Characterizing Metabolic Shifts in Septic Murine Kidney Tissue Using 2P-FLIM for Early Sepsis Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Handling and Thin Section Preparation
2.2. Measurement Setup
3. Results
3.1. Behavioral Scores
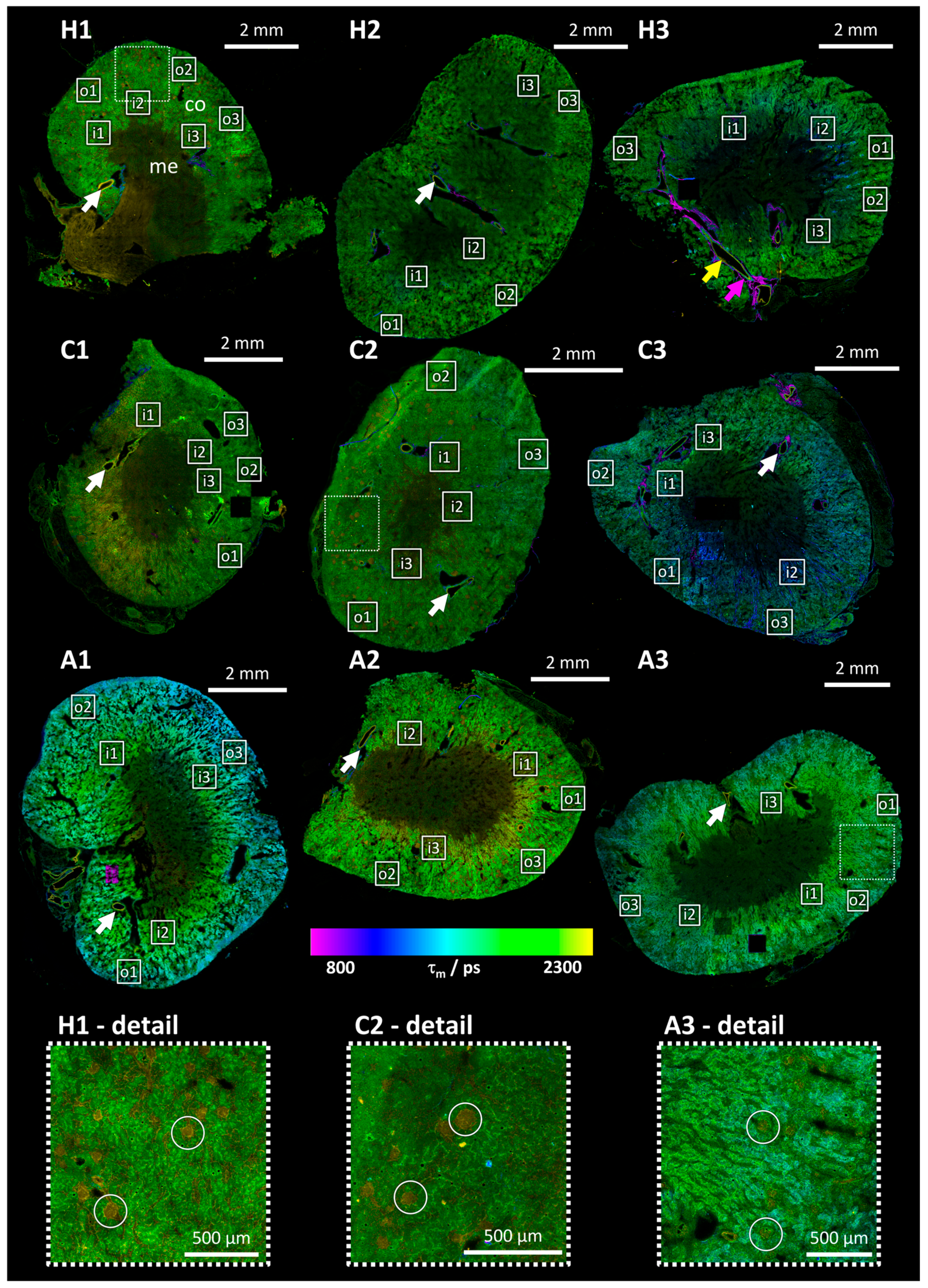
3.2. Mean Photon Arrival Time Overview Images
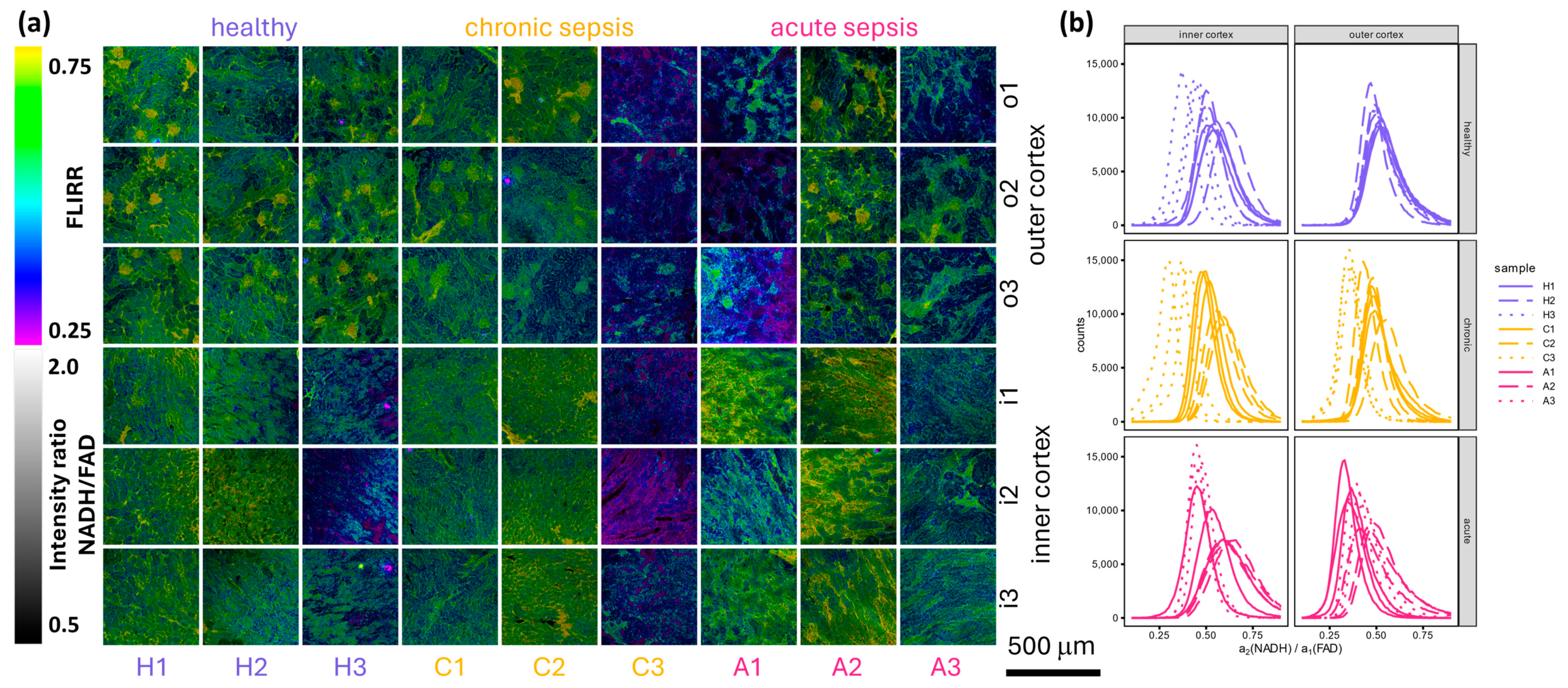
3.3. Spectral Scan
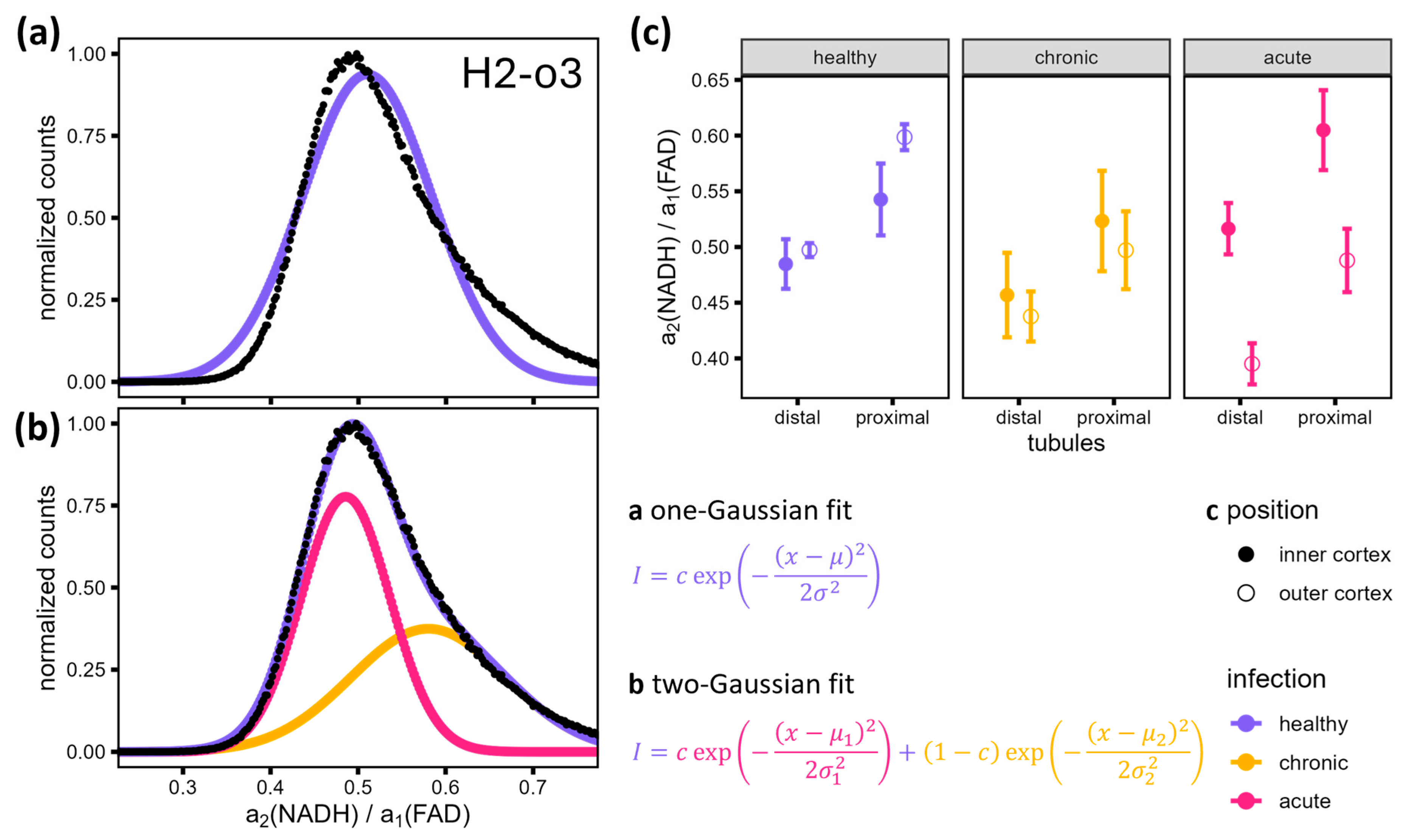
3.4. Detailed FLIM Images
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Samples | Δλ/nm | Ps/mW | θ/° | Accumulations |
|---|---|---|---|---|
| H1. H2, H3, C1, C2, C3 | 0.7 | 27 | 100 | 1000 |
| spectral scan | 0.9 | 27 | 100 | 2000 |
| A1, A2, A3 | 0.7 | 19 | 3 | 1000 |
Appendix B
| Parameter | Min | Max | Start | Min | Max | Start | Min | Max | Start |
|---|---|---|---|---|---|---|---|---|---|
| 0.5 | 1 | 0.95 | 60 | 90 | 65 | 1 | 20 | 5 | |
| FLIRR | 0.5 | 1 | 0.95 | 0.25 | 0.75 | 0.5 | 0.01 | 0.4 | 0.08 |
| min | 0.8 | 0.0 | μ-2σ | 0.3 σ |
| max | 1.3 | 1.0 | μ+2σ | 3.0 σ |
| start | 1.0 | 0.5 | μ (FLIRR) 0.95μ, 1.05μ () | σ |
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, M.; Evans, L.; Levy, M.; Rhodes, A. Sepsis and septic shock. Lancet 2018, 392, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef]
- World Health Organization. Global Report on the Epidemiology and Burden of Sepsis. Available online: https://www.who.int/publications/i/item/9789240010789 (accessed on 5 February 2025).
- Krafft, C.; Popp, J. Opportunities of optical and spectral technologies in intraoperative histopathology. Optica 2023, 10, 214. [Google Scholar] [CrossRef]
- Shaked, N.T.; Boppart, S.A.; Wang, L.V.; Popp, J. Label-free biomedical optical imaging. Nat. Photonics 2023, 17, 1031–1041. [Google Scholar] [CrossRef]
- Kim, J.A.; Wales, D.J.; Yang, G.-Z. Optical spectroscopy for in vivo medical diagnosis—A review of the state of the art and future perspectives. Prog. Biomed. Eng. 2020, 2, 42001. [Google Scholar] [CrossRef]
- Liang, W.; Chen, D.; Guan, H.; Park, H.-C.; Li, K.; Li, A.; Li, M.-J.; Gannot, I.; Li, X. Label-Free Metabolic Imaging In Vivo by Two-Photon Fluorescence Lifetime Endomicroscopy. ACS Photonics 2022, 9, 4017–4029. [Google Scholar] [CrossRef]
- Wang, X.F.; Periasamy, A.; Herman, B.; Coleman, D.M. Fluorescence Lifetime Imaging Microscopy (FLIM): Instrumentation and Applications. Crit. Rev. Anal. Chem. 1992, 23, 369–395. [Google Scholar] [CrossRef]
- Luu, P.; Fraser, S.E.; Schneider, F. More than double the fun with two-photon excitation microscopy. Commun. Biol. 2024, 7, 364. [Google Scholar] [CrossRef]
- Chorvat, D.; Chorvatova, A. Spectrally resolved time-correlated single photon counting: A novel approach for characterization of endogenous fluorescence in isolated cardiac myocytes. Eur. Biophys. J. 2006, 36, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Becker, W.; Bergmann, A.; Braun, L. Metabolic Imaging with the DCS-120 Confocal FLIM System: Simultaneous FLIM of NAD(P)H and FAD; Application Note; Becker & Hickl GmbH: Berlin, Germany, 2018. [Google Scholar]
- Chorvat, D.; Mateasik, A.; Cheng, Y.; Poirier, N.; Miró, J.; Dahdah, N.S.; Chorvatova, A. Rejection of transplanted hearts in patients evaluated by the component analysis of multi-wavelength NAD(P)H fluorescence lifetime spectroscopy. J. Biophotonics 2010, 3, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Chorvat, D.; Chorvatova, A. Multi-wavelength fluorescence lifetime spectroscopy: A new approach to the study of endogenous fluorescence in living cells and tissues. Laser Phys. Lett. 2009, 6, 175–193. [Google Scholar] [CrossRef]
- Schweitzer, D.; Schenke, S.; Hammer, M.; Schweitzer, F.; Jentsch, S.; Birckner, E.; Becker, W.; Bergmann, A. Towards metabolic mapping of the human retina. Microsc. Res. Tech. 2007, 70, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Snyder, G.A.; Kumar, S.; Lewis, G.K.; Ray, K. Two-photon fluorescence lifetime imaging microscopy of NADH metabolism in HIV-1 infected cells and tissues. Front. Immunol. 2023, 14, 1213180. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, M.A.; Sakadžić, S.; Wu, W.; Becker, W.; Kasischke, K.A.; Boas, D.A. In vivo imaging of cerebral energy metabolism with two-photon fluorescence lifetime microscopy of NADH. Biomed. Opt. Express 2013, 4, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Ranawat, H.; Pal, S.; Mazumder, N. Recent trends in two-photon auto-fluorescence lifetime imaging (2P-FLIM) and its biomedical applications. Biomed. Eng. Lett. 2019, 9, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Wallrabe, H.; Periasamy, A. Multiphoton FLIM imaging of NAD(P)H and FAD with one excitation wavelength. J. Biomed. Opt. 2020, 25, 014510. [Google Scholar] [CrossRef] [PubMed]
- Blacker, T.S.; Sewell, M.D.E.; Szabadkai, G.; Duchen, M.R. Metabolic Profiling of Live Cancer Tissues Using NAD(P)H Fluorescence Lifetime Imaging. Methods Mol. Biol. 2019, 1928, 365–387. [Google Scholar] [CrossRef]
- Wallrabe, H.; Svindrych, Z.; Alam, S.R.; Siller, K.H.; Wang, T.; Kashatus, D.; Hu, S.; Periasamy, A. Segmented cell analyses to measure redox states of autofluorescent NAD(P)H, FAD & Trp in cancer cells by FLIM. Sci. Rep. 2018, 8, 79. [Google Scholar] [CrossRef]
- Leppert, J.; Krajewski, J.; Kantelhardt, S.R.; Schlaffer, S.; Petkus, N.; Reusche, E.; Hüttmann, G.; Giese, A. Multiphoton excitation of autofluorescence for microscopy of glioma tissue. Neurosurgery 2006, 58, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Alobaidi, R.; Basu, R.K.; Goldstein, S.L.; Bagshaw, S.M. Sepsis-Associated Acute Kidney Injury. Semin. Nephrol. 2015, 35, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.-T.; Herrmann, K.-H.; Sibgatulin, R.; Nahardani, A.; Krämer, M.; Heitplatz, B.; van Marck, V.; Reuter, S.; Reichenbach, J.R.; Hoerr, V. Perfusion and T2 Relaxation Time as Predictors of Severity and Outcome in Sepsis-Associated Acute Kidney Injury: A Preclinical MRI Study. J. Magn. Reson. Imaging 2023, 58, 1954–1963. [Google Scholar] [CrossRef] [PubMed]
- Gonnert, F.A.; Recknagel, P.; Seidel, M.; Jbeily, N.; Dahlke, K.; Bockmeyer, C.L.; Winning, J.; Lösche, W.; Claus, R.A.; Bauer, M. Characteristics of clinical sepsis reflected in a reliable and reproducible rodent sepsis model. J. Surg. Res. 2011, 170, e123–e134. [Google Scholar] [CrossRef] [PubMed]
- Missbach-Guentner, J.; Pinkert-Leetsch, D.; Dullin, C.; Ufartes, R.; Hornung, D.; Tampe, B.; Zeisberg, M.; Alves, F. 3D virtual histology of murine kidneys –high resolution visualization of pathological alterations by micro computed tomography. Sci. Rep. 2018, 8, 1407. [Google Scholar] [CrossRef] [PubMed]
- Krstic, R.V. Human Microscopic Anatomy: An Atlas for Students of Medicine and Biology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1994. [Google Scholar]
- Lutz, V.; Sattler, M.; Gallinat, S.; Wenck, H.; Poertner, R.; Fischer, F. Impact of collagen crosslinking on the second harmonic generation signal and the fluorescence lifetime of collagen autofluorescence. Ski. Res. Technol. 2012, 18, 168–179. [Google Scholar] [CrossRef]
- Vazquez-Portalatin, N.; Alfonso-Garcia, A.; Liu, J.C.; Marcu, L.; Panitch, A. Physical, Biomechanical, and Optical Characterization of Collagen and Elastin Blend Hydrogels. Ann. Biomed. Eng. 2020, 48, 2924–2935. [Google Scholar] [CrossRef] [PubMed]
- Hato, T.; Winfree, S.; Day, R.; Sandoval, R.M.; Molitoris, B.A.; Yoder, M.C.; Wiggins, R.C.; Zheng, Y.; Dunn, K.W.; Dagher, P.C. Two-Photon Intravital Fluorescence Lifetime Imaging of the Kidney Reveals Cell-Type Specific Metabolic Signatures. J. Am. Soc. Nephrol. 2017, 28, 2420–2430. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.R.; Wallrabe, H.; Svindrych, Z.; Chaudhary, A.K.; Christopher, K.G.; Chandra, D.; Periasamy, A. Investigation of Mitochondrial Metabolic Response to Doxorubicin in Prostate Cancer Cells: An NADH, FAD and Tryptophan FLIM Assay. Sci. Rep. 2017, 7, 10451. [Google Scholar] [CrossRef] [PubMed]
- Cojocel, C.; Maita, K.; Pasino, D.A.; Kuo, C.-H.; Hook, J.B. Metabolic heterogeneity of the proximal and distal kidney tubules. Life Sci. 1983, 33, 855–861. [Google Scholar] [CrossRef]
- Gewin, L.S. Sugar or Fat? Renal Tubular Metabolism Reviewed in Health and Disease. Nutrients 2021, 13, 1580. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Stallons, L.J.; Schnellmann, R.G. Renal cortical hexokinase and pentose phosphate pathway activation through the EGFR/Akt signaling pathway in endotoxin-induced acute kidney injury. Am. J. Physiol. Ren. Physiol. 2014, 307, F435–F444. [Google Scholar] [CrossRef] [PubMed]
- Gómez, H.; Kellum, J.A.; Ronco, C. Metabolic reprogramming and tolerance during sepsis-induced AKI. Nat. Rev. Nephrol. 2017, 13, 143–151. [Google Scholar] [CrossRef]
- Li, Y.; Nourbakhsh, N.; Pham, H.; Tham, R.; Zuckerman, J.E.; Singh, P. Evolution of altered tubular metabolism and mitochondrial function in sepsis-associated acute kidney injury. Am. J. Physiol. Ren. Physiol. 2020, 319, F229–F244. [Google Scholar] [CrossRef]
- Waltz, P.; Carchman, E.; Gomez, H.; Zuckerbraun, B. Sepsis results in an altered renal metabolic and osmolyte profile. J. Surg. Res. 2016, 202, 8–12. [Google Scholar] [CrossRef]
- Han, S.H.; Malaga-Dieguez, L.; Chinga, F.; Kang, H.M.; Tao, J.; Reidy, K.; Susztak, K. Deletion of Lkb1 in Renal Tubular Epithelial Cells Leads to CKD by Altering Metabolism. J. Am. Soc. Nephrol. 2016, 27, 439–453. [Google Scholar] [CrossRef]
- Shane, A.L.; Stoll, B.J. Neonatal sepsis: Progress towards improved outcomes. J. Infect. 2014, 68 (Suppl. S1), S24–S32. [Google Scholar] [CrossRef]
- Kumar, S.; Wang, L.; Fan, J.; Kraft, A.; Bose, M.E.; Tiwari, S.; van Dyke, M.; Haigis, R.; Luo, T.; Ghosh, M.; et al. Detection of 11 common viral and bacterial pathogens causing community-acquired pneumonia or sepsis in asymptomatic patients by using a multiplex reverse transcription-PCR assay with manual (enzyme hybridization) or automated (electronic microarray) detection. J. Clin. Microbiol. 2008, 46, 3063–3072. [Google Scholar] [CrossRef] [PubMed]
- Preibisch, S.; Saalfeld, S.; Tomancak, P. Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics 2009, 25, 1463–1465. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Wallrabe, H.; Siller, K.; Periasamy, A. Optimization of FLIM imaging, fitting and analysis for auto-fluorescent NAD(P)H and FAD in cells and tissues. Methods Appl. Fluoresc. 2020, 8, 24001. [Google Scholar] [CrossRef]
- Elzhov, T.V.; Mullen, K.M.; Spiess, A.-N.; Bolker, B.R.; Mullen, M.K. Interface to the Levenberg-Marquardt Nonlinear Least-Squares Algorithm Found in MINPACK, Plus Support for Bounds. Available online: https://cran.r-project.org/web/packages/minpack.lm/minpack.lm.pdf (accessed on 5 February 2025).

| Technique | Sensitivity to Metabolism | Label-Free? | Resolution | Clinical Applicability |
|---|---|---|---|---|
| 2P-FLIM | High (FLIRR enables quantitative analysis) | Yes | High (subcellular) | Potential for sepsis diagnostics |
| Raman spectroscopy | Medium | Yes | Medium | Tissue characterization |
| Immuno- fluorescence | High | No | High | Marker-specific diagnostics |
| Infection | Sample | Average Score | Time After Infection/h | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 | 12 | 18 | 24 | 30 | 36 | 42 | 48 | 54 | 60 | 66 | 72 | 84 | 96+ | |||
| Healthy * | H1 | 0 | 0 | 0 | ||||||||||||
| H2 | 0 | 0 | 0 | |||||||||||||
| H3 | 0 | 0 | 0 | |||||||||||||
| Chronic Sepsis | C1 | 3.55 | 4 | 7 | 7 | 7 | 5 | 3 | 3 | 2 | 0 | 0 | 1 | 0 | 0 | 0 |
| C2 | 4.36 | 5 | 6 | 7 | 7 | 6 | 4 | 5 | 4 | 1 | 1 | 2 | 0 | 0 | 0 | |
| C3 | 4.44 | 4 | 6 | 7 | 7 | 6 | 3 | 3 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | |
| Acute Sepsis | A1 | 7.50 | 5 | 8 | 8 | 9 | ||||||||||
| A2 | 1.00 | 1 | 1 | 0 | 0 | |||||||||||
| A3 | 7.75 | 6 | 8 | 8 | 9 | |||||||||||
| Fluorophore | λex/nm | λdet/nm | a1/% | τ1/ps | τ2/ps |
|---|---|---|---|---|---|
| FAD | 876 | 550–650 | 66.1 | 400 | 2650 |
| NADH | 711 | 380–450 | 63.8 | 420 | 2170 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greiner, S.; Ebrahimi, M.; Rodewald, M.; Urbanek, A.; Meyer-Zedler, T.; Schmitt, M.; Neugebauer, U.; Popp, J. Characterizing Metabolic Shifts in Septic Murine Kidney Tissue Using 2P-FLIM for Early Sepsis Detection. Bioengineering 2025, 12, 170. https://doi.org/10.3390/bioengineering12020170
Greiner S, Ebrahimi M, Rodewald M, Urbanek A, Meyer-Zedler T, Schmitt M, Neugebauer U, Popp J. Characterizing Metabolic Shifts in Septic Murine Kidney Tissue Using 2P-FLIM for Early Sepsis Detection. Bioengineering. 2025; 12(2):170. https://doi.org/10.3390/bioengineering12020170
Chicago/Turabian StyleGreiner, Stella, Mahyasadat Ebrahimi, Marko Rodewald, Annett Urbanek, Tobias Meyer-Zedler, Michael Schmitt, Ute Neugebauer, and Jürgen Popp. 2025. "Characterizing Metabolic Shifts in Septic Murine Kidney Tissue Using 2P-FLIM for Early Sepsis Detection" Bioengineering 12, no. 2: 170. https://doi.org/10.3390/bioengineering12020170
APA StyleGreiner, S., Ebrahimi, M., Rodewald, M., Urbanek, A., Meyer-Zedler, T., Schmitt, M., Neugebauer, U., & Popp, J. (2025). Characterizing Metabolic Shifts in Septic Murine Kidney Tissue Using 2P-FLIM for Early Sepsis Detection. Bioengineering, 12(2), 170. https://doi.org/10.3390/bioengineering12020170









