.H.; Narasimhan, B. Nano-enabled delivery of diverse payloads across complex biological barriers. J. Control. Release 2015, 219, 548–559. [Google Scholar] [CrossRef]
- Rizvi, S.A.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi. Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef]
- Shi, J.; Votruba, A.R.; Farokhzad, O.C.; Langer, R. Nanotechnology in Drug Delivery and Tissue Engineering: From Discovery to Applications. Nano Lett. 2010, 10, 3223–3230. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Q.; Xia, G.; Adilijiang, N.; Li, Y.; Hou, Z.; Fan, Z.; Li, J. Recent Advances in Targeted Drug Delivery Strategy for Enhancing Oncotherapy. Pharmaceutics 2023, 15, 2233. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.D.; Claypool, S.E.; Liu, R. The smart targeting of nanoparticles. Curr. Pharm. Des. 2013, 19, 6315–6329. [Google Scholar] [CrossRef]
- Sanna, V.; Sechi, M. Therapeutic Potential of Targeted Nanoparticles and Perspective on Nanotherapies. ACS Med. Chem. Lett. 2020, 11, 1069–1073. [Google Scholar] [CrossRef]
- Liu, H.; Pietersz, G.; Peter, K.; Wang, X. Nanobiotechnology approaches for cardiovascular diseases: Site-specific targeting of drugs and nanoparticles for atherothrombosis. J. Nanobiotechnology 2022, 20, 75. [Google Scholar] [CrossRef]
- Aparna, V.A.; Biswas, R.; Jayakumar, R. Chapter 12-Targeted nanoparticles for treating infectious diseases. In Biomimetic Nanoengineered Materials for Advanced Drug Delivery; Unnithan, A.R., Sasikala, A.R.K., Park, C.H., Kim, C.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 169–185. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef]
- Wood, H. FDA approves patisiran to treat hereditary transthyretin amyloidosis. Nat. Rev. Neurol. 2018, 14, 570. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-J.; Wu, J.-Y.; Liu, J.; Xu, W.; Qiu, X.; Huang, S.; Hu, X.-B.; Xiang, D.-X. Artificial exosomes for translational nanomedicine. J. Nanobiotechnol. 2021, 19, 242. [Google Scholar] [CrossRef] [PubMed]
- Varenne, F.; Vauthier, C. Practical Guidelines for the Characterization and Quality Control of Nanoparticles in the Pharmaceutical Industry. In Emerging Technologies for Nanoparticle Manufacturing; Patel, J.K., Pathak, Y.V., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 487–508. [Google Scholar] [CrossRef]
- Bahadar, H.; Maqbool, F.; Niaz, K.; Abdollahi, M. Toxicity of Nanoparticles and an Overview of Current Experimental Models. Iran Biomed. J. 2016, 20, 1–11. [Google Scholar] [CrossRef]
- Yildirimer, L.; Thanh, N.T.K.; Loizidou, M.; Seifalian, A.M. Toxicology and clinical potential of nanoparticles. Nano Today 2011, 6, 585–607. [Google Scholar] [CrossRef] [PubMed]
- Crist, R.M.; Grossman, J.H.; Patri, A.K.; Stern, S.T.; Dobrovolskaia, M.A.; Adiseshaiah, P.P.; Clogston, J.D.; McNeil, S.E. Common pitfalls in nanotechnology: Lessons learned from NCI’s Nanotechnology Characterization Laboratory. Integr. Biol. 2013, 5, 66–73. [Google Scholar] [CrossRef]
- Adiseshaiah, P.P.; Hall, J.B.; McNeil, S.E. Nanomaterial standards for efficacy and toxicity assessment. WIREs Nanomed. Nanobiotechnol. 2010, 2, 99–112. [Google Scholar] [CrossRef]
- Amini, Y.; Amel Jamehdar, S.; Sadri, K.; Zare, S.; Musavi, D.; Tafaghodi, M. Different methods to determine the encapsulation efficiency of protein in PLGA nanoparticles. Biomed. Mater. Eng. 2017, 28, 613–620. [Google Scholar] [CrossRef]
- Sharifi, S.; Mahmoud, N.N.; Voke, E.; Landry, M.P.; Mahmoudi, M. Importance of Standardizing Analytical Characterization Methodology for Improved Reliability of the Nanomedicine Literature. Nanomicro. Lett. 2022, 14, 172. [Google Scholar] [CrossRef]
- Naftaly, A.; Izgilov, R.; Omari, E.; Benayahu, D. Revealing Advanced Glycation end Products Associated Structural Changes in Serum Albumin. ACS Biomater. Sci. Eng. 2021, 7, 3179–3189. [Google Scholar] [CrossRef]
- Li, Y.; Struwe, W.B.; Kukura, P. Single molecule mass photometry of nucleic acids. Nucleic Acids. Res. 2020, 48, e97. [Google Scholar] [CrossRef]
- Lebedeva, M.A.; Palmieri, E.; Kukura, P.; Fletcher, S.P. Emergence and Rearrangement of Dynamic Supramolecular Aggregates Visualized by Interferometric Scattering Microscopy. ACS Nano 2020, 14, 11160–11168. [Google Scholar] [CrossRef]
- Wu, D.; Hwang, P.; Li, T.; Piszczek, G. Rapid characterization of adeno-associated virus (AAV) gene therapy vectors by mass photometry. Gene Ther. 2022, 29, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Brun, J.; Vasiljevic, S.; Gangadharan, B.; Hensen, M.; Chandran, A.V.; Hill, M.L.; Kiappes, J.L.; Dwek, R.A.; Alonzi, D.S.; Struwe, W.B.; et al. Assessing Antigen Structural Integrity through Glycosylation Analysis of the SARS-CoV-2 Viral Spike. ACS Cent. Sci. 2021, 7, 586–593. [Google Scholar] [CrossRef]
- Yin, V.; Lai, S.-H.; Caniels, T.G.; Brouwer, P.J.M.; Brinkkemper, M.; Aldon, Y.; Liu, H.; Yuan, M.; Wilson, I.A.; Sanders, R.W.; et al. Probing Affinity, Avidity, Anticooperativity, and Competition in Antibody and Receptor Binding to the SARS-CoV-2 Spike by Single Particle Mass Analyses. ACS Cent. Sci. 2021, 7, 1863–1873. [Google Scholar] [CrossRef]
- Higuchi, Y.; Suzuki, T.; Arimori, T.; Ikemura, N.; Mihara, E.; Kirita, Y.; Ohgitani, E.; Mazda, O.; Motooka, D.; Nakamura, S.; et al. Engineered ACE2 receptor therapy overcomes mutational escape of SARS-CoV-2. Nat. Commun. 2021, 12, 3802. [Google Scholar] [CrossRef]
- Gooding, J.J.; Gaus, K. Single-Molecule Sensors: Challenges and Opportunities for Quantitative Analysis. Angew. Chem. Int. Ed. 2016, 55, 11354–11366. [Google Scholar] [CrossRef]
- Garoli, D.; Yamazaki, H.; Maccaferri, N.; Wanunu, M. Plasmonic Nanopores for Single-Molecule Detection and Manipulation: Toward Sequencing Applications. Nano Lett. 2019, 19, 7553–7562. [Google Scholar] [CrossRef]
- Spitzberg, J.D.; Zrehen, A.; van Kooten, X.F.; Meller, A. Plasmonic-Nanopore Biosensors for Superior Single-Molecule Detection. Adv. Mater. 2019, 31, 1900422. [Google Scholar] [CrossRef]
- Bazak, R.; Houri, M.; Achy, S.E.; Hussein, W.; Refaat, T. Passive targeting of nanoparticles to cancer: A comprehensive review of the literature. Mol. Clin. Oncol. 2014, 2, 904–908. [Google Scholar] [CrossRef]
- Anjum, S.; Naseer, F.; Ahmad, T.; Jahan, F.; Qadir, H.; Gul, R.; Kousar, K.; Sarwar, A.; Shabbir, A. Enhancing therapeutic efficacy: Sustained delivery of 5-fluorouracil (5-FU) via thiolated chitosan nanoparticles targeting CD44 in triple-negative breast cancer. Sci. Rep. 2024, 14, 11431. [Google Scholar] [CrossRef]
- Zhang, Y.; Ni, Q.; Xu, C.; Wan, B.; Geng, Y.; Zheng, G.; Yang, Z.; Tao, J.; Zhao, Y.; Wen, J.; et al. Smart Bacterial Magnetic Nanoparticles for Tumor-Targeting Magnetic Resonance Imaging of HER2-Positive Breast Cancers. ACS Appl. Mater. Interfaces 2019, 11, 3654–3665. [Google Scholar] [CrossRef]
- Yaman, S.; Ramachandramoorthy, H.; Iyer, P.; Chintapula, U.; Nguyen, T.; Sabnani, M.; Kotadia, T.; Ghaffari, S.; Pop, L.M.; Hannan, R.; et al. Targeted chemotherapy via HER2-based chimeric antigen receptor (CAR) engineered T-cell membrane coated polymeric nanoparticles. Bioact. Mater. 2024, 34, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, K.; Li, C.; Guo, Q.; Chen, Q.; He, X.; Liu, L.; Zhang, Y.; Lu, Y.; Chen, X.; et al. Macrophage-Membrane-Coated Nanoparticles for Tumor-Targeted Chemotherapy. Nano Lett. 2018, 18, 1908–1915. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lin, S.; Wang, S.; Jiang, Z.; Ding, T.; Wei, X.; Lu, Y.; Yang, F.; Zhan, C. Folic Acid Enables Targeting Delivery of Lipodiscs by Circumventing IgM-Mediated Opsonization. Nano Lett. 2022, 22, 6516–6522. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Xin, H.; Guo, Y.; Zhu, B.; Su, L.; Wang, S.; Zeng, J.; Chen, Q.; Deng, R.; et al. Mitochondria-targeting folic acid-modified nanoplatform based on mesoporous carbon and a bioactive peptide for improved colorectal cancer treatment. Acta Biomater. 2022, 152, 453–472. [Google Scholar] [CrossRef]
- Jang, E.H.; Shim, M.K.; Kim, G.L.; Kim, S.; Kang, H.; Kim, J.H. Hypoxia-responsive folic acid conjugated glycol chitosan nanoparticle for enhanced tumor targeting treatment. Int. J. Pharm. 2020, 580, 119237. [Google Scholar] [CrossRef]
- Banik, B.; Surnar, B.; Askins, B.W.; Banerjee, M.; Dhar, S. Dual-Targeted Synthetic Nanoparticles for Cardiovascular Diseases. ACS Appl. Mater. Interfaces 2020, 12, 6852–6862. [Google Scholar] [CrossRef]
- Li, Y.; Che, J.; Chang, L.; Guo, M.; Bao, X.; Mu, D.; Sun, X.; Zhang, X.; Lu, W.; Xie, J. CD47- and Integrin α4/β1-Comodified-Macrophage-Membrane-Coated Nanoparticles Enable Delivery of Colchicine to Atherosclerotic Plaque. Adv. Healthc. Mater. 2022, 11, e2101788. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, Z.; Hu, C.; Maitz, M.F.; Yang, L.; Luo, R.; Wang, Y. Platelet Membrane-Coated Nanocarriers Targeting Plaques to Deliver Anti-CD47 Antibody for Atherosclerotic Therapy. Research 2022, 2022, 9845459. [Google Scholar] [CrossRef]
- Wen, C.; Xu, X.; Zhang, Y.; Xia, J.; Liang, Y.; Xu, L. Bone Targeting Nanoparticles for the Treatment of Osteoporosis. Int. J. Nanomed. 2024, 19, 1363–1383. [Google Scholar] [CrossRef]
- Thurner, G.C.; Haybaeck, J.; Debbage, P. Targeting Drug Delivery in the Elderly: Are Nanoparticles an Option for Treating Osteoporosis? Int. J. Mol. Sci. 2021, 22, 8932. [Google Scholar] [CrossRef]
- Zheng, L.; Zhuang, Z.; Li, Y.; Shi, T.; Fu, K.; Yan, W.; Zhang, L.; Wang, P.; Li, L.; Jiang, Q. Bone targeting antioxidative nano-iron oxide for treating postmenopausal osteoporosis. Bioact. Mater. 2022, 14, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Su, C.M.; Yeh, J.C.; Wu, P.R.; Tsai, T.Y.; Lou, S.L. Synthesis of composite magnetic nanoparticles Fe(3)O(4) with alendronate for osteoporosis treatment. Int. J. Nanomed. 2016, 11, 4583–4594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, W.; Zhu, D.; Li, Z.; Wang, Z.; Li, J.; Mei, X.; Xu, W.; Cheng, K.; Zhong, B. Nanoparticles functionalized with stem cell secretome and CXCR4-overexpressing endothelial membrane for targeted osteoporosis therapy. J. Nanobiotechnol. 2022, 20, 35. [Google Scholar] [CrossRef]
- Sun, Y.; Ye, X.; Cai, M.; Liu, X.; Xiao, J.; Zhang, C.; Wang, Y.; Yang, L.; Liu, J.; Li, S.; et al. Osteoblast-Targeting-Peptide Modified Nanoparticle for siRNA/microRNA Delivery. ACS Nano 2016, 10, 5759–5768. [Google Scholar] [CrossRef]
- Pandey, R.P.; Vidic, J.; Mukherjee, R.; Chang, C.M. Experimental Methods for the Biological Evaluation of Nanoparticle-Based Drug Delivery Risks. Pharmaceutics 2023, 15, 612. [Google Scholar] [CrossRef]
- Zou, Z.; Li, H.; Xu, G.; Hu, Y.; Zhang, W.; Tian, K. Current Knowledge and Future Perspectives of Exosomes as Nanocarriers in Diagnosis and Treatment of Diseases. Int. J. Nanomed. 2023, 18, 4751–4778. [Google Scholar] [CrossRef]
- Lee, B.R.; Jo, E.; Yoon, H.Y.; Yoon, C.J.; Lee, H.J.; Kwon, K.C.; Kim, T.W.; Lee, J. Nonimmunogenetic Viral Capsid Carrier with Cancer Targeting Activity. Adv. Sci. 2018, 5, 1800494. [Google Scholar] [CrossRef]
- Joudeh, N.; Linke, D. Nanoparticle classification, physicochemical properties, characterization, and applications: A comprehensive review for biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar]
- Azzi, S.; Hebda, J.K.; Gavard, J. Vascular permeability and drug delivery in cancers. Front. Oncol. 2013, 3, 211. [Google Scholar] [CrossRef]
- Al Bostami, R.D.; Abuwatfa, W.H.; Husseini, G.A. Recent Advances in Nanoparticle-Based Co-Delivery Systems for Cancer Therapy. Nanomaterials 2022, 12, 2672. [Google Scholar] [CrossRef] [PubMed]
- Municoy, S.; Álvarez Echazú, M.I.; Antezana, P.E.; Galdopórpora, J.M.; Olivetti, C.; Mebert, A.M.; Foglia, M.L.; Tuttolomondo, M.V.; Alvarez, G.S.; Hardy, J.G.; et al. Stimuli-Responsive Materials for Tissue Engineering and Drug Delivery. Int. J. Mol. Sci. 2020, 21, 4724. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef]
- Behr, M.; Zhou, J.; Xu, B.; Zhang, H. In vivo delivery of CRISPR-Cas9 therapeutics: Progress and challenges. Acta Pharm. Sin. B 2021, 11, 2150–2171. [Google Scholar] [CrossRef]
- Liu, S.; Chen, X.; Bao, L.; Liu, T.; Yuan, P.; Yang, X.; Qiu, X.; Gooding, J.J.; Bai, Y.; Xiao, J.; et al. Treatment of infarcted heart tissue via the capture and local delivery of circulating exosomes through antibody-conjugated magnetic nanoparticles. Nat. Biomed. Eng. 2020, 4, 1063–1075. [Google Scholar] [CrossRef]
- Tian, H.; Yu, L.; Zhang, M.; He, J.; Sun, X.; Ni, P. Dextran-doxorubicin prodrug nanoparticles conjugated with CD147 monoclonal antibody for targeted drug delivery in hepatoma therapy. Colloids Surf. B Biointerfaces 2023, 228, 113400. [Google Scholar] [CrossRef]
- Bayram, B.; Ozgur, A.; Tutar, L.; Tutar, Y. Tumor Targeting of Polymeric Nanoparticles Conjugated with Peptides, Saccharides, and Small Molecules for Anticancer Drugs. Curr. Pharm. Des. 2017, 23, 5349–5357. [Google Scholar] [CrossRef]
- Moon, Y.; Shim, M.K.; Choi, J.; Yang, S.; Kim, J.; Yun, W.S.; Cho, H.; Park, J.Y.; Kim, Y.; Seong, J.K.; et al. Anti-PD-L1 peptide-conjugated prodrug nanoparticles for targeted cancer immunotherapy combining PD-L1 blockade with immunogenic cell death. Theranostics 2022, 12, 1999–2014. [Google Scholar] [CrossRef]
- Zhu, X.; Li, C.; Lu, Y.; Liu, Y.; Wan, D.; Zhu, D.; Pan, J.; Ma, G. Tumor microenvironment-activated therapeutic peptide-conjugated prodrug nanoparticles for enhanced tumor penetration and local T cell activation in the tumor microenvironment. Acta Biomater. 2021, 119, 337–348. [Google Scholar] [CrossRef]
- Kato, N.; Sato, T.; Fuchigami, Y.; Suga, T.; Geng, L.; Tsurumaru, M.; Hagimori, M.; Mukai, H.; Kawakami, S. Synthesis and evaluation of a novel adapter lipid derivative for preparation of cyclic peptide-modified PEGylated liposomes: Application of cyclic RGD peptide. Eur. J. Pharm. Sci. 2022, 176, 106239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, J.; Ren, J.; Xue, Z.; Qi, X.; Si, Q. Cyclic RGD functionalized PLGA nanoparticles loaded with noncovalent complex of indocyanine green with urokinase for synergistic thrombolysis. Front. Bioeng. Biotechnol. 2022, 10, 945531. [Google Scholar] [CrossRef]
- Qin, W.; Chandra, J.; Abourehab, M.A.S.; Gupta, N.; Chen, Z.S.; Kesharwani, P.; Cao, H.L. New opportunities for RGD-engineered metal nanoparticles in cancer. Mol. Cancer 2023, 22, 87. [Google Scholar] [CrossRef]
- Sakhtianchi, R.; Darvishi, B.; Mirzaie, Z.; Dorkoosh, F.; Shanehsazzadeh, S.; Dinarvand, R. Pegylated magnetic mesoporous silica nanoparticles decorated with AS1411 Aptamer as a targeting delivery system for cytotoxic agents. Pharm. Dev. Technol. 2019, 24, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Abraham, T.; McGovern, C.O.; Linton, S.S.; Wilczynski, Z.; Adair, J.H.; Matters, G.L. Aptamer-Targeted Calcium Phosphosilicate Nanoparticles for Effective Imaging of Pancreatic and Prostate Cancer. Int. J. Nanomed. 2021, 16, 2297–2309. [Google Scholar] [CrossRef]
- Liu, H.; Su, Y.Y.; Jiang, X.C.; Gao, J.Q. Cell membrane-coated nanoparticles: A novel multifunctional biomimetic drug delivery system. Drug Deliv. Transl. Res. 2023, 13, 716–737. [Google Scholar] [CrossRef]
- Jiang, Y.; Krishnan, N.; Zhou, J.; Chekuri, S.; Wei, X.; Kroll, A.V.; Yu, C.L.; Duan, Y.; Gao, W.; Fang, R.H.; et al. Engineered Cell-Membrane-Coated Nanoparticles Directly Present Tumor Antigens to Promote Anticancer Immunity. Adv. Mater. 2020, 32, e2001808. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.M.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef]
- Rao, L.; Meng, Q.F.; Bu, L.L.; Cai, B.; Huang, Q.; Sun, Z.J.; Zhang, W.F.; Li, A.; Guo, S.S.; Liu, W.; et al. Erythrocyte Membrane-Coated Upconversion Nanoparticles with Minimal Protein Adsorption for Enhanced Tumor Imaging. ACS Appl. Mater. Interfaces 2017, 9, 2159–2168. [Google Scholar] [CrossRef]
- Zhuang, J.; Gong, H.; Zhou, J.; Zhang, Q.; Gao, W.; Fang, R.H.; Zhang, L. Targeted gene silencing in vivo by platelet membrane-coated metal-organic framework nanoparticles. Sci. Adv. 2020, 6, eaaz6108. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, L.; Li, X.; Liu, F.; Cheng, X.; Ran, H.; Wang, Z.; Li, Y.; Feng, Y.; Liang, L.; et al. Anti-CXCR2 antibody-coated nanoparticles with an erythrocyte-platelet hybrid membrane layer for atherosclerosis therapy. J. Control. Release 2023, 356, 610–622. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, C.; Zhang, R.; Huang, L. Macrophage membrane-coated nanoparticles for the treatment of infectious diseases. Biomed. Mater. 2024, 19, 042003. [Google Scholar] [CrossRef]
- Oroojalian, F.; Beygi, M.; Baradaran, B.; Mokhtarzadeh, A.; Shahbazi, M.A. Immune Cell Membrane-Coated Biomimetic Nanoparticles for Targeted Cancer Therapy. Small 2021, 17, e2006484. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Yang, C.; Shi, K.; Liu, Y.; Hu, D.; He, X.; Yang, Y.; Chu, B.; Peng, J.; Zhou, Z.; et al. Activated macrophage membrane-coated nanoparticles relieve osteoarthritis-induced synovitis and joint damage. Biomaterials 2023, 295, 122036. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Pedersen, J.N.; Marie, R. Size and surface charge characterization of nanoparticles with a salt gradient. Nat. Commun. 2020, 11, 2337. [Google Scholar] [CrossRef]
- Awad, N.S.; Salkho, N.M.; Abuwatfa, W.H.; Paul, V.; AlSawaftah, N.M.; Husseini, G.A. Tumor vasculature vs tumor cell targeting: Understanding the latest trends in using functional nanoparticles for cancer treatment. OpenNano 2023, 11, 100136. [Google Scholar] [CrossRef]
- Choi, C.H.J.; Zuckerman, J.E.; Webster, P.; Davis, M.E. Targeting kidney mesangium by nanoparticles of defined size. Proc. Natl. Acad. Sci. USA 2011, 108, 6656–6661. [Google Scholar] [CrossRef]
- Aljabali, A.A.; Obeid, M.A.; Bashatwah, R.M.; Serrano-Aroca, Á.; Mishra, V.; Mishra, Y.; El-Tanani, M.; Hromić-Jahjefendić, A.; Kapoor, D.N.; Goyal, R.; et al. Nanomaterials and Their Impact on the Immune System. Int. J. Mol. Sci. 2023, 24, 2008. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, S.; Yang, D.; Fang, Y.; Lin, X.; Jin, X.; Liu, Y.; Liu, X.; Su, K.; Shi, K. Influencing factors and strategies of enhancing nanoparticles into tumors in vivo. Acta Pharm. Sin. B 2021, 11, 2265–2285. [Google Scholar] [CrossRef]
- Obeid, M.A.; Tate, R.J.; Mullen, A.B.; Ferro, V.A. Chapter 8-Lipid-based nanoparticles for cancer treatment. In Lipid Nanocarriers for Drug Targeting; Grumezescu, A.M., Ed.; William Andrew Publishing: Amsterdam, The Netherlands, 2018; pp. 313–359. [Google Scholar] [CrossRef]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef]
- Carvalho, P.M.; Felício, M.R.; Santos, N.C.; Gonçalves, S.; Domingues, M.M. Application of Light Scattering Techniques to Nanoparticle Characterization and Development. Front. Chem. 2018, 6, 237. [Google Scholar] [CrossRef]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates. Pharm. Res. 2010, 27, 796–810. [Google Scholar] [CrossRef]
- Hoo, C.M.; Starostin, N.; West, P.; Mecartney, M.L. A comparison of atomic force microscopy (AFM) and dynamic light scattering (DLS) methods to characterize nanoparticle size distributions. J. Nanoparticle Res. 2008, 10, 89–96. [Google Scholar] [CrossRef]
- Li, T.; Senesi, A.J.; Lee, B. Small Angle X-ray Scattering for Nanoparticle Research. Chem. Rev. 2016, 116, 11128–11180. [Google Scholar] [CrossRef] [PubMed]
- Pauw, B.R.; Kästner, C.; Thünemann, A.F. Nanoparticle size distribution quantification: Results of a small-angle X-ray scattering inter-laboratory comparison. J. Appl. Crystallogr. 2017, 50, 1280–1288. [Google Scholar] [CrossRef]
- Da Vela, S.; Svergun, D.I. Methods, development and applications of small-angle X-ray scattering to characterize biological macromolecules in solution. Curr. Res. Struct. Biol. 2020, 2, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Agbabiaka, A.; Wiltfong, M.; Park, C. Small Angle X-Ray Scattering Technique for the Particle Size Distribution of Nonporous Nanoparticles. J. Nanoparticles 2013, 2013, 640436. [Google Scholar] [CrossRef]
- Minelli, C.; Sikora, A.; Garcia-Diez, R.; Sparnacci, K.; Gollwitzer, C.; Krumrey, M.; Shard, A.G. Measuring the size and density of nanoparticles by centrifugal sedimentation and flotation. Anal. Methods 2018, 10, 1725–1732. [Google Scholar] [CrossRef]
- Perez-Potti, A.; Lopez, H.; Pelaz, B.; Abdelmonem, A.; Soliman, M.G.; Schoen, I.; Kelly, P.M.; Dawson, K.A.; Parak, W.J.; Krpetic, Z.; et al. In depth characterisation of the biomolecular coronas of polymer coated inorganic nanoparticles with differential centrifugal sedimentation. Sci. Rep. 2021, 11, 6443. [Google Scholar] [CrossRef]
- D’Atri, V.; Imiołek, M.; Quinn, C.; Finny, A.; Lauber, M.; Fekete, S.; Guillarme, D. Size exclusion chromatography of biopharmaceutical products: From current practices for proteins to emerging trends for viral vectors, nucleic acids and lipid nanoparticles. J. Chromatogr. A 2024, 1722, 464862. [Google Scholar] [CrossRef]
- Huang, C.; Neupane, Y.R.; Lim, X.C.; Shekhani, R.; Czarny, B.; Wacker, M.G.; Pastorin, G.; Wang, J.-W. Chapter Two-Extracellular vesicles in cardiovascular disease. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 103, pp. 47–95. [Google Scholar]
- Liau, B.; Zhang, L.; Ang, M.J.Y.; Ng, J.Y.; CV, S.B.; Schneider, S.; Gudihal, R.; Bae, K.H.; Yang, Y.Y. Quantitative analysis of mRNA-lipid nanoparticle stability in human plasma and serum by size-exclusion chromatography coupled with dual-angle light scattering. Nanomedicine 2024, 58, 102745. [Google Scholar] [CrossRef] [PubMed]
- Caputo, F.; Arnould, A.; Bacia, M.; Ling, W.L.; Rustique, E.; Texier, I.; Mello, A.P.; Couffin, A.-C. Measuring Particle Size Distribution by Asymmetric Flow Field Flow Fractionation: A Powerful Method for the Preclinical Characterization of Lipid-Based Nanoparticles. Mol. Pharm. 2019, 16, 756–767. [Google Scholar] [CrossRef]
- Klein, M.; Menta, M.; Dacoba, T.G.; Crecente-Campo, J.; Alonso, M.J.; Dupin, D.; Loinaz, I.; Grassl, B.; Séby, F. Advanced nanomedicine characterization by DLS and AF4-UV-MALS: Application to a HIV nanovaccine. J. Pharm. Biomed. Anal. 2020, 179, 113017. [Google Scholar] [CrossRef]
- Pei, Y.; Vogel, R.; Minelli, C. Chapter 3.1.4-Tunable resistive pulse sensing (TRPS). In Characterization of Nanoparticles; Hodoroaba, V.-D., Unger, W.E.S., Shard, A.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 117–136. [Google Scholar] [CrossRef]
- Willmott, G.R. Tunable Resistive Pulse Sensing: Better Size and Charge Measurements for Submicrometer Colloids. Anal. Chem. 2018, 90, 2987–2995. [Google Scholar] [CrossRef] [PubMed]
- Malatesta, M. Transmission electron microscopy for nanomedicine: Novel applications for long-established techniques. Eur. J. Histochem. 2016, 60, 2751. [Google Scholar] [CrossRef]
- Baxa, U. Imaging of Liposomes by Transmission Electron Microscopy. In Characterization of Nanoparticles Intended for Drug Delivery; McNeil, S.E., Ed.; Springer: New York, NY, USA, 2018; pp. 73–88. [Google Scholar] [CrossRef]
- Robson, A.-L.; Dastoor, P.C.; Flynn, J.; Palmer, W.; Martin, A.; Smith, D.W.; Woldu, A.; Hua, S. Advantages and Limitations of Current Imaging Techniques for Characterizing Liposome Morphology. Front. Pharmacol. 2018, 9, 80. [Google Scholar] [CrossRef]
- Kuntsche, J.; Horst, J.C.; Bunjes, H. Cryogenic transmission electron microscopy (cryo-TEM) for studying the morphology of colloidal drug delivery systems. Int. J. Pharm. 2011, 417, 120–137. [Google Scholar] [CrossRef]
- Yuana, Y.; Koning, R.I.; Kuil, M.E.; Rensen, P.C.; Koster, A.J.; Bertina, R.M.; Osanto, S. Cryo-electron microscopy of extracellular vesicles in fresh plasma. J. Extracell Vesicles 2013, 2, 21494. [Google Scholar] [CrossRef]
- Foreman-Ortiz, I.U.; Ma, T.F.; Hoover, B.M.; Wu, M.; Murphy, C.J.; Murphy, R.M.; Pedersen, J.A. Nanoparticle tracking analysis and statistical mixture distribution analysis to quantify nanoparticle–vesicle binding. J. Colloid Interface Sci. 2022, 615, 50–58. [Google Scholar] [CrossRef]
- Vogel, R.; Savage, J.; Muzard, J.; Della Camera, G.; Vella, G.; Law, A.; Marchioni, M.; Mehn, D.; Geiss, O.; Peacock, B.; et al. Measuring particle concentration of multimodal synthetic reference materials and extracellular vesicles with orthogonal techniques: Who is up to the challenge? J. Extracell. Vesicles 2021, 10, e12052. [Google Scholar] [CrossRef]
- Grobelny, J.; DelRio, F.W.; Pradeep, N.; Kim, D.I.; Hackley, V.A.; Cook, R.F. Size measurement of nanoparticles using atomic force microscopy. Methods Mol. Biol. 2011, 697, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Palacio, P.L.; Pleet, M.L.; Reátegui, E.; Magaña, S.M. Emerging role of extracellular vesicles in multiple sclerosis: From cellular surrogates to pathogenic mediators and beyond. J. Neuroimmunol. 2023, 377, 578064. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, C. Precise Analysis of Nanoparticle Size Distribution in TEM Image. Methods Protoc. 2023, 6. [Google Scholar] [CrossRef]
- Shen, S.; Wu, Y.; Liu, Y.; Wu, D. High drug-loading nanomedicines: Progress, current status, and prospects. Int. J. Nanomed. 2017, 12, 4085–4109. [Google Scholar] [CrossRef]
- Zheng, C.H.; Liang, W.Q.; Yu, H.Y.; Chen, H.L. Evaluation of different methods to determine the loading of proteins in PLGA microspheres. Pharmazie 2004, 59, 232–233. [Google Scholar]
- Iyer, R.; Kuriakose, A.E.; Yaman, S.; Su, L.C.; Shan, D.; Yang, J.; Liao, J.; Tang, L.; Banerjee, S.; Xu, H.; et al. Nanoparticle eluting-angioplasty balloons to treat cardiovascular diseases. Int. J. Pharm. 2019, 554, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Gomez, A.; Syed, S.; Marshall, K.; Hosseinidoust, Z. Liposomal Nanovesicles for Efficient Encapsulation of Staphylococcal Antibiotics. ACS Omega 2019, 4, 10866–10876. [Google Scholar] [CrossRef] [PubMed]
- Menon, J.U.; Kuriakose, A.; Iyer, R.; Hernandez, E.; Gandee, L.; Zhang, S.; Takahashi, M.; Zhang, Z.; Saha, D.; Nguyen, K.T. Dual-Drug Containing Core-Shell Nanoparticles for Lung Cancer Therapy. Sci. Rep. 2017, 7, 13249. [Google Scholar] [CrossRef]
- Alshawwa, S.Z.; Kassem, A.A.; Farid, R.M.; Mostafa, S.K.; Labib, G.S. Nanocarrier Drug Delivery Systems: Characterization, Limitations, Future Perspectives and Implementation of Artificial Intelligence. Pharmaceutics 2022, 14, 883. [Google Scholar] [CrossRef]
- Mendes, B.B.; Conniot, J.; Avital, A.; Yao, D.; Jiang, X.; Zhou, X.; Sharf-Pauker, N.; Xiao, Y.; Adir, O.; Liang, H.; et al. Nanodelivery of nucleic acids. Nat. Rev. Methods Primers 2022, 2, 24. [Google Scholar] [CrossRef]
- Shishparenok, A.N.; Furman, V.V.; Zhdanov, D.D. DNA-Based Nanomaterials as Drug Delivery Platforms for Increasing the Effect of Drugs in Tumors. Cancers 2023, 15, 2151. [Google Scholar] [CrossRef] [PubMed]
- Bruno, B.J.; Miller, G.D.; Lim, C.S. Basics and recent advances in peptide and protein drug delivery. Ther. Deliv. 2013, 4, 1443–1467. [Google Scholar] [CrossRef]
- Mirón-Barroso, S.; Domènech, E.B.; Trigueros, S. Nanotechnology-Based Strategies to Overcome Current Barriers in Gene Delivery. Int. J. Mol. Sci. 2021, 22, 8537. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.Y.; Costumbrado, J.; Hsu, C.Y.; Kim, Y.H. Agarose gel electrophoresis for the separation of DNA fragments. J. Vis. Exp. 2012, 62, 3923. [Google Scholar] [CrossRef]
- Dimitrov, D.S. Therapeutic proteins. Methods Mol. Biol. 2012, 899, 1–26. [Google Scholar] [CrossRef]
- Jain, A.; Singh, S.K.; Arya, S.K.; Kundu, S.C.; Kapoor, S. Protein Nanoparticles: Promising Platforms for Drug Delivery Applications. ACS Biomater. Sci. Eng. 2018, 4, 3939–3961. [Google Scholar] [CrossRef]
- Yu, M.; Wu, J.; Shi, J.; Farokhzad, O.C. Nanotechnology for protein delivery: Overview and perspectives. J. Control. Release 2016, 240, 24–37. [Google Scholar] [CrossRef]
- Morton, R.E.; Evans, T.A. Modification of the bicinchoninic acid protein assay to eliminate lipid interference in determining lipoprotein protein content. Anal. Biochem. 1992, 204, 332–334. [Google Scholar] [CrossRef]
- Gates, R.E. Elimination of interfering substances in the presence of detergent in the bicinchoninic acid protein assay. Anal. Biochem. 1991, 196, 290–295. [Google Scholar] [CrossRef]
- Noble, J.E.; Bailey, M.J. Quantitation of protein. Methods Enzym. 2009, 463, 73–95. [Google Scholar] [CrossRef]
- Matthessen, R.; Van Pottelberge, R.; Goffin, B.; De Winter, G. Impact of mixing and shaking on mRNA-LNP drug product quality characteristics. Sci. Rep. 2024, 14, 19590. [Google Scholar] [CrossRef]
- Li, Y.; Ye, Z.; Yang, H.; Xu, Q. Tailoring combinatorial lipid nanoparticles for intracellular delivery of nucleic acids, proteins, and drugs. Acta Pharm. Sin. B 2022, 12, 2624–2639. [Google Scholar] [CrossRef] [PubMed]
- Ban, E.; Kim, A. PicoGreen assay for nucleic acid quantification-Applications, challenges, and solutions. Anal. Biochem. 2024, 692, 115577. [Google Scholar] [CrossRef]
- Ahmad, R.M.; Greish, Y.E.; El-Maghraby, H.F.; Lubbad, L.; Makableh, Y.; Hammad, F.T. Preparation and Characterization of Blank and Nerolidol-Loaded Chitosan-Alginate Nanoparticles. Nanomaterials 2022, 12, 1183. [Google Scholar] [CrossRef]
- Anthis, N.J.; Clore, G.M. Sequence-specific determination of protein and peptide concentrations by absorbance at 205 nm. Protein. Sci. 2013, 22, 851–858. [Google Scholar] [CrossRef]
- Mirjalili, F.; Mahmoodi, M. Controlled release of protein from gelatin/chitosan hydrogel containing platelet-rich fibrin encapsulated in chitosan nanoparticles for accelerated wound healing in an animal model. Int. J. Biol. Macromol. 2023, 225, 588–604. [Google Scholar] [CrossRef]
- Iranpour, S.; Nejati, V.; Delirezh, N.; Biparva, P.; Shirian, S. Enhanced stimulation of anti-breast cancer T cells responses by dendritic cells loaded with poly lactic-co-glycolic acid (PLGA) nanoparticle encapsulated tumor antigens. J. Exp. Clin. Cancer Res. 2016, 35, 168. [Google Scholar] [CrossRef]
- Gaur, M.; Maurya, S.; Akhtar, M.S.; Yadav, A.B. Synthesis and Evaluation of BSA-Loaded PLGA-Chitosan Composite Nanoparticles for the Protein-Based Drug Delivery System. ACS Omega 2023, 8, 18751–18759. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, P.; Liu, L.; Ma, G.; Ma, J.; Liu, X.; Liu, Y.; Lin, W.; Zhu, Y. Evaluation of PLGA containing anti-CTLA4 inhibited endometriosis progression by regulating CD4+CD25+Treg cells in peritoneal fluid of mouse endometriosis model. Eur. J. Pharm. Sci. 2017, 96, 542–550. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, J.; Yang, Z.; Xu, J.; Xu, L.; Liang, C.; Han, X.; Liu, Z. Nanoparticle-Enhanced Radiotherapy to Trigger Robust Cancer Immunotherapy. Adv. Mater. 2019, 31, e1802228. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, V.P.; Ibrahim, S.; Zahedi, R.P.; Borchers, C.H. Utility, promise, and limitations of liquid chromatography-mass spectrometry-based therapeutic drug monitoring in precision medicine. J. Mass Spectrom. 2021, 56, e4788. [Google Scholar] [CrossRef]
- Khajavinia, A.; Yarahmadi, M.; El-Aneed, A.; Haddadi, A. Development of a liquid chromatography-tandem mass spectrometry method for the analysis of docetaxel-loaded Poly(lactic-co-glycolic acid) nanoparticles. J. Pharm. Biomed. Anal. 2023, 223, 115114. [Google Scholar] [CrossRef]
- Amani, A.; Kabiri, T.; Shafiee, S.; Hamidi, A. Preparation and Characterization of PLA-PEG-PLA/PEI/DNA Nanoparticles for Improvement of Transfection Efficiency and Controlled Release of DNA in Gene Delivery Systems. Iran J. Pharm. Res. 2019, 18, 125–141. [Google Scholar]
- Senapati, S.; Upadhyaya, A.; Dhruw, S.; Giri, D.; Maiti, P. Controlled DNA Delivery Using Poly(lactide) Nanoparticles and Understanding the Binding Interactions. J. Phys. Chem. B 2021, 125, 10009–10017. [Google Scholar] [CrossRef] [PubMed]
- Schober, G.B.; Story, S.; Arya, D.P. A careful look at lipid nanoparticle characterization: Analysis of benchmark formulations for encapsulation of RNA cargo size gradient. Sci. Rep. 2024, 14, 2403. [Google Scholar] [CrossRef] [PubMed]
- Messerschmidt, V.L.; Chintapula, U.; Kuriakose, A.E.; Laboy, S.; Truong, T.T.D.; Kydd, L.A.; Jaworski, J.; Pan, Z.; Sadek, H.; Nguyen, K.T.; et al. Notch Intracellular Domain Plasmid Delivery via Poly(Lactic-Co-Glycolic Acid) Nanoparticles to Upregulate Notch Pathway Molecules. Front. Cardiovasc. Med. 2021, 8, 707897. [Google Scholar] [CrossRef] [PubMed]
- Amir Kalvanagh, P.; Ebtekara, M.; Kokhaei, P.; Soleimanjahi, H. Preparation and Characterization of PLGA Nanoparticles Containing Plasmid DNA Encoding Human IFN-lambda-1/IL-29. Iran. J. Pharm. Res. IJPR 2019, 18, 156–167. [Google Scholar]
- Patil, Y.; Panyam, J. Polymeric nanoparticles for siRNA delivery and gene silencing. Int. J. Pharm. 2009, 367, 195–203. [Google Scholar] [CrossRef]
- Cai, W.; Luo, T.; Chen, X.; Mao, L.; Wang, M. A Combinatorial Library of Biodegradable Lipid Nanoparticles Preferentially Deliver mRNA into Tumor Cells to Block Mutant RAS Signaling. Adv. Funct. Mater. 2022, 32, 2204947. [Google Scholar] [CrossRef]
- Swingle, K.L.; Billingsley, M.M.; Bose, S.K.; White, B.; Palanki, R.; Dave, A.; Patel, S.K.; Gong, N.; Hamilton, A.G.; Alameh, M.-G.; et al. Amniotic fluid stabilized lipid nanoparticles for in utero intra-amniotic mRNA delivery. J. Control. Release 2022, 341, 616–633. [Google Scholar] [CrossRef]
- Kim, J.; Jozic, A.; Lin, Y.; Eygeris, Y.; Bloom, E.; Tan, X.; Acosta, C.; MacDonald, K.D.; Welsher, K.D.; Sahay, G. Engineering Lipid Nanoparticles for Enhanced Intracellular Delivery of mRNA through Inhalation. ACS Nano 2022, 16, 14792–14806. [Google Scholar] [CrossRef]
- Patel, S.K.; Billingsley, M.M.; Frazee, C.; Han, X.; Swingle, K.L.; Qin, J.; Alameh, M.-G.; Wang, K.; Weissman, D.; Mitchell, M.J. Hydroxycholesterol substitution in ionizable lipid nanoparticles for mRNA delivery to T cells. J. Control. Release 2022, 347, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Lowenthal, M.S.; Antonishek, A.S.; Phinney, K.W. Quantification of mRNA in Lipid Nanoparticles Using Mass Spectrometry. Anal. Chem. 2024, 96, 1214–1222. [Google Scholar] [CrossRef]
- Fan, Y.; Marioli, M.; Zhang, K. Analytical characterization of liposomes and other lipid nanoparticles for drug delivery. J. Pharm. Biomed. Anal. 2021, 192, 113642. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Ma, R.; Xu, G.; Chen, Z.; Zhang, D.; Wang, Q.; Hei, L.; Ma, W. Development and In Vitro Release of Isoniazid and Rifampicin-Loaded Bovine Serum Albumin Nanoparticles. Med. Sci. Monit. 2018, 24, 473–478. [Google Scholar] [CrossRef]
- Mickaela Martinez, S.; Inda, A.; Marina Garcia, A.; María Bermúdez, J.; Emilio Gonzo, E.; Herrero-Vanrell, R.; Domingo Luna, J.; Alberto Allemandi, D.; Alejandra Quinteros, D. Development of melatonin-loaded, human-serum-albumin nanoparticles formulations using different methods of preparation for ophthalmic administration. Int. J. Pharm. 2022, 628, 122308. [Google Scholar] [CrossRef] [PubMed]
- Saruchi; Kumar, M.; Kumar, V.; Ghfar, A.A.; Pandey, S. A Green Approach for the Synthesis of Silver Nanoparticle-Embedded Chitosan Bionanocomposite as a Potential Device for the Sustained Release of the Itraconazole Drug and Its Antibacterial Characteristics. Polymers 2022, 14, 1911. [Google Scholar] [CrossRef]
- Boersma, B.; Möller, K.; Wehl, L.; Puddinu, V.; Huard, A.; Fauteux-Daniel, S.; Bourquin, C.; Palmer, G.; Bein, T. Inhibition of IL-1β release from macrophages targeted with necrosulfonamide-loaded porous nanoparticles. J. Control. Release 2022, 351, 989–1002. [Google Scholar] [CrossRef]
- Iyer, R.; Nguyen, T.; Padanilam, D.; Xu, C.; Saha, D.; Nguyen, K.T.; Hong, Y. Glutathione-responsive biodegradable polyurethane nanoparticles for lung cancer treatment. J. Control. Release 2020, 321, 363–371. [Google Scholar] [CrossRef]
- Li, X.; Xu, T.; Wu, C.; Fan, G.; Li, T.; Wang, Y.; Zhou, D. Fabrication and characterization of self-assembled whey protein isolate/short linear glucan core-shell nanoparticles for sustained release of curcumin. Food Chem. 2023, 407, 135124. [Google Scholar] [CrossRef] [PubMed]
- Watcharadulyarat, N.; Rattanatayarom, M.; Ruangsawasdi, N.; Patikarnmonthon, N. PEG-PLGA nanoparticles for encapsulating ciprofloxacin. Sci. Rep. 2023, 13, 266. [Google Scholar] [CrossRef]
- Díaz-Saldívar, P.; Huidobro-Toro, J.P. ATP-loaded biomimetic nanoparticles as controlled release system for extracellular drugs in cancer applications. Int. J. Nanomed. 2019, 14, 2433–2447. [Google Scholar] [CrossRef]
- Qazi, R.E.M.; Sajid, Z.; Zhao, C.; Hussain, I.; Iftikhar, F.; Jameel, M.; Rehman, F.U.; Mian, A.A. Lyophilization Based Isolation of Exosomes. Int. J. Mol. Sci. 2023, 24, 10477. [Google Scholar] [CrossRef]
- Kwon, Y.; Park, J. Methods to analyze extracellular vesicles at single particle level. Micro Nano Syst. Lett. 2022, 10, 14. [Google Scholar] [CrossRef]
- Arab, T.; Mallick, E.R.; Huang, Y.; Dong, L.; Liao, Z.; Zhao, Z.; Gololobova, O.; Smith, B.; Haughey, N.J.; Pienta, K.J.; et al. Characterization of extracellular vesicles and synthetic nanoparticles with four orthogonal single-particle analysis platforms. J. Extracell. Vesicles 2021, 10, e12079. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Lázaro-Ibáñez, E.; Gunnarsson, A.; Dhande, A.; Daaboul, G.; Peacock, B.; Osteikoetxea, X.; Salmond, N.; Friis, K.P.; Shatnyeva, O.; et al. Quantification of protein cargo loading into engineered extracellular vesicles at single-vesicle and single-molecule resolution. J. Extracell Vesicles 2021, 10, e12130. [Google Scholar] [CrossRef]
- Liu, H.; Tian, Y.; Xue, C.; Niu, Q.; Chen, C.; Yan, X. Analysis of extracellular vesicle DNA at the single-vesicle level by nano-flow cytometry. J. Extracell Vesicles 2022, 11, e12206. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.P.d.; Zigon, E.; Rogers, G.; Davodian, D.; Lu, S.; Jovanovic-Talisman, T.; Jones, J.; Tigges, J.; Tyagi, S.; Ghiran, I.C. Detection of Extracellular Vesicle RNA Using Molecular Beacons. iScience 2020, 23, 100782. [Google Scholar] [CrossRef]
- McCarthy Riley, B.F.; Mai, H.T.; Linz, T.H. Microfluidic Digital Quantitative PCR to Measure Internal Cargo of Individual Liposomes. Anal. Chem. 2022, 94, 7433–7441. [Google Scholar] [CrossRef]
- Liu, K.J.; Wang, T.H. Cylindrical illumination confocal spectroscopy: Rectifying the limitations of confocal single molecule spectroscopy through one-dimensional beam shaping. Biophys. J. 2008, 95, 2964–2975. [Google Scholar] [CrossRef]
- Li, S.; Hu, Y.; Lin, J.; Schneiderman, Z.; Shao, F.; Wei, L.; Li, A.; Hsieh, K.; Kokkoli, E.; Curk, T.; et al. Single-Particle Spectroscopic Chromatography Reveals Heterogeneous RNA Loading and Size Correlations in Lipid Nanoparticles. ACS Nano 2024, 18, 15729–15743. [Google Scholar] [CrossRef] [PubMed]
- Saha, T.; Fojtů, M.; Nagar, A.V.; Thurakkal, L.; Srinivasan, B.B.; Mukherjee, M.; Sibiyon, A.; Aggarwal, H.; Samuel, A.; Dash, C.; et al. Antibody nanoparticle conjugate–based targeted immunotherapy for non–small cell lung cancer. Sci. Adv. 2024, 10, eadi2046. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.J.; Bu, J.; Kubiatowicz, L.J.; Chen, S.S.; Kim, Y.; Hong, S. Peptide-nanoparticle conjugates: A next generation of diagnostic and therapeutic platforms? Nano Converg. 2018, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, X.; Ye, M.; Jiang, J.; Yang, R.; Fu, T.; Chen, Y.; Wang, K.; Liu, C.; Tan, W. Aptamer-conjugated nanomaterials and their applications. Adv. Drug Deliv. Rev. 2011, 63, 1361–1370. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Zhu, L.; Weller, H.; Mews, A.; Parak, W.J.; Barz, M.; Feliu, N. Ligand density on nanoparticles: A parameter with critical impact on nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 22–36. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, D.; Salmain, M.; Liedberg, B.; Boujday, S. Direct quantification of surface coverage of antibody in IgG-Gold nanoparticles conjugates. Talanta 2019, 204, 875–881. [Google Scholar] [CrossRef]
- Filbrun, S.L.; Driskell, J.D. A fluorescence-based method to directly quantify antibodies immobilized on gold nanoparticles. Analyst 2016, 141, 3851–3857. [Google Scholar] [CrossRef]
- Tripathi, K.; Driskell, J.D. Quantifying Bound and Active Antibodies Conjugated to Gold Nanoparticles: A Comprehensive and Robust Approach To Evaluate Immobilization Chemistry. ACS Omega 2018, 3, 8253–8259. [Google Scholar] [CrossRef]
- Jayawardena, H.S.N.; Liyanage, S.H.; Rathnayake, K.; Patel, U.; Yan, M. Analytical Methods for Characterization of Nanomaterial Surfaces. Anal. Chem. 2021, 93, 1889–1911. [Google Scholar] [CrossRef]
- Perera, Y.R.; Hill, R.A.; Fitzkee, N.C. Protein Interactions with Nanoparticle Surfaces: Highlighting Solution NMR Techniques. Isr. J. Chem. 2019, 59, 962–979. [Google Scholar] [CrossRef]
- Woythe, L.; Madhikar, P.; Feiner-Gracia, N.; Storm, C.; Albertazzi, L. A Single-Molecule View at Nanoparticle Targeting Selectivity: Correlating Ligand Functionality and Cell Receptor Density. ACS Nano 2022, 16, 3785–3796. [Google Scholar] [CrossRef] [PubMed]
- García-Fernández, L.; Garcia-Pardo, J.; Tort, O.; Prior, I.; Brust, M.; Casals, E.; Lorenzo, J.; Puntes, V.F. Conserved effects and altered trafficking of Cetuximab antibodies conjugated to gold nanoparticles with precise control of their number and orientation. Nanoscale 2017, 9, 6111–6121. [Google Scholar] [CrossRef] [PubMed]
- Herda, L.M.; Hristov, D.R.; Lo Giudice, M.C.; Polo, E.; Dawson, K.A. Mapping of Molecular Structure of the Nanoscale Surface in Bionanoparticles. J. Am. Chem. Soc. 2017, 139, 111–114. [Google Scholar] [CrossRef]
- Delcanale, P.; Miret-Ontiveros, B.; Arista-Romero, M.; Pujals, S.; Albertazzi, L. Nanoscale Mapping Functional Sites on Nanoparticles by Points Accumulation for Imaging in Nanoscale Topography (PAINT). ACS Nano 2018, 12, 7629–7637. [Google Scholar] [CrossRef]
- Jazayeri, M.H.; Amani, H.; Pourfatollah, A.A.; Pazoki-Toroudi, H.; Sedighimoghaddam, B. Various methods of gold nanoparticles (GNPs) conjugation to antibodies. Sens. Bio-Sens. Res. 2016, 9, 17–22. [Google Scholar] [CrossRef]
- Zhenxi, Z.; Sijia, W.; Hao, X.; Bo, W.; Cuiping, Y. Role of 5-aminolevulinic acid-conjugated gold nanoparticles for photodynamic therapy of cancer. J. Biomed. Opt. 2015, 20, 051043. [Google Scholar] [CrossRef]
- Desai, N.; Tambe, V.; Pofali, P.; Vora, L.K. Cell Membrane-Coated Nanoparticles: A New Frontier in Immunomodulation. Adv. NanoBiomed Res. 2024, 4, 2400012. [Google Scholar] [CrossRef]
- Yaman, S.; Ramachandramoorthy, H.; Oter, G.; Zhukova, D.; Nguyen, T.; Sabnani, M.K.; Weidanz, J.A.; Nguyen, K.T. Melanoma Peptide MHC Specific TCR Expressing T-Cell Membrane Camouflaged PLGA Nanoparticles for Treatment of Melanoma Skin Cancer. Front. Bioeng. Biotechnol. 2020, 8, 343. [Google Scholar] [CrossRef]
- Zheng, P.; Zhou, C.; Ding, Y.; Liu, B.; Lu, L.; Zhu, F.; Duan, S. Nanopore sequencing technology and its applications. MedComm 2023, 4, e316. [Google Scholar] [CrossRef]
- Singh, A. Nanopores for sequencing proteins. Nat. Methods 2023, 20, 1870. [Google Scholar] [CrossRef]
- Scott, R.; Minjun, K.; George, A. Plasmonic nanopore sensing with continuous AC modulation. In Optical Trapping and Optical Micromanipulation; SPIE: Bellingham, WA, USA, 2023; p. 1264905. [Google Scholar]
- Lee, J.S.; Saharia, J.; Bandara, Y.M.N.D.Y.; Karawdeniya, B.I.; Goyal, G.; Darvish, A.; Wang, Q.; Kim, M.J.; Kim, M.J. Stiffness measurement of nanosized liposomes using solid-state nanopore sensor with automated recapturing platform. Electrophoresis 2019, 40, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Stranik, O.; McEvoy, H.M.; McDonagh, C.; MacCraith, B.D. Plasmonic enhancement of fluorescence for sensor applications. Sens. Actuators B Chem. 2005, 107, 148–153. [Google Scholar] [CrossRef]
- Ural, M.S.; Dartois, E.; Mathurin, J.; Desmaële, D.; Collery, P.; Dazzi, A.; Deniset-Besseau, A.; Gref, R. Quantification of drug loading in polymeric nanoparticles using AFM-IR technique: A novel method to map and evaluate drug distribution in drug nanocarriers. Analyst 2022, 147, 5564–5578. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Deng, D.; Liu, R.; Lv, Y. Single nanoparticle analysis by ICPMS: A potential tool for bioassay. J. Anal. At. Spectrom. 2018, 33, 57–67. [Google Scholar] [CrossRef]
| Category | Methods | Detection Range | Parameters That Can Be Measured | Advantages | Disadvantages | Sources |
|---|---|---|---|---|---|---|
| Bulk analysis | Dynamic light scattering (DLS) | 0.3 nm–10 µm | Size, zeta potential, polydispersity | Can be conducted on a wide range of sample buffers, temperatures and concentrations. Non-invasive technique. Low amount of sample required [83]. Low peak resolution, can only resolve particle size at least by a factor of 3 [85]. | Low resolution. Only for transparent sample preparation. Concentration needs to be optimized to produce reliable data [83]. | [83,85,90] |
| Small angle X-ray scattering | 1–1000 nm [89] | Size, size distribution, shape, structure parameter, internal structure, crystallinity | Can determine various types of nanoparticles [90]. Little sample preparation time [90]. | Low resolution, complexity in data interpretation, can be affected by solvent, sample preparation has to be dispersed. | [89,90] | |
| Field flow fractionation (FFF) | 1 nm–hundreds of µm | Size, particle size distribution, molecular weight, shape, morphology, density, concentration | High separation efficiency, minimal sample requirement, real-time monitoring. | Not effective in differentiating small molecules. | [108] | |
| Size-exclusion chromatography (SEC) | 10 kDa–1000 kDa | Size, size distribution | Preserve biological activity, fast, easy sample preparation. | Hard to differentiate populations of samples with similar sizes. | ||
| Centrifugal Sedimentation | 0.01–40 µm | Size, size distribution, density, shape, concentration | High resolution, minimal sample preparation, rapid analysis, real-time monitoring | Causes damage to particles. | [91] | |
| Single-molecule characterization | Tunable resistive pulse sensing | 40 nm–11 µm (Izon) 40–20 µM [106] | Size, size distribution, zeta potential | More accurate. Can also be used to measure charge. Single-molecule analysis. | Clogging of analytes. Cannot measure large particles. | [106] |
| Scanning Electron Microscopy (SEM) | Resolution: 3–20 nm | Size, polydispersity | High resolution. | Can only obtain surface information of nanoparticles [83]. | [83] | |
| Transmission Electron Microscopy (TEM), Cryo-TEM | 0.1 nm–10 µm | Size, polydispersity | Direct visualization. Can visualize the interior of the specimen. Cryo-TEM: can keep analytes in native form. | Expensive equipment. Complicated, time-consuming sample process. Only provides static and 2-dimensional information. | [103,109] | |
| Nanoparticle tracking analysis (NTA) | 30–1000 nm [85] 30–600 nm [106] | Size, polydispersity, concentration | Enables sample visualization. Provides approximate concentration. | Requires optimization. More time-consuming than DLS. | [85,106] | |
| Atomic Force Microscopy (AFM)–Single molecule | 0.5–50 nm | Size, deformability | Can see analytes’ topography. Allows 3D visualization [86]. | Sample needs to be fixed. Deposition method will alter the size distribution [86]. | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, V.; Nguyen, N.; Renkes, S.; Nguyen, K.T.; Nguyen, T.; Alexandrakis, G. Current and Near-Future Technologies to Quantify Nanoparticle Therapeutic Loading Efficiency and Surface Coating Efficiency with Targeted Moieties. Bioengineering 2025, 12, 362. https://doi.org/10.3390/bioengineering12040362
Tran V, Nguyen N, Renkes S, Nguyen KT, Nguyen T, Alexandrakis G. Current and Near-Future Technologies to Quantify Nanoparticle Therapeutic Loading Efficiency and Surface Coating Efficiency with Targeted Moieties. Bioengineering. 2025; 12(4):362. https://doi.org/10.3390/bioengineering12040362
Chicago/Turabian StyleTran, Vy, Na Nguyen, Scott Renkes, Kytai T. Nguyen, Tam Nguyen, and George Alexandrakis. 2025. "Current and Near-Future Technologies to Quantify Nanoparticle Therapeutic Loading Efficiency and Surface Coating Efficiency with Targeted Moieties" Bioengineering 12, no. 4: 362. https://doi.org/10.3390/bioengineering12040362
APA StyleTran, V., Nguyen, N., Renkes, S., Nguyen, K. T., Nguyen, T., & Alexandrakis, G. (2025). Current and Near-Future Technologies to Quantify Nanoparticle Therapeutic Loading Efficiency and Surface Coating Efficiency with Targeted Moieties. Bioengineering, 12(4), 362. https://doi.org/10.3390/bioengineering12040362








