A Design of Experiments Approach for Enhancing Room Temperature Stability of a Lyophilised and Paper-Based Bacterial Cell-Free System
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Growth Condition
2.2. Cell-Free Extract Preparation
2.3. Cell-Free Protein Synthesis
2.4. CFPS on Cellulose Stacks
2.5. Design of Experiments (DoE)
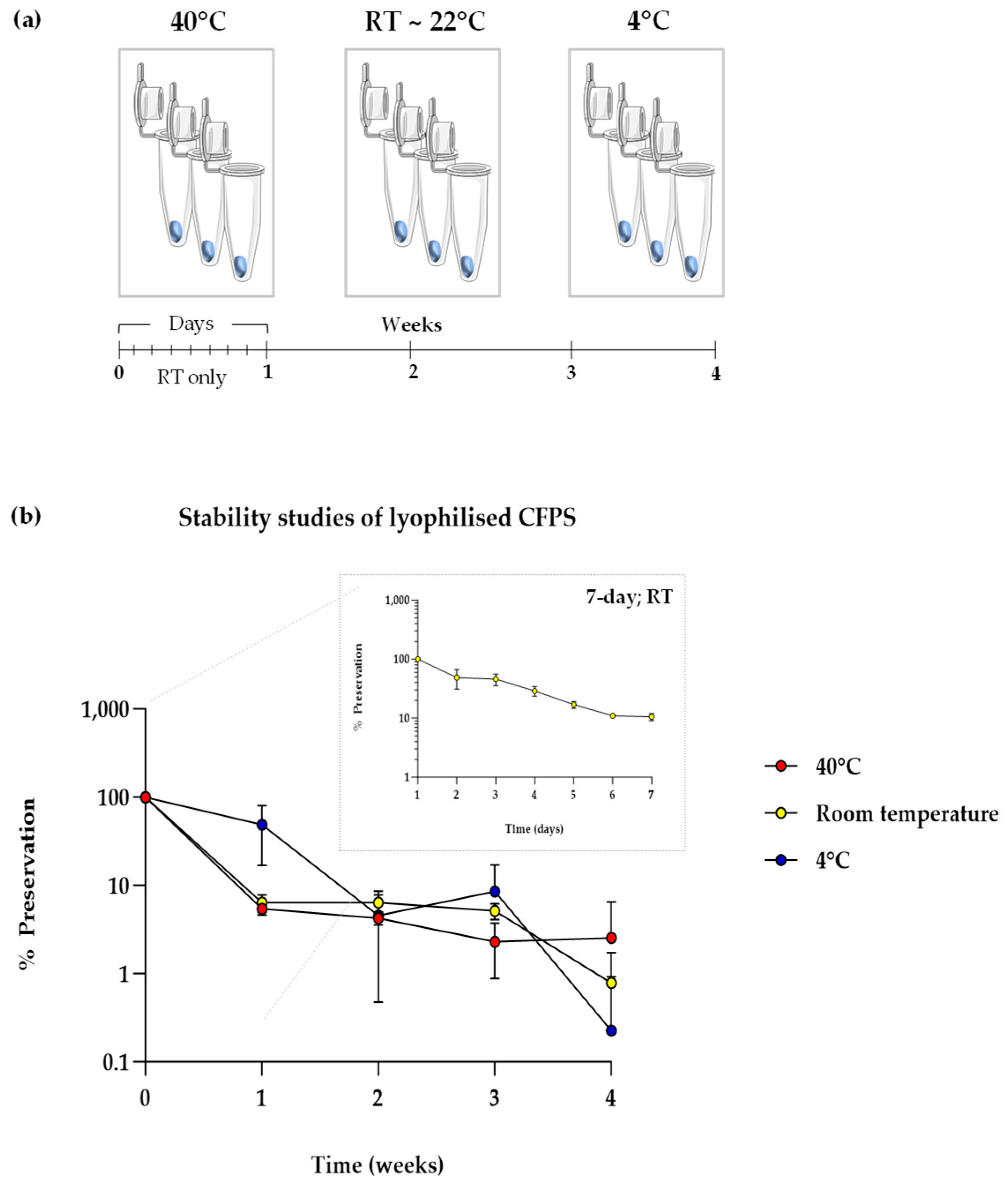
2.6. Stability Studies
2.7. SDS-PAGE and Western Blotting
2.8. Statistical Analysis
3. Results
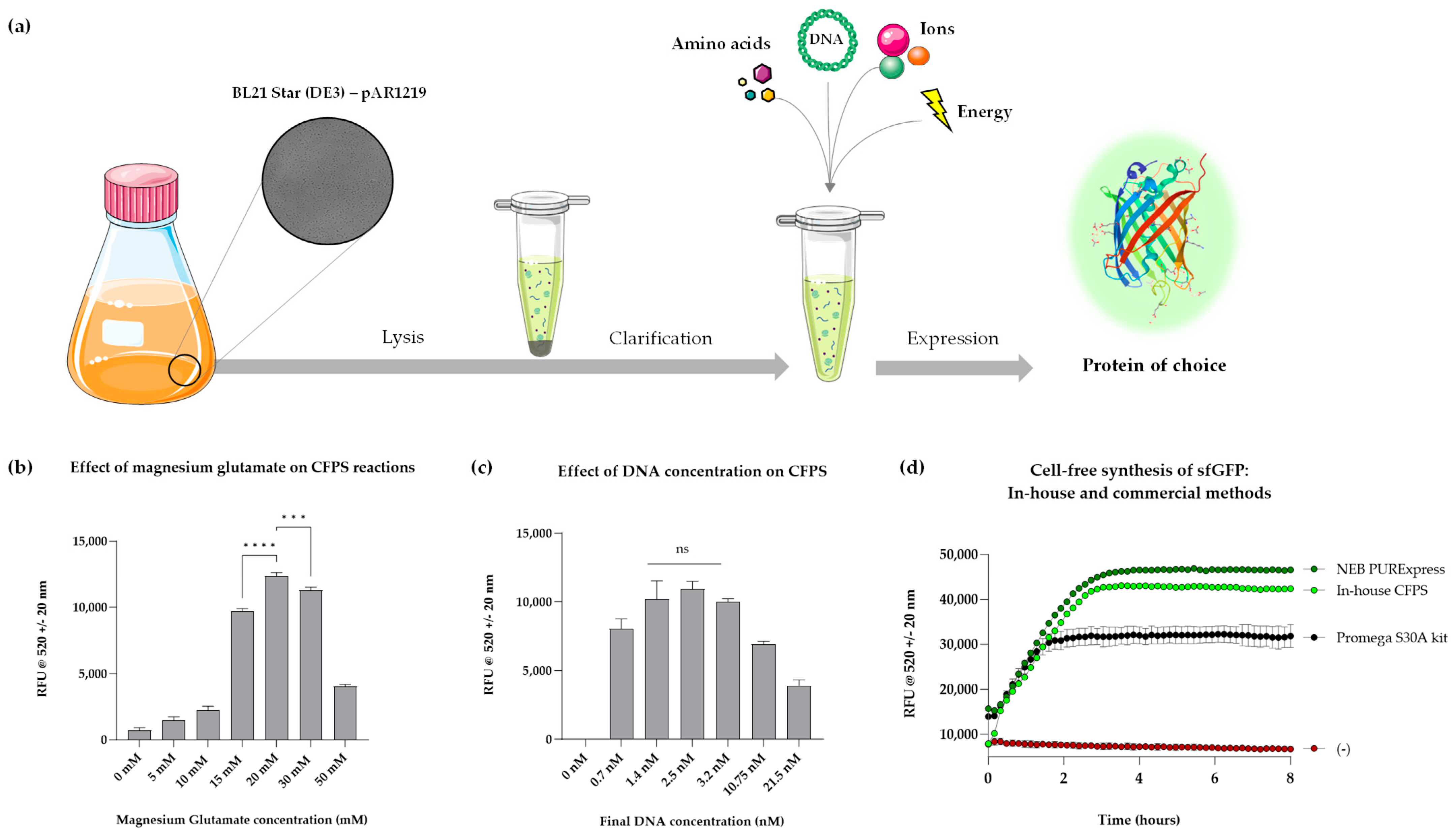
3.1. Development and Optimisation of Bacterial Cell-Free System
3.2. On-Demand Synthesis Enabled by Lyophilised and Paper-Based Formats
3.3. Lyophilised Cell-Free Systems Perform Poorly in Pharma-Grade Stability Tests
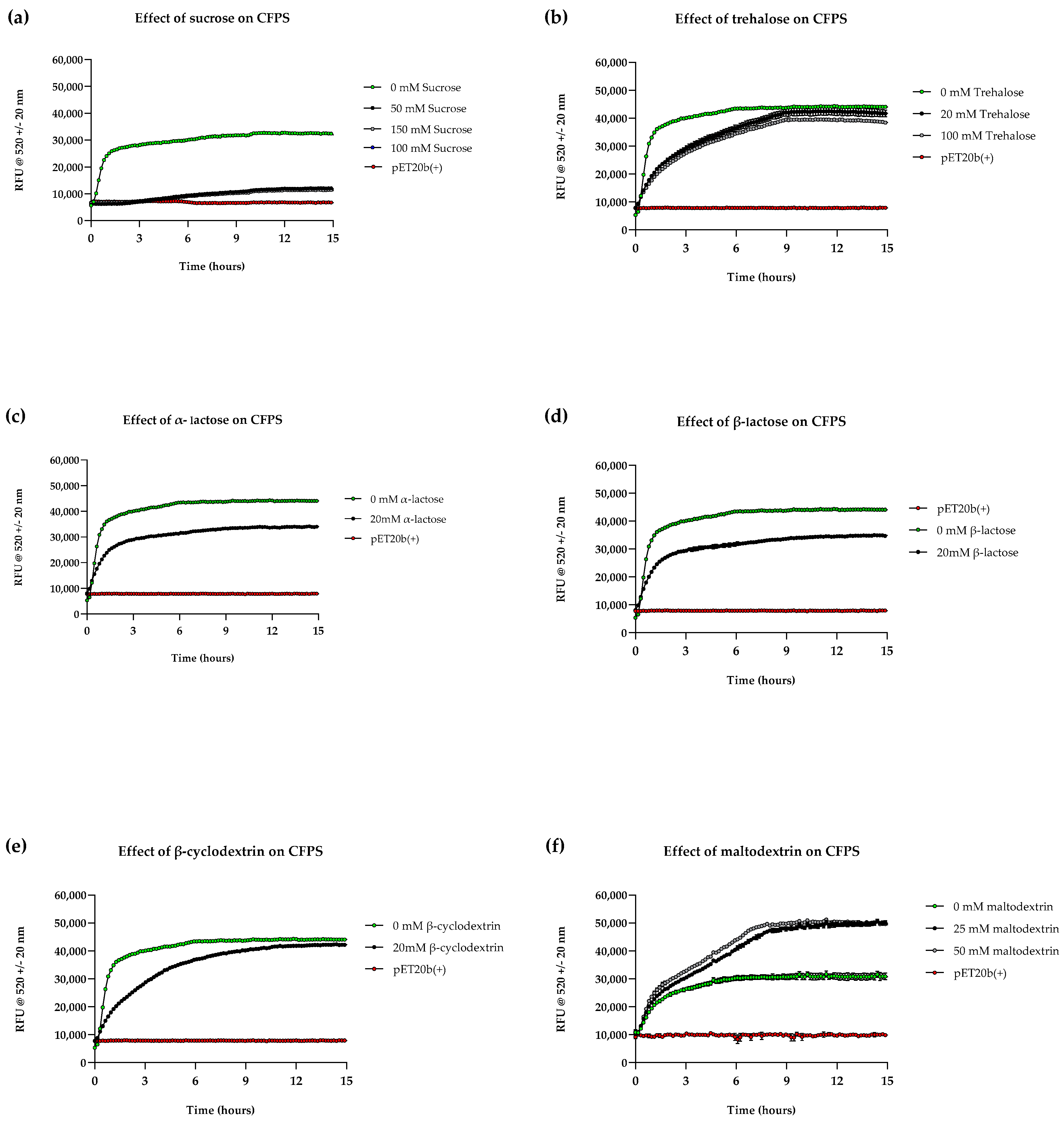
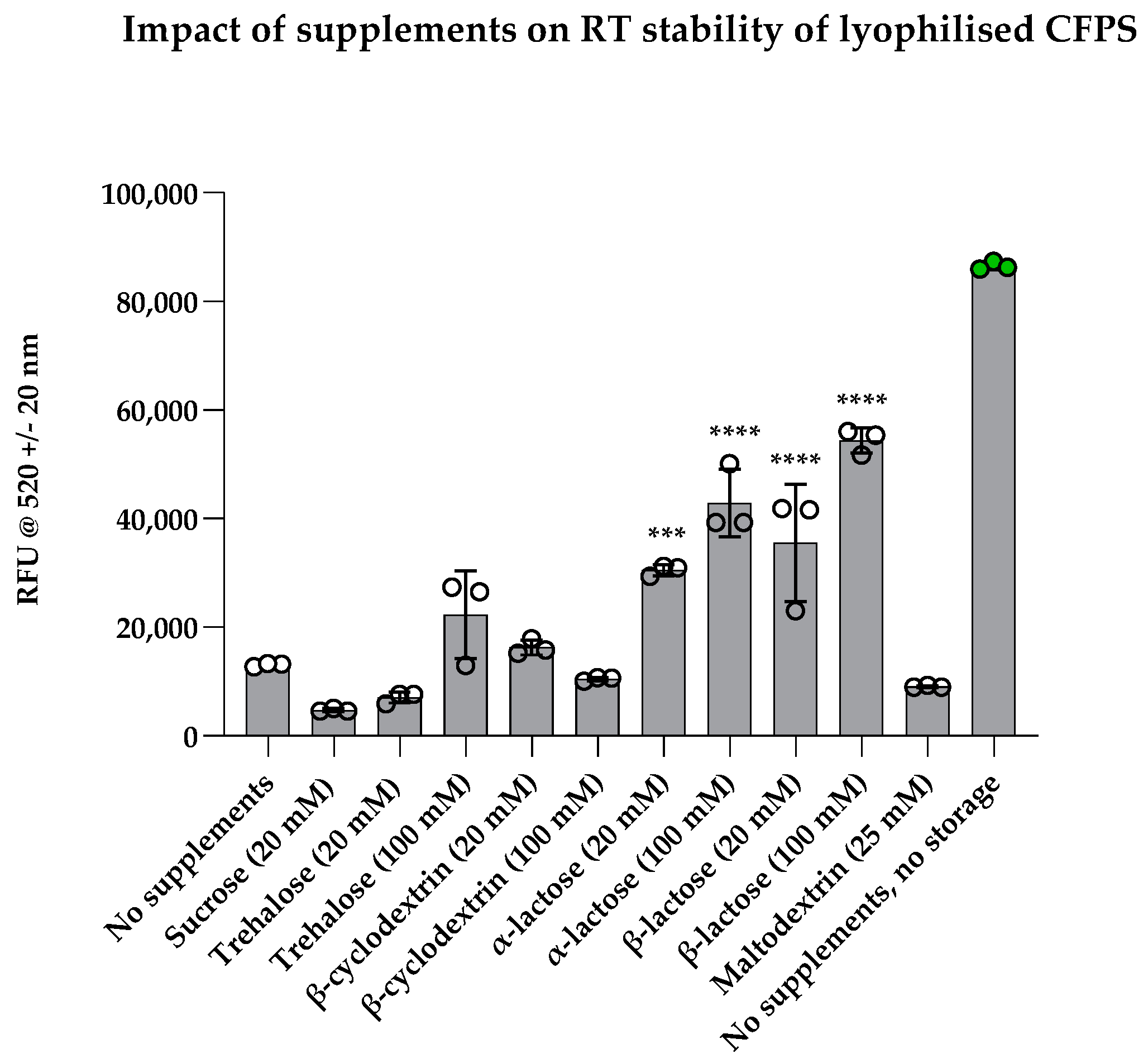
3.4. Effects of Various Lyoprotectants and Stabilisers on CFPS Kinetics and Stability at Room Temperature
3.5. DoE Strategy Enables the Identification of Combinations of Protectants That Enhance Room-Temperature Stability of Lyophilised Cell-Free System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ezure, T.; Suzuki, T.; Higashide, S.; Shintani, E.; Endo, K.; Kobayashi, S.; Shikata, M.; Ito, M.; Tanimizu, K.; Nishimura, O. Cell-free protein synthesis system prepared from insect cells by freeze-thawing. Biotechnol. Prog. 2006, 22, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Mikami, S.; Kobayashi, T.; Masutani, M.; Yokoyama, S.; Imataka, H. A human cell-derived in vitro coupled transcription/translation system optimized for production of recombinant proteins. Protein Expr. Purif. 2008, 62, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Goering, A.W.; Li, J.; McClure, R.A.; Thomson, R.J.; Jewett, M.C.; Kelleher, N.L. In Vitro Reconstruction of Nonribosomal Peptide Biosynthesis Directly from DNA Using Cell-Free Protein Synthesis. ACS Synth. Biol. 2017, 6, 39–44. [Google Scholar] [CrossRef]
- Choi, E.J.; Ling, G.S. Battlefield medicine: Paradigm shift for pharmaceuticals manufacturing. PDA J. Pharm. Sci. Technol. 2014, 68, 312. [Google Scholar] [CrossRef]
- Adiga, R.; Al-adhami, M.; Andar, A.; Borhani, S.; Brown, S.; Burgenson, D.; Cooper, M.A.; Deldari, S.; Frey, D.D.; Ge, X.; et al. Point-of-care production of therapeutic proteins of good-manufacturing-practice quality. Nat. Biomed. Eng. 2018, 2, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Stark, J.C.; Jaroentomeechai, T.; Moeller, T.D.; Hershewe, J.M.; Warfel, K.F.; Moricz, B.S.; Martini, A.M.; Dubner, R.S.; Hsu, K.J.; Stevenson, T.C.; et al. On-demand biomanufacturing of protective conjugate vaccines. Sci. Adv. 2021, 7, eabe9444. [Google Scholar] [CrossRef]
- Tang, C.; Wang, L.; Zang, L.; Wang, Q.; Qi, D.; Dai, Z. On-demand biomanufacturing through synthetic biology approach. Mater. Today Bio 2023, 18, 100518. [Google Scholar] [CrossRef] [PubMed]
- Pardee, K.; Slomovic, S.; Nguyen, P.Q.; Lee, J.W.; Donghia, N.; Burrill, D.; Ferrante, T.; McSorley, F.R.; Furuta, Y.; Vernet, A.; et al. Portable, On-Demand Biomolecular Manufacturing. Cell 2016, 167, 248–259.e212. [Google Scholar] [CrossRef] [PubMed]
- Salehi, A.S.; Smith, M.T.; Bennett, A.M.; Williams, J.B.; Pitt, W.G.; Bundy, B.C. Cell-free protein synthesis of a cytotoxic cancer therapeutic: Onconase production and a just-add-water cell-free system. Biotechnol. J. 2016, 11, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Gregorio, N.E.; Kao, W.Y.; Williams, L.C.; Hight, C.M.; Patel, P.; Watts, K.R.; Oza, J.P. Unlocking Applications of Cell-Free Biotechnology through Enhanced Shelf Life and Productivity of E. coli Extracts. ACS Synth. Biol. 2020, 9, 766–778. [Google Scholar] [CrossRef] [PubMed]
- Pardee, K.; Green, A.A.; Ferrante, T.; Cameron, D.E.; DaleyKeyser, A.; Yin, P.; Collins, J.J. Paper-based synthetic gene networks. Cell 2014, 159, 940–954. [Google Scholar] [CrossRef]
- Jovanović, N.; Bouchard, A.; Hofland, G.W.; Witkamp, G.J.; Crommelin, D.J.; Jiskoot, W. Distinct effects of sucrose and trehalose on protein stability during supercritical fluid drying and freeze-drying. Eur. J. Pharm. Sci. 2006, 27, 336–345. [Google Scholar] [CrossRef]
- Brom, J.A.; Petrikis, R.G.; Pielak, G.J. How Sugars Protect Dry Protein Structure. Biochemistry 2023, 62, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.T.; Berkheimer, S.D.; Werner, C.J.; Bundy, B.C. Lyophilized Escherichia coli-based cell-free systems for robust, high-density, long-term storage. Biotechniques 2014, 56, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Ding, X.; Lu, Y. Development of a robust Escherichia coli-based cell-free protein synthesis application platform. Biochem. Eng. J. 2021, 165, 107830. [Google Scholar] [CrossRef]
- Warfel, K.F.; Williams, A.; Wong, D.A.; Sobol, S.E.; Desai, P.; Li, J.; Chang, Y.F.; DeLisa, M.P.; Karim, A.S.; Jewett, M.C. A Low-Cost, Thermostable, Cell-Free Protein Synthesis Platform for On-Demand Production of Conjugate Vaccines. ACS Synth. Biol. 2023, 12, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Spice, A.J.; Aw, R.; Bracewell, D.G.; Polizzi, K.M. Improving the reaction mix of a Pichia pastoris cell-free system using a design of experiments approach to minimise experimental effort. Synth. Syst. Biotechnol. 2020, 5, 137–144. [Google Scholar] [CrossRef]
- Au-Levine, M.Z.; Au-Gregorio, N.E.; Au-Jewett, M.C.; Au-Watts, K.R.; Au-Oza, J.P. Escherichia coli-Based Cell-Free Protein Synthesis: Protocols for a robust, flexible, and accessible platform technology. J. Vis. Exp. 2019, 144, e58882. [Google Scholar] [CrossRef]
- Krinsky, N.; Kaduri, M.; Shainsky-Roitman, J.; Goldfeder, M.; Ivanir, E.; Benhar, I.; Shoham, Y.; Schroeder, A. A Simple and Rapid Method for Preparing a Cell-Free Bacterial Lysate for Protein Synthesis. PLoS ONE 2016, 11, e0165137. [Google Scholar] [CrossRef]
- Kim, J.; Copeland, C.E.; Padumane, S.R.; Kwon, Y.C. A Crude Extract Preparation and Optimization from a Genomically Engineered Escherichia coli for the Cell-Free Protein Synthesis System: Practical Laboratory Guideline. Methods Protoc. 2019, 2, 68. [Google Scholar] [CrossRef] [PubMed]
- Dopp, J.L.; Jo, Y.R.; Reuel, N.F. Methods to reduce variability in E. Coli-based cell-free protein expression experiments. Synth. Syst. Biotechnol. 2019, 4, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.Z.; So, B.; Mullin, A.C.; Watts, K.R.; Oza, J.P. Redesigned upstream processing enables a 24-hour workflow from E. coli cells to cell-free protein synthesis. bioRxiv 2019, 2019, 729699. [Google Scholar] [CrossRef]
- Jewett, M.C.; Miller, M.L.; Chen, Y.; Swartz, J.R. Continued protein synthesis at low [ATP] and [GTP] enables cell adaptation during energy limitation. J. Bacteriol. 2009, 191, 1083–1091. [Google Scholar] [CrossRef]
- Guo, X.; Zhu, Y.; Bai, L.; Yang, D. The Protection Role of Magnesium Ions on Coupled Transcription and Translation in Lyophilized Cell-Free System. ACS Synth. Biol. 2020, 9, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Petrov, A.S.; Bernier, C.R.; Hsiao, C.; Okafor, C.D.; Tannenbaum, E.; Stern, J.; Gaucher, E.; Schneider, D.; Hud, N.V.; Harvey, S.C.; et al. RNA-magnesium-protein interactions in large ribosomal subunit. J. Phys. Chem. B 2012, 116, 8113–8120. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Kim, D.M.; Choi, C.Y. Rapid production of milligram quantities of proteins in a batch cell-free protein synthesis system. J. Biotechnol. 2006, 124, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Silverman, A.D.; Kelley-Loughnane, N.; Lucks, J.B.; Jewett, M.C. Deconstructing Cell-Free Extract Preparation for in Vitro Activation of Transcriptional Genetic Circuitry. ACS Synth. Biol. 2019, 8, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, A.M.; Jan, E.; Sarnow, P. Initiation factor-independent translation mediated by the hepatitis C virus internal ribosome entry site. Rna 2006, 12, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, V.H.; Greene, J.M.; Sengupta, A.M.; Sontag, E.D. Translation inhibition and resource balance in the TX-TL cell-free gene expression system. Synth. Biol. 2017, 2, ysx005. [Google Scholar] [CrossRef] [PubMed]
- Zubay, G. In vitro synthesis of protein in microbial systems. Annu. Rev. Genet. 1973, 7, 267–287. [Google Scholar] [CrossRef]
- Zubay, G. The isolation and properties of CAP, the catabolite gene activator. Methods Enzymol. 1980, 65, 856–877. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Inoue, A.; Tomari, Y.; Suzuki, T.; Yokogawa, T.; Nishikawa, K.; Ueda, T. Cell-free translation reconstituted with purified components. Nat. Biotechnol. 2001, 19, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Wang, W. Lyophilization and development of solid protein pharmaceuticals. Int. J. Pharm. 2000, 203, 1–60. [Google Scholar] [CrossRef] [PubMed]
- Kasper, J.C.; Winter, G.; Friess, W. Recent advances and further challenges in lyophilization. Eur. J. Pharm. Biopharm. 2013, 85, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, L.; Ren, G.-y.; Duan, X.; Guo, J.; Liu, W.-C.; Ang, Y.; Zhu, L.; Ren, X. A comprehensive review on stability of therapeutic proteins treated by freeze-drying: Induced stresses and stabilization mechanisms involved in processing. Dry. Technol. 2022, 40, 3373–3388. [Google Scholar] [CrossRef]
- Wilding, K.M.; Hunt, J.P.; Wilkerson, J.W.; Funk, P.J.; Swensen, R.L.; Carver, W.C.; Christian, M.L.; Bundy, B.C. Endotoxin-Free E. coli-Based Cell-Free Protein Synthesis: Pre-Expression Endotoxin Removal Approaches for on-Demand Cancer Therapeutic Production. Biotechnol. J. 2019, 14, e1800271. [Google Scholar] [CrossRef] [PubMed]
- Des Soye, B.J.; Gerbasi, V.R.; Thomas, P.M.; Kelleher, N.L.; Jewett, M.C. A Highly Productive, One-Pot Cell-Free Protein Synthesis Platform Based on Genomically Recoded Escherichia coli. Cell Chem. Biol. 2019, 26, 1743–1754.e1749. [Google Scholar] [CrossRef] [PubMed]
- Merivaara, A.; Zini, J.; Koivunotko, E.; Valkonen, S.; Korhonen, O.; Fernandes, F.M.; Yliperttula, M. Preservation of biomaterials and cells by freeze-drying: Change of paradigm. J. Control. Release 2021, 336, 480–498. [Google Scholar] [CrossRef]
- Gräwe, A.; Dreyer, A.; Vornholt, T.; Barteczko, U.; Buchholz, L.; Drews, G.; Ho, U.L.; Jackowski, M.E.; Kracht, M.; Lüders, J.; et al. A paper-based, cell-free biosensor system for the detection of heavy metals and date rape drugs. PLoS ONE 2019, 14, e0210940. [Google Scholar] [CrossRef]
- Lin, X.; Li, Y.; Li, Z.; Hua, R.; Xing, Y.; Lu, Y. Portable environment-signal detection biosensors with cell-free synthetic biosystems. RSC Adv. 2020, 10, 39261–39265. [Google Scholar] [CrossRef]
- Patkar, A.Y.; Bowen, B.D.; Piret, J.M. Protein adsorption in polysulfone hollow fiber bioreactors used for serum-free mammalian cell culture. Biotechnol. Bioeng. 1993, 42, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cui, Y.; Cao, Z.; Ma, S.; Lu, Y. Strategy exploration for developing robust lyophilized cell-free systems. Biotechnol. Notes 2021, 2, 44–50. [Google Scholar] [CrossRef]
- Karig, D.K.; Bessling, S.; Thielen, P.; Zhang, S.; Wolfe, J. Preservation of protein expression systems at elevated temperatures for portable therapeutic production. J. R. Soc. Interface 2017, 14, 20161039. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Chavez, F.; Arce, A.; Adhikari, A.; Vadhin, S.; Pedroza-Garcia, J.A.; Gandini, C.; Ajioka, J.W.; Molloy, J.; Sanchez-Nieto, S.; Varner, J.D.; et al. Constructing Cell-Free Expression Systems for Low-Cost Access. ACS Synth. Biol. 2022, 11, 1114–1128. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.M.; Swartz, J.R. Prolonging cell-free protein synthesis by selective reagent additions. Biotechnol. Prog. 2000, 16, 385–390. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.H.P. Cell-free protein synthesis energized by slowly-metabolized maltodextrin. BMC Biotechnol. 2009, 9, 58. [Google Scholar] [CrossRef]
- Gamble, J.F.; Chiu, W.S.; Gray, V.; Toale, H.; Tobyn, M.; Wu, Y. Investigation into the degree of variability in the solid-state properties of common pharmaceutical excipients-anhydrous lactose. AAPS PharmSciTech 2010, 11, 1552–1557. [Google Scholar] [CrossRef]



| Component | Final Concentration | Procurement |
|---|---|---|
| E. coli cell-free extract (40% v/v) | 40% v/v | Prepared in-house |
| Energy components master mix | 160 mM HEPES, 4.8 mM ATP sodium salt, 4.8 mM ATP potassium salt, 4.15 mM GTP, 3.2 mM UTP, 3.2 mM CTP, 0.95 mM Coenzyme A, 1.3 mM NAD+, 2.5 mM cAMP, 0.27 mM Folinic acid, 3.3 mM Spermidine, 118.7 mM 3-PGA | Prepared in-house |
| Amino acid mixture | 2.5 mM each | Prepared in-house |
| Plasmid DNA | 10 nM | Prepared in-house |
| Magnesium glutamate | 20 mM | Sigma Aldrich |
| Potassium glutamate | 50 mM | Sigma Aldrich |
| RNase inhibitor | 4 units | Roche |
| Polyethylene glycol (PEG) 8000 | 2% | Sigma Aldrich |
| Nuclease-free water | to 50 μL | Thermo Scientific |
| Formulation # | MDX (mM) | β-Lactose (mM) | PEG (%) | PEG (MW) |
|---|---|---|---|---|
| Control | 0 | 0 | 0 | n/a |
| 1 | 0 | 200 | 0.5 | 6000 |
| 2 | 25 | 200 | 5 | 6000 |
| 3 | 25 | 20 | 5 | 6000 |
| 4 | 0 | 20 | 5 | 600 |
| 5 | 25 | 200 | 0.5 | 6000 |
| 6 | 0 | 20 | 0.5 | 6000 |
| 7 | 0 | 200 | 5 | 600 |
| 8 | 25 | 20 | 0.5 | 600 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shivakumar, T.; Clark, J.; Goode, A.; Anyanwu, V.E.; Williams, P.M. A Design of Experiments Approach for Enhancing Room Temperature Stability of a Lyophilised and Paper-Based Bacterial Cell-Free System. Bioengineering 2025, 12, 223. https://doi.org/10.3390/bioengineering12030223
Shivakumar T, Clark J, Goode A, Anyanwu VE, Williams PM. A Design of Experiments Approach for Enhancing Room Temperature Stability of a Lyophilised and Paper-Based Bacterial Cell-Free System. Bioengineering. 2025; 12(3):223. https://doi.org/10.3390/bioengineering12030223
Chicago/Turabian StyleShivakumar, Tejasvi, Joshua Clark, Alice Goode, Valentine E. Anyanwu, and Philip M. Williams. 2025. "A Design of Experiments Approach for Enhancing Room Temperature Stability of a Lyophilised and Paper-Based Bacterial Cell-Free System" Bioengineering 12, no. 3: 223. https://doi.org/10.3390/bioengineering12030223
APA StyleShivakumar, T., Clark, J., Goode, A., Anyanwu, V. E., & Williams, P. M. (2025). A Design of Experiments Approach for Enhancing Room Temperature Stability of a Lyophilised and Paper-Based Bacterial Cell-Free System. Bioengineering, 12(3), 223. https://doi.org/10.3390/bioengineering12030223







