Clinical and Technological Evaluation of the Remineralising Effect of Biomimetic Hydroxyapatite in a Population Aged 6 to 18 Years: A Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Participants
- Aged between 6 and 18 years.
- Permanent first molars erupted and completely healthy.
- Patients presenting C1 values (0–12) and C2 values (13–24) of the DIAGNOdent Pen of first molars.
- Patients with DIAGNOdent Pen score on first molars > 25.
- Patients with groove sealings of sealed permanent first molars or composite restorations.
- Permanent first molars with extensive demineralisations (Molar Incisor Hypomineralization, fluorosis, white/brown spots).
2.3. Interventions and Outcomes
- -
- Trial group: Biorepair Total Protective toothpaste containing zinc carbonate-substituted hydroxyapatite crystals [20].
- -
- Control group: Curasept Biosmalto Caries & Erosion toothpaste containing fluorohydroxyapatite and magnesium strontium carbonate hydroxyapatite conjugated with chitosan [13]. This toothpaste was considered the control group because it is a fluoro-based formula and it is considered the gold standard for remineralization [13,25].
2.4. Sample Size
2.5. Randomisation and Blinding
2.6. Statistical Methods
3. Results
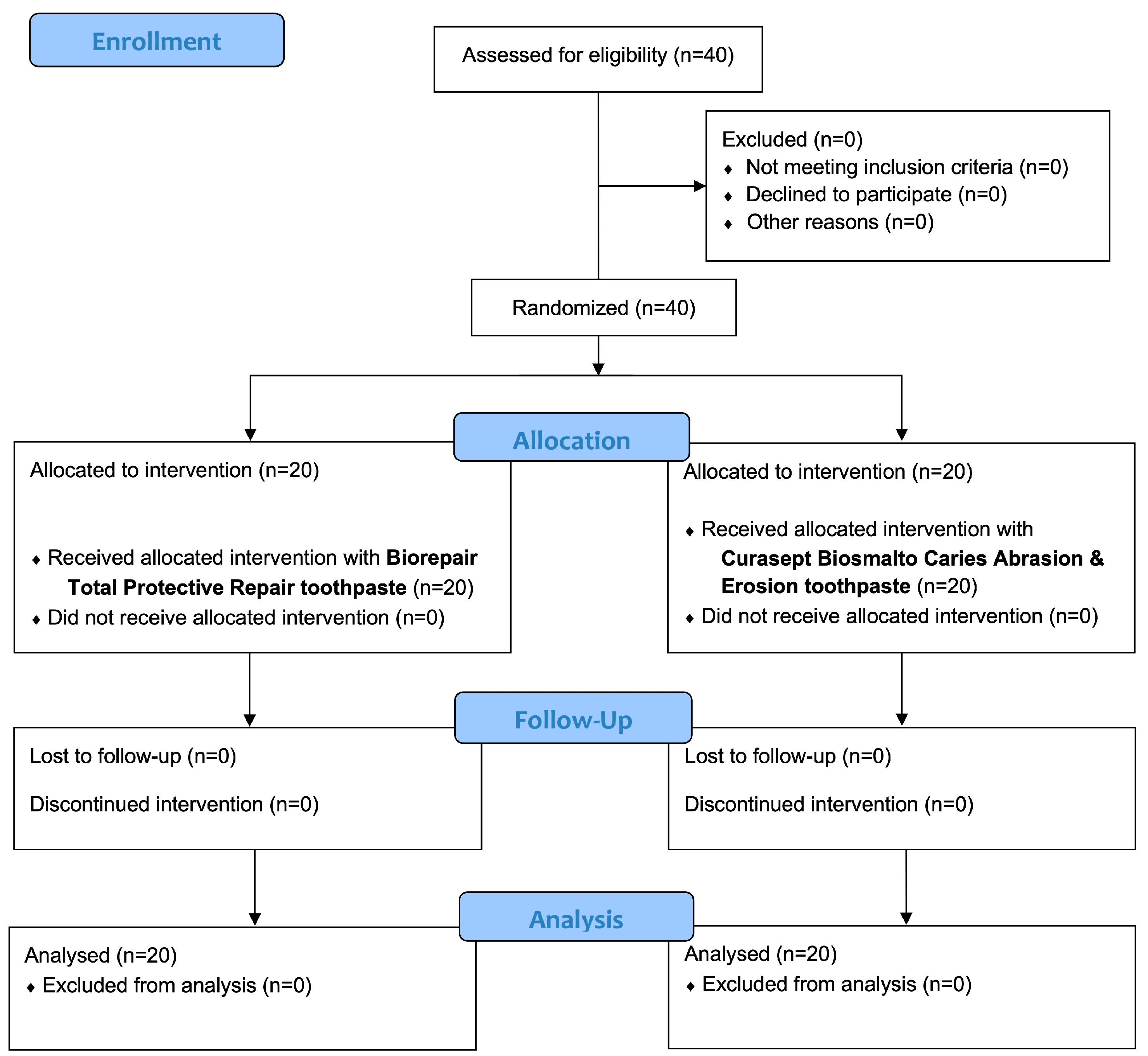
3.1. Participant Flow and Baseline Data
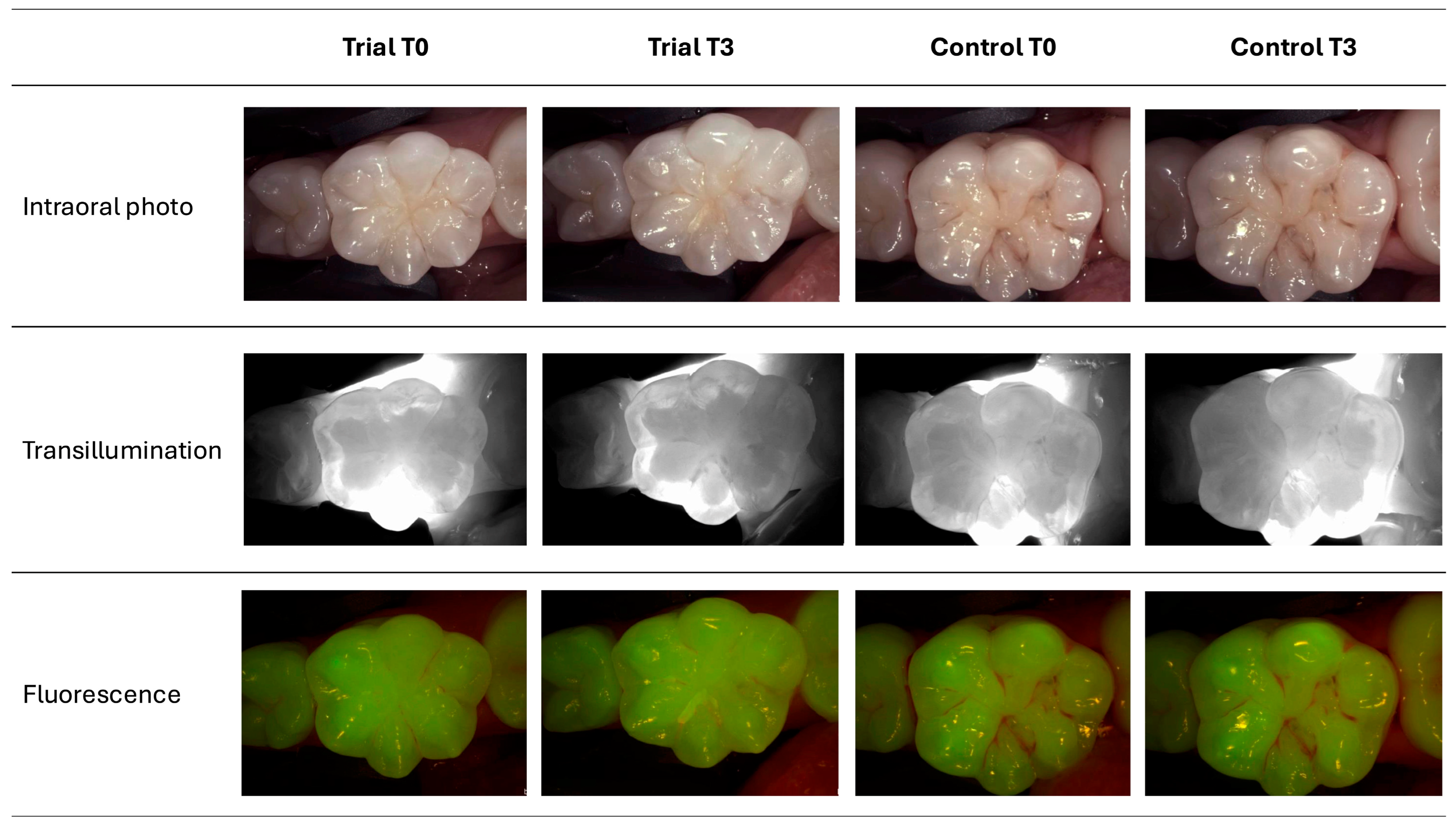
3.2. Study Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lacruz, R.S.; Habelitz, S.; Wright, J.T.; Paine, M.L. Dental Enamel Formation and Implications for Oral Health and Disease. Physiol. Rev. 2017, 97, 939–993. [Google Scholar] [CrossRef] [PubMed]
- Pandya, M.; Diekwisch, T.G.H. Amelogenesis: Transformation of a Protein-Mineral Matrix into Tooth Enamel. J. Struct. Biol. 2021, 213, 107809. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, L.; Yue, L.; Ling, J.; Fan, M.; Yang, D.; Huang, Z.; Niu, Y.; Liu, J.; Zhao, J.; et al. Expert Consensus on Dental Caries Management. Int. J. Oral Sci. 2022, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- Struzycka, I. The Oral Microbiome in Dental Caries. Pol. J. Microbiol. 2014, 63, 127–135. [Google Scholar] [CrossRef] [PubMed]
- da Costa Rosa, T.; de Almeida Neves, A.; Azcarate-Peril, M.A.; Divaris, K.; Wu, D.; Cho, H.; Moss, K.; Paster, B.J.; Chen, T.; Freitas-Fernandes, L.B.; et al. The Bacterial Microbiome and Metabolome in Caries Progression and Arrest. J. Oral Microbiol. 2021, 13, 1886748. [Google Scholar] [CrossRef] [PubMed]
- Farooq, I.; Bugshan, A. The Role of Salivary Contents and Modern Technologies in the Remineralization of Dental Enamel: A Narrative Review. F1000Research 2020, 9, 171. [Google Scholar] [CrossRef]
- Orsini, G.; Orilisi, G.; Notarstefano, V.; Monterubbianesi, R.; Vitiello, F.; Tosco, V.; Belloni, A.; Putignano, A.; Giorgini, E. Vibrational Imaging Techniques for the Characterization of Hard Dental Tissues: From Bench-Top to Chair-Side. Appl. Sci. 2021, 11, 11953. [Google Scholar] [CrossRef]
- Gokce, A.N.P.; Kelesoglu, E.; Sagır, K.; Kargul, B. Remineralization Potential of a Novel Varnish: An In Vitro Comparative Evaluation. J. Clin. Pediatr. Dent. 2024, 48, 173–180. [Google Scholar]
- ElGhandour, R.K.; ElTekeya, M.M.H.; Sharaf, A.A. Effectiveness of Silver Diamine Fluoride in Arresting Early Childhood Caries: A Randomised Controlled Clinical Trial. Eur. J. Paediatr. Dent. 2024, 25, 202–207. [Google Scholar]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Montasser, M.A.; Abd El Latief, M.H.; Modica, G.G.; Scribante, A. Home Oral Care with Biomimetic Hydroxyapatite vs. Conventional Fluoridated Toothpaste for the Remineralization and Desensitizing of White Spot Lesions: Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 8676. [Google Scholar] [CrossRef]
- Patel, M.K.; Milano, M.; Messer, R.L. Acceptance and Awareness of Southeastern and Western Private Practice Pediatric Dentists of Fluoride-Free Toothpastes: A Survey Study. J. Clin. Pediatr. Dent. 2023, 47, 73–80. [Google Scholar]
- Alamoudi, N.M.; Khan, J.A.; El-Ashiry, E.A.; Felemban, O.M.; Bagher, S.M.; Al-Tuwirqi, A.A. Accuracy of the DIAGNOcam and Bitewing Radiographs in the Diagnosis of Cavitated Proximal Carious Lesions in Primary Molars. Niger. J. Clin. Pract. 2019, 22, 1576–1582. [Google Scholar] [CrossRef]
- Butera, A.; Maiorani, C.; Gallo, S.; Pascadopoli, M.; Quintini, M.; Lelli, M.; Tarterini, F.; Foltran, I.; Scribante, A. Biomimetic Action of Zinc Hydroxyapatite on Remineralization of Enamel and Dentin: A Review. Biomimetics 2023, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, A.D.P.; Mora, V.S.A.; Dávila, M.; Montesinos-Guevara, C. Dental Caries Prevention in Pediatric Patients with Molar Incisor Hypomineralization: A Scoping Review. J. Clin. Pediatr. Dent. 2023, 47, 9–15. [Google Scholar] [PubMed]
- Giammarinaro, E.; Cosola, S.; Oldoini, G.; Gulia, F.; Peñarrocha-Oltra, D.; Marconcini, S.; Genovesi, A.M. Local Formula with Mucoadhesive Property: A Randomized Clinical Trial of a Therapeutic Agent for the Treatment of Oral Aphthous Ulcers. J. Contemp. Dent. Pract. 2019, 20, 1249–1253. [Google Scholar] [CrossRef]
- Butera, A.; Folini, E.; Cosola, S.; Russo, G.; Scribante, A.; Gallo, S.; Stablum, G.; Menchini Fabris, G.B.; Covani, U.; Genovesi, A. Evaluation of the Efficacy of Probiotics Domiciliary Protocols for the Management of Periodontal Disease, in Adjunction of Non-Surgical Periodontal Therapy (NSPT): A Systematic Literature Review. Appl. Sci. 2023, 13, 663. [Google Scholar] [CrossRef]
- Meng, N.; Liu, Q.; Dong, Q.; Gu, J.; Yang, Y. Effects of Probiotics on Preventing Caries in Preschool Children: A Systematic Review and Meta-Analysis. J. Clin. Pediatr. Dent. 2023, 47, 85–100. [Google Scholar]
- Butera, A.; Pascadopoli, M.; Nardi, M.G.; Ogliari, C.; Chiesa, A.; Preda, C.; Perego, G.; Scribante, A. Clinical Use of Paraprobiotics for Pregnant Women with Periodontitis: Randomized Clinical Trial. Dent. J. 2024, 12, 116. [Google Scholar] [CrossRef]
- Latifi-Xhemajli, B.; Kutllovci, T.; Begzati, A.; Rexhepi, A.; Ahmeti, D. A Prospective Longitudinal Cohort Study of the Effectiveness of 25% Xylitol Toothpaste on Mutans Streptococci in High Caries-Risk Young Children. Eur. J. Paediatr. Dent. 2023, 24, 188–193. [Google Scholar]
- Scribante, A.; Pascadopoli, M.; Bergomi, P.; Licari, A.; Marseglia, G.L.; Bizzi, F.M.; Butera, A. Evaluation of Two Different Remineralising Toothpastes in Children with Drug-Controlled Asthma and Allergic Rhinitis: A Randomized Clinical Trial. Eur. J. Paediatr. Dent. 2024, 25, 137–142. [Google Scholar] [PubMed]
- Degli Esposti, L.; Ionescu, A.C.; Brambilla, E.; Tampieri, A.; Iafisco, M. Characterization of a Toothpaste Containing Bioactive Hydroxyapatites and In Vitro Evaluation of Its Efficacy to Remineralize Enamel and to Occlude Dentinal Tubules. Materials 2020, 13, 2928. [Google Scholar] [CrossRef]
- Sürme, K.; Kara, N.B.; Yilmaz, Y. In Vitro Evaluation of Occlusal Caries Detection Methods in Primary and Permanent Teeth: A Comparison of CarieScan PRO, DIAGNOdent Pen, and DIAGNOcam Methods. Photobiomodul. Photomed. Laser Surg. 2020, 38, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Arshad, S.; Zaidi, S.J.A.; Farooqui, W.A. Comparative Efficacy of BioMin-F, Colgate Sensitive Pro-Relief and Sensodyne Rapid Action in Relieving Dentin Hypersensitivity: A Randomized Controlled Trial. BMC Oral Health 2021, 21, 498. [Google Scholar] [CrossRef] [PubMed]
- Giuca, M.R.; Pasini, M.; Giuca, G.; Caruso, S.; Necozione, S.; Gatto, R. Investigation of Periodontal Status in Type 1 Diabetic Adolescents. Eur. J. Paediatr. Dent. 2015, 16, 319–323. [Google Scholar]
- Marqués Martínez, L.; Leyda Menéndez, A.M.; Ribelles Llop, M.; Segarra Ortells, C.; Aiuto, R.; Garcovich, D. Dental Erosion. Etiologic Factors in a Sample of Valencian Children and Adolescents. Cross-Sectional Study. Eur. J. Paediatr. Dent. 2019, 20, 189–193. [Google Scholar]
- Mollabashi, V.; Heydarpour, M.; Farhadifard, H.; Alafchi, B. DIAGNOdent Pen Quantification of the Synergy of NovaMin® in Fluoride Toothpaste to Remineralize White Spot Lesions in Patients with Fixed Orthodontic Appliances: A Double-Blind, Randomized, Controlled Clinical Trial. Int. Orthod. 2022, 20, 100632. [Google Scholar] [CrossRef] [PubMed]
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral Diseases: A Global Public Health Challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Moussa, D.G.; Sharma, A.K.; Mansour, T.A.; Witthuhn, B.; Perdigão, J.; Rudney, J.D.; Aparicio, C.; Gomez, A. Functional Signatures of Ex-Vivo Dental Caries Onset. J. Oral Microbiol. 2022, 14, 2123624. [Google Scholar] [CrossRef]
- Li, S.; Fan, L.; Zhou, S. Analysis of the incidence and influencing factors of dental caries and periodontitis in children aged 5-12 in Jinhua, Zhejiang province. J. Clin. Pediatr. Dent. 2024, 48, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, H.N. Assessment of Early Childhood Caries Using ICDAS and Snyder Caries Activity Test among Preschool Children: A Cross-Sectional Study. J. Clin. Pediatr. Dent. 2023, 47, 163–170. [Google Scholar] [PubMed]
- Pretty, I.A. Caries Detection and Diagnosis: Novel Technologies. J. Dent. 2006, 34, 727–739. [Google Scholar] [CrossRef]
- Pelliccioni, G.A.; Gatto, M.R.A.; Bolognesi, S.; Dal Fiume, D.; Sebold, M.; Breschi, L. Clinical Analysis of the Diagnostic Accuracy and Time of Execution of a Transillumination Caries Detection Method Compared to Bitewing Radiographs. J. Clin. Med. 2021, 10, 4780. [Google Scholar] [CrossRef]
- Yehua, D.; Yiyuan, Y.; Yihao, L.; Jianjun, Z.; Shanshan, L.; Rourong, C.; Han, J.; Baojun, T.; Minquan, D.; Chang, L. Evaluation of DIAGNOdent Pen for Initial Occlusal Caries Diagnosis in Permanent Teeth. BMC Oral Health 2024, 24, 1111. [Google Scholar] [CrossRef] [PubMed]
- Pawinska, M.; Paszynska, E.; Amaechi, B.T.; Meyer, F.; Enax, J.; Limeback, H. Clinical evidence of caries prevention by hydroxyapatite: An updated systematic review and meta-analysis. J. Dent. 2024, 151, 105429. [Google Scholar] [CrossRef]
- Perdiou, A.; Fratila, A.D.; Sava-Rosianu, R.; Alexa, V.T.; Lalescu, D.; Jumanca, D.; Galuscan, A. In Vivo Performance of Visual Criteria, Laser-Induced Fluorescence, and Light-Induced Fluorescence for Early Caries Detection. Diagnostics 2023, 13, 317. [Google Scholar] [CrossRef] [PubMed]
- Foros, P.; Oikonomou, E.; Koletsi, D.; Rahiotis, C. Detection Methods for Early Caries Diagnosis: A Systematic Review and Meta-Analysis. Caries Res. 2021, 55, 247–259. [Google Scholar] [CrossRef]
- Davidopoulou, S.; Topitsoglou, V.; Berdouses, E.D.; Arapostathis, K.; Kavvadia, K.; Oulis, C.J. Tooth-Surface Distribution of Caries in Greek Schoolchildren, Using ICDAS-II Index. A National Pathfinder Survey. Eur. J. Paediatr. Dent. 2022, 23, 204–212. [Google Scholar] [PubMed]
- Lee, J.W.; Lee, E.S.; Kim, B.I. Optical Diagnosis of Dentin Caries Lesions Using Quantitative Light-Induced Fluorescence Technology. Photodiagn. Photodyn. Ther. 2018, 23, 68–70. [Google Scholar] [CrossRef]
- Puleio, F.; Di Spirito, F.; Lo Giudice, G.; Pantaleo, G.; Rizzo, D.; Lo Giudice, R. Long-Term Chromatic Durability of White Spot Lesions through Employment of Infiltration Resin Treatment. Medicina 2023, 59, 749. [Google Scholar] [CrossRef]
- Carvalho, F.B.; Barbosa, A.F.; Zanin, F.A.; Brugnera Júnior, A.; Silveira Júnior, L.; Pinheiro, A.L. Use of Laser Fluorescence in Dental Caries Diagnosis: A Fluorescence x Biomolecular Vibrational Spectroscopic Comparative Study. Braz. Dent. J. 2013, 24, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Alharthy, H.; Elkhodary, H.; Nahdreen, A.; Al Tuwirqi, A.; Baghlaf, K.; Alamoudi, N. Clinical Evaluation of Hydrophilic and Hydrophobic Resin-Based Sealants in Uncooperative Children: A Randomized Controlled Clinical Trial. J. Clin. Pediatr. Dent. 2024, 48, 149–159. [Google Scholar] [PubMed]
- Khan, A.A.; Al-Khureif, A.A.; Al-Mutairi, M.; Al-Majed, I.; Aftab, S. Physical and Mechanical Characterizations of Experimental Pit and Fissure Sealants Based on Bioactive Glasses. J. Clin. Pediatr. Dent. 2024, 48, 69–77. [Google Scholar] [PubMed]
| Toothpaste | Manufacturer | Composition |
|---|---|---|
| Biorepair Total Protective Repair | Coswell S.p.A., Funo di Argelato, BO, Italy | Zinc hydroxyapatite, aqua, aroma, glycerin, sorbitol, hydrated silica, silica, cellulose gum, tetrapotassium pyrophosphate, sodium methyl cocoyl taurate, sodium myristoyl sarcosinate, sodium saccharin, citric acid, phenoxyethanol, benzyl alcohol, sodium benzoate. |
| Curasept Biosmalto Caries Abrasion & Erosion | Curasept S.p.A, Saronno, VA, Italy | Purified water, glycerin, xylitol, fluoro hydroxyapatite, magnesium-strontium-carbonate hydroxyapatite conjugated with chitosan, cellulose gum, cocamidopropyl betaine, potassium acesulfame, xanthan gum, aroma, phenoxyethanol, sodium benzoate, aroma, citric acid. |
| Group | Time | DP Mean (SD) | DC Mean (SD) |
|---|---|---|---|
| Trial | T0 | 17.06 (10.25) a | 0.64 (0.77) a,b |
| T1 | 10.39 (7.63) b,c,d | 0.63 (0.75) a,b | |
| T2 | 8.39 (6.58) c,d | 0.48 (0.62) a,b | |
| T3 | 8.19 (4.77) c,d | 0.38 (0.56) a | |
| Control | T0 | 12.46 (6.93) a,b | 0.78 (0.83) b |
| T1 | 10.79 (6.21) b,c | 0.78 (0.83) b | |
| T2 | 9.53 (5.41) b,c,d | 0.64 (0.75) a,b | |
| T3 | 8.98 (5.05) b,c,d | 0.56 (0.65) a,b |
| Group | Time | SAI Mean (SD) | PI Mean (SD) | BoP Mean (SD) | BEWE Mean (SD) |
|---|---|---|---|---|---|
| Trial | T0 | 0.21 (0.69) a | 70.4 (29.67) a | 7.05 (17.14) a | 0.74 (0.52) a |
| T1 | 0.18 (0.57) a | 41.65 (18.92) a,b,c | 0.50 (2.24) a | 0.63 (0.54) a | |
| T2 | 0.14 (0.44) a | 37.6 (16.62) b,c,d | 0.00 (0.00) a | 0.48 (0.53) a | |
| T3 | 0.11 (0.39) a | 39.45 (21.79) c,d | 1.10 (3.08) a | 0.45 (0.53) a | |
| Control | T0 | 0.11 (0.45) a | 67.5 (31.53) a,c | 6.45 (11.56) a | 0.59 (00.59) a |
| T1 | 0.11 (0.45) a | 34.5 (13.93) b,d | 0.60 (02.26) a | 0.56 (0.59) a | |
| T2 | 0.08 (0.31) a | 37.4 (15.19) b,c,d | 1.00 (3.08) a | 0.53 (0.55) a | |
| T3 | 0.06 (0.29) a | 39.5 (20.63) b,c,d | 3.15 (8.31) a | 0.50 (0.55) a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scribante, A.; Cosola, S.; Pascadopoli, M.; Genovesi, A.; Battisti, R.A.; Butera, A. Clinical and Technological Evaluation of the Remineralising Effect of Biomimetic Hydroxyapatite in a Population Aged 6 to 18 Years: A Randomized Clinical Trial. Bioengineering 2025, 12, 152. https://doi.org/10.3390/bioengineering12020152
Scribante A, Cosola S, Pascadopoli M, Genovesi A, Battisti RA, Butera A. Clinical and Technological Evaluation of the Remineralising Effect of Biomimetic Hydroxyapatite in a Population Aged 6 to 18 Years: A Randomized Clinical Trial. Bioengineering. 2025; 12(2):152. https://doi.org/10.3390/bioengineering12020152
Chicago/Turabian StyleScribante, Andrea, Saverio Cosola, Maurizio Pascadopoli, Annamaria Genovesi, Rebecca Andrea Battisti, and Andrea Butera. 2025. "Clinical and Technological Evaluation of the Remineralising Effect of Biomimetic Hydroxyapatite in a Population Aged 6 to 18 Years: A Randomized Clinical Trial" Bioengineering 12, no. 2: 152. https://doi.org/10.3390/bioengineering12020152
APA StyleScribante, A., Cosola, S., Pascadopoli, M., Genovesi, A., Battisti, R. A., & Butera, A. (2025). Clinical and Technological Evaluation of the Remineralising Effect of Biomimetic Hydroxyapatite in a Population Aged 6 to 18 Years: A Randomized Clinical Trial. Bioengineering, 12(2), 152. https://doi.org/10.3390/bioengineering12020152










