Can Preoperative Blood Inflammatory Biomarkers Predict Early Dental Implant Outcomes in Systemically Healthy Patients?
Abstract
1. Introduction
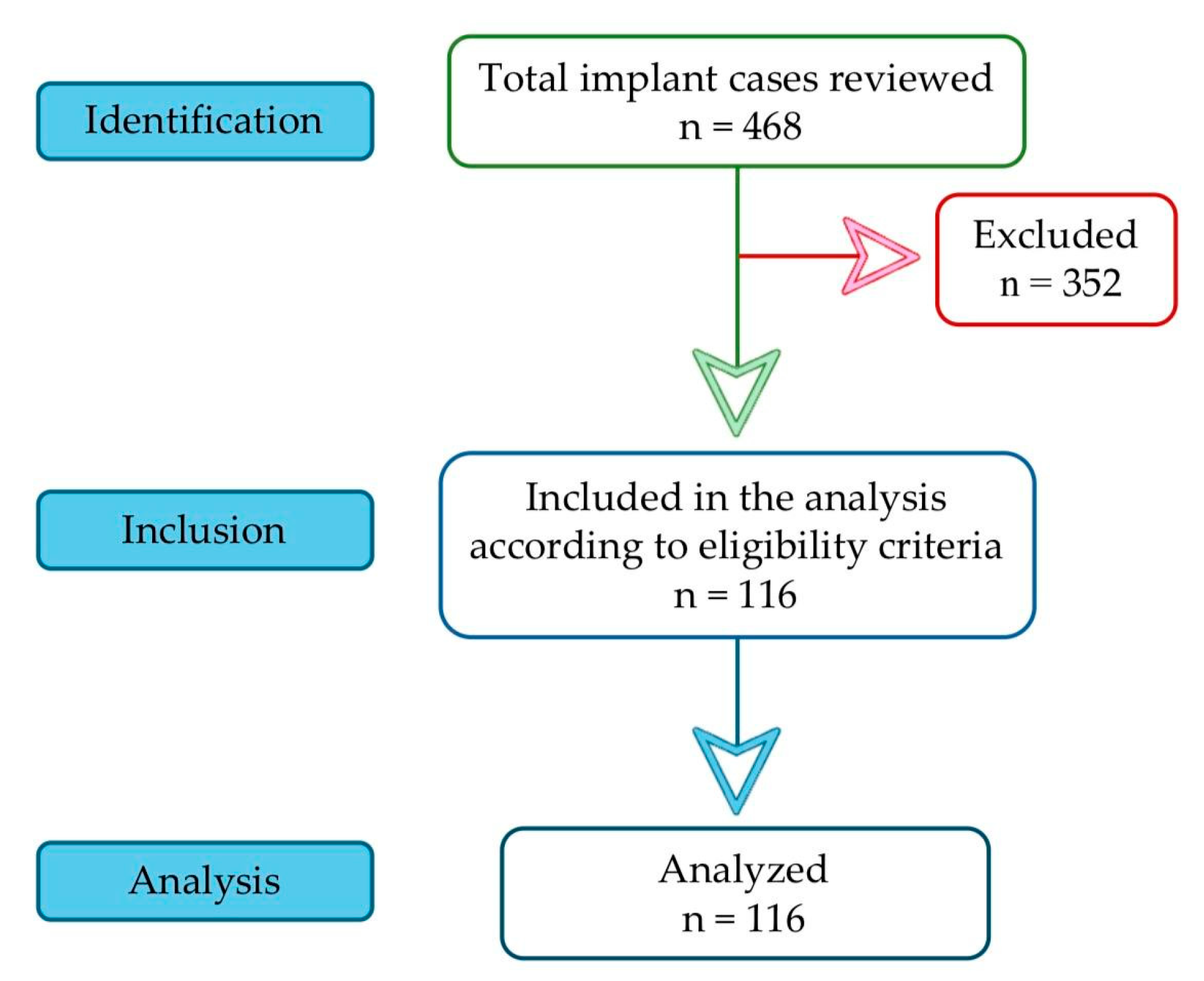
2. Materials and Methods
2.1. Research Design
2.2. Criteria for Patient Selection
- -
- age: 20–50 years;
- -
- diagnostic records: availability of radiographic (orthopantomography—OPG; cone beam computed tomography CBCT) and photographic documentation before and after treatment;
- -
- periodontal status: either periodontal health or stable periodontal status, defined by a history of periodontitis with <10% bleeding sites and probing depths ≤ 3 mm over the past 6 months [29];
- -
- non-smokers;
- -
- no systemic diseases;
- -
- no history of allergies (including food and metal allergies);
- -
- good treatment adherence and maintenance of satisfactory postoperative oral hygiene;
- -
- ethics: signed informed consent.
- -
- incomplete or missing clinical documentation (radiographic or photographic records) before or after treatment;
- -
- history of smoking, alcohol dependence, or substance abuse;
- -
- periodontal status matching stage III or IV;
- -
- history of periodontitis treatment within the past six months;
- -
- presence of critical anatomical limitations requiring sinus lift, bone additions, or immediate postextraction implantation;
- -
- use of antibacterial or anti-inflammatory medication within four weeks prior to blood sample collection;
- -
- systemic diseases or conditions affecting bone metabolism, including uncontrolled diabetes, allergies, coronary heart disease, pulmonary disease, malignant tumours, osteoporosis, or ongoing bisphosphonate therapy.
2.3. Operative Technique and Postoperative Care
2.4. Statistical Analysis
3. Results
- -
- SII (AUC = 0.821, SE = 0.066, 95% CI 0.692–0.951, p = 0.015) demonstrated good discrimination; the result was statistically significant.
- -
- NLR (AUC = 0.728, SE = 0.117, 95% CI 0.498–0.958, p = 0.085) demonstrated fair discrimination, but the result was not statistically significant.
- -
- PLR (AUC = 0.706, SE = 0.102, 95% CI 0.507–0.905, p = 0.12) also showed fair discrimination; not significant.
- -
- CRP (AUC = 0.581, SE = 0.095, 95% CI 0.395–0.767, p = 0.541) showed poor discrimination; not significant.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CBCT | Cone beam computed tomography |
| CRP | C-reactive protein level |
| L | Lymphocyte level |
| N | Neutrophil level |
| NLR | Neutrophil-to-lymphocyte ratio |
| OPG | Orthopantomography |
| PLR | Platelet-to-lymphocyte ratio |
| PLT | Platelet level |
| SD | Standard deviation |
| SII | Systemic immune-inflammatory index |
References
- Addy, L.D. An introduction to dental implants. Br. Dent. J. 2024, 236, 753–757. [Google Scholar] [CrossRef]
- Gallucci, G.O.; Benic, G.I.; Eckert, S.E.; Papaspyridakos, P.; Schimmel, M.; Schrott, A.; Weber, H.P. Consensus statements and clinical recommendations for implant loading protocols. Int. J. Oral Maxillofac. Implant. 2014, 29, 287–290. [Google Scholar] [CrossRef]
- De Souza, A.B.; Papaspyridakos, P.; Weber, H.P.; Vazouras, K.; Matarazzo, F. Effect of dental implant therapy on the preservation of orofacial tissues: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2023, 34, 240–256. [Google Scholar] [CrossRef]
- AlRowis, R.; Albelaihi, F.; Alquraini, H.; Almojel, S.; Alsudais, A.; Alaqeely, R. Factors affecting dental implant failure: A retrospective analysis. Healthcare 2025, 13, 1356. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Persson, L.; Klinge, B. A systematic review of the incidence of biological and technical complications in implant dentistry reported in prospective longitudinal studies of at least 5 years. J. Clin. Periodontol. 2002, 29, 197–212. [Google Scholar] [CrossRef]
- Esposito, M.; Hirsch, J.; Lekholm, U.; Thomsen, P. Biological factors contributing to failures of osseointegrated oral implants, (I). Success criteria and epidemiology. Eur. J. Oral Sci. 1998, 106, 527–551. [Google Scholar] [CrossRef]
- Insua, A.; Monje, A.; Wang, H.-L.; Miron, R.J. Basis of bone metabolism around dental implants during osseointegration and peri-implant bone loss. J. Biomed. Mater. Res. Part A 2017, 105, 2075–2089. [Google Scholar] [CrossRef] [PubMed]
- Vinhas, A.S.; Aroso, C.; Salazar, F.; López-Jarana, P.; Ríos-Santos, J.V.; Herrero-Climent, M. Review of the mechanical behavior of different implant–abutment connections. Int. J. Environ. Res. Public Health 2020, 17, 8685. [Google Scholar] [CrossRef]
- Mavrogenis, A.F.; Dimitriou, R.; Parvizi, J.; Babis, G.C. Biology of implant osseointegration. J. Musculoskelet. Neuronal Interact. 2009, 9, 61–71. [Google Scholar] [PubMed]
- Brånemark, P.I. Vital microscopy of bone marrow in rabbit. Scand. J. Clin. Lab. Investig. 1959, 11, 1–82. [Google Scholar]
- Brånemark, P.I. Osseointegration and its experimental background. J. Prosthet. Dent. 1983, 50, 399–410. [Google Scholar] [CrossRef]
- MacLeod, A.S.; Mansbridge, J.N. The innate immune system in acute and chronic wounds. Adv. Wound Care 2016, 5, 65–78. [Google Scholar] [CrossRef]
- Kondo, T.; Yamada, M.; Egusa, H. Innate immune regulation in dental implant osseointegration. J. Prosthodont. Res. 2024, 68, 511–521. [Google Scholar] [CrossRef]
- Amengual-Peñafiel, L.; Córdova, L.A.; Constanza Jara-Sepúlveda, M.; Brañes-Aroca, M.; Marchesani-Carrasco, F.; Cartes-Velásquez, R. Osteoimmunology drives dental implant osseointegration: A new paradigm for implant dentistry. Jpn. Dent. Sci. Rev. 2021, 57, 12–19. [Google Scholar] [CrossRef]
- Abaricia, J.O.; Farzad, N.; Heath, T.J.; Simmons, J.; Morandini, L.; Olivares-Navarrete, R. Control of innate immune response by biomaterial surface topography, energy, and stiffness. Acta Biomater. 2021, 133, 58–73. [Google Scholar] [CrossRef]
- Mirza, R.; El Rabbany, M.; Ali, D.S.; Tetradis, S.; Morrison, A.; Ruggiero, S.; Alnajimi, R.; Khan, A.A.; Guyatt, G. Dental implant failure and medication-related osteonecrosis of the jaw related to dental implants in patients taking antiresorptive therapy for osteoporosis: A systematic review and meta-analysis. Endocr. Pract. 2025, 31, 1189–1196. [Google Scholar] [CrossRef]
- Onică, N.; Budală, D.G.; Baciu, E.-R.; Onică, C.A.; Gelețu, G.L.; Murariu, A.; Balan, M.; Pertea, M.; Stelea, C. Long-term clinical outcomes of 3D-printed subperiosteal titanium implants: A 6-year follow-up. J. Pers. Med. 2024, 14, 541. [Google Scholar] [CrossRef]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef] [PubMed]
- Bastian, O.W.; Koenderman, L.; Alblas, J.; Leenen, L.P.H.; Blokhuis, T.J. Neutrophils contribute to fracture healing by synthesizing fibronectin+ extracellular matrix rapidly after injury. Clin. Immunol. 2016, 164, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Fest, J.; Ruiter, R.; Ikram, M.A.; Voortman, T.; van Eijck, C.H.J.; Stricker, B.H. Reference values for white blood-cell-based inflammatory markers in the Rotterdam Study: A population-based prospective cohort study. Sci. Rep. 2018, 8, 10566. [Google Scholar] [CrossRef] [PubMed]
- Bansal, T.; Pandey, A.; Deepa, D.; Asthana, A.K. C-reactive protein (CRP) and its association with periodontal disease: A brief review. J. Clin. Diagn. Res. 2014, 8, ZE21-4. [Google Scholar] [CrossRef]
- Lee, J.H.; Mun, S.J. Relationship between C-reactive protein level and periodontitis and systemic diseases. J. Periodontol. 2024, 95, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Suri, P.; Patil, P.B.; Rajguru, J.P.; Gupta, P.; Patel, N. Comparative evaluation of role of hs C-reactive protein as a diagnostic marker in chronic periodontitis patients. J. Fam. Med. Prim. Care 2020, 9, 1340–1347. [Google Scholar] [CrossRef]
- Esteves-Lima, R.P.; Reis, C.S.; Santirocchi-Júnior, F.; Abreu, L.G.; Costa, F.O. Association between periodontitis and serum C-reactive protein levels. J. Clin. Exp. Dent. 2020, 12, e838–e843. [Google Scholar] [CrossRef]
- Walther, K.A.; Gröger, S.; Vogler, J.A.H.; Wöstmann, B.; Meyle, J. Inflammation indices in association with periodontitis and cancer. Periodontol. 2000 2024, 96, 281–315. [Google Scholar] [CrossRef] [PubMed]
- Almășan, O.; Leucuța, D.C.; Hedeșiu, M. Blood cell count inflammatory markers as prognostic indicators of periodontitis: A systematic review and meta-analysis. J. Pers. Med. 2022, 12, 992. [Google Scholar] [CrossRef]
- Lu, R.; Li, W.; Wang, X.; Shi, D.; Meng, H. Elevated neutrophil-to-lymphocyte ratio but not platelet-to-lymphocyte ratio is associated with generalized aggressive periodontitis in a Chinese population. J. Periodontol. 2021, 92, 507–513. [Google Scholar] [CrossRef]
- Bhattacharya, H.S.; Srivastava, R.; Gummaluri, S.S.; Agarwal, M.C.; Bhattacharya, P.; Astekar, M.S. Comparison of blood parameters between periodontitis patients and healthy participants: A cross-sectional hematological study. J. Oral Maxillofac. Pathol. 2022, 26, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S74–S84. [Google Scholar] [CrossRef]
- Chhina, M.S. Is there a correlation between peri-implantitis and systemic inflammation? Evid. Based Dent. 2024, 25, 69–70. [Google Scholar] [CrossRef]
- Yan, Y.; Orlandi, M.; Suvan, J.; Harden, S.; Smith, J.; D’Aiuto, F. Association between peri-implantitis and systemic inflammation: A systematic review. Front. Immunol. 2023, 14, 1235155. [Google Scholar] [CrossRef] [PubMed]
- Wåhlberg, R.D.; Stenport, V.F.; Wennerberg, A.; Hjalmarsson, L. A multicenter study of factors related to early implant failures-Part 2: Patient factors. Clin. Implant. Dent. Relat. Res. 2025, 27, e70081. [Google Scholar] [CrossRef]
- Kupka, J.R.; König, J.; Al-Nawas, B.; Sagheb, K.; Schiegnitz, E. How far can we go? A 20-year meta-analysis of dental implant survival rates. Clin. Oral Investig. 2024, 28, 541. [Google Scholar] [CrossRef] [PubMed]
- Howe, M.S.; Keys, W.; Richards, D. Long-term (10-year) dental implant survival: A systematic review and sensitivity meta-analysis. J. Dent. 2019, 84, 9–21. [Google Scholar] [CrossRef]
- Hjalmarsson, L.; Gheisarifar, M.; Jemt, T. A systematic review of survival of single implants as presented in longitudinal studies with a follow-up of at least 10 years. Eur. J. Oral Implantol. 2016, 9, S155–S162. [Google Scholar] [PubMed]
- Jemt, T. Implant survival in the edentulous jaw-30 years of experience. Part I: A retro-prospective multivariate regression analysis of overall implant failure in 4,585 consecutively treated arches. Int. J. Prosthodont. 2018, 31, 425–435. [Google Scholar] [CrossRef]
- Hultin, M.; Gustafsson, A.; Hallström, H.; Johansson, L.A.; Ekfeldt, A.; Klinge, B. Microbiological findings and host response in patients with peri-implantitis. Clin. Oral Implant. Res. 2002, 13, 349–358. [Google Scholar] [CrossRef]
- Sharma, M.; Singh, A.P.; Kumar, B.; Girdhar, P.; Brar, A.S.; Mittal, P. Evaluation of C-reactive proteins levels in peri-implantitis patients. J. Pharm. Bioallied Sci. 2024, 16, S2800–S2802. [Google Scholar] [CrossRef]
- Khichy, A.; Khichy, R.; Singh, R.; Bali, Y.; Kaur, S.; Gill, T.K. Assessment of levels of C-reactive proteins and interleukin 6 in patients with peri-implantitis: A case-control study. J. Pharm. Bioallied Sci. 2021, 13, S444–S447. [Google Scholar] [CrossRef]



| Patient Characteristics | Count (N) | Percentage (%) | Tests of Normality | ||
|---|---|---|---|---|---|
| Kolmogorov–Smirnov | Shapiro–Wilk | ||||
| Variable | Gender | p = 0.000 | p = 0.000 | ||
| Male | 54 | 46.6 | |||
| Female | 62 | 53.4 | |||
| Age | p = 0.000 | p 0.000 | |||
| 20–30 years old | 22 | 19.0 | |||
| 31–40 years old | 41 | 35.3 | |||
| 41–50 years old | 53 | 45.7 | |||
| Mean ± SD (Minimum−Maximum) | 38.42 ± 8.255 (20–50) | ||||
| Periodontal status | p = 0.000 | p = 0.000 | |||
| Clinically healthy | 57 | 49.1 | |||
| Stable periodontal status | 59 | 50.9 | |||
| Neutrophil level (103/µL) | p = 0.007 | p = 0.000 | |||
| Mean ± SD (Minimum−Maximum) | 3.80 ± 1.41 (1.24–11.08) | ||||
| Reference range: 2–8 ×103/µL | |||||
| Lymphocyte level (103/µL) | p = 0.085 | p = 0.030 | |||
| Mean ± SD (Minimum−Maximum) | 2.33 ± 0.71 (0.90–4.48) | ||||
| Reference range: 1–4 ×103/µL | |||||
| Platelet level (103/µL) | p = 0.008 | p = 0.000 | |||
| Mean ± SD (Minimum−Maximum) | 263.55 ± 58.91 (162–454) | ||||
| Reference range: 150–450 ×103/µL | |||||
| C-reactive protein level (mg/L) | p = 0.011 | p = 0.000 | |||
| Mean ± SD (Minimum−Maximum) | 0.85 ± 0.54 (0.02–2.7) | ||||
| Reference range: 0–5 mg/L | |||||
| Neutrophil-to-lymphocyte ratio | p = 0.000 | p = 0.000 | |||
| Mean ± SD (Minimum−Maximum) | 1.73 ± 0.75 (0.52−6) | ||||
| Platelet-to-lymphocyte ratio | p = 0.000 | p = 0.000 | |||
| Mean ± SD (Minimum−Maximum) | 123.50 ± 51.62 (54.73–328.88) | ||||
| Systemic immune-inflammatory index | p = 0.000 | p = 0.000 | |||
| Mean ± SD (Minimum−Maximum) | 463.02 ± 258.74 (127.12–1776) | ||||
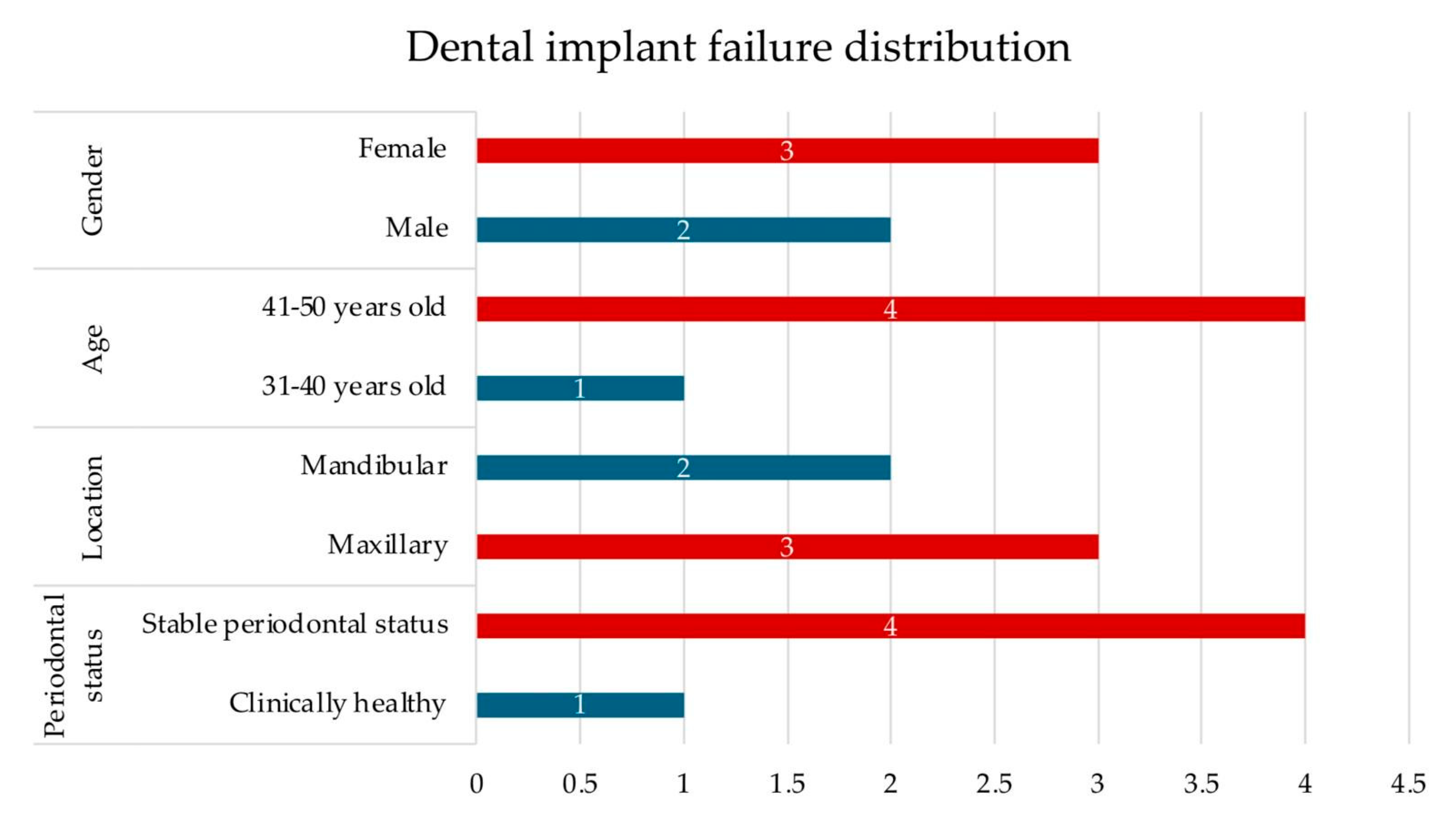
| Postoperative outcomes | p = 0.000 | p = 0.000 | |||
| Dental implant survival Proper implant osseointegration | 111 | 95.7 | |||
| Early dental implant failure Inadequate implant osseointegration | 5 | 4.3 | |||
| Age (Mean ± SD) | Gender (Mean ± SD) | Periodontal Status (Mean ± SD) | |||||
|---|---|---|---|---|---|---|---|
| 20–30 Years Old | 31–40 Years Old | 41–50 Years Old | Male | Female | Healthy | Stable | |
| N 103/µL | 3.88 ± 1.29 | 3.70 ± 1.09 | 3.84 ± 1.67 | 3.77 ± 1.45 | 3.83 ± 1.39 | 3.64 ± 1.08 | 3.93 ± 1.67 |
| Kruskal–Wallis Test; p = 0.756 | Mann–Whitney test; p = 0.897 | Mann–Whitney test; p = 0.522 | |||||
| L 103/µL | 2.55 ± 0.89 | 2.24 ± 0.64 | 2.31 ± 0.68 | 2.42 ± 0.71 | 2.26 ± 0.71 | 2.34 ± 0.73 | 2.33 ± 0.70 |
| Kruskal–Wallis Test; p = 0.438 | Mann–Whitney test; p = 0.141 | Mann–Whitney test; p = 0.667 | |||||
| PLT 103/µL | 251.68 ± 52.17 | 260.49 ± 62.22 | 270.84 ± 58.96 | 253.05 ± 54.35 | 272.69 ± 61.58 | 254.21 ± 52.55 | 272.57 ± 63.60 |
| Kruskal–Wallis Test; p = 0.461 | Mann–Whitney test; p = 0.055 | Mann–Whitney test; p = 0.126 | |||||
| NLR | 1.70 ± 1.06 | 1.74 ± 0.66 | 1.73 ± 0.67 | 1.66 ± 0.80 | 1.79 ± 0.70 | 1.70 ± 0.87 | 1.75 ± 0.62 |
| Kruskal–Wallis Test; p = 0.539 | Mann–Whitney test; p = 0.161 | Mann–Whitney test; p = 0.228 | |||||
| PLR | 111.51 ± 55.86 | 127.56 ± 59.61 | 125.33 ± 42.70 | 113.16 ± 43.72 | 132.50 ± 56.45 | 122.54 ± 62.16 | 124.42 ± 39.39 |
| Kruskal–Wallis Test; p = 0.193 | Mann–Whitney test; p = 0.041 * | Mann–Whitney test; p = 0.105 | |||||
| SII | 436.37 ± 322.80 | 462.73 ± 240.85 | 474.29 ± 246.82 | 420.59 ± 243.31 | 499.97 ± 267.95 | 446.76 ± 293.13 | 478.72 ± 221.96 |
| Kruskal–Wallis Test; p = 0.410 | Mann–Whitney test; p = 0.058 | Mann–Whitney test; p = 0.036 * | |||||
| CRP mg/L | 0.98 ± 0.51 | 0.80 ± 0.60 | 0.84 ± 0.50 | 0.81 ± 0.48 | 0.89 ± 0.59 | 0.83 ± 0.52 | 0.87 ± 0.57 |
| Kruskal–Wallis Test; p = 0.303 | Mann–Whitney test; p = 0.059 | Mann–Whitney test; p = 0.866 | |||||
| Baseline Clinical Parameters | Dental Implant Survival | Dental Implant Failure | ||||
|---|---|---|---|---|---|---|
| Mean ± SD | Minimum | Maximum | Mean ± SD | Minimum | Maximum | |
| N 103/µL | 3.77 ± 1.43 | 1.24 | 11.08 | 4.37± 0.49 | 3.8 | 5.1 |
| Mann–Whitney test, U = 404.000, p = 0.085 | ||||||
| L 103/µL | 2.34 ± 0.71 | 0.90 | 4.48 | 2.19± 0.84 | 1.30 | 3.54 |
| Mann–Whitney test, U = 234.500, p = 0.559 | ||||||
| PLT 103/µL | 260.99 ± 57.26 | 162 | 454 | 320.4± 73.25 | 231 | 427 |
| Mann–Whitney test, U = 417.500, p = 0.057 | ||||||
| NLR | 1.71 ± 0.75 | 0.52 | 6 | 2.18 ± 0.66 | 1.27 | 2.92 |
| Mann–Whitney test, U = 404.000, p = 0.085 | ||||||
| PLR | 121.57 ± 48.87 | 54.73 | 328.88 | 166.38 ± 92.41 | 97.17 | 328.46 |
| Mann–Whitney test, U = 392.000, p = 0.12 | ||||||
| SII | 452.05 ± 251.73 | 127.12 | 1776 | 706.42 ± 323.66 | 439.23 | 1248.15 |
| Mann–Whitney test, U = 456.000, p = 0.015 * | ||||||
| CRP mg/L | 0.85 ± 0.55 | 0.02 | 2.70 | 0.96 ± 0.44 | 0.6 | 1.72 |
| Mann–Whitney test, U = 322.500, p = 0.541 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baciu, E.-R.; Onică, C.A.; Gelețu, G.L.; Onică, N.; Toma, B.F.; Teodorescu, A.C.; Lupu, C.I.; Murariu, A. Can Preoperative Blood Inflammatory Biomarkers Predict Early Dental Implant Outcomes in Systemically Healthy Patients? Bioengineering 2025, 12, 1208. https://doi.org/10.3390/bioengineering12111208
Baciu E-R, Onică CA, Gelețu GL, Onică N, Toma BF, Teodorescu AC, Lupu CI, Murariu A. Can Preoperative Blood Inflammatory Biomarkers Predict Early Dental Implant Outcomes in Systemically Healthy Patients? Bioengineering. 2025; 12(11):1208. https://doi.org/10.3390/bioengineering12111208
Chicago/Turabian StyleBaciu, Elena-Raluca, Cezara Andreea Onică, Gabriela Luminița Gelețu, Neculai Onică, Bogdan Florin Toma, Alexandra Cornelia Teodorescu, Costin Iulian Lupu, and Alice Murariu. 2025. "Can Preoperative Blood Inflammatory Biomarkers Predict Early Dental Implant Outcomes in Systemically Healthy Patients?" Bioengineering 12, no. 11: 1208. https://doi.org/10.3390/bioengineering12111208
APA StyleBaciu, E.-R., Onică, C. A., Gelețu, G. L., Onică, N., Toma, B. F., Teodorescu, A. C., Lupu, C. I., & Murariu, A. (2025). Can Preoperative Blood Inflammatory Biomarkers Predict Early Dental Implant Outcomes in Systemically Healthy Patients? Bioengineering, 12(11), 1208. https://doi.org/10.3390/bioengineering12111208








