Predictive Model of Gemtuzumab Ozogamicin Response in Childhood Acute Myeloid Leukemia on Event-Free Survival: Data Analysis Based on Trial AAML0531
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Study Design
2.3. Cytogenetic and Molecular Profiling
2.4. Treatment Response and Follow-Up
2.5. Statistical Analyses
3. Results
3.1. Univariable and Multivariable Analyses
3.2. Development and Assessment of the Predictive Nomogram
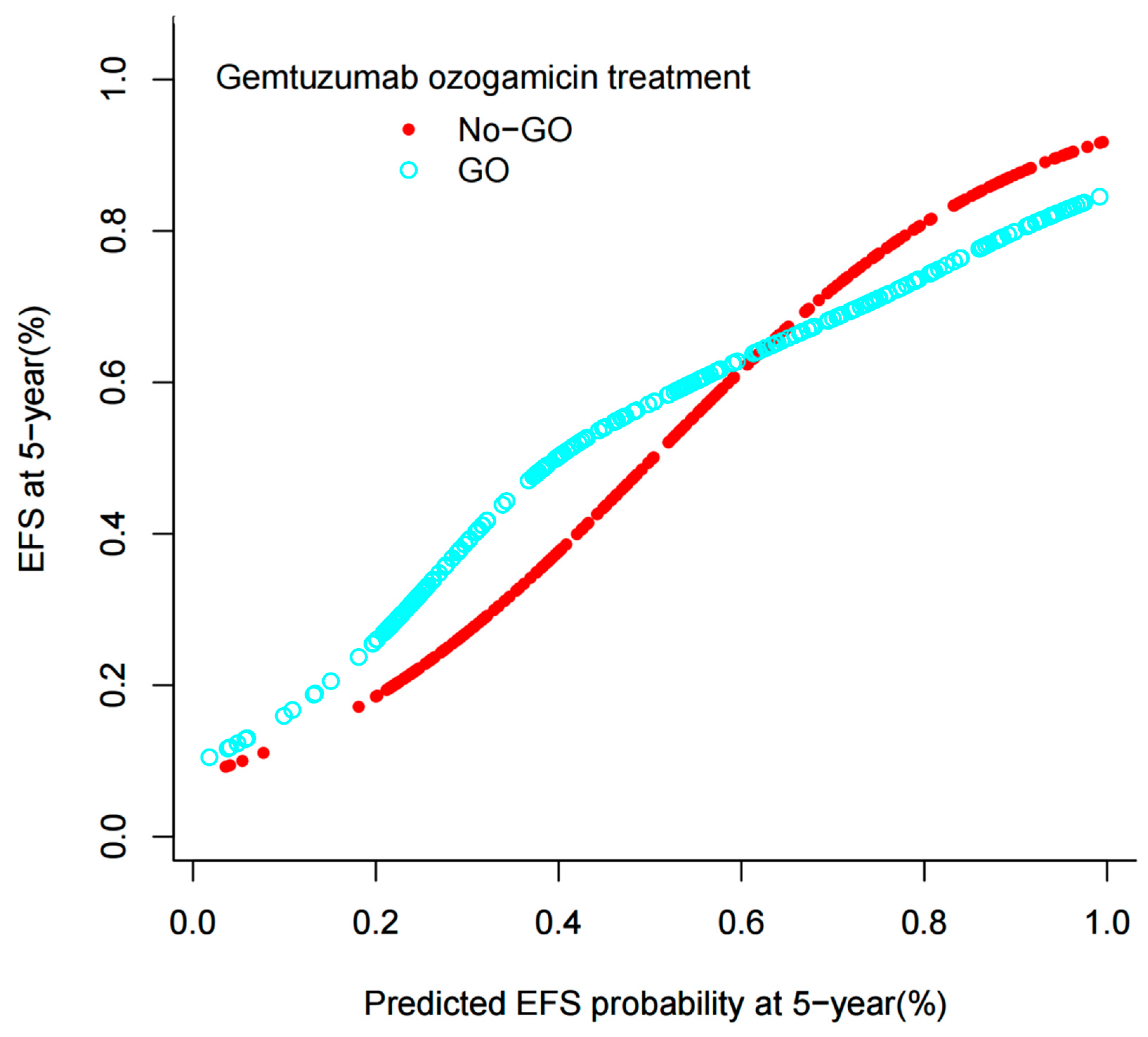
3.2.1. Model Development
3.2.2. Model Validation
3.3. Survival Analysis for the Entire Cohort
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Döhner, H.; Wei, A.H.; Löwenberg, B. Towards precision medicine for AML. Nat. Rev. Clin. Oncol. 2021, 18, 577–590. [Google Scholar] [CrossRef]
- Daver, N.; Schlenk, R.F.; Russell, N.H.; Levis, M.J. Targeting FLT3 mutations in AML: Review of current knowledge and evidence. Leukemia 2019, 33, 299–312. [Google Scholar] [CrossRef]
- Kayser, S.; Levis, M.J. Updates on targeted therapies for acute myeloid leukaemia. Br. J. Haematol. 2022, 196, 316–328. [Google Scholar] [CrossRef]
- Röllig, C. Gemtuzumab ozogamicin in AML: The next chapter. Blood 2023, 142, 1673–1674. [Google Scholar] [CrossRef]
- Lambert, J.; Pautas, C.; Terré, C.; Raffoux, E.; Turlure, P.; Caillot, D.; Legrand, O.; Thomas, X.; Gardin, C.; Gogat-Marchant, K.; et al. Gemtuzumab ozogamicin for de novo acute myeloid leukemia: Final efficacy and safety updates from the open-label, phase III ALFA-0701 trial. Haematologica 2019, 104, 113–119. [Google Scholar] [CrossRef]
- Freeman, S.D.; Thomas, A.; Thomas, I.; Hills, R.K.; Vyas, P.; Gilkes, A.F.; Metzner, M.; Jakobsen, N.A.; Kennedy, A.; Moore, R.; et al. Fractionated vs single-dose gemtuzumab ozogamicin with determinants of benefit in older patients with AML: The UK NCRI AML18 trial. Blood 2023, 142, 1697–1707. [Google Scholar] [CrossRef]
- Dhillon, S. Inotuzumab Ozogamicin. First Pediatric Approval. Paediatr. Drugs 2024, 26, 59–467. [Google Scholar] [CrossRef]
- Fenwarth, L.; Fournier, E.; Cheok, M.; Boyer, T.; Gonzales, F.; Castaigne, S.; Boissel, N.; Lambert, J.; Dombret, H.; Preudhomme, C.; et al. Biomarkers of Gemtuzumab Ozogamicin Response for Acute Myeloid Leukemia Treatment. Int. J. Mol. Sci. 2020, 21, 5626. [Google Scholar] [CrossRef]
- Wang, H.; Yang, S.; Chen, L.; Li, Y.; He, P.; Wang, G.; Dong, H.; Ma, P.; Ding, G. Tumor diagnosis using carbon-based quantum dots: Detection based on the hallmarks of cancer. Bioact. Mater. 2023, 33, 174–222. [Google Scholar] [CrossRef]
- Wijnen, N.E.; Koedijk, J.B.; Klein, K.; Luesink, M.; Goemans, B.F.; Zwaan, C.M.; Kaspers, G.J. Treating CD33-Positive de novo Acute Myeloid Leukemia in Pediatric Patients: Focus on the Clinical Value of Gemtuzumab Ozogamicin. Onco Targets Ther. 2023, 16, 297–308. [Google Scholar] [CrossRef]
- Aplenc, R.; Alonzo, T.A.; Gerbing, R.B.; Lange, B.J.; Hurwitz, C.A.; Wells, R.J.; Bernstein, I.; Buckley, P.; Krimmel, K.; Smith, F.O.; et al. Safety and efficacy of gemtuzumab ozogamicin in combination with chemotherapy for pediatric acute myeloid leukemia: A report from the Children’s Oncology Group. J. Clin. Oncol. 2008, 26, 2390–3295. [Google Scholar] [CrossRef] [PubMed]
- Cooper, T.M.; Franklin, J.; Gerbing, R.B.; Alonzo, T.A.; Hurwitz, C.; Raimondi, S.C.; Hirsch, B.; Smith, F.O.; Mathew, P.; Arceci, R.J.; et al. AAML03P1, a pilot study of the safety of gemtuzumab ozogamicin in combination with chemotherapy for newly diagnosed childhood acute myeloid leukemia: A report from the Children’s Oncology Group. Cancer 2012, 118, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.M.; DiNardo, C.D.; Kadia, T.M.; Daver, N.G.; Altman, J.K.; Stein, E.M.; Jabbour, E.; Schiffer, C.A.; Lang, A.; Ravandi, F. Acute myeloid leukemia management and research in 2025. CA Cancer J. Clin. 2025, 75, 46–67. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Thota, S.; Bradley, T.; Griffiths, E.A.; Faber, M.G.; Sadek, S.; Przespolewski, A.; Thompson, J.E.; Baron, J.; Cronin, T.; et al. Outcomes of Adult Acute Myeloid Leukemia Treated with Gemtuzumab-Ozogamicin: Cue to Optimized Chemotherapy Backbone. Clin. Lymphoma Myeloma Leuk. 2021, 21, 613–620. [Google Scholar] [CrossRef]
- Petersdorf, S.H.; Kopecky, K.J.; Slovak, M.; Willman, C.; Nevill, T.; Brandwein, J.; Larson, R.A.; Erba, H.P.; Stiff, P.J.; Stuart, R.K.; et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood 2013, 121, 4854–4860. [Google Scholar] [CrossRef]
- Burnett, A.; Cavenagh, J.; Russell, N.; Hills, R.; Kell, J.; Jones, G.; Nielsen, O.J.; Khwaja, A.; Thomas, I.; Clark, R. Defining the dose of gemtuzumab ozogamicin in combination with induction chemotherapy in acute myeloid leukemia: A comparison of 3 mg/m2 with 6 mg/m2 in the NCRI AML17 Trial. Haematologica 2016, 101, 724–731. [Google Scholar] [CrossRef]
- Bornhäuser, M.; Schliemann, C.; Schetelig, J.; Röllig, C.; Kramer, M.; Glass, B.; Platzbecker, U.; Burchert, A.; Hänel, M.; Müller, L.P.; et al. Allogeneic Hematopoietic Cell Transplantation vs Standard Consolidation Chemotherapy in Patients With Intermediate-Risk Acute Myeloid Leukemia: A Randomized Clinical Trial. JAMA Oncol. 2023, 9, 519–526. [Google Scholar] [CrossRef]
- Burnett, A.K.; Hills, R.K.; Grimwade, D.; Jovanovic, J.V.; Craig, J.; McMullin, M.F.; Kell, J.; Wheatley, K.; Yin, J.A.L.; Hunter, A.; et al. Inclusion of chemotherapy in addition to anthracycline in the treatment of acute promyelocytic leukaemia does not improve outcomes: Results of the MRC AML15 trial. Leukemia 2013, 27, 843–851. [Google Scholar] [CrossRef]
- Burnett, A.K.; Hills, R.K.; E Hunter, A.; Milligan, D.; Kell, W.J.; Wheatley, K.; Yin, J.; McMullin, M.F.; Dignum, H.; Bowen, D. The addition of gemtuzumab ozogamicin to low-dose Ara-C improves remission rate but does not significantly prolong survival in older patients with acute myeloid leukaemia: Results from the LRF AML14 and NCRI AML16 pick-a-winner comparison. Leukemia 2013, 27, 75–81. [Google Scholar] [CrossRef]
- Burnett, A.K.; Hills, R.K.; Milligan, D.; Kjeldsen, L.; Kell, J.; Russell, N.H.; Yin, J.A.; Hunter, A.; Goldstone, A.H.; Wheatley, K. Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: Results of the MRC AML15 trial. J. Clin. Oncol. 2011, 29, 369–377. [Google Scholar] [CrossRef]
- Burnett, A.K.; Russell, N.H.; Hills, R.K.; Kell, J.; Freeman, S.; Kjeldsen, L.; Hunter, A.E.; Yin, J.; Craddock, C.F.; Dufva, I.H.; et al. Addition of gemtuzumab ozogamicin to induction chemotherapy improves survival in older patients with acute myeloid leukemia. J. Clin. Oncol. 2012, 30, 3924–3931. [Google Scholar] [CrossRef] [PubMed]
- Castaigne, S.; Pautas, C.; Terré, C.; Raffoux, E.; Bordessoule, D.; Bastie, J.N.; Legrand, O.; Thomas, X.; Turlure, P.; Reman, O.; et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): A randomised, open-label, phase 3 study. Lancet 2012, 379, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Delaunay, J.; Recher, C.; Pigneux, A.; Witz, F.; Vey, N.; Blanchet, O.; Lefebvre, P.; Luquet, I.; Guillerme, I.; Volteau, C.; et al. Addition of gemtuzumab ozogamycin to chemotherapy improves event-free survival but not overall survival of AML patients with intermediate cytogenetics not eligible for allogeneic transplantation: Results of the GOELAMS AML. Blood 2011, 118, 79. [Google Scholar] [CrossRef]
- Hills, R.K.; Castaigne, S.; Appelbaum, F.R.; Delaunay, J.; Petersdorf, S.; Othus, M.; Estey, E.H.; Dombret, H.; Chevret, S.; Ifrah, N.; et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: A meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014, 15, 986–996. [Google Scholar] [CrossRef] [PubMed]
- Mort, J.F.; Brighton, D.; DiBenedetto, S.; Wells, L.; Clark, S.M.; Reid, J.; Patel, I.; Jackson, C.; Yelvington, B.; Miller, R.; et al. Evaluation of Gemtuzumab Ozogamicin and Anthracycline Dosing for Favorable Risk Acute Myeloid Leukemia. Eur. J. Haematol. 2025, 114, 481–494. [Google Scholar] [CrossRef]
- Gamis, A.S.; Alonzo, T.A.; Meshinchi, S.; Sung, L.; Gerbing, R.B.; Raimondi, S.C.; Hirsch, B.A.; Kahwash, S.B.; Heerema-McKenney, A.; Winter, L.; et al. Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: Results from the randomized phase III Children’s Oncology Group trial AAML0531. J. Clin. Oncol. 2014, 32, 3021–3032. [Google Scholar] [CrossRef] [PubMed]
- Jen, E.Y.; Ko, C.-W.; Lee, J.E.; Del Valle, P.L.; Aydanian, A.; Jewell, C.; Norsworthy, K.J.; Przepiorka, D.; Nie, L.; Liu, J. FDA Approval: Gemtuzumab Ozogamicin for the Treatment of Adults with Newly Diagnosed CD33-Positive Acute Myeloid Leukemia. Clin. Cancer Res. 2018, 24, 3242–3246. [Google Scholar] [CrossRef]
- Norsworthy, K.J.; Ko, C.-W.; Lee, J.E.; Liu, J.; John, C.S.; Przepiorka, D.; Farrell, A.T.; Pazdur, R. FDA Approval Summary: Mylotarg for Treatment of Patients with Relapsed or Refractory CD33-Positive Acute Myeloid Leukemia. Oncologist 2018, 23, 1103–1108. [Google Scholar] [CrossRef]
- Bolouri, H.; Farrar, J.E.; Triche, T., Jr.; Ries, R.E.; Lim, E.L.; Alonzo, T.A.; Ma, Y.; Moore, R.; Mungall, A.J.; Marra, M.A.; et al. The molecular landscape of pediatric acute myeloid leukemia reveals recurrent structural alterations and age-specific mutational interactions. Nat. Med. 2018, 24, 103–112. [Google Scholar] [CrossRef]
- Elgarten, C.W.; Aplenc, R. Pediatric acute myeloid leukemia: Updates on biology, risk stratification, and therapy. Curr. Opin. Pediatr. 2020, 32, 57–66. [Google Scholar] [CrossRef]
- Liao, X.Y.; Fang, J.P.; Zhou, D.H.; Qiu, K.Y. CEBPA are independent good prognostic factors in pediatric acute myeloid leukemia. Hematol. Oncol. 2022, 40, 258–268. [Google Scholar] [CrossRef]
- Qiu, K.-Y.; Liao, X.-Y.; Li, Y.; Huang, K.; Xu, H.-G.; Fang, J.-P.; Zhou, D.-H. Outcome and prognostic factors of CBF pediatric AML patients with t(8;21) differ from patients with inv(16). BMC Cancer 2023, 23, 476. [Google Scholar] [CrossRef] [PubMed]
- Qiu, K.Y.; Liao, X.Y.; Liu, Y.; Huang, K.; Li, Y.; Fang, J.P.; Zhou, D.H. Poor outcome of pediatric patients with acute myeloid leukemia harboring high FLT3/ITD allelic ratios. Nat. Commun. 2022, 13, 3679. [Google Scholar] [CrossRef] [PubMed]
- Mądry, K.; Lis, K.; Biecek, P.; Młynarczyk, M.; Rytel, J.; Górka, M.; Kacprzyk, P.; Dutka, M.; Rodzaj, M.; Bołkun, Ł.; et al. Predictive Model for Infection Risk in Myelodysplastic Syndromes, Acute Myeloid Leukemia, and Chronic Myelomonocytic Leukemia Patients Treated with Azacitidine; Azacitidine Infection Risk Model: The Polish Adult Leukemia Group Study. Clin. Lymphoma Myeloma Leuk. 2019, 19, 264–274.e4. [Google Scholar] [CrossRef]
- Chen, M.; Zeng, Z.; Qin, W.; Cai, X.; Lu, X.; Chen, S. A novel prognostic model of methylation-associated genes in acute myeloid leukemia. Clin. Transl. Oncol. 2023, 25, 1719–1728. [Google Scholar] [CrossRef]

| Characteristics | Total | GO Status | p Value | |
|---|---|---|---|---|
| No-GO (n = 358) | GO (n = 347) | |||
| Gender, n (%) | 0.248 | |||
| Male | 363 (51.5%) | 192 (53.6%) | 171 (49.3%) | |
| Female | 342 (48.5%) | 166 (46.4%) | 176 (50.7%) | |
| Age (y), median (range) | 10.0 (0.0–18.0) | 10.2 (0.0–18.0) | 9.7 (0.0–17.9) | 0.442 |
| FAB category | 0.798 | |||
| M0 | 24 (4.1%) | 12 (4.2%) | 12 (4.0%) | |
| M1 | 78 (13.3%) | 42 (14.6%) | 36 (12.0%) | |
| M2 | 156 (26.6%) | 77 (26.8%) | 79 (26.3%) | |
| M4 | 161 (27.4%) | 78 (27.2%) | 83 (27.7%) | |
| M5 | 128 (21.8%) | 56 (19.5%) | 72 (24.0%) | |
| M6 | 13 (2.2%) | 8 (2.8%) | 5 (1.7%) | |
| M7 | 27 (4.6%) | 14 (4.9%) | 13 (4.3%) | |
| Initial WBC (×109/L), median (range) | 33.5 (0.2–519.0) | 35.3 (0.2–473.1) | 32.7 (0.8–519.0) | 0.612 |
| PB blast (%) | 48.0 (0.0–98.0) | 47.0 (0.0–98.0) | 49.0 (0.0–98.0) | 0.645 |
| BM blast (%) | 71.0 (0.0–100.0) | 71.0 (0.0–99.0) | 71.0 (3.0–100.0) | 0.331 |
| Karyotype | 0.526 | |||
| Normal | 167 (24.3%) | 81 (23.1%) | 86 (25.6%) | |
| inv(16) | 91 (13.3%) | 46 (13.1%) | 45 (13.4%) | |
| MLL | 125 (18.2%) | 62 (17.7%) | 63 (18.8%) | |
| t(8;21) | 109 (15.9%) | 64 (18.3%) | 45 (13.4%) | |
| Other | 194 (28.3%) | 97 (27.7%) | 97 (28.9%) | |
| Risk group, n (%) | 0.568 | |||
| Low risk | 262 (38.2%) | 140 (40.1%) | 122 (36.2%) | |
| Standard risk | 305 (44.5%) | 151 (43.3%) | 154 (45.7%) | |
| High risk | 119 (17.3%) | 58 (16.6%) | 61 (18.1%) | |
| CNSL, n (%) | 0.541 | |||
| No | 654 (92.8%) | 330 (92.2%) | 324 (93.4%) | |
| Yes | 51 (7.2%) | 28 (7.8%) | 23 (6.6%) | |
| FLT3-ITD status, n (%) | 0.879 | |||
| FLT3-ITD wild-type | 555 (78.7%) | 281 (78.5%) | 274 (79.0%) | |
| FLT3-ITD mutation | 150 (21.3%) | 77 (21.5%) | 73 (21.0%) | |
| CEBPA status, n (%) | 0.898 | |||
| CEBPA wild-type | 1743 (94.5%) | 342 (96.6%) | 323 (94.4%) | |
| CEBPA mutation | 101 (5.5%) | 12 (3.4%) | 19 (5.6%) | |
| NPM1 status, n (%) | 0.480 | |||
| NPM1 wild-type | 639 (91.4%) | 321 (90.7%) | 318 (92.2%) | |
| NPM1 mutation | 60 (8.6%) | 33 (9.3%) | 27 (7.8%) | |
| WT1 status, n (%) | 0.923 | |||
| WT1 wild-type | 647 (92.6%) | 328 (92.7%) | 319 (92.5%) | |
| WT1 mutation | 52 (7.4%) | 26 (7.3%) | 26 (7.5%) | |
| End of IND1 response | 0.103 | |||
| CR | 513 (73.6%) | 257 (72.2%) | 256 (75.1%) | |
| Not in CR | 176 (25.3%) | 92 (25.8%) | 84 (24.6%) | |
| Death | 8 (1.1%) | 7 (2.0%) | 1 (0.3%) | |
| End of IND2 response | 0.099 | |||
| CR | 600 (86.8%) | 300 (84.5%) | 300 (89.3%) | |
| Not in CR | 79 (11.4%) | 46 (13.0%) | 33 (9.8%) | |
| Death | 12 (1.7%) | 9 (2.5%) | 3 (0.9%) | |
| SCT in first CR | 0.633 | |||
| No | 518 (81.8%) | 265 (82.6%) | 253 (81.1%) | |
| Yes | 115 (18.2%) | 56 (17.4%) | 59 (18.9%) | |
| Variables | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| HR (95%CI) | p Value | HR (95%CI) | p Value | |
| Gender | ||||
| Male | Ref. | Ref. | ||
| Female | 0.9 (0.7, 1.1) | 0.143 | 0.8 (0.6, 1.0) | 0.107 |
| Age | 1.0 (1.0, 1.0) | 0.995 | 1.0 (1.0, 1.0) | 0.084 |
| Risk group | ||||
| Low risk | Ref. | Ref. | ||
| Standard risk | 3.1 (2.4, 4.0) | <0.001 | 1.7 (0.7, 3.9) | 0.225 |
| High risk | 3.3 (2.4, 4.4) | <0.001 | 1.1 (0.5, 2.6) | 0.800 |
| WBC | 1.0 (1.0, 1.0) | <0.001 | 1.0 (1.0, 1.0) | <0.001 |
| BM blast (%) | 1.0 (1.0, 1.0) | <0.001 | 1.0 (1.0, 1.0) | 0.109 |
| Peripheral blasts (%) | 1.0 (1.0, 1.0) | 0.092 | 1.0 (1.0, 1.0) | 0.989 |
| CNSL | ||||
| No | Ref. | Ref. | ||
| Yes | 0.9 (0.6, 1.4) | 0.649 | 1.2 (0.8, 1.9) | 0.437 |
| FAB category | ||||
| M0 | Ref. | Ref. | ||
| M1 | 0.5 (0.3, 0.9) | 0.012 | 0.8 (0.4, 1.4) | 0.418 |
| M2 | 0.3 (0.2, 0.6) | <0.001 | 0.9 (0.5, 1.8) | 0.854 |
| M4 | 0.5 (0.3, 0.8) | 0.005 | 0.9 (0.5, 1.7) | 0.804 |
| M5 | 0.6 (0.4, 0.9) | 0.024 | 0.6 (0.3, 1.0) | 0.065 |
| M6 | 0.8 (0.3, 1.7) | 0.497 | 0.7 (0.3, 1.7) | 0.416 |
| M7 | 0.1 (0.1, 0.4) | <0.001 | 0.3 (0.1, 0.8) | 0.017 |
| Karyotype | ||||
| Normal | Ref. | Ref. | ||
| inv(16) | 0.5 (0.3, 0.8) | <0.001 | 0.3 (0.1, 0.8) | 0.014 |
| MLL | 1.2 (0.9, 1.7) | 0.138 | 0.8 (0.5, 1.3) | 0.316 |
| t(8;21) | 0.4 (0.3, 0.6) | <0.001 | 0.3 (0.1, 0.7) | 0.008 |
| Other | 1.2 (0.9, 1.6) | 0.122 | 0.8 (0.5, 1.1) | 0.190 |
| FLT3-ITD status | ||||
| FLT3-ITD wild-type | Ref. | Ref. | ||
| FLT3-ITD mutation | 1.6 (1.2, 2.0) | <0.001 | 1.0 (0.6, 1.5) | 0.875 |
| NPM1 status | ||||
| NPM1 wild-type | Ref. | Ref. | ||
| NPM1 mutation | 0.5 (0.3, 0.8) | 0.003 | 0.5 (0.2, 1.0) | 0.055 |
| CEBPA status | ||||
| CEBPA wild-type | Ref. | Ref. | ||
| CEBPA mutation | 0.4 (0.2, 0.8) | 0.008 | 0.2 (0.1, 0.7) | 0.011 |
| WT1 status | ||||
| WT1 wild-type | Ref. | Ref. | ||
| WT1 mutation | 2.3 (1.7, 3.2) | <0.001 | 2.1 (1.4, 3.2) | <0.001 |
| Gemtuzumab ozogamicin treatment | ||||
| No-GO | Ref. | Ref. | ||
| GO | 0.9 (0.8, 1.1) | 0.538 | 1.0 (0.8, 1.3) | 0.818 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, K.-Y.; Liao, X.-Y.; Fang, J.-P.; Zhou, D.-H. Predictive Model of Gemtuzumab Ozogamicin Response in Childhood Acute Myeloid Leukemia on Event-Free Survival: Data Analysis Based on Trial AAML0531. Bioengineering 2025, 12, 297. https://doi.org/10.3390/bioengineering12030297
Qiu K-Y, Liao X-Y, Fang J-P, Zhou D-H. Predictive Model of Gemtuzumab Ozogamicin Response in Childhood Acute Myeloid Leukemia on Event-Free Survival: Data Analysis Based on Trial AAML0531. Bioengineering. 2025; 12(3):297. https://doi.org/10.3390/bioengineering12030297
Chicago/Turabian StyleQiu, Kun-Yin, Xiong-Yu Liao, Jian-Pei Fang, and Dun-Hua Zhou. 2025. "Predictive Model of Gemtuzumab Ozogamicin Response in Childhood Acute Myeloid Leukemia on Event-Free Survival: Data Analysis Based on Trial AAML0531" Bioengineering 12, no. 3: 297. https://doi.org/10.3390/bioengineering12030297
APA StyleQiu, K.-Y., Liao, X.-Y., Fang, J.-P., & Zhou, D.-H. (2025). Predictive Model of Gemtuzumab Ozogamicin Response in Childhood Acute Myeloid Leukemia on Event-Free Survival: Data Analysis Based on Trial AAML0531. Bioengineering, 12(3), 297. https://doi.org/10.3390/bioengineering12030297







