1. Introduction
Whole-blood analysis is widely recognized as the gold standard in clinical diagnostics, offering a comprehensive assessment of a patient’s health status. However, the intense red coloration of hemoglobin can interfere with diagnostic techniques that rely on optical or colorimetric detection. Consequently, plasma separation from whole blood is often necessary—a process traditionally achieved through centrifugation. While effective, centrifugation requires specialized and bulky equipment, which limits its use in low-resource settings where laboratory infrastructure is not available or for Point of Care (POC) testing.
In many clinical scenarios, there is a pressing need for diagnostic tools that are rapid, simple, and reliable for the detection and quantification of biomarkers. These tools enable early medical decision-making, which can significantly influence patient outcomes. Delays in biomarker analysis may lead to increased risks of complications, long-term disability, or even death. Within this context, biosensors designed for Point-of-Care (POC) testing have gained considerable attention due to their ability to support timely and informed clinical decisions [
1].
Among the most critical biomarkers is glucose, a key metabolite involved in numerous physiological processes. Blood glucose levels are essential indicators in diseases such as diabetes, where continuous monitoring is crucial for effective disease management. Additionally, low glucose levels can result in hypoglycemia, a condition triggered by extended fasting or intense physical activity. Therefore, accurate and frequent glucose monitoring is vital for both diagnosis and the prevention of complications related to hyperglycemia and hypoglycemia [
2].
To support the development of accessible and effective diagnostic tools, the World Health Organization (WHO) established the ASSURED criteria for POC technologies: Affordable, Sensitive, Specific, User-friendly, Rapid and Robust, Equipment-free, and Deliverable to end users. These guidelines are particularly important in developing regions, where cost and ease of use are key factors driving the adoption of diagnostic innovations [
3,
4].
In this context, paper-based analytical devices (PADs) have emerged as promising platforms for low-cost, rapid diagnostic testing. Thanks to their porous structure and the ability to transport fluids through capillary action, paper is an ideal material for creating devices that operate without external pumps or instrumentation. Since their introduction by the Whitesides group in 2007—using photolithographic patterning techniques—microfluidic paper-based analytical devices (µPADs) have demonstrated significant potential in fields ranging from clinical diagnostics and environmental monitoring to food safety and biohazard detection [
5,
6].
Among the detection methods integrated into PADs, colorimetric assays are particularly appealing due to their ability to visually display results without requiring complex equipment. Although commercial test strips offer qualitative or semi-quantitative analysis, they often lack the precision and reproducibility needed for clinical reliability. While colorimeters can enhance measurement accuracy, they are typically expensive and require trained personnel to operate [
7,
8].
Beyond colorimetric sensing, smartphone integration has enabled alternative diagnostic modalities that exploit embedded cameras, inertial sensors, and artificial intelligence algorithms to extract physiological information without dedicated laboratory instruments. Examples include contactless optical detection of blood oxygen saturation using facial imaging [
9], biomechanical analysis of movement through smartphone inertial sensors coupled with machine learning [
10], and AI-driven mobile applications for pediatric diagnostics [
11]. These studies collectively demonstrate the versatility of smartphones as biomedical platforms, extending well beyond traditional colorimetric biosensing.
Recent advances in technology have enabled the use of smartphones as powerful diagnostic tools at the point-of-care [
12,
13]. With high-resolution cameras and robust processing capabilities, smartphones have facilitated the development of mobile biosensors that overcome many limitations of traditional colorimetric devices—providing more affordable, portable, and user-friendly solutions for detecting biomarkers in blood and other biological fluids [
14,
15].
A critical step in many diagnostic assays is the separation of plasma from whole blood, as plasma contains essential biomarkers that reflect physiological and pathological states. Studies have demonstrated the successful integration of plasma separation into μPADs through different mechanisms [
16,
17]. One approach involves using blood separation membranes, such as LF1, which effectively trap blood cells while allowing plasma to wick into detection zones, enabling protein quantification in under two minutes [
18]. Another strategy leverages red blood cell (RBC) agglutination, where antibodies immobilized on the paper matrix retain RBCs at the sample site, allowing clean plasma to migrate toward colorimetric readout zones [
19,
20]. Both methods have demonstrated the ability to deliver plasma with high purity and sufficient yield for accurate detection, achieving results comparable to conventional laboratory techniques. These integrated designs, combined with smartphone-based detection, represent a significant advancement in the development of fully self-contained, low-cost diagnostic tools suitable for resource-limited settings.
Another major challenge in the development of μPADs for electrochemical biomarker detection is the ability to collect sufficient plasma volume while preserving the biomarker integrity. A solution to this was proposed in a μPAD designed for the quantification of the S100B protein biomarker, relevant in neurological diagnostics [
21]. Using NaCl-functionalized VF2 paper for blood collection and MF1 paper for plasma retention, the device achieved efficient plasma separation (50 µL from 300 µL of whole blood) in under four minutes [
22].
This work presents the development of a paper-based device for colorimetric detection of glucose in whole-blood samples, using a smartphone for quantitative determination.
3. Results
For the comparative evaluation of the different types of paper, the main criterion was the ratio of paper pore size to the cellular dimensions of the blood components, primarily erythrocytes (~7 µm), leukocytes (8–20 µm), and platelets (~3 µm). Both pore size and paper thickness were considered critical parameters to ensure efficient cell retention without compromising capillary flow.
Different membrane materials were evaluated to optimize plasma separation: polycarbonate (3 µm pore size), glass fiber (3 µm pore size, 785 µm thickness), and Whatman No. 1 filter paper (11 µm pore size, 180 µm thickness). The polycarbonate membrane retained excessive sample due to platelet accumulation, which blocked capillary flow. The glass fiber membrane showed higher absorption capacity but required larger sample volumes and exhibited slower flow because of its high liquid retention.
When using whole blood, it contains many formed elements, and this probably clogs the pores, which is why it was very difficult for whole blood to flow through those papers. It is important to note that when using whole blood, separation must be rapid to prevent clotting. Therefore, if the flow is stopped by the accumulation of cells, the sample clots, and the device is unable to separate the blood.
When using heparinized blood, the effect is the opposite. Everything passes through the pores, as can be seen in
Figure S1 in the Supplementary Material. For this reason, Whatman No. 1 paper was used despite having a pore size of 11 µm. The use of NaCl allows the erythrocytes, when crenated, to change their globular shape to a structure with spicules. These tend to accumulate and become trapped in the structure of the paper. Nilghaz et al. used, for example, Whatman No. 4 paper, with a pore size of 20–25 µm, and also managed to separate and retain the erythrocytes in the structure of the paper [
23]. Whatman No. 1 provided the best balance between capillary velocity and partial cell retention, resulting in a stable flow. This paper proved to be the most efficient compared to the other materials evaluated.
The combined action of both agents, NaCl and sodium heparin, seeks to induce cellular deformation and prevent coagulation [
29]. NaCl generates an osmotic gradient that causes erythrocyte crenation, favoring their aggregation and arrest in the initial areas of the paper; meanwhile, sodium heparin acts as an anticoagulant by inhibiting the action of thrombin [
23].
The drop-casting method was selected for enzyme immobilization. Although there are advanced methods, such as covalent immobilization of enzymes, this simple procedure was chosen to avoid possible loss of enzyme activity that such complex processes can cause. The use of chitosan as a biopolymer was also explored to improve the distribution of enzymes on paper. Its ability to form uniform films and its compatibility with proteins position it as a promising candidate for further optimization [
30].
One of the main challenges associated with paper-based colorimetric assays is the poor color uniformity across detection zones, which is mainly attributed to the uncontrolled flow of reagents [
29]. Several strategies have been reported to enhance color uniformity in these colorimetric assays, such as the covalent immobilization of enzymes. Despite the advantages offered by these strategies, their application has not been universal due to potential enzyme inactivation and the complexity of multiple experimental steps. Chitosan is a biocompatible and biodegradable substance with excellent film-forming ability and a high specific surface area. Paper modification with chitosan has been shown to improve color uniformity in such assays [
23].
TMB is a chromogenic substrate commonly used in biochemical assays such as ELISA. Upon oxidation, it produces a blue color, making it an ideal choice for differentiation from blood. However, TMB is sensitive to environmental factors such as pH and the presence of certain metal ions, which can act as catalysts or inhibitors in redox reactions.
To improve performance, the cellulose-based detection zones were modified with chitosan, leveraging electrostatic interactions between the negatively charged cellulose and the positively charged chitosan, as well as additional interactions such as hydrogen bonding.
The osmotic action induced by NaCl favors the physical retention of red blood cells in the paper fibers, facilitated by the compression of the electrical double layer surrounding the cells, which increases interactions with the cellulose matrix. Concentrations were selected so as not to compromise the integrity of cellular components. This passive serum separation occurs by capillarity in less than five minutes and without the need for external centrifugation devices. The following figure shows the result of the separation:
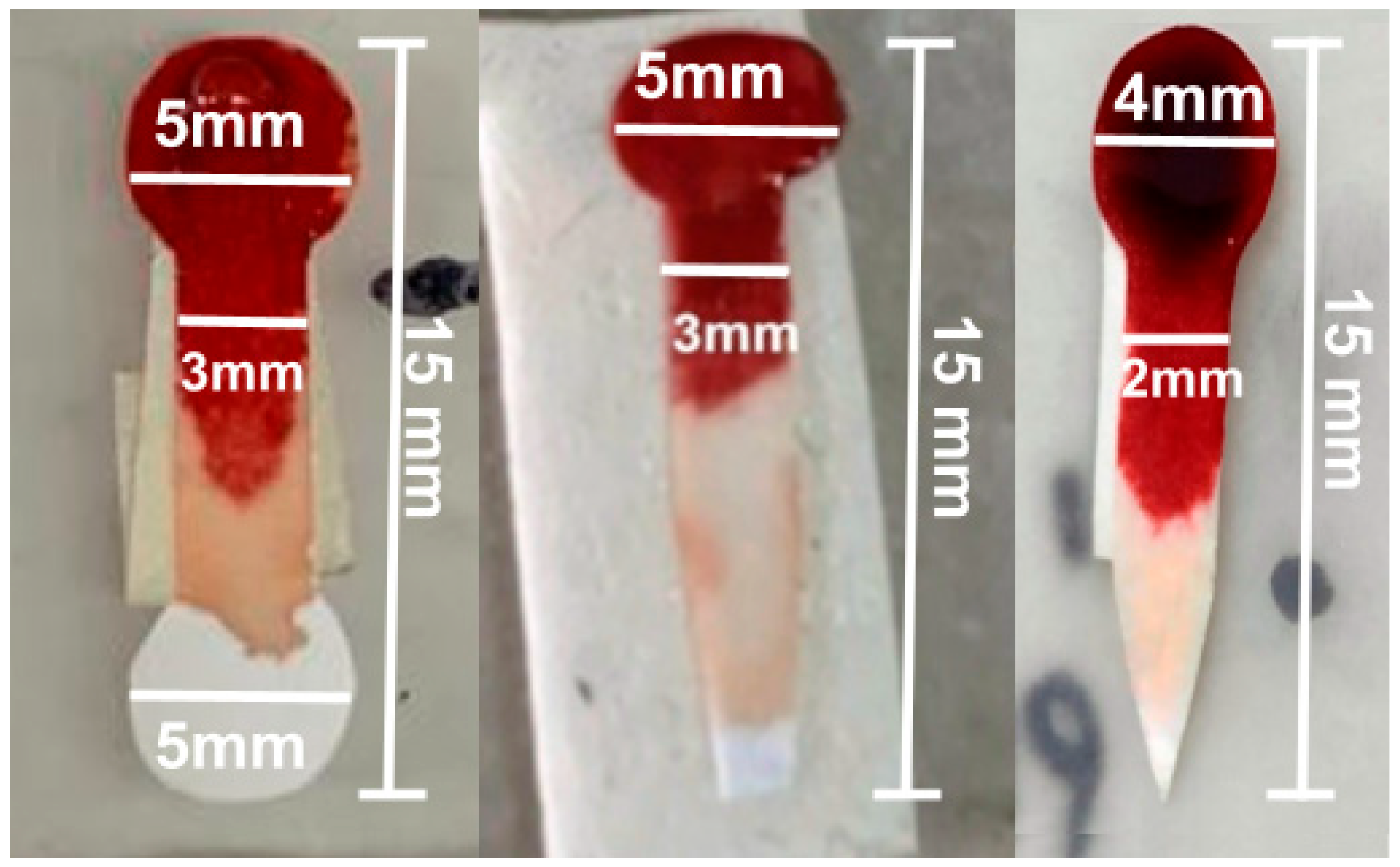
The final design included a 5 mm diameter sample collection zone and an analysis zone, connected by a 10 mm long and 3 mm wide channel, but the plasma distribution in the analysis zone was uneven. To improve this distribution, the diameter of the test zone was reduced to 4 mm, and the channel was narrowed to 2 mm, allowing for greater uniformity in plasma distribution for colorimetric analysis, as shown in
Figure 2.
The improved design introduced a pointed termination at the end of the analysis zone, which concentrates the plasma flow and promotes a more uniform distribution across the detection area. This geometry ensures that the filtered plasma reaches the analysis zone in a controlled manner, minimizing accumulation or gaps that were observed in the previous circular design, as illustrated in
Figure 2. Another important point to take into account is the sample volume. The goal was to minimize it to prioritize its use as a POC device, so that the sample could be obtained from a single drop of blood, without the need for extraction. The pointed shape thus serves a functional role in optimizing capillary flow of minimal sample volume within the device, rather than being a mere geometric modification.
During whole-blood tests, the effectiveness of different saline concentrations in separating blood plasma was evaluated (0.15 M, 0.68 M, and 1 M). The 1 M concentration was the most effective, achieving adequate plasma separation without hemoglobin contamination. Additionally, sodium heparin was incorporated into the paper to improve plasma flow and prevent clotting. The tests also evaluated the use of smaller blood volumes, adapting the design to work with smaller samples, which is crucial for Point-of-Care applications. The best concentration was 1 M, which was used for all subsequent measurements. These results can be seen in a congress communication of our group [
31].
During preliminary tests, shown in
Figure 3, mild hemolysis was specifically checked visually and by color uniformity in the separation zone. No red dye or absorbance background was observed in the plasma zone, confirming that 1 M NaCl did not cause any noticeable hemoglobin leakage. Additional tests were performed in microtubes using NaCl concentrations of 0.5, 1, 2.5, and 5 M, as can be seen in
Figure S2 in the Supplementary Material. The 0.5 M and 1 M solutions showed no signs of hemolysis, while at 2.5 M and, especially, at 5 M, clear signs of hemolysis were observed, with the latter showing intense reddish coloration in the plasma.
Glucose solutions with concentrations of 2 mM and 6 mM were tested, representing glucose levels under hypoglycemic and hyperglycemic conditions. The results demonstrated a direct correlation between glucose concentration and the intensity of the color generated in the enzymatic reaction, confirming the device’s sensitivity in measuring glucose levels.
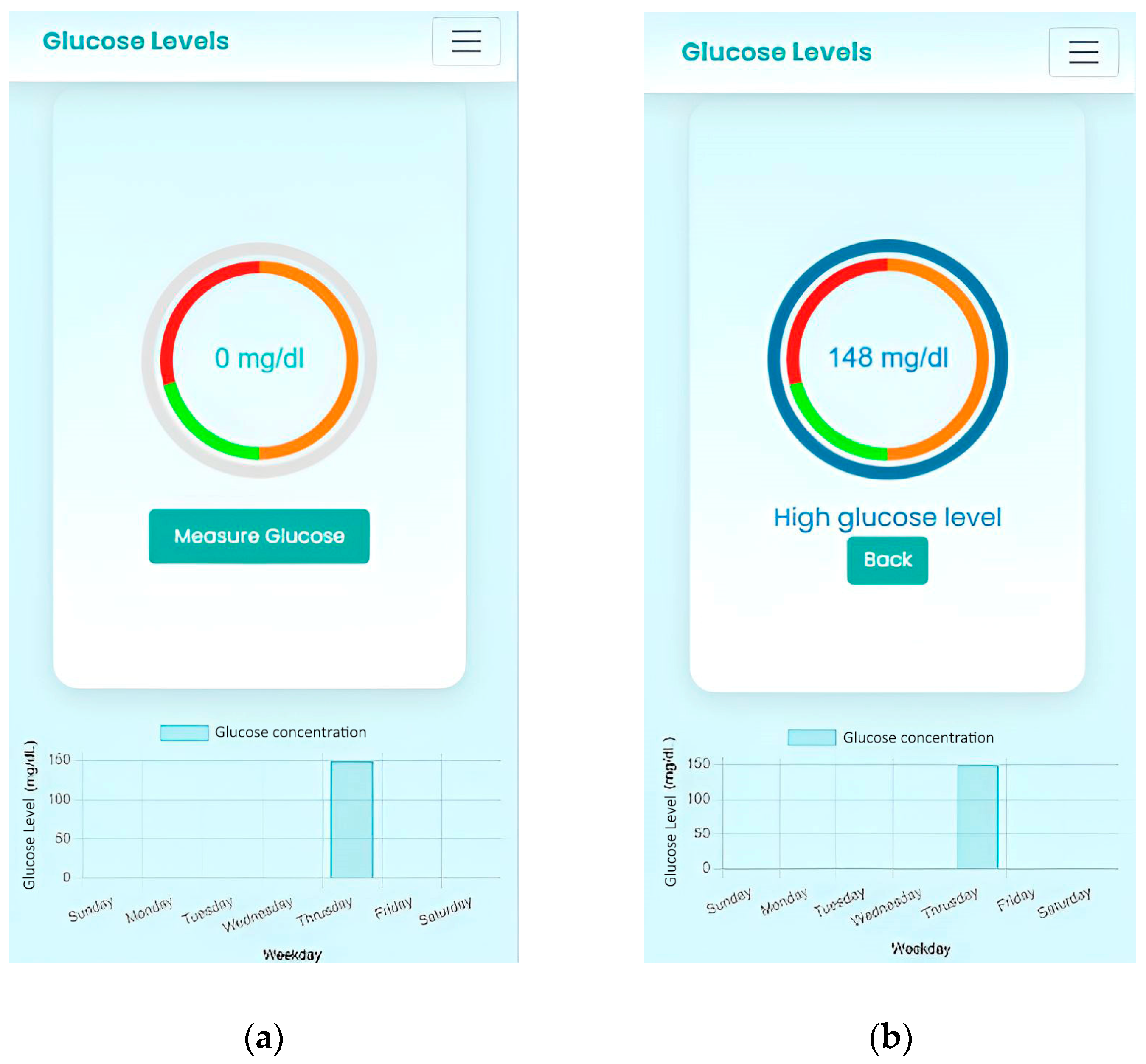
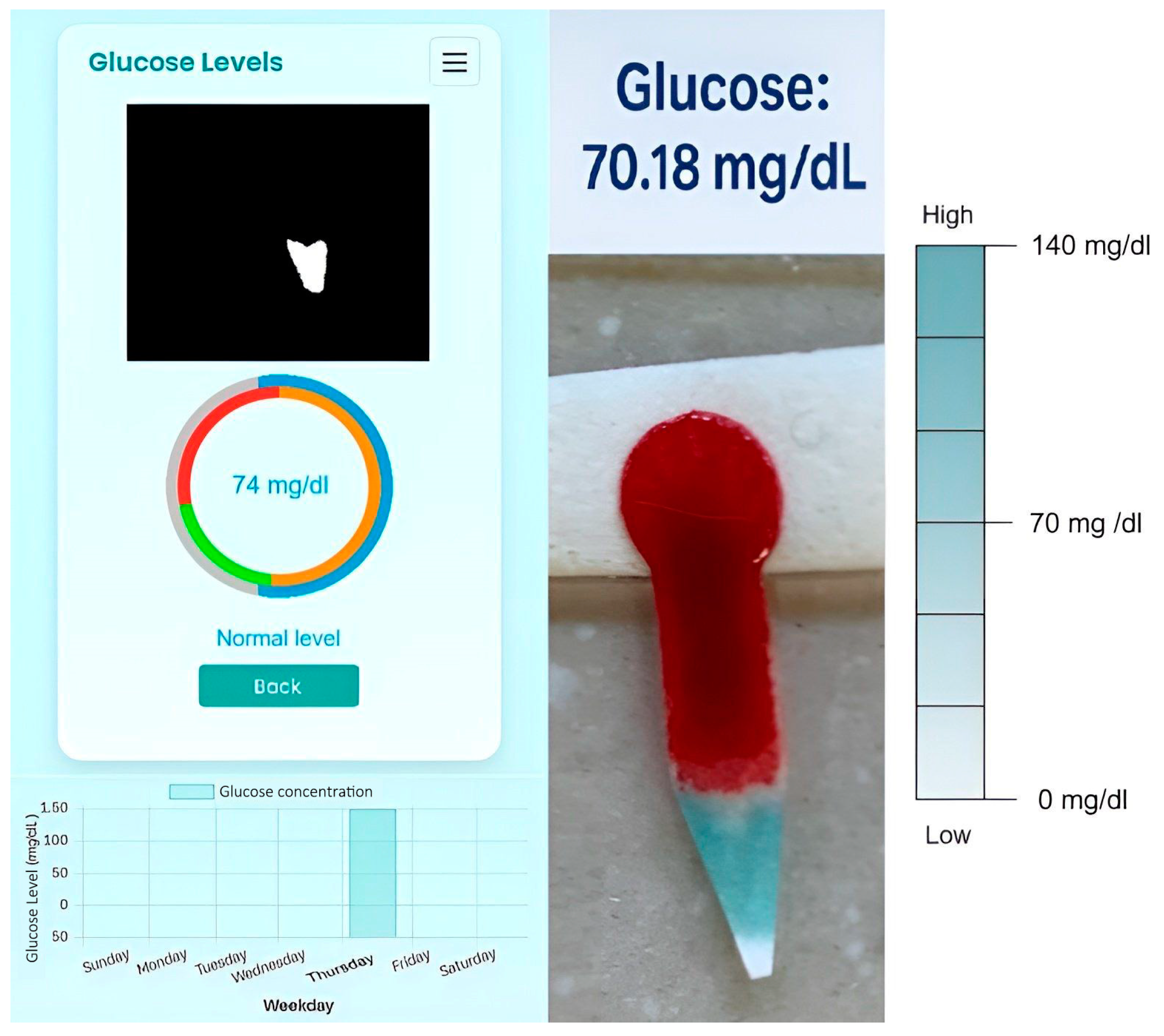
Following the measurement and color reaction, the results were analyzed by processing images of the test paper captured with the smartphone using the specially designed app. For optimal image acquisition, the test paper was placed under good lighting conditions without using the flash, which significantly improved the distinction between the colored areas and enhanced the sensitivity and accuracy of the assay. The analysis was performed using the HSV color model, which was chosen after preliminary comparisons with the RGB model because it offered better discrimination of hue variations during the TMB color transition (from blue to green) and was less affected by variations in light intensity. A semi-automatic calibration was performed. This consists of manually selecting the samples of interest within the expected color range and determining appropriate HSV (Hue, Saturation, Value) ranges that allow reliable color to define the color boundaries. Using these parameters, the OpenCV library automatically constructs the complete expected color range. This process enables the system to perform color detection efficiently by generating a binary mask that includes all pixels whose HSV values fall within the specified limits. Consequently, the calibration step ensures that the target color is accurately segmented, even under varying illumination conditions.
It is important to note that no external or internal color reference was used during image capture, so the color measurements depended entirely on the software implementation and the consistency of the imaging conditions, highlighting the role of the app in standardizing image processing.
As was mentioned in Step 2 of the Materials and Methods, once the areas are detected, those corresponding to the desired color are shown in white, and the areas that do not match are shown in black, as can be seen in
Figure 4.
The measurement protocol consists of collecting an 8 μL blood sample; waiting 10 min; performing the test in a well-lit area, preferably under cold LED lighting or daylight; and taking the photo from a distance of 10 cm without using a flash.
To quantify the flow rate until the reaction is complete, two stages are considered. The first stage runs from the start of the channel to the start of the reaction zone (10 mm channel). The second stage comprises the reaction zone, where the flow rate is measured until the color change is complete. In Whatman No. 1 paper modified with sodium heparin, the capillary flow typically in the first stage is 286.5 ± 15.2 s, corresponding to an average flow rate of 0.035 ± 0.002 mm s−1. The total separation was completed in 538.8 ± 109.4 s, corresponding to a total average flow rate of 0.019 ± 0.004 mm s−1.
Calibration is performed through a linear regression between the color intensity recorded by the smartphone and the glucose concentration obtained with the commercial blood glucose test kit. The obtained calibration equation allows the app to adjust an unknown glucose concentration based on color intensity. Glucose determination in the lab is normally determined by using plasma samples. To evaluate if the measurement and the application are consistent when using either whole blood or plasma, a parallel evaluation was conducted. Data collected from several samples (whole blood (a), and plasma (b) from the same sample,
Figure 5) in triplicate are organized in a table that includes the glucose concentration measured with the blood glucose test kit, the average of the three color intensities detected for each sample, and the standard deviation of each measurement, as can be seen in
Table 1. Each sample was analyzed using the smartphone application. A scatter plot and a calibration equation were obtained by linear regression.
Figure 5 shows the resulting chips for each measurement. The non-uniform color distribution observed, even with chitosan modification, can be attributed to several factors. Although the solutions are applied manually on paper, care is always taken to place the drop in the same spot. The porosity of the paper and the viscosity of the different solutions can lead to uneven distribution. Yet, the rapid solvent evaporation can exacerbate these differences by causing uneven reagent spreading and drying. On the other hand, it can be observed that the area of the greatest color forms a V shape following the shape of the paper at the tip. This is not a problem for determination with the smartphone application, as it detects and segments the region of greatest intensity.
The chitosan film (1 mg/mL in 0.25% acetic acid) forms a weakly acidic matrix that stabilizes GOx and HRP, maintaining an effective local pH of ≈6–6.5, within their optimal range. During the tests, only the separated serum was applied to the reaction zone, which does not change pH value. Wang et al. studied the activity of the enzyme immobilized in chitosan with buffer at different pH levels by evaluating the reaction with
TMB, and concluded that, for glucose detection, color intensity reached the highest value when the pH was 6.0 [
25].
The resulting equation allows the application to predict the glucose concentration of a sample simply from the color intensity values, making the system self-contained and efficient for future analysis.
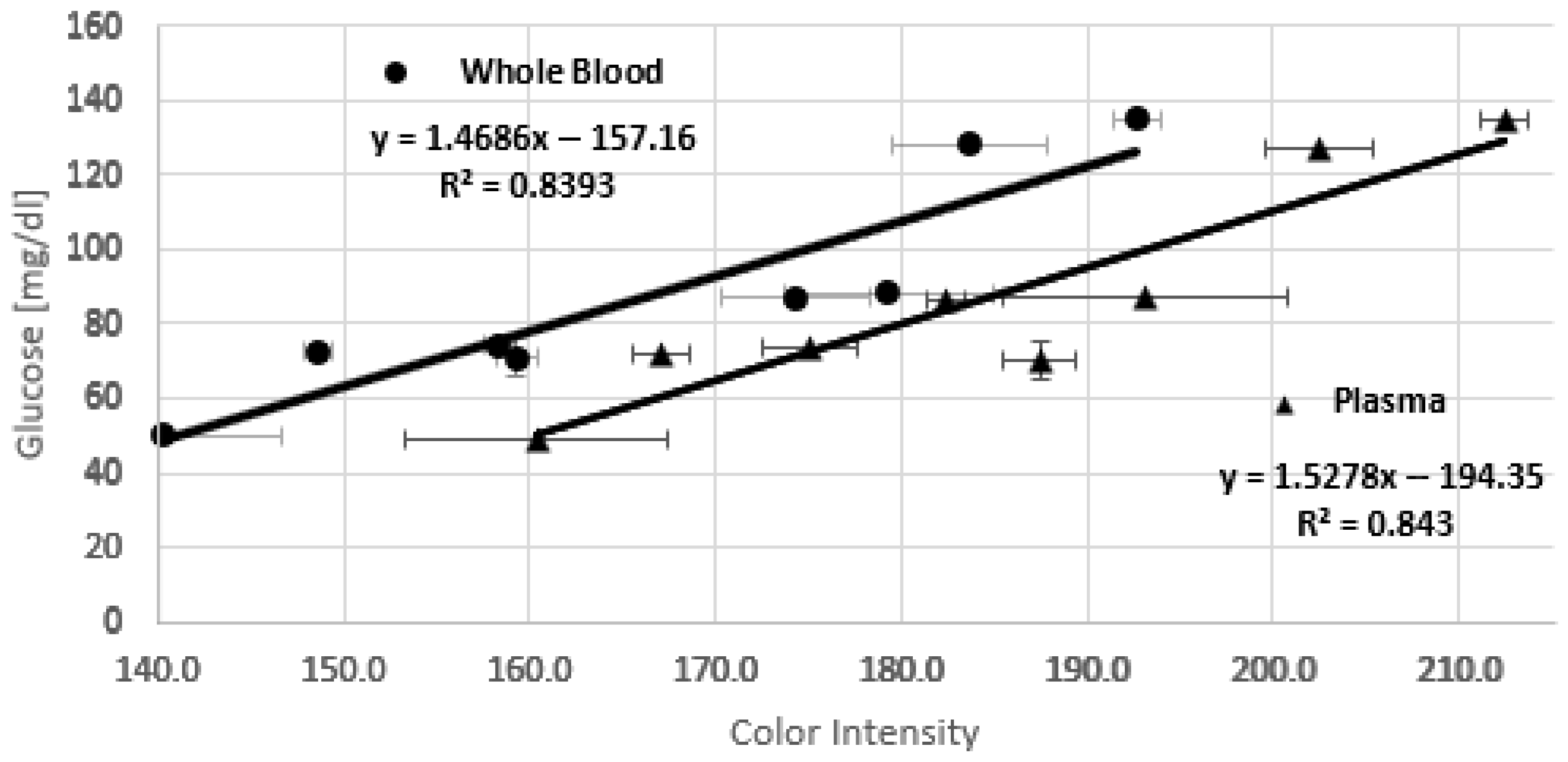
Figure 6 shows the calibration curves for whole blood and plasma, respectively. The large error bars observed in some points of
Figure 6 likely result from a combination of experimental and material-related factors affecting color uniformity and reproducibility across test strips, as was discussed previously.
The following equations describe the behavior of glucose concentration as a function of color intensity for whole blood (1) and plasma (2) samples.
As can be seen, the lines are parallel, i.e., they have almost the same slope, but there is a constant offset. It is noticeable that the chip overestimates the color intensity, possibly because, in the case of whole blood, the serum is not completely filtered. This issue could be related to the fact that the pore size of Whatman No. 1 paper is larger than the diameter of red blood cells, allowing some of them to pass through and mix with the serum. Additionally, it is important to note that at high salt concentrations, red blood cells could rupture, releasing hemoglobin and generating a faint pinkish color that interferes with the measurement by intensifying the final color.
To evaluate the performance of the system, it was tested with an unknown blood sample. The separated plasma was analyzed using a commercial glucose determination kit, in order to compare the results with those obtained from the developed device. This comparison is shown in
Figure 7.
To evaluate the error in the curves, we used the RMSE (root mean square error), a metric widely used to measure the accuracy of predictions. It was calculated for both curves, and its value was approximately 10.83, while the standard deviations of the different subsets of data ranged from 1.5 to 15.4. Since the RMSE is lower than the maximum standard deviation (15.4), it can be inferred that the model presents a reasonable average error compared to the overall variability of the data. However, the RMSE exceeds some of the lower standard deviations, suggesting that the model may have less precise fitting for certain subsets with lower variability. Overall, these results indicate that the model has acceptable performance and adequate predictive capability, although it could benefit from further adjustment to improve accuracy in cases with lower dispersion.
A clear linear trend is observed in both calibration curves, with similar R2 and RMSE values, confirming that the measurements obtained with the device using whole-blood samples are comparable to those obtained with plasma.
The detection range of the glucose test strip extends from approximately 50 to 140 mg/dL, as shown in the calibration curves for both whole blood and plasma samples. In terms of stability, the test strips can be stored for up to one week in a freezer (−20 °C), maintaining both chemical and colorimetric stability. When stored at room temperature (20–25 °C), the strips remain stable for at least one day if stored in a cooler, in a dry environment, protected from light and moisture.