Sex-Specific Analysis of Carotid Artery Through Bilateral 3D Modeling via MRI and DICOM Processing
Abstract
1. Introduction
2. Materials and Methods
2.1. Participant Characteristics
2.2. Setup of 3D Slicer
2.3. DICOM File Compiling
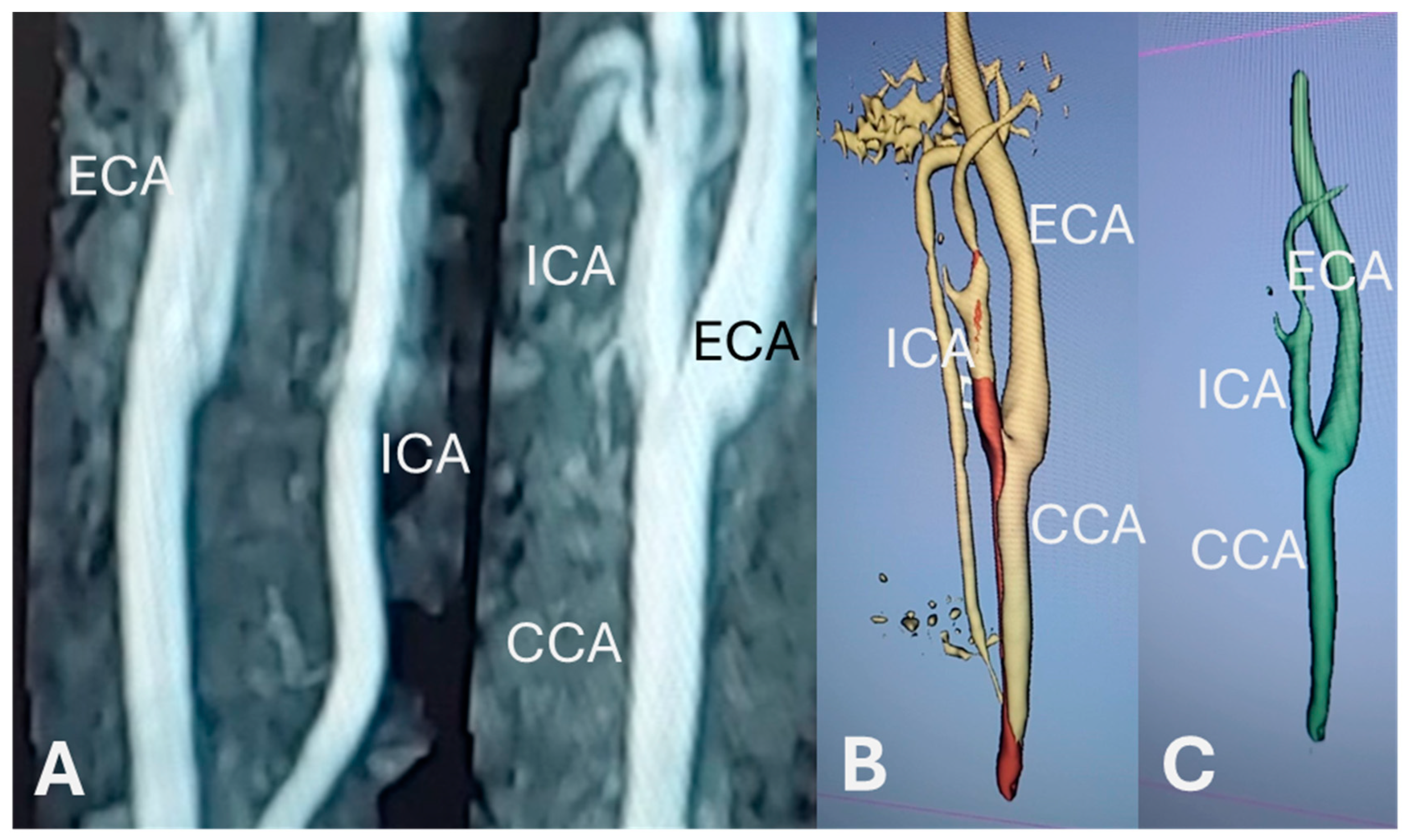
2.4. Vessel Segmentation
2.5. Segmentation Processing
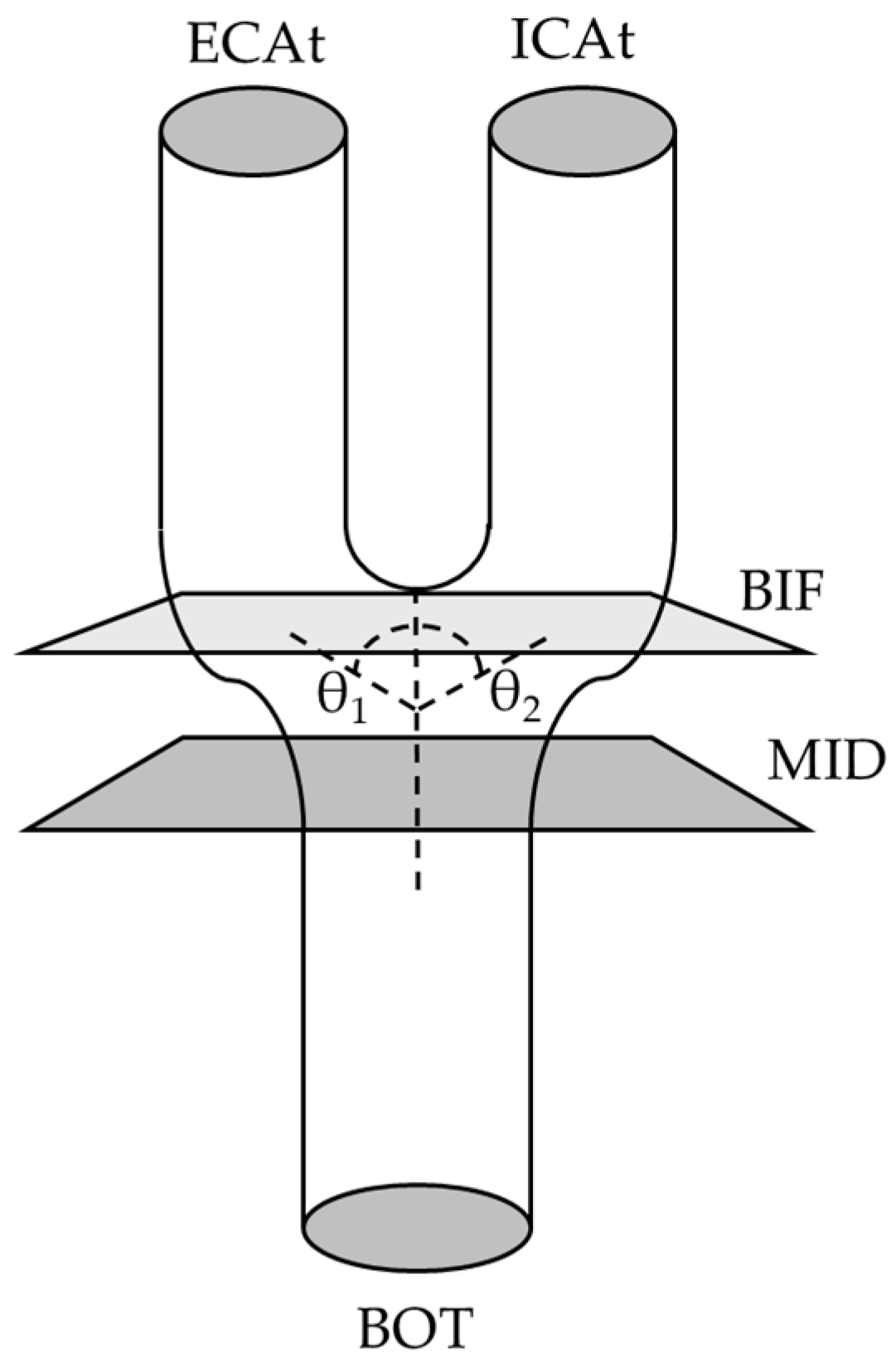
2.6. Landmark Determination
2.6.1. Midpoint (MID)
2.6.2. Bifurcation (BIF) Point and Angle and ECA and ICA Angles
2.6.3. TOP
2.6.4. Other Considerations
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Curtin, S.C. Stroke Death Rates among Adults Ages 45–64 by Region and Race and Hispanic Origin: United States, 2002–2022. NCHS Data Brief 2024. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics—2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and Atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Gurovich, A.N.; Rodriguez, L.; Morales-Acuna, F. There Are No Differences in Brachial Artery Endothelial Shear Stress and Blood Flow Patterns between Males and Females during Exercise. Clin. Physiol. Funct. Imaging 2021, 41, 471–479. [Google Scholar] [CrossRef]
- Cheng, C.; Tempel, D.; Van Haperen, R.; Van Der Baan, A.; Grosveld, F.; Daemen, M.J.; Krams, R.; De Crom, R. Atherosclerotic Lesion Size and Vulnerability Are Determined by Patterns of Fluid Shear Stress. Circulation 2006, 113, 2744–2753. [Google Scholar] [CrossRef]
- Davies, P.F. Hemodynamic Shear Stress and the Endothelium in Cardiovascular Pathophysiology. Nat. Clin. Pract. Cardiovasc. Med. 2009, 6, 16–26. [Google Scholar] [CrossRef]
- Montalvo, S.; Gomez, M.; Lozano, A.; Arias, S.; Rodriguez, L.; Morales-Acuna, F.; Gurovich, A.N. Differences in Blood Flow Patterns and Endothelial Shear Stress at the Carotid Artery Using Different Exercise Modalities and Intensities. Front. Physiol. 2022, 13, 857816. [Google Scholar] [CrossRef]
- Gurovich, A.N.; Montalvo, S.; Hassan, P.F.; Gomez, M. Carotid Arterial Compliance during Different Intensities of Submaximal Endurance Exercise. J. Clin. Med. 2024, 13, 3316. [Google Scholar] [CrossRef]
- Tavakol, M.; Ashraf, S.; Brener, S.J. Risks and Complications of Coronary Angiography: A Comprehensive Review. Glob. J. Health Sci. 2012, 4, 65. [Google Scholar] [CrossRef]
- Wardlaw, J.; Chappell, F.; Best, J.; Wartolowska, K.; Berry, E. Non-Invasive Imaging Compared with Intra-Arterial Angiography in the Diagnosis of Symptomatic Carotid Stenosis: A Meta-Analysis. Lancet 2006, 367, 1503–1512. [Google Scholar] [CrossRef]
- Ali, Z.A.; Karimi Galougahi, K.; Nazif, T.; Maehara, A.; Hardy, M.A.; Cohen, D.J.; Ratner, L.E.; Collins, M.B.; Moses, J.W.; Kirtane, A.J. Imaging-and Physiology-Guided Percutaneous Coronary Intervention without Contrast Administration in Advanced Renal Failure: A Feasibility, Safety, and Outcome Study. Eur. Heart J. 2016, 37, 3090–3095. [Google Scholar] [CrossRef] [PubMed]
- Gurm, H.S.; Dixon, S.R.; Smith, D.E.; Share, D.; LaLonde, T.; Greenbaum, A.; Moscucci, M.; BMC2 (Blue Cross Blue Shield of Michigan Cardiovascular Consortium) Registry. Renal Function-Based Contrast Dosing to Define Safe Limits of Radiographic Contrast Media in Patients Undergoing Percutaneous Coronary Interventions. J. Am. Coll. Cardiol. 2011, 58, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Silenzi, S.; Scalone, G.; di Vito, L.; Mariani, L.; Fraccaro, C.; Travaglini, F.; Grossi, P. Appropriate Use Criteria for Coronary Angiography: A Single Centre Experience. IJC Heart Vasc. 2020, 31, 100677. [Google Scholar] [CrossRef] [PubMed]
- Hartung, M.P.; Grist, T.M.; François, C.J. Magnetic Resonance Angiography: Current Status and Future Directions. J. Cardiovasc. Magn. Reson. 2011, 13, 19. [Google Scholar] [CrossRef]
- Debrey, S.M.; Yu, H.; Lynch, J.K.; Lövblad, K.-O.; Wright, V.L.; Janket, S.-J.D.; Baird, A.E. Diagnostic Accuracy of Magnetic Resonance Angiography for Internal Carotid Artery Disease: A Systematic Review and Meta-Analysis. Stroke 2008, 39, 2237–2248. [Google Scholar] [CrossRef]
- Wutke, R.; Lang, W.; Fellner, C.; Janka, R.; Denzel, C.; Lell, M.; Bautz, W.; Fellner, F.A. High-Resolution, Contrast-Enhanced Magnetic Resonance Angiography with Elliptical Centric k-Space Ordering of Supra-Aortic Arteries Compared with Selective X-Ray Angiography. Stroke 2002, 33, 1522–1529. [Google Scholar] [CrossRef]
- Srichai, M.B.; Lim, R.P.; Wong, S.; Lee, V.S. Cardiovascular Applications of Phase-Contrast MRI. Am. J. Roentgenol. 2009, 192, 662–675. [Google Scholar] [CrossRef]
- Lim, R.P.; Shapiro, M.; Wang, E.; Law, M.; Babb, J.; Rueff, L.; Jacob, J.; Kim, S.; Carson, R.; Mulholland, T. 3D Time-Resolved MR Angiography (MRA) of the Carotid Arteries with Time-Resolved Imaging with Stochastic Trajectories: Comparison with 3D Contrast-Enhanced Bolus-Chase MRA and 3D Time-of-Flight MRA. Am. J. Neuroradiol. 2008, 29, 1847–1854. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, L.; Lu, M.; Zhao, X.; Li, F.; Cai, J.; Yuan, C.; CARE-II investigators. Comparison of Carotid Plaque Characteristics Between Men and Women Using Magnetic Resonance Vessel Wall Imaging: A Chinese Atherosclerosis Risk Evaluation Study. J. Magn. Reson. Imaging 2021, 54, 646–654. [Google Scholar] [CrossRef]
- Schulz, U.G.R.; Rothwell, P.M. Sex Differences in Carotid Bifurcation Anatomy and the Distribution of Atherosclerotic Plaque. Stroke 2001, 32, 1525–1531. [Google Scholar] [CrossRef]
- Krejza, J.; Arkuszewski, M.; Kasner, S.E.; Weigele, J.; Ustymowicz, A.; Hurst, R.W.; Cucchiara, B.L.; Messe, S.R. Carotid Artery Diameter in Men and Women and the Relation to Body and Neck Size. Stroke 2006, 37, 1103–1105. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Hu, N.; Yuan, Y.; Xia, C.; Liu, X.; Zuo, P.; Stalder, A.F.; Schmidt, M.; Zhou, X.; Song, B.; et al. Accelerated Time-of-Flight Magnetic Resonance Angiography with Sparse Undersampling and Iterative Reconstruction for the Evaluation of Intracranial Arteries. Korean J. Radiol. 2019, 20, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.-C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an Image Computing Platform for the Quantitative Imaging Network. Quant. Imaging Cancer 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [PubMed]
- Bressloff, N.W. Parametric Geometry Exploration of the Human Carotid Artery Bifurcation. J. Biomech. 2007, 40, 2483–2491. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Phan, T.G.; Beare, R.J.; Jolley, D.; Das, G.; Ren, M.; Wong, K.; Chong, W.; Sinnott, M.D.; Hilton, J.E.; Srikanth, V. Carotid Artery Anatomy and Geometry as Risk Factors for Carotid Atherosclerotic Disease. Stroke 2012, 43, 1596–1601. [Google Scholar] [CrossRef]
- Abhilash, H.; Yanagita, Y.; Pai, R.; Zuber, M.; Tamagawa, M.; Prakashini, K.; Kamath, G.; Padmakumar, R.; Barboza, A.; Rao, V. Effect of Vascular Geometry on Haemodynamic Changes in a Carotid Artery Bifurcation Using Numerical Simulation. Clin. Neurol. Neurosurg. 2024, 237, 108153. [Google Scholar] [CrossRef]
- Ratcliffe, B.; Pawlak, R.; Morales, F.; Harrison, C.; Gurovich, A.N. Internal Validation of an Automated System for Brachial and Femoral Flow Mediated Dilation. Clin. Hypertens. 2017, 23, 17. [Google Scholar] [CrossRef]
- Goubergrits, L.; Affeld, K.; Fernandez-Britto, J.; Falcon, L. Geometry of the Human Common Carotid Artery. A Vessel Cast Study of 86 Specimens. Pathol. Res. Pract. 2002, 198, 543–551. [Google Scholar] [CrossRef]
- Thomas, J.B.; Antiga, L.; Che, S.L.; Milner, J.S.; Hangan Steinman, D.A.; Spence, J.D.; Rutt, B.K.; Steinman, D.A. Variation in the Carotid Bifurcation Geometry of Young Versus Older Adults. Stroke 2005, 36, 2450–2456. [Google Scholar] [CrossRef]
- Urquiza, S.A.; Blanco, P.J.; Vénere, M.J.; Feijóo, R.A. Multidimensional Modelling for the Carotid Artery Blood Flow. Comput. Methods Appl. Mech. Eng. 2006, 195, 4002–4017. [Google Scholar] [CrossRef]
| Overall (n = 20) | Females (n = 10) | Males (n = 10) | p | |
|---|---|---|---|---|
| Age (years) Mean (SD) | 26.9 (3.4) | 26.3 (2.4) | 27.4 (4.2) | 0.48 |
| Height (m) Mean (SD) | 1.68 (0.10) | 1.62 (0.06) | 1.73 (0.10) | 0.01 |
| Weight (kg) Mean (SD) | 68.9 (14.3) | 62.2 (10.0) | 75.7 (15.1) | 0.03 |
| BMI (kg/m2) Mean (SD) | 24.5 (4.0) | 23.6 (3.7) | 25.3 (4.3) | 0.38 |
| Resting systolic BP (mmHg) Mean (SD) | 113 (9) | 109 (9) | 117 (8) | 0.03 |
| Resting diastolic BP (mmHg) Mean (SD) | 73 (14) | 71 (11) | 76 (17) | 0.41 |
| Females | Males | Side Effect | Sex Effect | Side × Sex Interaction | |||
|---|---|---|---|---|---|---|---|
| Left (n = 10) | Right (n = 10) | Left (n = 10) | Right (n = 10) | ANOVA (F, p) | ANOVA (F, p) | ANOVA (F, p) | |
| MID (mm) Mean (SD) | 3.29 (1.02) | 3.27 (0.62) | 3.52 (0.77) | 3.47 (0.52) | 0.081 0.779 | 0.490 0.493 | 0.012 0.913 |
| BIF (mm) Mean (SD) | 6.02 ** (0.64) | 6.68 (1.19) | 7.20 ** (1.34) | 7.26 (1.27) | 2.373 0.141 | 3.770 0.068 | 1.617 0.220 |
| ICA (mm) Mean (SD) | 3.29 (0.39) | 3.25 (0.44) | 3.61 (0.48) | 3.54 (0.56) | 0.244 0.627 | 3.030 0.099 | 0.010 0.923 |
| ECA (mm) Mean (SD) | 3.00 * (0.62) | 2.98 (0.34) | 3.68 * (1.28) | 3.36 (0.50) | 0.656 0.429 | 3.730 0.069 | 0.518 0.481 |
| BIF angle (°) Mean (SD) | 90.3 (11.7) | 89.0 (12.0) | 88.4 (13.1) | 87.4 (11.8) | 1.856 0.190 | 0.106 0.748 | 0.022 0.883 |
| ICA angle (°) Mean (SD) | 37.8 (13.9) | 38.5 (13.3) | 41.1 (8.2) | 40.4 (8.7) | 0.001 0.976 | 0.268 0.611 | 0.860 0.366 |
| ECA angle (°) Mean (SD) | 52.5 (7.7) | 50.5 (10.2) | 47.3 (7.8) | 47.0 (6.6) | 1.454 0.244 | 1.511 0.235 | 0.685 0.419 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez, P.; Torres, J.R.; Conde, D.; Gomez, M.; Gurovich, A.N. Sex-Specific Analysis of Carotid Artery Through Bilateral 3D Modeling via MRI and DICOM Processing. Bioengineering 2025, 12, 142. https://doi.org/10.3390/bioengineering12020142
Martinez P, Torres JR, Conde D, Gomez M, Gurovich AN. Sex-Specific Analysis of Carotid Artery Through Bilateral 3D Modeling via MRI and DICOM Processing. Bioengineering. 2025; 12(2):142. https://doi.org/10.3390/bioengineering12020142
Chicago/Turabian StyleMartinez, Pedro, Jose Roberto Torres, Daniel Conde, Manuel Gomez, and Alvaro N. Gurovich. 2025. "Sex-Specific Analysis of Carotid Artery Through Bilateral 3D Modeling via MRI and DICOM Processing" Bioengineering 12, no. 2: 142. https://doi.org/10.3390/bioengineering12020142
APA StyleMartinez, P., Torres, J. R., Conde, D., Gomez, M., & Gurovich, A. N. (2025). Sex-Specific Analysis of Carotid Artery Through Bilateral 3D Modeling via MRI and DICOM Processing. Bioengineering, 12(2), 142. https://doi.org/10.3390/bioengineering12020142







