Comparison of Tissue-Engineered Dermis with Micronized Adipose Tissue and Artificial Dermis for Facial Reconstruction Following Skin Cancer Resection
Abstract
:1. Introduction
2. Patients and Methods
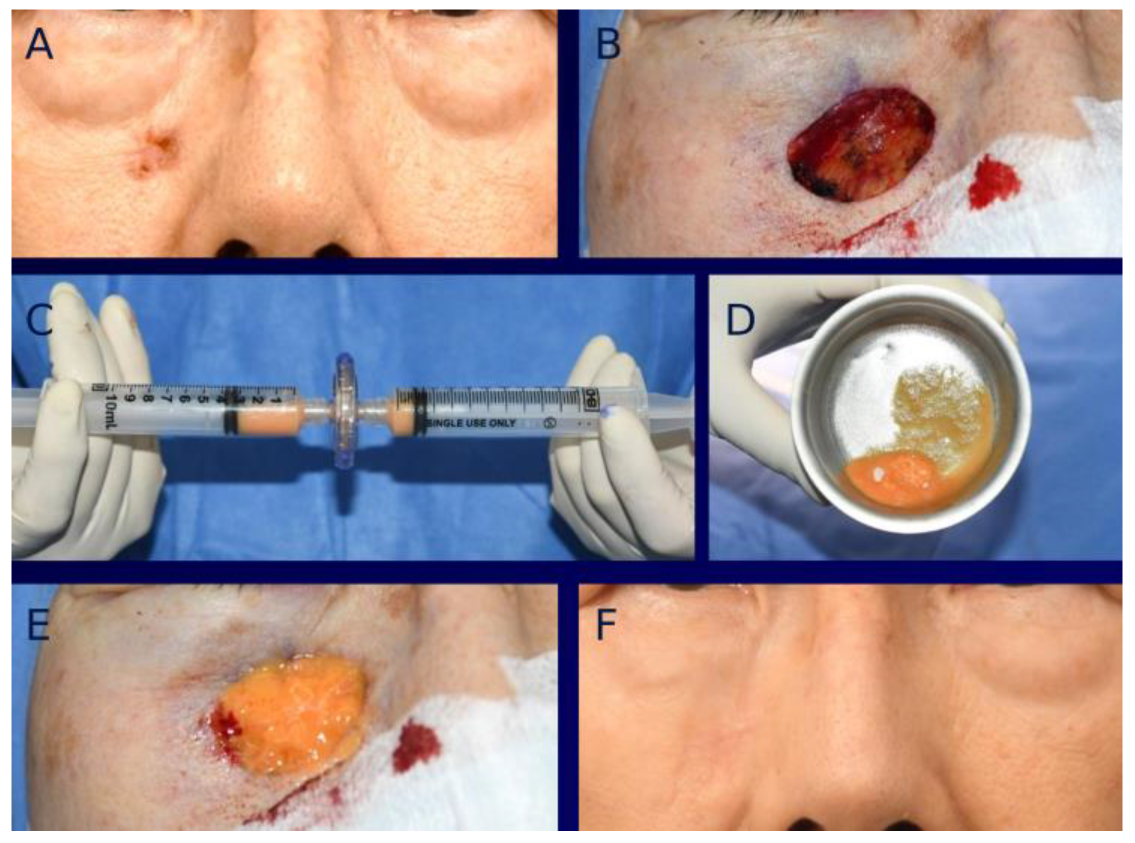
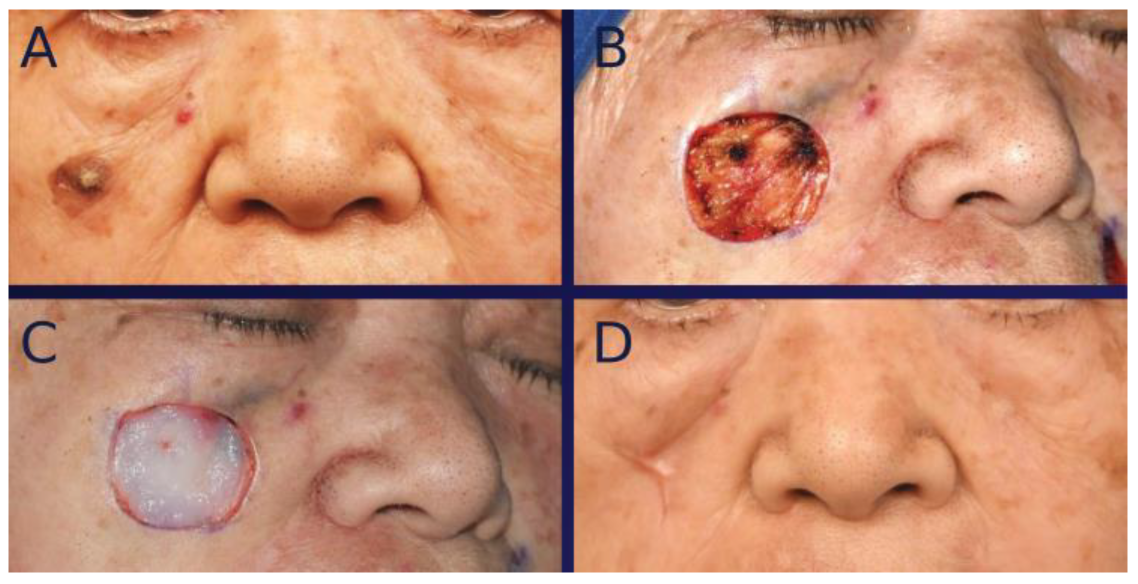
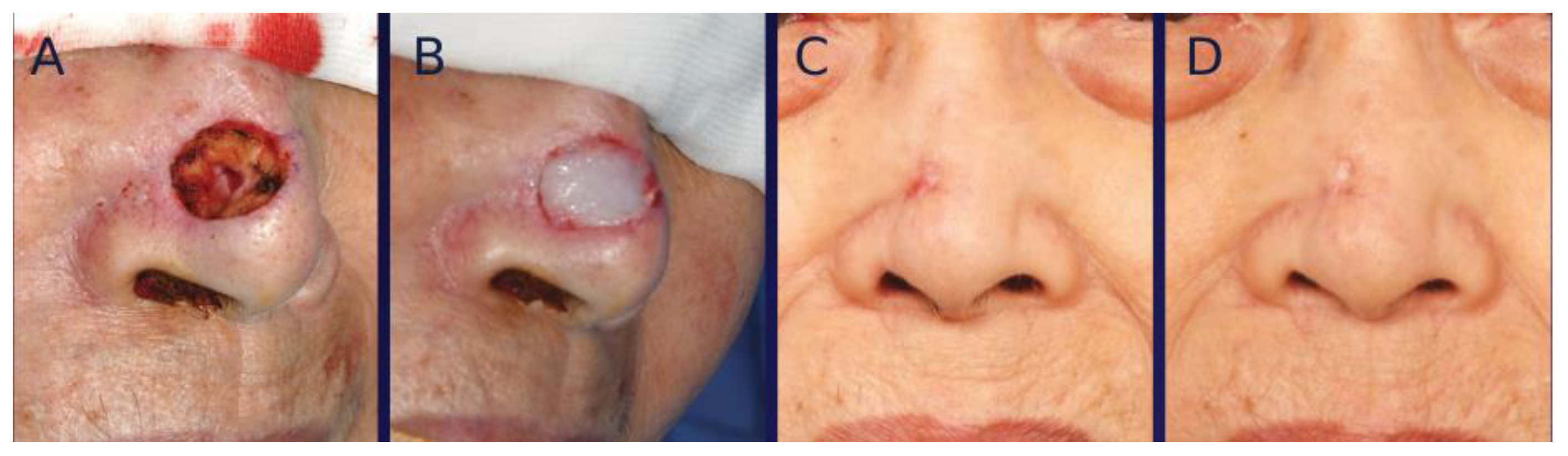
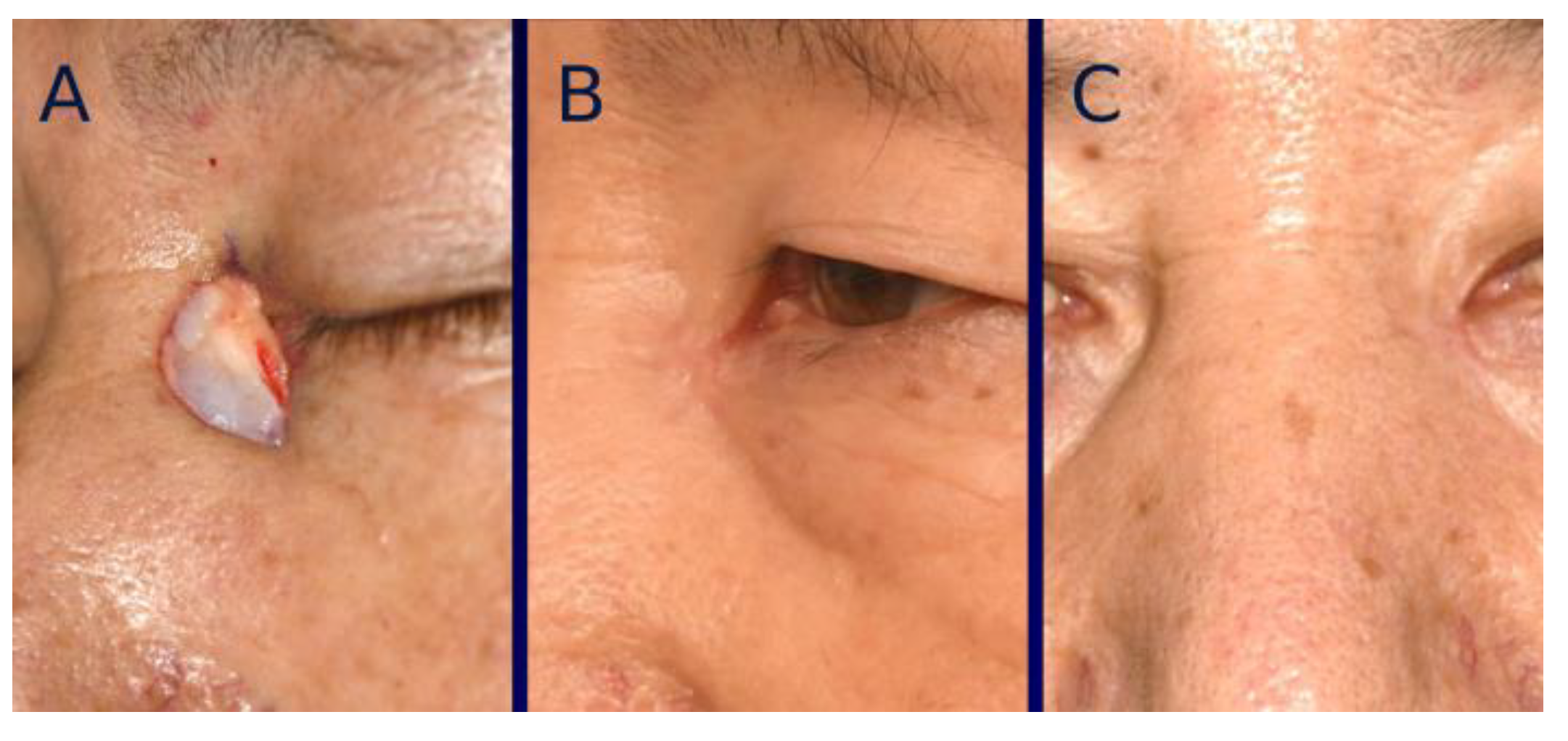
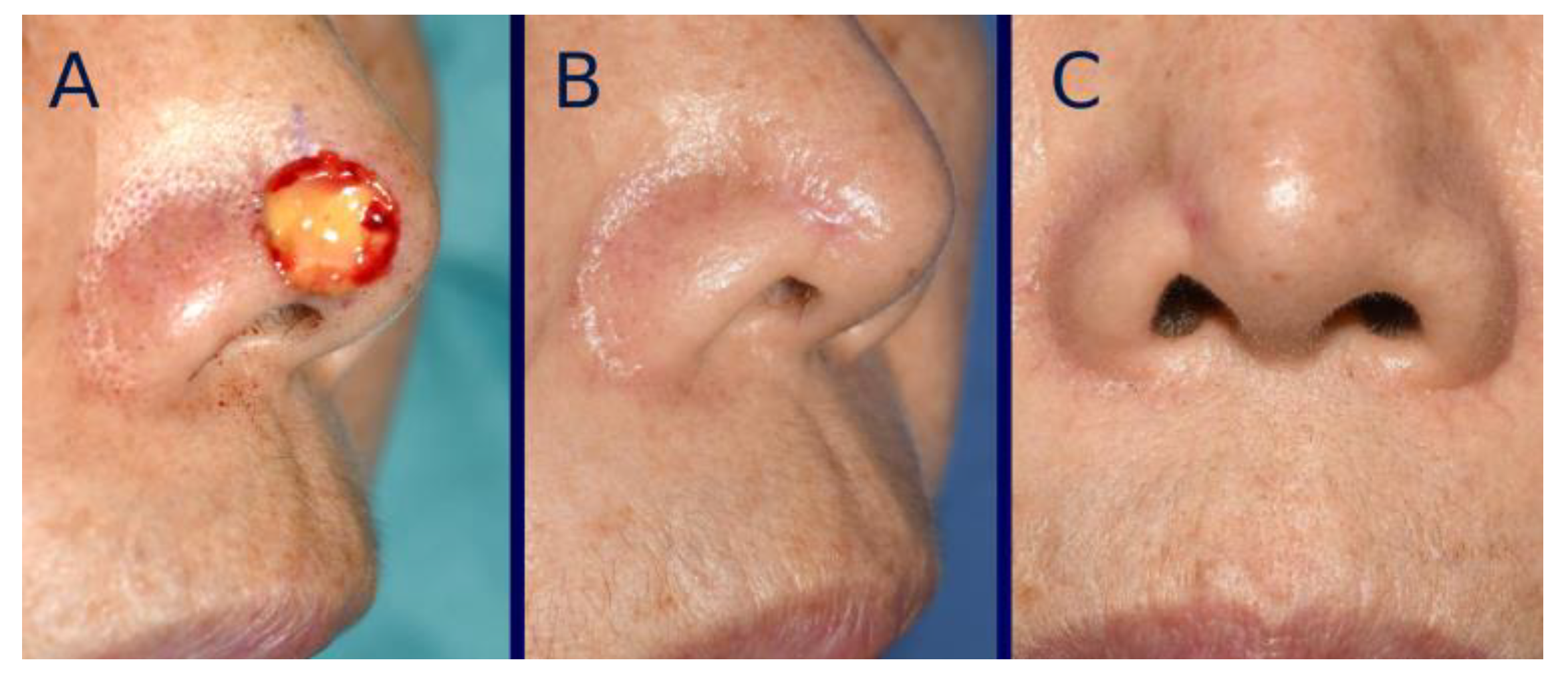
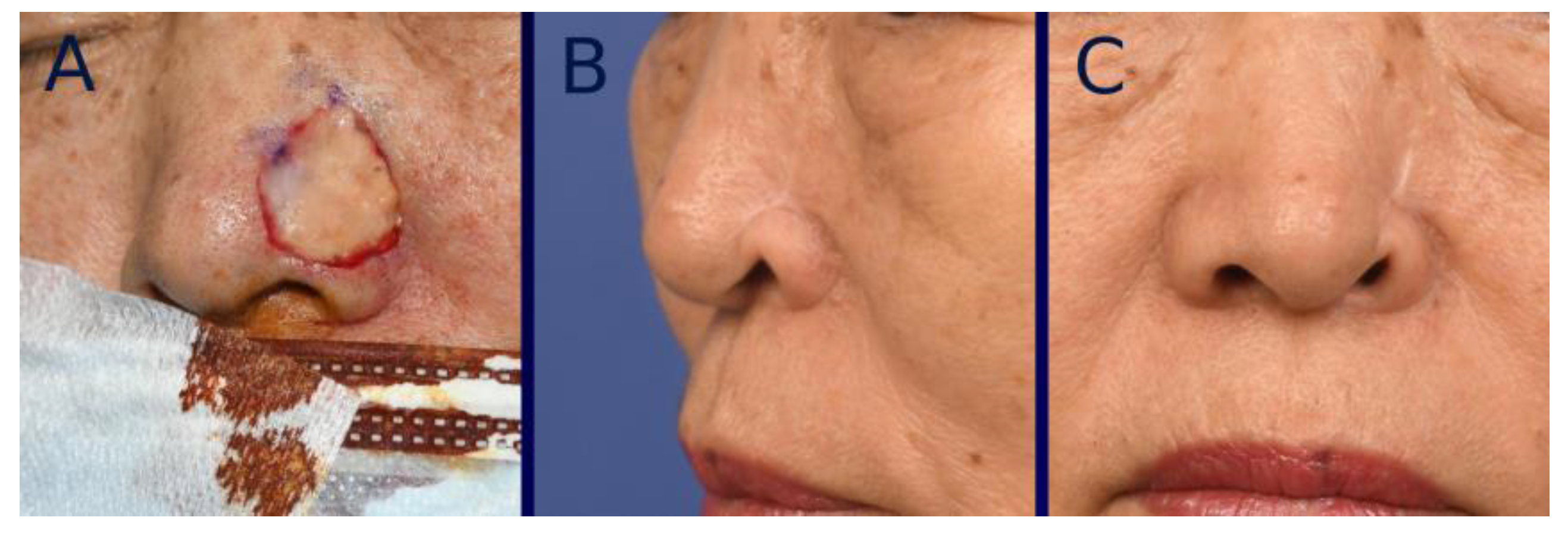
3. Surgical Technique
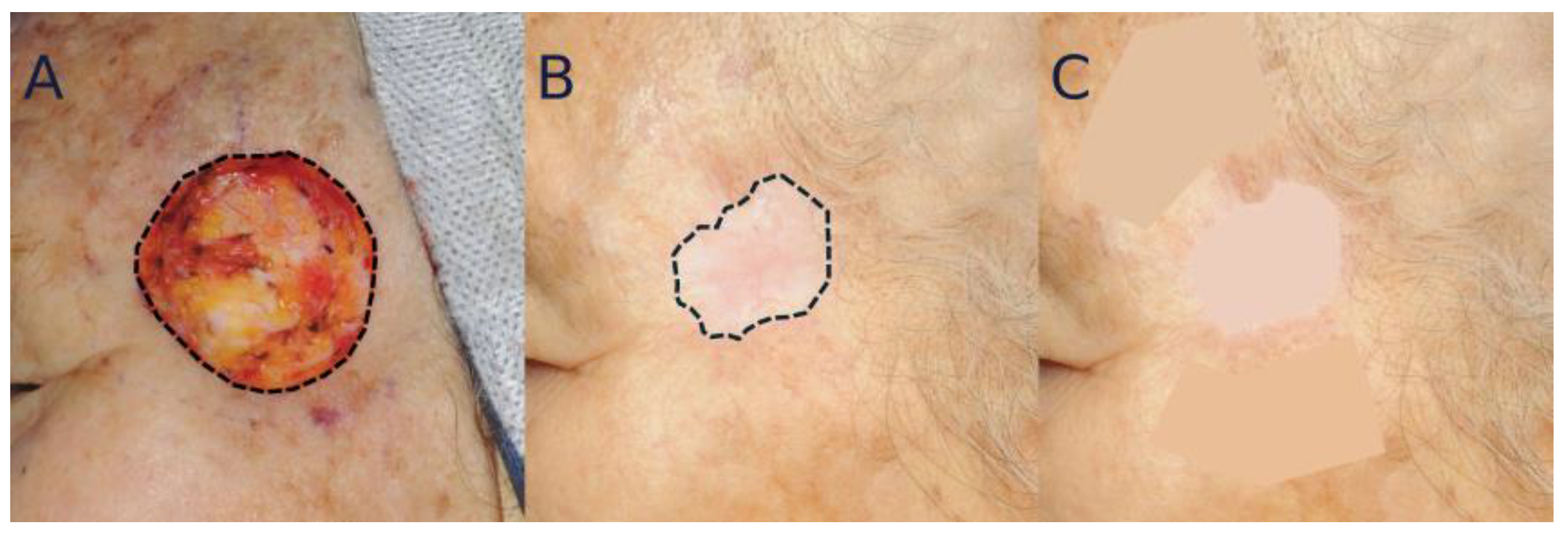
4. Standardization of Clinical Photographs
5. Evaluation
5.1. Analysis of Color Mismatching
5.2. Analysis of Scar Contraction
6. Statistical Analyses
7. Results
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lomas, A.; Leonardi-Bee, J.; Bath-Hextall, F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br. J. Dermatol. 2012, 166, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.C.; Jin, A.; Koh, W.P. Trends of cutaneous basal cell carcinoma, squamous cell carcinoma, and melanoma among the Chinese, Malays, and Indians in Singapore from 1968–2016. JAAD Int 2021, 4, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Sherris, D.A.; Larrabee, W.F. Principles of Facial Reconstruction: A Subunit Approach to Cutaneous Repair, 2nd ed.; Thieme: New York, NY, USA, 2010. [Google Scholar]
- Patel, K.G.; Sykes, J.M. Concepts in local flap design and classification. Oper. Tech. Otolaryngol.-Head Neck Surg. 2011, 22, 13–23. [Google Scholar] [CrossRef]
- Lawrence, J.W.; Mason, S.T.; Schomer, K.; Klein, M.B. Epidemiology and impact of scarring after burn injury: A systematic review of the literature. J. Burn Care Res. 2012, 33, 136–146. [Google Scholar] [CrossRef]
- Han, S.K.; Kim, S.Y.; Choi, R.J.; Jeong, S.H.; Kim, W.K. Comparison of tissue-engineered and artificial dermis grafts after removal of basal cell carcinoma on face—A pilot study. Dermatol. Surg. 2014, 40, 460–467. [Google Scholar] [CrossRef]
- Moon, K.C.; Chung, H.Y.; Han, S.K.; Jeong, S.H.; Dhong, E.S. Tissue-engineered dermis grafts using stromal vascular fraction cells on the nose: A retrospective case-control study. J. Plast. Reconstr. Aesthet. Surg. 2020, 73, 965–974. [Google Scholar] [CrossRef]
- Ceserani, V.; Ferri, A.; Berenzi, A.; Benetti, A.; Ciusani, E.; Pascucci, L.; Bazzucchi, C.; Coccè, V.; Bonomi, A.; Pessina, A.; et al. Angiogenic and anti-inflammatory properties of micro-fragmented fat tissue and its derived mesenchymal stromal cells. Vasc. Cell 2016, 8, 3. [Google Scholar] [CrossRef]
- Nava, S.; Sordi, V.; Pascucci, L.; Tremolada, C.; Ciusani, E.; Zeira, O.; Cadei, M.; Soldati, G.; Pessina, A.; Parati, E.; et al. Long-Lasting Anti-Inflammatory Activity of Human Microfragmented Adipose Tissue. Stem Cells Int. 2019, 2019, 5901479. [Google Scholar] [CrossRef]
- Dermody, S.M.; Kahng, P.W.; Rao, V.M.; Casper, K.A.; Malloy, K.M.; Rosko, A.J.; Stucken, C.L.; Chinn, S.B.; Spector, M.E.; Feng, A.L. Color Match following Free Flap Surgery in Head and Neck Reconstruction: A Colorimetric and Aesthetic Analysis. Plast. Reconstr. Surg. 2024, 153, 1142–1150. [Google Scholar] [CrossRef]
- Plonowska-Hirschfeld, K.A.; Eltawil, Y.; Soroudi, D.; Patel, N.N.; Park, A.M.; Knott, P.D. Assessment of Variability in Free Flap Color Match to Facial Skin by Donor Site and Race. Laryngoscope 2024, 134, 3581–3586. [Google Scholar] [CrossRef]
- Luo, M.R.; Cui, G.; Rigg, B. The development of the CIE 2000 colour-difference formula: CIEDE2000. Color Res. Appl. 2001, 26, 340–350. [Google Scholar] [CrossRef]
- Knott, P.D.; Alemi, S.A.; Han, M.; Seth, R.; Park, A.M.; Heaton, C.M.; Grekin, R.C.; Arron, S.T.; Neuhaus, I.; Yu, S.S.; et al. Skin Color Match in Head and Neck Reconstructive Surgery. Laryngoscope 2022, 132, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Ruszczak, Z. Effect of collagen matrices on dermal wound healing. Adv. Drug Deliv. Rev. 2003, 55, 1595–1611. [Google Scholar] [CrossRef] [PubMed]
- Houdek, M.T.; Wyles, C.C.; Stalboerger, P.G.; Terzic, A.; Behfar, A.; Moran, S.L. Collagen and Fractionated Platelet-Rich Plasma Scaffold for Dermal Regeneration. Plast. Reconstr. Surg. 2016, 137, 1498–1506. [Google Scholar] [CrossRef]
- Jeschke, M.G.; Sandmann, G.; Schubert, T.; Klein, D. Effect of oxidized regenerated cellulose/collagen matrix on dermal and epidermal healing and growth factors in an acute wound. Wound Repair. Regen. 2005, 13, 324–331. [Google Scholar] [CrossRef]
- Jang, J.C.; Choi, R.J.; Han, S.K.; Jeong, S.H.; Kim, W.K. Effect of fibroblast-seeded artificial dermis on wound healing. Ann. Plast. Surg. 2015, 74, 501–507. [Google Scholar] [CrossRef]
- Robert, S.; Gicquel, T.; Victoni, T.; Valença, S.; Barreto, E.; Bailly-Maître, B.; Boichot, E.; Lagente, V. Involvement of matrix metalloproteinases (MMPs) and inflammasome pathway in molecular mechanisms of fibrosis. Biosci. Rep. 2016, 36, e00360. [Google Scholar] [CrossRef]
- El-Ghalbzouri, A.; Gibbs, S.; Lamme, E.; Van Blitterswijk, C.A.; Ponec, M. Effect of fibroblasts on epidermal regeneration. Br. J. Dermatol. 2002, 147, 230–243. [Google Scholar] [CrossRef]
- Uysal, C.A.; Tobita, M.; Hyakusoku, H.; Mizuno, H. The Effect of Bone-Marrow-Derived Stem Cells and Adipose-Derived Stem Cells on Wound Contraction and Epithelization. Adv. Wound Care 2014, 3, 405–413. [Google Scholar] [CrossRef]
- Zonari, A.; Martins, T.M.; Paula, A.C.; Boeloni, J.N.; Novikoff, S.; Marques, A.P.; Correlo, V.M.; Reis, R.L.; Goes, A.M. Polyhydroxybutyrate-co-hydroxyvalerate structures loaded with adipose stem cells promote skin healing with reduced scarring. Acta Biomater. 2015, 17, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.J.; Tholpady, A.; Tholpady, S.S.; Shang, H.; Ogle, R.C. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells 2005, 23, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Namgoong, S.; Yoon, I.J.; Han, S.K.; Son, J.W.; Kim, J. A Pilot Study Comparing a Micronized Adipose Tissue Niche versus Standard Wound Care for Treatment of Neuropathic Diabetic Foot Ulcers. J. Clin. Med. 2022, 11, 5887. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.K.; Han, S.K.; Yoon, I.J.; Namgoong, S.; Jeong, S.H.; Dhong, E.S.; Kim, J.H.; Lee, M.C. Evaluating micronized adipose tissue niche and artificial dermis grafts following nonmelanoma skin cancer excision: A pilot study. Wounds 2024, 36, 129–136. [Google Scholar] [CrossRef]
- Vaidya, T.S.; Mori, S.; Dusza, S.W.; Rossi, A.M.; Nehal, K.S.; Lee, E.H. Appearance-related psychosocial distress following facial skin cancer surgery using the FACE-Q Skin Cancer. Arch. Dermatol. Res. 2019, 311, 691–696. [Google Scholar] [CrossRef]
- Sobanko, J.F.; Sarwer, D.B.; Zvargulis, Z.; Miller, C.J. Importance of physical appearance in patients with skin cancer. Dermatol. Surg. 2015, 41, 183–188. [Google Scholar] [CrossRef]
- ASTM D4303-10(2022); Standard Test Methods for Lightfastness of Colorants Used in Artists’ Materials. ASTM International: West Conshohocken, PA, USA, 2022.
- Greenhalgh, D.G. A primer on pigmentation. J. Burn Care Res. 2015, 36, 247–257. [Google Scholar] [CrossRef]
- Bond, J.S.; Duncan, J.A.L.; Mason, T.; Sattar, A.; Boanas, A.; O’Kane, S.; Ferguson, M.W.J. Scar redness in humans: How long does it persist after incisional and excisional wounding? Plast. Reconstr. Surg. 2008, 121, 487–496. [Google Scholar] [CrossRef]
- Ananta, M.; Brown, R.A.; Mudera, V. A rapid fabricated living dermal equivalent for skin tissue engineering: An in vivo evaluation in an acute wound model. Tissue Eng. Part. A 2012, 18, 353–361. [Google Scholar] [CrossRef]

| Group | AD (n = 35) | MAT (n = 30) | p-Value |
|---|---|---|---|
| Age (years) | 73.5 ± 10.0 | 71.4 ± 8.6 | 0.362 |
| Sex | |||
| Male (%) | 16 (45.7%) | 15 (50.0%) | 0.924 |
| Female (%) | 19 (54.3%) | 15 (50.0%) | |
| Defect size (cm2) | 3.4 ± 2.3 | 3.7 ± 2.6 | 0.807 |
| Cancer subtype | 1.000 | ||
| Basal cell carcinoma | 24 (68.6%) | 20 (66.7%) | |
| Squamous cell carcinoma | 11 (31.4%) | 10 (33.3%) | |
| Location | 0.906 | ||
| Nose | 16 (45.7%) | 15 (50.0%) | |
| Orbit | 6 (17.1%) | 5 (16.7%) | |
| Temple | 6 (17.1%) | 6 (20.0%) | |
| Others | 7 (20.0%) | 4 (13.3%) |
| Group | AD (n = 35) | MAT (n = 30) | p-Value |
|---|---|---|---|
| Healing time (days) | 34.3 ± 5.4 | 30.0 ± 4.3 | 0.001 |
| Scar contraction (%) | 23.2 ± 12.8 | 16.2 ± 12.3 | 0.030 |
| Landmark distortion (score) | 0.50 ± 0.71 | 0.20 ± 0.50 | 0.043 |
| Color mismatch (score) | 5.1 ± 1.7 | 4.9 ± 1.7 | 0.567 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.-I.; Song, W.-S.; Han, S.-K.; Moon, K.-C.; Jeong, S.-H.; Dhong, E.-S. Comparison of Tissue-Engineered Dermis with Micronized Adipose Tissue and Artificial Dermis for Facial Reconstruction Following Skin Cancer Resection. Bioengineering 2025, 12, 145. https://doi.org/10.3390/bioengineering12020145
Lee K-I, Song W-S, Han S-K, Moon K-C, Jeong S-H, Dhong E-S. Comparison of Tissue-Engineered Dermis with Micronized Adipose Tissue and Artificial Dermis for Facial Reconstruction Following Skin Cancer Resection. Bioengineering. 2025; 12(2):145. https://doi.org/10.3390/bioengineering12020145
Chicago/Turabian StyleLee, Kyu-Il, Won-Seok Song, Seung-Kyu Han, Kyung-Chul Moon, Seong-Ho Jeong, and Eun-Sang Dhong. 2025. "Comparison of Tissue-Engineered Dermis with Micronized Adipose Tissue and Artificial Dermis for Facial Reconstruction Following Skin Cancer Resection" Bioengineering 12, no. 2: 145. https://doi.org/10.3390/bioengineering12020145
APA StyleLee, K.-I., Song, W.-S., Han, S.-K., Moon, K.-C., Jeong, S.-H., & Dhong, E.-S. (2025). Comparison of Tissue-Engineered Dermis with Micronized Adipose Tissue and Artificial Dermis for Facial Reconstruction Following Skin Cancer Resection. Bioengineering, 12(2), 145. https://doi.org/10.3390/bioengineering12020145








