The Secretome of the Inductive Tooth Germ Exhibits Signals Required for Tooth Development
Abstract
1. Introduction
2. Results
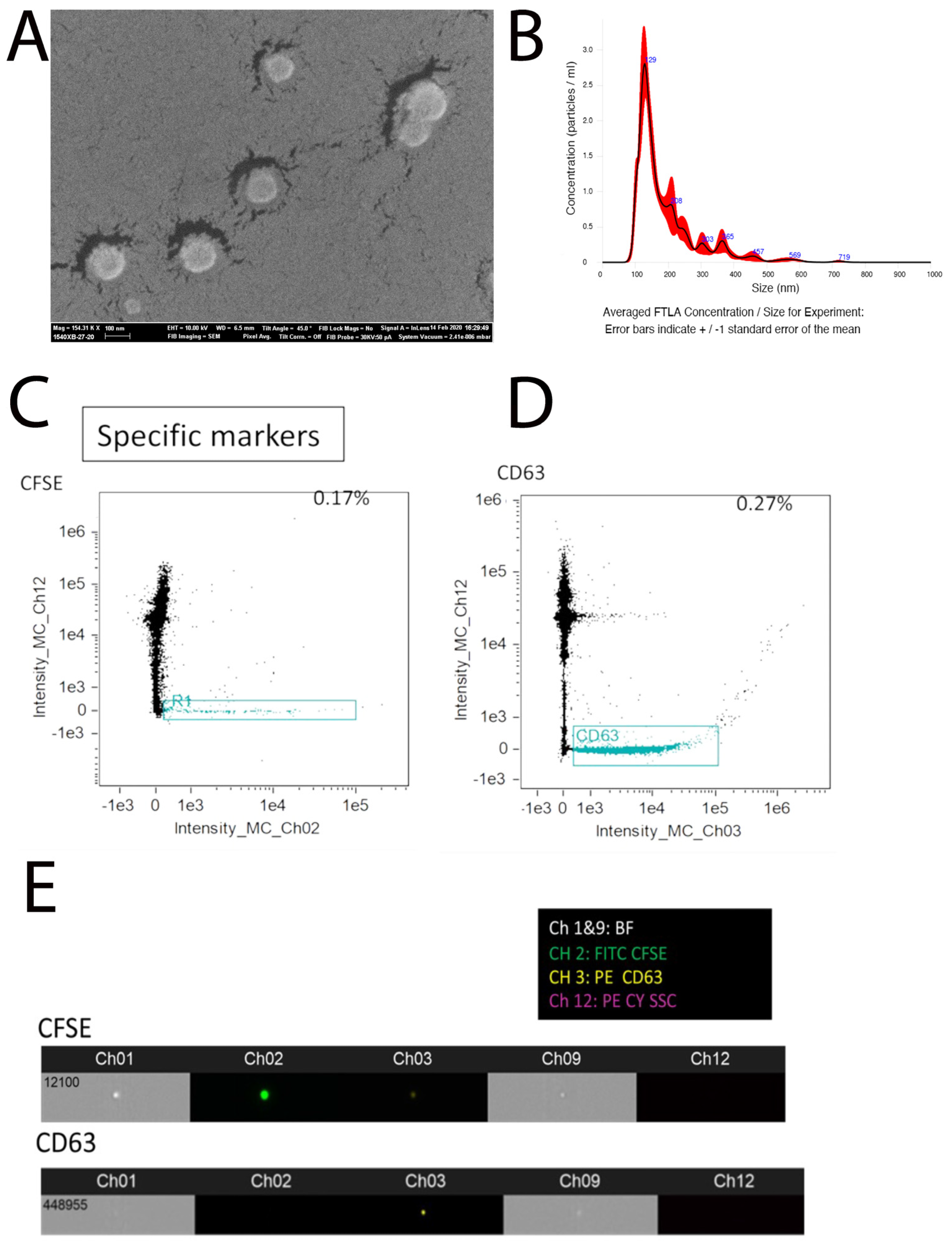
2.1. Purification of Extracellular Vesicles from Embryonic Tooth Germ
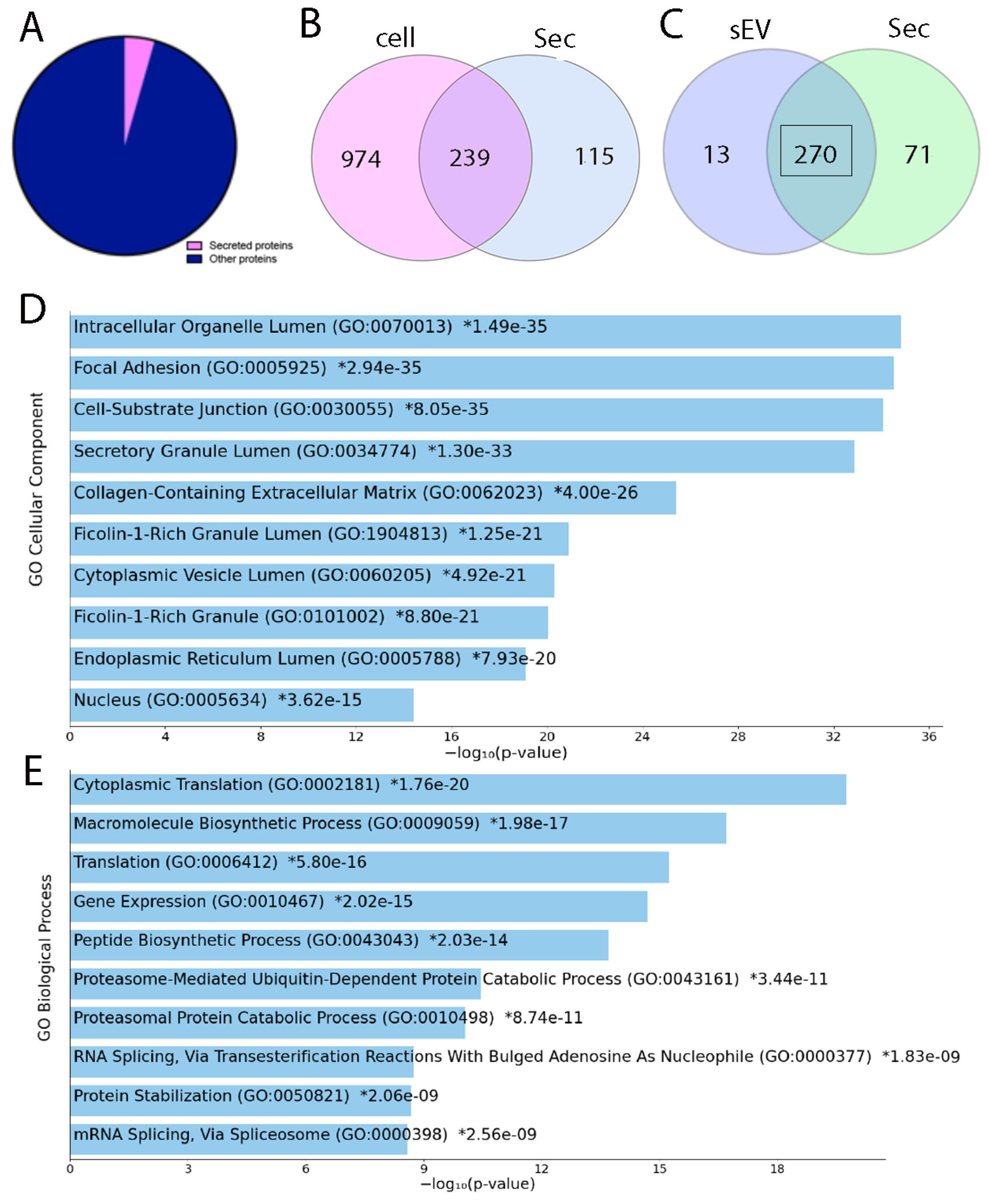
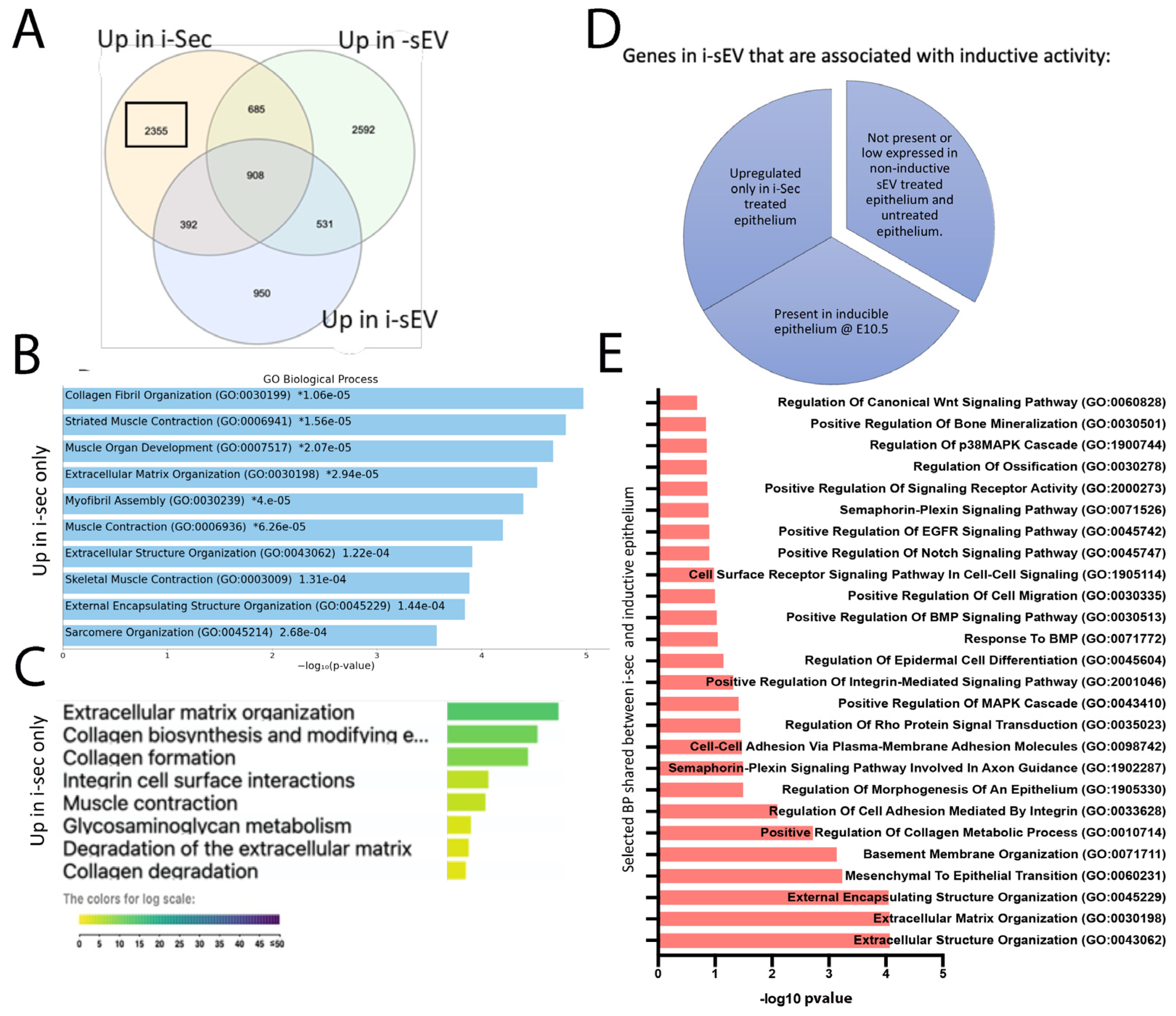
2.2. LC-MS/MS Analysis of Inductive Tooth Germ Mesenchyme Cells and Their Secretome
- E14.5 mesenchymal cells isolated from tooth germ;
- Secretome purified from E14.5 mesenchymal cells;
- s-EV purified from E14.5 mesenchymal cells.
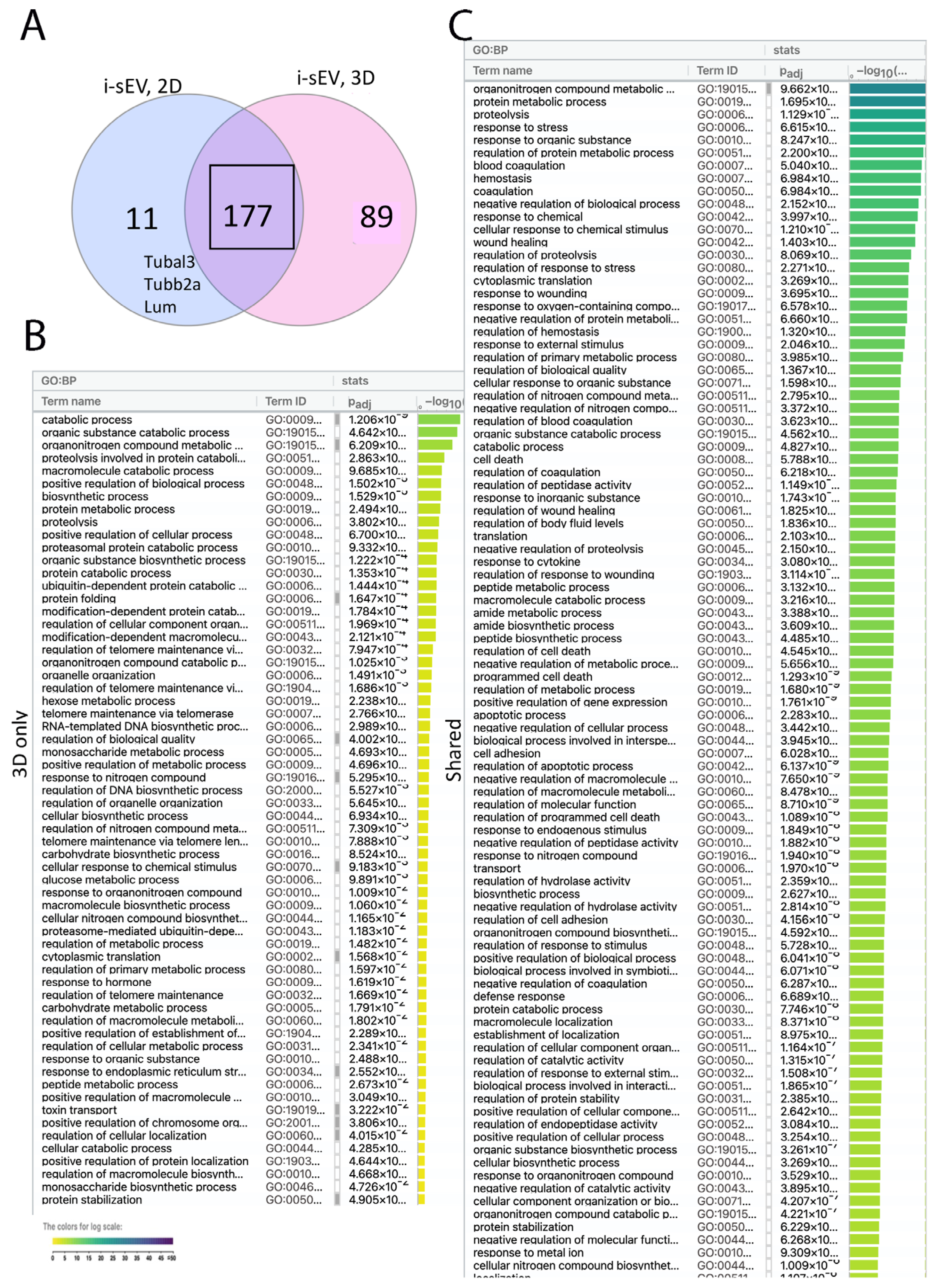
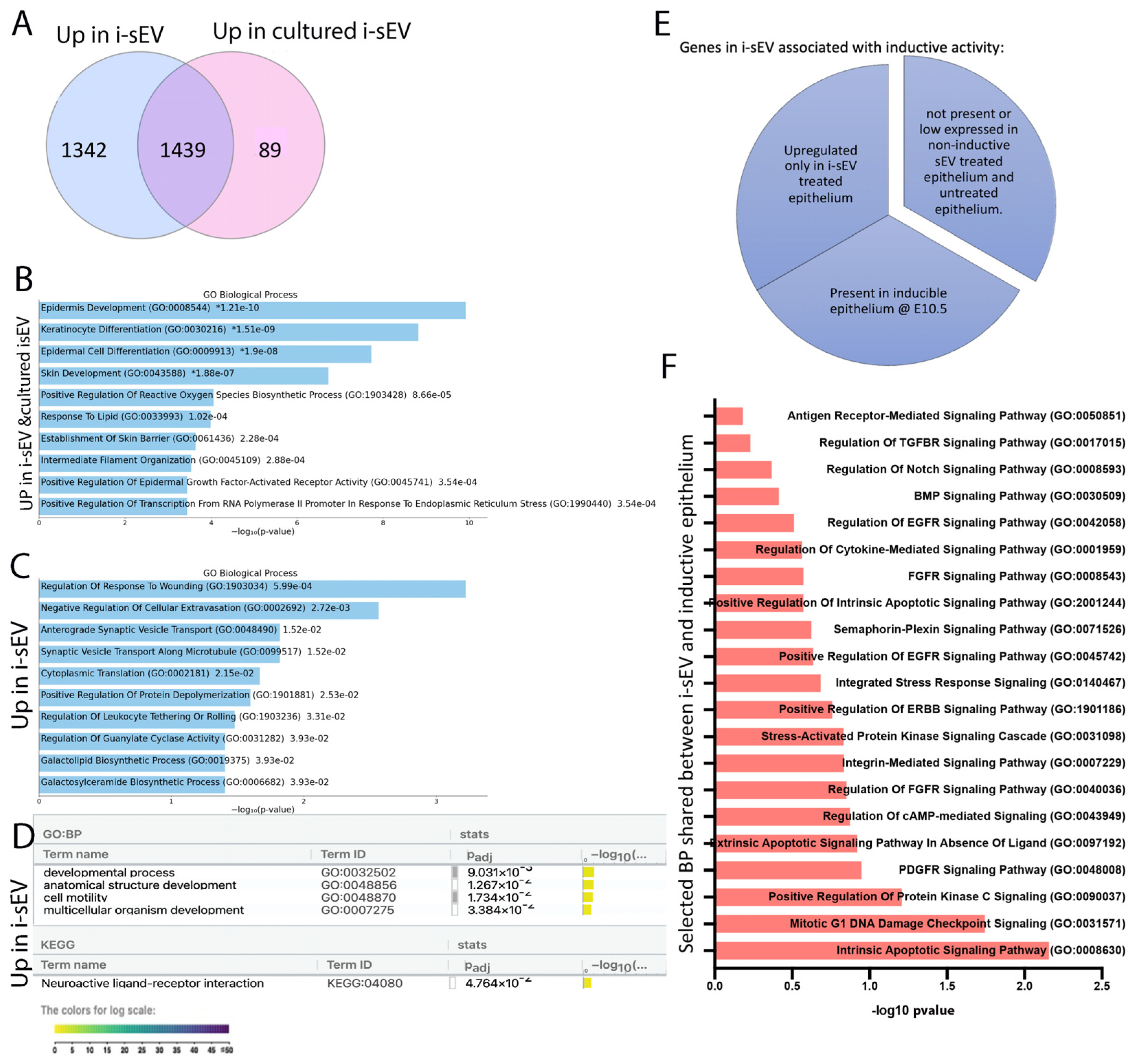
2.3. Impact of Culture Conditions on the Proteome of sEV Purified from Inductive Mesenchyme
- i-sEV derived from E14.5 tooth mesenchyme cultured for 24 h in 3D culture wells;
- i-sEV derived from E14.5 tooth mesenchyme cultured for 24 h in 2D culture wells.
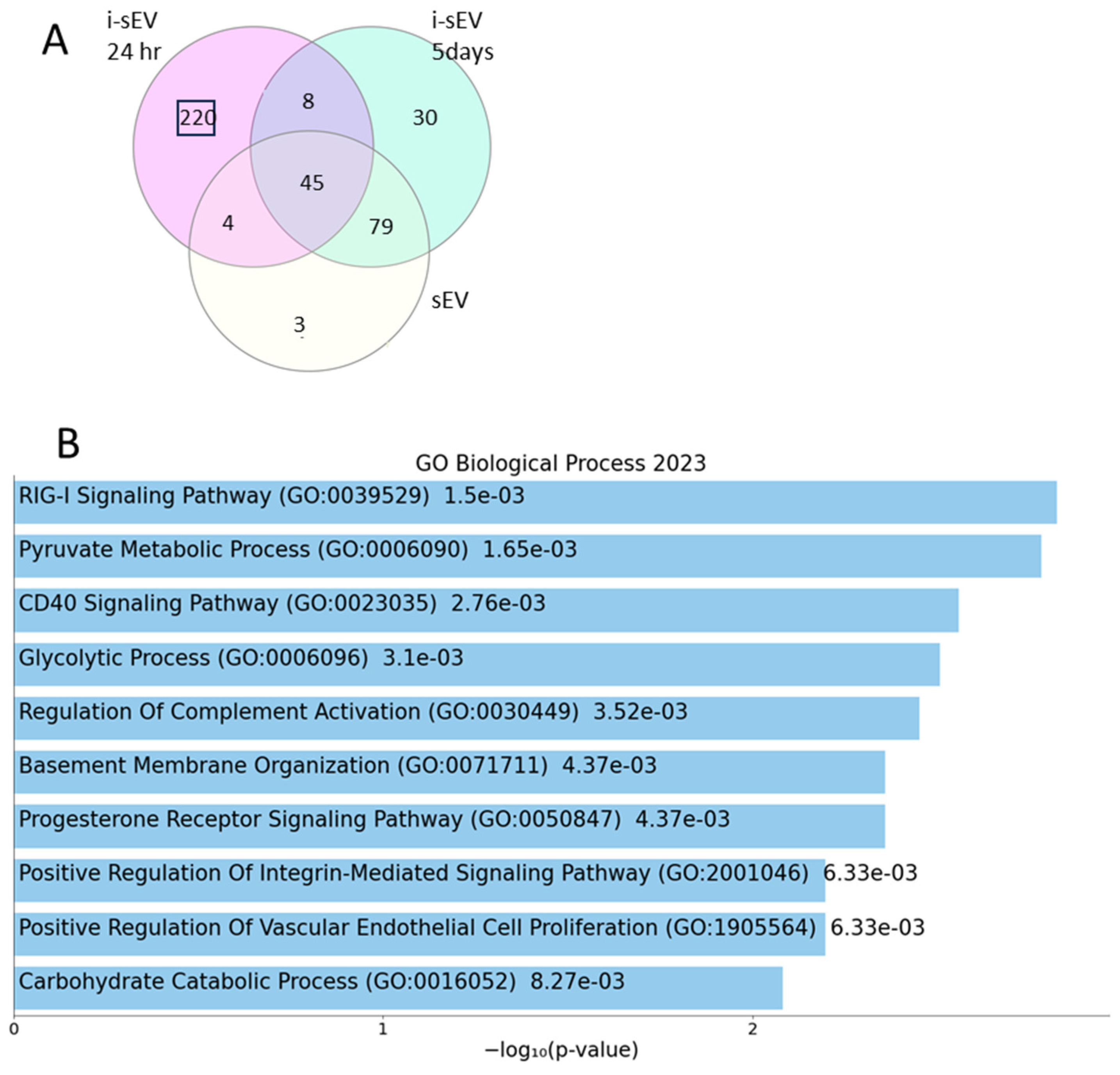
2.4. Proteome Unique to Inductive sEV Shows Association with Multiple Signaling Pathway Related to Tooth Development
- sEV derived from E14.5 tooth mesenchyme cultured for 24 h in 3D culture wells (i-sEV 24 h);
- sEV derived from E14.5 tooth mesenchyme cultured for 5 days in 3D culture wells (i-sEV 5 days or cultured i-sEV);
- sEV derived from mesenchyme of E10.5 branchial arch cultured for 24 h as non-inductive control (sEV).
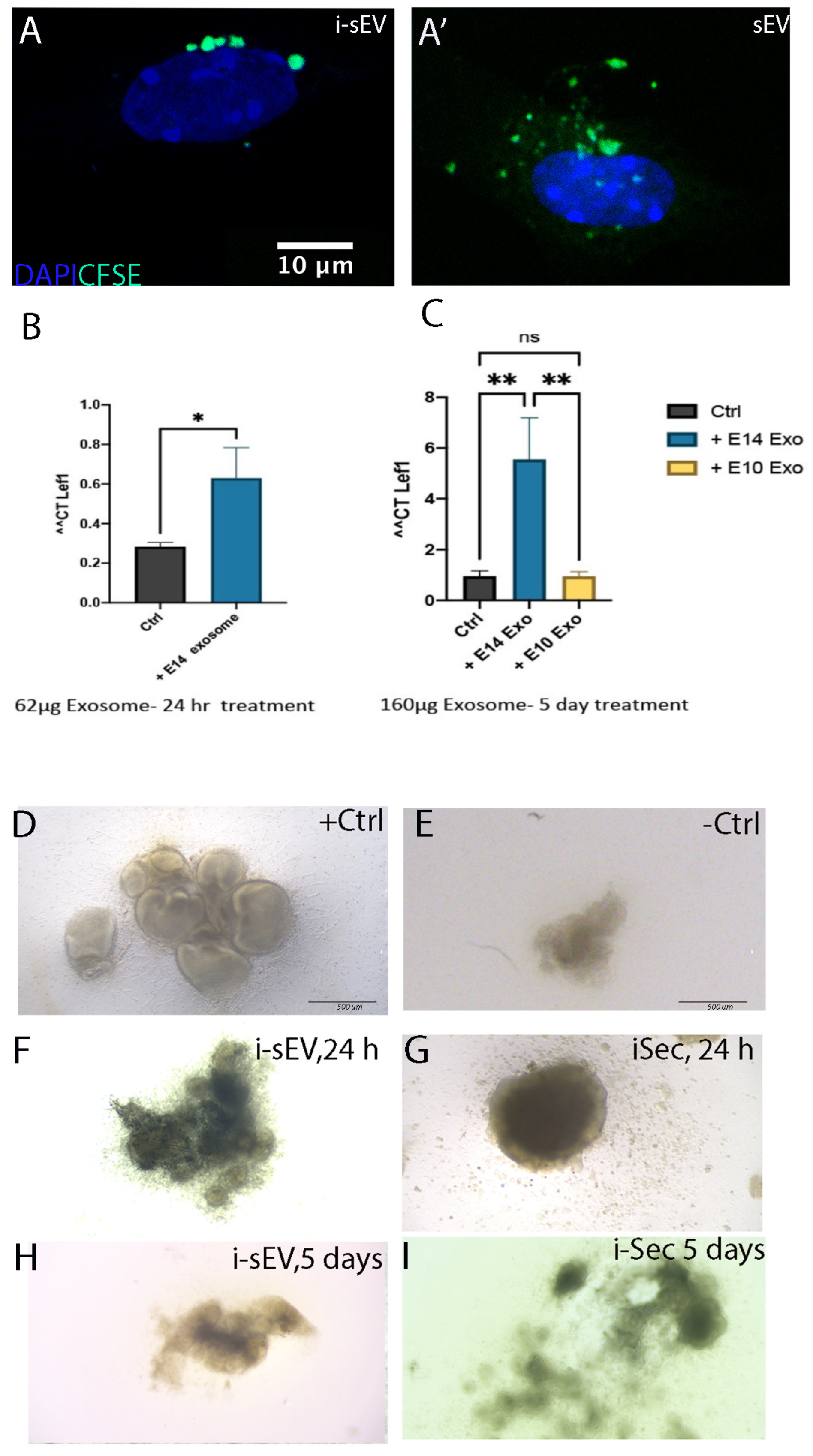
2.5. Treatment of Tooth Germ Epithelium with sEV from Inductive Mesenchyme
- E14.5 tooth germ epithelium treated with i-sEV (derived from 24 h cultured E14.5 tooth germ mesenchyme),
- E14.5 tooth germ epithelium treated with cultured i-sEV (derived from 5-day-cultured E14.5 tooth germ mesenchyme),
- untreated E14.5 tooth germ epithelium as our negative control,
- inductive epithelium from E10.5 branchial arch as our positive control.
2.6. Treatment of Tooth Germ Epithelium with Secretome from Inductive Mesenchyme
2.7. Generation of Bioengineered Teeth Using Secretome of Inductive Tooth Germ Mesenchyme
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Purification and Characterization of Small Extracellular Vesicles
- sEV derived from E14.5 tooth germ mesenchyme cultured for 24 h in 3D culture wells;
- sEV derived from E14.5 tooth germ mesenchyme cultured for 24 h in 2D culture wells;
- sEV derived from E14.5 tooth germ mesenchyme cultured for 5 days in 2D culture wells.
4.3. Quantitative PCR
4.4. Proteomics
4.5. RNA Sequencing
4.6. Generation of Tooth Ex Vivo
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thesleff, I.; Keranen, S.; Jernvall, J. Enamel Knots as Signaling Centers Linking Tooth Morphogenesis and Odontoblast Differentiation. Adv. Dent. Res. 2001, 15, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.D.; Chen, Z.; Song, Y.Q.; Liu, C.; Chen, Y.P. Making a tooth: Growth factors, transcription factors, and stem cells. Cell Res. 2005, 15, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Vaahtokari, A.; Åberg, T.; Jernvall, J.; Keränen, S.; Thesleff, I. The enamel knot as a signaling center in the developing mouse tooth. Mech. Dev. 1996, 54, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, M.; Tian, W. An inductive signalling network regulates mammalian tooth morphogenesis with implications for tooth regeneration. Cell Prolif. 2013, 46, 501–508. [Google Scholar] [CrossRef]
- Hallikas, O.; Das Roy, R.; Christensen, M.M.; Renvoisé, E.; Sulic, A.; Jernvall, J. System-level analyses of keystone genes required for mammalian tooth development. J. Exp. Zool. B Mol. Dev. Evol. 2021, 336, 7–17. [Google Scholar] [CrossRef]
- Chen, Y.; Bei, M.; Woo, I.; Satokata, I.; Maas, R. Msx1 controls inductive signaling in mammalian tooth morphogenesis. Development 1996, 122, 3035–3044. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, Y.; Lan, Y.; Jia, S.; Jiang, R. Pax9 regulates a molecular network involving Bmp4, Fgf10, Shh signaling and the Osr2 transcription factor to control palate morphogenesis. Development 2013, 140, 4709–4718. [Google Scholar] [CrossRef]
- Vainio, S. Identification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell 1993, 75, 45–58. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Zhao, X.; Yu, X.; Hu, Y.; Geronimo, B.; Fromm, S.H.; Chen, Y. A new function of BMP4: Dual role for BMP4 in regulation of Sonic hedgehog expression in the mouse tooth germ. Development 2000, 127, 1431–1443. [Google Scholar] [CrossRef]
- Jia, S.; Kwon, H.-J.E.; Lan, Y.; Zhou, J.; Liu, H.; Jiang, R. Bmp4-Msx1 signaling and Osr2 control tooth organogenesis through antagonistic regulation of secreted Wnt antagonists. Dev. Biol. 2016, 420, 110–119. [Google Scholar] [CrossRef]
- Zhang, Z.; Lan, Y.; Chai, Y.; Jiang, R. Antagonistic Actions of Msx1 and Osr2 Pattern Mammalian Teeth into a Single Row. Science 2009, 323, 1232–1234. [Google Scholar] [CrossRef] [PubMed]
- Ohazama, A.; Modino, S.; Miletich, I.; Sharpe, P.T. Stem-cell-based tissue engineering of murine teeth. J. Dent. Res. 2004, 83, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Volponi, A.A.; Kawasaki, M.; Sharpe, P.T. Adult Human Gingival Epithelial Cells as a Source for Whole-tooth Bioengineering. J. Dent. Res. 2013, 92, 329–334. [Google Scholar] [CrossRef]
- Mammoto, T.; Mammoto, A.; Torisawa, Y.-S.; Tat, T.; Gibbs, A.; Derda, R.; Mannix, R.; de Bruijn, M.; Yung, C.W.; Huh, D.; et al. Mechanochemical Control of Mesenchymal Condensation and Embryonic Tooth Organ Formation. Dev. Cell 2011, 21, 758–769. [Google Scholar] [CrossRef]
- Balic, A. Concise Review: Cellular and Molecular Mechanisms Regulation of Tooth Initiation. Stem Cells 2019, 37, 26–32. [Google Scholar] [CrossRef]
- Yang, L.; Volponi, A.A.; Pang, Y.; Sharpe, P.T. Mesenchymal Cell Community Effect in Whole Tooth Bioengineering. J. Dent. Res. 2017, 96, 186–191. [Google Scholar] [CrossRef]
- Rosen, J.M.; Roarty, K. Paracrine signaling in mammary gland development: What can we learn about intratumoral heterogeneity? Breast Cancer Res. 2014, 16, 202. [Google Scholar] [CrossRef]
- Itoh, N.; Ohta, H. Fgf10: A Paracrine-Signaling Molecule in Development, Disease, and Regenerative Medicine. Curr. Mol. Med. 2014, 14, 504–509. [Google Scholar] [CrossRef]
- Hartig, S.M.; Cox, A.R. Paracrine signaling in islet function and survival. J. Mol. Med. 2020, 98, 451–467. [Google Scholar] [CrossRef]
- Kim, J.W.; Luo, J.Z.Q.; Luo, L. Bone Marrow Mesenchymal Stem Cells as a New Therapeutic Approach for Diabetes Mellitus. In A Roadmap to Non-Hematopoietic Stem Cell-Based Therapeutics; Elsevier: Amsterdam, The Netherlands, 2019; pp. 251–273. [Google Scholar] [CrossRef]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 2015, 65, 783–797. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Liu, M.; Tan, T.; Yang, Q.; Wang, Y.; Men, L.; Zhao, L.; Zhang, H.; Wang, S.; Xie, T.; et al. Emerging Significance and Therapeutic Potential of Extracellular vesicles. Int. J. Biol. Sci. 2021, 17, 2476–2486. [Google Scholar] [CrossRef] [PubMed]
- Piazza, N.; Dehghani, M.; Gaborski, T.R.; Wuertz-Kozak, K. Therapeutic Potential of Extracellular Vesicles in Degenerative Diseases of the Intervertebral Disc. Front. Bioeng. Biotechnol. 2020, 8, 311. [Google Scholar] [CrossRef] [PubMed]
- Kodam, S.P.; Ullah, M. Diagnostic and Therapeutic Potential of Extracellular Vesicles. Technol. Cancer Res. Treat. 2021, 20, 153303382110412. [Google Scholar] [CrossRef] [PubMed]
- Bicer, M.; Cottrell, G.S.; Widera, D. Impact of 3D cell culture on bone regeneration potential of mesenchymal stromal cells. Stem Cell Res. Ther. 2021, 12, 31. [Google Scholar] [CrossRef]
- Du, J.; Zu, Y.; Li, J.; Du, S.; Xu, Y.; Zhang, L.; Jiang, L.; Wang, Z.; Chien, S.; Yang, C. Extracellular Matrix Stiffness Dictates Wnt Expression Through Integrin Pathway. Sci. Rep. 2016, 6, 20395. [Google Scholar] [CrossRef]
- Tejeda-Muñoz, N.; Morselli, M.; Moriyama, Y.; Sheladiya, P.; Pellegrini, M.; De Robertis, E.M. Canonical Wnt signaling induces focal adhesion and Integrin beta-1 endocytosis. IScience 2022, 25, 104123. [Google Scholar] [CrossRef]
- Hall, B.K.; Miyake, T. All for one and one for all: Condensations and the initiation of skeletal development. BioEssays 2000, 22, 138–147. [Google Scholar] [CrossRef]
- Clark, A.T.; Bertram, J.F. Advances in renal development. Curr. Opin. Nephrol. Hypertens. 2000, 9, 247–251. [Google Scholar] [CrossRef]
- DeLise, A.M.; Fischer, L.; Tuan, R.S. Cellular interactions and signaling in cartilage development. Osteoarthr. Cartil. 2000, 8, 309–334. [Google Scholar] [CrossRef]
- Rosowski, J.; Bräunig, J.; Amler, A.-K.; Strietzel, F.P.; Lauster, R.; Rosowski, M. Emulating the early phases of human tooth development in vitro. Sci. Rep. 2019, 9, 7057. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.K.; Miyake, T. Divide, accumulate, differentiate: Cell condensation in skeletal development revisited. Int. J. Dev. Biol. 1995, 39, 881–893. [Google Scholar] [PubMed]
- Kalra, H.; Simpson, R.J.; Ji, H.; Aikawa, E.; Altevogt, P.; Askenase, P.; Bond, V.C.; Borràs, F.E.; Breakefield, X.; Budnik, V.; et al. Vesiclepedia: A Compendium for Extracellular Vesicles with Continuous Community Annotation. PLoS Biol. 2012, 10, e1001450. [Google Scholar] [CrossRef]
- Poornima, K.; Francis, A.P.; Hoda, M.; Eladl, M.A.; Subramanian, S.; Veeraraghavan, V.P.; El-Sherbiny, M.; Asseri, S.M.; Hussamuldin, A.B.A.; Surapaneni, K.M.; et al. Implications of Three-Dimensional Cell Culture in Cancer Therapeutic Research. Front. Oncol. 2022, 12, 891673. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef]
- Afewerki, S.; Sheikhi, A.; Kannan, S.; Ahadian, S.; Khademhosseini, A. Gelatin-polysaccharide composite scaffolds for 3D cell culture and tissue engineering: Towards natural therapeutics. Bioeng. Transl. Med. 2019, 4, 96–115. [Google Scholar] [CrossRef]
- Zheng, Y.; Cai, J.; Hutchins, A.P.; Jia, L.; Liu, P.; Yang, D.; Chen, S.; Ge, L.; Pei, D.; Wei, S. Remission for Loss of Odontogenic Potential in a New Micromilieu In Vitro. PLoS ONE 2016, 11, e0152893. [Google Scholar] [CrossRef]
- Matsumiya, T.; Stafforini, D.M. Function and Regulation of Retinoic Acid-Inducible Gene-I. Crit. Rev. Immunol. 2010, 30, 489–513. [Google Scholar] [CrossRef]
- Seritrakul, P.; Samarut, E.; Lama, T.T.S.; Gibert, Y.; Laudet, V.; Jackman, W.R. Retinoic acid expands the evolutionarily reduced dentition of zebrafish. FASEB J. 2012, 26, 5014–5024. [Google Scholar] [CrossRef]
- Morkmued, S.; Laugel-Haushalter, V.; Mathieu, E.; Schuhbaur, B.; Hemmerlé, J.; Dollé, P.; Bloch-Zupan, A.; Niederreither, K. Retinoic Acid Excess Impairs Amelogenesis Inducing Enamel Defects. Front. Physiol. 2017, 7, 673. [Google Scholar] [CrossRef]
- Dolan, M.-J.; Therrien, M.; Jereb, S.; Kamath, T.; Gazestani, V.; Atkeson, T.; Marsh, S.E.; Goeva, A.; Lojek, N.M.; Murphy, S.; et al. Exposure of iPSC-derived human microglia to brain substrates enables the generation and manipulation of diverse transcriptional states in vitro. Nat. Immunol. 2023, 24, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Fukumoto, E.; Yamada, A.; Yuasa, K.; Yoshizaki, K.; Iwamoto, T.; Saito, M.; Nakamura, T.; Fukumoto, S. Interaction between Fibronectin and β1 Integrin Is Essential for Tooth Development. PLoS ONE 2015, 10, e0121667. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, S.; Miner, J.H.; Ida, H.; Fukumoto, E.; Yuasa, K.; Miyazaki, H.; Hoffman, M.P.; Yamada, Y. Laminin α5 Is Required for Dental Epithelium Growth and Polarity and the Development of Tooth Bud and Shape. J. Biol. Chem. 2006, 281, 5008–5016. [Google Scholar] [CrossRef] [PubMed]
- Salmivirta, K.; Gullberg, D.; Hirsch, E.; Altruda, F.; Ekblom, P. Integrin subunit expression associated with epithelial-mesenchymal interactions during murine tooth development. Dev. Dyn. 1996, 205, 104–113. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, M.; Yang, Z.; Qin, W.; Guo, S.; Ma, J.; Chen, W. GATA Binding Protein 4 Regulates Tooth Root Dentin Development via FBP1. Int. J. Biol. Sci. 2020, 16, 181–193. [Google Scholar] [CrossRef]
- Nijakowski, K.; Ortarzewska, M.; Jankowski, J.; Lehmann, A.; Surdacka, A. The Role of Cellular Metabolism in Maintaining the Function of the Dentine-Pulp Complex: A Narrative Review. Metabolites 2023, 13, 520. [Google Scholar] [CrossRef]
- Ida-Yonemochi, H.; Nakatomi, M.; Harada, H.; Takata, H.; Baba, O.; Ohshima, H. Glucose uptake mediated by glucose transporter 1 is essential for early tooth morphogenesis and size determination of murine molars. Dev. Biol. 2012, 363, 52–61. [Google Scholar] [CrossRef]
- Xu, X.; Bringas, P.; Soriano, P.; Chai, Y. PDGFR-? signaling is critical for tooth cusp and palate morphogenesis. Dev. Dyn. 2005, 232, 75–84. [Google Scholar] [CrossRef]
- Chai, Y.; Bringas, P.; Mogharei, A.; Shuler, C.F.; Slavkin, H.C. PDGF-A and PDGFR-α regulate tooth formation via autocrine mechanism during mandibular morphogenesis in vitro. Dev. Dyn. 1998, 213, 500–511. [Google Scholar] [CrossRef]
- Pieles, O.; Reichert, T.E.; Morsczeck, C. Classical isoforms of protein kinase C (PKC) and Akt regulate the osteogenic differentiation of human dental follicle cells via both β-catenin and NF-κB. Stem Cell Res. Ther. 2021, 12, 242. [Google Scholar] [CrossRef]
- Novak, A.; Hsu, S.-C.; Leung-Hagesteijn, C.; Radeva, G.; Papkoff, J.; Montesano, R.; Roskelley, C.; Grosschedl, R.; Dedhar, S. Cell adhesion and the integrin-linked kinase regulate the LEF-1 and β-catenin signaling pathways. Proc. Natl. Acad. Sci. USA 1998, 95, 4374–4379. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, J.; Jiang, H.; Hu, Q.; Chen, J.; Zhang, J.; Zhu, R.; Liu, W.; Li, B. Wnt3a activates β1-integrin and regulates migration and adhesion of vascular smooth muscle cells. Mol. Med. Rep. 2014, 9, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Millar, S.E. Wnt/beta-catenin signaling in oral tissue development and disease. J. Dent. Res. 2010, 89, 318–330. [Google Scholar] [CrossRef]
- Catón, J.; Tucker, A.S. Current knowledge of tooth development: Patterning and mineralization of the murine dentition. J. Anat. 2009, 214, 502–515. [Google Scholar] [CrossRef]
- Tucker, A.S.; Matthews, K.L.; Sharpe, P.T. Transformation of Tooth Type Induced by Inhibition of BMP Signaling. Science 1998, 282, 1136–1138. [Google Scholar] [CrossRef]
- Rothova, M.; Thompson, H.; Lickert, H.; Tucker, A.S. Lineage tracing of the endoderm during oral development. Dev. Dyn. 2012, 241, 1183–1191. [Google Scholar] [CrossRef]
- Jing, J.; Feng, J.; Yuan, Y.; Guo, T.; Lei, J.; Pei, F.; Ho, T.-V.; Chai, Y. Spatiotemporal single-cell regulatory atlas reveals neural crest lineage diversification and cellular function during tooth morphogenesis. Nat. Commun. 2022, 13, 4803. [Google Scholar] [CrossRef]
- Driscoll, J.; Patel, T. The mesenchymal stem cell secretome as an acellular regenerative therapy for liver disease. J. Gastroenterol. 2019, 54, 763–773. [Google Scholar] [CrossRef]
- D’arrigo, D.; Roffi, A.; Cucchiarini, M.; Moretti, M.; Candrian, C.; Filardo, G. Secretome and Extracellular Vesicles as New Biological Therapies for Knee Osteoarthritis: A Systematic Review. J. Clin. Med. 2019, 8, 1867. [Google Scholar] [CrossRef]
- Műzes, G.; Sipos, F. Mesenchymal Stem Cell-Derived Secretome: A Potential Therapeutic Option for Autoimmune and Immune-Mediated Inflammatory Diseases. Cells 2022, 11, 2300. [Google Scholar] [CrossRef]
- Brennan, K.; Martin, K.; Fitzgerald, S.P.; O’Sullivan, J.; Wu, Y.; Blanco, A.; Richardson, C.; Mc Gee, M.M. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci. Rep. 2020, 10, 1039. [Google Scholar] [CrossRef] [PubMed]
- Małys, M.S.; Aigner, C.; Schulz, S.M.; Schachner, H.; Rees, A.J.; Kain, R. Isolation of Small Extracellular Vesicles from Human Sera. Int. J. Mol. Sci. 2021, 22, 4653. [Google Scholar] [CrossRef] [PubMed]
- Welton, J.L.; Webber, J.P.; Botos, L.; Jones, M.; Clayton, A. Ready-made chromatography columns for extracellular vesicle isolation from plasma. J. Extracell. Vesicles 2015, 4, 27269. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Wang, J.T.-W.; Yavuz, E.; Zam, A.; Rouatbi, N.; Utami, R.N.; Liam-Or, R.; Griffiths, A.; Dickson, W.; Sosabowski, J.; et al. Spatiotemporal tracking of gold nanorods after intranasal administration for brain targeting. J. Control Release 2023, 357, 606–619. [Google Scholar] [CrossRef]
- Knight, H.; Abis, G.; Kaur, M.; Green, H.L.; Krasemann, S.; Hartmann, K.; Lynham, S.; Clark, J.; Zhao, L.; Ruppert, C.; et al. Cyclin D-CDK4 Disulfide Bond Attenuates Pulmonary Vascular Cell Proliferation. Circ. Res. 2023, 133, 966–988. [Google Scholar] [CrossRef]
- Zhao, Y.; Ehteramyan, M.; Li, Y.; Bai, X.; Huang, L.; Gao, Y.; Angbohang, A.; Yang, X.; Lynham, S.; Margariti, A.; et al. A novel nested gene Aff3ir participates in vascular remodeling by enhancing endothelial cell differentiation in mice. Genes. Dis. 2024, 12, 101339. [Google Scholar] [CrossRef]
- Eng, J.K.; McCormack, A.L.; Yates, J.R. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass. Spectrom. 1994, 5, 976–989. [Google Scholar] [CrossRef]
- Paizal, J.P.; Au, S.H.; Bakal, C. Nuclear rupture induced by capillary constriction forces promotes differential effects on metastatic and normal breast cells. Sci. Rep. 2024, 14, 14793. [Google Scholar] [CrossRef]
- Huang, H.; Ren, Z.; Gao, X.; Hu, X.; Zhou, Y.; Jiang, J.; Lu, H.; Yin, S.; Ji, J.; Zhou, L.; et al. Integrated analysis of microbiome and host transcriptome reveals correlations between gut microbiota and clinical outcomes in HBV-related hepatocellular carcinoma. Genome Med. 2020, 12, 102. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birjandi, A.A.; Sharpe, P. The Secretome of the Inductive Tooth Germ Exhibits Signals Required for Tooth Development. Bioengineering 2025, 12, 96. https://doi.org/10.3390/bioengineering12020096
Birjandi AA, Sharpe P. The Secretome of the Inductive Tooth Germ Exhibits Signals Required for Tooth Development. Bioengineering. 2025; 12(2):96. https://doi.org/10.3390/bioengineering12020096
Chicago/Turabian StyleBirjandi, Anahid A, and Paul Sharpe. 2025. "The Secretome of the Inductive Tooth Germ Exhibits Signals Required for Tooth Development" Bioengineering 12, no. 2: 96. https://doi.org/10.3390/bioengineering12020096
APA StyleBirjandi, A. A., & Sharpe, P. (2025). The Secretome of the Inductive Tooth Germ Exhibits Signals Required for Tooth Development. Bioengineering, 12(2), 96. https://doi.org/10.3390/bioengineering12020096





