Dynamic Surface Topography for Thoracic and Lumbar Pain Patients—Applicability and First Results
Abstract
1. Introduction
2. Methods
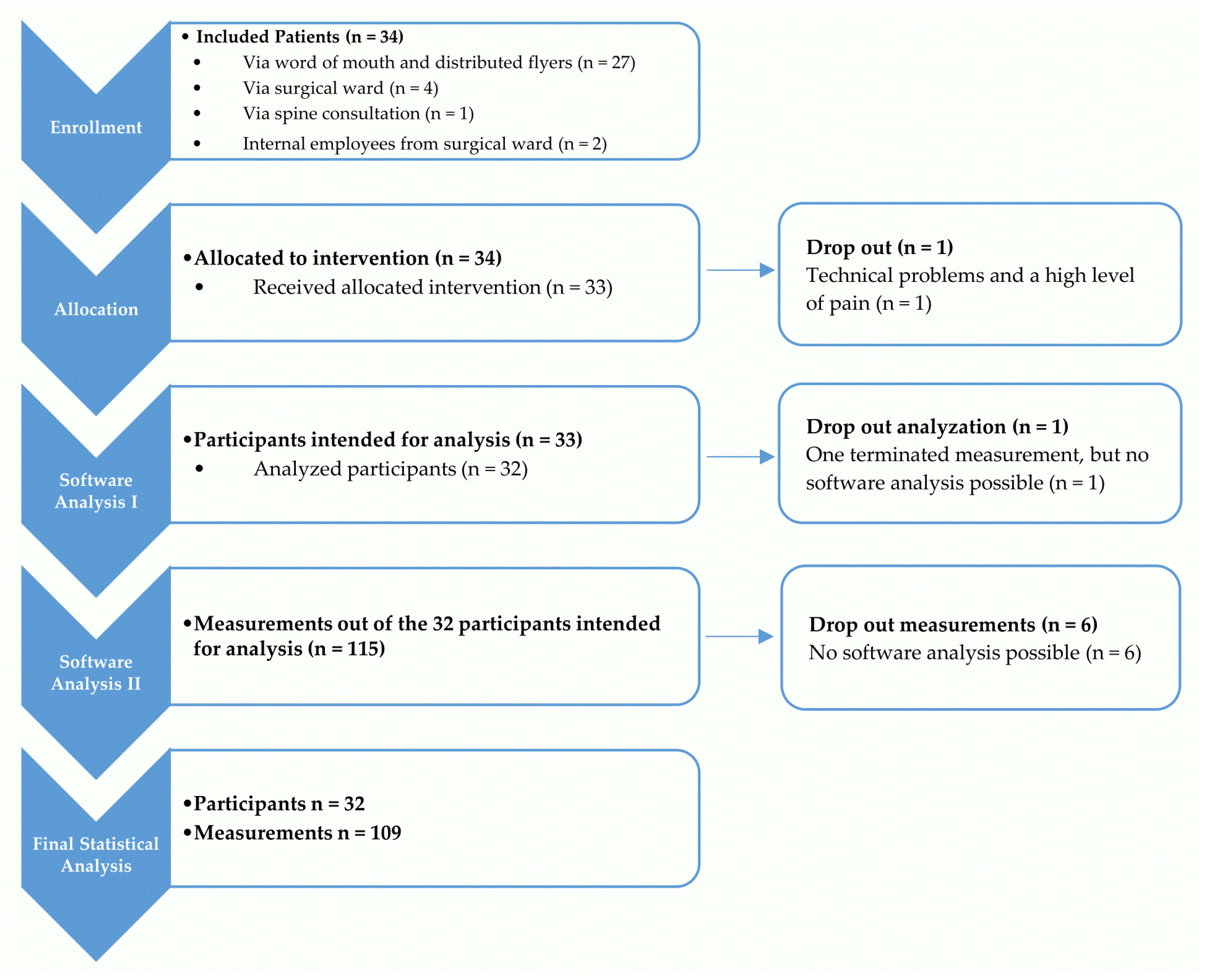
2.1. Inclusion and Exclusion Criteria of the Study Population
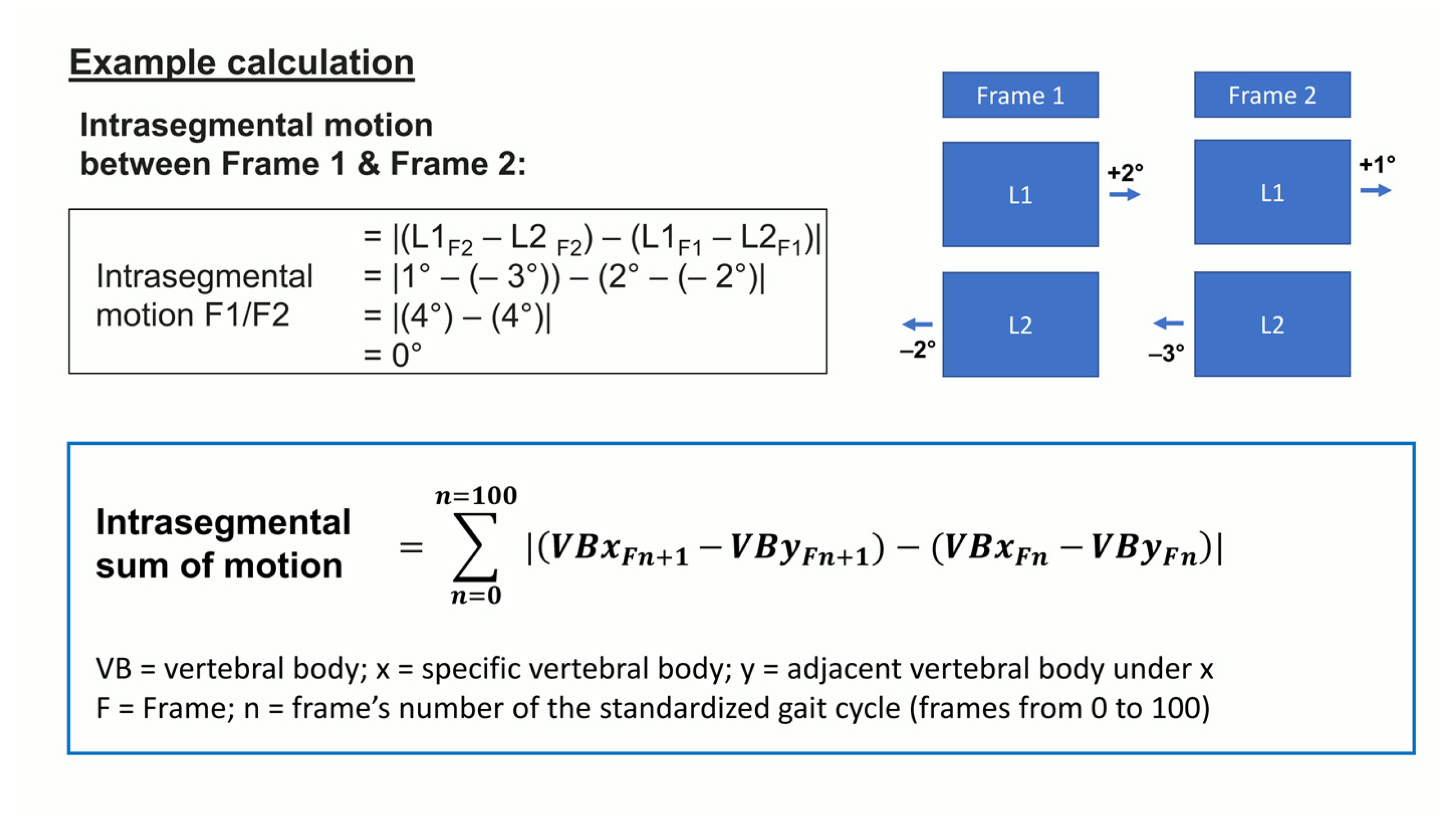
2.2. Measuring Device
2.3. Measuring Procedure
2.4. Healthy Reference Group
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Applicability of Dynamic Surface Topography
3.3. Data of Dynamic Spinal Rotation
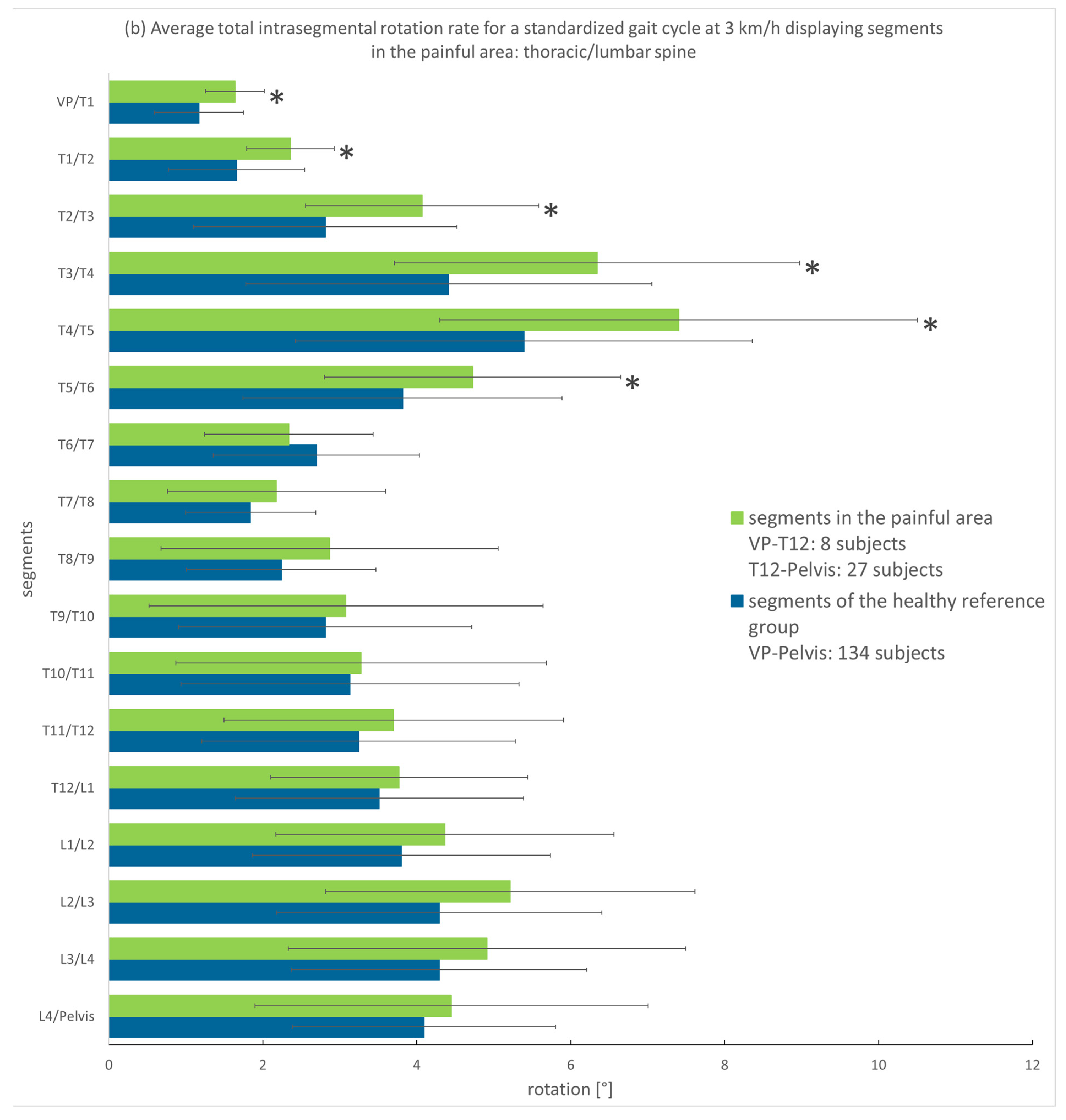
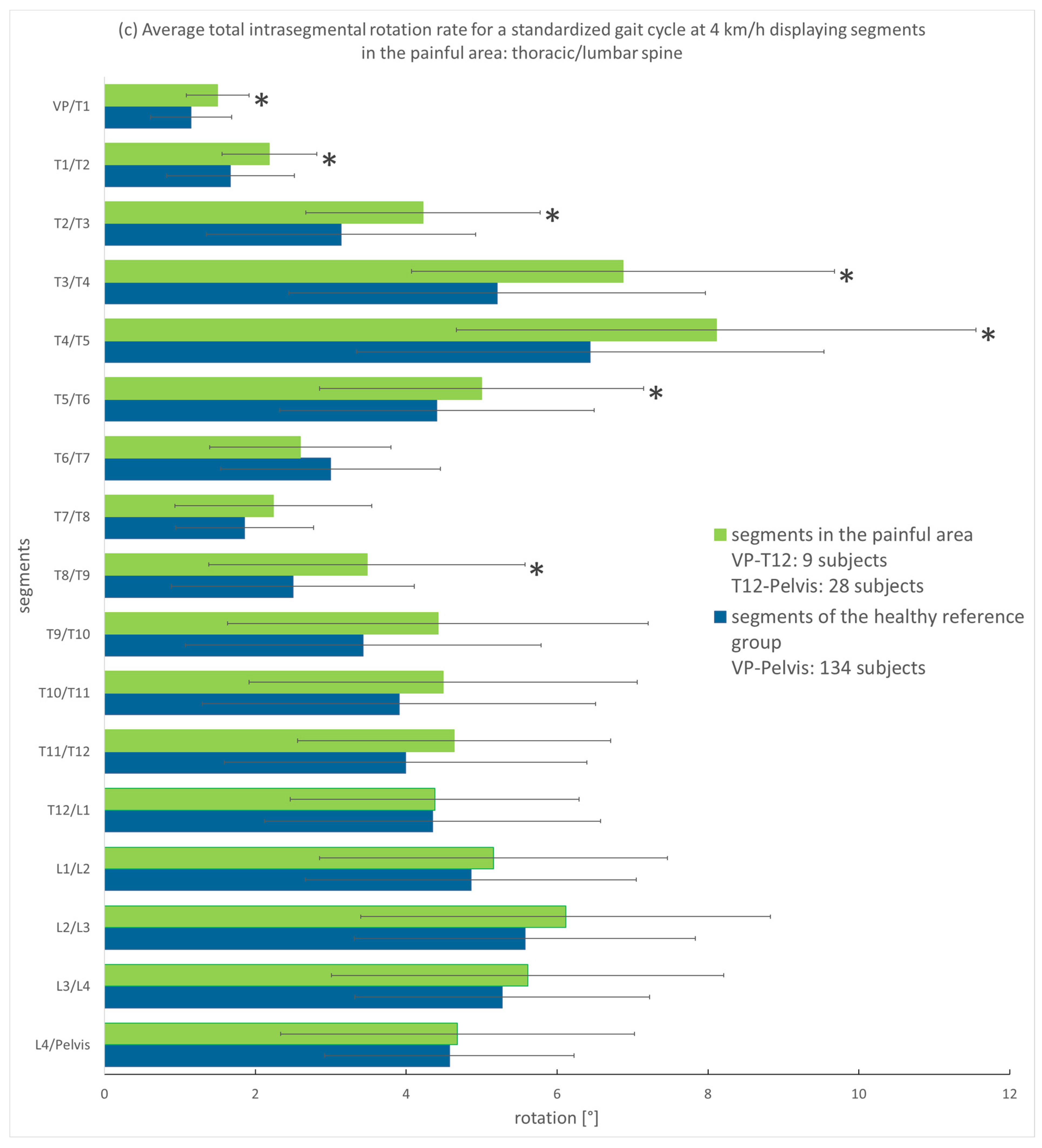
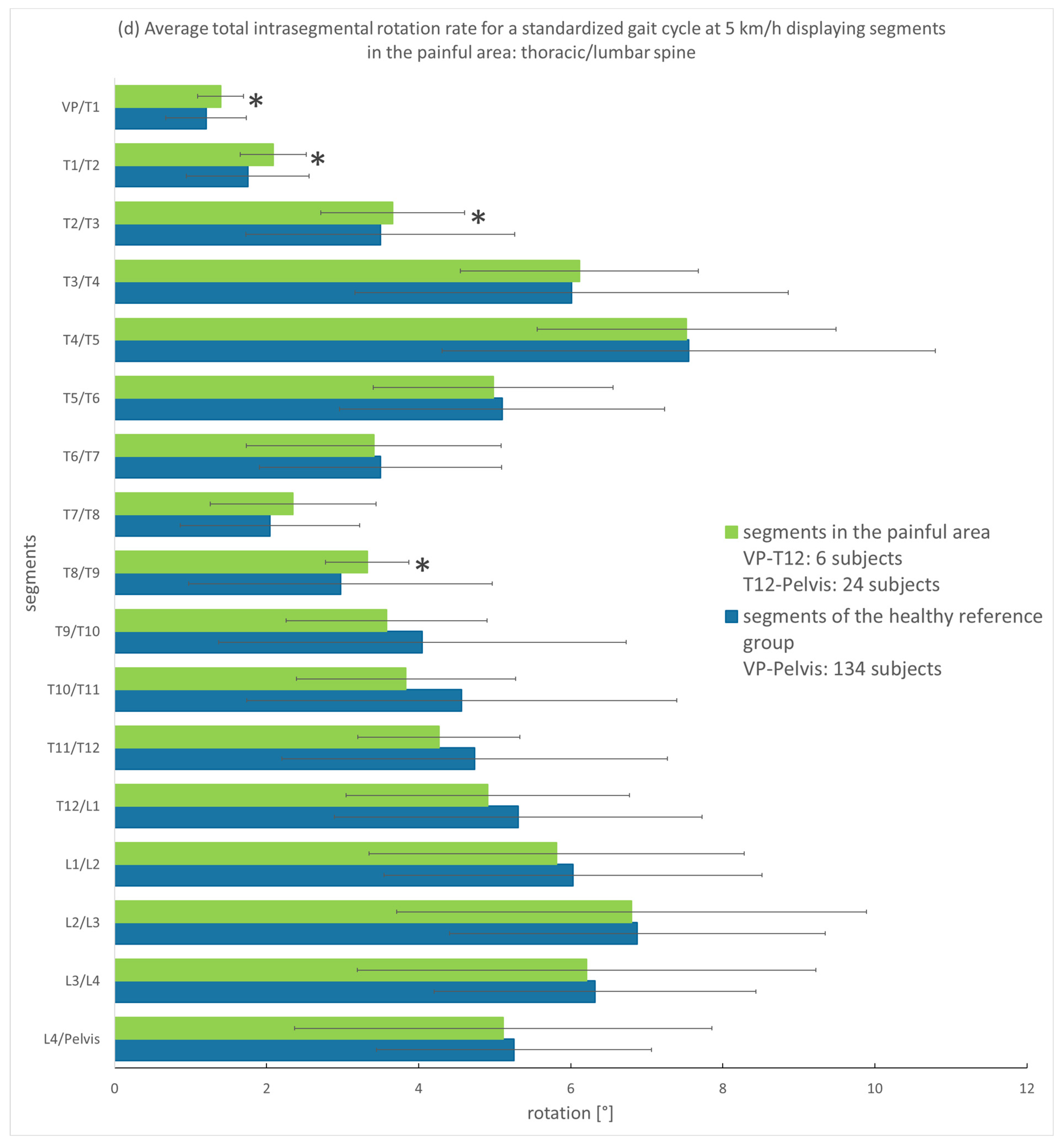
3.3.1. Analysis of the Entire Group
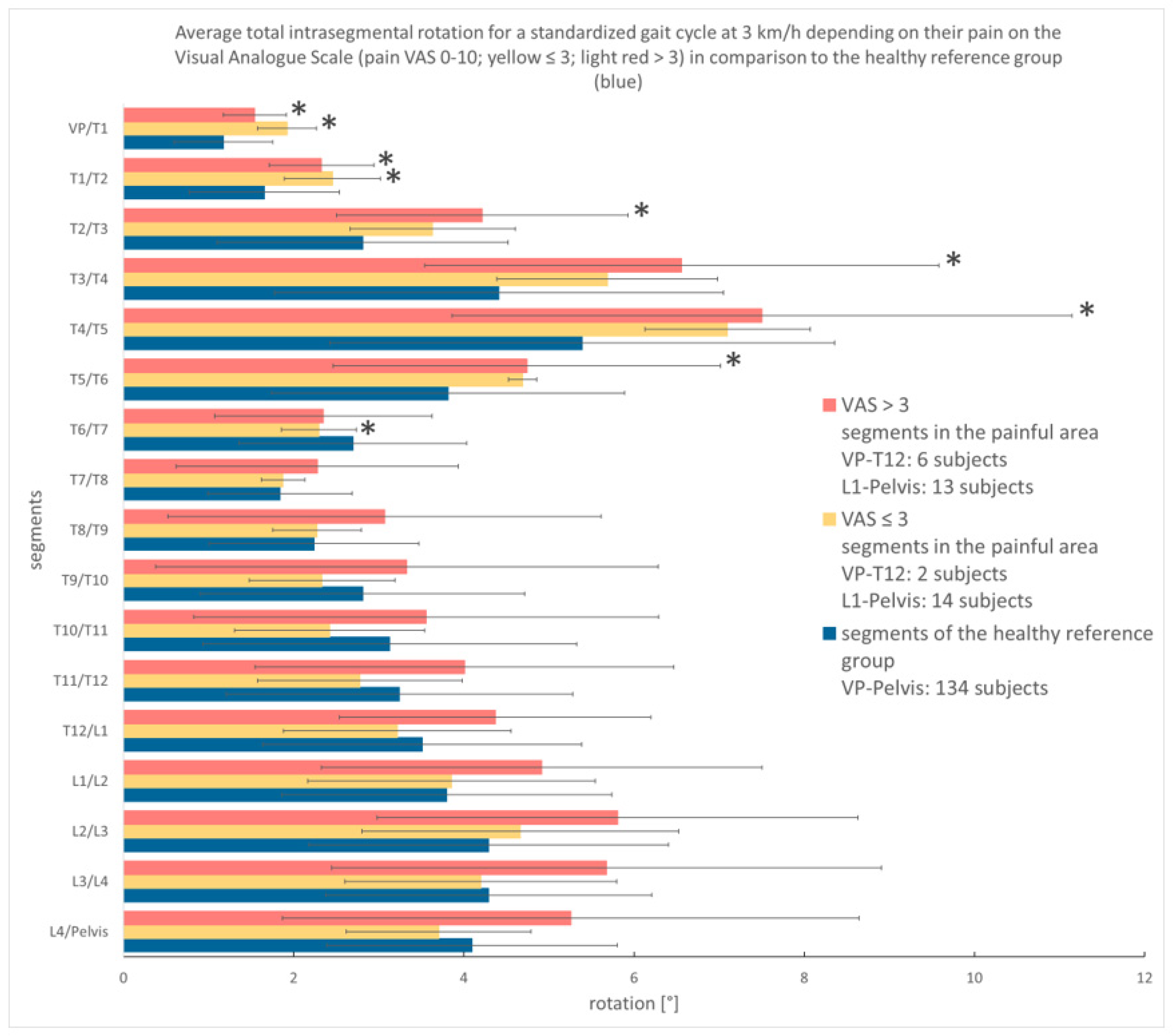
3.3.2. Analysis According to Pain Intensity
4. Discussion
4.1. Spinal Motion of Thoracic and Lumbar Pain Patients
4.2. Limitations of the Measuring System and the Study Design
4.3. Implications of the Findings for Clinical Practice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2MWT | Two-minute Walk Test |
| BMI | Body Mass Index |
| DST | Dynamic Surface Topography |
| SoMpGC | Sum of motion per gait cycle |
| VAS | Visual Analogue Scale |
| VP | Vertebra prominens |
References
- GBD 2021 Low Back Pain Collaborators. Global, regional, and national burden of low back pain, 1990–2020, its attributable risk factors, and projections to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e316–e329. [Google Scholar] [CrossRef] [PubMed]
- Maher, C.; Underwood, M.; Buchbinder, R. Non-specific low back pain. Lancet 2017, 389, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Ract, I.; Meadeb, J.M.; Mercy, G.; Cueff, F.; Husson, J.L.; Guillin, R. A review of the value of MRI signs in low back pain. Diagn. Interv. Imaging 2015, 96, 239–249. [Google Scholar] [CrossRef]
- Bayer, T.L.; Baer, P.E.; Early, C. Situational and psychophysiological factors in psychologically induced pain. Pain 1991, 44, 45–50. [Google Scholar] [CrossRef]
- Jensen, M.C.; Brant-Zawadzki, M.N.; Obuchowski, N.; Modic, M.T.; Malkasian, D.; Ross, J.S. Magnetic resonance imaging of the lumbar spine in people without back pain. N. Engl. J. Med. 1994, 331, 69–73. [Google Scholar] [CrossRef]
- O’Sullivan, P. Diagnosis and classification of chronic low back pain disorders: Maladaptive movement and motor control impairments as underlying mechanism. Man. Ther. 2005, 10, 242–255. [Google Scholar] [CrossRef]
- Lamoth, C.J.C.; Meijer, O.G.; Daffertshofer, A.; Wuisman, P.I.J.M.; Beek, P.J. Effects of chronic low back pain on trunk coordination and back muscle activity during walking: Changes in motor control. Eur. Spine J. 2006, 15, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Stabbert, H.; Bagwell, J.J.; Teng, H.-L.; Wade, V.; Lee, S.-P. Do people with low back pain walk differently? A systematic review and meta-analysis. J. Sport Health Sci. 2022, 11, 450–465. [Google Scholar] [CrossRef]
- Goodvin, C.; Park, E.J.; Huang, K.; Sakaki, K. Development of a real-time three-dimensional spinal motion measurement system for clinical practice. Med. Biol. Eng. Comput. 2006, 44, 1061–1075. [Google Scholar] [CrossRef]
- Fernandes, R.S.; Armada-da-Silva, P.; Pool-Goudzwaard, A.L.; Moniz-Pereira, V.; Veloso, A.N.P. Corrigendum to “Test-retest reliability and minimal detectable change of three-dimensional gait analysis in chronic low back pain patients” [Gait Posture 42 (2015) 491-497]. Gait Posture 2016, 48, 268, Erratum in Gait Posture 2015, 42, 491–497. [Google Scholar] [CrossRef]
- Betsch, M.; Wild, M.; Johnstone, B.; Jungbluth, P.; Hakimi, M.; Kuhlmann, B.; Rapp, W. Evaluation of a novel spine and surface topography system for dynamic spinal curvature analysis during gait. PLoS ONE 2013, 8, e70581. [Google Scholar] [CrossRef]
- Betsch, M.; Wild, M.; Rath, B.; Tingart, M.; Schulze, A.; Quack, V. Radiation-free diagnosis of scoliosis: An overview of the surface and spine topography. Orthopade 2015, 44, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Drerup, B.; Ellger, B.; Meyer zu Bentrup, F.M.; Hierholzer, E. Functional rasterstereographic images. A new method for biomechanical analysis of skeletal geometry. Orthopade 2001, 30, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Drerup, B.; Hierholzer, E. Evaluation of frontal radiographs of scoliotic spines—Part II. Relations between lateral deviation, lateral tilt and axial rotation of vertebrae. J. Biomech. 1992, 25, 1443–1450. [Google Scholar] [CrossRef]
- Drerup, B.; Hierholzer, E. Evaluation of frontal radiographs of scoliotic spines—Part I. Measurement of position and orientation of vertebrae and assessment of clinical shape parameters. J. Biomech. 1992, 25, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Drerup, B.; Hierholzer, E. Back shape measurement using video rasterstereography and three-dimensional reconstruction of spinal shape. Clin. Biomech. 1994, 9, 28–36. [Google Scholar] [CrossRef]
- Turner-Smith, A.R. A television/computer three-dimensional surface shape measurement system. J. Biomech. 1988, 21, 515–529. [Google Scholar] [CrossRef]
- Drerup, B.; Hierholzer, E. Assessment of scoliotic deformity from back shape asymmetry using an improved mathematical model. Clin. Biomech. 1996, 11, 376–383. [Google Scholar] [CrossRef]
- Mohokum, M.; Mendoza, S.; Udo, W.; Sitter, H.; Paletta, J.R.; Skwara, A. Reproducibility of rasterstereography for kyphotic and lordotic angles, trunk length, and trunk inclination: A reliability study. Spine 2010, 35, 1353–1358. [Google Scholar] [CrossRef]
- Gipsman, A.; Rauschert, L.; Daneshvar, M.; Knott, P. Evaluating the Reproducibility of Motion Analysis Scanning of the Spine during Walking. Adv. Med. 2014, 2014, 721829. [Google Scholar] [CrossRef]
- Dindorf, C.; Konradi, J.; Wolf, C.; Taetz, B.; Bleser, G.; Huthwelker, J.; Drees, P.; Fröhlich, M.; Betz, U. General method for automated feature extraction and selection and its application for gender classification and biomechanical knowledge discovery of sex differences in spinal posture during stance and gait. Comput. Methods Biomech. Biomed. Eng. 2020, 24, 299–307. [Google Scholar] [CrossRef]
- Dindorf, C.; Konradi, J.; Wolf, C.; Taetz, B.; Bleser, G.; Huthwelker, J.; Werthmann, F.; Bartaguiz, E.; Kniepert, J.; Drees, P.; et al. Classification and Automated Interpretation of Spinal Posture Data Using a Pathology-Independent Classifier and Explainable Artificial Intelligence (XAI). Sensors 2021, 21, 6323. [Google Scholar] [CrossRef]
- Frobin, W.; Hierholzer, E. Automatic measurement of body surfaces using rasterstereography. Part I: Image scan and control point measurement. Photogramm. Eng. Rem. S 1983, 49, 377–384. [Google Scholar]
- Frobin, W.; Hierholzer, E. Automatic measurement of body surfaces using rasterstereography. Part II: Analysis of the rasterstereographic line pattern and three-dimensional surface reconstruction. Photogramm. Eng. Rem. S 1983, 49, 1443–1452. [Google Scholar]
- Huthwelker, J.; Konradi, J.; Wolf, C.; Westphal, R.; Schmidtmann, I.; Schubert, P.; Drees, P.; Betz, U. Reference values and functional descriptions of transverse plane spinal dynamics during gait based on surface topography. Hum. Mov. Sci. 2023, 88, 103054. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Wang, Y.C.; Gershon, R.C. Two-minute walk test performance by adults 18 to 85 years: Normative values, reliability, and responsiveness. Arch. Phys. Med. Rehabil. 2015, 96, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Asamoah, V.; Mellerowicz, H.; Venus, J.; Klöckner, C. Oberflächenvermessung des Rückens. Der Orthopäde 2000, 29, 480–489. [Google Scholar] [CrossRef]
- Huthwelker, J.; Konradi, J.; Wolf, C.; Westphal, R.; Schmidtmann, I.; Drees, P.; Betz, U. Reference Values for 3D Spinal Posture Based on Videorasterstereographic Analyses of Healthy Adults. Bioengineering 2022, 9, 809. [Google Scholar] [CrossRef]
- Kniepert, J. 4D-Bewegungsanalyse der Wirbelsäule im Gang nach mono-und Multisegmentaler Versteifung der Wirbelsäule Sowie bei Patienten mit Rückenschmerzen. Ph.D. Thesis, Johannes Gutenberg-Universität Mainz, Mainz, Germany, 2019. [Google Scholar]
- Haimerl, M.; Nebel, I.; Linkerhagner, A.; Konradi, J.; Wolf, C.; Drees, P.; Betz, U. Comprehensive visualization of spinal motion in gait sequences based on surface topography. Hum. Mov. Sci. 2022, 81, 102919. [Google Scholar] [CrossRef]
- Dindorf, C.; Konradi, J.; Wolf, C.; Taetz, B.; Bleser, G.; Huthwelker, J.; Werthmann, F.; Drees, P.; Frohlich, M.; Betz, U. Machine learning techniques demonstrating individual movement patterns of the vertebral column: The fingerprint of spinal motion. Comput. Methods Biomech. Biomed. Eng. 2021, 25, 821–831. [Google Scholar] [CrossRef]
- Konradi, J. SPSS syntax script to create graphs of spinal motion relative to phases of gait. Mendeley Data 2022, V1. [Google Scholar] [CrossRef]
- Konradi, J. SPSS syntax script to create graphs of spinal motion for a Standardized Gait Cycle. Mendeley Data 2022, V1. [Google Scholar] [CrossRef]
- Konradi, J.; Betz, U. Validation of automatic detection of gait phases. Mendeley Data 2022, V1. [Google Scholar] [CrossRef]
- Schmidtmann, I.; Konradi, J. SAS syntax script for merging export files. Mendeley Data 2022, V1. [Google Scholar] [CrossRef]
- Westphal, R.; Konradi, J. SAS syntax script for creation of a Standardized Gait Cycle. Mendeley Data 2022, V1. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioural Sciences, 2nd ed.; Lawrence Erlbaum: Hillsdale, MI, USA, 1988. [Google Scholar]
- Hedges, L.V.; Olkin, I. Statistical Methods for Meta-Analysis, 1st ed.; Academic Press: New York, NY, USA, 1985; p. 369. [Google Scholar]
- Dickey, J.P.; Pierrynowski, M.R.; Bednar, D.A.; Yang, S.X. Relationship between pain and vertebral motion in chronic low-back pain subjects. Clin. Biomech. 2002, 17, 345–352. [Google Scholar] [CrossRef]
- Blankenbaker, D.G.; Haughton, V.M.; Rogers, B.P.; Meyerand, M.E.; Fine, J.P. Axial rotation of the lumbar spinal motion segments correlated with concordant pain on discography: A preliminary study. AJR Am. J. Roentgenol. 2006, 186, 795–799. [Google Scholar] [CrossRef]
- Passias, P.G.; Wang, S.; Kozanek, M.; Xia, Q.; Li, W.; Grottkau, B.; Wood, K.B.; Li, G. Segmental lumbar rotation in patients with discogenic low back pain during functional weight-bearing activities. J. Bone Jt. Surg. 2011, 93, 29–37. [Google Scholar] [CrossRef]
- Feipel, V.; De Mesmaeker, T.; Klein, P.; Rooze, M. Three-dimensional kinematics of the lumbar spine during treadmill walking at different speeds. Eur. Spine J. 2001, 10, 16–22. [Google Scholar] [CrossRef]
- Dvorak, J.; Vajda, E.G.; Grob, D.; Panjabi, M.M. Normal motion of the lumbar spine as related to age and gender. Eur. Spine J. 1995, 4, 18–23. [Google Scholar] [CrossRef]
- Ochia, R.S.; Inoue, N.; Takatori, R.; Andersson, G.B.; An, H.S. In vivo measurements of lumbar segmental motion during axial rotation in asymptomatic and chronic low back pain male subjects. Spine 2007, 32, 1394–1399. [Google Scholar] [CrossRef]
- Gombatto, S.P.; Brock, T.; DeLork, A.; Jones, G.; Madden, E.; Rinere, C. Lumbar spine kinematics during walking in people with and people without low back pain. Gait Posture 2015, 42, 539–544. [Google Scholar] [CrossRef]
- Crosbie, J.; de Faria Negrao Filho, R.; Nascimento, D.P.; Ferreira, P. Coordination of spinal motion in the transverse and frontal planes during walking in people with and without recurrent low back pain. Spine 2013, 38, E286–E292. [Google Scholar] [CrossRef] [PubMed]
- Schmölz, W.; Bostelmann, R. 2.1 Biomechanik der Wirbelsäule. In Wirbelsäule Interdisziplinär: Operative und Konservative Therapie; Börm, W., Meyer, F., Bullmann, V., Knop, C., Eds.; Schatthauer: Stuttgart, Germany, 2017; pp. 21–28. [Google Scholar]
- van der Hulst, M.; Vollenbroek-Hutten, M.M.; Rietman, J.S.; Schaake, L.; Groothuis-Oudshoorn, K.G.; Hermens, H.J. Back muscle activation patterns in chronic low back pain during walking: A “guarding” hypothesis. Clin. J. Pain 2010, 26, 30–37. [Google Scholar] [CrossRef]
- Beauchet, O.; Annweiler, C.; Lecordroch, Y.; Allali, G.; Dubost, V.; Herrmann, F.R.; Kressig, R.W. Walking speed-related changes in stride time variability: Effects of decreased speed. J. Neuroeng. Rehabil. 2009, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Akl, A.R.; Gonçalves, P.; Fonseca, P.; Hassan, A.; Vilas-Boas, J.P.; Conceição, F. Muscle Co-Activation around the Knee during Different Walking Speeds in Healthy Females. Sensors 2021, 21, 677. [Google Scholar] [CrossRef]
- Hodges, P.W.; Moseley, G.L.; Gabrielsson, A.; Gandevia, S.C. Experimental muscle pain changes feedforward postural responses of the trunk muscles. Exp. Brain Res. 2003, 151, 262–271. [Google Scholar] [CrossRef]
- Vlaeyen, J.W.S.; Linton, S.J. Fear-avoidance model of chronic musculoskeletal pain: 12 years on. Pain 2012, 153, 1144–1147. [Google Scholar] [CrossRef] [PubMed]
- Arendt-Nielsen, L.; Graven-Nielsen, T.; Svarrer, H.; Svensson, P. The influence of low back pain on muscle activity and coordination during gait: A clinical and experimental study. Pain 1996, 64, 231–240. [Google Scholar] [CrossRef]
- Mörl, F.; Blickhan, R. Three-dimensional relation of skin markers to lumbar vertebrae of healthy subjects in different postures measured by open MRI. Eur. Spine J. 2006, 15, 742–751. [Google Scholar] [CrossRef]
- Colyer, S.L.; Evans, M.; Cosker, D.P.; Salo, A.I.T. A Review of the Evolution of Vision-Based Motion Analysis and the Integration of Advanced Computer Vision Methods Towards Developing a Markerless System. Sports Med. Open 2018, 4, 24. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Analyzed Participants (n = 32) | |
|---|---|---|
| 1a | Age (years): average (SD), range | 44.53 (14.84), 19–68 |
| Male sex n (%) | 14 (44%) | |
| BMI average (SD), range | 26.01 (4.79), 16.76–37.56 | |
| 2MWT (distance in meters): average (SD), range | 190.72 (35.60), 81.28–243.84 | |
| 2MWT (speed in km/h): average (SD), range | 5.72 (1.07), 2.4–7.3 | |
| 1b | Location of back pain | Number of subjects (percentage) |
| Thoracic spine | 2 (6%) | |
| Lumbar spine | 23 (72%) | |
| Thoracic and lumbar spine | 7 (22%) |
| Pain Characteristics | Number of Subjects (Percentage) |
|---|---|
| Rest pain | 32/32 (100%) |
| Pain when walking | 23/32 (72%) |
| Pain level (VAS: 0–10) | average: 3.67/10 (minimum: 1, maximum: 8; SD: 1.83) median: 3.25; range: 1–8 SD: 1.83 |
| Time period of pain | |
| <1 month | 5 (15%) |
| 1–6 month | 4 (12%) |
| 6–12 months | 3 (9%) |
| 12–24 months | 6 (18%) |
| 24–60 months | 2 (6%) |
| >60 months | 13 (39%) |
| Pain medication | |
| Yes | 6 (18%) |
| None | 27 (82%) |
| Radiation to the legs | |
| Right | 5 (15%) |
| Left | 9 (27%) |
| None | 19 (58%) |
| Paresthesia/Reduction in strength | |
| Right | 2 (6%) |
| Left | 6 (18%) |
| Both sides | 2 (6%) |
| Cervical spine | 1 (3%) |
| None | 22 (67%) |
| Walking Speed on the Treadmill | Number of Subjects Examined (Percentage) |
|---|---|
| 2 km/h | 33 (100%) |
| 3 km/h | 31 (94%) |
| 4 km/h | 30 (91%) |
| 5 km/h | 28 (85%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kniepert, J.; Rönsch, H.; Betz, U.; Konradi, J.; Huthwelker, J.; Wolf, C.; Westphal, R.; Drees, P. Dynamic Surface Topography for Thoracic and Lumbar Pain Patients—Applicability and First Results. Bioengineering 2025, 12, 289. https://doi.org/10.3390/bioengineering12030289
Kniepert J, Rönsch H, Betz U, Konradi J, Huthwelker J, Wolf C, Westphal R, Drees P. Dynamic Surface Topography for Thoracic and Lumbar Pain Patients—Applicability and First Results. Bioengineering. 2025; 12(3):289. https://doi.org/10.3390/bioengineering12030289
Chicago/Turabian StyleKniepert, Johanna, Henriette Rönsch, Ulrich Betz, Jürgen Konradi, Janine Huthwelker, Claudia Wolf, Ruben Westphal, and Philipp Drees. 2025. "Dynamic Surface Topography for Thoracic and Lumbar Pain Patients—Applicability and First Results" Bioengineering 12, no. 3: 289. https://doi.org/10.3390/bioengineering12030289
APA StyleKniepert, J., Rönsch, H., Betz, U., Konradi, J., Huthwelker, J., Wolf, C., Westphal, R., & Drees, P. (2025). Dynamic Surface Topography for Thoracic and Lumbar Pain Patients—Applicability and First Results. Bioengineering, 12(3), 289. https://doi.org/10.3390/bioengineering12030289






