Study on the Polarization of Astrocytes in the Optic Nerve Head of Rats Under High Intraocular Pressure: In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
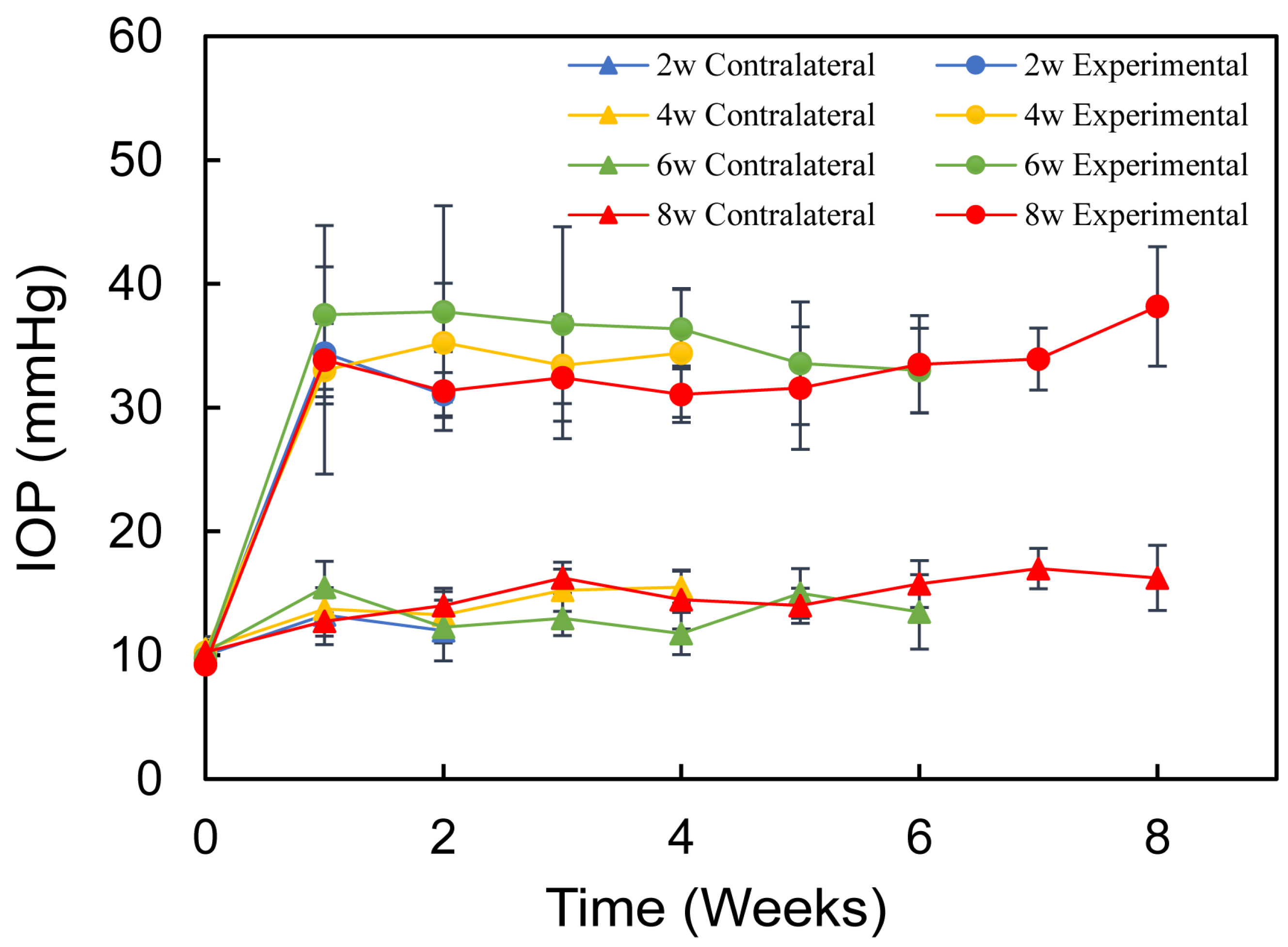
2.2. Model Induction and IOP Measurement
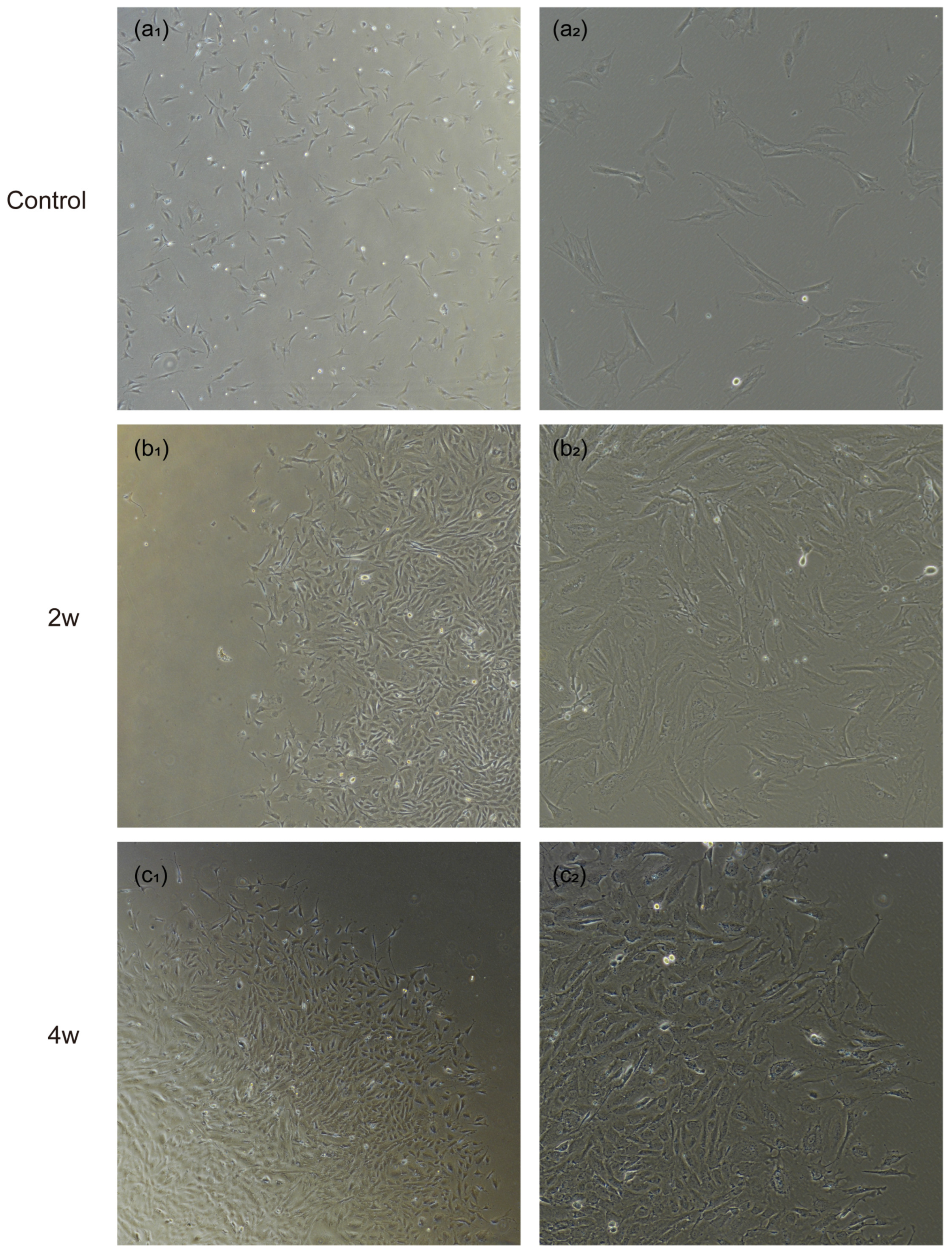
2.3. Isolation of Astrocytes from Adult Rat Lamina Cribrosa
2.4. Cell Passaging and Purification
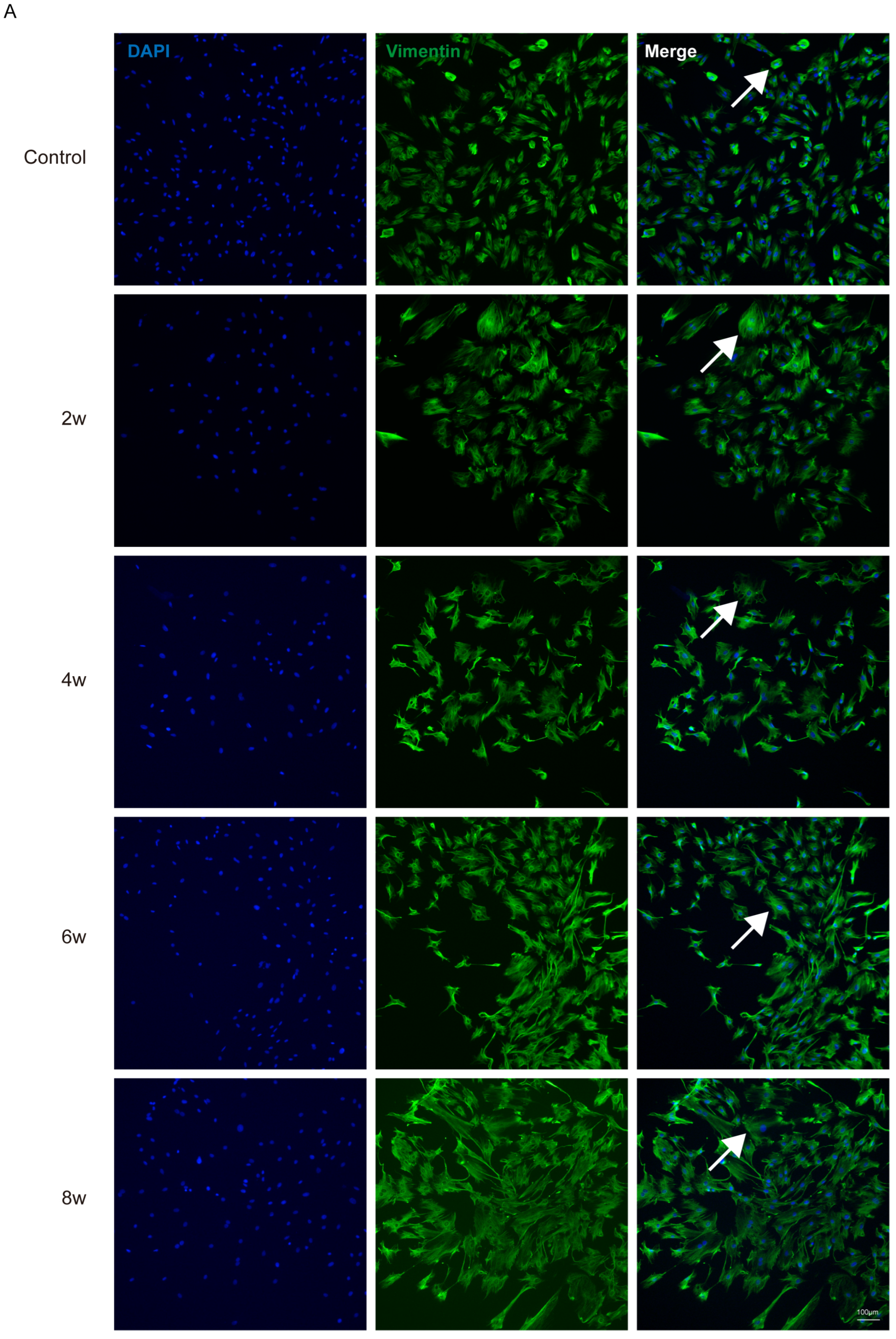
2.5. Cell Immunofluorescence Staining
2.6. Protein Extraction and Digestion
2.7. Data-Dependent Acquisition (DDA) and Data-Independent Acquisition (DIA) Analysis by Nanoscale Liquid Chromatography Coupled with Tandem Mass Spectrometry
2.8. DIA-MS Data Analysis
2.9. Western Blotting
2.10. Messenger RNA Extraction and qPCR
2.11. Statistical Analyses
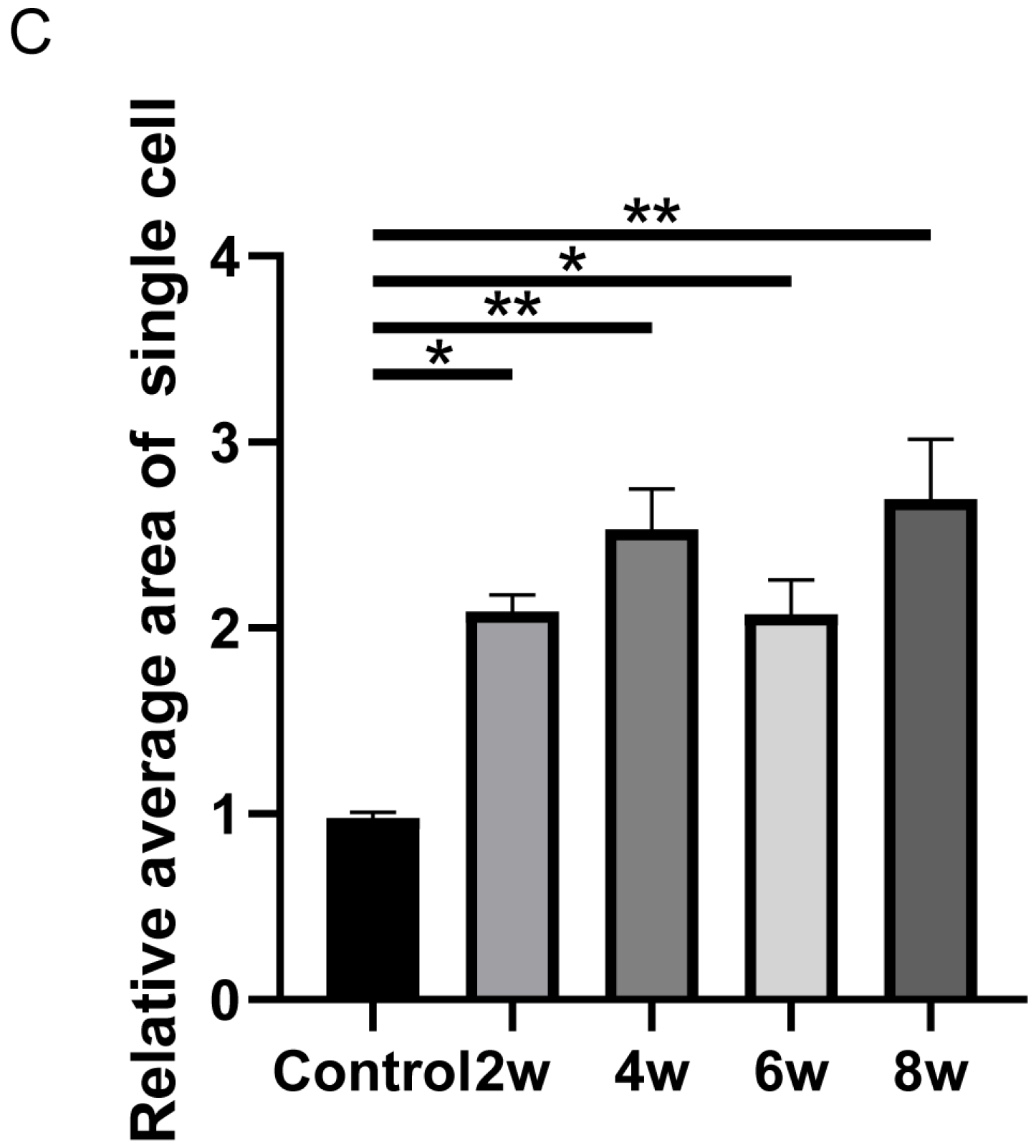
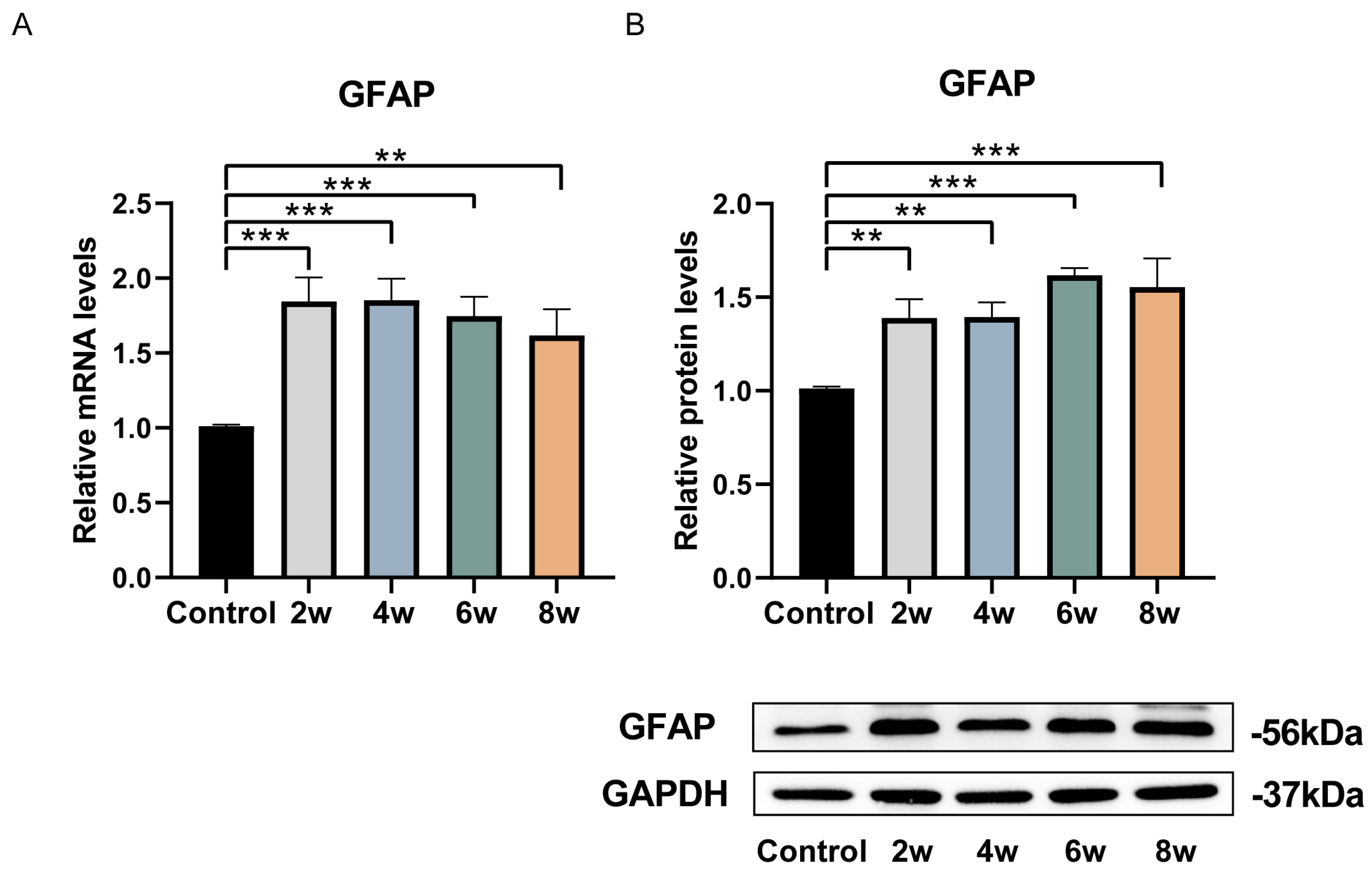
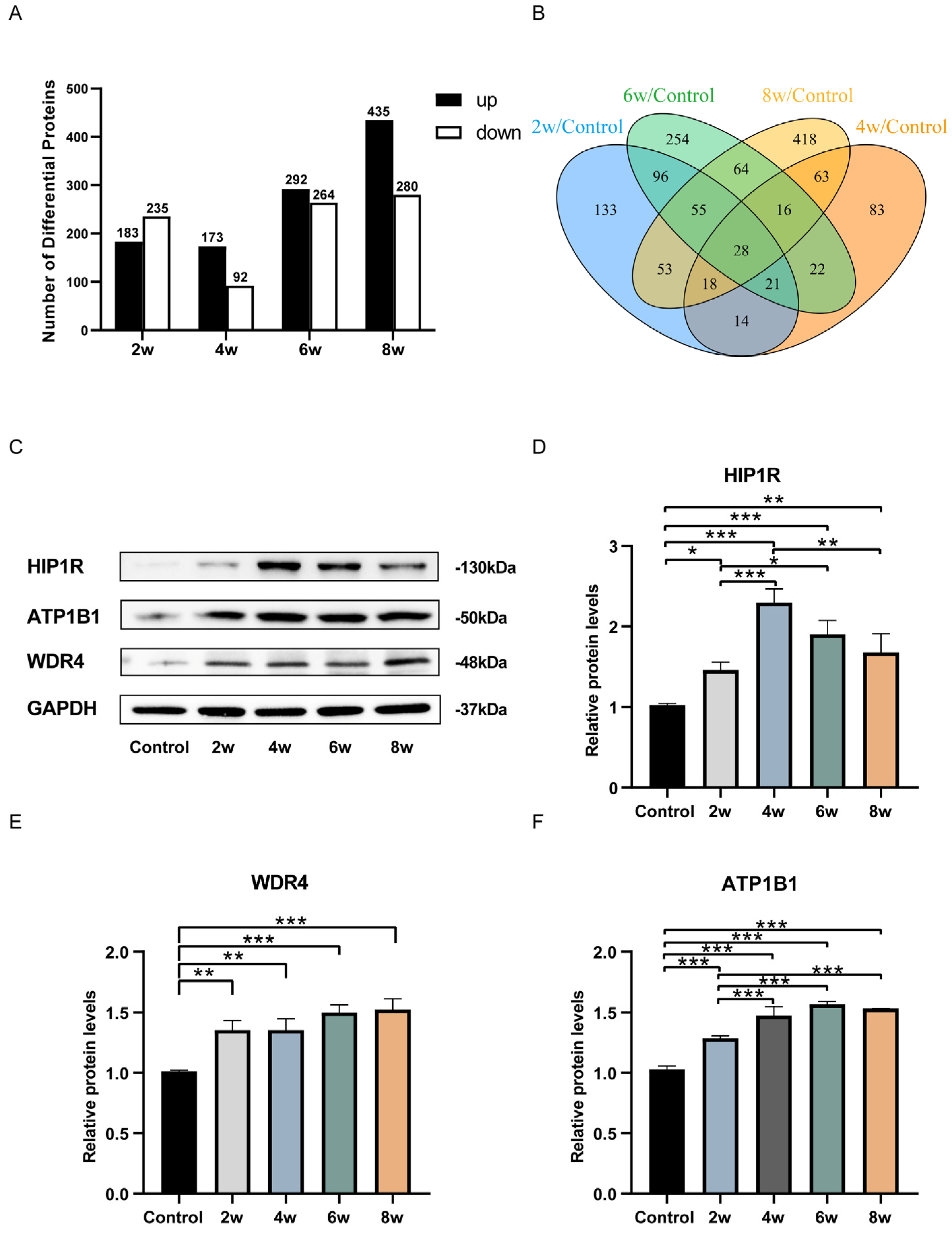
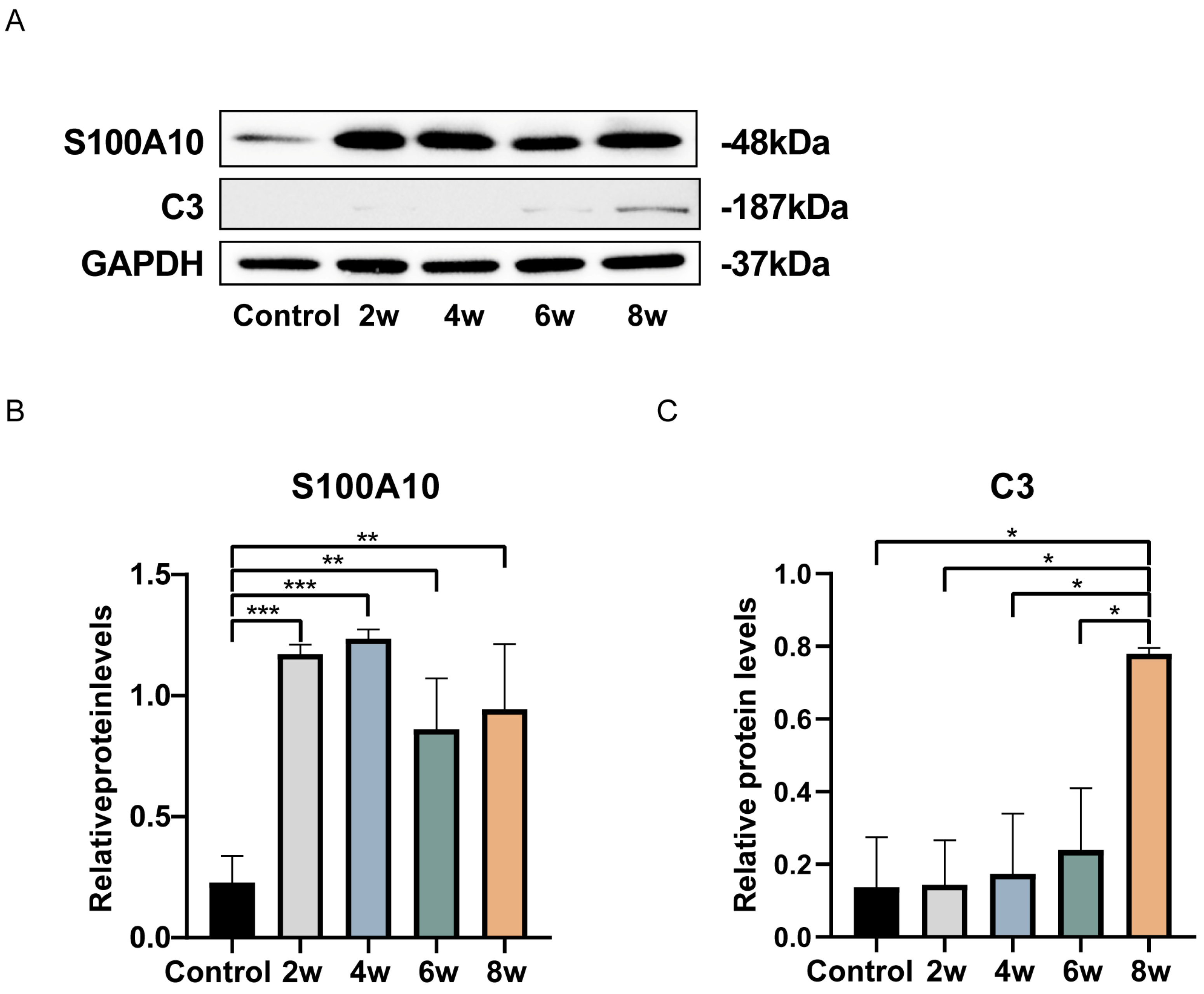
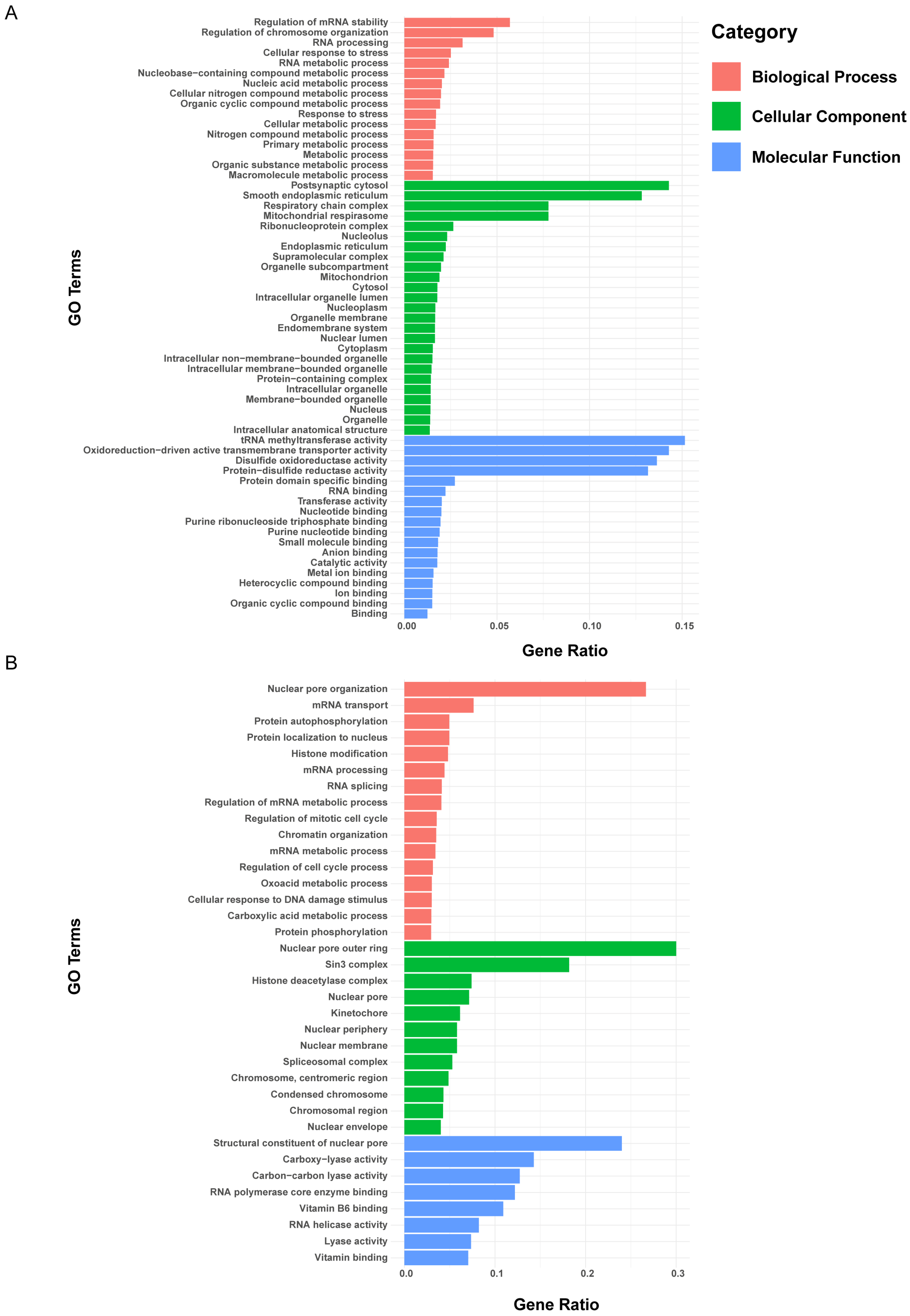
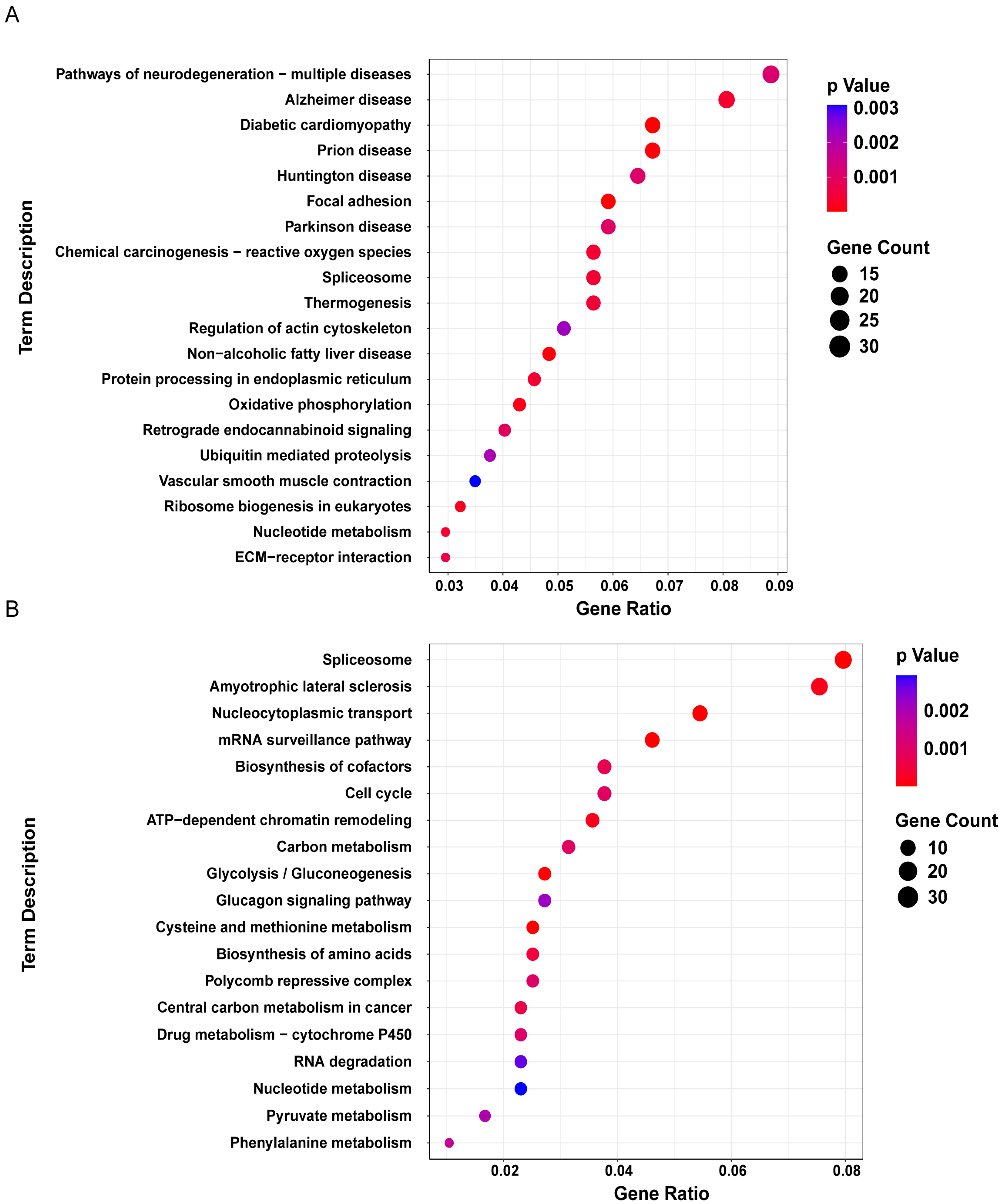
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A. The Number of Nuclei and Vimentin-Positive Cells in Vimentin Immunofluorescence Staining of Cells Extracted at Different Time Points Under High IOP
| Control | 2w | 4w | 6w | 8w | |
|---|---|---|---|---|---|
| The number of cell nuclei | 253 | 86 | 77 | 151 | 132 |
| The number of vimentin−positive cells | 253 | 86 | 77 | 151 | 132 |
References
- Tham, Y.-C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.-Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Report on Vision; World Health Organization: Geneva, Switzerland, 2019; Volume 214, ISBN 9789241516570. [Google Scholar]
- Quigley, H.A.; Addicks, E.M.; Green, W.R.; Maumenee, A.E. Optic Nerve Damage in Human Glaucoma: II. The Site of Injury and Susceptibility to Damage. Arch. Ophthalmol. 1981, 99, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, B.P.; Inman, D.M.; Lambert, W.; Oglesby, E.; Calkins, D.J.; Steele, M.R.; Vetter, M.L.; Marsh-Armstrong, N.; Horner, P.J. Progressive Ganglion Cell Degeneration Precedes Neuronal Loss in a Mouse Model of Glaucoma. J. Neurosci. 2008, 28, 2735–2744. [Google Scholar] [CrossRef]
- Soto, I.; Oglesby, E.; Buckingham, B.P.; Son, J.L.; Roberson, E.D.O.; Steele, M.R.; Inman, D.M.; Vetter, M.L.; Horner, P.J.; Marsh-Armstrong, N. Retinal Ganglion Cells Downregulate Gene Expression and Lose Their Axons within the Optic Nerve Head in a Mouse Glaucoma Model. J. Neurosci. 2008, 28, 548–561. [Google Scholar] [CrossRef]
- Chidlow, G.; Ebneter, A.; Wood, J.P.M.; Casson, R.J. The Optic Nerve Head Is the Site of Axonal Transport Disruption, Axonal Cytoskeleton Damage and Putative Axonal Regeneration Failure in a Rat Model of Glaucoma. Acta Neuropathol. 2011, 121, 737–751. [Google Scholar] [CrossRef]
- Mélik Parsadaniantz, S.; Réaux-le Goazigo, A.; Sapienza, A.; Habas, C.; Baudouin, C. Glaucoma: A Degenerative Optic Neuropathy Related to Neuroinflammation? Cells 2020, 9, 535. [Google Scholar] [CrossRef]
- Midgett, D.E.; Jefferys, J.L.; Quigley, H.A.; Nguyen, T.D. The Inflation Response of the Human Lamina Cribrosa and Sclera: Analysis of Deformation and Interaction. Acta Biomater. 2020, 106, 225–241. [Google Scholar] [CrossRef]
- Schneider, M.; Fuchshofer, R. The Role of Astrocytes in Optic Nerve Head Fibrosis in Glaucoma. Exp. Eye Res. 2016, 142, 49–55. [Google Scholar] [CrossRef]
- Zhao, G.; Zhou, H.; Guo, Y.; Zhong, S.; Zhou, H.; Li, F.; Lei, B.; Wang, Z.; Miao, Y. Modulation of Rac1/PAK1/Connexin43-mediated ATP Release from Astrocytes Contributes to Retinal Ganglion Cell Survival in Experimental Glaucoma. GLIA 2023, 71, 1502–1521. [Google Scholar] [CrossRef]
- Waxman, S.; Quinn, M.; Donahue, C.; Falo, L.D., Jr.; Sun, D.; Jakobs, T.C.; Sigal, I.A. Individual Astrocyte Morphology in the Collagenous Lamina Cribrosa Revealed by Multicolor DiOlistic Labeling. Exp. Eye Res. 2023, 230, 109458. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Molecular Dissection of Reactive Astrogliosis and Glial Scar Formation. Trends Neurosci. 2009, 32, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, S.; Davis, L.; Cepurna, W.O.; Delf, R.K.; Lozano, D.C.; Choe, T.E.; Johnson, E.C.; Morrison, J.C. Optic Nerve Head Astrocytes Display Axon-Dependent and -Independent Reactivity in Response to Acutely Elevated Intraocular Pressure. Investig. Ophthalmol. Vis. Sci. 2019, 60, 312. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Parpura, V.; Verkhratsky, A. Neuroglia as a Central Element of Neurological Diseases: An Underappreciated Target for Therapeutic Intervention. Curr. Neuropharmacol. 2014, 12, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Lozano, D.C.; Choe, T.E.; Cepurna, W.O.; Morrison, J.C.; Johnson, E.C. Early Optic Nerve Head Glial Proliferation and Jak-Stat Pathway Activation in Chronic Experimental Glaucoma. Investig. Ophthalmol. Vis. Sci. 2019, 60, 921. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Seifert, P.; Jakobs, T.C. Astrocytes in the Optic Nerve Head of Glaucomatous Mice Display a Characteristic Reactive Phenotype. Investig. Ophthalmol. Vis. Sci. 2017, 58, 924. [Google Scholar] [CrossRef]
- Hartmann, K.; Sepulveda-Falla, D.; Rose, I.V.L.; Madore, C.; Muth, C.; Matschke, J.; Butovsky, O.; Liddelow, S.; Glatzel, M.; Krasemann, S. Complement 3+-Astrocytes Are Highly Abundant in Prion Diseases, but Their Abolishment Led to an Accelerated Disease Course and Early Dysregulation of Microglia. Acta Neuropathol. Commun. 2019, 7, 83. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, H.; Guo, W. Astrocyte Polarization in Glaucoma: A New Opportunity. Neural Regen. Res. 2022, 17, 2582. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Liu, L.; Li, L.; Qian, X. Morphological Changes of Glial Lamina Cribrosa of Rats Suffering from Chronic High Intraocular Pressure. Bioengineering 2022, 9, 741. [Google Scholar] [CrossRef]
- Zamanian, J.L.; Xu, L.; Foo, L.C.; Nouri, N.; Zhou, L.; Giffard, R.G.; Barres, B.A. Genomic Analysis of Reactive Astrogliosis. J. Neurosci. 2012, 32, 6391–6410. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Joshi, A.U.; Minhas, P.S.; Liddelow, S.A.; Haileselassie, B.; Andreasson, K.I.; Dorn, G.W.; Mochly-Rosen, D. Fragmented Mitochondria Released from Microglia Trigger A1 Astrocytic Response and Propagate Inflammatory Neurodegeneration. Nat. Neurosci. 2019, 22, 1635–1648. [Google Scholar] [CrossRef]
- Hou, B.; Zhang, Y.; Liang, P.; He, Y.; Peng, B.; Liu, W.; Han, S.; Yin, J.; He, X. Inhibition of the NLRP3-Inflammasome Prevents Cognitive Deficits in Experimental Autoimmune Encephalomyelitis Mice via the Alteration of Astrocyte Phenotype. Cell Death Dis. 2020, 11, 377. [Google Scholar] [CrossRef]
- Barbar, L.; Jain, T.; Zimmer, M.; Kruglikov, I.; Sadick, J.S.; Wang, M.; Kalpana, K.; Rose, I.V.L.; Burstein, S.R.; Rusielewicz, T.; et al. CD49f Is a Novel Marker of Functional and Reactive Human iPSC-Derived Astrocytes. Neuron 2020, 107, 436–453.e12. [Google Scholar] [CrossRef]
- Guttenplan, K.A.; Weigel, M.K.; Prakash, P.; Wijewardhane, P.R.; Hasel, P.; Rufen-Blanchette, U.; Münch, A.E.; Blum, J.A.; Fine, J.; Neal, M.C.; et al. Neurotoxic Reactive Astrocytes Induce Cell Death via Saturated Lipids. Nature 2021, 599, 102–107. [Google Scholar] [CrossRef]
- Anderson, M.A.; Burda, J.E.; Ren, Y.; Ao, Y.; O’Shea, T.M.; Kawaguchi, R.; Coppola, G.; Khakh, B.S.; Deming, T.J.; Sofroniew, M.V. Astrocyte Scar Formation Aids Central Nervous System Axon Regeneration. Nature 2016, 532, 195–200. [Google Scholar] [CrossRef]
- Su, Y.; Chen, Z.; Du, H.; Liu, R.; Wang, W.; Li, H.; Ning, B. Silencing miR-21 Induces Polarization of Astrocytes to the A2 Phenotype and Improves the Formation of Synapses by Targeting Glypican 6 via the Signal Transducer and Activator of Transcription-3 Pathway after Acute Ischemic Spinal Cord Injury. FASEB J. 2019, 33, 10859–10871. [Google Scholar] [CrossRef]
- Diniz, L.P.; Tortelli, V.; Garcia, M.N.; Araújo, A.P.B.; Melo, H.M.; Seixas Da Silva, G.S.; De Felice, F.G.; Alves-Leon, S.V.; De Souza, J.M.; Romão, L.F.; et al. Astrocyte Transforming Growth Factor Beta 1 Promotes Inhibitory Synapse Formation via CaM Kinase II Signaling. GLIA 2014, 62, 1917–1931. [Google Scholar] [CrossRef]
- Kang, W.; Balordi, F.; Su, N.; Chen, L.; Fishell, G.; Hébert, J.M. Astrocyte Activation Is Suppressed in Both Normal and Injured Brain by FGF Signaling. Proc. Natl. Acad. Sci. USA 2014, 111, E2987–E2995. [Google Scholar] [CrossRef]
- Norden, D.M.; Fenn, A.M.; Dugan, A.; Godbout, J.P. TGFβ Produced by IL-10 Redirected Astrocytes Attenuates Microglial Activation. GLIA 2014, 62, 881–895. [Google Scholar] [CrossRef]
- Wang, J.; Sareddy, G.R.; Lu, Y.; Pratap, U.P.; Tang, F.; Greene, K.M.; Meyre, P.L.; Tekmal, R.R.; Vadlamudi, R.K.; Brann, D.W. Astrocyte-Derived Estrogen Regulates Reactive Astrogliosis and Is Neuroprotective Following Ischemic Brain Injury. J. Neurosci. 2020, 40, 9751–9771. [Google Scholar] [CrossRef]
- Wang, J.; Hou, Y.; Zhang, L.; Liu, M.; Zhao, J.; Zhang, Z.; Ma, Y.; Hou, W. Estrogen Attenuates Traumatic Brain Injury by Inhibiting the Activation of Microglia and Astrocyte-Mediated Neuroinflammatory Responses. Mol. Neurobiol. 2021, 58, 1052–1061. [Google Scholar] [CrossRef]
- Li, Y.; Xie, Y.; Liu, R.; Wang, Z.; Chen, P.; Wang, M.; Yu, Z.; Wang, W.; Luo, X. Knockout of Microglial Hv1 Proton Channel Reduces Neurotoxic A1 Astrocytes and Neuronal Damage via the ROS/STAT3 Pathway after Spinal Cord Injury. GLIA 2023, 71, 2418–2436. [Google Scholar] [CrossRef]
- Cameron, E.G.; Nahmou, M.; Toth, A.B.; Heo, L.; Tanasa, B.; Dalal, R.; Yan, W.; Nallagatla, P.; Xia, X.; Hay, S.; et al. A Molecular Switch for Neuroprotective Astrocyte Reactivity. Nature 2024, 626, 574–582. [Google Scholar] [CrossRef]
- Li, T.; Li, L.; Liu, Z. Time Course Changes of the Mechanical Properties of the Iris Pigment Epithelium in a Rat Chronic Ocular Hypertension Model. BioMed Res. Int. 2018, 2018, 4862309. [Google Scholar] [CrossRef]
- Peng, F.; Ma, L.; Liu, L.; Li, L.; Qian, X. Preliminary Study on the Blockade of Axonal Transport by Activated Astrocytes in Optic Nerve Head under Chronic Ocular Hypertension. J. Mech. Med. Biol. 2019, 19, 1940040. [Google Scholar] [CrossRef]
- Ernst, O.; Zor, T. Linearization of the Bradford Protein Assay. JoVE 2010, 38, 1918. [Google Scholar] [CrossRef]
- Ma, J.; Liu, M.; Wang, Y.; Xin, C.; Zhang, H.; Chen, S.; Zheng, X.; Zhang, X.; Xiao, F.; Yang, S. Quantitative Proteomics Analysis of Young and Elderly Skin with DIA Mass Spectrometry Reveals New Skin Aging-Related Proteins. Aging 2020, 12, 13529–13554. [Google Scholar] [CrossRef]
- Zhu, W.; Cheng, X.; Ren, C.; Chen, J.; Zhang, Y.; Chen, Y.; Jia, X.; Wang, S.; Sun, Z.; Zhang, R.; et al. Proteomic Characterization and Comparison of Ram (Ovis aries) and Buck (Capra hircus) Spermatozoa Proteome Using a Data Independent Acquisition Mass Spectometry (DIA-MS) Approach. PLoS ONE 2020, 15, e0228656. [Google Scholar] [CrossRef]
- Cox, J.; Mann, M. MaxQuant Enables High Peptide Identification Rates, Individualized p.p.b.-Range Mass Accuracies and Proteome-Wide Protein Quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef]
- Bruderer, R.; Bernhardt, O.M.; Gandhi, T.; Reiter, L. High-precision iRT Prediction in the Targeted Analysis of Data-independent Acquisition and Its Impact on Identification and Quantitation. Proteomics 2016, 16, 2246–2256. [Google Scholar] [CrossRef]
- Choi, M.; Chang, C.-Y.; Clough, T.; Broudy, D.; Killeen, T.; MacLean, B.; Vitek, O. MSstats: An R Package for Statistical Analysis of Quantitative Mass Spectrometry-Based Proteomic Experiments. Bioinformatics 2014, 30, 2524–2526. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Dai, Y.; Chen, G.; Cui, S. Dissecting the Dual Role of the Glial Scar and Scar-Forming Astrocytes in Spinal Cord Injury. Front. Cell. Neurosci. 2020, 14, 78. [Google Scholar] [CrossRef]
- Dai, C.; Khaw, P.T.; Yin, Z.Q.; Li, D.; Raisman, G.; Li, Y. Structural Basis of Glaucoma: The Fortified Astrocytes of the Optic Nerve Head Are the Target of Raised Intraocular Pressure. GLIA 2012, 60, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Wahane, S.; Sofroniew, M.V. Loss-of-Function Manipulations to Identify Roles of Diverse Glia and Stromal Cells during CNS Scar Formation. Cell Tissue Res. 2022, 387, 337–350. [Google Scholar] [CrossRef]
- Bush, T.G.; Puvanachandra, N.; Horner, C.H.; Polito, A.; Ostenfeld, T.; Svendsen, C.N.; Mucke, L.; Johnson, M.H.; Sofroniew, M.V. Leukocyte Infiltration, Neuronal Degeneration, and Neurite Outgrowth after Ablation of Scar-Forming, Reactive Astrocytes in Adult Transgenic Mice. Neuron 1999, 23, 297–308. [Google Scholar] [CrossRef]
- Herrmann, J.E.; Imura, T.; Song, B.; Qi, J.; Ao, Y.; Nguyen, T.K.; Korsak, R.A.; Takeda, K.; Akira, S.; Sofroniew, M.V. STAT3 Is a Critical Regulator of Astrogliosis and Scar Formation after Spinal Cord Injury. J. Neurosci. 2008, 28, 7231–7243. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, R.; Pappas, A.C.; Seifert, P.; Savol, A.; Sadreyev, R.I.; Sun, D.; Jakobs, T.C. Astrocytes in the Optic Nerve Are Heterogeneous in Their Reactivity to Glaucomatous Injury. Cells 2023, 12, 2131. [Google Scholar] [CrossRef]
- Sun, D.; Moore, S.; Jakobs, T.C. Optic Nerve Astrocyte Reactivity Protects Function in Experimental Glaucoma and Other Nerve Injuries. J. Exp. Med. 2017, 214, 1411–1430. [Google Scholar] [CrossRef]
- Guttenplan, K.A.; Stafford, B.K.; El-Danaf, R.N.; Adler, D.I.; Münch, A.E.; Weigel, M.K.; Huberman, A.D.; Liddelow, S.A. Neurotoxic Reactive Astrocytes Drive Neuronal Death after Retinal Injury. Cell Rep. 2020, 31, 107776. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, Y.; Chen, D. The Heterogeneity of Astrocytes in Glaucoma. Front. Neuroanat. 2022, 16, 995369. [Google Scholar] [CrossRef]
- Sun, D.; Lye-Barthel, M.; Masland, R.H.; Jakobs, T.C. The Morphology and Spatial Arrangement of Astrocytes in the Optic Nerve Head of the Mouse. J. Comp. Neurol. 2009, 516, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Oberheim, N.A.; Tian, G.-F.; Han, X.; Peng, W.; Takano, T.; Ransom, B.; Nedergaard, M. Loss of Astrocytic Domain Organization in the Epileptic Brain. J. Neurosci. 2008, 28, 3264–3276. [Google Scholar] [CrossRef] [PubMed]
- Wanner, I.B.; Anderson, M.A.; Song, B.; Levine, J.; Fernandez, A.; Gray-Thompson, Z.; Ao, Y.; Sofroniew, M.V. Glial Scar Borders Are Formed by Newly Proliferated, Elongated Astrocytes That Interact to Corral Inflammatory and Fibrotic Cells via STAT3-Dependent Mechanisms after Spinal Cord Injury. J. Neurosci. 2013, 33, 12870–12886. [Google Scholar] [CrossRef]
- Morizawa, Y.M.; Hirayama, Y.; Ohno, N.; Shibata, S.; Shigetomi, E.; Sui, Y.; Nabekura, J.; Sato, K.; Okajima, F.; Takebayashi, H.; et al. Reactive Astrocytes Function as Phagocytes after Brain Ischemia via ABCA1-Mediated Pathway. Nat. Commun. 2017, 8, 28. [Google Scholar] [CrossRef]
- Mazumder, A.G.; Julé, A.M.; Sun, D. Astrocytes of the Optic Nerve Exhibit a Region-Specific and Temporally Distinct Response to Elevated Intraocular Pressure. Mol. Neurodegener. 2023, 18, 68. [Google Scholar] [CrossRef]
- Phatnani, H.; Maniatis, T. Astrocytes in Neurodegenerative Disease: Table 1. Cold Spring Harb. Perspect. Biol. 2015, 7, a020628. [Google Scholar] [CrossRef]
- Amirian, R.; Badrbani, M.A.; Derakhshankhah, H.; Izadi, Z.; Shahbazi, M.-A. Targeted Protein Degradation for the Treatment of Parkinson’s Disease: Advances and Future Perspective. Biomed. Pharmacother. 2023, 166, 115408. [Google Scholar] [CrossRef]
- Eva, I.-T.; Maliha, S.; Nelson, W.; Silvia, V.; Philipp, S.; Erich, W.E.; Bieschke, J. Unconventional Chaperone Inhibits Amyloid Formation by Promoting Off-Pathway Aggregation. Biophys. J. 2016, 110, 551a–552a. [Google Scholar] [CrossRef][Green Version]
- Ziff, O.J.; Taha, D.M.; Crerar, H.; Clarke, B.E.; Chakrabarti, A.M.; Kelly, G.; Neeves, J.; Tyzack, G.E.; Luscombe, N.M.; Patani, R. Reactive Astrocytes in ALS Display Diminished Intron Retention. Nucleic Acids Res. 2021, 49, 3168–3184. [Google Scholar] [CrossRef]
- Shelke, G.V.; Lässer, C.; Gho, Y.S.; Lötvall, J. Importance of Exosome Depletion Protocols to Eliminate Functional and RNA-containing Extracellular Vesicles from Fetal Bovine Serum. J. Extracell. Vesicles 2014, 3, 24783. [Google Scholar] [CrossRef]
- Aswad, H.; Jalabert, A.; Rome, S. Depleting Extracellular Vesicles from Fetal Bovine Serum Alters Proliferation and Differentiation of Skeletal Muscle Cells in Vitro. BMC Biotechnol. 2016, 16, 32. [Google Scholar] [CrossRef] [PubMed]
- Kirikae, T.; Tamura, H.; Hashizume, M.; Kirikae, F.; Uemura, Y.; Tanaka, S.; Yokochi, T.; Nakano, M. Endotoxin Contamination in Fetal Bovine Serum and Its Influence on Tumor Necrosis Factor Production by Macrophage-like Cells J774.1 Cultured in the Presence of the Serum. Int. J. Immunopharmacol. 1997, 19, 255–262. [Google Scholar] [CrossRef] [PubMed]



| Protein_ID | Gene_ID | Fold Change | |||
|---|---|---|---|---|---|
| 2w | 4w | 6w | 8w | ||
| tr_A0A0G2K779_A0A0G2K779_RAT | CEP76 | 2.83 | 2.15 | 2.49 | 2.55 |
| tr_B5DFK5_B5DFK5_RAT | HIP1R | 1.09 | 1.08 | 1.11 | 1.22 |
| tr_A0A0G2K1G5_A0A0G2K1G5_RAT | LTBP2 | 1.98 | 1.19 | 2.04 | 1.50 |
| sp_Q63531_KS6A1_RAT | RPS6KA1 | 2.85 | 1.41 | 1.48 | 3.29 |
| tr_D3ZIT4_D3ZIT4_RAT | ANAPC7 | 1.26 | 1.31 | 1.16 | 1.62 |
| tr_B0BN35_B0BN35_RAT | ELP4 | 1.32 | 1.64 | 1.74 | 1.89 |
| sp_P31977_EZRI_RAT | EZR | 1.09 | 1.12 | 1.34 | 1.01 |
| tr_D3ZY40_D3ZY40_RAT | PCF11 | 1.79 | 2.17 | 1.66 | 1.95 |
| tr_D4ACM1_D4ACM1_RAT | ELP3 | 1.15 | 1.27 | 1.00 | 1.82 |
| tr_B2RZB8_B2RZB8_RAT | PSMG4 | 1.53 | 1.35 | 1.31 | 1.48 |
| tr_D4A0Q3_D4A0Q3_RAT | WDR4 | 3.01 | 1.84 | 2.66 | 2.18 |
| tr_D4A435_D4A435_RAT | ICAM5 | 1.95 | 1.30 | 2.49 | 1.82 |
| tr_D4A6W4_D4A6W4_RAT | RFT1 | 1.12 | 1.25 | 1.38 | 1.20 |
| sp_P07340_AT1B1_RAT | ATP1B1 | 1.19 | 1.67 | 2.27 | 1.19 |
| sp_O88484_PDP2_RAT | PDP2 | 2.87 | 2.29 | 2.58 | 2.72 |
| sp_Q6MG60_DDAH2_RAT | DDAH2 | −1.07 | −1.01 | −2.17 | −1.01 |
| tr_A0A0G2K0J7_A0A0G2K0J7_RAT | CTDP1 | −1.14 | −1.39 | −1.06 | −1.27 |
| sp_Q2YDU8_SPNS1_RAT | SPNS1 | −1.25 | −1.16 | −2.37 | −1.28 |
| tr_M0RA39_M0RA39_RAT | MXRA7 | −1.40 | −1.05 | −1.22 | −1.25 |
| sp_Q923W4_HDGR3_RAT | HDGFL3 | −1.40 | −1.11 | −1.37 | −1.52 |
| sp_P23764_GPX3_RAT | GPX3 | −1.46 | −1.00 | −1.17 | −1.39 |
| tr_F1LLW8_F1LLW8_RAT | IDS | −1.53 | −1.14 | −1.64 | −1.16 |
| sp_Q6IRK9_CBPQ_RAT | CPQ | −1.54 | −1.54 | −1.57 | −2.38 |
| sp_P30919_ASPG_RAT | AGA | −1.72 | −1.04 | −1.78 | −2.25 |
| tr_I6LBX8_I6LBX8_RAT | PCDHGC3 | −1.90 | −1.01 | −1.57 | −1.14 |
| tr_F1LMV9_F1LMV9_RAT | CORO2B | −2.04 | −1.34 | −1.63 | −2.48 |
| sp_Q6AY80_NQO2_RAT | NQO2 | −3.31 | −1.71 | −4.41 | −2.66 |
| tr_Q9JKB7_Q9JKB7_RAT | GDA | −2.04 | −2.60 | −2.36 | 1.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, B.; Ren, J.; Qian, X. Study on the Polarization of Astrocytes in the Optic Nerve Head of Rats Under High Intraocular Pressure: In Vitro. Bioengineering 2025, 12, 104. https://doi.org/10.3390/bioengineering12020104
Ma B, Ren J, Qian X. Study on the Polarization of Astrocytes in the Optic Nerve Head of Rats Under High Intraocular Pressure: In Vitro. Bioengineering. 2025; 12(2):104. https://doi.org/10.3390/bioengineering12020104
Chicago/Turabian StyleMa, Bochao, Jifeng Ren, and Xiuqing Qian. 2025. "Study on the Polarization of Astrocytes in the Optic Nerve Head of Rats Under High Intraocular Pressure: In Vitro" Bioengineering 12, no. 2: 104. https://doi.org/10.3390/bioengineering12020104
APA StyleMa, B., Ren, J., & Qian, X. (2025). Study on the Polarization of Astrocytes in the Optic Nerve Head of Rats Under High Intraocular Pressure: In Vitro. Bioengineering, 12(2), 104. https://doi.org/10.3390/bioengineering12020104






