Neuro-Nutrition and Exercise Synergy: Exploring the Bioengineering of Cognitive Enhancement and Mental Health Optimization
Abstract
1. Introduction
2. Neuro-Nutrition: Key Nutrients for Brain Health
2.1. The Role of Omega-3 Fatty Acids in Brain Health
2.2. B Vitamins and Their Critical Role in Brain Health
2.3. Antioxidants and Neuroprotection: The Role of Vitamins C and E
2.4. Polyphenols and Cognitive Function
2.5. Amino Acids: Building Blocks for Neurotransmitter Synthesis
3. The Role of Exercise in Cognitive Enhancement
3.1. Exercise and Brain Plasticity
3.2. Neurogenesis and Brain Health
3.3. Neuroinflammation and Exercise
3.4. Exercise and Cognitive Function in Aging
3.5. Mental Health and Cognitive Function
4. Mechanisms of Neuroplasticity: Nutrition and Exercise
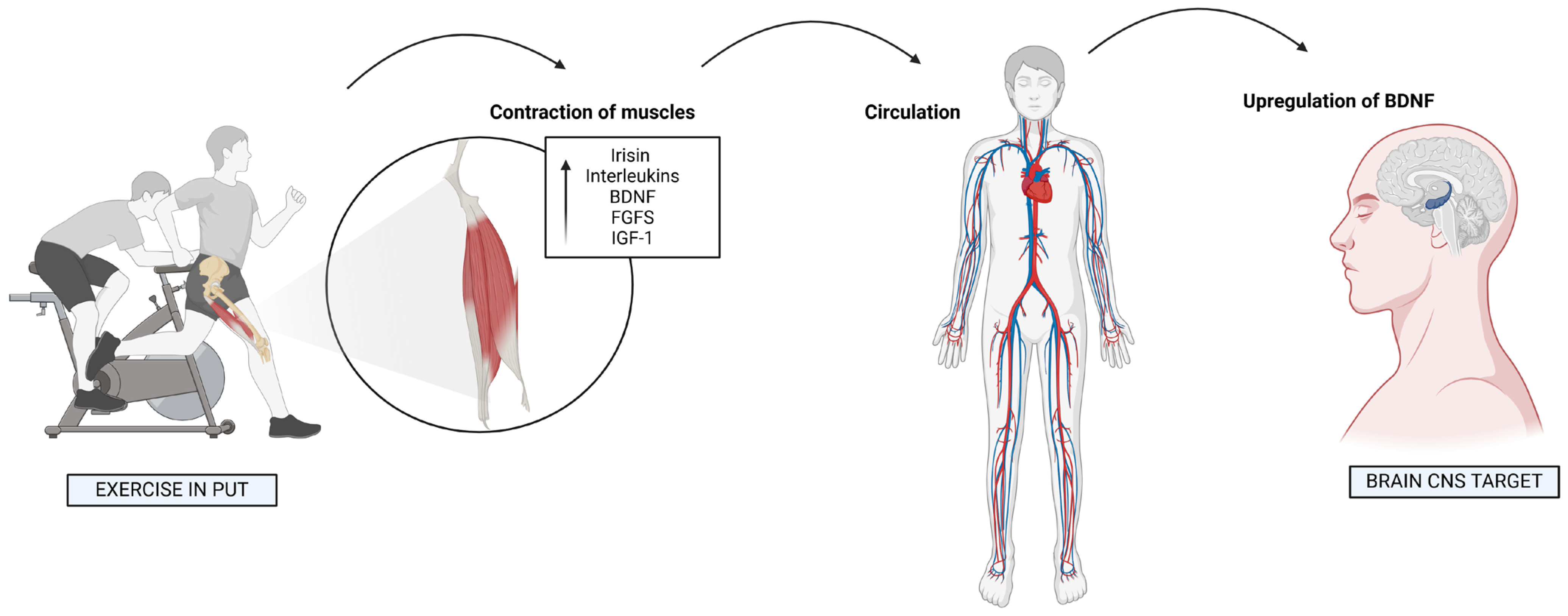
5. Myokines and Neurotrophins: Exercise-Induced Brain Factors
6. Gut–Brain Axis: Nutrition and Cognitive Function
7. Oxidative Stress and Neuroprotection: Antioxidants in Action
8. Neurogenesis and Synaptic Plasticity: Interventions and Insights
8.1. The Role of Exercise
8.2. Key Nutrients
8.3. Synergy of Exercise and Nutrition
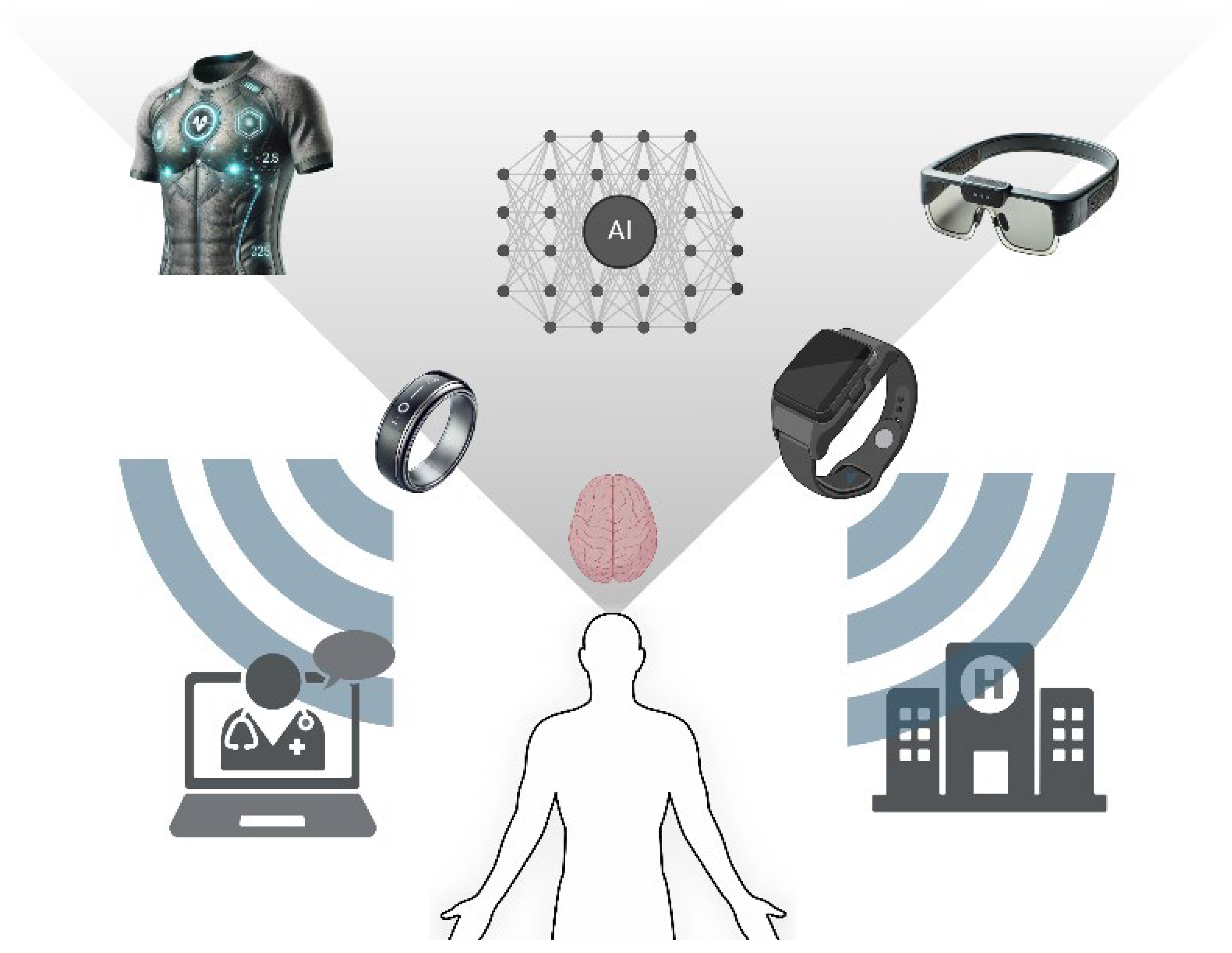
9. Wearable Bioelectronics in Monitoring Mental Health
9.1. Monitoring Mental Health
9.2. Key Innovations
10. Bioengineering and Personalized Neuro-Nutritional Strategies
11. Integrating Exercise with Nutrition for Stress Resilience
12. Translational Approaches to Address Neurodegeneration
13. Practical Applications: Bridging Science and Everyday Health
- Personalized Intervention Design: Leveraging wearable bioelectronics, individuals can monitor physiological and cognitive markers to fine-tune their nutritional and exercise regimens. For example, using devices to track heart rate variability and stress biomarkers enables real-time adjustments to maintain optimal mental and physical health.
- Mental Health Optimization: The integration of exercise and targeted nutritional strategies—such as omega-3 fatty acids, polyphenols, and B vitamins—can serve as complementary therapies for managing stress, anxiety, and depression. Practitioners can design structured interventions combining dietary guidance with aerobic or resistance training programs.
- Cognitive Enhancement Programs: For populations ranging from students to aging adults, incorporating brain-boosting nutrients alongside regular physical activity can enhance learning, memory, and executive function. This is particularly relevant for addressing age-related cognitive decline and neurodegenerative diseases.
- Corporate Wellness Initiatives: Organizations can incorporate these insights into workplace wellness programs, promoting cognitive resilience and emotional well-being through guided exercise sessions and on-site nutritional support.
- Community Outreach and Education: Public health campaigns can disseminate simplified guidelines on combining balanced diets with exercise to optimize mental health and cognitive performance, empowering individuals to take proactive steps toward well-being.
14. Conclusions
14.1. Limitations
14.2. Future Studies
- −
- BDNF-TrkB Signaling Pathway: Future studies should explore how neuro-nutrition and exercise can optimize BDNF expression to enhance neuroplasticity and cognitive resilience. The impact of tailored interventions on TrkB receptor activation and downstream effects warrants further research.
- −
- PI3K/Akt/mTOR Pathway: Research should focus on the combined effects of exercise and specific dietary components, such as omega-3 fatty acids and polyphenols, in modulating this pathway to promote neuronal growth and prevent cognitive decline.
- −
- ERK/CREB Pathway: Investigating the role of dietary antioxidants and structured exercise programs in enhancing CREB-mediated memory formation and synaptic remodeling could provide new insights into cognitive optimization strategies.
- −
- Nrf2-Antioxidant Response Pathway: Further studies should assess how antioxidant-rich diets and exercise-induced mild oxidative stress synergistically activate Nrf2, potentially reducing neuroinflammation and age-related cognitive decline.
- −
- Gut–Brain Axis and Neuroinflammation: The influence of gut microbiota on brain health via metabolites like SCFAs and serotonin precursors requires deeper investigation, with an emphasis on dietary and exercise interventions tailored to individual microbiome profiles.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Jia, R.X.; Liang, J.H.; Xu, Y.; Wang, Y.Q. Effects of Physical Activity and Exercise on the Cognitive Function of Patients with Alzheimer Disease: A Meta-Analysis. BMC Geriatr. 2019, 19, 181. [Google Scholar] [CrossRef] [PubMed]
- Mahalakshmi, B.; Maurya, N.; Lee, S.D.; Kumar, V.B. Possible Neuroprotective Mechanisms of Physical Exercise in Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 5895. [Google Scholar] [CrossRef] [PubMed]
- Hillman, C.H.; Erickson, K.I.; Kramer, A.F. Be Smart, Exercise Your Heart: Exercise Effects on Brain and Cognition. Nat. Rev. Neurosci. 2008, 9, 58–65. [Google Scholar] [CrossRef]
- Phillips, C. Lifestyle Modulators of Neuroplasticity: How Physical Activity, Mental Engagement, and Diet Promote Cognitive Health during Aging. Neural Plast. 2017, 2017, 3589271. [Google Scholar] [CrossRef]
- Schuch, F.B.; Vancampfort, D.; Firth, J.; Rosenbaum, S.; Ward, P.B.; Silva, E.S.; Hallgren, M.; De Leon, A.P.; Dunn, A.L.; Deslandes, A.C.; et al. Physical Activity and Incident Depression: A Meta-Analysis of Prospective Cohort Studies. Am. J. Psychiatry 2018, 175, 631–648. [Google Scholar] [CrossRef]
- Gómez-Pinilla, F. Brain Foods: The Effects of Nutrients on Brain Function. Nat. Rev. Neurosci. 2008, 9, 568–578. [Google Scholar] [CrossRef]
- Hötting, K.; Röder, B. Beneficial Effects of Physical Exercise on Neuroplasticity and Cognition. Neurosci. Biobehav. Rev. 2013, 37 Pt B, 2243–2257. [Google Scholar] [CrossRef]
- Gomez-Pinilla, F.; Hillman, C. The Influence of Exercise on Cognitive Abilities. Compr. Physiol. 2013, 3, 403–428. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.F.; Wang, J. Wearable Biosensors for Healthcare Monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Wang, J. Non-Invasive Wearable Electrochemical Sensors: A Review. Trends Biotechnol. 2014, 32, 363–371. [Google Scholar] [CrossRef]
- Heikenfeld, J.; Jajack, A.; Rogers, J.; Gutruf, P.; Tian, L.; Pan, T.; Li, R.; Khine, M.; Kim, J.; Wang, J.; et al. Wearable Sensors: Modalities, Challenges, and Prospects. Lab Chip 2018, 18, 217–248. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, A.; Su, H.W.; Heneghan, C. Assessment of Physiological Signs Associated with COVID-19 Measured Using Wearable Devices. NPJ Digit. Med. 2020, 3, 156. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients 2023, 15, 2749. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Yáñez-Sepúlveda, R.; Tornero-Aguilera, J.F. Neuro-Vulnerability in Energy Metabolism Regulation: A Comprehensive Narrative Review. Nutrients 2023, 15, 3106. [Google Scholar] [CrossRef]
- Martín-Rodríguez, A.; Bustamante-Sánchez, Á.; Martínez-Guardado, I.; Navarro-Jiménez, E.; Plata-SanJuan, E.; Tornero-Aguilera, J.F.; Clemente-Suárez, V.J. Infancy Dietary Patterns, Development, and Health: An Extensive Narrative Review. Children 2022, 9, 1072. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Bustamante-Sanchez, Á.; Tornero-Aguilera, J.F.; Ruisoto, P.; Mielgo-Ayuso, J. Inflammation in COVID-19 and the Effects of Non-Pharmacological Interventions during the Pandemic: A Review. Int. J. Mol. Sci. 2022, 23, 15584. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Martínez-González, M.B.; Benitez-Agudelo, J.C.; Navarro-Jiménez, E.; Beltran-Velasco, A.I.; Ruisoto, P.; Diaz Arroyo, E.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. The Impact of the COVID-19 Pandemic on Mental Disorders. A Critical Review. Int. J. Environ. Res. Public Health 2021, 18, 10041. [Google Scholar] [CrossRef]
- Bayliak, M.M.; Gospodaryov, D.V.; Lushchak, V.I. Homeostasis of Carbohydrates and Reactive Oxygen Species Is Critically Changed in the Brain of Middle-Aged Mice: Molecular Mechanisms and Functional Reasons. BBA Adv. 2023, 3, 100077. [Google Scholar] [CrossRef]
- Puente-González, A.S.; Sánchez-González, F.; Hernández-Xumet, J.E.; Sánchez-Sánchez, M.C.; Barbero-Iglesias, F.J.; Méndez-Sánchez, R. Short and Medium-Term Effects of a Multicomponent Physical Exercise Program with a Mediterranean Diet on Bone Mineral Density, Gait, Balance, and Fall Risk for Patients with Alzheimer Disease: Randomized Controlled Clinical Trial Study Protocol. Medicine 2020, 99, e22385. [Google Scholar] [CrossRef]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Bennett, D.A.; Aggarwal, N.T. MIND Diet Associated with Reduced Incidence of Alzheimer’s Disease. Alzheimer’s Dement. 2015, 11, 1007–1014. [Google Scholar] [CrossRef]
- Wei, B.Z.; Li, L.; Dong, C.W.; Tan, C.C.; Xu, W. The Relationship of Omega-3 Fatty Acids with Dementia and Cognitive Decline: Evidence from Prospective Cohort Studies of Supplementation, Dietary Intake, and Blood Markers. Am. J. Clin. Nutr. 2023, 117, 1096–1109. [Google Scholar] [CrossRef] [PubMed]
- Ellouze, I.; Sheffler, J.; Nagpal, R.; Arjmandi, B. Dietary Patterns and Alzheimer’s Disease: An Updated Review Linking Nutrition to Neuroscience. Nutrients 2023, 15, 3204. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Park, M.; Lee, E.; Jung, J.; Kim, T. The Role of Vitamin D in Alzheimer’s Disease: A Transcriptional Regulator of Amyloidopathy and Gliopathy. Biomedicines 2022, 10, 1824. [Google Scholar] [CrossRef] [PubMed]
- Harding, S.L.; Bishop, J. The Gut Microbiome, Mental Health, and Cognitive and Neurodevelopmental Disorders: A Scoping Review. J. Nurse Pr. 2022, 18, 719–725. [Google Scholar] [CrossRef]
- Jin, J.; Jin, Q.; Wang, X.; Akoh, C.C. High Sn-2 Docosahexaenoic Acid Lipids for Brain Benefits, and Their Enzymatic Syntheses: A Review. Engineering 2020, 6, 424–431. [Google Scholar] [CrossRef]
- Derbyshire, E. Brain Health across the Lifespan: A Systematic Review on the Role of Omega-3 Fatty Acid Supplements. Nutrients 2018, 10, 1094. [Google Scholar] [CrossRef]
- Scheinman, S.B.; Sugasini, D.; Zayed, M.; Yalagala, P.C.R.; Marottoli, F.M.; Subbaiah, P.V.; Tai, L.M. LPC-DHA/EPA-Enriched Diets Increase Brain DHA and Modulate Behavior in Mice That Express Human APOE4. Front. Neurosci. 2021, 15, 690410. [Google Scholar] [CrossRef]
- Kocyigit, A.; Selek, S. Exogenous Antioxidants Are Double-Edged Swords. Bezmialem Sci. 2016, 4, 70–75. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y.; Gao, M.; Bai, X.; Chen, Z. Neuroprotective Effect of Several Phytochemicals and Its Potential Application in the Prevention of Neurodegenerative Diseases. Geriatrics 2016, 1, 29. [Google Scholar] [CrossRef]
- McGurran, H.; Glenn, J.; Madero, E.; Bott, N. Risk Reduction and Prevention of Alzheimer’s Disease: Biological Mechanisms of Diet. Curr. Alzheimer Res. 2020, 17, 407–427. [Google Scholar] [CrossRef]
- Tofighi, N.; Asle-Rousta, M.; Rahnema, M.; Amini, R. The Anxiolytic Effect of Alpha-Linoleic Acid in Alzheimer’s Disease Model Rats Is Mediated by Enhanced Brain-Derived Neurotrophic Factor Expression. J. Knowl. Health Basic Med. Sci. 2021, 16, 9–14. [Google Scholar] [CrossRef]
- Wysoczański, T.; Sokoła-Wysoczańska, E.; Pękala, J.; Lochyński, S.; Czyż, K.; Bodkowski, R.; Herbinger, G.; Patkowska-Sokoła, B.; Librowski, T. Omega-3 Fatty Acids and Their Role in Central Nervous System—A Review. Curr. Med. Chem. 2016, 23, 816–831. [Google Scholar] [CrossRef] [PubMed]
- Regner-Nelke, L.; Nelke, C.; Schroeter, C.B.; Dziewas, R.; Warnecke, T.; Ruck, T.; Meuth, S.G. Enjoy Carefully: The Multifaceted Role of Vitamin e in Neuro-Nutrition. Int. J. Mol. Sci. 2021, 22, 10087. [Google Scholar] [CrossRef] [PubMed]
- Kangisser, L.; Tan, E.; Bellomo, R.; Deane, A.M.; Plummer, M.P. Neuroprotective Properties of Vitamin C: A Scoping Review of Pre-Clinical and Clinical Studies. J. Neurotrauma 2021, 38, 2194–2205. [Google Scholar] [CrossRef]
- Tucker, K.L.; Qiao, N.; Scott, T.; Rosenberg, I.; Spiro, A. High Homocysteine and Low B Vitamins Predict Cognitive Decline in Aging Men: The Veterans Affairs Normative Aging Study. Am. J. Clin. Nutr. 2005, 82, 627–635. [Google Scholar] [CrossRef]
- McNulty, H.; Ward, M.; Hoey, L.; Hughes, C.F.; Pentieva, K. Addressing Optimal Folate and Related B-Vitamin Status through the Lifecycle: Health Impacts and Challenges. Proc. Nutr. Soc. 2019, 78, 449–462. [Google Scholar] [CrossRef]
- García, R.M.M.; Ortega, A.I.J.; López-Sobaler, A.M.; Ortega, R.M. Nutrition Strategies That Improve Cognitive Function. Nutr. Hosp. 2018, 35, 16–19. [Google Scholar] [CrossRef]
- Abrego-Guandique, D.M.; Bonet, M.L.; Caroleo, M.C.; Cannataro, R.; Tucci, P.; Ribot, J.; Cione, E. The Effect of Beta-Carotene on Cognitive Function: A Systematic Review. Brain Sci. 2023, 13, 1468. [Google Scholar] [CrossRef]
- Lauer, A.A.; Grimm, H.S.; Apel, B.; Golobrodska, N.; Kruse, L.; Ratanski, E.; Schulten, N.; Schwarze, L.; Slawik, T.; Sperlich, S.; et al. Mechanistic Link between Vitamin B12 and Alzheimer’s Disease. Biomolecules 2022, 12, 129. [Google Scholar] [CrossRef]
- Li, S.; Guo, Y.; Men, J.; Fu, H.; Xu, T. The Preventive Efficacy of Vitamin B Supplements on the Cognitive Decline of Elderly Adults: A Systematic Review and Meta-Analysis. BMC Geriatr. 2021, 21, 367. [Google Scholar] [CrossRef]
- Maurya, V.K.; Shakya, A.; McClements, D.J.; Srinivasan, R.; Bashir, K.; Ramesh, T.; Lee, J.; Sathiyamoorthi, E. Vitamin C Fortification: Need and Recent Trends in Encapsulation Technologies. Front. Nutr. 2023, 10, 1229243. [Google Scholar] [CrossRef] [PubMed]
- Bournival, J.; Quessy, P.; Martinoli, M.G. Protective Effects of Resveratrol and Quercetin against MPP+ -Induced Oxidative Stress Act by Modulating Markers of Apoptotic Death in Dopaminergic Neurons. Cell. Mol. Neurobiol. 2009, 29, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Gardener, S.L.; Rainey-Smith, S.R.; Weinborn, M.; Bondonno, C.P.; Martins, R.N. Intake of Products Containing Anthocyanins, Flavanols, and Flavanones, and Cognitive Function: A Narrative Review. Front. Aging Neurosci. 2021, 13, 640381. [Google Scholar] [CrossRef] [PubMed]
- Rezai-Zadeh, K.; Arendash, G.W.; Hou, H.; Fernandez, F.; Jensen, M.; Runfeldt, M.; Shytle, R.D.; Tan, J. Green Tea Epigallocatechin-3-Gallate (EGCG) Reduces β-Amyloid Mediated Cognitive Impairment and Modulates Tau Pathology in Alzheimer Transgenic Mice. Brain Res. 2008, 1214, 177–187. [Google Scholar] [CrossRef]
- Jenkins, T.; Nguyen, J.; Polglaze, K.; Bertrand, P. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients 2016, 8, 56. [Google Scholar] [CrossRef]
- Meeusen, R.; Decroix, L. Nutritional Supplements and the Brain. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 200–211. [Google Scholar] [CrossRef]
- Young, S.N. How to Increase Serotonin in the Human Brain without Drugs. J. Psychiatry Neurosci. 2024, 32, 394. [Google Scholar]
- Freir, D.B.; Fedriani, R.; Scully, D.; Smith, I.M.; Selkoe, D.J.; Walsh, D.M.; Regan, C.M. Aβ Oligomers Inhibit Synapse Remodelling Necessary for Memory Consolidation. Neurobiol. Aging 2011, 32, 2211–2218. [Google Scholar] [CrossRef]
- Clark, A.; Mach, N. Exercise-Induced Stress Behavior, Gut-Microbiota-Brain Axis and Diet: A Systematic Review for Athletes. J. Int. Soc. Sports Nutr. 2016, 13, 43. [Google Scholar] [CrossRef]
- Jonasson, L.S.; Nyberg, L.; Kramer, A.F.; Lundquist, A.; Riklund, K.; Boraxbekk, C.J. Aerobic Exercise Intervention, Cognitive Performance, and Brain Structure: Results from the Physical Influences on Brain in Aging (PHIBRA) Study. Front. Aging Neurosci. 2017, 8, 336. [Google Scholar] [CrossRef]
- Su, H.M. Mechanisms of N-3 Fatty Acid-Mediated Development and Maintenance of Learning Memory Performance. J. Nutr. Biochem. 2010, 21, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Zerbes, G.; Kausche, F.M.; Schwabe, L. Stress-Induced Cortisol Modulates the Control of Memory Retrieval towards the Dorsal Striatum. Eur. J. Neurosci. 2020, 55, 2699–2713. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Noble, J.M.; Marder, K.; Hartman, J.S.; Gu, Y.; Scarmeas, N. Dietary Patterns, Physical Activity, Sleep, and Risk for Dementia and Cognitive Decline. Curr. Nutr. Rep. 2018, 7, 335–345. [Google Scholar] [CrossRef]
- Li, Q.; Gong, B.; Zhao, Y.; Wu, C. Effect of Exercise Cognitive Combined Training on Physical Function in Cognitively Healthy Older Adults: A Systematic Review and Meta-Analysis. J. Aging Phys. Act. 2023, 31, 155–170. [Google Scholar] [CrossRef]
- Peng, J.; Yuan, Y.; Zhao, Y.; Ren, H. Effects of Exercise on Patients with Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 10845. [Google Scholar] [CrossRef]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise Training Increases Size of Hippocampus and Improves Memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise Induces Hippocampal BDNF through a PGC-1α/FNDC5 Pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef]
- Liu, P.Z.; Nusslock, R. Exercise-Mediated Neurogenesis in the Hippocampus via BDNF. Front. Neurosci. 2018, 12, 52. [Google Scholar] [CrossRef]
- Stillman, C.M.; Cohen, J.; Lehman, M.E.; Erickson, K.I. Mediators of Physical Activity on Neurocognitive Function: A Review at Multiple Levels of Analysis. Front. Hum. Neurosci. 2016, 10, 626. [Google Scholar] [CrossRef]
- Mattson, M.P. Energy Intake and Exercise as Determinants of Brain Health and Vulnerability to Injury and Disease. Cell Metab. 2012, 16, 706–722. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Castillo-García, A.; Morales, J.S.; de la Villa, P.; Hampel, H.; Emanuele, E.; Lista, S.; Lucia, A. Exercise Benefits on Alzheimer’s Disease: State-of-the-Science. Ageing Res. Rev. 2020, 62, 101108. [Google Scholar] [CrossRef] [PubMed]
- Tarumi, T.; Zhang, R. The Role of Exercise-Induced Cardiovascular Adaptation in Brain Health. Exerc. Sport Sci. Rev. 2015, 43, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, M.G.; Kyparos, A.; Spanou, C.; Paschalis, V.; Theodorou, A.A.; Vrabas, I.S. Redox Biology of Exercise: An Integrative and Comparative Consideration of Some Overlooked Issues. J. Exp. Biol. 2012, 215, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Meeusen, R. Exercise, Nutrition and the Brain. Sports Med. 2014, 44, 47–56. [Google Scholar] [CrossRef]
- Griñán-Ferré, C.; Sarroca, S.; Ivanova, A.; Puigoriol-Illamola, D.; Aguado, F.; Camins, A.; Sanfeliu, C.; Pallàs, M. Epigenetic Mechanisms Underlying Cognitive Impairment and Alzheimer Disease Hallmarks in 5XFAD Mice. Aging 2016, 8, 664–684. [Google Scholar] [CrossRef]
- Kauppinen, A.; Suuronen, T.; Ojala, J.; Kaarniranta, K.; Salminen, A. Antagonistic Crosstalk between NF-ΚB and SIRT1 in the Regulation of Inflammation and Metabolic Disorders. Cell. Signal. 2013, 25, 1939–1948. [Google Scholar] [CrossRef]
- Coker, S.J.; Smith-Díaz, C.C.; Dyson, R.M.; Vissers, M.C.M.; Berry, M.J. The Epigenetic Role of Vitamin C in Neurodevelopment. Int. J. Mol. Sci. 2022, 23, 1208. [Google Scholar] [CrossRef]
- Fernandes, J.; Arida, R.M.; Gomez-Pinilla, F. Physical Exercise as an Epigenetic Modulator of Brain Plasticity and Cognition. Neurosci. Biobehav. Rev. 2017, 80, 443–456. [Google Scholar] [CrossRef]
- de Meireles, L.C.F.; Galvão, F.; Walker, D.M.; Cechinel, L.R.; de Souza Grefenhagen, Á.I.; Andrade, G.; Palazzo, R.P.; Lovatel, G.A.; Basso, C.G.; Nestler, E.J.; et al. Exercise Modalities Improve Aversive Memory and Survival Rate in Aged Rats: Role of Hippocampal Epigenetic Modifications. Mol. Neurobiol. 2019, 56, 8408–8419. [Google Scholar] [CrossRef]
- Baek, S.-S. Role of Exercise on the Brain. J. Exerc. Rehabil. 2016, 12, 380–385. [Google Scholar] [CrossRef]
- Neeper, S.A.; Góauctemez-Pinilla, F.; Choi, J.; Cotman, C. Exercise and Brain Neurotrophins. Nature 1995, 373, 109. [Google Scholar] [CrossRef] [PubMed]
- Van Praag, H.; Kempermann, G.; Gage, F.H. Running Increases Cell Proliferation and Neurogenesis in the Adult Mouse Dentate Gyrus. Nat. Neurosci. 1999, 2, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Mizuuchi, D.; Omura, K.; Lee, M.; Oharazawa, A.; Yook, J.S.; Inoue, K.; Soya, H. High-Intensity Intermittent Training Enhances Spatial Memory and Hippocampal Neurogenesis Associated with BDNF Signaling in Rats. Cerebral Cortex 2021, 31, 4386–4397. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.C.; Huddleston, D.E.; Brickman, A.M.; Sosunov, A.A.; Hen, R.; McKhann, G.M.; Sloan, R.; Gage, F.H.; Brown, T.R.; Small, S.A. An in Vivo Correlate of Exercise-Induced Neurogenesis in the Adult Dentate Gyrus. Proc. Natl. Acad. Sci. USA 2007, 104, 5638–5643. [Google Scholar] [CrossRef]
- Firth, J.; Stubbs, B.; Vancampfort, D.; Schuch, F.; Lagopoulos, J.; Rosenbaum, S.; Ward, P.B. Effect of Aerobic Exercise on Hippocampal Volume in Humans: A Systematic Review and Meta-Analysis. Neuroimage 2018, 166, 230–238. [Google Scholar] [CrossRef]
- Meeusen, R.; De Meirleir, K. Exercise and Brain Neurotransmission. Sports Med. 1995, 20, 160–188. [Google Scholar] [CrossRef]
- Strasser, B.; Gostner, J.M.; Fuchs, D. Mood, Food, and Cognition: Role of Tryptophan and Serotonin. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 55–61. [Google Scholar] [CrossRef]
- Xie, Y.; Wu, Z.; Sun, L.; Zhou, L.; Wang, G.; Xiao, L.; Wang, H. The Effects and Mechanisms of Exercise on the Treatment of Depression. Front. Psychiatry 2021, 12, 705559. [Google Scholar] [CrossRef]
- Foster, P.P. Role of Physical and Mental Training in Brain Network Configuration. Front. Aging Neurosci. 2015, 7, 117. [Google Scholar] [CrossRef][Green Version]
- Rothman, S.M.; Mattson, M.P. Activity-Dependent, Stress-Responsive BDNF Signaling and the Quest for Optimal Brain Health and Resilience throughout the Lifespan. Neuroscience 2013, 239, 228–240. [Google Scholar] [CrossRef]
- Cullen, J.M.A.; Shahzad, S.; Dhillon, J. A Systematic Review on the Effects of Exercise on Gut Microbial Diversity, Taxonomic Composition, and Microbial Metabolites: Identifying Research Gaps and Future Directions. Front. Physiol. 2023, 14, 1292673. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Velasco, A.I.; Clemente-Suárez, V.J. Harnessing Gut Microbiota for Biomimetic Innovations in Health and Biotechnology. Biomimetics 2025, 10, 73. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscles, Exercise and Obesity: Skeletal Muscle as a Secretory Organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Rubio-Zarapuz, A.; Belinchón-deMiguel, P.; Beltrán-Velasco, A.I.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Impact of Physical Activity on Cellular Metabolism Across Both Neurodegenerative and General Neurological Conditions: A Narrative Review. Cells 2024, 13, 1940. [Google Scholar] [CrossRef]
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levälahti, E.; Ahtiluoto, S.; Antikainen, R.; Bäckman, L.; Hänninen, T.; Jula, A.; Laatikainen, T.; et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet Neurol. 2015, 14, 333–343. [Google Scholar] [CrossRef]
- Keawtep, P.; Sungkarat, S.; Boripuntakul, S.; Sa-Nguanmoo, P.; Wichayanrat, W.; Chattipakorn, S.C.; Worakul, P. Effects of combined dietary intervention and physical-cognitive exercise on cognitive function and cardiometabolic health of postmenopausal women with obesity: A randomized controlled trial. Int. J. Behav. Nutr. Phys. Act. 2024, 21, 28. [Google Scholar] [CrossRef]
- Cermak, N.M.; Res, P.T.; De Groot, L.C.P.G.M.; Saris, W.H.M.; Van Loon, L.J.C. Protein Supplementation Augments the Adaptive Response of Skeletal Muscle to Resistance-Type Exercise Training: A Meta-Analysis. Am. J. Clin. Nutr. 2012, 96, 1454–1464. [Google Scholar] [CrossRef]
- Wang, B.; Liang, J.; Lu, C.; Lu, A.; Wang, C. Exercise Regulates Myokines in Aging-Related Diseases through Muscle-Brain Crosstalk. Gerontology 2024, 70, 193–209. [Google Scholar] [CrossRef]
- Gomez-Pinilla, F. The Influences of Diet and Exercise on Mental Health through Hormesis. Ageing Res. Rev. 2008, 7, 49–62. [Google Scholar] [CrossRef]
- Saucedo Marquez, C.M.; Vanaudenaerde, B.; Troosters, T.; Wenderoth, N. High-Intensity Interval Training Evokes Larger Serum BDNF Levels Compared with Intense Continuous Exercise. J. Appl. Physiol. 2015, 119, 1363–1373. [Google Scholar] [CrossRef]
- Cotman, C.W.; Berchtold, N.C. Exercise: A Behavioral Intervention to Enhance Brain Health and Plasticity. Trends Neurosci. 2002, 25, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Kendig, M.D.; Leigh, S.J.; Morris, M.J. Unravelling the Impacts of Western-Style Diets on Brain, Gut Microbiota and Cognition. Neurosci. Biobehav. Rev. 2021, 128, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Callow, D.D.; Kommula, Y.; Stark, C.E.L.; Smith, J.C. Acute Cycling Exercise and Hippocampal Subfield Function and Microstructure in Healthy Older Adults. Hippocampus 2023, 33, 1123–1138. [Google Scholar] [CrossRef] [PubMed]
- Gothe, N.P.; Khan, I.; Hayes, J.; Erlenbach, E.; Damoiseaux, J.S. Yoga Effects on Brain Health: A Systematic Review of the Current Literature. Brain Plast. 2019, 5, 105–122. [Google Scholar] [CrossRef]
- Neuvonen, E.; Lehtisalo, J.; Solomon, A.; Antikainen, R.; Havulinna, S.; Hänninen, T.; Laatikainen, T.; Lindström, J.; Rautio, N.; Soininen, H.; et al. Psychosocial Determinants for Adherence to a Healthy Lifestyle and Intervention Participation in the FINGER Trial: An Exploratory Analysis of a Randomised Clinical Trial. Aging Clin. Exp. Res. 2022, 34, 1793–1805. [Google Scholar] [CrossRef]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef]
- Hoban, A.E.; Moloney, R.D.; Golubeva, A.V.; McVey Neufeld, K.A.; O’Sullivan, O.; Patterson, E.; Stanton, C.; Dinan, T.G.; Clarke, G.; Cryan, J.F. Behavioural and Neurochemical Consequences of Chronic Gut Microbiota Depletion during Adulthood in the Rat. Neuroscience 2016, 339, 463–477. [Google Scholar] [CrossRef]
- Tillisch, K.; Labus, J.; Kilpatrick, L.; Jiang, Z.; Stains, J.; Ebrat, B.; Guyonnet, D.; Legrain-Raspaud, S.; Trotin, B.; Naliboff, B.; et al. Consumption of Fermented Milk Product with Probiotic Modulates Brain Activity. Gastroenterology 2013, 144, 1394–1401.e4. [Google Scholar] [CrossRef]
- Shi, H.; Ge, X.; Ma, X.; Zheng, M.; Cui, X.; Pan, W.; Zheng, P.; Yang, X.; Zhang, P.; Hu, M.; et al. A Fiber-Deprived Diet Causes Cognitive Impairment and Hippocampal Microglia-Mediated Synaptic Loss through the Gut Microbiota and Metabolites. Microbiome 2021, 9, 223. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The Role of Short-Chain Fatty Acids in Microbiota–Gut–Brain Communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Moloney, R.D.; Dinan, T.G.; Cryan, J.F. Stress & the Microbiota–Gut–Brain Axis in Visceral Pain. Psychoneuroendocrinology 2015, 61, 8. [Google Scholar] [CrossRef]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus Strain Regulates Emotional Behavior and Central GABA Receptor Expression in a Mouse via the Vagus Nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D. Dietary Polyphenols as Modulators of Brain Functions: Biological Actions and Molecular Mechanisms Underpinning Their Beneficial Effects. Oxidative Med. Cell. Longev. 2012, 2012, 914273. [Google Scholar] [CrossRef]
- Berding, K.; Vlckova, K.; Marx, W.; Schellekens, H.; Stanton, C.; Clarke, G.; Jacka, F.; Dinan, T.G.; Cryan, J.F. Diet and the Microbiota-Gut-Brain Axis: Sowing the Seeds of Good Mental Health. Adv. Nutr. Int. Rev. J. 2021, 12, 1239–1285. [Google Scholar] [CrossRef]
- Dumitrescu, L.; Popescu-Olaru, I.; Cozma, L.; Tulbǎ, D.; Hinescu, M.E.; Ceafalan, L.C.; Gherghiceanu, M.; Popescu, B.O. Oxidative Stress and the Microbiota-Gut-Brain Axis. Oxid. Med. Cell. Longev. 2018, 2018, 2406594. [Google Scholar] [CrossRef]
- Sordillo, J.E.; Korrick, S.; Laranjo, N.; Carey, V.; Weinstock, G.M.; Gold, D.R.; O’Connor, G.; Sandel, M.; Bacharier, L.B.; Beigelman, A.; et al. Association of the Infant Gut Microbiome With Early Childhood Neurodevelopmental Outcomes. JAMA Netw. Open 2019, 2, e190905. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Mind-Altering Microorganisms: The Impact of the Gut Microbiota on Brain and Behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Sampson, T.R.; Mazmanian, S.K. Control of Brain Development, Function, and Behavior by the Microbiome. Cell Host Microbe 2015, 17, 565–576. [Google Scholar] [CrossRef]
- Akbari, E.; Asemi, Z.; Daneshvar Kakhaki, R.; Bahmani, F.; Kouchaki, E.; Tamtaji, O.R.; Hamidi, G.A.; Salami, M. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer’s Disease: A Randomized, Double-Blind and Controlled Trial. Front. Aging Neurosci. 2016, 8, 256. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean Diet Intervention Alters the Gut Microbiome in Older People Reducing Frailty and Improving Health Status: The NU-AGE 1-Year Dietary Intervention across Five European Countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. Melancholic Microbes: A Link between Gut Microbiota and Depression? Neurogastroenterol. Motil. 2013, 25, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lapiscina, E.H.; Clavero, P.; Toledo, E.; Estruch, R.; Salas-Salvadó, J.; San Julián, B.; Sanchez-Tainta, A.; Ros, E.; Valls-Pedret, C.; Martinez-Gonzalez, M.Á. Mediterranean Diet Improves Cognition: The PREDIMED-NAVARRA Randomised Trial. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, K.R.; Hauck, L.; Jeffrey, B.M.; Elias, V.; Humphrey, A.; Nath, R.; Perrone, A.; Bermudez, L.E. Relationships between Diet-Related Changes in the Gut Microbiome and Cognitive Flexibility. Neuroscience 2015, 300, 128–140. [Google Scholar] [CrossRef]
- Halliwell, B. Oxidative Stress and Neurodegeneration: Where Are We Now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen Peroxide as a Central Redox Signaling Molecule in Physiological Oxidative Stress: Oxidative Eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Halliwell, B. Oxidative Stress, Dysfunctional Glucose Metabolism and Alzheimer Disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef]
- Puri, S.; Shaheen, M.; Grover, B. Nutrition and Cognitive Health: A Life Course Approach. Front. Public Health 2023, 11, 1023907. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxid. Med. Cell. Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef]
- Mattson, M.P. Energy Intake, Meal Frequency, and Health: A Neurobiological Perspective. Annu. Rev. Nutr. 2005, 25, 237–260. [Google Scholar] [CrossRef]
- Sena, L.A.; Chandel, N.S. Physiological Roles of Mitochondrial Reactive Oxygen Species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.A.; Shukitt-Hale, B.; Lau, F.C. Fruit Polyphenols and Their Effects on Neuronal Signaling and Behavior in Senescence. Ann. New York Acad. Sci. 2007, 1100, 470–485. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative Stress, Mitochondrial Damage and Neurodegenerative Diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Tramutola, A.; Lanzillotta, C.; Perluigi, M.; Butterfield, D.A. Oxidative Stress, Protein Modification and Alzheimer Disease. Brain Res. Bull. 2017, 133, 88–96. [Google Scholar] [CrossRef]
- Sun, M.; Ma, K.; Wen, J.; Wang, G.; Zhang, C.; Li, Q.; Bao, X.; Wang, H. A Review of the Brain-Gut-Microbiome Axis and the Potential Role of Microbiota in Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 73, 849–865. [Google Scholar] [CrossRef]
- Vauzour, D.; Vafeiadou, K.; Rodriguez-Mateos, A.; Rendeiro, C.; Spencer, J.P.E. The Neuroprotective Potential of Flavonoids: A Multiplicity of Effects. Genes Nutr. 2008, 3, 115–126. [Google Scholar] [CrossRef]
- Scarmeas, N.; Stern, Y.; Tang, M.; Mayeux, R.; Luchsinger, J.A. Mediterranean Diet and Risk for Alzheimer’s Disease. Ann. Neurol. 2006, 59, 912–921. [Google Scholar] [CrossRef]
- Sang, L.; Liu, C.; Wang, L.; Zhang, J.; Zhang, Y.; Li, P.; Qiao, L.; Li, C.; Qiu, M. Disrupted Brain Structural Connectivity Network in Subcortical Ischemic Vascular Cognitive Impairment With No Dementia. Front. Aging Neurosci. 2020, 12, 6. [Google Scholar] [CrossRef]
- Radak, Z.; Hart, N.; Sarga, L.; Koltai, E.; Atalay, M.; Ohno, H.; Boldogh, I. Exercise Plays a Preventive Role Against Alzheimer’s Disease. J. Alzheimer’s Dis. 2010, 20, 777–783. [Google Scholar] [CrossRef]
- Phillips, M.C.L. Fasting as a Therapy in Neurological Disease. Nutrients 2019, 11, 2501. [Google Scholar] [CrossRef]
- Dias, V.; Junn, E.; Mouradian, M.M. The Role of Oxidative Stress in Parkinson’s Disease. J. Park. Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef] [PubMed]
- Sekikawa, A.; Cui, C.; Sugiyama, D.; Fabio, A.; Harris, W.S.; Zhang, X. Effect of High-Dose Marine Omega-3 Fatty Acids on Atherosclerosis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Nutrients 2019, 11, 2599. [Google Scholar] [CrossRef] [PubMed]
- Calis, Z.; Mogulkoc, R.; Baltaci, A.K. The Roles of Flavonols/Flavonoids in Neurodegeneration and Neuroinflammation. Mini Rev. Med. Chem. 2019, 20, 1475–1488. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Murawska-Ciałowicz, E.; Wiatr, M.; Ciałowicz, M.; de Assis, G.G.; Borowicz, W.; Rocha-Rodrigues, S.; Paprocka-Borowicz, M.; Marques, A. Bdnf Impact on Biological Markers of Depression—Role of Physical Exercise and Training. Int. J. Environ. Res. Public Health 2021, 18, 7553. [Google Scholar] [CrossRef]
- Müller, P.; Duderstadt, Y.; Lessmann, V.; Müller, N.G. Lactate and BDNF: Key Mediators of Exercise Induced Neuroplasticity? J. Clin. Med. 2020, 9, 1136. [Google Scholar] [CrossRef]
- Xing, Y.; Bai, Y. A Review of Exercise-Induced Neuroplasticity in Ischemic Stroke: Pathology and Mechanisms. Mol. Neurobiol. 2020, 57, 4218–4231. [Google Scholar] [CrossRef]
- von Bohlen und Halbach, O. Editorial: Cellular and Molecular Responses to Changes in Nutrition and Exercise. Front. Cell. Neurosci. 2022, 16, 1102308. [Google Scholar] [CrossRef]
- Nct. Neural Changes of Exercise: A Functional MRI Study. 2015. Available online: https://clinicaltrials.gov/show/NCT02541136 (accessed on 3 January 2025).
- Pickersgill, J.W.; Turco, C.V.; Ramdeo, K.; Rehsi, R.S.; Foglia, S.D.; Nelson, A.J. The Combined Influences of Exercise, Diet and Sleep on Neuroplasticity. Front. Psychol. 2022, 13, 831819. [Google Scholar] [CrossRef]
- Lin, T.-W.; Tsai, S.-F.; Kuo, Y.-M. Physical Exercise Enhances Neuroplasticity and Delays Alzheimer’s Disease. Brain Plast. 2018, 4, 95–110. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Belinchón-deMiguel, P.; Ramos-Campo, D.J.; Curiel-Regueros, A.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. The Interplay of Sports and Nutrition in Neurological Health and Recovery. J. Clin. Med. 2024, 13, 2065. [Google Scholar] [CrossRef] [PubMed]
- Schmolesky, M.T.; Webb, D.L.; Hansen, R.A. The Effects of Aerobic Exercise Intensity and Duration on Levels of Brain- Derived Neurotrophic Factor in Healthy Men. J. Sports Sci. Med. 2013, 12, 502–511. [Google Scholar] [PubMed]
- Hao, Z.; Zhang, X.; Wang, Y. Evidence of the Long-Term Protective Effect of Moderate-Intensity Physical Activity on Cognitive Function in Middle-Aged and Elderly Individuals: A Predictive Analysis of Longitudinal Studies. Life 2024, 14, 1343. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, H.; Miao, X.; Li, H.; Pan, H.; Zhou, D.; Liu, Y.; Li, Z.; Wang, J.; Liu, X.; et al. High-Intensity Physical Activity Is Not Associated with Better Cognition in the Elder: Evidence from the China Health and Retirement Longitudinal Study. Alzheimer’s Res. Ther. 2021, 13, 182. [Google Scholar] [CrossRef] [PubMed]
- Levinger, I.; Goodman, C.; Matthews, V.; Hare, D.L.; Jerums, G.; Garnham, A.; Selig, S. BDNF, Metabolic Risk Factors, and Resistance Training in Middle-Aged Individuals. Med. Sci. Sports Exerc. 2008, 40, 535–541. [Google Scholar] [CrossRef] [PubMed]
- De Sousa Fernandes, M.S.; Ordônio, T.F.; Santos, G.C.J.; Santos, L.E.R.; Calazans, C.T.; Gomes, D.A.; Santos, T.M. Effects of Physical Exercise on Neuroplasticity and Brain Function: A Systematic Review in Human and Animal Studies. Neural Plast. 2020, 2020, 8856621. [Google Scholar] [CrossRef]
- Baek, J.-E.; Hyeon, S.-J.; Kim, M.; Cho, H.; Hahm, S.-C. Effects of Dual-Task Resistance Exercise on Cognition, Mood, Depression, Functional Fitness, and Activities of Daily Living in Older Adults with Cognitive Impairment: A Single-Blinded, Randomized Controlled Trial. BMC Geriatr. 2024, 24, 369. [Google Scholar] [CrossRef]
- Jackson, P.A.; Pialoux, V.; Corbett, D.; Drogos, L.; Erickson, K.I.; Eskes, G.A.; Poulin, M.J. Promoting Brain Health through Exercise and Diet in Older Adults: A Physiological Perspective. J. Physiol. 2016, 594, 4485–4498. [Google Scholar] [CrossRef]
- Ogoh, S.; Ainslie, P.N. Cerebral Blood Flow during Exercise: Mechanisms of Regulation. J. Appl. Physiol. 2009, 107, 1370–1380. [Google Scholar] [CrossRef]
- Olivo, G.; Nilsson, J.; Garzón, B.; Lebedev, A.; Wåhlin, A.; Tarassova, O.; Ekblom, M.; Lövdén, M. Immediate Effects of a Single Session of Physical Exercise on Cognition and Cerebral Blood Flow: A Randomized Controlled Study of Older Adults. NeuroImage 2021, 225, 117500. [Google Scholar] [CrossRef]
- Melgar-Locatelli, S.; de Ceglia, M.; Mañas-Padilla, M.C.; Rodriguez-Pérez, C.; Castilla-Ortega, E.; Castro-Zavala, A.; Rivera, P. Nutrition and Adult Neurogenesis in the Hippocampus: Does What You Eat Help You Remember? Front. Neurosci. 2023, 17, 1147269. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Medoro, A.; Ali, S.; Passarella, D.; Intrieri, M.; Scapagnini, G. Dietary Flavonoids and Adult Neurogenesis: Potential Implications for Brain Aging. Curr. Neuropharmacol. 2022, 21, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Anbari-Nogyni, Z.; Bidaki, R.; Madadizadeh, F.; Sangsefidi, Z.S.; Fallahzadeh, H.; Karimi-Nazari, E.; Nadjarzadeh, A. Relationship of Zinc Status with Depression and Anxiety among Elderly Population. Clin. Nutr. ESPEN 2020, 37, 233–239. [Google Scholar] [CrossRef]
- Asigbee, F.M.; Whitney, S.D.; Peterson, C.E. The Link Between Nutrition and Physical Activity in Increasing Academic Achievement. J. Sch. Health 2018, 88, 407–415. [Google Scholar] [CrossRef]
- Antonopoulou, M.; Mantzorou, M.; Serdari, A.; Bonotis, K.; Vasios, G.; Pavlidou, E.; Trifonos, C.; Vadikolias, K.; Petridis, D.; Giaginis, C. Evaluating Mediterranean Diet Adherence in University Student Populations: Does This Dietary Pattern Affect Students’ Academic Performance and Mental Health? Int. J. Heal. Plan. Manag. 2020, 35, 5–21. [Google Scholar] [CrossRef]
- Aune, D. Plant Foods, Antioxidant Biomarkers, and the Risk of Cardiovascular Disease, Cancer, and Mortality: A Review of the Evidence. Adv. Nutr. Int. Rev. J. 2019, 10, S404–S421. [Google Scholar] [CrossRef]
- Craig, W.J.; Mangels, A.R.; Fresán, U.; Marsh, K.; Miles, F.L.; Saunders, A.V.; Haddad, E.H.; Heskey, C.E.; Johnston, P.; Larson-Meyer, E.; et al. The Safe and Effective Use of Plant-Based Diets with Guidelines for Health Professionals. Nutrients 2021, 13, 4144. [Google Scholar] [CrossRef]
- Nehlig, A. The Neuroprotective Effects of Cocoa Flavanol and Its Influence on Cognitive Performance. Br. J. Clin. Pharmacol. 2013, 75, 716–727. [Google Scholar] [CrossRef]
- Arts, I.C.W.; Hollman, P.C.H. Polyphenols and Disease Risk in Epidemiologic Studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [Google Scholar] [CrossRef]
- Cho, J.A.; Park, E. Curcumin Utilizes the Anti-Inflammatory Response Pathway to Protect the Intestine against Bacterial Invasion. Nutr. Res. Pract. 2015, 9, 117–122. [Google Scholar] [CrossRef]
- Sun, G.Y.; Simonyi, A.; Fritsche, K.L.; Chuang, D.Y.; Hannink, M.; Gu, Z.; Greenlief, C.M.; Yao, J.K.; Lee, J.C.; Beversdorf, D.Q. Docosahexaenoic Acid (DHA): An Essential Nutrient and a Nutraceutical for Brain Health and Diseases. Prostaglandins Leukot. Essent. Fatty Acids 2018, 136, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Sambra, V.; Echeverria, F.; Valenzuela, A.; Chouinard-Watkins, R.; Valenzuela, R. Docosahexaenoic and Arachidonic Acids as Neuroprotective Nutrients throughout the Life Cycle. Nutrients 2021, 13, 986. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.B.; Franceschini, M.A.; Silver, R.E.; Taylor, S.F.; De Sa, A.B.; Có, R.; Sonco, A.; Krauss, A.; Taetzsch, A.; Webb, P.; et al. Effects of Food Supplementation on Cognitive Function, Cerebral Blood Flow, and Nutritional Status in Young Children at Risk of Undernutrition: Randomized Controlled Trial. BMJ 2020, 370, m2397. [Google Scholar] [CrossRef] [PubMed]
- Martínez, V.G.; Salas, A.A.; Ballestín, S.S. Vitamin Supplementation and Dementia: A Systematic Review. Nutrients 2022, 14, 1033. [Google Scholar] [CrossRef]
- McCaddon, A. Vitamin B12 in Neurology and Ageing; Clinical and Genetic Aspects. Biochimie 2013, 95, 1066–1076. [Google Scholar] [CrossRef]
- McGeown, J.P.; Hume, P.A.; Theadom, A.; Quarrie, K.L.; Borotkanics, R. Nutritional Interventions to Improve Neurophysiological Impairments Following Traumatic Brain Injury: A Systematic Review. J. Neurosci. Res. 2021, 99, 573–603. [Google Scholar] [CrossRef]
- Muscaritoli, M. The Impact of Nutrients on Mental Health and Well-Being: Insights From the Literature. Front. Nutr. 2021, 8, 656290. [Google Scholar] [CrossRef]
- Hutton, C.P.; Déry, N.; Rosa, E.; Lemon, J.A.; Rollo, C.D.; Boreham, D.R.; Fahnestock, M.; de Catanzaro, D.; Wojtowicz, J.M.; Becker, S. Synergistic Effects of Diet and Exercise on Hippocampal Function in Chronically Stressed Mice. Neuroscience 2015, 308, 180–193. [Google Scholar] [CrossRef]
- Aguiló, A.; Tauler, P.; Sureda, A.; Cases, N.; Tur, J.; Pons, A. Antioxidant Diet Supplementation Enhances Aerobic Performance in Amateur Sportsmen. J. Sports Sci. 2007, 25, 1203–1210. [Google Scholar] [CrossRef]
- Mabrey, G.; Koozehchian, M.S.; Newton, A.T.; Naderi, A.; Forbes, S.C.; Haddad, M. The Effect of Creatine Nitrate and Caffeine Individually or Combined on Exercise Performance and Cognitive Function: A Randomized, Crossover, Double-Blind, Placebo-Controlled Trial. Nutrients 2024, 16, 766. [Google Scholar] [CrossRef]
- Wang, J.; Rang, Y.; Liu, C. Effects of Caloric Restriction and Intermittent Fasting and Their Combined Exercise on Cognitive Functioning: A Review. Curr. Nutr. Rep. 2024, 13, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Veronese, N.; Vernuccio, L.; Catanese, G.; Inzerillo, F.; Salemi, G.; Barbagallo, M. Nutrition, Physical Activity, and Other Lifestyle Factors in the Prevention of Cognitive Decline and Dementia. Nutrients 2021, 13, 4080. [Google Scholar] [CrossRef] [PubMed]
- Pastor, R.; Tur, J.A. Response to Exercise in Older Adults Who Take Supplements of Antioxidants and/or Omega-3 Polyunsaturated Fatty Acids: A Systematic Review. Biochem. Pharmacol. 2020, 173, 113649. [Google Scholar] [CrossRef] [PubMed]
- Köbe, T.; Witte, A.V.; Schnelle, A.; Lesemann, A.; Fabian, S.; Tesky, V.A.; Pantel, J.; Flöel, A. Combined Omega-3 Fatty Acids, Aerobic Exercise and Cognitive Stimulation Prevents Decline in Gray Matter Volume of the Frontal, Parietal and Cingulate Cortex in Patients with Mild Cognitive Impairment. Neuroimage 2016, 131, 226–238. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Vellas, B.; Rizzoli, R.; Kressig, R.W.; Da Silva, J.A.P.; Blauth, M.; Felson, D.T.; McCloskey, E.V.; Watzl, B.; Hofbauer, L.C.; et al. Effect of Vitamin D Supplementation, Omega-3 Fatty Acid Supplementation, or a Strength-Training Exercise Program on Clinical Outcomes in Older Adults: The DO-HEALTH Randomized Clinical Trial. JAMA J. Am. Med. Assoc. 2020, 324, 1855–1868. [Google Scholar] [CrossRef]
- Mundell, N.L.; Owen, P.J.; Dalla Via, J.; MacPherson, H.; Daly, R.; Livingston, P.M.; Rantalainen, T.; Foulkes, S.; Millar, J.; Murphy, D.G.; et al. Effects of a Multicomponent Resistance-Based Exercise Program with Protein, Vitamin D and Calcium Supplementation on Cognition in Men with Prostate Cancer Treated with ADT: Secondary Analysis of a 12-Month Randomised Controlled Trial. BMJ Open 2022, 12, e060189. [Google Scholar] [CrossRef]
- Formica, M.B.; Gianoudis, J.; Nowson, C.A.; O’Connell, S.L.; Milte, C.; Ellis, K.A.; Daly, R.M. Effect of Lean Red Meat Combined with a Multicomponent Exercise Program on Muscle and Cognitive Function in Older Adults: A 6-Month Randomized Controlled Trial. Am. J. Clin. Nutr. 2020, 112, 113–128. [Google Scholar] [CrossRef]
- Mischley, L.K.; Lau, R.C.; Bennett, R.D. Role of Diet and Nutritional Supplements in Parkinson’s Disease Progression. Oxid. Med. Cell. Longev. 2017, 2017, 6405278. [Google Scholar] [CrossRef]
- Aragon, A.A.; Schoenfeld, B.J. Nutrient Timing Revisited: Is There a Post-Exercise Anabolic Window? J. Int. Soc. Sports Nutr. 2013, 10, 5. [Google Scholar] [CrossRef]
- Takahashi, Y.; Matsunaga, Y.; Banjo, M.; Takahashi, K.; Sato, Y.; Seike, K.; Nakano, S.; Hatta, H. Effects of Nutrient Intake Timing on Post-Exercise Glycogen Accumulation and Its Related Signaling Pathways in Mouse Skeletal Muscle. Nutrients 2019, 11, 2555. [Google Scholar] [CrossRef]
- Ahalli, S.; Fort, E.; Bridai, Y.; Baborier, N.; Charbotel, B. Mental Health and Working Constraints of First-Year PhD Students in Health and Science in a French University: A Cross-Sectional Study in the Context of Occupational Health Monitoring. BMJ Open 2022, 12, e057679. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, Y.; Jiang, N.; Yetisen, A.K. Biosensors for Psychiatric Biomarkers in Mental Health Monitoring. Biosens. Bioelectron. 2024, 256, 116242. [Google Scholar] [CrossRef] [PubMed]
- Bonato, P. Wearable Sensors and Systems. IEEE Eng. Med. Biol. Mag. 2010, 29, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Masoumian Hosseini, M.; Masoumian Hosseini, S.T.; Qayumi, K.; Hosseinzadeh, S.; Sajadi Tabar, S.S. Smartwatches in Healthcare Medicine: Assistance and Monitoring; a Scoping Review. BMC Med. Inform. Decis. Mak. 2023, 23, 248. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Chai, K. Wearable Sensing Systems for Monitoring Mental Health. Sensors 2022, 22, 994. [Google Scholar] [CrossRef]
- Hickey, B.A.; Chalmers, T.; Newton, P.; Lin, C.T.; Sibbritt, D.; McLachlan, C.S.; Clifton-Bligh, R.; Morley, J.; Lal, S. Smart Devices and Wearable Technologies to Detect and Monitor Mental Health Conditions and Stress: A Systematic Review. Sensors 2021, 21, 3461. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.S.; Mahmood, M.; Kwon, S.; Epps, F.; Rim, Y.S.; Yeo, W.H. Wireless, Continuous Monitoring of Daily Stress and Management Practice via Soft Bioelectronics. Biosens. Bioelectron. 2021, 173, 112764. [Google Scholar] [CrossRef]
- Alshurafa, N.; Sideris, C.; Pourhomayoun, M.; Kalantarian, H.; Sarrafzadeh, M.; Eastwood, J.A. Remote Health Monitoring Outcome Success Prediction Using Baseline and First Month Intervention Data. IEEE J. Biomed. Health Inform. 2017, 21, 507–514. [Google Scholar] [CrossRef]
- Aranki, D.; Kurillo, G.; Yan, P.; Liebovitz, D.M.; Bajcsy, R. Real-Time Tele-Monitoring of Patients with Chronic Heart-Failure Using a Smartphone: Lessons Learned. IEEE Trans. Affect. Comput. 2016, 7, 206–219. [Google Scholar] [CrossRef]
- Dewa, L.H.; Lavelle, M.; Pickles, K.; Kalorkoti, C.; Jaques, J.; Pappa, S.; Aylin, P. Young Adults’ Perceptions of Using Wearables, Social Media and Other Technologies to Detect Worsening Mental Health: A Qualitative Study. PLoS ONE 2019, 14, e0222655. [Google Scholar] [CrossRef]
- Osmani, V. Smartphones in Mental Health: Detecting Depressive and Manic Episodes. IEEE Pervasive Comput. 2015, 14, 10–13. [Google Scholar] [CrossRef]
- Behar, J.A.; Oster, J.; De Vos, M.; Clifford, G.D. Wearables and MHealth in Mental Health and Neurological Disorders. Physiol. Meas. 2019, 40, 070401. [Google Scholar] [CrossRef] [PubMed]
- Reinertsen, E.; Osipov, M.; Liu, C.; Kane, J.M.; Petrides, G.; Clifford, G.D. Continuous Assessment of Schizophrenia Using Heart Rate and Accelerometer Data. Physiol. Meas. 2017, 38, 1456–1471. [Google Scholar] [CrossRef]
- Greco, A.; Benvenuti, S.M.; Gentili, C.; Palomba, D.; Scilingo, E.P.; Valenza, G. Assessment of Linear and Nonlinear/Complex Heartbeat Dynamics in Subclinical Depression (Dysphoria). Physiol. Meas. 2018, 39, 034004. [Google Scholar] [CrossRef]
- Prince, J.; Arora, S.; De Vos, M. Big Data in Parkinson’s Disease: Using Smartphones to Remotely Detect Longitudinal Disease Phenotypes. Physiol. Meas. 2018, 39, 044005. [Google Scholar] [CrossRef]
- Gomes, N.; Pato, M.; Lourenço, A.R.; Datia, N. A Survey on Wearable Sensors for Mental Health Monitoring. Sensors 2023, 23, 1330. [Google Scholar] [CrossRef]
- Robinson, T.; Condell, J.; Ramsey, E.; Leavey, G. Self-Management of Subclinical Common Mental Health Disorders (Anxiety, Depression and Sleep Disorders) Using Wearable Devices. Int. J. Environ. Res. Public Health 2023, 20, 2636. [Google Scholar] [CrossRef]
- Owens, A.P. The Role of Heart Rate Variability in the Future of Remote Digital Biomarkers. Front. Neurosci. 2020, 14, 582145. [Google Scholar] [CrossRef]
- Kaushik, A.; Vasudev, A.; Arya, S.K.; Pasha, S.K.; Bhansali, S. Recent Advances in Cortisol Sensing Technologies for Point-of-Care Application. Biosens. Bioelectron. 2014, 53, 499–512. [Google Scholar] [CrossRef]
- Abd-Alrazaq, A.; AlSaad, R.; Aziz, S.; Ahmed, A.; Denecke, K.; Househ, M.; Farooq, F.; Sheikh, J. Wearable Artificial Intelligence for Anxiety and Depression: Scoping Review. J. Med Internet Res. 2023, 25, e42672. [Google Scholar] [CrossRef]
- Jacobson, N.C.; Feng, B. Digital Phenotyping of Generalized Anxiety Disorder: Using Artificial Intelligence to Accurately Predict Symptom Severity Using Wearable Sensors in Daily Life. Transl. Psychiatry 2022, 12, 336. [Google Scholar] [CrossRef] [PubMed]
- Meneses do Rêgo, A.C.; Araújo-Filho, I. Leveraging Artificial Intelligence to Enhance the Quality of Life for Patients with Autism Spectrum Disorder: A Comprehensive Review. Eur. J. Clin. Med. 2024, 5, 28–38. [Google Scholar] [CrossRef]
- Mone, V.; Shakhlo, F. Health Data on the Go: Navigating Privacy Concerns with Wearable Technologies. Leg. Inf. Manag. 2023, 23, 179–188. [Google Scholar] [CrossRef]
- Suryawanshi, N.S. Predicting Mental Health Outcomes Using Wearable Device Data and Machine Learning. Int. J. Innov. Sci. Res. Technol. 2024, 6, 1334–1341. [Google Scholar] [CrossRef]
- Canali, S.; Schiaffonati, V.; Aliverti, A. Challenges and Recommendations for Wearable Devices in Digital Health: Data Quality, Interoperability, Health Equity, Fairness. PLoS Digit. Health 2022, 1, e0000104. [Google Scholar] [CrossRef]
- Novikov, V.N.; Badaeva, A.V.; Danilov, A.B.; Vorobyeva, Y.D. Precision Neuronutrition: Personalized Approaches for Optimizing Brain Health. Biol. Life Sci. Forum 2023, 29, 20. [Google Scholar] [CrossRef]
- Bhatia, D.; Paul, S.; Acharjee, T.; Ramachairy, S.S. Biosensors and Their Widespread Impact on Human Health. Sens. Int. 2024, 5, 100257. [Google Scholar] [CrossRef]
- Mishra, A.; Singh, P.K.; Chauhan, N.; Roy, S.; Tiwari, A.; Gupta, S.; Tiwari, A.; Patra, S.; Das, T.R.; Mishra, P.; et al. Emergence of Integrated Biosensing-Enabled Digital Healthcare Devices. Sens. Diagn. 2024, 3, 718–744. [Google Scholar] [CrossRef]
- Hernández-Mustieles, M.A.; Lima-Carmona, Y.E.; Pacheco-Ramírez, M.A.; Mendoza-Armenta, A.A.; Romero-Gómez, J.E.; Cruz-Gómez, C.F.; Rodríguez-Alvarado, D.C.; Arceo, A.; Cruz-Garza, J.G.; Ramírez-Moreno, M.A.; et al. Wearable Biosensor Technology in Education: A Systematic Review. Sensors 2024, 24, 2437. [Google Scholar] [CrossRef]
- Olyanasab, A.; Annabestani, M. Leveraging Machine Learning for Personalized Wearable Biomedical Devices: A Review. J. Pers. Med. 2024, 14, 203. [Google Scholar] [CrossRef]
- Kim, D.; Min, J.; Ko, S.H. Recent Developments and Future Directions of Wearable Skin Biosignal Sensors. Adv. Sens. Res. 2024, 3, 2300118. [Google Scholar] [CrossRef]
- Sidhu, J.S.; Jamwal, A.; Mehta, D.; Gautam, A. Integration of IoT and AI in Bioengineering of Natural Materials. In Calcium-Based Materials; CRC Press: Boca Raton, FL, USA, 2024; pp. 168–188. [Google Scholar] [CrossRef]
- Mahato, K. Implantable Biosensors for Personalized Healthcare. In Biosensors for Personalized Healthcare; Springer Nature: Singapore, 2024; pp. 375–392. [Google Scholar] [CrossRef]
- Meng, Z.; Zhang, Y.; Yang, L.; Yuan, F.; Wang, J.; Chen, J.; Liu, J.; Wang, G.; Zang, G. Application of Advanced Biosensors in Nervous System Diseases. Interdiscip. Med. 2024, 2, e20240024. [Google Scholar] [CrossRef]
- Kussmann, M.; Fay, L.B. Nutrigenomics and Personalized Nutrition: Science and Concept. Per. Med. 2008, 5, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Ebright, B.; Duro, M.V.; Chen, K.; Louie, S.; Yassine, H.N. Effects of APOE4 on Omega-3 Brain Metabolism across the Lifespan. Trends Endocrinol. Metab. 2024, 35, 745–757. [Google Scholar] [CrossRef]
- Lutz, M.; Moya, P.R.; Gallorio, S.; Ríos, U.; Arancibia, M. Effects of Dietary Fiber, Phenolic Compounds, and Fatty Acids on Mental Health: Possible Interactions with Genetic and Epigenetic Aspects. Nutrients 2024, 16, 2578. [Google Scholar] [CrossRef]
- Kaput, J.; Monteiro, J.P. Human Nutrition Research in the Data Era: Results of 11 Reports on the Effects of a Multiple-Micronutrient-Intervention Study. Nutrients 2024, 16, 188. [Google Scholar] [CrossRef]
- Gáll, Z.; Székely, O. Role of Vitamin D in Cognitive Dysfunction: New Molecular Concepts and Discrepancies between Animal and Human Findings. Nutrients 2021, 13, 3672. [Google Scholar] [CrossRef]
- Aquili, L. The Role of Tryptophan and Tyrosine in Executive Function and Reward Processing. Int. J. Tryptophan Res. 2020, 13, 1178646920964825. [Google Scholar] [CrossRef]
- Suganya, K.; Koo, B.-S. Gut–Brain Axis: Role of Gut Microbiota on Neurological Disorders and How Probiotics/Prebiotics Beneficially Modulate Microbial and Immune Pathways to Improve Brain Functions. Int. J. Mol. Sci. 2020, 21, 7551. [Google Scholar] [CrossRef]
- Chudzik, A.; Orzyłowska, A.; Rola, R.; Stanisz, G.J. Probiotics, Prebiotics and Postbiotics on Mitigation of Depression Symptoms: Modulation of the Brain–Gut–Microbiome Axis. Biomolecules 2021, 11, 1000. [Google Scholar] [CrossRef]
- Lobo, F.; Haase, J.; Brandhorst, S. The Effects of Dietary Interventions on Brain Aging and Neurological Diseases. Nutrients 2022, 14, 5086. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.E.; Ortega-Santos, C.P.; Whisner, C.M.; Klein-Seetharaman, J.; Jasbi, P. Navigating Challenges and Opportunities in Multi-Omics Integration for Personalized Healthcare. Biomedicines 2024, 12, 1496. [Google Scholar] [CrossRef]
- Brancato, V.; Esposito, G.; Coppola, L.; Cavaliere, C.; Mirabelli, P.; Scapicchio, C.; Borgheresi, R.; Neri, E.; Salvatore, M.; Aiello, M. Standardizing Digital Biobanks: Integrating Imaging, Genomic, and Clinical Data for Precision Medicine. J. Transl. Med. 2024, 22, 136. [Google Scholar] [CrossRef]
- Chbeir, S.; Carrión, V. Resilience by Design: How Nature, Nurture, Environment, and Microbiome Mitigate Stress and Allostatic Load. World J. Psychiatry 2023, 13, 144–159. [Google Scholar] [CrossRef]
- Hantsoo, L.; Jagodnik, K.M.; Novick, A.M.; Baweja, R.; di Scalea, T.L.; Ozerdem, A.; McGlade, E.C.; Simeonova, D.I.; Dekel, S.; Kornfield, S.L.; et al. The Role of the Hypothalamic-Pituitary-Adrenal Axis in Depression across the Female Reproductive Lifecycle: Current Knowledge and Future Directions. Front. Endocrinol. 2023, 14, 1295261. [Google Scholar] [CrossRef]
- Lapp, H.E.; Ahmed, S.; Moore, C.L.; Hunter, R.G. Toxic Stress History and Hypothalamic-Pituitary-Adrenal Axis Function in a Social Stress Task: Genetic and Epigenetic Factors. Neurotoxicol. Teratol. 2019, 71, 41–49. [Google Scholar] [CrossRef]
- Nowacka-Chmielewska, M.; Grabowska, K.; Grabowski, M.; Meybohm, P.; Burek, M.; Małecki, A. Running from Stress: Neurobiological Mechanisms of Exercise-Induced Stress Resilience. Int. J. Mol. Sci. 2022, 23, 13348. [Google Scholar] [CrossRef]
- Ignácio, Z.M.; da Silva, R.S.; Plissari, M.E.; Quevedo, J.; Réus, G.Z. Physical Exercise and Neuroinflammation in Major Depressive Disorder. Mol. Neurobiol. 2019, 56, 8323–8335. [Google Scholar] [CrossRef]
- Numakawa, T.; Kajihara, R. Involvement of Brain-Derived Neurotrophic Factor Signaling in the Pathogenesis of Stress-Related Brain Diseases. Front. Mol. Neurosci. 2023, 16, 1247422. [Google Scholar] [CrossRef]
- Zühtü Birinci, Y. The Potential Role of Exercise-Induced Neurotrophic Factors for Mental Health. In Mental Health—Preventive Strategies; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar] [CrossRef]
- Molina-Hidalgo, C.; Stillman, C.M.; Collins, A.M.; Velazquez-Diaz, D.; Ripperger, H.S.; Drake, J.A.; Gianaros, P.J.; Marsland, A.L.; Erickson, K.I. Changes in Stress Pathways as a Possible Mechanism of Aerobic Exercise Training on Brain Health: A Scoping Review of Existing Studies. Front. Physiol. 2023, 14, 1273981. [Google Scholar] [CrossRef]
- Fiocco, A.J.; D’Amico, D.; De Beaumont, L.; Poirier, J.; Lupien, S. Association between BDNF Polymorphism and Hypothalamic-Pituitary-Adrenal Activity in Later Adulthood. Gerontology 2020, 66, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, T.; Nakano, D.; Hashida, R.; Sano, T.; Kawaguchi, M.; Amano, K.; Kawaguchi, T. The Inter-Organ Crosstalk Reveals an Inevitable Link between MAFLD and Extrahepatic Diseases. Nutrients 2023, 15, 1123. [Google Scholar] [CrossRef] [PubMed]
- Estrada, J.A.; Contreras, I. Nutritional Modulation of Immune and Central Nervous System Homeostasis: The Role of Diet in Development of Neuroinflammation and Neurological Disease. Nutrients 2019, 11, 1076. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Ospina, C.A.; Nava-Mesa, M.O. B Vitamins in the Nervous System: Current Knowledge of the Biochemical Modes of Action and Synergies of Thiamine, Pyridoxine, and Cobalamin. CNS Neurosci. Ther. 2020, 26, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Holton, K. The Potential Role of Dietary Intervention for the Treatment of Neuroinflammation. In Translational Neuroimmunology: Neuroinflammation; Elsevier: Amsterdam, The Netherlands, 2023; Volume 7. [Google Scholar] [CrossRef]
- Benmelouka, A.Y.; Shah, M.A.; Saleem, U.; Elshanbary, A.A.; Meshref, M.; Shah, G.M.; Alsharif, I.; Althobaiti, N.A.; Alhasani, R.H. Therapeutic Role of Nutraceuticals in the Management of Brain Disorders. In The Role of Phytonutrients in Metabolic Disorders; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar] [CrossRef]
- Donati Zeppa, S.; Ferrini, F.; Agostini, D.; Amatori, S.; Barbieri, E.; Piccoli, G.; Sestili, P.; Stocchi, V. Nutraceuticals and Physical Activity as Antidepressants: The Central Role of the Gut Microbiota. Antioxidants 2022, 11, 236. [Google Scholar] [CrossRef]
- Liaqat, H.; Parveen, A.; Kim, S.Y. Antidepressive Effect of Natural Products and Their Derivatives Targeting BDNF-TrkB in Gut–Brain Axis. Int. J. Mol. Sci. 2022, 23, 14968. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fülling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short Chain Fatty Acids: Microbial Metabolites for Gut-Brain Axis Signalling. Mol. Cell Endocrinol. 2022, 546, 111572. [Google Scholar] [CrossRef]
- Gubert, C.; Kong, G.; Renoir, T.; Hannan, A.J. Exercise, Diet and Stress as Modulators of Gut Microbiota: Implications for Neurodegenerative Diseases. Neurobiol. Dis. 2020, 134, 104621. [Google Scholar] [CrossRef]
- Belbin, O.; Lehmann, S.; Sabidó, E.; Hirtz, C. Editorial: Proteomics as a Tool for Biomarker and Drug Target Discovery: Improving the Diagnosis and Treatment of Neurodegenerative Diseases. Front. Aging Neurosci. 2020, 12, 232. [Google Scholar] [CrossRef]
- Akselrod, S.; Collins, T.E.; Hoe, C.; Seyer, J.; Tulenko, K.; Ortenzi, F.; Berlina, D.; Sobel, H. Building an Interdisciplinary Workforce for Prevention and Control of Non-Communicable Diseases: The Role of e-Learning. BMJ 2023, 381, e071071. [Google Scholar] [CrossRef]
- Koníčková, D.; Menšíková, K.; Tučková, L.; Hényková, E.; Strnad, M.; Friedecký, D.; Stejskal, D.; Matěj, R.; Kaňovský, P. Biomarkers of Neurodegenerative Diseases: Biology, Taxonomy, Clinical Relevance, and Current Research Status. Biomedicines 2022, 10, 1760. [Google Scholar] [CrossRef] [PubMed]
- Park, S.A.; Jang, Y.J.; Kim, M.K.; Lee, S.M.; Moon, S.Y. Promising Blood Biomarkers for Clinical Use in Alzheimer’s Disease: A Focused Update. J. Clin. Neurol. 2022, 18, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Drzezga, A.; Barthel, H.; Minoshima, S.; Sabri, O. Potential Clinical Applications of PET/MR Imaging in Neurodegenerative Diseases. J. Nucl. Med. 2014, 55 (Suppl. S2), 47S–55S. [Google Scholar] [CrossRef] [PubMed]
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A Review of the Common Neurodegenerative Disorders: Current Therapeutic Approaches and the Potential Role of Nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef]
- Parlak, O.; Keene, S.T.; Marais, A.; Curto, V.F.; Salleo, A. Molecularly Selective Nanoporous Membrane-Based Wearable Organic Electrochemical Device for Noninvasive Cortisol Sensing. Sci. Adv. 2018, 4, eaar2904. [Google Scholar] [CrossRef]
- Watson, C.N.; Belli, A.; Di Pietro, V. Small Non-Coding RNAs: New Class of Biomarkers and Potential Therapeutic Targets in Neurodegenerative Disease. Front. Genet. 2019, 10, 364. [Google Scholar] [CrossRef]

| Key Aspect | Cellular and Molecular Mechanisms | Synaptic and Structural Plasticity | Role of Nutrition (Nutrients, Dietary Patterns) | Role of Exercise (Type, Intensity, Frequency) | Evidence (Preclinical, Clinical, Imaging) |
|---|---|---|---|---|---|
| Neurotrophic Factors (BDNF, NGF) | Molecular: Exercise and omega-3 fatty acids, polyphenols (resveratrol, catechins), and flavonoids enhance BDNF, IGF-1, and NGF expression. Mechanisms involve the AMPK–PGC-1α–FNDC5 pathway leading to BDNF upregulation. BDNF signaling via TrkB activates downstream MAPK/ERK and PI3K/Akt cascades, enhancing synaptic protein synthesis [6,56]. | Synaptic Plasticity: Increased BDNF levels facilitate long-term potentiation, synaptogenesis, dendritic spine density, and spine maturation in hippocampal and cortical circuits. This results in improved cognitive functions (learning, memory) [57,58]. | Nutritional Interventions: Diets rich in long-chain omega-3 (DHA/EPA), berries, green tea, and other polyphenol sources upregulate BDNF. Caloric restriction and intermittent fasting can amplify neurotrophic signaling by promoting metabolic hormesis and mitochondrial efficiency [59]. | Exercise Modalities: Aerobic training (moderate-to-vigorous intensity) and resistance training enhance circulating BDNF and facilitate its transport across the blood–brain barrier. High-intensity interval training (HIIT) also increases BDNF acutely. Combined exercise plus enriched diets synergistically boost BDNF expression [57]. | Evidence: Rodent models show robust hippocampal BDNF increases correlating with enhanced long-term potentiation and spatial memory. Human RCTs demonstrate improved cognitive performance and hippocampal volume after aerobic interventions. Neuroimaging (MRI) shows increased gray matter volume in the hippocampus in older adults practicing regular exercise and healthy diets [56]. |
| Metabolic and Mitochondrial Efficiency | Molecular: Exercise enhances mitochondrial biogenesis (via PGC-1α, NRF1, TFAM) and improves bioenergetics. Nutrients with antioxidant and anti-inflammatory properties reduce ROS and maintain mitochondrial integrity. Ketone bodies and certain dietary patterns (Mediterranean, plant-based) support efficient ATP generation and mitigate oxidative stress [60]. | Synaptic and Structural Impact: Optimized mitochondrial function sustains synaptic transmission, buffering metabolic stress, and thereby stabilizing synaptic connectivity and network efficiency. Enhanced mitochondrial density supports synaptic remodeling and plasticity [61]. | Nutritional Patterns: Mediterranean-type diets, rich in monounsaturated and polyunsaturated fats, whole grains, legumes, fruits, and vegetables, enhance metabolic flexibility and reduce systemic inflammation. Certain micronutrients (vitamin E, polyphenols) and intermittent fasting regimens promote mitochondrial health and neuroplasticity [62]. | Exercise Types: Regular aerobic exercise increases hippocampal and cortical mitochondrial density and capacity for oxidative phosphorylation. Resistance training improves insulin sensitivity and reduces metabolic dysfunction, indirectly supporting neuronal energetics [63]. | Evidence: Animal studies link improved mitochondrial function with robust synaptic resilience and delayed neurodegenerative processes. Human imaging studies (fMRI, MRS) suggest enhanced cerebral blood flow and metabolic efficiency in exercising individuals, correlating with better cognitive performance [64]. |
| Epigenetic Regulation (DNA Methylation, Histone Modifications, miRNAs) | Molecular: Dietary polyphenols (resveratrol, curcumin), B vitamins, and exercise modulate epigenetic regulators such as SIRT1, HDACs, DNMTs, and miRNAs. Exercise-induced changes in histone acetylation and DNA methylation patterns favor genes involved in synaptic plasticity (BDNF, synapsin I, CREB) [65]. | Synaptic Plasticity: Epigenetically driven gene expression shifts promote synaptogenesis, dendritic remodeling, and long-term stabilization of synaptic connections. Enhances adaptive responses to cognitive challenges and stressors [66]. | Nutritional Factors: Nutraceuticals and diets rich in polyphenols, folate, and choline influence DNA methylation and histone modification, bolstering the transcription of plasticity-related genes. Omega-3s also modulate miRNA profiles linked to neural plasticity [67]. | Exercise Parameters: Chronic aerobic exercise modifies epigenetic markers that regulate the expression of neurotrophic and synaptic genes. Resistance training may alter histone acetylation patterns, further enhancing neuron structural integrity [68]. | Evidence: Animal models show that exercise-induced epigenetic modifications improve memory persistence. Human studies have associated physical activity with beneficial epigenetic signatures (reduced hypermethylation of BDNF promoter) [69]. |
| Adult Hippocampal Neurogenesis | Molecular/Cellular: Exercise increases levels of VEGF, IGF-1, and BDNF, stimulating proliferation and differentiation of neural progenitor cells in the dentate gyrus. Nutrients like flavonoids and omega-3s enhance survival and maturation of newborn neurons [70]. | Structural Impact: Enhanced neurogenesis leads to greater hippocampal volume, improved pattern separation, spatial navigation, and memory consolidation. Increases synaptic integration of new neurons into functional circuits [71]. | Dietary Enhancers: Diets rich in cocoa flavanols, green tea catechins, blueberries, and DHA have been linked to increased neurogenesis. Ketogenic diets and intermittent fasting strategies may also support regenerative capacity by modulating insulin and growth factor pathways [72]. | Exercise Interventions: Sustained aerobic exercise (running, cycling) robustly enhances neurogenesis. HIIT protocols can also boost neurogenesis, though more research is needed. Combined nutritional and exercise interventions can synergistically amplify hippocampal neurogenesis [73]. | Evidence: Rodent studies consistently show increased dentate gyrus neurogenesis after exercise and flavonoid supplementation. Human imaging (MRI) has correlated higher fitness with increased hippocampal volume and memory performance in older adults [74]. |
| Neurotransmitter System Modulation | Molecular: Exercise and balanced nutrition modulate dopaminergic, serotonergic, glutamatergic, and GABAergic systems. Adequate iron, B vitamins, and amino acids are essential for optimal neurotransmitter synthesis. Exercise may increase serotonin availability and receptor sensitivity, and modulate dopamine release in reward circuits [75]. | Synaptic Balancing: Improved neurotransmitter homeostasis refines excitatory–inhibitory balance, enhancing signal-to-noise ratio in neuronal processing. This leads to better mood regulation, attention, and executive control [76]. | Nutrient Contributions: Iron, folate, vitamin B12, and amino acids (tryptophan, tyrosine) support neurotransmitter synthesis and receptor function. Polyphenol-rich diets can modulate GABA and glutamate signaling, improving cognitive flexibility and emotional stability [77]. | Exercise Modalities: Aerobic and resistance training can elevate serotonin and dopamine levels, improve receptor sensitivity, and modulate hippocampal and cortical glutamatergic signaling. The frequency and intensity of exercise influence the magnitude of these effects [64]. | Evidence: Preclinical models reveal exercise-induced increases in dopamine turnover and serotonin release. Clinical studies link regular physical activity and quality diets to reduced depressive symptoms and enhanced cognitive–emotional integration [78]. |
| Inflammation and Oxidative Stress Reduction | Molecular: Physical exercise reduces proinflammatory cytokines (TNF-α, IL-6) and enhances antioxidant enzyme activities (SOD, CAT). Nutrients rich in antioxidants (vitamin C, E, polyphenols) and anti-inflammatory compounds (curcumin, n-3 fatty acids) lower neuroinflammation and oxidative stress, preserving neuronal integrity [75]. | Structural Stability: Reduced inflammation and oxidative damage maintain synaptic integrity, prevent neuronal atrophy, and support dendritic complexity. This stable environment fosters robust adaptive plasticity and decreases vulnerability to neurodegeneration [76]. | Anti-inflammatory Diets: Mediterranean, DASH, and Nordic diets, as well as specific compounds (alpha-lipoic acid, sulforaphane), attenuate systemic and neuroinflammation. Lower systemic inflammation correlates with improved cognitive trajectories and reduced risk of dementia [77]. | Exercise Approach: Moderate, regular aerobic exercise exerts anti-inflammatory and antioxidative effects, while high-intensity exercise might transiently increase ROS but subsequently enhance endogenous antioxidant defenses. The long-term net effect is neuroprotective [75]. | Evidence: Animal studies show combined diet–exercise protocols reduce microglial activation and oxidative damage. Clinical trials link exercise plus dietary intervention with lowered inflammatory biomarkers and improved executive functions [77]. |
| Hormonal and Peripheral Factor Regulation | Molecular: Exercise-induced release of irisin, adiponectin, and decreased cortisol levels reshape the metabolic and neurotrophic environment. Nutritional interventions maintaining glycemic control and insulin sensitivity support optimal hormone signaling to the brain [57]. | Synaptic and Network Effects: Balanced hormonal signaling influences synaptic plasticity indirectly by regulating energy availability, neurotrophin circulation, and inflammatory status, thereby optimizing conditions for neuroplastic adaptation [79]. | Diet–Hormone Interplay: Balanced diets stabilizing insulin and leptin signaling prevent metabolic stress on neurons. Protein-rich diets and adequate micronutrients ensure proper hormone synthesis and receptor functionality [57]. | Exercise Synergy: Aerobic and resistance training improve peripheral insulin sensitivity, enhance irisin and adiponectin levels, and reduce cortisol, collectively promoting a more neurotrophic and less catabolic environment [79]. | Evidence: Animal models demonstrate that exercise-driven irisin increases BDNF expression and supports neuroplastic changes. Human interventions show exercise and diet synergy improves metabolic profiles and correlates with better cognitive outcomes [79]. |
| Functional Connectivity and Network Integration | Network-Level: Exercise and nutrient-rich diets improve functional connectivity within key neural networks (default mode, frontoparietal, hippocampo-cortical circuits). Enhanced vascularization (via VEGF and nitric oxide) supports network-level efficiency [80]. | Structural Connectivity: Strengthening of white matter integrity and synaptic pruning leads to more efficient neural communication. Enhanced cerebrovascular perfusion supports neurovascular coupling and network resilience [3]. | Nutritional Quality: Adherence to the Mediterranean diet, or diets with high polyphenolic and healthy fat content, is associated with reduced brain atrophy and improved functional connectivity, delaying cognitive decline [80]. | Exercise Integration: Endurance training, HIIT, and combined aerobic–resistance regimens are linked with improved white matter integrity, resting-state functional connectivity, and reduced brain atrophy in aging populations [3]. | Evidence: Neuroimaging studies (fMRI, DTI) in humans reveal that physically fit individuals with high diet quality display increased hippocampal volume, stronger connectivity in cognition-related networks, and slower age-related cognitive decline [3,80]. |
| Gut-Brain Axis and Metabolites (Novel Aspect) | Molecular/Systems: The gut microbiota, modulated by diet and exercise, produces short-chain fatty acids (SCFAs), vitamins, and neurotransmitter precursors influencing brain function. Exercise alters gut microbiome composition, increasing beneficial bacteria that produce neuroprotective metabolites [81]. | Synaptic/Structural: Improved gut barrier integrity and SCFA availability support synaptic plasticity, reduce neuroinflammation, and enhance BDNF expression. This fosters a more adaptive and resilient neural circuitry [82]. | Dietary Influence: Fiber-rich, plant-based diets and fermented foods promote healthy microbiota that supports neural health. Polyphenols and probiotics can modulate microbial communities, influencing neurotransmitter metabolism and synaptic plasticity [81]. | Exercise Interactions: Endurance training modulates gut flora towards a more anti-inflammatory profile, synergizing with diet to bolster neuroplasticity. Preclinical studies show exercise-induced increases in Lactobacillus and Bifidobacterium linked to improved cognition [82]. | Evidence: Animal research links exercise- and diet-induced microbiome shifts with improved learning and stress resilience. Human studies (observational and intervention-based) correlate better gut flora diversity with enhanced cognitive performance and lower risk of neurological disorders [81,82,83]. |
| Exercise Modality | Stimulus Characteristics | Molecular Pathways (Myokines, Neurotrophins) | Cognitive and Neural Effects |
|---|---|---|---|
| Aerobic (Endurance) Training | Continuous moderate-to-vigorous-intensity, extended-duration sessions (≥30 min) | Robust elevations in BDNF and irisin; enhanced mitochondrial function and blood flow; improved inflammatory profile | Increases hippocampal neurogenesis, improves memory, executive function, and slows age-related cognitive decline |
| High, Intensity Interval Training (HIIT) | Short, intense bouts (30 s–4 min) interspersed with low-intensity recovery | Acute, sometimes pronounced spikes in BDNF; transient metabolic stress; potential rapid induction of beneficial inflammatory and metabolic mediators | May yield immediate improvements in attention, processing speed; long-term effects on sustained cognitive function still under investigation |
| Resistance Training | Repetitive, high-load, low-to-moderate repetition schemes; focuses on muscular strength and hypertrophy | Potential moderate BDNF increases; improved insulin sensitivity, hormonal balance, and anti-inflammatory environment indirectly support neurotrophic signaling | Potential protective effect on cognitive aging; may enhance executive functions over time and support brain volume maintenance, especially in older adults |
| Mind, Body Exercises | Low-to-moderate intensity, emphasis on breathing, balance, proprioception, and mindfulness | May modulate stress hormones, inflammatory markers, and possibly support balanced myokine and neurotrophin levels, though evidence at the molecular level is less extensive | Associated with improved attention, emotional regulation, and memory consolidation; may indirectly foster a neuroprotective environment |
| Combined/Multimodal Training | Integrates multiple stimulus types (endurance + strength) within a weekly program | Potential additive effects on BDNF and irisin; improved metabolic and vascular profiles; enhanced overall systemic homeostasis | May yield comprehensive cognitive gains (memory, executive function, processing speed), improved resilience against neurodegeneration, and synergistic brain benefits |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clemente-Suárez, V.J.; Martín-Rodríguez, A.; Curiel-Regueros, A.; Rubio-Zarapuz, A.; Tornero-Aguilera, J.F. Neuro-Nutrition and Exercise Synergy: Exploring the Bioengineering of Cognitive Enhancement and Mental Health Optimization. Bioengineering 2025, 12, 208. https://doi.org/10.3390/bioengineering12020208
Clemente-Suárez VJ, Martín-Rodríguez A, Curiel-Regueros A, Rubio-Zarapuz A, Tornero-Aguilera JF. Neuro-Nutrition and Exercise Synergy: Exploring the Bioengineering of Cognitive Enhancement and Mental Health Optimization. Bioengineering. 2025; 12(2):208. https://doi.org/10.3390/bioengineering12020208
Chicago/Turabian StyleClemente-Suárez, Vicente Javier, Alexandra Martín-Rodríguez, Agustín Curiel-Regueros, Alejandro Rubio-Zarapuz, and José Francisco Tornero-Aguilera. 2025. "Neuro-Nutrition and Exercise Synergy: Exploring the Bioengineering of Cognitive Enhancement and Mental Health Optimization" Bioengineering 12, no. 2: 208. https://doi.org/10.3390/bioengineering12020208
APA StyleClemente-Suárez, V. J., Martín-Rodríguez, A., Curiel-Regueros, A., Rubio-Zarapuz, A., & Tornero-Aguilera, J. F. (2025). Neuro-Nutrition and Exercise Synergy: Exploring the Bioengineering of Cognitive Enhancement and Mental Health Optimization. Bioengineering, 12(2), 208. https://doi.org/10.3390/bioengineering12020208









