A Sensor-Based Classification for Neuromotor Robot-Assisted Rehabilitation
Abstract
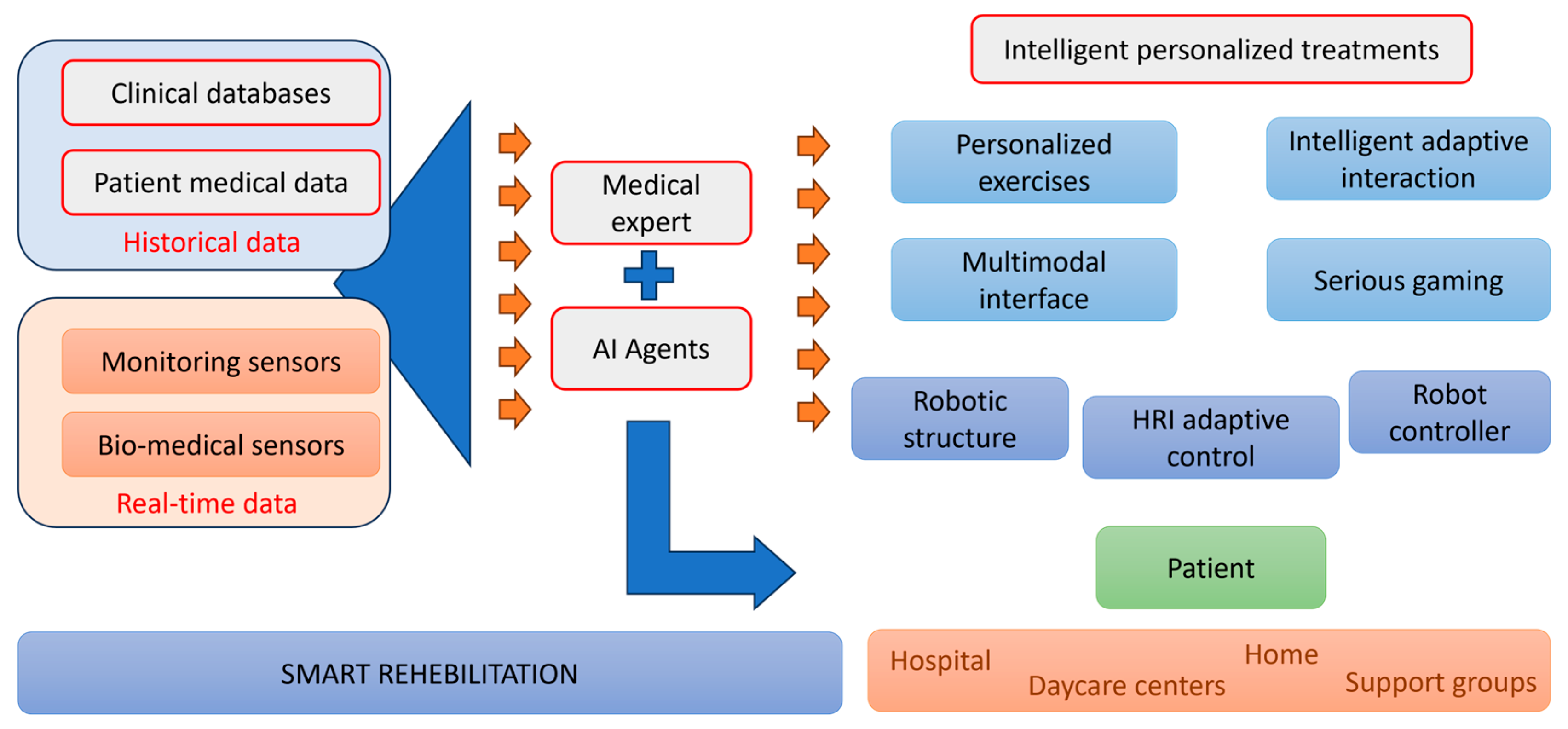
1. Introduction
- (i)
- Analyzes the methods and reliability in collecting specific and non-specific parameters in rehabilitation treatment.
- (ii)
- Classifies the sensors according to their types, the data they collect, their usability, and ergonomics.
- (iii)
- Assesses the impact of comorbidities on treatment rehabilitation.
- (iv)
- Evaluates the most efficient methods for data acquisition and utilization.
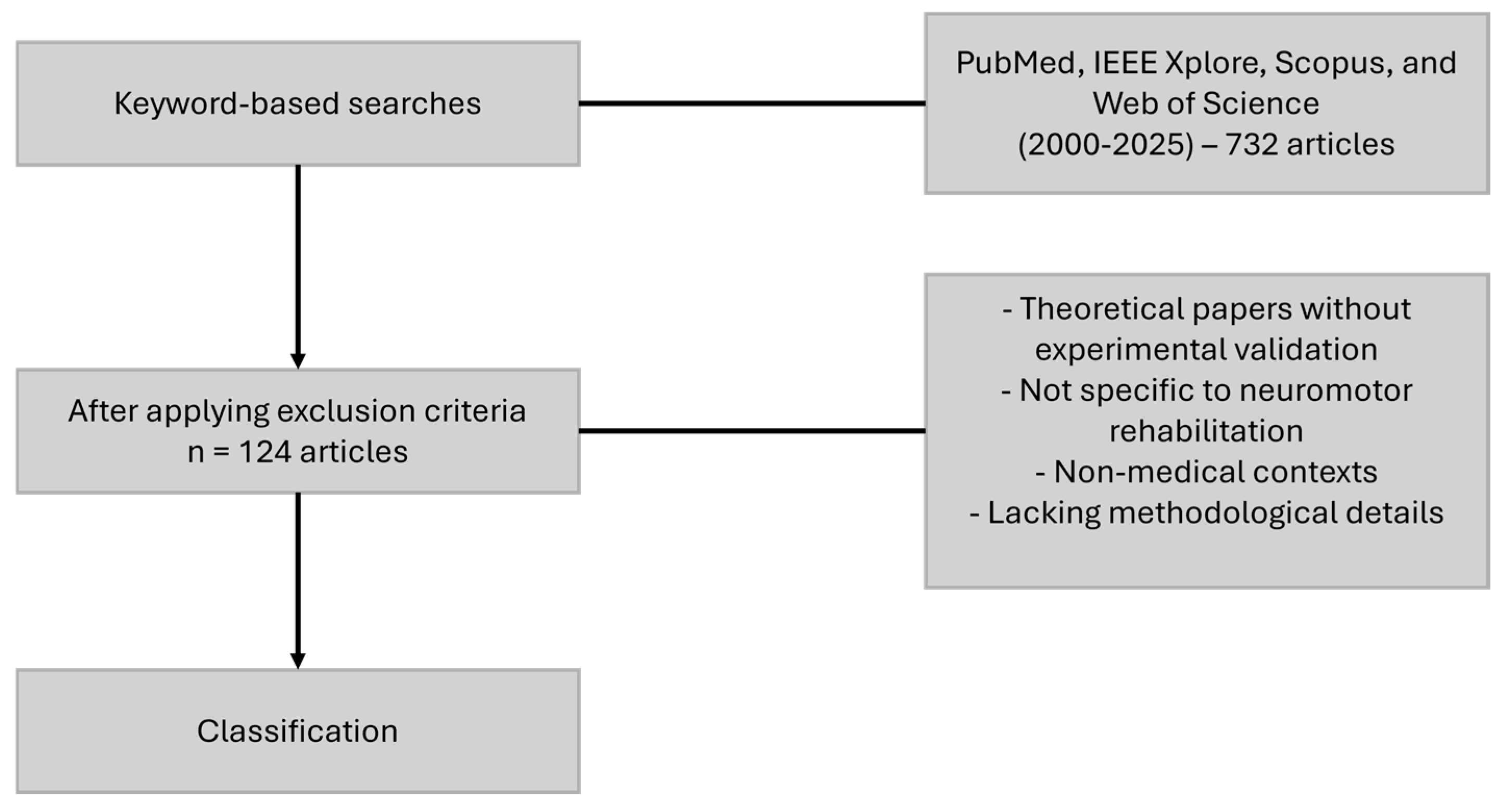
2. Methodology
2.1. Literature Review Methodology
- Sensor-based rehabilitation (“rehabilitation sensors“, “robot-assisted rehabilitation”, “sensor technology in rehabilitation”)
- Wearable and non-wearable sensors (“wearable rehabilitation devices”, “non-invasive sensors”, “implantable biosensors”)
- Motion and force tracking (“motion tracking sensors”, “force sensors in rehabilitation”, “gait analysis sensors”)
- Bioelectrical and neurophysiological sensors (“electromyography in rehabilitation”, “EEG for neurorehabilitation”)
- Real-time data processing and AI-driven feedback (“real-time rehabilitation feedback”, “AI in rehabilitation sensors”, “sensor fusion in neurorehabilitation”)
- Comorbidity-driven sensor applications (“sensors for metabolic disorders”, “neurological disease monitoring”, “rehabilitation in chronic pain conditions”)
- Physiological and metabolic monitoring in rehabilitation (“oxygen saturation in rehabilitation”, “cardiac monitoring for therapy adaptation”, “sweat analysis for patient assessment”)
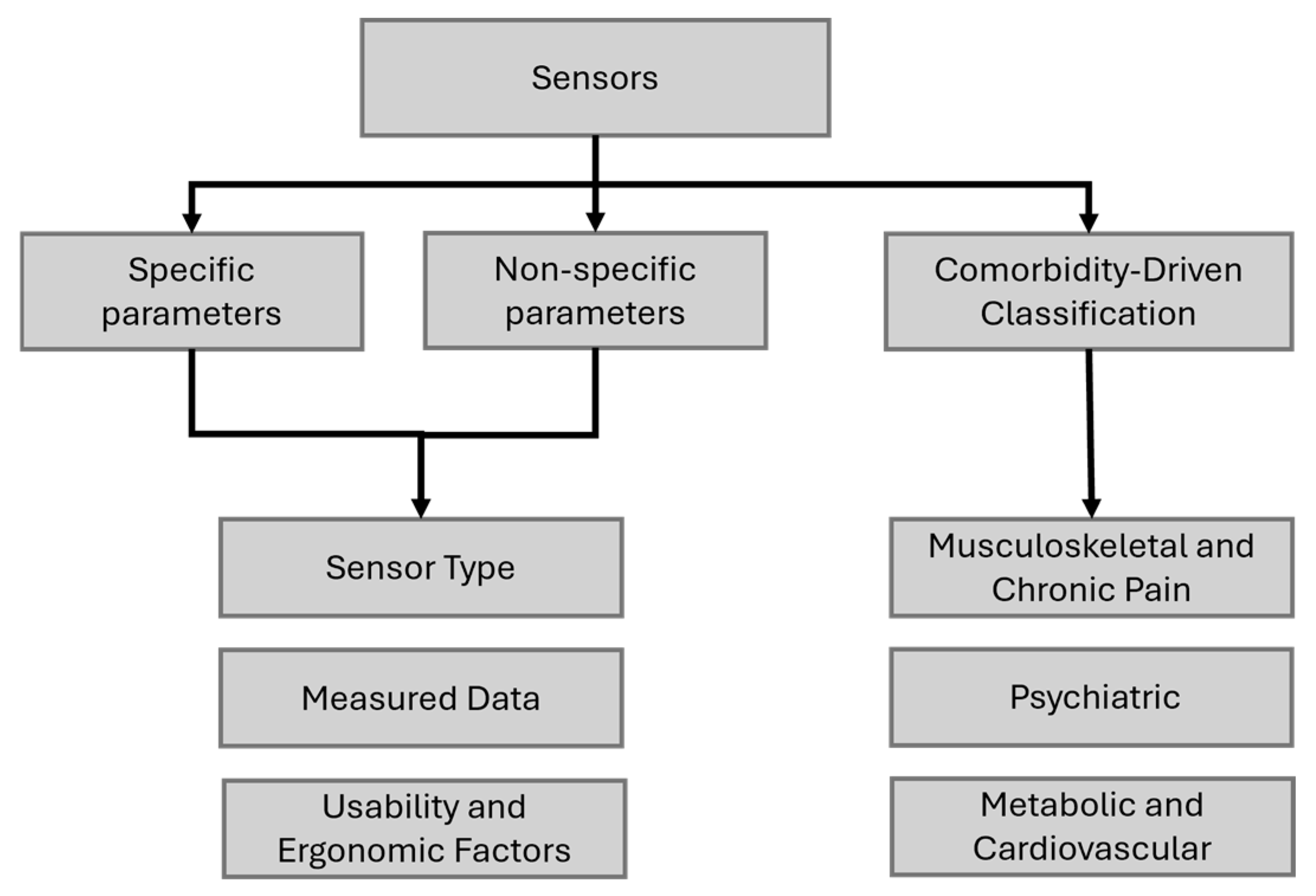
2.2. Analysis and Categorization Process
2.2.1. Data Extraction and Organization
2.2.2. Classification Development
- (a)
- Specific Parameters
- (b)
- Non-Specific Parameters
- (c)
- Comorbidity-Driven Classification
3. Classification of Sensors for Specific Motor Parameters in Rehabilitation: Type, Measured Data, Usability, and Ergonomics
3.1. Classification Based on Type
- ➢
- ➢
- ➢
- Gyroscopes are used to measure orientation and angular velocity, providing information about the joint movements and changes in posture [32].
- ➢
- ➢
- ➢
- ➢
- ➢
- ➢
- Piezoresistive—these types of sensors are often encountered in applications where detecting subtle movements and pressures during rehabilitation exercises is crucial and can be used in applications such as measurement and analysis of the plantar pressure force, manipulator soft grabbing, and human movement monitoring or touch-based game systems for upper-limb rehabilitation [50,51,52].
- ➢
- ➢
- ➢
- Electromyography (EEG) sensors: Similarly, electroencephalography (EEG) sensors are integral to BCI systems, facilitating direct communication between the brain and external devices. They offer various applications including neurofeedback training for cognitive rehabilitation, assistance in telerehabilitation, and interaction with gaming or virtual reality environments [59,60,61].
3.2. Classification Based on Measured Data
3.3. Classification Based on Usability and Ergonomics
- ➢
- Sensors: Inertial measurement unit (IMU)s, optical motion tracker systems, capacitive sensors, piezoelectric sensors, EMG sensors, EEG sensors.
- ➢
- Sensors: IMUs, optical motion tracker systems.
- ➢
- Sensors: Optical sensors, force sensors, EMG sensors, EEG sensors.
- ➢
- Sensors: IMUs, optical sensors, force sensors, EMG sensors, EEG sensors, gyroscopes, accelerometers.
- ➢
- Sensors: IMUs sensors, accelerometers, gyroscopes, optical motion tracker systems, pressure sensors, capacitive sensors, triboelectric sensors, piezoresistive sensors, piezoelectric sensors, EMG sensors (electromyography), EEG sensors (electroencephalography).
- ➢
- Sensors: Piezoresistive, capacitive, triboelectric, gyroscopes, accelerometers.
- ➢
- Sensors: Accelerometers, gyroscopes, pressure sensors, capacitive sensors, triboelectric sensors.
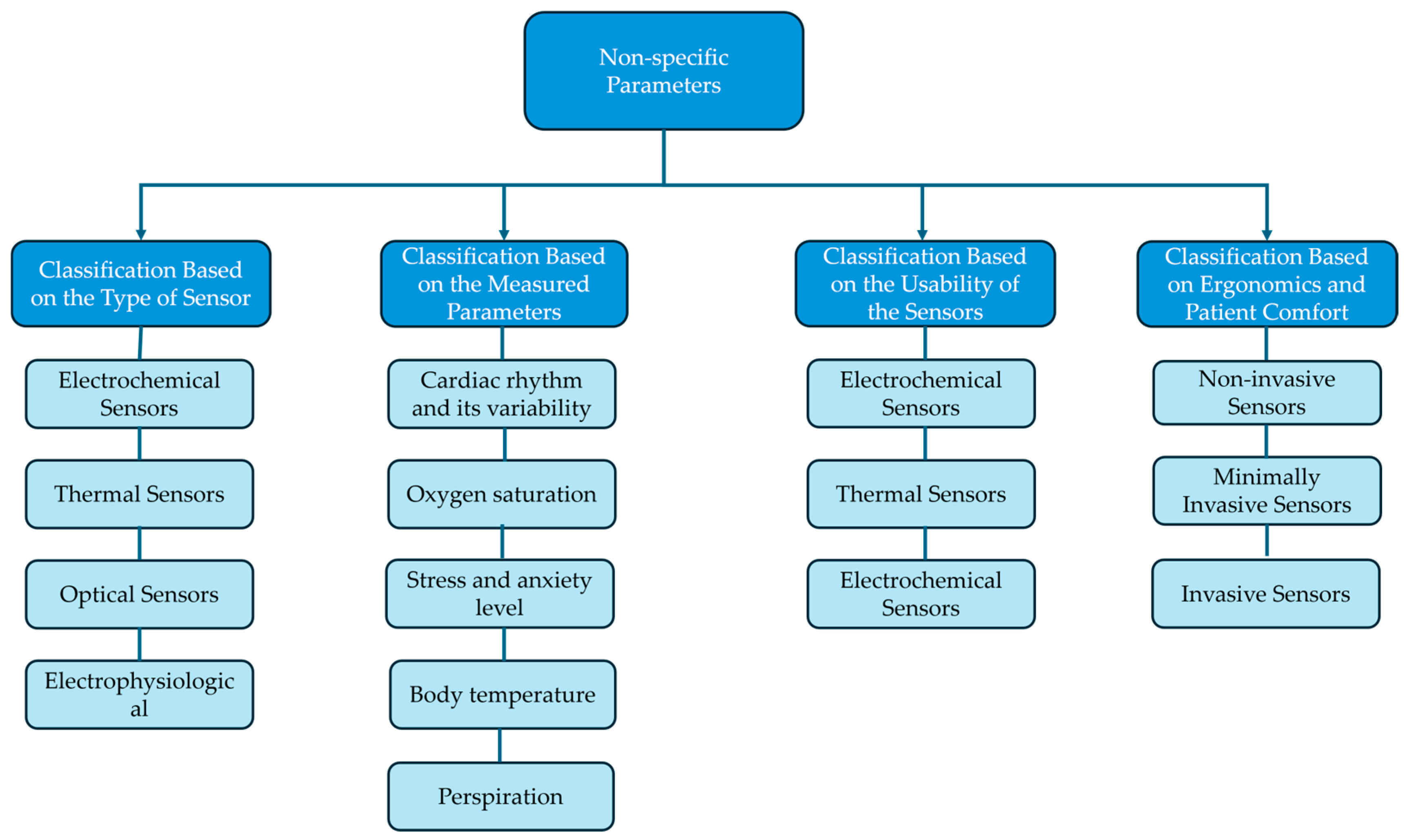
4. Sensors for Non-Specific Parameters in Rehabilitation: A Classification Based on Type, Measured Data, Usability, and Ergonomics
4.1. Classification Based on the Type of Sensor
- ➢
- ➢
- Photoplethysmography (PPG) sensors measure blood volume changes in tissues using light, detecting heart rate, oxygen saturation, and vascular health, and they can be used in neurorehabilitation for heart rate variability (HRV) monitoring, biofeedback training, sleep and fatigue tracking, and blood flow assessment [73,74,75].
- ➢
- ➢
- ➢
- ➢
4.2. Classification Based on the Measured Parameters
4.3. Classification Based on the Usability of the Sensors
- ➢
- Sensors: ECG patches, wrist-worn PPG sensors, smart textiles.
- ➢
- Sensors: Force plates, EMG sensors, infrared motion capture systems, fixed PPG sensors, NIRS sensors, thermal cameras, fixed sweat analysis sensors
- ➢
- Sensors: Infrared thermometers, camera-based respiration monitors, infrared motion capture systems.
4.4. Classification Based on Ergonomics and Patient Comfort
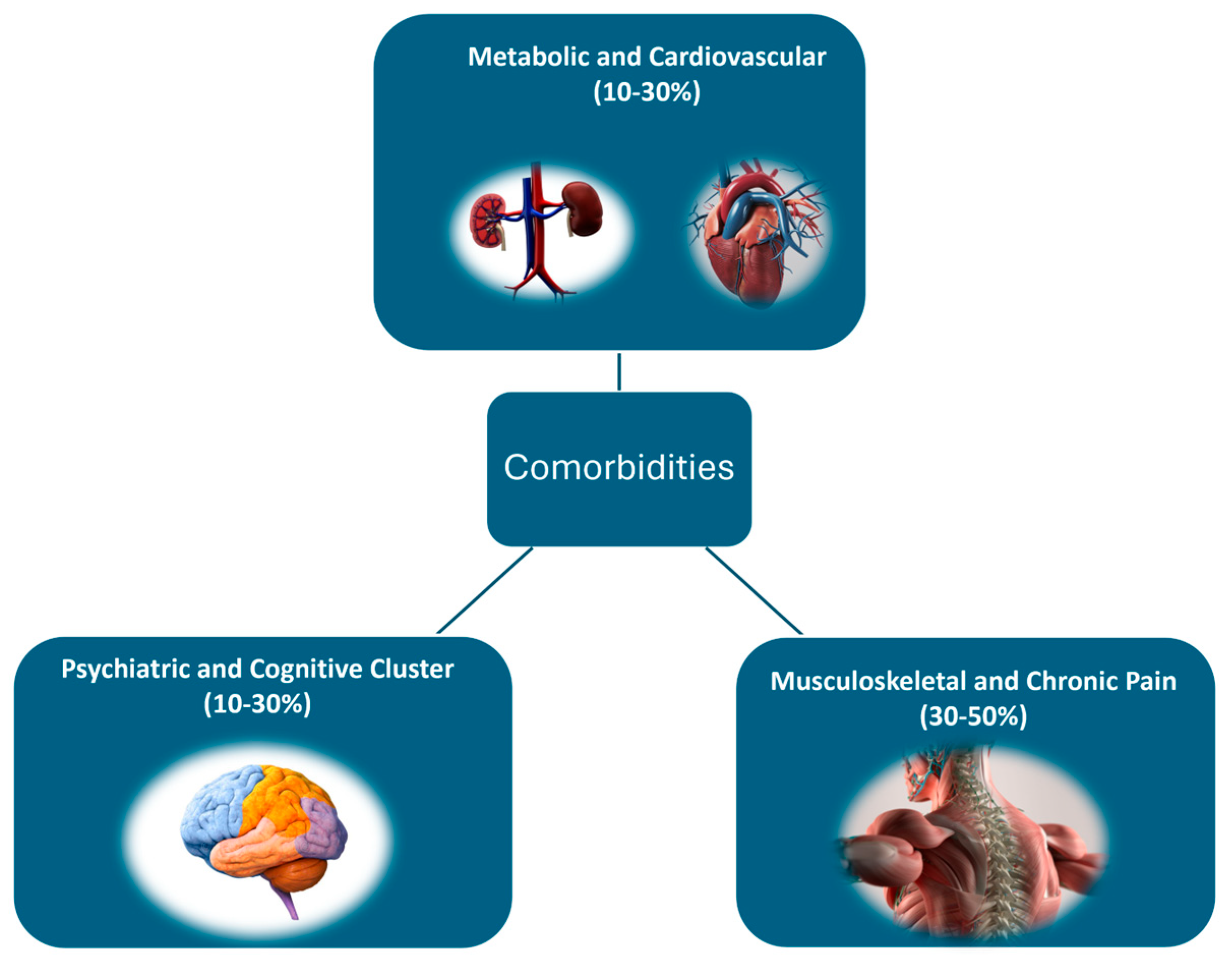
5. Classification Based on Comorbidities and Their Impact on Treatment Personalization
5.1. Metabolic and Cardiovascular Comorbidities
- ➢
- Diabetes (Type 2 diabetes, blood sugar imbalances) → Peripheral neuropathy, decreased muscle strength, and delayed reaction time.
- ➢
- Cardiovascular diseases (e.g., hypertension, heart failure) → Reduced exercise tolerance, increased risk of complications.
5.2. Musculoskeletal and Chronic Pain Comorbidities
- ➢
- Neuropathic Pain—Pain resulting from nerve damage due to conditions such as diabetes, spinal cord injury, or multiple sclerosis. Neuropathic pain can alter the way a patient perceives touch, movement, and pressure, making certain movements or exercises intolerable or extremely painful.
- -
- Force Sensors: To measure how much force is being applied during exercises, ensuring it does not aggravate the pain.
- ➢
- Osteoporosis—A condition characterized by weak, brittle bones, commonly seen in the elderly [93]. Osteoporosis increases the risk of fractures even with minimal pressure or movement, which can make physical rehabilitation difficult.
- -
- Accelerometers: To monitor movement intensity and avoid high-impact actions that could lead to fractures.
- -
- Force Sensors: To track the amount of pressure being placed on bones during exercises, that it remains within safe limits.
- ➢
- Muscle Weakness—Weakness in specific muscle groups, often due to neurological conditions like stroke, spinal cord injury, or chronic diseases such as muscular dystrophy. Muscle weakness can severely limit a patient’s ability to perform daily activities and participate in rehabilitation exercises
- -
- Goniometers: To track joint movement and range of motion, ensuring that exercises do not strain weakened muscles.
- -
- Force Sensors: To measure the applied force during exercises, ensuring that muscle activity is gradual and within the patient’s capacity.
5.3. Psychiatric Comorbidities
- ➢
- Depression can arise due to chronic pain, limited mobility, uncertainty about recovery, fatigue, social isolation, medication side effects, and the psychological stress of dealing with long-term illness and rehabilitation. Depression can significantly affect a patient’s motivation to engage in physical activities and adhere to a treatment plan [94].
- -
- Wearable EEG monitors brainwave activity to detect depressive patterns and guide adjustments in therapy or mental health support.
- -
- Sleep-Monitoring Devices help assess the impact of depression on sleep patterns, as poor sleep can worsen depressive symptoms and overall physical function.
- ➢
- Anxiety—anxiety can create heightened physical responses such as muscle tension, increased heart rate, and shortness of breath, which can interfere with therapy.
- -
- Wearable EEG: this is used to monitor brain activity, particularly during moments of stress or anxiety, helping to identify anxiety patterns and optimize the treatment program.
- -
- Sleep-Monitoring Devices: Anxiety can severely affect sleep, and these devices can monitor sleep disturbances, which are common in anxious individuals.
- ➢
- Sleep disorders: Due to chronic pain, anxiety, depression, medication side effects, and physical limitations, all of which disrupt normal sleep patterns, sleep disorders can lead to fatigue, reduced recovery time, and difficulty concentrating or performing physical exercises [95].
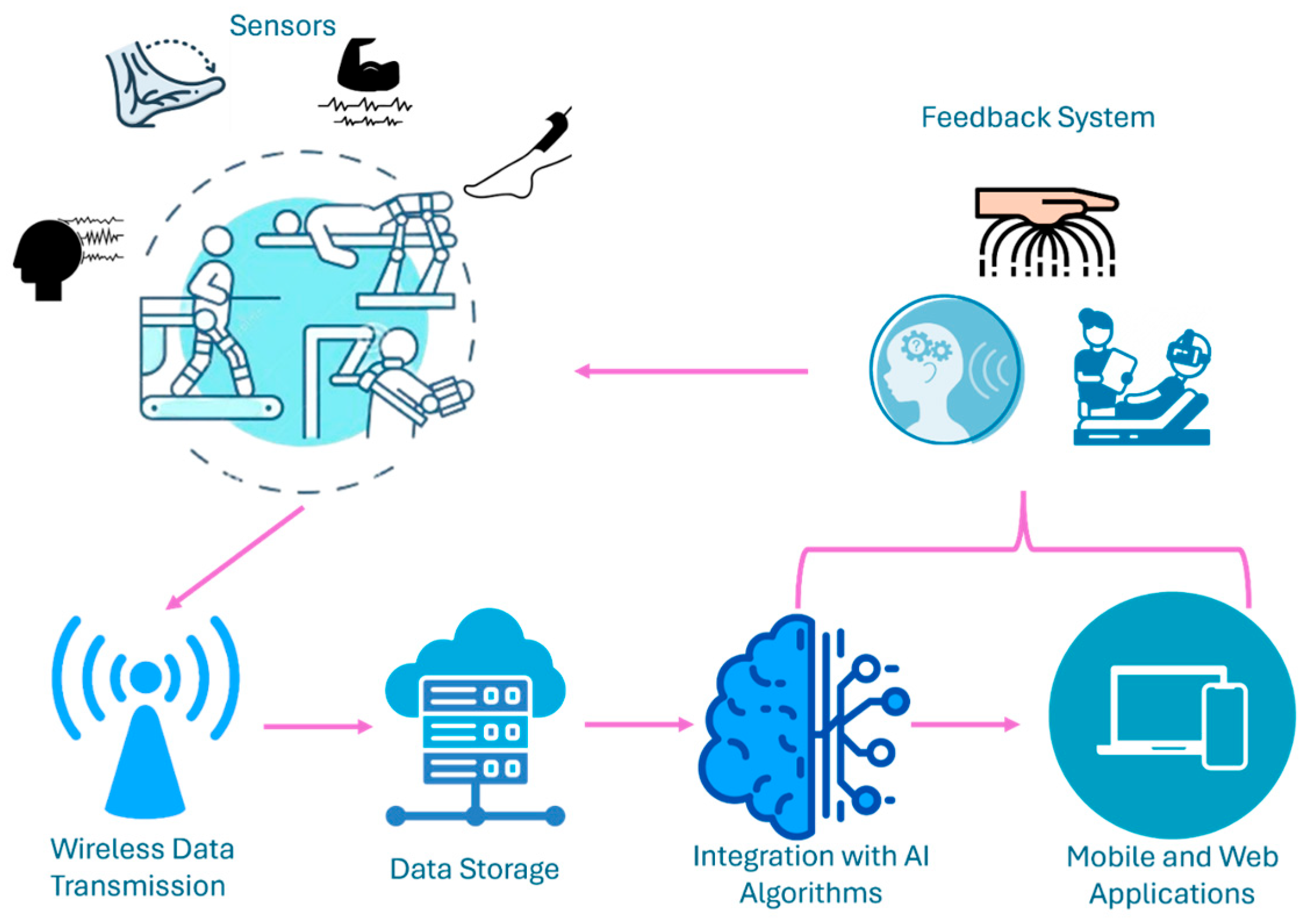
6. Real-Time Data Acquisition—Collection Strategies
7. Discussion
7.1. Sensor-Driven Personalization of Rehabilitation Strategies
7.2. Addressing Comorbidities in Sensor-Assisted Rehabilitation
7.3. Enhancing Treatment Outcomes Through AI-Driven Data Processing
7.4. Future Considerations in Sensor-Assisted Neuromotor Robot-Assisted Rehabilitation
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, A.; Vardalakis, N.; Wagner, F.B. Neuroprosthetics: From sensorimotor to cognitive disorders. Commun. Biol. 2023, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Wareham, L.K.; Liddelow, S.A.; Temple, S.; Benowitz, L.I.; Di Polo, A.; Wellington, C.; Goldberg, J.L.; He, Z.; Duan, X.; Bu, G.; et al. Solving neurodegeneration: Common mechanisms and strategies for new treatments. Mol. Neurodegener. 2022, 17, 23. [Google Scholar] [CrossRef]
- Major, Z.Z.; Vaida, C.; Major, K.A.; Tucan, P.; Brusturean, E.; Gherman, B.; Birlescu, I.; Craciunaș, R.; Ulinici, I.; Simori, G.; et al. Comparative assessment of robotic versus classical physical therapy using muscle strength and ranges of motion testing in neurological diseases. J. Pers. Med. 2021, 11, 953. [Google Scholar] [CrossRef]
- World Health Organization. World Population Prospects: The 2019 Revision. Available online: https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/undesa_pd_kf_wpp2019_10keyfindings.pdf (accessed on 23 April 2024).
- Intersectoral Global Action Plan on Epilepsy and Other Neurological Disorders. 2022. Available online: https://apps.who.int/iris/handle/10665/325293 (accessed on 23 April 2024).
- Ding, C.; Wu, Y.; Chen, X.; Chen, Y.; Wu, Z.; Lin, Z.; Kang, D.; Fang, W.; Chen, F. Global, regional, and national burden and attributable risk factors of neurological disorders: The Global Burden of Disease study 1990–2019. Front. Public Health 2022, 10, 952161. [Google Scholar] [CrossRef] [PubMed]
- Kavaliunas, A.; Danylaitė Karrenbauer, V.; Binzer, S.; Hillert, J. Systematic Review of the Socioeconomic Consequences in Patients with Multiple Sclerosis with Different Levels of Disability and Cognitive Function. Front. Neurol. 2022, 12, 737211. [Google Scholar] [CrossRef]
- Hrastelj, J.; Robertson, N.P. Socioeconomic status in neurological disorders: A modifiable risk factor? J. Neurol. 2022, 269, 3385–3386. [Google Scholar] [CrossRef] [PubMed]
- Towfighi, A.; Berger, R.P.; Corley, A.M.S.; Glymour, M.M.; Manly, J.J.; Skolarus, L.E. Recommendations on Social Determinants of Health in Neurologic Disease. Neurology 2023, 101, S17–S26. [Google Scholar] [CrossRef]
- Zillner, S.; Bisset, D.; Milano, M.; Curry, E.; García Robles, A.; Hahn, T.; Irgens, M.; Lafrenz, R.; Liepert, B.; O’Sullivan, B.; et al. (Eds.) Joint Strategic Research Innovation and Deployment Agenda (SRIDA) for the AI, Data and Robotics Partnership; BDVA/euRobotics/ELLIS/EurAI/CLAIRE: Brussels, Belgium, 2020. [Google Scholar]
- Vaida, C.; Rus, G.; Lupu, D.; Gherman, B.; Tucan, P.; Horvath, D.; Machado, J.; Pisla, D. Assessment of Different Biosignals with Potential Benefits in Robotic Assisted Neuromotor Rehabilitation. In Proceedings of the 11th IEEE International Conference on E-Health and Bioengineering-EHB 2023, Bucharest, Romania, 9–10 November 2023. [Google Scholar]
- Pan, L.; Song, A.; Duan, S.; Yu, Z. Patient-Centered Robot-Aided Passive Neurorehabilitation Exercise Based on Safety-Motion Decision-Making Mechanism. BioMed Res. Int. 2017, 2017, 4185939. [Google Scholar] [CrossRef] [PubMed]
- Tanev, T.; Dachkinov, P.; Valayil, T.; Dimitrova, M.; Kostova, S.; Lekova, A. Implementation of robotic and assistive technologies in the patientcentered physical rehabilitation. J. Tech. Univ. Gabrovo 2023, 66, 11–15. [Google Scholar] [CrossRef]
- Vaida, C.; Birlescu, I.; Pisla, A.; Ulinici, I.-M.; Tarnita, D.; Carbone, G.; Pisla, D. Systematic Design of a Parallel Robotic System for Lower Limb Rehabilitation. IEEE Access 2020, 8, 34522–34537. [Google Scholar] [CrossRef]
- Vaida, C.; Plitea, N.; Carbone, G.; Birlescu, I.; Ulinici, I.; Pisla, A.; Pisla, D. Innovative development of a spherical parallel robot for upper limb rehabilitation. Int. J. Mech. Robot. Syst. 2018, 4, 256. [Google Scholar] [CrossRef]
- Das, R.; Paul, S.; Mourya, G.K.; Kumar, N.; Hussain, M. Recent Trends and Practices Toward Assessment and Rehabilitation of Neurodegenerative Disorders: Insights from Human Gait. Front. Neurosci. 2022, 16, 859298. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, B.; Chen, W.; Liu, W.; Zhang, S. Current development of wearable sensors based on nanosheets and applications. TrAC Trends Anal. Chem. 2021, 143, 116334. [Google Scholar] [CrossRef]
- De Fazio, R.; Mastronardi, V.M.; De Vittorio, M.; Visconti, P. Wearable Sensors and Smart Devices to Monitor Rehabilitation Parameters and Sports Performance: An Overview. Sensors 2023, 23, 1856. [Google Scholar] [CrossRef] [PubMed]
- Kristoffersson, A.; Lindén, M. A Systematic Review of Wearable Sensors for Monitoring Physical Activity. Sensors 2022, 22, 573. [Google Scholar] [CrossRef] [PubMed]
- Scano, A.; Guanziroli, E.; Brambilla, C.; Amendola, C.; Pirovano, I.; Gasperini, G.; Molteni, F.; Spinelli, L.; Molinari Tosatti, L.; Rizzo, G.; et al. A Narrative Review on Multi-Domain Instrumental Approaches to Evaluate Neuromotor Function in Rehabilitation. Healthcare 2023, 11, 2282. [Google Scholar] [CrossRef] [PubMed]
- Freitas, L.; de Araújo Val, S.; Magalhaes, F.; Marinho, V.; Ayres, C.; Teixeira, S.; Bastos, V.H. Virtual reality exposure therapy for neuro-psychomotor recovery in adults: A systematic review. Disabil. Rehabil. Assist. Technol. 2021, 16, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Tohanean, N.; Tucan, P.; Vanta, O.-M.; Abrudan, C.; Pintea, S.; Gherman, B.; Burz, A.; Banica, A.; Vaida, C.; Neguran, D.A.; et al. The Efficacity of the NeuroAssist Robotic System for Motor Rehabilitation of the Upper Limb—Promising Results from a Pilot Study. J. Clin. Med. 2023, 12, 425. [Google Scholar] [CrossRef]
- Polsinelli, G.; Rodio, A.; Federico, B. Estimation of cardiovascular drift through ear temperature during prolonged steady-state cycling: A study protocol. BMJ Open Sport. Exerc. Med. 2021, 7, e000907. [Google Scholar] [CrossRef] [PubMed]
- Roffe, C.; Sills, S.; Wilde, K.; Crome, P. Effect of Hemiparetic Stroke on Pulse Oximetry Readings on the Affected Side. Stroke 2025, 32, 1808–1810. [Google Scholar] [CrossRef]
- Boyne, P.; Meyrose, C.; Westover, J.; Whitesel, D.; Hatter, K.; Reisman, D.S.; Cunningham, D.; Carl, D.; Jansen, C.; Khoury, J.C.; et al. Exercise intensity affects acute neurotrophic and neurophysiological responses poststroke. J. Appl. Physiol. 2019, 126, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Lin, W.; He, X.; Zhang, L.; Zhang, M. IMU-based motion capture system for rehabilitation applications: A systematic review. Biomim. Intell. Robot. 2023, 3, 100097. [Google Scholar] [CrossRef]
- Barraza Madrigal, J.A.; Contreras Rodríguez, L.A.; Cardiel Pérez, E.; Hernández Rodríguez, P.R.; Sossa, H. Title: Hip and lower limbs 3D motion tracking using a double-stage data fusion algorithm for IMU/MARG-based wearables sensors. Biomed. Signal Process Control 2023, 86, 104938. [Google Scholar] [CrossRef]
- Felius, R.A.W.; Geerars, M.; Bruijn, S.M.; Wouda, N.C.; Van Dieën, J.H.; Punt, M. Reliability of IMU-based balance assessment in clinical stroke rehabilitation. Gait Posture 2022, 98, 62–68. [Google Scholar] [CrossRef]
- Bai, L.; Pepper, M.G.; Yan, Y.; Phillips, M.; Sakel, M. Low Cost Inertial Sensors for the Motion Tracking and Orientation Estimation of Human Upper Limbs in Neurological Rehabilitation. IEEE Access 2020, 8, 54254–54268. [Google Scholar] [CrossRef]
- Mielnik, P.; Hjelle, A.M.; Pollen, B.; Tokarz, K.; Fojcik, M. Identification and authorization with single accelerometer data—Implications from “Wearables in Arthritis” project. Procedia Comput. Sci. 2023, 225, 374–383. [Google Scholar] [CrossRef]
- Ellis, F.; Hancock, N.; Kennedy, N.; Clark, A.; Wells, J.; Chandler, E.; Payne, D.; Pomeroy, V.M. Consideration-of-concept of EvolvRehab-Body for upper limb virtual rehabilitation at home for people late after stroke. Physiotherapy 2022, 116, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Celik, Y.; Stuart, S.; Woo, W.L.; Godfrey, A. Gait analysis in neurological populations: Progression in the use of wearables. Med. Eng. Phys. 2021, 87, 9–29. [Google Scholar] [CrossRef]
- Leal-Junior, A.; Avellar, L.; Jaimes, J.; Díaz, C.; dos Santos, W.; Siqueira, A.A.G.; Pontes, M.J.; Marques, C.; Frizera, A. Polymer optical fiber-based integrated instrumentation in a robot-assisted rehabilitation smart environment: A proof of concept. Sensors 2020, 20, 3199. [Google Scholar] [CrossRef]
- Slade, P.; Habib, A.; Hicks, J.L.; Delp, S.L. An Open-Source and Wearable System for Measuring 3D Human Motion in Real-Time. IEEE Trans. Biomed. Eng. 2022, 69, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Antico, M.; Balletti, N.; Laudato, G.; Lazich, A.; Notarantonio, M.; Oliveto, R.; Ricciardi, S.; Scalabrino, S.; Simeone, J. Postural control assessment via Microsoft Azure Kinect DK: An evaluation study. Comput. Methods Programs Biomed. 2021, 209, 106324. [Google Scholar] [CrossRef] [PubMed]
- Husty, M.; Birlescu, I.; Tucan, P.; Vaida, C.; Pisla, D. An algebraic parameterization approach for parallel robots analysis. Mech. Mach. Theory 2019, 140, 245–257. [Google Scholar] [CrossRef]
- Cha, K.; Wang, J.; Li, Y.; Shen, L.; Chen, Z.; Long, J. A novel upper-limb tracking system in a virtual environment for stroke rehabilitation. J. Neuroeng. Rehabil. 2021, 18, 166. [Google Scholar] [CrossRef] [PubMed]
- Casile, A.; Fregna, G.; Boarini, V.; Paoluzzi, C.; Manfredini, F.; Lamberti, N.; Baroni, A.; Straudi, S. Quantitative Comparison of Hand Kinematics Measured with a Markerless Commercial Head-Mounted Display and a Marker-Based Motion Capture System in Stroke Survivors. Sensors 2023, 23, 7906. [Google Scholar] [CrossRef]
- Bai, J.; Li, G.; Lu, X.; Wen, X. Automatic rehabilitation assessment method of upper limb motor function based on posture and distribution force. Front. Neurosci. 2024, 18, 1362495. [Google Scholar] [CrossRef] [PubMed]
- Shull, P.B.; Jiang, S.; Zhu, Y.; Zhu, X. Hand Gesture Recognition and Finger Angle Estimation via Wrist-Worn Modified Barometric Pressure Sensing. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Bucinskas, V.; Dzedzickis, A.; Rozene, J.; Subaciute-Zemaitiene, J.; Satkauskas, I.; Uvarovas, V.; Bobina, R. Wearable feet pressure sensor for human gait and falling diagnosis. Sensors 2021, 21, 5240. [Google Scholar] [CrossRef]
- Esposito, D.; Centracchio, J.; Andreozzi, E.; Savino, S.; Gargiulo, G.D.; Naik, G.R.; Bifulco, P. Design of a 3D-Printed Hand Exoskeleton Based on Force-Myography Control for Assistance and Rehabilitation. Machines 2022, 10, 57. [Google Scholar] [CrossRef]
- Miramand, L.; Richard, V.; McFadyen, B.J.; Turcot, K. Three dimensional validation of an instrumented handrail for stair gait. Med. Eng. Phys. 2020, 86, 16–19. [Google Scholar] [CrossRef]
- Lan, R.; Zhang, J.; Chen, J.; Tang, W.; Wu, Q.; Zhou, X.; Kang, X.; Wang, J.; Wang, H.; Li, H. High-Sensitivity Flexible Capacitive Pressure Sensors Based on Biomimetic Hibiscus Flower Microstructures. ACS Omega 2024, 9, 13704–13713. [Google Scholar] [CrossRef]
- Chen, L.; Lu, M.; Yang, H.; Salas Avila, J.R.; Shi, B.; Ren, L.; Wei, G.; Liu, X.; Yin, W. Textile-Based Capacitive Sensor for Physical Rehabilitation via Surface Topological Modification. ACS Nano 2020, 14, 8191–8201. [Google Scholar] [CrossRef] [PubMed]
- Galli, V.; Ahmadizadeh, C.; Kunz, R.; Menon, C. Textile-Based Body Capacitive Sensing for Knee Angle Monitoring. Sensors 2023, 23, 9657. [Google Scholar] [CrossRef]
- Zeng, Y.; Xiang, H.; Zheng, N.; Cao, X.; Wang, N.; Wang, Z.L. Flexible triboelectric nanogenerator for human motion tracking and gesture recognition. Nano Energy 2022, 91, 106601. [Google Scholar] [CrossRef]
- Pu, X.; An, S.; Tang, Q.; Guo, H.; Hu, C. Wearable triboelectric sensors for biomedical monitoring and human-machine interface. iScience 2021, 24, 102027. [Google Scholar] [CrossRef] [PubMed]
- Pisla, D.; Nadas, I.; Tucan, P.; Albert, S.; Carbone, G.; Antal, T.; Banica, A.; Gherman, B. Development of a control system and functional validation of a parallel robot for lower limb rehabilitation. Actuators 2021, 10, 277. [Google Scholar] [CrossRef]
- Garcia-Hernandez, N.; Huerta-Cervantes, K.; Muñoz-Pepi, I.; Parra-Vega, V. Personalized Touch-Based Exergame System for Unilateral and Bilateral Rehabilitation Training. Games Health J. 2022, 11, 157–167. [Google Scholar] [CrossRef]
- Wolterink, G.; Sanders, R.; van Beijnum, B.J.; Veltink, P.; Krijnen, G. A 3D-printed soft fingertip sensor for providing information about normal and shear components of interaction forces. Sensors 2021, 21, 4271. [Google Scholar] [CrossRef]
- Guo, X.; Hong, W.; Hu, B.; Zhang, T.; Jin, C.; Yao, X.; Li, H.; Yan, Z.; Jiao, Z.; Wang, M.; et al. Human touch sensation-inspired, ultrawide-sensing-range, and high-robustness flexible piezoresistive sensor based on CB/MXene/SR/fiber nanocomposites for wearable electronics. Compos. Struct. 2023, 321, 117329. [Google Scholar] [CrossRef]
- Ali, M.; Hoseyni, S.M.; Das, R.; Awais, M.; Basdogan, I.; Beker, L. A Flexible and Biodegradable Piezoelectric-Based Wearable Sensor for Non-Invasive Monitoring of Dynamic Human Motions and Physiological Signals. Adv. Mater. Technol. 2023, 8, 2300347. [Google Scholar] [CrossRef]
- Pan, C.T.; Chang, C.C.; Yang, Y.S.; Yen, C.K.; Kao, Y.H.; Shiue, Y.L. Development of MMG sensors using PVDF piezoelectric electrospinning for lower limb rehabilitation exoskeleton. Sens. Actuators A Phys. 2020, 301, 111708. [Google Scholar] [CrossRef]
- Ogul, O.E.; Coskunsu, D.K.; Akcay, S.; Akyol, K.; Hanoglu, L.; Ozturk, N. The effect of Electromyography (EMG)-driven Robotic Treatment on the recovery of the hand Nine years after stroke. J. Hand Ther. 2023, 36, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Lai, K.W.C. Dynamic gripping force estimation and reconstruction in EMG-based human-machine interaction. Biomed. Signal Process Control 2023, 80, 104216. [Google Scholar] [CrossRef]
- Wang, B.; Ou, C.; Xie, N.; Wang, L.; Yu, T.; Fan, G.; Chu, J. Lower limb motion recognition based on surface electromyography signals and its experimental verification on a novel multi-posture lower limb rehabilitation robots. Comput. Electr. Eng. 2022, 101, 108067. [Google Scholar] [CrossRef]
- McDonald, C.G.; Sullivan, J.L.; Dennis, T.A.; O’Malley, M.K. A Myoelectric Control Interface for Upper-Limb Robotic Rehabilitation following Spinal Cord Injury. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Halder Roy, A. EEG sensor driven assistive device for elbow and finger rehabilitation using deep learning. Expert. Syst. Appl. 2024, 244, 122954. [Google Scholar] [CrossRef]
- Das, S.; Adhikary, A.; Laghari, A.A.; Mitra, S. Eldo-care: EEG with Kinect sensor based telehealthcare for the disabled and the elderly. Neurosci. Inform. 2023, 3, 100130. [Google Scholar] [CrossRef]
- Khan, M.A.; Das, R.; Iversen, H.K.; Puthusserypady, S. Review on motor imagery based BCI systems for upper limb post-stroke neurorehabilitation: From designing to application. Comput. Biol. Med. 2020, 123, 103843. [Google Scholar] [CrossRef]
- Mohebbi, A. Human-Robot Interaction in Rehabilitation and Assistance: A Review. Curr. Robot. Rep. 2020, 1, 131–144. [Google Scholar] [CrossRef]
- Geonea, I.D.; Tarnita, D.; Pisla, D.; Carbone, G.; Bolcu, A.; Tucan, P.; Georgescu, M.; Tarniță, D.N. Dynamic analysis of a spherical parallel robot used for brachial monoparesis rehabilitation. Appl. Sci. 2021, 11, 11849. [Google Scholar] [CrossRef]
- Pisla, D.; Tarnita, D.; Tucan, P.; Tohanean, N.; Vaida, C.; Geonea, I.D.; Bogdan, G.; Abrudan, C.; Carbone, G.; Plitea, N. A parallel robot with torque monitoring for brachial monoparesis rehabilitation tasks. Appl. Sci. 2021, 11, 9932. [Google Scholar] [CrossRef]
- Chellal, A.A.; Lima, J.; Gonçalves, J.; Fernandes, F.P.; Pacheco, F.; Monteiro, F.; Brito, T.; Soares, S. Robot-Assisted Rehabilitation Architecture Supported by a Distributed Data Acquisition System. Sensors 2022, 22, 9532. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.P.; Liu, Y.Y.; Hsu, W.H.; Lai, L.J.; Lee, M.S. Monitoring and assessment of rehabilitation progress on range of motion after total knee replacement by sensor-based system. Sensors 2020, 20, 1703. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Jamwal, P.K.; Vliet, P.V.; Brown, N.A.T. Robot Assisted Ankle Neuro-Rehabilitation: State of the art and Future Challenges. Expert. Rev. Neurother. 2021, 21, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Vaida, C.; Birlescu, I.; Pisla, A.; Carbone, G.; Plitea, N.; Ulinici, I.; Gherman, B.; Puskas, F.; Tucan, P.; Pisla, D. RAISE—An Innovative Parallel Robotic System for Lower Limb Rehabilitation. In New Trends in Medical and Service Robotics; Carbone, G., Ceccarelli, M., Pisla, D., Eds.; Mechanisms and Machine Science; Springer: Cham, Switzerland, 2018; pp. 293–302. [Google Scholar]
- Prill, R.; Walter, M.; Królikowska, A.; Becker, R. A systematic review of diagnostic accuracy and clinical applications of wearable movement sensors for knee joint rehabilitation. Sensors 2021, 21, 8221. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Saibene, M.; Das, R.; Brunner, I.; Puthusserypady, S. Emergence of flexible technology in developing advanced systems for post-stroke rehabilitation: A comprehensive review. J. Neural Eng. 2021, 18, 061003. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Vargas, P.; Flor, O.; Salvador-Acosta, B.; Suárez-Carreño, F.; Santórum, M.; Solorzano, S.; Salvador-Ullauri, L. Inertial Sensors for Hip Arthroplasty Rehabilitation: A Scoping Review. Sensors 2023, 23, 5048. [Google Scholar] [CrossRef]
- Khalid, U.; Naeem, M.; Stasolla, F.; Syed, M.; Abbas, M.; Coronato, A. Impact of AI-Powered Solutions in Rehabilitation Process: Recent Improvements and Future Trends. Int. J. Gen. Med. 2024, 17, 943–969. [Google Scholar] [CrossRef] [PubMed]
- Elgendi, M.; Fletcher, R.; Liang, Y.; Howard, N.; Lovell, N.H.; Abbott, D.; Lim, K.; Ward, R. The use of photoplethysmography for assessing hypertension. NPJ Digit. Med. 2019, 2, 60. [Google Scholar] [CrossRef]
- Ullah, H.; Wahab, M.A.; Will, G.; Karim, M.R.; Pan, T.; Gao, M.; Lai, D.; Lin, Y.; Miraz, M.H. Recent. Recent Advances in Stretchable and Wearable Capacitive Electrophysiological Sensors for Long-Term Health Monitoring. Biosensors 2022, 12, 630. [Google Scholar] [CrossRef]
- Park, J.; Seok, H.S.; Kim, S.S.; Shin, H. Photoplethysmogram Analysis and Applications: An Integrative Review. Front. Physiol. 2022, 12, 808451. [Google Scholar] [CrossRef]
- Wick, K.D.; Matthay, M.A.; Ware, L.B. Pulse oximetry for the diagnosis and management of acute respiratory distress syndrome. Lancet Respir. Med. 2022, 10, 1086–1098. [Google Scholar] [CrossRef] [PubMed]
- Quaresima, V.; Ferrari, M.; Scholkmann, F. Ninety years of pulse oximetry: History, current status, and outlook. J. Biomed. Opt. 2024, 29, S33307. [Google Scholar] [CrossRef] [PubMed]
- Agrafiotis, D.K.; Yang, E.; Littman, G.S.; Byttebier, G.; Dipietro, L.; DiBernardo, A.; Chavez, J.C.; Rykman, A.; McArthur, K.; Hajjar, K.; et al. Accurate prediction of clinical stroke scales and improved biomarkers of motor impairment from robotic measurements. PLoS ONE 2021, 16, e0245874. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, H.; Wang, Y.; Lin, H.; Zhu, X.; Wang, Y. Use of non-contact infrared thermometers in rehabilitation patients: A randomized controlled study. J. Int. Med. Res. 2021, 49, 0300060520984617. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Bergmann, J.H.M. Non-Contact Infrared Thermometers and Thermal Scanners for Human Body Temperature Monitoring: A Systematic Review. Sensors 2023, 23, 7439. [Google Scholar] [CrossRef]
- Li, F.; Xue, H.; Lin, X.; Zhao, H.; Zhang, T. Wearable Temperature Sensor with High Resolution for Skin Temperature Monitoring. ACS Appl. Mater. Interfaces 2022, 14, 43844–43852. [Google Scholar] [CrossRef]
- Lu, Y.; Fujita, Y.; Honda, S.; Yang, H.; Xuan, Y.; Xu, K.; Arie, T.; Akita, S.; Takei, K. Wireless and Flexible Skin Moisture and Temperature Sensor Sheets toward the Study of Thermoregulator Center. Adv. Healthc. Mater. 2021, 10, 2100103. [Google Scholar] [CrossRef]
- Qiao, Y.; Qiao, L.; Chen, Z.; Liu, B.; Gao, L.; Zhang, L. Wearable Sensor for Continuous Sweat Biomarker Monitoring. Chemosensors 2022, 10, 273. [Google Scholar] [CrossRef]
- Bi, Y. Universal Fully Integrated Wearable Sensor Arrays for the Multiple Electrolyte and Metabolite Monitoring in Raw Sweat, Saliva, or Urine. Anal. Chem. 2023, 95, 6690–6699. [Google Scholar] [CrossRef]
- Kukkar, D.; Zhang, D.; Jeon, B.H.; Kim, K.H. Recent advances in wearable biosensors for non-invasive monitoring of specific metabolites and electrolytes associated with chronic kidney disease: Performance evaluation and future challenges. TrAC—Trends Anal. Chem. 2022, 150, 116570. [Google Scholar] [CrossRef]
- Jiang, D.; Zhu, Y.; Sun, Z.; Zhu, Z.; He, Q.; Huang, X.; Yang, Y.; Ge, Y.; Zhang, Q.; Wang, Y. A silver nanowires@Prussian blue composite aerogel-based wearable sensor for noninvasive and dynamic monitoring of sweat uric acid. Chem. Eng. J. 2024, 486, 150220. [Google Scholar] [CrossRef]
- Cao, H.; Lin, R.; Long, Z.; Xing, L.; Xue, X. A self-powered wireless sweat-analysis patch for real-time monitoring physiological status. Nano Energy 2024, 123, 109411. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, W.; Shen, C.; Jiang, G.; Yang, C. Flexible and printable integrated biosensors for monitoring sweat and skin condition. Anal. Biochem. 2023, 661, 114985. [Google Scholar] [CrossRef] [PubMed]
- Hegedűs, B. The Potential Role of Thermography in Determining the Efficacy of Stroke Rehabilitation. J. Stroke Cerebrovasc. Dis. 2018, 27, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Yogev, D.; Goldberg, T.; Arami, A.; Tejman-Yarden, S.; Winkler, T.E.; Maoz, B.M. Current state of the art and future directions for implantable sensors in medical technology: Clinical needs and engineering challenges. APL Bioeng. 2023, 7, 031506. [Google Scholar] [CrossRef]
- Pasluosta, C.F.; Popovic, M.R.; Eskofier, B.M.; Stieglitz, T. Editorial: Wearable and Implantable Technologies in the Rehabilitation of Patients with Sensory Impairments. Front. Neurosci. 2021, 15, 740263. [Google Scholar] [CrossRef]
- Salminger, S.; Sturma, A.; Hofer, C.; Evangelista, M.; Perrin, M.; Bergmeister, K.D.; Roche, A.D.; Hasenoehrl, T.; Dietl, H.; Farina, D.; et al. Long-term implant of intramuscular sensors and nerve transfers for wireless control of robotic arms in above-elbow amputees. Sci. Robot. 2019, 4, eaaw6306. [Google Scholar] [CrossRef]
- Nicholson, B.; Verma, S. Comorbidities in chronic neuropathic pain. Pain Med. 2024, 5 (Suppl. S1), S9–S27. [Google Scholar] [CrossRef]
- Oliver Tobin, W. Management of Multiple Sclerosis Symptoms and Comorbidities. Continuum 2019, 25, 753–772. [Google Scholar]
- Kumar, R.G.; Ketchum, J.M.; Corrigan, J.D.; Hammond, F.M.; Sevigny, M.; Dams-O’Connor, K. The Longitudinal Effects of Comorbid Health Burden on Functional Outcomes for Adults with Moderate to Severe Traumatic Brain Injury. J. Head Trauma Rehabil. 2020, 35, 372–381. [Google Scholar] [CrossRef]
- Davie, G.; Samaranayaka, A.; Derrett, S. The role of pre-existing comorbidity on the rate of recovery following injury: A longitudinal cohort study. PLoS ONE 2018, 13, e0193019. [Google Scholar] [CrossRef] [PubMed]
- Fakolade, A.; Bisson, E.J.; Pétrin, J.; Lamarre, J.; Finlayson, M. Effect of comorbidities on outcomes of neurorehabilitation interventions in multiple sclerosis: A scoping review. Int. J. MS Care 2016, 18, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Simić-Panić, D.; Bošković, K.; Milićević, M.; Rabi Žikić, T.; Cvjetković Bošnjak, M.; Tomašević-Todorović, S.; Jovićević, M. The impact of comorbidity on rehabilitation outcome after ischemic stroke. Acta Clin. Croat. 2018, 57, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.; Fiedeldey, L.K.; Kim, B.; Clemens, F.; Irvine, M.A.; Hosseini, S.H.; Smolina, K.; Wister, A. Systematic review and meta-analysis of disease clustering in multimorbidity: A study protocol. BMJ Open 2023, 13, e076496. [Google Scholar] [CrossRef] [PubMed]
- Maliszewski, K.; Feldmann, A.; McCully, K.K.; Julian, R. A systematic review of the relationship between muscle oxygen dynamics and energy rich phosphates. Can NIRS help? BMC Sports Sci. Med. Rehabil. 2024, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Kurkin, S.; Badarin, A.; Grubov, V.; Maksimenko, V.; Hramov, A. The oxygen saturation in the primary motor cortex during a single hand movement: Functional near-infrared spectroscopy (fNIRS) study. Eur. Phys. J. Plus 2021, 136, 548. [Google Scholar] [CrossRef]
- Longatelli, V.; Torricelli, D.; Tornero, J.; Pedrocchi, A.; Molteni, F.; Pons, J.L.; Gandolla, M. A unified scheme for the benchmarking of upper limb functions in neurological disorders. J. Neuroeng. Rehabil. 2022, 19, 102. [Google Scholar] [CrossRef]
- Guerrini, A.; Siotto, M.; Germanotta, M.; Cipollini, V.; Cortellini, L.; Pavan, A.; Insalaco, S.; Khazrai, Y.M.; Aprile, I. Muscle quality improvement in subacute post-stroke patients after rehabilitation: Usefulness of segmental phase angle from bioelectrical impedance analysis. Clin. Nutr. 2024, 43, 224–231. [Google Scholar] [CrossRef]
- Sunny, M.S.H.; Hernandez, J.; Rulik, I.; Wang, I.; Rahman, M. Functionality and Performance Assessment of Assistive Robots’ Grippers Performing Activities of Daily Livings. Arch. Phys. Med. Rehabil. 2022, 103, e147. [Google Scholar] [CrossRef]
- Wei, X.; Guo, H.; Wang, X.; Wang, X.; Qiu, M. Reliable Data Collection Techniques in Underwater Wireless Sensor Networks: A Survey. IEEE Commun. Surv. Tutor. 2022, 24, 404–431. [Google Scholar] [CrossRef]
- Vijayan, V.; Connolly, J.; Condell, J.; McKelvey, N.; Gardiner, P. Review of wearable devices and data collection considerations for connected health. Sensors 2021, 21, 5589. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Bian GBin Tian, Z. Removal of artifacts from EEG signals: A review. Sensors 2019, 19, 987. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhang, S.; Yang, Y.; Liu, X.; Li, J.; Bao, B.; Liu, C.; Yang, H.; Guo, K.; Cheng, H. Conformal, stretchable, breathable, wireless epidermal surface electromyography sensor system for hand gesture recognition and rehabilitation of stroke hand function. Mater. Des. 2024, 243, 113029. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, J.; Liu, C.; Shi, M. A portable EEG signal acquisition system and a limited-electrode channel classification network for SSVEP. Front. Neurorobot. 2024, 18, 1502560. [Google Scholar] [CrossRef]
- Palumbo, A.; Vizza, P.; Calabrese, B.; Ielpo, N. Biopotential signal monitoring systems in rehabilitation: A review. Sensors 2021, 21, 7172. [Google Scholar] [CrossRef]
- Do, X.P.; Phan TT, T.; Nguyen HV, K.; Phan HT, T.; Pham, H.T. A New Design of Electromyography Sensor Using the High-Pass and Low-Pass Filters for Classification of Upper-Limb Movements. Available online: https://ssrn.com/abstract=4992906 (accessed on 12 January 2025).
- Liu, Z.; Kong, J.; Qu, M.; Zhao, G.; Zhang, C. Progress in Data Acquisition of Wearable Sensors. Biosensors 2022, 12, 889. [Google Scholar] [CrossRef]
- Bailey, N.W.; Hill, A.T.; Godfrey, K.; Perera, M.P.N.; Rogasch, N.C.; Fitzgibbon, B.M.; Fitzgerald, P.B. EEG is better when cleaning effectively targets artifacts. bioRxiv 2024. [Google Scholar] [CrossRef]
- Zou, Y.; Zhao, X.; Chu, Y.; Xu, W.; Han, J.; Li, W. A supervised independent component analysis algorithm for motion imagery-based brain computer interface. Biomed. Signal Process Control 2022, 75, 103576. [Google Scholar] [CrossRef]
- Kumaravel, V.P.; Farella, E. IMU-integrated Artifact Subspace Reconstruction for Wearable EEG Devices. In Proceedings of the IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Istanbul, Turkiye, 5–8 December 2023; pp. 2508–2514. [Google Scholar]
- Do Nascimento, L.M.S.; Bonfati, L.V.; Freitas, M.L.B.; Mendes Junior, J.J.A.; Siqueira, H.V.; Stevan, S.L. Sensors and systems for physical rehabilitation and health monitoring—A review. Sensors 2020, 20, 4063. [Google Scholar] [CrossRef]
- Al Abiad, N.; Houdry, E.; El Khoury, C.; Renaudin, V.; Robert, T. A method for calculating fall risk parameters from discrete stride time series regardless of sensor placement. Gait Posture 2024, 111, 182–184. [Google Scholar] [CrossRef]
- Donisi, L.; Pagano, G.; Cesarelli, G.; Coccia, A.; Amitrano, F.; D’Addio, G. Benchmarking between two wearable inertial systems for gait analysis based on a different sensor placement using several statistical approaches. Measurement 2021, 173, 108642. [Google Scholar] [CrossRef]
- Li, T.; Dong, T. Monocular camera-based online sensor-to-segment calibration for upper body pose estimation. Sens. Actuators A Phys. 2023, 364, 114829. [Google Scholar] [CrossRef]
- Giangrande, A.; Botter, A.; Piitulainen, H.; Cerone, G.L. Motion Artifacts in Dynamic EEG Recordings: Experimental Observations, Electrical Modelling, and Design Considerations. Sensors 2024, 24, 6363. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, R.; Sahana, B.C.; Bhandari, A.K. Motion Artifacts Suppression from EEG Signals Using an Adaptive Signal Denoising Method. IEEE Trans. Instrum. Meas. 2022, 71, 4000410. [Google Scholar] [CrossRef]
- Wang, K.C.; Liu, K.C.; Peng, S.Y.; Tsao, Y. ECG Artifact Removal from Single-Channel Surface EMG Using Fully Convolutional Networks. arXiv 2022, arXiv:2210.13271. [Google Scholar]
- Yamini, B.; Pradeep, G.; Kalaiyarasi, D.; Jayaprakash, M.; Janani, G.; Uthayakumar, G.S. Theoretical study and analysis of advanced wireless sensor network techniques in Internet of Things (IoT). Meas. Sens. 2024, 33, 101098. [Google Scholar] [CrossRef]
- Sofi, A.; Jane Regita, J.; Rane, B.; Lau, H.H. Structural health monitoring using wireless smart sensor network—An overview. Mech. Syst. Signal Process 2022, 163, 108113. [Google Scholar] [CrossRef]
- Belov, V.; Tatarintsev, A.; Nikulchev, E. Choosing a data storage format in the apache hadoop system based on experimental evaluation using apache spark. Symmetry 2021, 13, 195. [Google Scholar] [CrossRef]
- Wang, K.; Xie, S.; Rodrigues, J. Medical data security of wearable tele-rehabilitation under internet of things. Internet Things Cyber-Phys. Syst. 2022, 2, 1–11. [Google Scholar] [CrossRef]
- Sumner, J.; Lim, H.W.; Chong, L.S.; Bundele, A.; Mukhopadhyay, A.; Kayambu, G. Artificial intelligence in physical rehabilitation: A systematic review. Artif. Intell. Med. 2023, 146, 102693. [Google Scholar] [CrossRef]
- Nikulchev, E.; Ilin, D.; Silaeva, A.; Kolyasnikov, P.; Belov, V.; Runtov, A.; Pushkin, P.; Laptev, N.; Alexeenko, A.; Magomedov, S.; et al. Digital Psychological Platform for Mass Web-Surveys. Mendeley Data 2020. [Google Scholar] [CrossRef]
- Adam, R.; Catchpoole, D.R.; Simoff, S.S.; Kennedy, P.J.; Nguyen, Q.V. Novel Hybrid Edge-Cloud Framework for Efficient and Sustainable Omics Data Management. Innov. Digit. Health Diagn. Biomark. 2024, 4, 81–88. [Google Scholar] [CrossRef]
- Gupta, M.; Bhatia, D.; Kumar, P. Virtual reality, augmented reality technologies, and rehabilitation. Mod. Interv. Tools Rehabil. 2023, 2023, 111–134. [Google Scholar] [CrossRef]
- Boumrah, M.; Garbaya, S.; Radgui, A. Real-Time Visual Analytics for Remote Monitoring of Patients’ Health. Comput. Sci. Res. Notes 2023, 31, 368–378. [Google Scholar] [CrossRef]
- Fornés, A.; Bensalah, A.; Carmona-Duarte, C.; Chen, J.; Ferrer, M.A.; Fischer, A.; Lladós, J.; Martín, C.; Opisso, E.; Plamondon, R.; et al. The RPM3D project: 3D Kinematics for Remote Patient Monitoring. In Proceedings of the 20th International Conference of the International Graphonomics Society, IGS 2021, Las Palmas de Gran Canaria, Spain, 7–9 June 2022. [Google Scholar]
- Proulx, C.E.; Louis Jean, M.T.; Higgins, J.; Gagnon, D.H.; Dancause, N. Somesthetic, Visual, and Auditory Feedback and Their Interactions Applied to Upper Limb Neurorehabilitation Technology: A Narrative Review to Facilitate Contextualization of Knowledge. Front. Rehabil. Sci. 2022, 3, 789479. [Google Scholar] [CrossRef]
- Du, Q.; Luo, J.; Cheng, Q.; Wang, Y.; Guo, S. Vibrotactile enhancement in hand rehabilitation has a reinforcing effect on sensorimotor brain activities. Front. Neurosci. 2022, 16, 935827. [Google Scholar] [CrossRef]
- Segear, S.; Chheang, V.; Baron, L.; Li, J.; Kim, K.; Barmaki, R.L. Visual feedback and guided balance training in an immersive virtual reality environment for lower extremity rehabilitation. Comput. Graph. 2024, 119, 103880. [Google Scholar] [CrossRef]
- Demolder, C.; Molina, A.; Hammond, F.L.; Yeo, W.H. Recent advances in wearable biosensing gloves and sensory feedback biosystems for enhancing rehabilitation, prostheses, healthcare, and virtual reality. Biosens. Bioelectron. 2021, 190, 113443. [Google Scholar] [CrossRef]
- Islam, M.S.; Lim, S. Vibrotactile feedback in virtual motor learning: A systematic review. Appl. Ergon. 2022, 101, 103694. [Google Scholar] [CrossRef]
- Guelmami, N.; Fekih-Romdhane, F.; Mechraoui, O.; Bragazzi, L.N. Injury Prevention, Optimized Training and Rehabilitation: How Is AI Reshaping the Field of Sports Medicine. New Asian J. Med. 2023, 1, 30–34. [Google Scholar] [CrossRef]

| Robot | Upper Limb | Lower Limb |
|---|---|---|
| Joint torque [62,63,64] | EEG—electrical activity of the brain [59,60,61] | EEG—electrical activity of the brain [59] |
| Joint angle [26,33] | EMG—muscle activity [55,56] | EMG—muscle activity [57] |
| Joint stiffness [65] | Range of motion [26] | Range of motion [27,66] |
| Pressure distribution [67,68] | Maximum isometric force [42] | Ground interaction force [41] |
| Force feedback [66] | Muscle contraction length [53] | Muscle contraction length [46] |
| Muscle activation [58] | Muscle activation [57] | |
| Angle for specific motions (abduction/adduction; rotation; pronation/supination; flexion extension) [26] | Inclination angle [28] | |
| Angular velocity [26] | Angular velocity [26] | |
| Linear acceleration [19] | Linear acceleration [19] | |
| Angular acceleration [29] | Angular acceleration [66] |
| Strategy | Key Steps and Considerations |
|---|---|
| Sensors | |
| Filtering and Artifact Removal |
|
| Wireless Data Transmission |
|
| Data Storage |
|
| Integration with AI Algorithms |
|
| Mobile and Web Applications |
|
| Feedback Systems |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaida, C.; Rus, G.; Pisla, D. A Sensor-Based Classification for Neuromotor Robot-Assisted Rehabilitation. Bioengineering 2025, 12, 287. https://doi.org/10.3390/bioengineering12030287
Vaida C, Rus G, Pisla D. A Sensor-Based Classification for Neuromotor Robot-Assisted Rehabilitation. Bioengineering. 2025; 12(3):287. https://doi.org/10.3390/bioengineering12030287
Chicago/Turabian StyleVaida, Calin, Gabriela Rus, and Doina Pisla. 2025. "A Sensor-Based Classification for Neuromotor Robot-Assisted Rehabilitation" Bioengineering 12, no. 3: 287. https://doi.org/10.3390/bioengineering12030287
APA StyleVaida, C., Rus, G., & Pisla, D. (2025). A Sensor-Based Classification for Neuromotor Robot-Assisted Rehabilitation. Bioengineering, 12(3), 287. https://doi.org/10.3390/bioengineering12030287








