Augmented Reality Navigation for Extreme Lateral Interbody Fusion with Posterior Instrumentation: Feasibility, Outcomes, and Surgical Technique
Abstract
1. Introduction
2. Methods
2.1. Patient Population
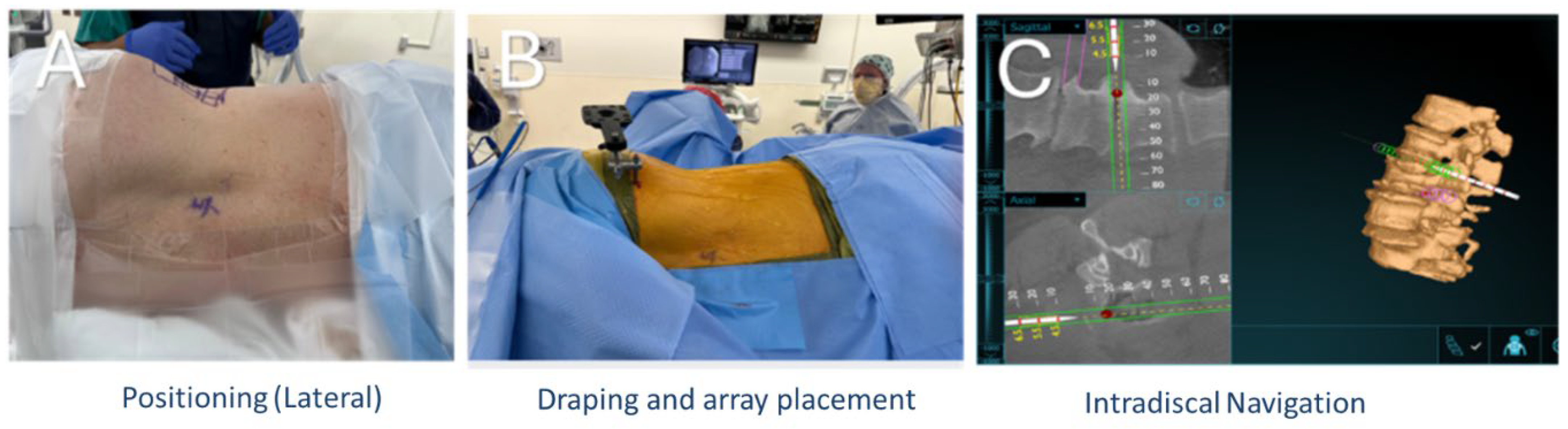
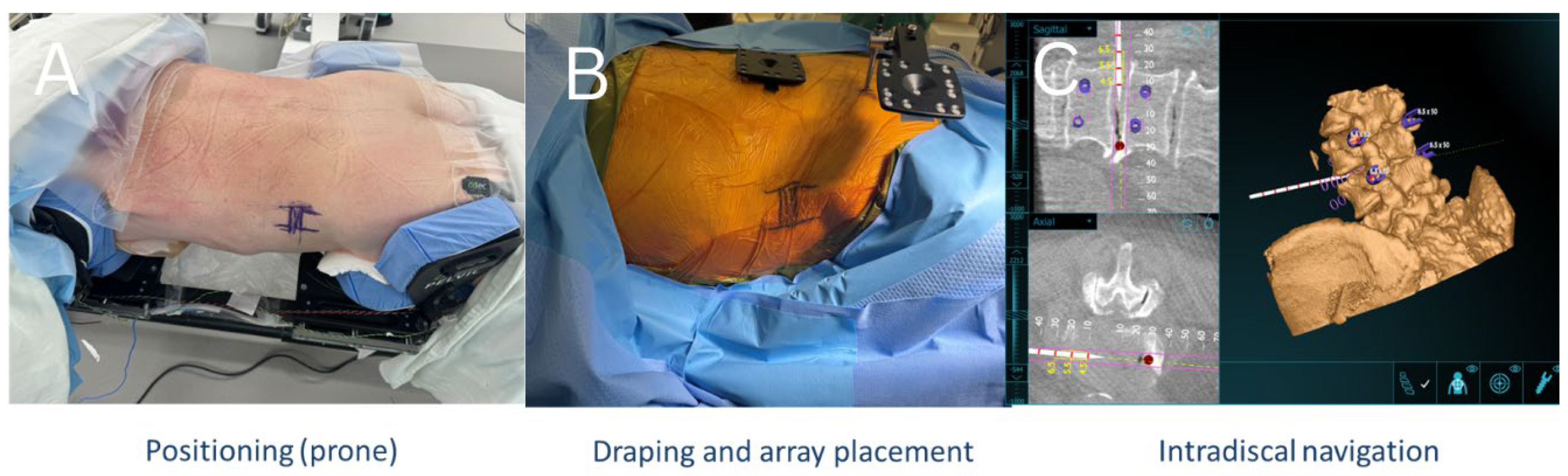
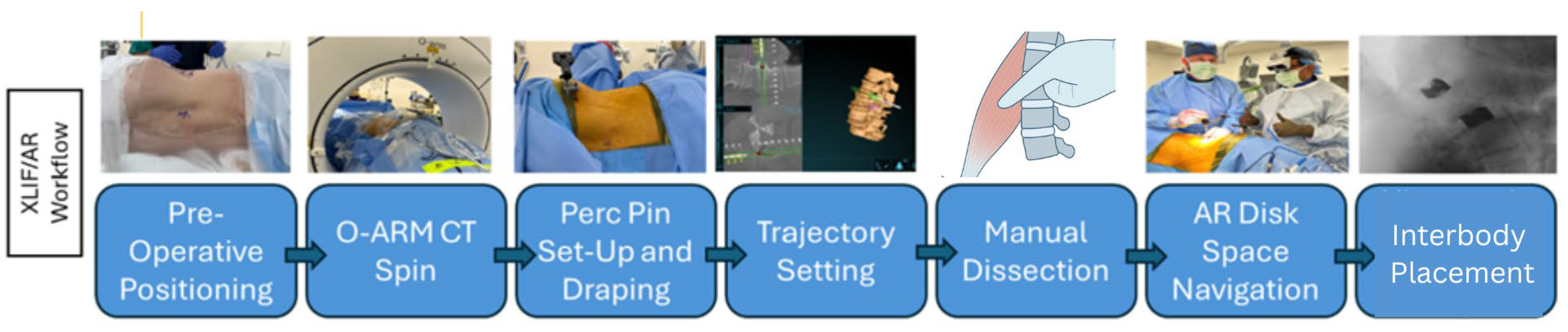
2.2. Operative Technique
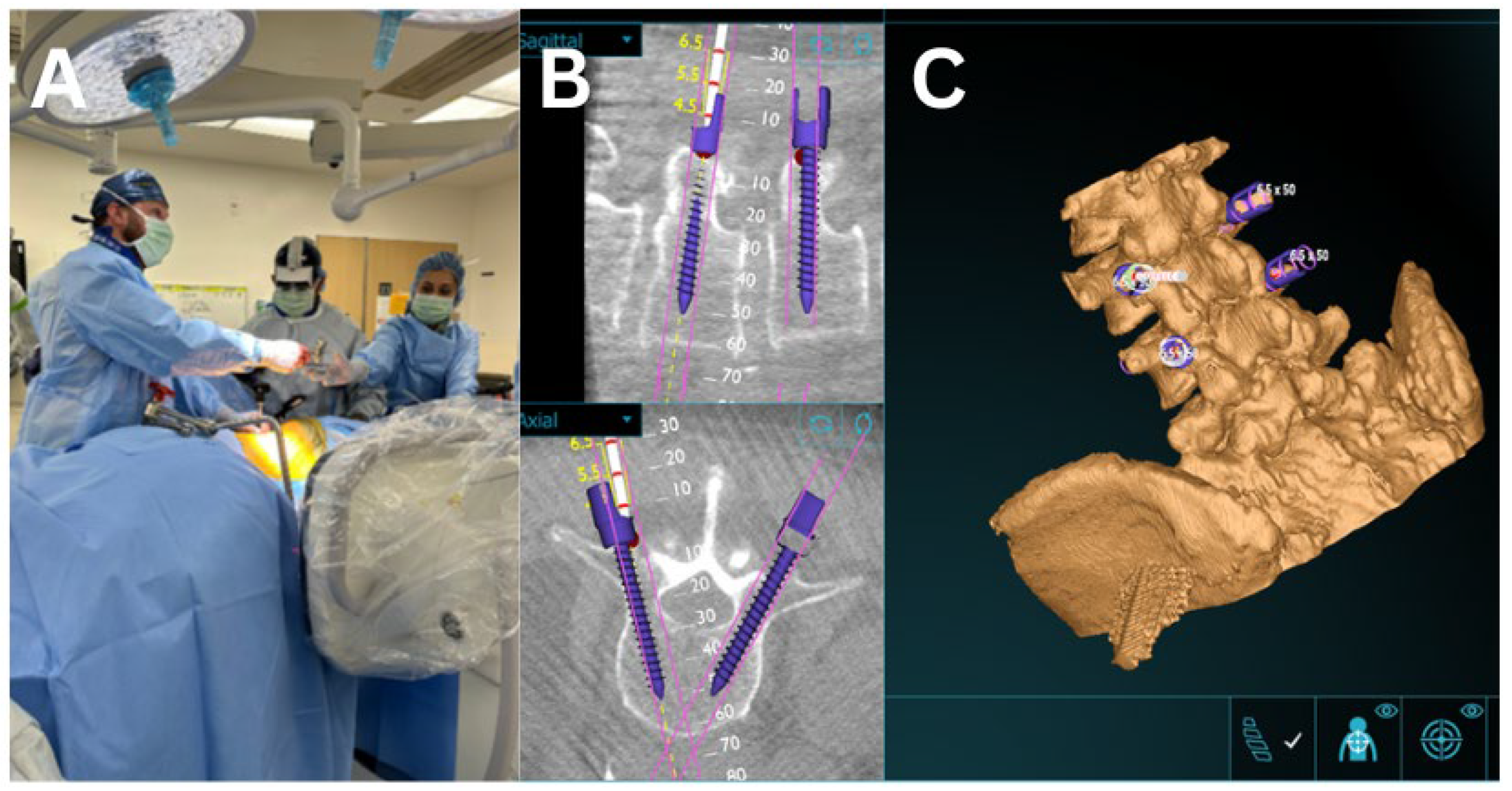
2.3. AR Guided Posterior Screw and Rod Placement
2.4. Augmented Reality Navigation
2.5. Surgical Outcome & Statistics
3. Results
4. Discussion
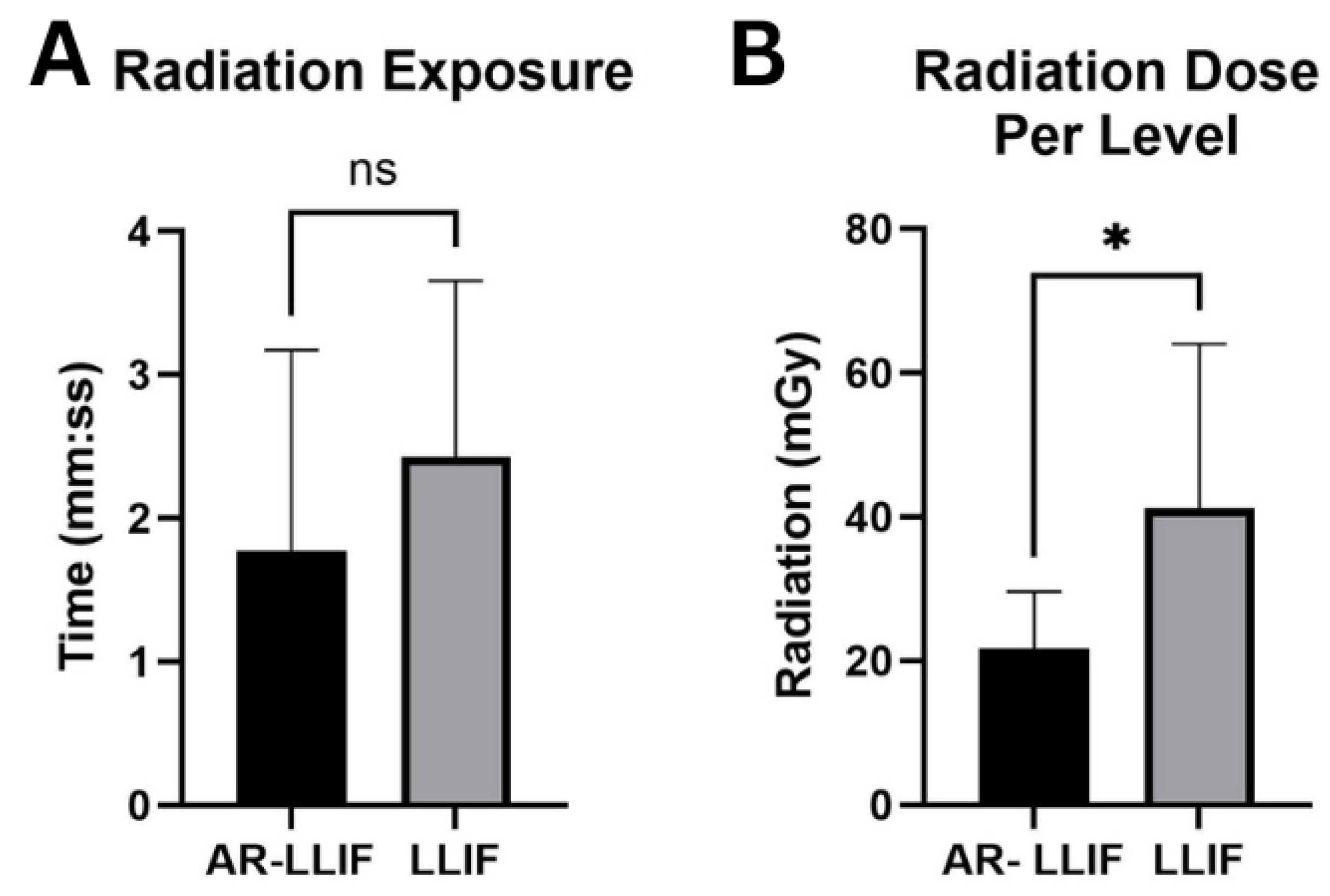
4.1. Radiation Exposure
4.2. Efficacy, Accuracy and Safety
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OBENCHAIN, T.G. Laparoscopic Lumbar Discectomy: Case Report. J. Laparoendosc. Surg. 1991, 1, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Palacios, P.; Palacios, I.; Palacios, A.; Gutiérrez, J.A.C.J.; Mariscal, G.; Lorente, A. Efficacy and Safety of the Extreme Lateral Interbody Fusion (XLIF) Technique in Spine Surgery: Meta-Analysis of 1409 Patients. J. Clin. Med. 2024, 13, 960. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.; Kim, D.H. Lateral Lumbar Interbody Fusion: Indications, Outcomes, and Complications. J. Am. Acad. Orthop. Surg. 2016, 24, 96. [Google Scholar] [CrossRef] [PubMed]
- Goodnough, L.H.; Koltsov, J.; Wang, T.; Xiong, G.; Nathan, K.; Cheng, I. Decreased estimated blood loss in lateral trans-psoas versus anterior approach to lumbar interbody fusion for degenerative spondylolisthesis. J. Spine Surg. 2019, 5, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.T.; Farber, S.H.; Cole, T.S.; Xu, D.S.; Godzik, J.; Whiting, A.C.; Hartman, C.; Porter, R.W.; Turner, J.D.; Uribe, J. Complications for minimally invasive lateral interbody arthrodesis: A systematic review and meta-analysis comparing prepsoas and transpsoas approaches. J. Neurosurg. Spine 2019, 30, 446–460. [Google Scholar] [CrossRef] [PubMed]
- Ozgur, B.M.; Aryan, H.E.; Pimenta, L.; Taylor, W.R. Extreme Lateral Interbody Fusion (XLIF): A novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006, 6, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Extreme Lateral Interbody Fusion (XLIF®): How I Do It|Acta Neurochirurgica. Available online: https://link.springer.com/article/10.1007/s00701-014-2248-9 (accessed on 16 September 2025).
- Pimenta, L.; Amaral, R.; Taylor, W.; Tohmeh, A.; Pokorny, G.; Rodrigues, R.; Arnoni, D.; Guirelli, T.; Batista, M. The prone transpsoas technique: Preliminary radiographic results of a multicenter experience. Eur. Spine J. 2021, 30, 108–113. [Google Scholar] [CrossRef] [PubMed]
- NaPier, Z. Prone Transpsoas Lateral Interbody Fusion (PTP LIF) with Anterior Docking: Preliminary functional and radiographic outcomes. N. Am. Spine Soc. J. 2023, 16, 100283. [Google Scholar] [CrossRef] [PubMed]
- Antes, S.; Moringlane, R.; von Eckardstein, K.L. Augmented Reality-Supported Rod Bending in Multilevel Spinal Fusion Using the ADVISE Software. World Neurosurg. 2023, 178, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Sato, S.; Kato, K.; Hyakumachi, T.; Yanagibashi, Y.; Ito, M.; Abumi, K. A novel 3D guidance system using augmented reality for percutaneous vertebroplasty. J. Neurosurg. 2013, 19, 492–501. Available online: https://thejns.org/spine/view/journals/j-neurosurg-spine/19/4/article-p492.xml (accessed on 4 December 2024). [CrossRef] [PubMed]
- Azad, T.D.; Warman, A.; Tracz, J.A.; Hughes, L.P.; Judy, B.F.; Witham, T.F. Augmented reality in spine surgery—Past, present, and future. Spine J. 2024, 24, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bindal, R.K.; Glaze, S.; Ognoskie, M.; Tunner, V.; Malone, R.; Ghosh, S. Surgeon and patient radiation exposure in minimally invasive transforaminal lumbar interbody fusion. J. Neurosurg. 2008, 9, 570–573. Available online: https://thejns.org/spine/view/journals/j-neurosurg-spine/9/6/article-p570.xml (accessed on 4 December 2024). [CrossRef] [PubMed]
- Carl, B.; Bopp, M.; Saß, B.; Voellger, B.; Nimsky, C. Implementation of augmented reality support in spine surgery. Eur. Spine J. 2019, 28, 1697–1711. [Google Scholar] [CrossRef] [PubMed]
- Striano, B. Comparison of Radiation Exposure Between Anterior, Lateral, and Posterior Interbody Fusion Techniques and the Influence of Patient and Procedural Factors. Spine 2021, 46, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Edström, E.; Burström, G.; Omar, A.; Nachabe, R.; Söderman, M.; Persson, O.; Gerdhem, P.; Elmi-Terander, A. Augmented Reality Surgical Navigation in Spine Surgery to Minimize Staff Radiation Exposure. Spine 2020, 45, E45. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Shi, H.; Zeng, B.; Chen, X. 2D/3D registration based on biplanar X-ray and CT images for surgical navigation. Comput. Methods Programs Biomed. 2024, 257, 108444. [Google Scholar] [CrossRef] [PubMed]
- Tessitore, E.; Molliqaj, G.; Schaller, K.; Gautschi, O.P. Extreme lateral interbody fusion (XLIF): A single-center clinical and radiological follow-up study of 20 patients. J. Clin. Neurosci. 2017, 36, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, W.B.; Gerber, E.J.; Patterson, J. Intraoperative and Early Postoperative Complications in Extreme Lateral Interbody Fusion: An Analysis of 600 Cases. Spine 2011, 36, 26. [Google Scholar] [CrossRef] [PubMed]
- Sharif, S.; Afsar, A. Learning Curve and Minimally Invasive Spine Surgery. World Neurosurg. 2018, 119, 472–478. [Google Scholar] [CrossRef] [PubMed]
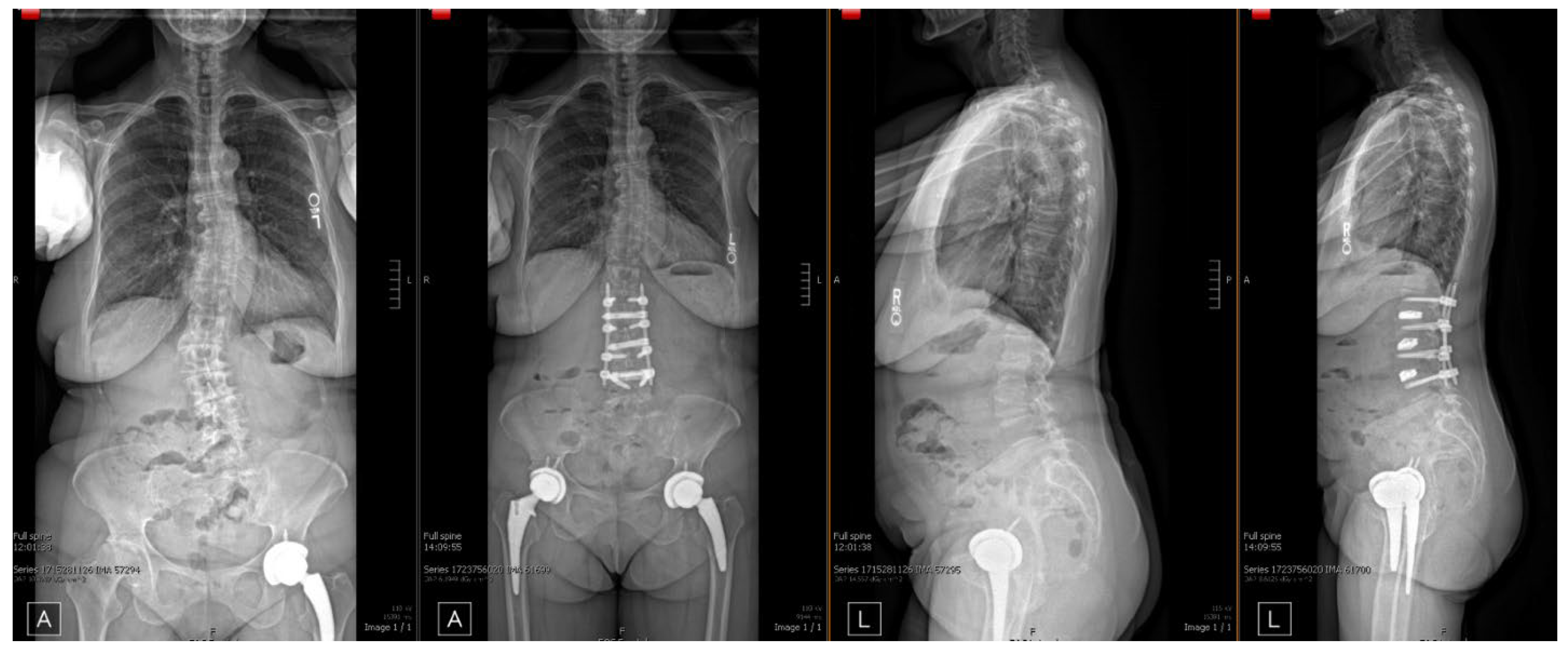
| Patient No. | Age | Sex | BMI | Pre-Operative Diagnosis | Procedure(s) |
|---|---|---|---|---|---|
| 1 | 75 | M | 21.9 | - Adjacent segment disease L2–L4 - Lumbar Radiculopathy | (1). Hardware Removal (2). L2-5 Revision MIS PSF (3). L2-L4 PTP XLIF |
| 2 | 66 | F | 27.4 | - Degenerative Scoliosis - L2–L5 Lumbar Stenosis | (1). L2-5 XLIF |
| 3 | 69 | F | 20.7 | - L3–L4 spondylolisthesis | (1). L3-4 XLIF (2). L2-L5 MIS PSF |
| 4 | 65 | F | 27 | - L3–L4 spondylolisthesis | (1). L3-4 PTP XLIF (2). L3-4 MIS PSF |
| 5 | 71 | F | 34.7 | - Spinal Stenosis w. neurogenic claudication | (1). L3-4 XLIF (2). L3-4 PSF |
| 6 | 64 | M | 37.2 | - Scoliosis of lumbar spine | (1). L2-5 XLIF (2). L2-5 PSF |
| 7 | 78 | M | 34.1 | - Spinal stenosis without neurogenic claudication | (1). L2-5 PSF (2). L2-5 XLIF |
| Patient No. | Surgery Time | EBL | LOS (Days) | Operative Complications | Hardware Adequacy/ Arthrodesis | Pain Score Pre-Op/Post-Op | Current Pain Meds |
|---|---|---|---|---|---|---|---|
| 1 | 6 h 45 min | 200 mL | 5 | None | Yes/Yes | 8/10/0/10 | Tylenol prn |
| 2 | 5 h 38 min | 150 mL | 10 | Planned diaphragm injury | Yes/Yes | - | None |
| 3 | 5 h 20 min | 20 mL | 5 | None | Yes/Yes | - | Methadone 10 mg BID |
| 4 | 2 h 8 min | 50 mL | 6 | None | Yes/Yes | 6/10/0/10 | None |
| 5 | 5 h 16 min | 50 mL | 2 | None | Yes/Yes | 8/10/2/10 | Gabapentin 600 mg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urreola, G.; Cranick, M.G.; Castillo, J.A., Jr.; Shahzad, H.; Martin, A.R.; Kim, K.; Khan, S.; Price, R.L. Augmented Reality Navigation for Extreme Lateral Interbody Fusion with Posterior Instrumentation: Feasibility, Outcomes, and Surgical Technique. Bioengineering 2025, 12, 1262. https://doi.org/10.3390/bioengineering12111262
Urreola G, Cranick MG, Castillo JA Jr., Shahzad H, Martin AR, Kim K, Khan S, Price RL. Augmented Reality Navigation for Extreme Lateral Interbody Fusion with Posterior Instrumentation: Feasibility, Outcomes, and Surgical Technique. Bioengineering. 2025; 12(11):1262. https://doi.org/10.3390/bioengineering12111262
Chicago/Turabian StyleUrreola, Gabriel, Matileen G. Cranick, Jose A. Castillo, Jr., Hania Shahzad, Allan R. Martin, Kee Kim, Safdar Khan, and Richard L. Price. 2025. "Augmented Reality Navigation for Extreme Lateral Interbody Fusion with Posterior Instrumentation: Feasibility, Outcomes, and Surgical Technique" Bioengineering 12, no. 11: 1262. https://doi.org/10.3390/bioengineering12111262
APA StyleUrreola, G., Cranick, M. G., Castillo, J. A., Jr., Shahzad, H., Martin, A. R., Kim, K., Khan, S., & Price, R. L. (2025). Augmented Reality Navigation for Extreme Lateral Interbody Fusion with Posterior Instrumentation: Feasibility, Outcomes, and Surgical Technique. Bioengineering, 12(11), 1262. https://doi.org/10.3390/bioengineering12111262





