A Novel Approach for Optimizing Molecularly Imprinted Polymer Composition in Electrochemical Detection of Collagen Peptides
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation of Collagen Peptides
2.2. Polymers Synthesis for Collagen Peptides Detection
2.3. Screen-Printed Electrode (SPE) Preparation
2.4. Cyclic Voltammogram Measuring
- Baseline Establishment: Initial CV scans were carried out on blank electrodes to determine background current levels.
- Binding Analysis: Upon introduction of collagen peptides, changes in the current response indicated successful molecular recognition by the MIPs.
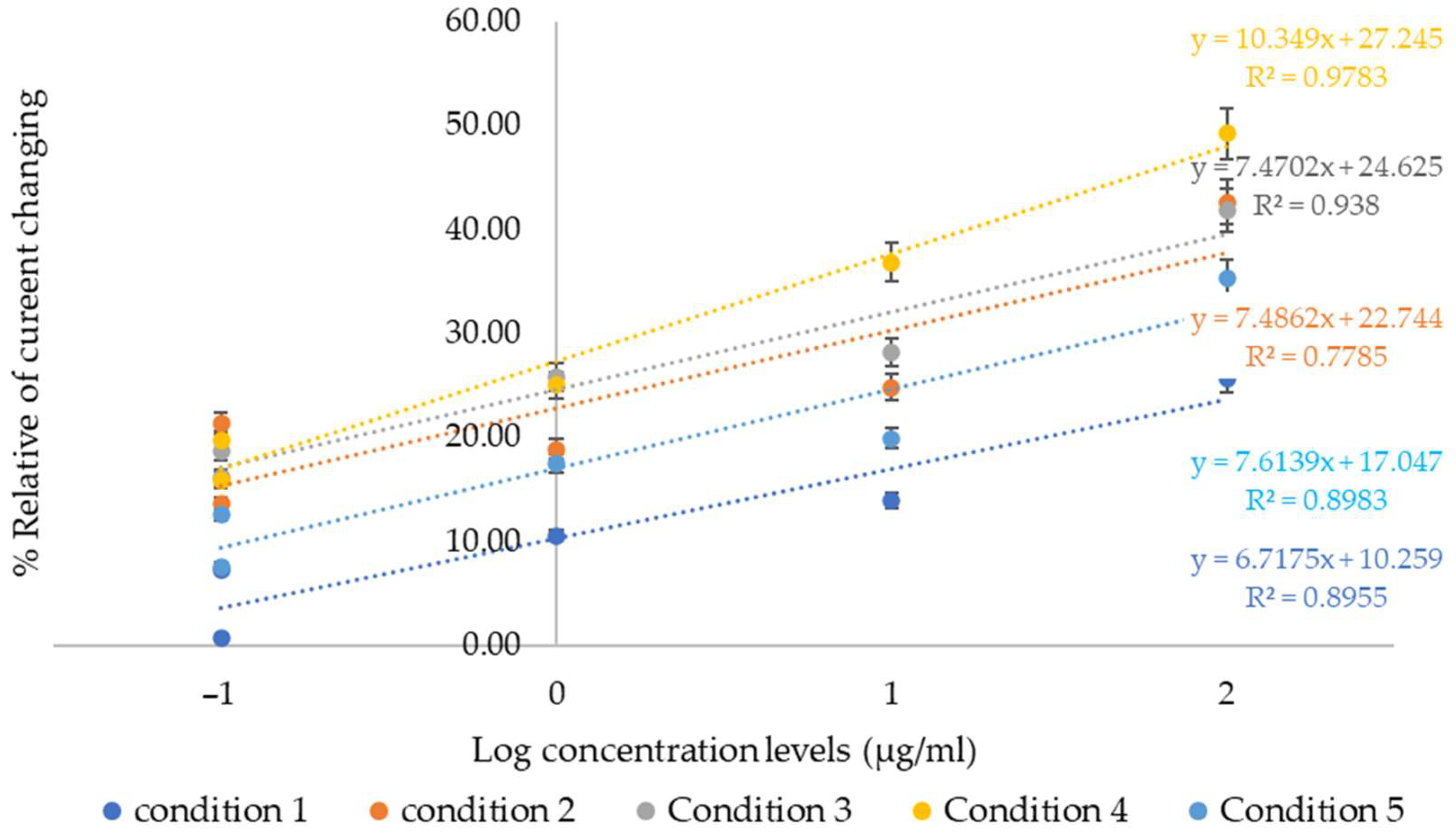
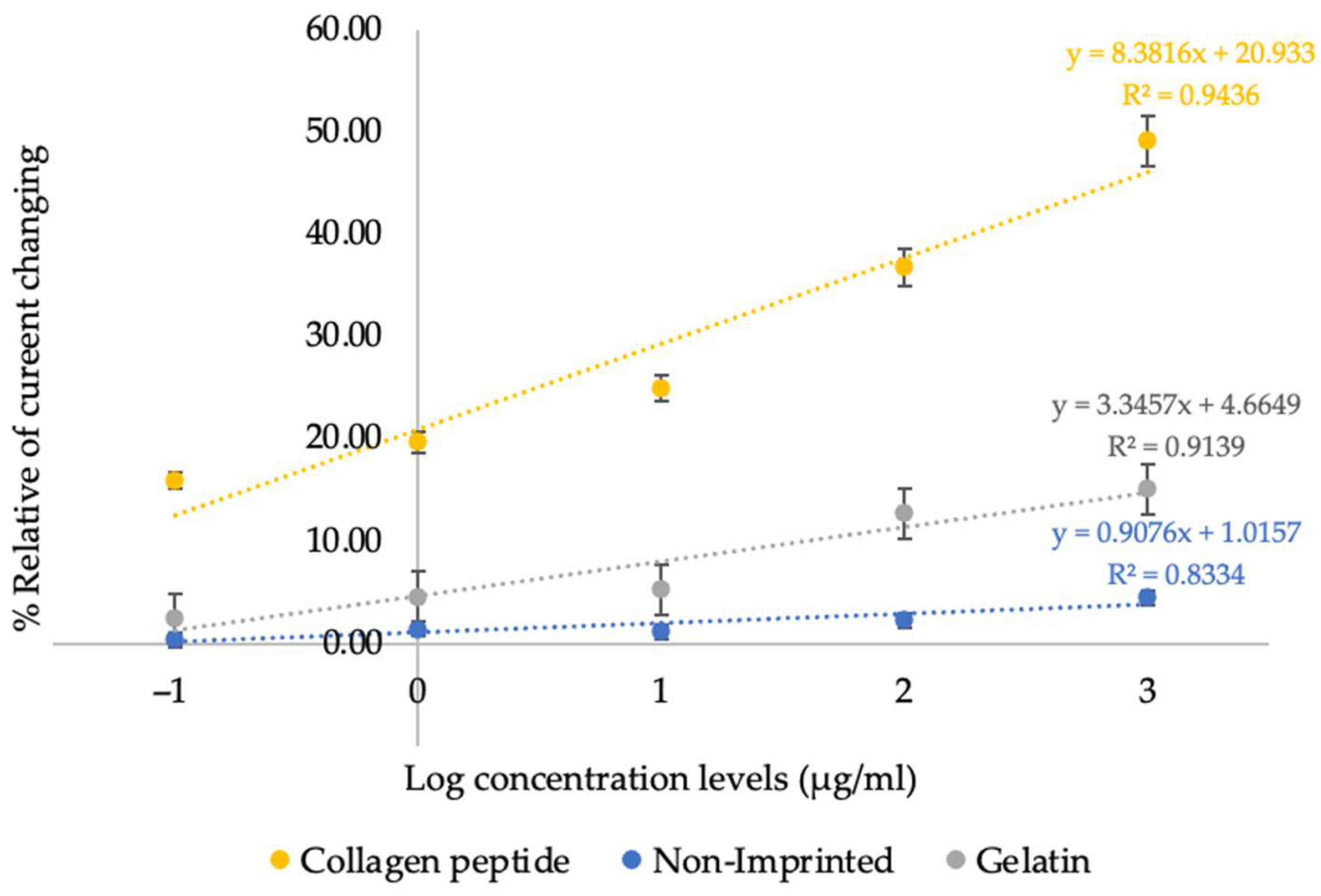
- Calibration Curve Construction: The relationship between the percentage of relative current change and the logarithmic scale of collagen peptide concentration was plotted to evaluate sensor sensitivity and determine the detection limit.
- = 1000 µg/mL (stock concentration);
- = Desired concentration (µg/mL);
- = 1000 µL (final volume);
- = Volume of stock needed;
- = .
2.5. Selectivity Test Preparation
3. Results
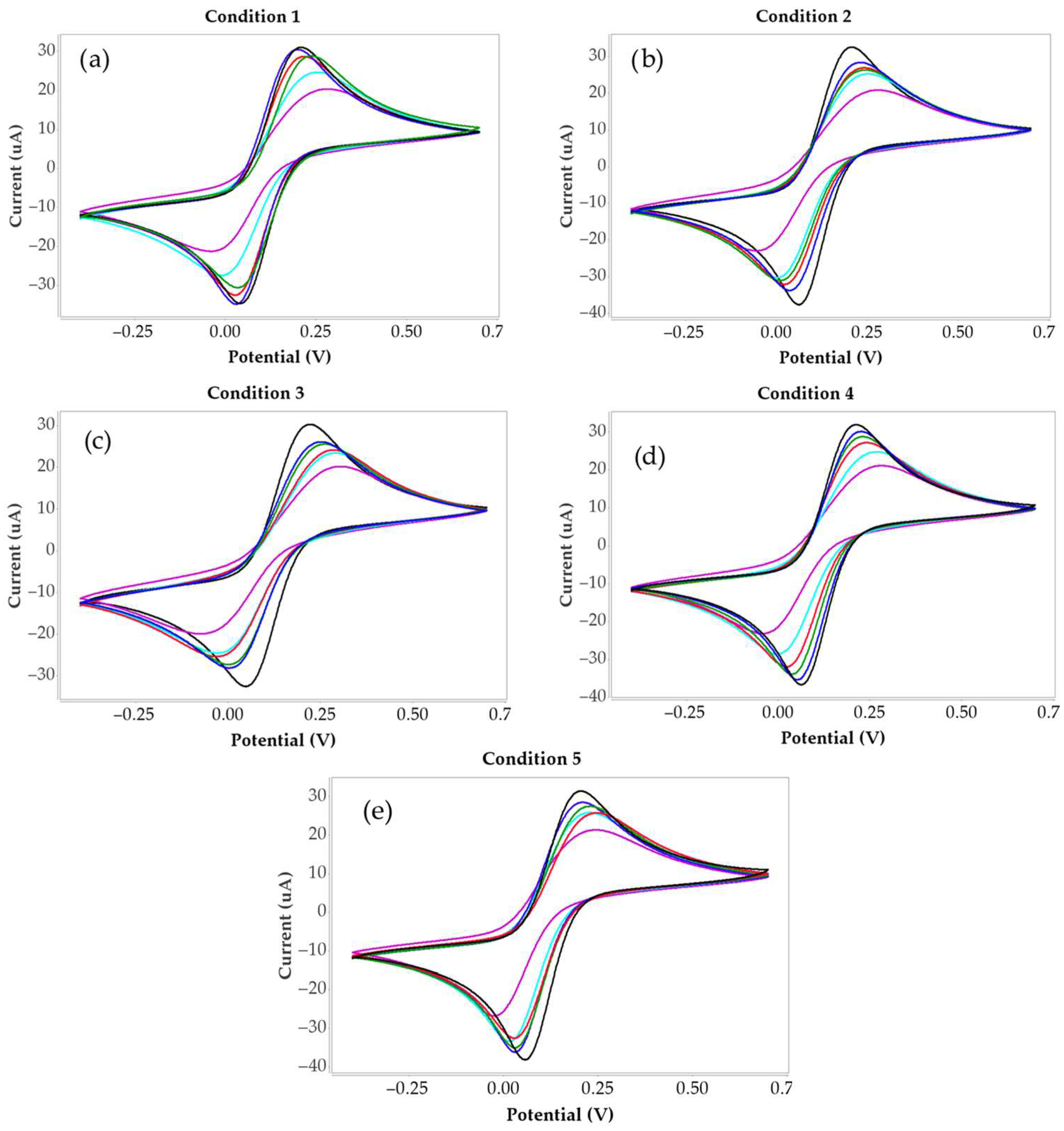
3.1. Cyclic Voltammogram of Carbon Electrode
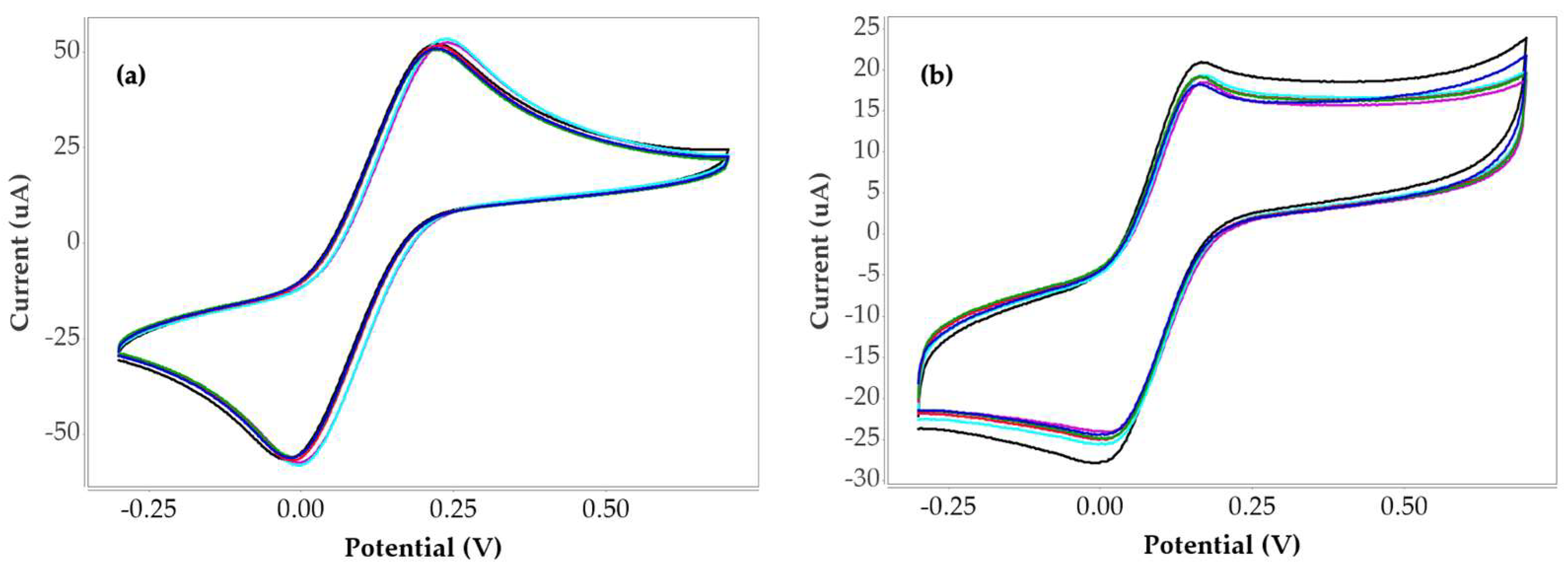
3.2. Selectivity Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cleveland Clinic. Osteoarthritis. Cleveland Clinic. What Is Type 2 Collagen? Benefits and How to Use. (n.d.). 26 November 2019. Available online: https://my.clevelandclinic.org/health/diseases/5599-osteoarthritis (accessed on 10 February 2023).
- Bolke, L.; Schlippe, G.; Gerß, J.; Voss, W. A Collagen Supplement Improves Skin Hydration, Elasticity, Roughness, and Den-sity: Results of a Randomized, Placebo-Controlled, Blind Study. Nutrients 2019, 11, 2494. [Google Scholar] [CrossRef] [PubMed]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Derm. Endocrinol. 2012, 4, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.A.-O. A Review of the Effects of Collagen Treatment in Clinical Studies. Polymers 2021, 13, 3868. [Google Scholar] [CrossRef]
- Elango, J.A.-O.; Zamora-Ledezma, C.A.-O.; Ge, B.; Hou, C.; Pan, Z.; Bao, B.; Pérez Albacete Martínez, C.A.-O.; Marín, J.M.G.; de Val, J.; Bao, C.; et al. Paradoxical Duel Role of Collagen in Rheumatoid Arthritis: Cause of Inflammation and Treatment. Bioengineering 2022, 9, 321. [Google Scholar] [CrossRef]
- Moreland, L.W.; Gay, R.E.; Gay, S. Collagen autoantibodies in patients with vasculitis and systemic lupus ery-thematosus. Clin. Immunol. Immunopathol. 1991, 60, 412–418. [Google Scholar] [CrossRef]
- Sawamura, S.; Jinnin, M.; Kajihara, I.; Makino, K.; Aoi, J.; Ichihara, A.; Makino, T.; Fukushima, S.; Ihn, H. Do scleroderma patients look young?: Evaluation by using facial imaging system. Drug Discov. Ther. 2017, 11, 342–345. [Google Scholar] [CrossRef]
- ScienceDirect. Type II Collagen—An Overview. ScienceDirect Topics. 2016. Available online: https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/type-ii-collagen (accessed on 10 February 2023).
- Afsarimanesh, N.; Zia, A.I.; Mukhopadhyay, S.C.; Kruger, M.; Yu, P.-L.; Kosel, J.; Kovacs, Z. Smart Sensing System for the Prognostic Monitoring of Bone Health. Sensors 2016, 16, 976. [Google Scholar] [CrossRef]
- Naresh, V.A.-O.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Shahrashoob, M.; Dehshiri, M.; Yousefi, V.; Moassesfar, M.; Saberi, H.; Molaabasi, F.; Zare, Y.; Rhee, K.Y. Optical and Elec-trochemical Biosensors for Detection of Pathogens Using Metal Nanoclusters: A Systematic Review. Biosensors 2025, 15, 460. [Google Scholar] [CrossRef]
- Senf, B.A.-O.; Yeo, W.A.-O.; Kim, J.A.-O. Recent Advances in Portable Biosensors for Biomarker Detection in Body Fluids. Biosensors 2020, 10, 127. [Google Scholar] [CrossRef]
- Xing, E.; Chen, H.; Xin, X.; Cui, H.; Dou, Y.; Song, S. Recent Advances in Smart Phone-Based Biosensors for Various Applica-tions. Chemosensors 2025, 13, 221. [Google Scholar] [CrossRef]
- Zabitler, D.; Ülker, E.; Turan, K.; Erdoğan, N.Ö.; Aydoğdu Tığ, G. Electrochemical Sensor for Biological Samples Monitoring. Top. Catal. 2025, 1–31. [Google Scholar] [CrossRef]
- Wardak, C.; Wólczyński, H.; Malinowski, S.; Paczosa-Bator, B.; Wardak, M. A Low-Cost and Environmentally Friendly Electrochemical Biosensor for the Determination of Estradiol. Materials 2025, 18, 2932. [Google Scholar] [CrossRef] [PubMed]
- Bunyakul, N.; Baeumner, A.J. Combining electrochemical sensors with miniaturized sample preparation for rapid detection in clinical samples. Sensors 2015, 15, 547–564. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhao, Y.; Huang, Q.; Huang, J.; Tao, Y.; Chen, J.; Li, H.-Y.; Liu, H. Electrochemical protein biosensors for disease marker detection: Progress and opportunities. Microsyst. Nanoeng. 2024, 10, 65. [Google Scholar] [CrossRef]
- Li, Y.E.; Lee, I.C. The Current Trends of Biosensors in Tissue Engineering. Biosensors 2020, 10, 88. [Google Scholar] [CrossRef]
- Fu, E.; Khederlou, K.; Lefevre, N.; Ramsey, S.A.; Johnston, M.L.; Wentland, L. Progress on Electrochemical Sensing of Phar-maceutical Drugs in Complex Biofluids. Chemosensors 2023, 11, 467. [Google Scholar] [CrossRef]
- Wang, F.; Xie, Y.; Zhu, W.; Wei, T. Recent Advances in Functionalization Strategies for Biosensor Interfaces, Especially the Emerging Electro-Click: A Review. Chemosensors 2023, 11, 481. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical Sensors and Biosensors Based on Nanomaterials and Nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, J.; Wang, H. Bioreceptors as the key components for electrochemical biosensing in medicine. Cell Rep. Phys. Sci. 2024, 5, 101801. [Google Scholar] [CrossRef]
- Li, Y.; Ho, D.J.; Meng, H.; Chan, T.R.; An, B.; Yu, H.; Brodsky, B.; Jun, A.S.; Yu, S.M. Direct Detection of Collagenous Proteins by Fluorescently Labeled Collagen Mimetic Peptides. Bioconjugate Chem. 2013, 24, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Göktürk, I.; Güler, K.Ç.; Yılmaz, F.; Oktar, C.; Yılmaz, G.E.; Denizli, A. Molecularly Imprinted Polymeric Biomaterials in Diagnosis and Medical Practice. Biomed. Mater. Devices 2024, 3, 299–316. [Google Scholar] [CrossRef]
- Agnishwaran, B.; Manivasagam, G.; Udduttula, A. Molecularly Imprinted Polymers: Shaping the Future of Early-Stage Bone Loss Detection─A Review. ACS Omega 2024, 9, 8730–8742. [Google Scholar] [CrossRef]
- Hasseb, A.A.; Abdel Ghani, N.d.T.; Shehab, O.R.; El Nashar, R.M. Application of molecularly imprinted polymers for electrochemical detection of some important biomedical markers and pathogens. Curr. Opin. Electrochem. 2022, 31, 100848. [Google Scholar] [CrossRef]
- Akgönüllü, S.; Kılıç, S.; Esen, C.; Denizli, A. Molecularly Imprinted Polymer- Based Sensors for Protein Detection. Polymers 2023, 15, 629. [Google Scholar] [CrossRef]
- Wu, Z.; Korntner, S.; Mullen, A.; Zeugolis, D. Collagen peptide complex: From and biosynthesis to advanced biomaterials for cartilage engineering. Biomater. Biosyst. 2021, 4, 100030. [Google Scholar] [CrossRef]
- Dar, K.K.; Shao, S.; Tan, T.; Lv, Y. Molecularly imprinted polymers for the selective recognition of microorganisms. Biotechnol. Adv. 2020, 45, 107640. [Google Scholar] [CrossRef]
- Pistone, A.; Scolaro, C.; Celesti, C.; Visco, A. Study of Protective Layers Based on Crosslinked Glutaraldehyde/3-aminopropyltriethoxysilane. Polymers 2022, 14, 801. [Google Scholar] [CrossRef]
- Mugnaini, G.; Gelli, R.; Mori, L.; Bonini, M. How to Cross-Link Gelatin: The Effect of Glutaraldehyde and Glyceraldehyde on the Hydrogel Properties. ACS Appl. Polym. Mater. 2023, 5, 9192–9202. [Google Scholar] [CrossRef]
- Chandra, A.; Singh, A.A.; Prasad, S.; Andersson, M.R.; Gedefaw, D. Crosslinking Approaches for Polyethylene Imine (PEI) and Its Uses in Adsorption of Heavy Metals, Dyes, and Carbon Dioxide. Appl. Sci. 2025, 15, 4767. [Google Scholar] [CrossRef]
- Figueiró, S.D.; Góes, J.C.; Moreira, R.A.; Sombra, A.S.B. On the physico-chemical and dielectric properties of glutaraldehyde crosslinked galactomannan–collagen films. Carbohydr. Polym. 2004, 56, 313–320. [Google Scholar] [CrossRef]
- Yu, Z.; Wu, J.; Zhang, T.; Chen, C.; Ma, Y.; Liu, H.; Chiou, B.-S.; Liu, F.; Li, J. Improving the crosslinking of collagen casing and glutaraldehyde by facilitating the formation of conjugate structure via pH. Collagen Leather 2024, 6, 29. [Google Scholar] [CrossRef]
- Gao, Y.; Kyratzis, I. Covalent Immobilization of Proteins on Carbon Nanotubes Using the Cross-Linker 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide—A Critical Assessment. Bioconjugate Chem. 2008, 19, 1945–1950. [Google Scholar] [CrossRef]
- Nordholm, S.; Bacskay, G.B. The Basics of Covalent Bonding in Terms of Energy and Dynamics. Molecules 2020, 25, 2667. [Google Scholar] [CrossRef]
- Zhao, Z.; Deng, J.; Fan, D. Green biomanufacturing in recombinant collagen biosynthesis: Trends and selection in various expression systems. Biomater Sci. 2023, 11, 5439–5461. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Liang, C.H.; Wu, H.T.; Pang, H.Y.; Chen, C.; Wang, G.H.; Chan, L.P. Antioxidant and anti-inflammatory capacities of collagen peptides from milkfish (Chanos chanos) scales. J. Food Sci. Technol. 2018, 55, 2310–2317. [Google Scholar] [CrossRef]
- Kim, D.U.; Chung, H.C.; Choi, J.; Sakai, Y.; Lee, B.Y. Oral Intake of Low-Molecular-Weight Collagen Peptide Improves Hydration, Elasticity, and Wrinkling in Human Skin: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2018, 10, 826. [Google Scholar] [CrossRef]
- Liu, L.; Huang, K.; Li, W.; Qiu, R.; Fang, Y.; Huang, Y.; Zhao, S.; Lv, H.; Zhang, K.; Shan, H.; et al. Molecular Imaging of Collagen Destruction of the Spine. ACS Nano 2021, 15, 19138–19149. [Google Scholar] [CrossRef]
- Lukin, I.; Erezuma, I.; Maeso, L.; Zarate, J.; Desimone, M.F.; Al-Tel, T.H.; Dolatshahi-Pirouz, A.; Orive, G. Progress in Gelatin as Biomaterial for Tissue Engineering. Pharmaceutics 2022, 14, 1177. [Google Scholar] [CrossRef]
- Naomi, R.; Bahari, H.; Ridzuan, P.M.; Othman, F. Natural-Based Biomaterial for Skin Wound Healing (Gelatin vs. Collagen): Expert Review. Polymers 2021, 13, 2319. [Google Scholar] [CrossRef]
- Nikkhah, M.; Akbari, M.; Paul, A.; Memic, A.; Dolatshahi-Pirouz, A.; Khademhosseini, A. Gelatin-Based Biomaterials for Tissue Engineering and Stem Cell Bioengineering. In Biomaterials from Nature for Advanced Devices and Therapies; Neves, N.M., Reis, R.L., Eds.; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar] [CrossRef]
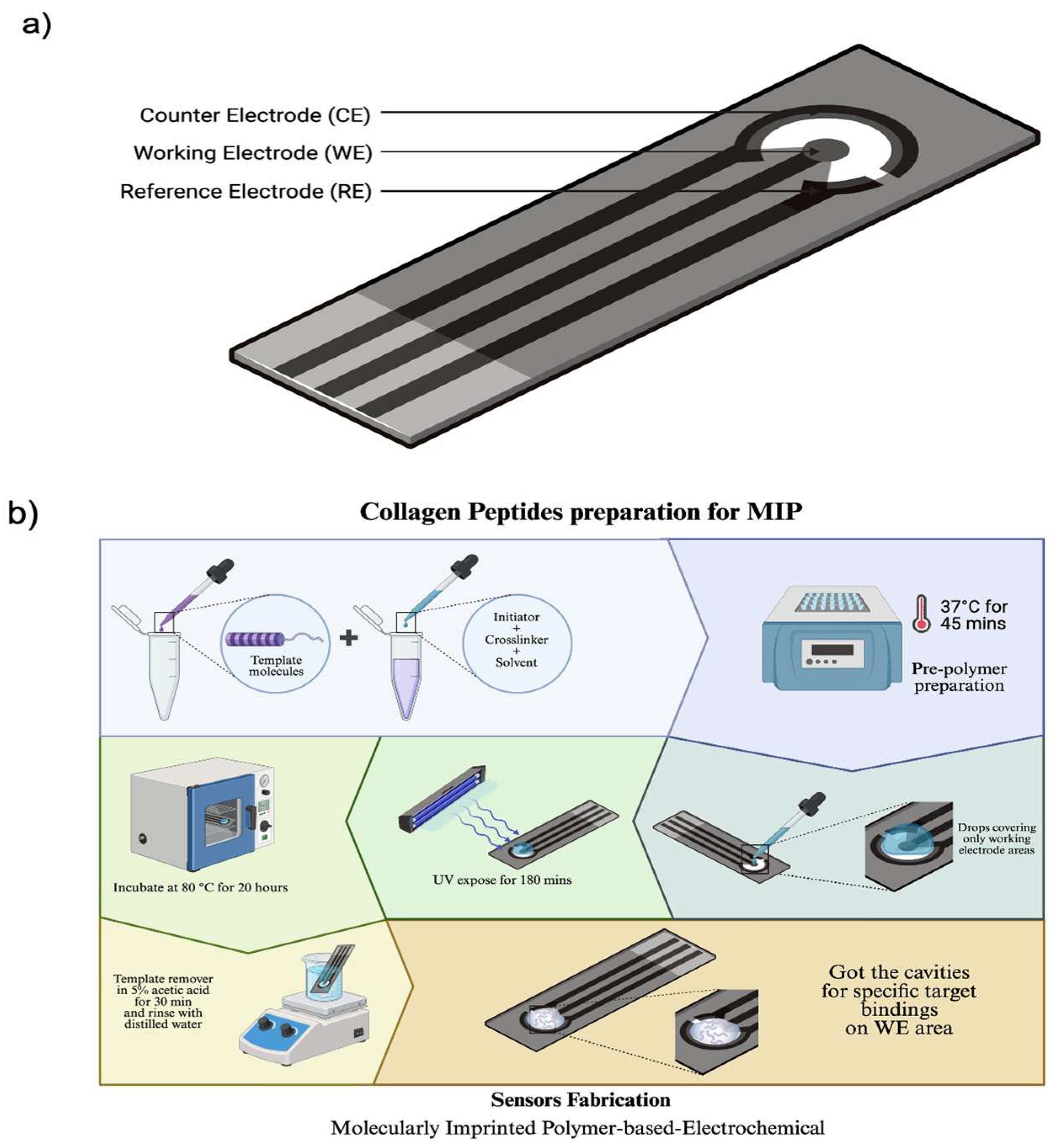
| Condition | Monomer Ratio (n:n) | HYP (mg) | AA (µL) | GA (µL) | DHEBA (mg) | AIBN (mg) | DMSO (µL) |
|---|---|---|---|---|---|---|---|
| 1 | 1:1 | 13.1 | 8.2 | 9.4 | 47 | 1.5 | 300 |
| 2 | 1:4 | 13.1 | 32.8 | 9.4 | 47 | 1.5 | 300 |
| 3 | 3:1 | 39.3 | 8.2 | 9.4 | 47 | 1.5 | 300 |
| 4 | 2:3 | 26.3 | 24.6 | 9.4 | 47 | 1.5 | 300 |
| 5 | 2:5 | 26.2 | 41 | 9.4 | 47 | 1.5 | 300 |
| Condition 1 | Current (µA) | ∆I (µA) | % Current changing |
| Blank | 32.74 | ||
| 0.1 µg/mL | 32.53 | 0.21 | 0.64 |
| 1 µg/mL | 30.36 | 2.39 | 7.28 |
| 10 µg/mL | 29.29 | 3.45 | 10.53 |
| 100 µg/mL | 28.19 | 4.55 | 13.90 |
| 1000 µg/mL | 24.34 | 8.40 | 25.66 |
| Condition 2 | Current (µA) | ∆I (µA) | % Current changing |
| Blank | 34.31 | ||
| 0.1 µg/mL | 29.66 | 4.66 | 13.57 |
| 1 µg/mL | 26.99 | 7.32 | 21.34 |
| 10 µg/mL | 27.83 | 6.48 | 18.88 |
| 100 µg/mL | 25.80 | 8.51 | 24.79 |
| 1000 µg/mL | 19.68 | 14.63 | 42.63 |
| Condition 3 | Current (µA) | ∆I (µA) | % Current changing |
| Blank | 31.46 | ||
| 0.1 µg/mL | 26.38 | 5.08 | 16.14 |
| 1 µg/mL | 25.58 | 5.89 | 18.71 |
| 10 µg/mL | 23.35 | 8.11 | 25.78 |
| 100 µg/mL | 22.60 | 8.86 | 28.18 |
| 1000 µg/mL | 18.31 | 13.15 | 41.80 |
| Condition 4 | Current (µA) | ∆I (µA) | % Current changing |
| Blank | 37.24 | ||
| 0.1 µg/mL | 31.33 | 5.91 | 15.88 |
| 1 µg/mL | 29.91 | 7.33 | 19.68 |
| 10 µg/mL | 27.93 | 9.31 | 24.99 |
| 100 µg/mL | 23.53 | 13.71 | 36.81 |
| 1000 µg/mL | 18.91 | 18.33 | 49.22 |
| Condition 5 | Current (µA) | ∆I (µA) | % Current changing |
| Blank | 32.86 | ||
| 0.1 µg/mL | 30.37 | 2.49 | 7.59 |
| 1 µg/mL | 28.74 | 4.12 | 12.54 |
| 10 µg/mL | 27.11 | 5.76 | 17.51 |
| 100 µg/mL | 26.31 | 6.55 | 19.93 |
| 1000 µg/mL | 21.27 | 11.59 | 35.27 |
| Reference | Target | Sensor Type | LOD | Linear Range | SAMPLE MATRIX | Key Advantage/Remark |
|---|---|---|---|---|---|---|
| [38] | Collagen fragment | Colorimetric paper sensor | 2 µg/mL | 1–50 µg/mL | Buffer | Cheap, but very narrow linear range, not reusable |
| [39] | Collagen peptide | Simple electrochemical sensor | 0.8 µg/mL | 0.5–100 µg/mL | Buffer | Moderate LOD but linear range limited; electrode instability reported |
| [40] | Collagen type I | Optical sensor | 5 µg/mL | 2–80 µg/mL | Buffer | Easy readout, but fabrication complex and not reusable |
| [41] | Collagen I peptide | Paper-based electrochemical | 3 µg/mL | 1–100 µg/mL | Buffer | Portable, low cost, but low reproducibility and narrow working range |
| This work | Collagen peptide | MIP-based electrochemical | 1.0106 µg/mL | 0.1–1000 µg/mL | Buffer | Broad range, simple fabrication, antibody-free, stable, cost-effective |
| Non-Imprinted | Current (µA) | ∆I (µA) | % Current changing |
| Blank | 52.38 | ||
| 0.1 µg/mL | 52.20 | 0.18 | 0.34 |
| 1 µg/mL | 51.64 | 0.74 | 1.41 |
| 10 µg/mL | 51.77 | 0.61 | 1.16 |
| 100 µg/mL | 51.20 | 1.18 | 2.25 |
| 1000 µg/mL | 50.05 | 2.34 | 4.46 |
| Gelatin | Current (µA) | ∆I (µA) | % Current changing |
| Blank | 18.89 | ||
| 0.1 µg/mL | 18.44 | 0.45 | 2.40 |
| 1 µg/mL | 18.02 | 0.87 | 4.58 |
| 10 µg/mL | 17.89 | 1.00 | 5.29 |
| 100 µg/mL | 16.49 | 2.40 | 12.71 |
| 1000 µg/mL | 16.04 | 2.85 | 15.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vongmanee, N.; Nampeng, J.; Rattanapithan, K.; Sriwichai, P.; Pintavirooj, C.; Visitsattapongse, S. A Novel Approach for Optimizing Molecularly Imprinted Polymer Composition in Electrochemical Detection of Collagen Peptides. Bioengineering 2025, 12, 1272. https://doi.org/10.3390/bioengineering12111272
Vongmanee N, Nampeng J, Rattanapithan K, Sriwichai P, Pintavirooj C, Visitsattapongse S. A Novel Approach for Optimizing Molecularly Imprinted Polymer Composition in Electrochemical Detection of Collagen Peptides. Bioengineering. 2025; 12(11):1272. https://doi.org/10.3390/bioengineering12111272
Chicago/Turabian StyleVongmanee, Naphatsawan, Jindapa Nampeng, Katesirin Rattanapithan, Phuritasinee Sriwichai, Chuchart Pintavirooj, and Sarinporn Visitsattapongse. 2025. "A Novel Approach for Optimizing Molecularly Imprinted Polymer Composition in Electrochemical Detection of Collagen Peptides" Bioengineering 12, no. 11: 1272. https://doi.org/10.3390/bioengineering12111272
APA StyleVongmanee, N., Nampeng, J., Rattanapithan, K., Sriwichai, P., Pintavirooj, C., & Visitsattapongse, S. (2025). A Novel Approach for Optimizing Molecularly Imprinted Polymer Composition in Electrochemical Detection of Collagen Peptides. Bioengineering, 12(11), 1272. https://doi.org/10.3390/bioengineering12111272









