Effect of Decorin and Aligned Collagen Fibril Topography on TGF-β1 Activation of Corneal Keratocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Micropatterning of Aligned Collagen Fibrils
2.2. Patterning of Random Collagen Fibrils and Collagen Monomer Coated Surfaces
2.3. Decorin Coating of Aligned Collagen Fibril Micropatterns and Other Collagen Surfaces
2.4. Corneal Cell Culture
2.5. Fluorescent Imaging of Collagen and Decorin Coating
2.6. Immunofluorescent Staining and Imaging
2.7. Cell Morphology Measurements
2.8. Statistics
3. Results
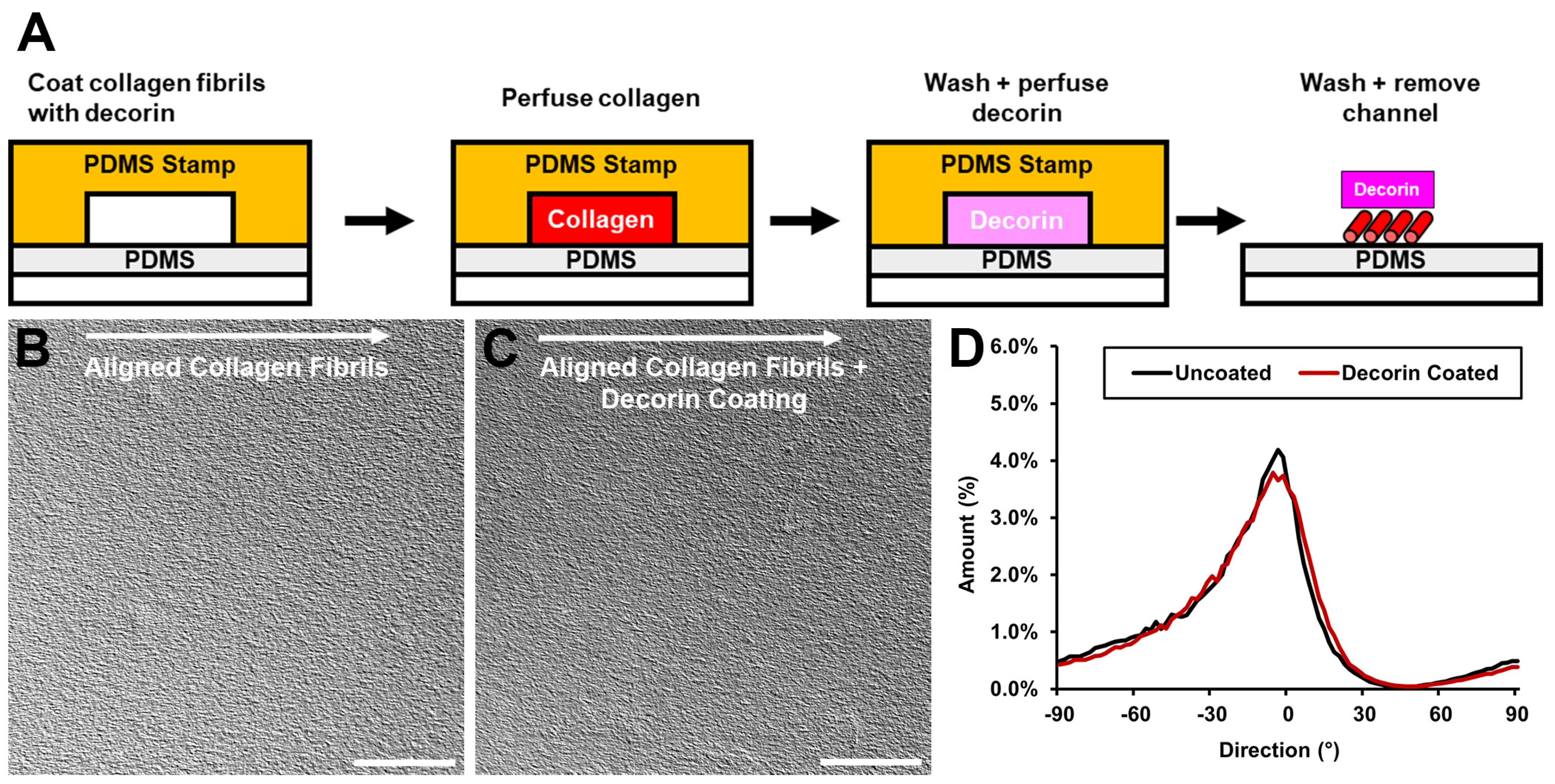
3.1. Incorporation of Decorin into Aligned Collagen Fibril Patterns
3.2. Decorin Coating Stability and Desorption
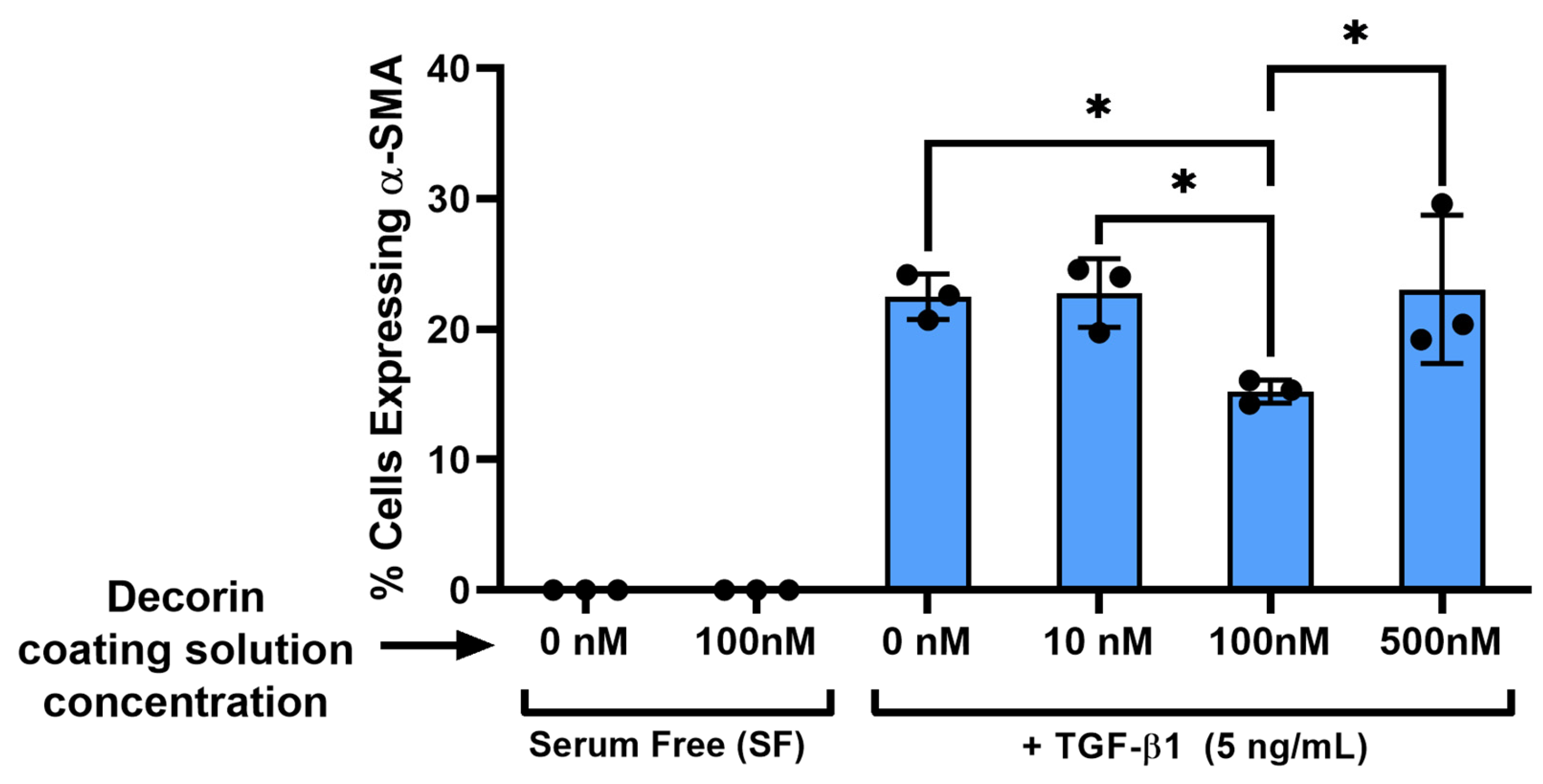
3.3. Effect of the Decorin Coating on Keratocyte Differentiation in Response to TGF-β1 Stimulation
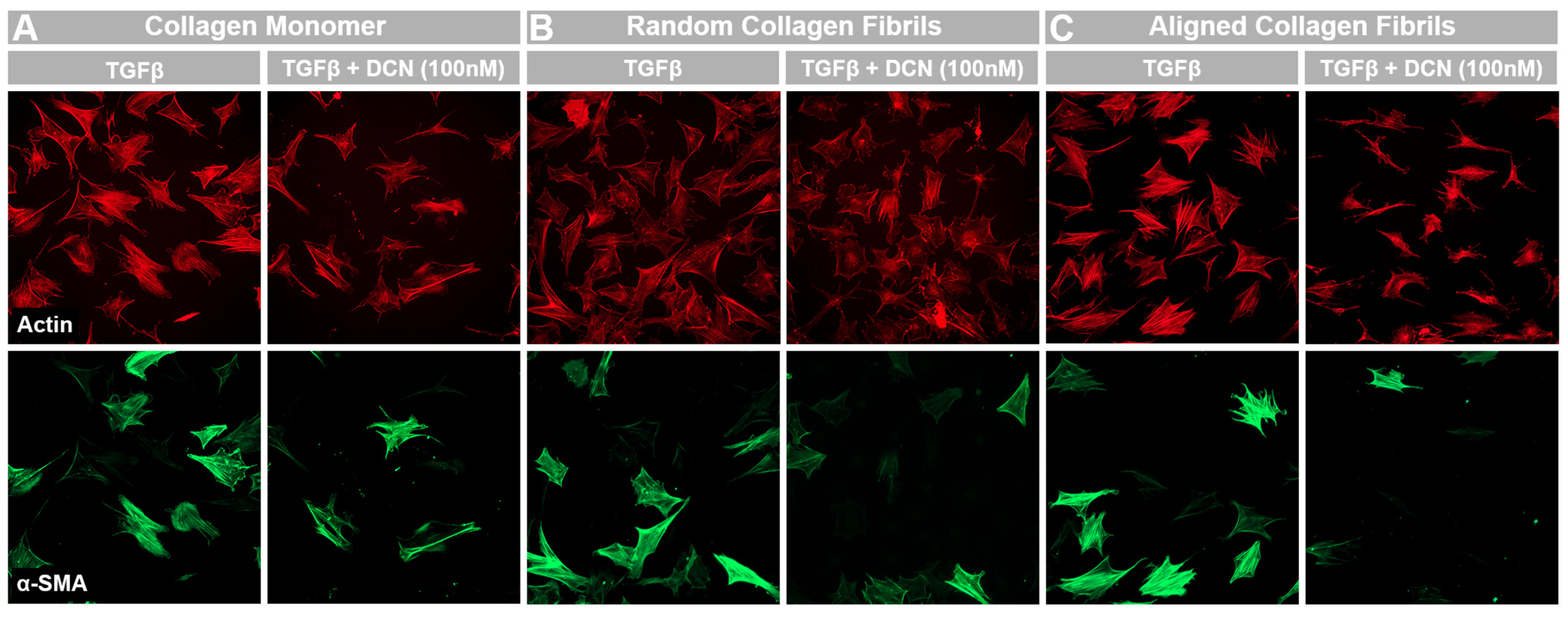
3.4. Effects of Topography and Fibril Alignment on Decorin Coating Inhibition of TGF-β1 Mediated Activation
3.5. Decorin Coating Inhibition of TGF-β1 Mediated Activation of Keratocytes Is Partially Independent from TGF-β1 Concentration on Aligned Collagen Fibrils
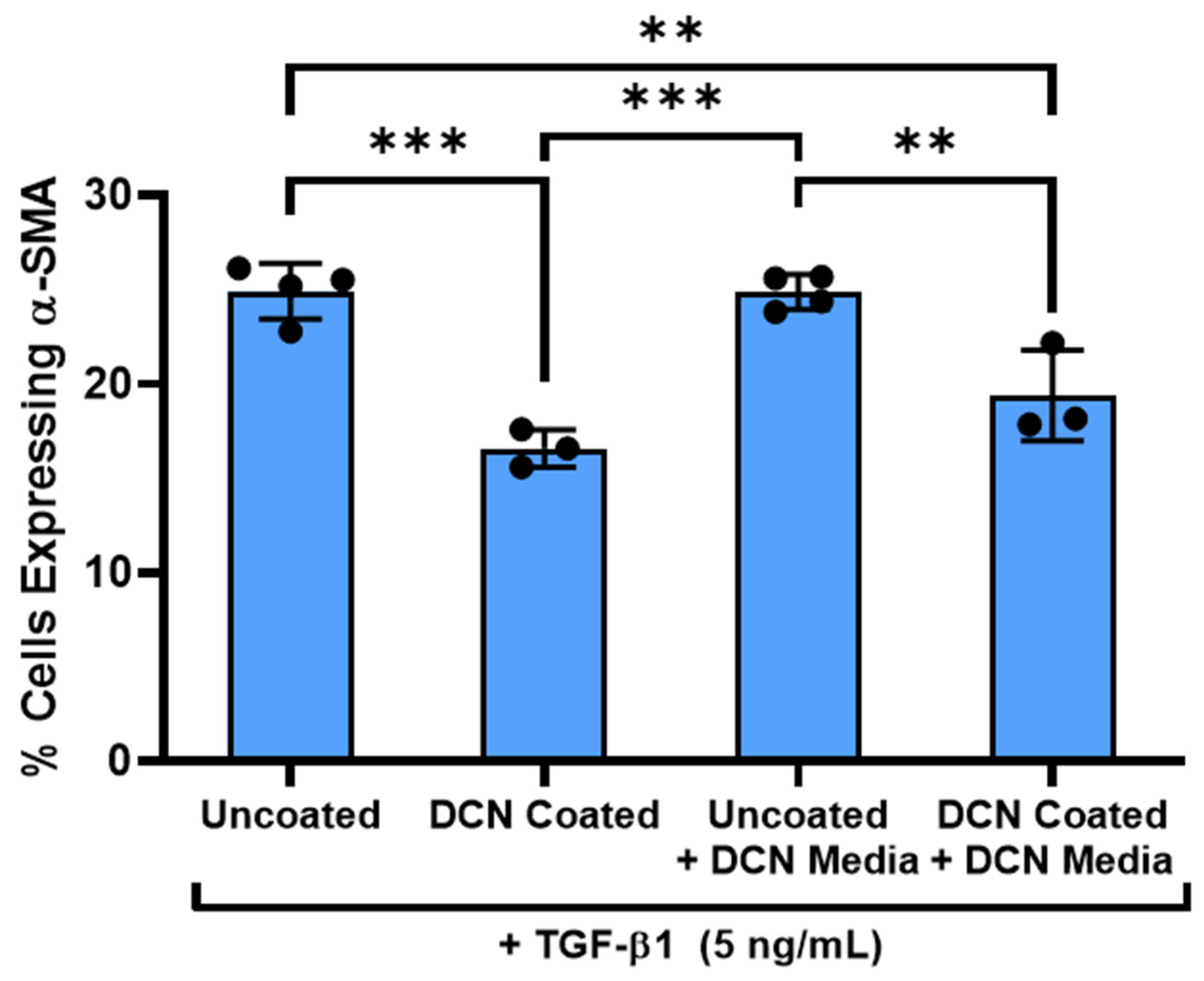
3.6. Decorin Coating Inhibition of TGF-β1 Mediated Activation of Keratocytes Is Dependent on Immobilization of Decorin to Aligned Collagen Fibrils
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| α-SMA | Alpha smooth muscle actin |
| CMOS | Complementary metal oxide semiconductor |
| DIC | Differential interference contrast |
| DTAF | 5-([4,6-Dichlorotriazin-2-yl]amino) fluorescein hydrochloride |
| ECM | Extracellular matrix |
| EGF | Epidermal growth factor |
| EGFR | Epidermal growth factor receptor |
| IGF | Insulin-like growth factor |
| LRP-1 | Low density lipoprotein related receptor one |
| MEM | Minimum essential media |
| NRK | Normal rabbit keratocytes |
| OI | Orientation index |
| PBS | Phosphate buffered saline |
| PDGF | Platelet derived growth factor |
| PDGFR | Platelet derived growth factor receptor |
| PDMS | Polydimethylsiloxane |
| TGF-β1 | Transforming growth factor beta one |
References
- Radner, W.; Zehetmayer, M.; Aufreiter, R.; Mallinger, R. Interlacing and Cross-Angle Distribution of Collagen Lamellae in the Human Cornea. Cornea 1998, 17, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Komai, Y.; Ushiki, T. The three-dimensional organization of collagen fibrils in the human cornea and sclera. Investig. Ophthalmol. Vis. Sci. 1991, 32, 2244–2258. [Google Scholar]
- Puri, S.; Coulson-Thomas, Y.M.; Gesteira, T.F.; Coulson-Thomas, V.J. Distribution and Function of Glycosaminoglycans and Proteoglycans in the Development, Homeostasis and Pathology of the Ocular Surface. Front. Cell Dev. Biol. 2020, 8, 731. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Phillip, J.M.; Majumdar, S.; Wu, P.-H.; Chen, J.; Calderón-Colón, X.; Schein, O.; Smith, B.J.; Trexler, M.M.; Wirtz, D.; et al. Modulation of keratocyte phenotype by collagen fibril nanoarchitecture in membranes for corneal repair. Biomaterials 2013, 34, 9365–9372. [Google Scholar] [CrossRef]
- Torbet, J.; Malbouyres, M.; Builles, N.; Justin, V.; Roulet, M.; Damour, O.; Oldberg, A.; Ruggiero, F.; Hulmes, D.J. Tissue Engineering of the Cornea: Orthogonal Scaffold of Magnetically Aligned Collagen Lamellae for Corneal Stroma Reconstruction. In Proceedings of the 29th Annual International Conference of the IEEE EMBS, Lyon, France, 23–26 August 2007. [Google Scholar]
- Wu, J.; Du, Y.; Watkins, S.C.; Funderburgh, J.L.; Wagner, W.R. The engineering of organized human corneal tissue through the spatial guidance of corneal stromal stem cells. Biomaterials 2012, 33, 1343–1352. [Google Scholar] [CrossRef]
- Lakshman, N.; Kim, A.; Petroll, W.M. Characterization of corneal keratocyte morphology and mechanical activity within 3-D collagen matrices. Exp. Eye Res. 2010, 90, 350–359. [Google Scholar] [CrossRef]
- Schmidinger, G.; Hanselmayer, G.; Pieh, S.; Lackner, B.; Kaminski, S.; Ruhswurm, I.; Skorpik, C. Effect of tenascin and fibronectin on the migration of human corneal fibroblasts. J. Cataract. Refract. Surg. 2003, 29, 354–360. [Google Scholar] [CrossRef]
- Andresen, J.L.; Ledet, T.; Hager, H.; Josephsen, K.; Ehlers, N. The influence of corneal stromal matrix proteins on the migration of human corneal fibroblasts. Exp. Eye Res. 2000, 71, 33–43. [Google Scholar] [CrossRef]
- Pot, S.A.; Liliensiek, S.J.; Myrna, K.E.; Bentley, E.; Jester, J.V.; Nealey, P.F.; Murphy, C.J. Nanoscale topography-induced modulation of fundamental cell behaviors of rabbit corneal keratocytes, fibroblasts, and myofibroblasts. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1373–1381. [Google Scholar] [CrossRef]
- Lam, K.H.; Shihabeddin, T.Z.; Awkal, J.A.; Najjar, A.M.; Miron-Mendoza, M.; Maruri, D.P.; Varner, V.D.; Petroll, W.M.; Schmidtke, D.W. Effects of Topography and PDGF on the Response of Corneal Keratocytes to Fibronectin-Coated Surfaces. J. Funct. Biomater. 2023, 14, 217. [Google Scholar] [CrossRef]
- Mellgren, A.E.C.; Bruland, O.; Vedeler, A.; Saraste, J.; Schönheit, J.; Bredrup, C.; Knappskog, P.M.; Rødahl, E. Development of congenital stromal corneal dystrophy is dependent on export and extracellular deposition of truncated decorin. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2909–2915. [Google Scholar] [CrossRef] [PubMed]
- Santra, M.; Reed, C.C.; Iozzo, R.V. Decorin binds to a narrow region of the epidermal growth factor (EGF) receptor, partially overlapping but distinct from the EGF-binding epitope. J. Biol. Chem. 2002, 277, 35671–35681. [Google Scholar] [CrossRef] [PubMed]
- Iozzo, R.V.; Moscatello, D.K.; McQuillan, D.J.; Eichstetter, I. Decorin Is a Biological Ligand for the Epidermal Growth Factor Receptor. J. Biol. Chem. 1999, 274, 4489–4492. [Google Scholar] [CrossRef] [PubMed]
- Baghy, K.; Horváth, Z.; Regős, E.; Kiss, K.; Schaff, Z.; Iozzo, R.V.; Kovalszky, I. Decorin interferes with platelet-derived growth factor receptor signaling in experimental hepatocarcinogenesis. FEBS J. 2013, 280, 2150–2164. [Google Scholar] [CrossRef] [PubMed]
- Gubbiotti, M.A.; Vallet, S.D.; Ricard-Blum, S.; Iozzo, R.V. Decorin Interacting Network: A Comprehensive Analysis of Decorin-Binding Partners and Their Versatile Functions; Elsevier B.V.: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Kubo, E.; Shibata, S.; Shibata, T.; Sasaki, H.; Singh, D.P. Role of Decorin in the Lens and Ocular Diseases. Cells 2023, 12, 74. [Google Scholar] [CrossRef]
- Nili, N.; Cheema, A.N.; Giordano, F.J.; Barolet, A.W.; Babaei, S.; Hickey, R.; Eskandarian, M.R.; Smeets, M.; Butany, J.; Pasterkamp, G.; et al. Decorin Inhibition of PDGF-Stimulated Vascular Smooth Muscle Cell Function Potential Mechanism for Inhibition of Intimal Hyperplasia after Balloon Angioplasty. Am. J. Pathol. 2003, 163, 869–878. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Mann, D.M.; Ruoslahti, E. Negative regulation of transforming growth factor-fJ by the proteoglycan decorin. Nature 1990, 346, 281–284. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, S.; Goldoni, S.; Calder, B.W.; Simpson, H.C.; Owens, R.T.; McQuillan, D.J.; Young, M.F.; Iozzo, R.V.; Birk, D.E. Genetic evidence for the coordinated regulation of collagen fibrillogenesis in the cornea by decorin and biglycan. J. Biol. Chem. 2009, 284, 8888–8897. [Google Scholar] [CrossRef]
- Stamov, D.R.; Müller, A.; Wegrowski, Y.; Brezillon, S.; Franz, C.M. Quantitative analysis of type I collagen fibril regulation by lumican and decorin using AFM. J. Struct. Biol. 2013, 183, 394–403. [Google Scholar] [CrossRef]
- Gupta, S.; Buyank, F.; Sinha, N.R.; Grant, D.G.; Sinha, P.R.; Iozzo, R.V.; Chaurasia, S.S.; Mohan, R.R. Decorin regulates collagen fibrillogenesis during corneal wound healing in mouse in vivo. Exp. Eye Res. 2022, 216, 108933. [Google Scholar] [CrossRef]
- Mohan, R.R.; Tandon, A.; Sharma, A.; Cowden, J.W.; Tovey, J.C.K. Significant inhibition of corneal scarring in vivo with tissue-selective, targeted AAV5 decorin gene therapy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4833–4841. [Google Scholar] [CrossRef] [PubMed]
- Hill, L.J.; Moakes, R.J.A.; Vareechon, C.; Butt, G.; Ng, A.; Brock, K.; Chouhan, G.; Vincent, R.C.; Abbondante, S.; Williams, R.L.; et al. Sustained release of decorin to the surface of the eye enables scarless corneal regeneration. NPJ Regen. Med. 2018, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.R.; Gupta, R.; Mehan, M.K.; Cowden, J.W.; Sinha, S. Decorin transfection suppresses profibrogenic genes and myofibroblast formation in human corneal fibroblasts. Exp. Eye Res. 2010, 91, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Dreier, B.; Thomasy, S.M.; Mendonsa, R.; Raghunathan, V.K.; Russell, P.; Murphy, C.J. Substratum compliance modulates corneal fibroblast to myofibroblast transformation. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5901–5907. [Google Scholar] [CrossRef]
- Lam, K.H.; Kivanany, P.B.; Grose, K.; Yonet-Tanyeri, N.; Alsmadi, N.; Varner, V.D.; Petroll, W.M.; Schmidtke, D.W. A high-throughput microfluidic method for fabricating aligned collagen fibrils to study Keratocyte behavior. Biomed. Microdevices 2019, 21, 99. [Google Scholar] [CrossRef]
- Subramanian, D.; Tjahjono, N.S.; Hernandez, P.A.; Varner, V.D.; Petroll, W.M.; Schmidtke, D.W. Fabrication of Micropatterns of Aligned Collagen Fibrils. Langmuir 2024, 40, 2551–2561. [Google Scholar] [CrossRef]
- Kivanany, P.B.; Grose, K.C.; Yonet-Tanyeri, N.; Manohar, S.; Sunkara, Y.; Lam, K.H.; Schmidtke, D.W.; Varner, V.D.; Petroll, W.M. An in vitro model for assessing corneal keratocyte spreading and migration on aligned fibrillar collagen. J. Funct. Biomater. 2018, 9, 54. [Google Scholar] [CrossRef]
- Jester, J.V.; Huang, J.; Petroll, W.M.; Cavanagh, H.D. TGFβ induced myofibroblast differentiation of rabbit keratocytes requires synergistic TGFβ, PDGF and integrin signaling. Exp. Eye Res. 2002, 75, 645–657. [Google Scholar] [CrossRef]
- Myrna, K.E.; Mendonsa, R.; Russell, P.; Pot, S.A.; Liliensiek, S.J.; Jester, J.V.; Nealey, P.F.; Brown, D.; Murphy, C.J. Substratum topography modulates corneal fibroblast to Myofibroblast transformation. Investig. Ophthalmol. Vis. Sci. 2012, 53, 811–816. [Google Scholar] [CrossRef]
- Ständer, M.; Naumann, U.; Wick, W.; Weller, M. Transforming growth factor-β and p-21: Multiple molecular targets of decorin-mediated suppression of neoplastic growth. Cell Tissue Res. 1999, 296, 221–227. [Google Scholar] [CrossRef]
- Zhang, Z.; Garron, T.M.; Li, X.-J.; Liu, Y.; Zhang, X.; Li, Y.-Y.; Xu, W.-S. Recombinant human decorin inhibits TGF-β1-induced contraction of collagen lattice by hypertrophic scar fibroblasts. Burns 2009, 35, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Cabello-Verrugio, C.; Brandan, E. A novel modulatory mechanism of transforming growth factor-β signaling through decorin and LRP-1. J. Biol. Chem. 2007, 282, 18842–18850. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.R.; Tripathi, R.; Sharma, A.; Sinha, P.R.; Giuliano, E.A.; Hesemann, N.P.; Chaurasia, S.S. Decorin antagonizes corneal fibroblast migration via caveolae-mediated endocytosis of epidermal growth factor receptor. Exp. Eye Res. 2019, 180, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, M.L.; Ratner, B.D. Collagen affinity coating for surface binding of decorin and other biomolecules: Surface characterization. Biointerphases 2017, 12, 02C419. [Google Scholar] [CrossRef]
- Bredrup, C.; Stang, E.; Bruland, O.; Palka, B.P.; Young, R.D.; Haavik, J.; Knappskog, P.M.; Rødahl, E. Decorin accumulation contributes to the stromal opacities found in congenital stromal corneal dystrophy. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5578–5582. [Google Scholar] [CrossRef]
- Kamil, S.; Mohan, R.R. Corneal Stromal Wound Healing: Major Regulators and Therapeutic Targets; Elsevier Inc.: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Xuan, M.; Wang, S.; Liu, X.; He, Y.; Li, Y.; Zhang, Y. Proteins of the Corneal Stroma: Importance in Visual Function; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- Geng, Y.; McQuillan, D.; Roughley, P.J. SLRP interaction can protect collagen fibrils from cleavage by collagenases. Matrix Biol. 2006, 25, 484–491. [Google Scholar] [CrossRef]
- Svensson, L.; Heinegard, D.; Oldbergf, A. Decorin-binding Sites for Collagen Type I Are Mainly Located in Leucine-rich Repeats 4–5*. J. Biol. Chem. 1995, 270, 20712–20716. [Google Scholar] [CrossRef]
- Bhide, V.M.; Laschinger, C.A.; Arora, P.D.; Lee, W.; Hakkinen, L.; Larjava, H.; Sodek, J.; McCulloch, C.A. Collagen phagocytosis by fibroblasts is regulated by decorin. J. Biol. Chem. 2005, 280, 23103–23113. [Google Scholar] [CrossRef]
- Petroll, W.M.; Kivanany, P.B.; Hagenasr, D.; Graham, E.K. Corneal fibroblast migration patterns during intrastromal wound healing correlate with ECM structure and alignment. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7352–7361. [Google Scholar] [CrossRef]
- English, E.J.; Samolyk, B.L.; Gaudette, G.R.; Pins, G.D. Micropatterned fibrin scaffolds increase cardiomyocyte alignment and contractility for the fabrication of engineered myocardial tissue. J. Biomed. Mater. Res. A 2023, 111, 1309–1321. [Google Scholar] [CrossRef]
- Yang, L.; Ge, L.; Zhou, Q.; Mokabber, T.; Pei, Y.; Bron, R.; van Rijn, P. Biomimetic Multiscale Hierarchical Topography Enhances Osteogenic Differentiation of Human Mesenchymal Stem Cells. Adv. Mater. Interfaces 2020, 7, 2000385. [Google Scholar] [CrossRef]
- Muthusubramaniam, L.; Zaitseva, T.; Paukshto, M.; Martin, G.; Desai, T. Effect of collagen nanotopography on keloid fibroblast proliferation and matrix synthesis: Implications for dermal wound healing. Tissue Eng. Part A 2014, 20, 2728–2736. [Google Scholar] [CrossRef] [PubMed]
- Orgel, J.P.R.O.; Eid, A.; Antipova, O.; Bella, J.; Scott, J.E. Decorin core protein (decoron) shape complements collagen fibril surface structure and mediates its binding. PLoS ONE 2009, 4, e7028. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Gor, J.; Perkins, S.J.; Ishikawa, Y.; Bächinger, H.P.; Hohenester, E. The concave face of decorin mediates reversible dimerization and collagen binding. J. Biol. Chem. 2013, 288, 35526–35533. [Google Scholar] [CrossRef]
- Mogensen, E.H.; Poulsen, E.T.; Thøgersen, I.B.; Yamamoto, K.; Brüel, A.; Enghild, J.J. The low-density lipoprotein receptor-related protein 1 (LRP1) interactome in the human cornea. Exp. Eye Res. 2022, 219, 109081. [Google Scholar] [CrossRef]
- Schaefer, L.; Iozzo, R.V. Biological functions of the small leucine-rich proteoglycans: From genetics to signal transduction. J. Biol. Chem. 2008, 283, 21305–21309. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tjahjono, N.S.; Subramanian, D.; Shihabeddin, T.Z.; Hicks, H.D.; Varner, V.D.; Petroll, W.M.; Schmidtke, D.W. Effect of Decorin and Aligned Collagen Fibril Topography on TGF-β1 Activation of Corneal Keratocytes. Bioengineering 2025, 12, 259. https://doi.org/10.3390/bioengineering12030259
Tjahjono NS, Subramanian D, Shihabeddin TZ, Hicks HD, Varner VD, Petroll WM, Schmidtke DW. Effect of Decorin and Aligned Collagen Fibril Topography on TGF-β1 Activation of Corneal Keratocytes. Bioengineering. 2025; 12(3):259. https://doi.org/10.3390/bioengineering12030259
Chicago/Turabian StyleTjahjono, Nathaniel S., Divya Subramanian, Tarik Z. Shihabeddin, Hudson D. Hicks, Victor D. Varner, W. Matthew Petroll, and David W. Schmidtke. 2025. "Effect of Decorin and Aligned Collagen Fibril Topography on TGF-β1 Activation of Corneal Keratocytes" Bioengineering 12, no. 3: 259. https://doi.org/10.3390/bioengineering12030259
APA StyleTjahjono, N. S., Subramanian, D., Shihabeddin, T. Z., Hicks, H. D., Varner, V. D., Petroll, W. M., & Schmidtke, D. W. (2025). Effect of Decorin and Aligned Collagen Fibril Topography on TGF-β1 Activation of Corneal Keratocytes. Bioengineering, 12(3), 259. https://doi.org/10.3390/bioengineering12030259






