Mesenchymal Stem Cell-Mediated Targeted Drug Delivery Systems for Hepatocellular Carcinoma: Current Advances and Future Directions
Abstract
1. Introduction
2. Current Landscape of Hepatocellular Carcinoma Therapy
3. Biological Basis of MSC-Based Delivery Systems
3.1. Source
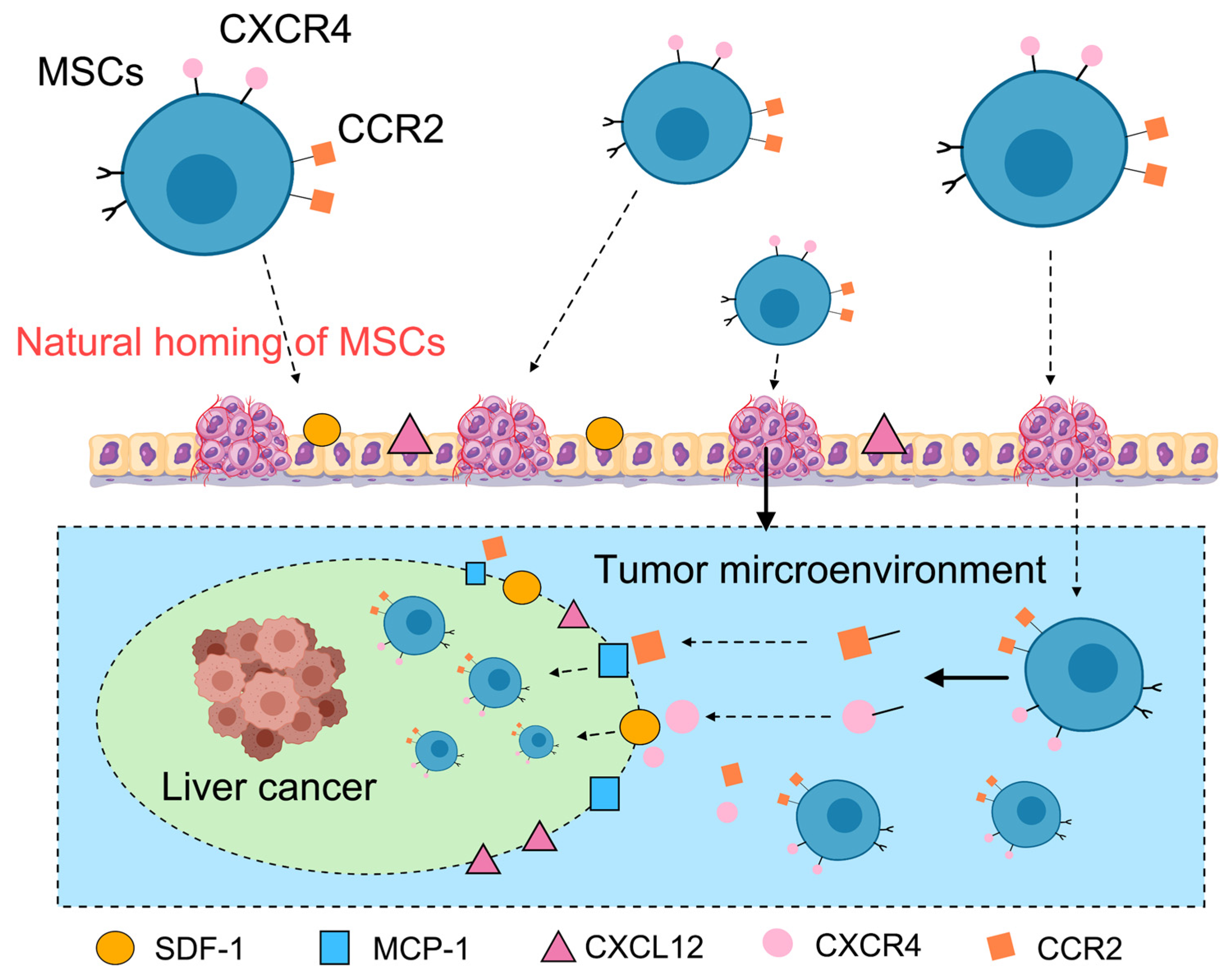
3.2. Homing Capacity and Drug Delivery
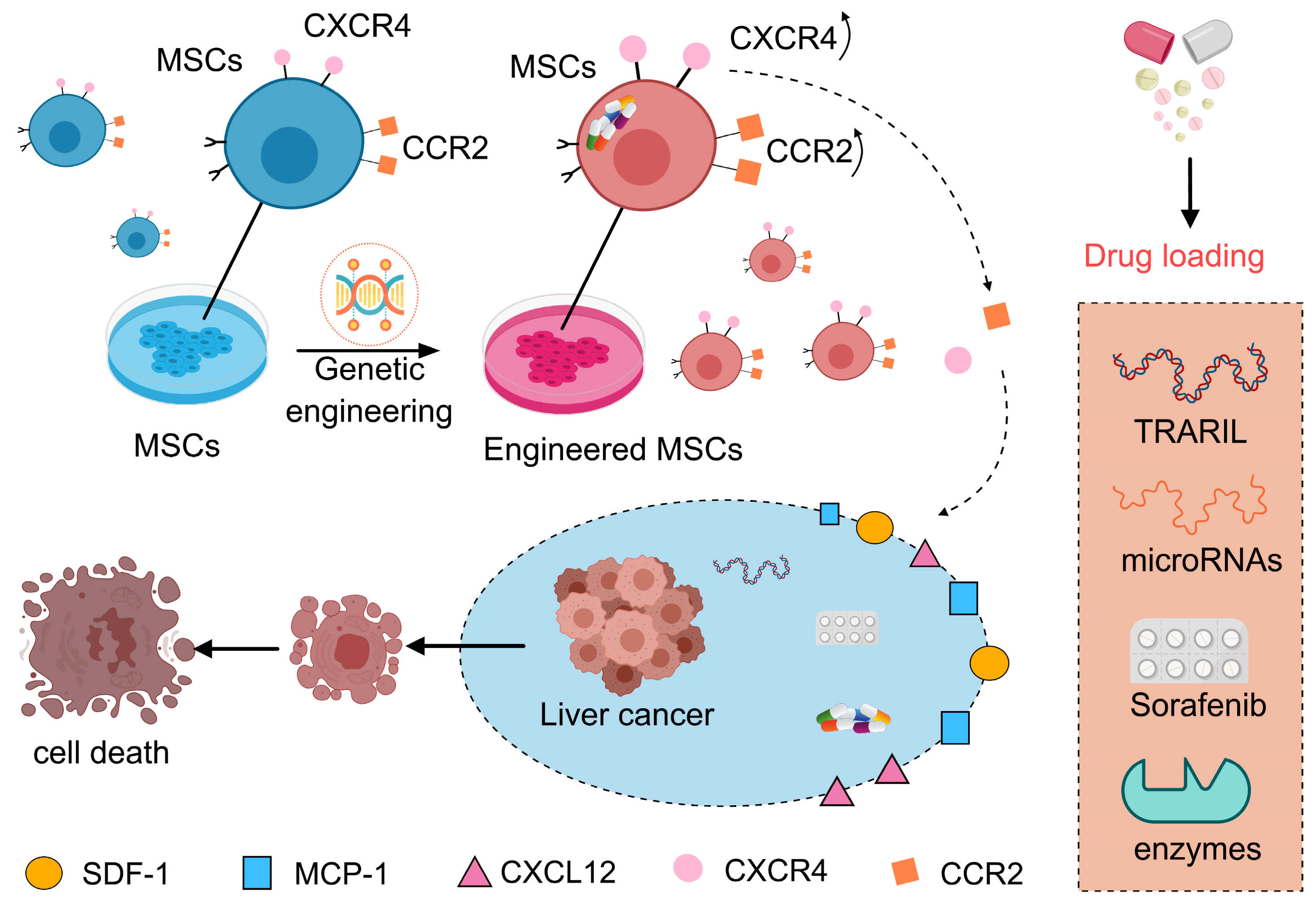
3.3. Loading Versatility of MSCs
3.4. Immunomodulatory Properties of MSCs
3.5. Safety and Reliability of MSCs
4. Advances in MSC-Based Drug Delivery Systems for HCC Therapy
4.1. MSC-Mediated Chemotherapy Delivery for Liver Cancer
4.2. Exosomes and Drug Delivery
4.3. MSC-Delivered Immunomodulators for Cancer Therapy
4.4. Breakthroughs in Genetically Engineered MSC Technologies
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Burden of Disease Cancer Collaboration. A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019, 5, 1749–1768. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Islami, F.; Ward, E.M.; Sung, H.; Cronin, K.A.; Tangka, F.K.L.; Sherman, R.L.; Zhao, J.; Anderson, R.N.; Henley, S.J.; Yabroff, K.R.; et al. Annual Report to the Nation on the Status of Cancer, Part 1: National Cancer Statistics. J. Natl. Cancer Inst. 2021, 113, 1648–1669. [Google Scholar] [CrossRef]
- Zhu, C.-Y.; Yu, P.-H.; Sun, Q.; Hong, D.-F.; Yang, C.; Naranmandura, H. Nuclear receptors in metabolism and diseases: Mechanistic and therapeutic insights. Pharmacol. Res. 2025, 218, 107862. [Google Scholar] [CrossRef]
- Jemal, A.; Ward, E.M.; Johnson, C.J.; Cronin, K.A.; Ma, J.; Ryerson, A.B.; Mariotto, A.; Lake, A.J.; Wilson, R.; Sherman, R.L.; et al. Annual Report to the Nation on the Status of Cancer, 1975–2014, Featuring Survival. JNCI J. Natl. Cancer Inst. 2017, 109, djx030. [Google Scholar] [CrossRef]
- Dimitroulis, D.; Damaskos, C.; Valsami, S.; Davakis, S.; Garmpis, N.; Spartalis, E.; Athanasiou, A.; Moris, D.; Sakellariou, S.; Kykalos, S.; et al. From diagnosis to treatment of hepatocellular carcinoma: An epidemic problem for both developed and developing world. World J. Gastroenterol. 2017, 23, 5282–5294. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, J.; Zheng, S.; Li, M.; Xu, W.; Shi, J.; Kamei, K.-I.; Tian, C. Hybrid adipocyte-derived exosome nano platform for potent chemo-phototherapy in targeted hepatocellular carcinoma. J. Control. Release 2024, 370, 168–181. [Google Scholar] [CrossRef]
- Qin, Y.-T.; Liu, X.; An, J.-X.; Chen, Z.; Niu, M.-T.; Yan, X.; Li, Q.-R.; Rao, Z.-Y.; Zhang, X.-Z. Oral Saccharomyces cerevisiae-Guided Enzyme Prodrug Therapy Combined with Immunotherapy for the Treatment of Orthotopic Colorectal Cancer. ACS Nano 2024, 18, 23497–23507. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.A. Pegylated Liposomal Doxorubicin: Metamorphosis of an Old Drug into a New Form of Chemotherapy. Cancer Investig. 2001, 19, 424–436. [Google Scholar] [CrossRef]
- Sun, R.; Ma, W.; Ling, M.; Tang, C.; Zhong, M.; Dai, J.; Zhu, M.; Cai, X.; Li, G.; Xu, Q.; et al. pH-activated nanoplatform for visualized photodynamic and ferroptosis synergistic therapy of tumors. J. Control. Release 2022, 350, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zhai, Y.; Li, C.T.; Liu, J.; Xu, X.; Chen, H.; Tse, H.; Lian, Q. Translating mesenchymal stem cell and their exosome research into GMP compliant advanced therapy products: Promises, problems and prospects. Med. Res. Rev. 2024, 44, 919–938. [Google Scholar] [CrossRef]
- Iaquinta, M.R.; Lanzillotti, C.; Mazziotta, C.; Bononi, I.; Frontini, F.; Mazzoni, E.; Oton-Gonzalez, L.; Rotondo, J.C.; Torreggiani, E.; Tognon, M.; et al. The role of microRNAs in the osteogenic and chondrogenic differentiation of mesenchymal stem cells and bone pathologies. Theranostics 2021, 11, 6573–6591. [Google Scholar] [CrossRef]
- Karp, J.M.; Teo, G.S.L. Mesenchymal Stem Cell Homing: The Devil Is in the Details. Cell Stem Cell 2009, 4, 206–216. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, Y.; Xu, Y.; Li, G.; Li, Z.; Liu, T. Mesenchymal stem cell-derived exosomes in cancer therapy resistance: Recent advances and therapeutic potential. Mol. Cancer 2022, 21, 179. [Google Scholar] [CrossRef]
- Gao, P.; Ding, Q.; Wu, Z.; Jiang, H.; Fang, Z. Therapeutic potential of human mesenchymal stem cells producing IL-12 in a mouse xenograft model of renal cell carcinoma. Cancer Lett. 2010, 290, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Tang, Q.; Yin, X.; Yan, D.; Tang, M.; Xin, J.; Pan, Q.; Ma, C.; Yan, S. The Therapeutic Potential of Adipose Tissue-Derived Mesenchymal Stem Cells to Enhance Radiotherapy Effects on Hepatocellular Carcinoma. Front. Cell Dev. Biol. 2019, 7, 267. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Liu, X.; Zhong, X.; Yan, R.; Shi, P. Nongenetic surface engineering of mesenchymal stromal cells with polyvalent antibodies to enhance targeting efficiency. Nat. Commun. 2023, 14, 5806. [Google Scholar] [CrossRef]
- Ho, K.; Chan, A. Liver transplantation for hepatocellular carcinoma: Current status in Hong Kong, China. Hepatobiliary Pancreat. Dis. Int. 2025, 24, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-J.; Chen, J.; Zhou, Z.; Hu, D.; Wang, J.; Pan, Y.; Fu, Y.; Hu, Z.; Xu, L.; Chen, M.-S. Transarterial Chemoembolization with Radiofrequency Ablation versus Surgical Resection for Small Late-Recurrence Hepatocellular Carcinoma. Radiology 2025, 314, e241096. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, J.M. Prediction models of hepatocellular carcinoma recurrence after liver transplantation: A comprehensive review. Clin. Mol. Hepatol. 2022, 28, 739–753. [Google Scholar] [CrossRef]
- Agarwal, P.D.; Lucey, M.R. Management of hepatocellular carcinoma recurrence after liver transplantation. Ann. Hepatol. 2022, 27, 100654. [Google Scholar] [CrossRef]
- Gorji, L.; Brown, Z.J.; Limkemann, A.; Schenk, A.D.; Pawlik, T.M. Liver Transplant as a Treatment of Primary and Secondary Liver Neoplasms. JAMA Surg. 2024, 159, 211–218. [Google Scholar] [CrossRef]
- Hu, Z.; Deng, M.; Fu, Y.; Zhou, Z.; Chen, H.; Chen, M.; Zhang, Y. Neoadjuvant hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin for resectable single large hepatocellular carcinoma. Int. J. Surg. 2025, 111, 3850–3858. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, H.; Lin, T.; Zhang, C.; Shen, J.; Chen, J.; Zhao, Y.; Xu, W.; Wang, G.; Huang, P. Ultrasound-activated prodrug-loaded liposome for efficient cancer targeting therapy without chemotherapy-induced side effects. J. Nanobiotechnol. 2024, 22, 2. [Google Scholar] [CrossRef]
- Sun, J.; Mao, F.; Liu, C.; Zhang, F.; Jiang, D.; Guo, W.; Huo, L.; Zhou, L.; Lau, W.Y.; Shi, J.; et al. Combined FOLFOX4 with all-trans retinoic acid versus FOLFOX4 with placebo in treatment of advanced hepatocellular carcinoma with extrahepatic metastasis: A randomized, double-blind comparative study. Signal Transduct. Target. Ther. 2023, 8, 368. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Zhu, X.; Fu, S.; Cao, G.; Li, W.-Q.; Xu, L.; Chen, H.; Wu, D.; Yang, R.; Wang, K.; et al. Sorafenib Plus Hepatic Arterial Infusion Chemotherapy versus Sorafenib for Hepatocellular Carcinoma with Major Portal Vein Tumor Thrombosis: A Randomized Trial. Radiology 2022, 303, 455–464. [Google Scholar] [CrossRef]
- Ding, M.; Shi, R.; Fu, F.; Li, M.; De, D.; Du, Y.; Li, Z. Paeonol protects against doxorubicin-induced cardiotoxicity by promoting Mfn2-mediated mitochondrial fusion through activating the PKCε-Stat3 pathway. J. Adv. Res. 2023, 47, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, H.; Zhang, L.; Zhu, A.X.; Bernards, R.; Qin, W.; Wang, C. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 203–222. [Google Scholar] [CrossRef]
- Xu, J.; Ji, L.; Ruan, Y.; Wan, Z.; Lin, Z.; Xia, S.; Tao, L.; Zheng, J.; Cai, L.; Wang, Y.; et al. UBQLN1 mediates sorafenib resistance through regulating mitochondrial biogenesis and ROS homeostasis by targeting PGC1β in hepatocellular carcinoma. Signal Transduct. Target. Ther. 2021, 6, 190. [Google Scholar] [CrossRef]
- Chon, Y.E.; Kim, D.Y.; Na Kim, M.; Kim, B.K.; Kim, S.U.; Park, J.Y.; Ahn, S.H.; Ha, Y.; Lee, J.H.; Lee, K.S.; et al. Sorafenib vs. Lenvatinib in advanced hepatocellular carcinoma after atezolizumab/bevacizumab failure: A real-world study. Clin. Mol. Hepatol. 2024, 30, 345–359. [Google Scholar] [CrossRef]
- Chen, Y.; Dai, S.; Cheng, C.-S.; Chen, L. Lenvatinib and immune-checkpoint inhibitors in hepatocellular carcinoma: Mechanistic insights, clinical efficacy, and future perspectives. J. Hematol. Oncol. 2024, 17, 130. [Google Scholar] [CrossRef]
- Tang, W.; Chen, Z.; Zhang, W.; Cheng, Y.; Zhang, B.; Wu, F.; Wang, Q.; Wang, S.; Rong, D.; Reiter, F.P.; et al. The mechanisms of sorafenib resistance in hepatocellular carcinoma: Theoretical basis and therapeutic aspects. Signal Transduct. Target. Ther. 2020, 5, 87. [Google Scholar] [CrossRef]
- Yang, T.; Zhu, C.Y.; Yu, P.H.; Yang, C.; Naranmandura, H. Arsenic trioxide could promote SARS-CoV-2 NSP12 protein degradation. J. Gen. Virol. 2025, 106, 002121. [Google Scholar] [CrossRef]
- Xu, W.; Zheng, J.; Zhang, J.; Shi, H.; Peng, W.; Liu, Y.; Feng, G.; Wang, Y.; Liang, Y.-J.; Chen, J. Dual-Mode Treatment of Hepatocellular Carcinoma Using RGD Cyclopeptide-Modified Liposomes Loaded with Ce6/DOX. Int. J. Nanomed. 2025, 20, 3845–3860. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Chan, S.L.; Gu, S.; Bai, Y.; Ren, Z.; Lin, X.; Chen, Z.; Jia, W.; Jin, Y.; Guo, Y.; et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): A randomised, open-label, international phase 3 study. Lancet 2023, 402, 1133–1146. [Google Scholar] [CrossRef]
- Su, D.; Wu, B.; Shi, L. Cost-effectiveness of Atezolizumab Plus Bevacizumab vs Sorafenib as First-Line Treatment of Unresectable Hepatocellular Carcinoma. JAMA Netw. Open 2021, 4, e210037. [Google Scholar] [CrossRef]
- Liu, X.; Lu, Y.; Zhou, W.; Peng, T.; Zhou, J.; Bi, H.; Xia, F.; Chen, X. Chinese Multidisciplinary Expert Consensus on Immune Checkpoint Inhibitor-Based Combination Therapy for Hepatocellular Carcinoma (2023 Edition). Liver Cancer 2024, 13, 355–375. [Google Scholar] [CrossRef]
- Shen, K.-Y.; Zhu, Y.; Xie, S.-Z.; Qin, L.-X. Immunosuppressive tumor microenvironment and immunotherapy of hepatocellular carcinoma: Current status and prospectives. J. Hematol. Oncol. 2024, 17, 25. [Google Scholar] [CrossRef]
- Zhang, B.; Tao, B.; Li, Y.; Yi, C.; Lin, Z.; Ma, Y.; Han, J.; Shao, W.; Chen, Z.; Lin, J.; et al. Dual immune checkpoint inhibitors or combined with anti-VEGF agents in advanced, unresectable hepatocellular carcinoma. Eur. J. Intern. Med. 2023, 111, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Ryoo, B.-Y.; Hsu, C.-H.; Numata, K.; Stein, S.; Verret, W.; Hack, S.P.; Spahn, J.; Liu, B.; Abdullah, H.; et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): An open-label, multicentre, phase 1b study. Lancet Oncol. 2020, 21, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Ryoo, B.-Y.; Hsu, C.-H.; Li, D.; Burgoyne, A.M.; Cotter, C.; Badhrinarayanan, S.; Wang, Y.; Yin, A.; Edubilli, T.R.; et al. Tiragolumab in combination with atezolizumab and bevacizumab in patients with unresectable, locally advanced or metastatic hepatocellular carcinoma (MORPHEUS-Liver): A randomised, open-label, phase 1b–2, study. Lancet Oncol. 2025, 26, 214–226. [Google Scholar] [CrossRef]
- Liu, A.; Wu, Q.; Peng, D.; Ares, I.; Anadón, A.; Lopez-Torres, B.; Martínez-Larrañaga, M.; Wang, X.; Martínez, M. A novel strategy for the diagnosis, prognosis, treatment, and chemoresistance of hepatocellular carcinoma: DNA methylation. Med. Res. Rev. 2020, 40, 1973–2018. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, J.; Wang, Z.; Liu, K.; Feng, K.; Wang, F.; Mei, Y. Energy Stress–Induced circEPB41(2) Promotes Lipogenesis in Hepatocellular Carcinoma. Cancer Res. 2025, 85, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef]
- Krawczenko, A.; Klimczak, A. Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells and Their Contribution to Angiogenic Processes in Tissue Regeneration. Int. J. Mol. Sci. 2022, 23, 2425. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.A.; Mohamed, A.H.; Rizaev, J.; Mallick, A.K.; Qasim, M.T.; Al Abdulmonem, W.; Jamal, A.; Hattiwale, H.M.; Kamal, M.A.; Ahmad, F. Application of mesenchymal stem cells derived from the umbilical cord or Wharton’s jelly and their extracellular vesicles in the treatment of various diseases. Tissue Cell 2024, 89, 102415. [Google Scholar] [CrossRef]
- Eirin, A.; Thaler, R.; Glasstetter, L.M.; Xing, L.; Zhu, X.-Y.; Osborne, A.C.; Mondesir, R.; Bhagwate, A.V.; Lerman, A.; van Wijnen, A.J.; et al. Obesity-driven mitochondrial dysfunction in human adipose tissue-derived mesenchymal stem/stromal cells involves epigenetic changes. Cell Death Dis. 2024, 15, 387. [Google Scholar] [CrossRef]
- Zhang, F.; Hu, G.; Chen, X.; Zhang, L.; Guo, L.; Li, C.; Zhao, H.; Cui, Z.; Guo, X.; Sun, F.; et al. Excessive branched-chain amino acid accumulation restricts mesenchymal stem cell-based therapy efficacy in myocardial infarction. Signal Transduct. Target. Ther. 2022, 7, 171. [Google Scholar] [CrossRef]
- Yan, W.; Diao, S.; Fan, Z. The role and mechanism of mitochondrial functions and energy metabolism in the function regulation of the mesenchymal stem cells. Stem Cell Res. Ther. 2021, 12, 140. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-G.; He, Z.-Y.; Liang, S.; Yang, Q.; Cheng, P.; Chen, A.-M. Comprehensive proteomic analysis of exosomes derived from human bone marrow, adipose tissue, and umbilical cord mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 511. [Google Scholar] [CrossRef]
- Yea, J.-H.; Bae, T.S.; Kim, B.J.; Cho, Y.W.; Jo, C.H. Regeneration of the rotator cuff tendon-to-bone interface using umbilical cord-derived mesenchymal stem cells and gradient extracellular matrix scaffolds from adipose tissue in a rat model. Acta Biomater. 2020, 114, 104–116. [Google Scholar] [CrossRef]
- Calcat-I-Cervera, S.; Rendra, E.; Scaccia, E.; Amadeo, F.; Hanson, V.; Wilm, B.; Murray, P.; O’bRien, T.; Taylor, A.; Bieback, K. Harmonised culture procedures minimise but do not eliminate mesenchymal stromal cell donor and tissue variability in a decentralised multicentre manufacturing approach. Stem Cell Res. Ther. 2023, 14, 120. [Google Scholar] [CrossRef]
- Wu, H.-H.; Zhou, Y.; Tabata, Y.; Gao, J.-Q. Mesenchymal stem cell-based drug delivery strategy: From cells to biomimetic. J. Control. Release 2019, 294, 102–113. [Google Scholar] [CrossRef]
- Yu, Y.; Tao, Y.; Ma, J.; Li, J.; Song, Z. Targeting the tumor microenvironment with mesenchymal stem cells based delivery approach for efficient delivery of anticancer agents: An updated review. Biochem. Pharmacol. 2025, 232, 116725. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, J.; Li, Y.; Feng, C.; Shao, C.; Shi, Y.; Fang, J. Engineered mesenchymal stem/stromal cells against cancer. Cell Death Dis. 2025, 16, 113. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Huang, Y.; Zhang, F.; Shi, W.; Cheng, Y.; Yang, K.; Tian, P.; Zhou, F.; Wang, Y.; Fang, X.; et al. Ultrasonic microbubbles promote mesenchymal stem cell homing to the fibrotic liver via upregulation of CXCR4 expression. Cell Div. 2024, 19, 7. [Google Scholar] [CrossRef]
- Yu, S.; Yu, S.; Liu, H.; Liao, N.; Liu, X. Enhancing mesenchymal stem cell survival and homing capability to improve cell engraftment efficacy for liver diseases. Stem Cell Res. Ther. 2023, 14, 235. [Google Scholar] [CrossRef]
- Harrell, C.R.; Volarevic, A.; Djonov, V.; Volarevic, V. Mesenchymal Stem-Cell-Derived Exosomes as Novel Drug Carriers in Anti-Cancer Treatment: A Myth or Reality? Cells 2025, 14, 202. [Google Scholar] [CrossRef]
- Hass, R.; von der Ohe, J.; Luo, T. Human mesenchymal stroma/stem-like cell-derived taxol-loaded EVs/exosomes transfer anti-tumor microRNA signatures and express enhanced SDF-1-mediated tumor tropism. Cell Commun. Signal. 2024, 22, 506. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Z.; Yue, C.; Ma, J.; Cao, L.; Lin, J.; Zhu, D.; An, R.; Lai, J.; Guo, Y.; et al. Human umbilical cord mesenchymal stem cell-derived exosomes carrying miR-1827 downregulate SUCNR1 to inhibit macrophage M2 polarization and prevent colorectal liver metastasis. Apoptosis 2023, 28, 549–565. [Google Scholar] [CrossRef]
- Ghaleh, H.E.G.; Vakilzadeh, G.; Zahiri, A.; Farzanehpour, M. Investigating the potential of oncolytic viruses for cancer treatment via MSC delivery. Cell Commun. Signal. 2023, 21, 228. [Google Scholar] [CrossRef]
- Yoon, A.-R.; Hong, J.; Li, Y.; Shin, H.C.; Lee, H.; Kim, H.S.; Yun, C.-O. Mesenchymal Stem Cell–Mediated Delivery of an Oncolytic Adenovirus Enhances Antitumor Efficacy in Hepatocellular Carcinoma. Cancer Res. 2019, 79, 4503–4514. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.-T.; Federspiel, M.J.; Guo, C.M.; Ooi, L.L.; Russell, S.J.; Peng, K.-W.; Hui, K.M. Systemically delivered measles virus-infected mesenchymal stem cells can evade host immunity to inhibit liver cancer growth. J. Hepatol. 2013, 59, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.; Ahmad, N.; Lei, D.; Ali, S. Emerging role of oncolytic viruses and stem cells in gene therapy: Should they be integrated? Drug Discov. Today 2022, 27, 2244–2251. [Google Scholar] [CrossRef]
- Ha, D.H.; Kim, H.-K.; Lee, J.; Kwon, H.H.; Park, G.-H.; Yang, S.H.; Jung, J.Y.; Choi, H.; Lee, J.H.; Sung, S.; et al. Mesenchymal Stem/Stromal Cell-Derived Exosomes for Immunomodulatory Therapeutics and Skin Regeneration. Cells 2020, 9, 1157. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, M.; Yao, L.; Xiong, Z.; Dai, K.; Liu, P.; Chen, P.; Sun, M.; Shu, K.; Xia, Y.; et al. Exosomal miR-499a-5p from human umbilical cord mesenchymal stem cells attenuates liver fibrosis via targeting ETS1/GPX4-mediated ferroptosis in hepatic stellate cells. J. Nanobiotechnol. 2025, 23, 222. [Google Scholar] [CrossRef]
- Wang, M.-L.; Pan, C.-M.; Chiou, S.-H.; Chen, W.-H.; Chang, H.-Y.; Lee, O.K.-S.; Hsu, H.-S.; Wu, C.-W. Oncostatin m modulates the mesenchymal-epithelial transition of lung a denocarcinoma cells by a mesenchymal stem cell-mediated paracrine effect. Cancer Res. 2012, 72, 6051–6064. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, Y.; Li, Q.; Liu, K.; Hou, J.; Shao, C.; Wang, Y. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat. Rev. Nephrol. 2018, 14, 493–507. [Google Scholar] [CrossRef]
- Zhang, B.; Han, F.; Wang, Y.; Sun, Y.; Zhang, M.; Yu, X.; Qin, C.; Zhang, H.; Wu, C. Cells-Micropatterning Biomaterials for Immune Activation and Bone Regeneration. Adv. Sci. 2022, 9, e2200670. [Google Scholar] [CrossRef]
- Cao, W.; Cao, K.; Cao, J.; Wang, Y.; Shi, Y. Mesenchymal stem cells and adaptive immune responses. Immunol. Lett. 2015, 168, 147–153. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, R.; Wu, H.; Jiang, X.; Gao, J. Mesenchymal stem cells: A living carrier for active tumor-targeted delivery. Adv. Drug Deliv. Rev. 2022, 185, 114300. [Google Scholar] [CrossRef]
- François, M.; Romieu-Mourez, R.; Stock-Martineau, S.; Boivin, M.-N.; Bramson, J.L.; Galipeau, J. Mesenchymal stromal cells cross-present soluble exogenous antigens as part of their antigen-presenting cell properties. Blood 2009, 114, 2632–2638. [Google Scholar] [CrossRef] [PubMed]
- Brennen, W.N.; Denmeade, S.R.; Isaacs, J.T. Mesenchymal stem cells as a vector for the inflammatory prostate microenvironment. Endocr.-Relat. Cancer 2013, 20, R269–R290. [Google Scholar] [CrossRef]
- Niess, H.; Bao, Q.; Conrad, C.; Zischek, C.; Notohamiprodjo, M.; Schwab, F.; Schwarz, B.; Huss, R.; Jauch, K.-W.; Nelson, P.J.; et al. Selective targeting of genetically engineered mesenchymal stem cells t o tumor stroma microenvironments using tissue-specific suicide gene ex pression suppresses growth of hepatocellular carcinoma. Ann. Surg. 2011, 254, 767–774; discussion 774–775. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Y.; Chi, Y.; Sun, T.; Gao, Y.; Dou, X.; Han, Z.; Xue, F.; Li, H.; Liu, W.; et al. Efficacy and safety of human umbilical cord-derived mesenchymal stem cells in the treatment of refractory immune thrombocytopenia: A prospective, single arm, phase I trial. Signal Transduct. Target. Ther. 2024, 9, 102. [Google Scholar] [CrossRef]
- Lynch, K.; Treacy, O.; Chen, X.; Murphy, N.; Lohan, P.; Islam, N.; Donohoe, E.; Griffin, M.D.; Watson, L.; McLoughlin, S.; et al. TGF-β1-Licensed Murine MSCs Show Superior Therapeutic Efficacy in Modulating Corneal Allograft Immune Rejection In Vivo. Mol. Ther. 2020, 28, 2023–2043. [Google Scholar] [CrossRef]
- Song, N.; Scholtemeijer, M.; Shah, K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol. Sci. 2020, 41, 653–664. [Google Scholar] [CrossRef]
- Oh, J.Y.; Lee, R.H. Mesenchymal stromal cells for the treatment of ocular autoimmune diseases. Prog. Retin. Eye Res. 2021, 85, 100967. [Google Scholar] [CrossRef]
- Li, H.; Dai, H.; Li, J. Immunomodulatory properties of mesenchymal stromal/stem cells: The link with metabolism. J. Adv. Res. 2023, 45, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Choi, Y.R.; Ko, J.H.; Ryu, J.S.; Oh, J.Y. Defining mesenchymal stem/stromal cell-induced myeloid-derived suppressor cells using single-cell transcriptomics. Mol. Ther. 2024, 32, 1970–1983. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, T.; Li, X.; Li, W.; Lei, Y.; Shang, Q.; Zheng, Z.; Fang, J.; Cao, L.; Yu, D.; et al. SOD2 promotes the immunosuppressive function of mesenchymal stem cells at the expense of adipocyte differentiation. Mol. Ther. 2024, 32, 1144–1157. [Google Scholar] [CrossRef]
- Malekpour, K.; Hazrati, A.; Soudi, S.; Hashemi, S.M. Mechanisms behind therapeutic potentials of mesenchymal stem cell mitochondria transfer/delivery. J. Control. Release 2023, 354, 755–769. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Feng, B.; Zhou, J.; Yang, J.; Pan, Q.; Yu, J.; Shang, D.; Li, L.; Cao, H. Mesenchymal stem cells alleviate mouse liver fibrosis by inhibiting pathogenic function of intrahepatic B cells. Hepatology 2025, 81, 1211–1227. [Google Scholar] [CrossRef]
- Pham, D.-V.; Shrestha, P.; Nguyen, T.-K.; Park, J.; Pandit, M.; Chang, J.-H.; Kim, S.Y.; Choi, D.-Y.; Han, S.S.; Choi, I.; et al. Modulation of NLRP3 inflammasomes activation contributes to improved survival and function of mesenchymal stromal cell spheroids. Mol. Ther. 2023, 31, 890–908. [Google Scholar] [CrossRef]
- Xia, L.; Zhang, C.; Lv, N.; Liang, Z.; Ma, T.; Cheng, H.; Xia, Y.; Shi, L. AdMSC-derived exosomes alleviate acute lung injury via transferring mitochondrial component to improve homeostasis of alveolar macrophages. Theranostics 2022, 12, 2928–2947. [Google Scholar] [CrossRef]
- Li, Y.; Jin, M.; Guo, D.; Shen, S.; Lu, K.; Pan, R.; Sun, L.; Zhang, H.; Shao, J.; Pan, G. Unveiling the immunogenicity of allogeneic mesenchymal stromal cells: Challenges and strategies for enhanced therapeutic efficacy. Biomed. Pharmacother. 2024, 180, 117537. [Google Scholar] [CrossRef]
- Dai, P.; Wu, Y.; Du, Q.; Du, J.; Wang, K.; Chen, R.; Feng, X.; Chen, C.; Zhang, X. Knockout of B2M in combination with PD-L1 overexpression protects MSC-derived new islet β cells from graft rejection in the treatment of canine diabetes mellitus. Stem Cell Res. Ther. 2024, 15, 458. [Google Scholar] [CrossRef]
- Chang, S.-H.; Kim, H.J.; Park, C.-G. Allogeneic ADSCs Induce the Production of Alloreactive Memory-CD8 T Cells through HLA-ABC Antigens. Cells 2020, 9, 1246. [Google Scholar] [CrossRef] [PubMed]
- Kalimuthu, S.; Oh, J.M.; Gangadaran, P.; Zhu, L.; Lee, H.W.; Jeon, Y.H.; Jeong, S.Y.; Lee, S.-W.; Lee, J.; Ahn, B.-C. Genetically engineered suicide gene in mesenchymal stem cells using a Tet-On system for anaplastic thyroid cancer. PLoS ONE 2017, 12, e0181318. [Google Scholar] [CrossRef] [PubMed]
- Lalu, M.M.; McIntyre, L.; Pugliese, C.; Fergusson, D.; Winston, B.W.; Marshall, J.C.; Granton, J.; Stewart, D.J.; Canadian Critical Care Trials Group. Safety of cell therapy with mesenchymal stromal cells (Safe Cell): A systematic review and meta-analysis of clinical trials. PLoS ONE 2012, 7, e47559. [Google Scholar] [CrossRef]
- Yang, C.; Guan, Z.; Pang, X.; Tan, Z.; Yang, X.; Li, X.; Guan, F. Desialylated Mesenchymal Stem Cells-Derived Extracellular Vesicles Loaded with Doxorubicin for Targeted Inhibition of Hepatocellular Carcinoma. Cells 2022, 11, 2642. [Google Scholar] [CrossRef]
- Hazrati, A.; Malekpour, K.; Soudi, S.; Hashemi, S.M. Mesenchymal Stromal/Stem Cells and Their Extracellular Vesicles Application in Acute and Chronic Inflammatory Liver Diseases: Emphasizing on the Anti-Fibrotic and Immunomodulatory Mechanisms. Front. Immunol. 2022, 13, 865888. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Yin, Z.; Wang, Z.; Xiong, Z.; Chen, P.; Yao, L.; Liu, P.; Sun, M.; Shu, K.; Li, L.; et al. Modification of MSCs with aHSCs-targeting peptide pPB for enhanced therapeutic efficacy in liver fibrosis. Biomaterials 2025, 321, 123295. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, C.; Zhao, X.; Lin, F.; Liu, S.; Bai, J. A pH-sensitive peptide amphiphilic-based drug delivery system inhibits hepatocellular carcinoma growth by suppressing hepatic stellate cell activation. Mater. Today Bio 2025, 32, 101821. [Google Scholar] [CrossRef]
- Park, H.W.; Lee, D.H.; Kim, S.; Park, H.; Jangid, A.K.; Lee, C.E.; Park, J.; Park, G.T.; Park, H.Y.; Kim, H.; et al. Amphiphilic lipid-peptide engineered placenta-derived mesenchymal stem cells for liver fibrosis treatment. Asian J. Pharm. Sci. 2025, 20, 101061. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, Y.K.; Kim, S.; Choi, Y.-S.; Lee, I.; Joo, H.; Kim, J.; Kwon, M.; Park, S.; Jo, M.K.; et al. Dual-mode action of scalable, high-quality engineered stem cell-derived SIRPα-extracellular vesicles for treating acute liver failure. Nat. Commun. 2025, 16, 1903. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-Y.; Liu, W.-T.; Hou, X.-J.; Zong, C.; Yu, W.; Shen, Z.-M.; Qu, S.-P.; Tao, M.; Xue, M.-M.; Zhou, D.-Y.; et al. CD34+CLDN5+ tumor associated senescent endothelial cells through IGF2-IGF2R signaling increased cholangiocellular phenotype in hepatocellular carcinoma. J. Adv. Res. 2024, 76, 511–528. [Google Scholar] [CrossRef]
- Lou, G.; Song, X.; Yang, F.; Wu, S.; Wang, J.; Chen, Z.; Liu, Y. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J. Hematol. Oncol. 2015, 8, 122. [Google Scholar] [CrossRef]
- Lou, G.; Chen, L.; Xia, C.; Wang, W.; Qi, J.; Li, A.; Zhao, L.; Chen, Z.; Zheng, M.; Liu, Y. MiR-199a-modified exosomes from adipose tissue-derived mesenchymal stem cells improve hepatocellular carcinoma chemosensitivity through mTOR pathway. J. Exp. Clin. Cancer Res. 2020, 39, 4. [Google Scholar] [CrossRef]
- Liang, L.; Zhao, L.; Wang, Y.; Wang, Y. Treatment for Hepatocellular Carcinoma Is Enhanced When Norcantharidin Is Encapsulated in Exosomes Derived from Bone Marrow Mesenchymal Stem Cells. Mol. Pharm. 2021, 18, 1003–1013. [Google Scholar] [CrossRef]
- Li, H.; Yang, C.; Shi, Y.; Zhao, L. Exosomes derived from siRNA against GRP78 modified bone-marrow-derived mesenchymal stem cells suppress Sorafenib resistance in hepatocellular carcinoma. J. Nanobiotechnol. 2018, 16, 103. [Google Scholar] [CrossRef]
- Szoor, A.; Vaidya, A.; Velasquez, M.P.; Mei, Z.; Galvan, D.L.; Torres, D.; Gee, A.; Heczey, A.; Gottschalk, S. T Cell-Activating Mesenchymal Stem Cells as a Biotherapeutic for HCC. Mol. Ther.-Oncolytics 2017, 6, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Yan, C.; Wan, H.; Wu, L.; Liu, J.; Zhu, Z.; Gao, D. Mesenchymal stem cell-derived exosomes block malignant behaviors of hepatocellular carcinoma stem cells through a lncRNA C5orf66-AS1/microRNA-127-3p/DUSP1/ERK axis. Hum. Cell 2021, 34, 1812–1829. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Zhang, Q.; Li, Z.; Zhang, X.; Bao, S.; Fan, D.; Ru, Y.; Dong, S.; Zhang, Y.; Zhang, Y.; et al. Mesenchymal stem cells deliver and release conditionally replicative adenovirus depending on hepatic differentiation to eliminate hepatocellular carcinoma cells specifically. Cancer Lett. 2016, 381, 85–95. [Google Scholar] [CrossRef]
- Yan, C.; Yang, M.; Li, Z.; Li, S.; Hu, X.; Fan, D.; Zhang, Y.; Wang, J.; Xiong, D. Suppression of orthotopically implanted hepatocarcinoma in mice by umbilical cord-derived mesenchymal stem cells with sTRAIL gene expression driven by AFP promoter. Biomaterials 2014, 35, 3035–3043. [Google Scholar] [CrossRef]
- Seyhoun, I.; Hajighasemlou, S.; Muhammadnejad, S.; Ai, J.; Nikbakht, M.; Alizadeh, A.A.; Hosseinzadeh, F.; Mirmoghtadaei, M.; Seyhoun, S.M.; Verdi, J. Combination therapy of sorafenib with mesenchymal stem cells as a novel cancer treatment regimen in xenograft models of hepatocellular carcinoma. J. Cell. Physiol. 2019, 234, 9495–9503. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-C.; Wang, R.; Zhao, X.; Wei, Y.-Q.; Hu, M.; Wang, Y.-S.; Zhang, X.-W.; Zhang, R.; Zhang, L.; Bin Yao, B.; et al. Prophylaxis against carcinogenesis in three kinds of unestablished tumor models via IL12-gene-engineered MSCs. Carcinogenesis 2006, 27, 2434–2441. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, Y.-L.; Wang, H.-T.; Li, D.-W.; Dai, H.-J.; Zhang, Q.-Q.; Zhang, J.; Ma, Y.; Xia, Q.; Bian, J.-M.; et al. Overexpression of hepatocyte nuclear factor 4α in human mesenchymal stem cells suppresses hepatocellular carcinoma development through Wnt/β-catenin signaling pathway downregulation. Cancer Biol. Ther. 2016, 17, 558–565. [Google Scholar] [CrossRef]
- Zhang, B.; Shan, H.; Li, D.; Li, Z.-R.; Zhu, K.-S.; Jiang, Z.-B. The inhibitory effect of MSCs expressing TRAIL as a cellular delivery vehicle in combination with cisplatin on hepatocellular carcinoma. Cancer Biol. Ther. 2012, 13, 1175–1184. [Google Scholar] [CrossRef]
- Zhang, J.; Hou, L.; Wu, X.; Zhao, D.; Wang, Z.; Hu, H.; Fu, Y.; He, J. Inhibitory effect of genetically engineered mesenchymal stem cells with Apoptin on hepatoma cells in vitro and in vivo. Mol. Cell. Biochem. 2016, 416, 193–203. [Google Scholar] [CrossRef]
- Lu, X.; Guo, H.; Wei, X.; Lu, D.; Shu, W.; Song, Y.; Qiu, N.; Xu, X. Current Status and Prospect of Delivery Vehicle Based on Mesenchymal Stem Cell-Derived Exosomes in Liver Diseases. Int. J. Nanomed. 2023, 18, 2873–2890. [Google Scholar] [CrossRef]
- Hu, C.; Wang, L. Advances in the treatment of liver injury based on mesenchymal stem cell-derived exosomes. Stem Cell Res. Ther. 2024, 15, 474. [Google Scholar] [CrossRef]
- Jahangiri, B.; Khalaj-Kondori, M.; Asadollahi, E.; Dizaj, L.P.; Sadeghizadeh, M. MSC-Derived exosomes suppress colorectal cancer cell proliferation and metastasis via miR-100/mTOR/miR-143 pathway. Int. J. Pharm. 2022, 627, 122214. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, Y.; Li, Y.; Shi, T.; Luan, Y.; Yin, C. Role of stem cell derivatives in inflammatory diseases. Front. Immunol. 2023, 14, 1153901. [Google Scholar] [CrossRef] [PubMed]
- Nikfarjam, S.; Rezaie, J.; Zolbanin, N.M.; Jafari, R. Mesenchymal stem cell derived-exosomes: A modern approach in translational medicine. J. Transl. Med. 2020, 18, 449. [Google Scholar] [CrossRef] [PubMed]
- Pascucci, L.; Coccè, V.; Bonomi, A.; Ami, D.; Ceccarelli, P.; Ciusani, E.; Viganò, L.; Locatelli, A.; Sisto, F.; Doglia, S.M.; et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: A new approach for drug delivery. J. Control. Release 2014, 192, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Kanchanapally, R.; Khan, M.A.; Deshmukh, S.K.; Srivastava, S.K.; Khushman, M.; Singh, S.; Singh, A.P. Exosomal Formulation Escalates Cellular Uptake of Honokiol Leading to the Enhancement of Its Antitumor Efficacy. ACS Omega 2020, 5, 23299–23307. [Google Scholar] [CrossRef]
- Ko, S.-F.; Yip, H.-K.; Zhen, Y.-Y.; Lee, C.-C.; Lee, C.-C.; Huang, C.-C.; Ng, S.-H.; Lin, J.-W. Adipose-Derived Mesenchymal Stem Cell Exosomes Suppress Hepatocellular Carcinoma Growth in a Rat Model: Apparent Diffusion Coefficient, Natural Killer T-Cell Responses, and Histopathological Features. Stem Cells Int. 2015, 2015, 853506. [Google Scholar] [CrossRef]
- Yu, P.-H.; Zhu, C.-Y.; Kang, Y.-Y.; Naranmandura, H.; Yang, C. Mutation in the Unrearranged PML Allele Confers Resistance to Arsenic Trioxide in Acute Promyelocytic Leukemia. Research 2025, 8, 0696. [Google Scholar] [CrossRef]
- Yang, C.; Kang, Y.-Y.; Zhu, C.-Y.; Ma, Y.; Yu, P.-H.; Yang, T.; Liu, Y.-Q.; Zhang, Z.-Y.; Suzuki, N.; Ogra, Y.; et al. Heat stress targets and degrades BCR::ABL1 oncoproteins to overcome drug-resistance in Philadelphia chromosome-positive acute lymphoblastic leukemia. Leukemia 2025, 39, 2140–2151. [Google Scholar] [CrossRef]
- Shi, C.-J.; Pang, F.-X.; Lei, Y.-H.; Deng, L.-Q.; Pan, F.-Z.; Liang, Z.-Q.; Xie, T.; Wu, X.-L.; Wang, Y.-Y.; Xian, Y.-F.; et al. 5-methylcytosine methylation of MALAT1 promotes resistance to sorafenib in hepatocellular carcinoma through ELAVL1/SLC7A11-mediated ferroptosis. Drug Resist. Updat. 2025, 78, 101181. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Liu, J.; Zhang, Z.; Ye, C.; Zhou, X.; Yi, Y.; Wu, Y.; Li, Y.; Zhang, Q.; Xiong, X.; et al. Strontium–Alix interaction enhances exosomal miRNA selectively loading in synovial MSCs for temporomandibular joint osteoarthritis treatment. Int. J. Oral Sci. 2025, 17, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-Y.; Wen, L.; Du, L.; Liu, T.T.; Sun, Y.; Chen, Y.-Z.; Lu, Y.-X.; Cheng, X.-C.; Sun, H.-Y.; Xiao, F.-J.; et al. S-RBD-modified and miR-486-5p-engineered exosomes derived from mesenchymal stem cells suppress ferroptosis and alleviate radiation-induced lung injury and long-term pulmonary fibrosis. J. Nanobiotechnol. 2024, 22, 662. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, T.; Wang, Q.; Yan, K.; Ma, S.; Lin, Y.; Zeng, G.; Liu, J.; Cao, J.; Wang, D. Dual-Synergistic Nanomodulator Alleviates Exosomal PD-L1 Expression Enabling Exhausted Cytotoxic T Lymphocytes Rejuvenation for Potentiated iRFA-Treated Hepatocellular Carcinoma Immunotherapy. ACS Nano 2024, 18, 32818–32833. [Google Scholar] [CrossRef]
- Yi, M.; Li, T.; Niu, M.; Wu, Y.; Zhao, B.; Shen, Z.; Hu, S.; Zhang, C.; Zhang, X.; Zhang, J.; et al. Blockade of CCR5+ T Cell Accumulation in the Tumor Microenvironment Optimizes Anti-TGF-β/PD-L1 Bispecific Antibody. Adv. Sci. 2024, 11, e2408598. [Google Scholar] [CrossRef]
- Lu, J.-C.; Zhang, P.-F.; Huang, X.-Y.; Guo, X.-J.; Gao, C.; Zeng, H.-Y.; Zheng, Y.-M.; Wang, S.-W.; Cai, J.-B.; Sun, Q.-M.; et al. Amplification of spatially isolated adenosine pathway by tumor–macrophage interaction induces anti-PD1 resistance in hepatocellular carcinoma. J. Hematol. Oncol. 2021, 14, 200. [Google Scholar] [CrossRef]
- Allard, B.; Jacoberger-Foissac, C.; Cousineau, I.; Bareche, Y.; Buisseret, L.; Chrobak, P.; Allard, D.; Pommey, S.; Ah-Pine, F.; Duquenne, S.; et al. Adenosine A2A receptor is a tumor suppressor of NASH-associated hepatocellular carcinoma. Cell Rep. Med. 2023, 4, 101188. [Google Scholar] [CrossRef]
- Schug, C.; Kitzberger, C.; Sievert, W.; Spellerberg, R.; Tutter, M.; Schmohl, K.A.; Eberlein, B.; Biedermann, T.; Steiger, K.; Zach, C.; et al. Radiation-Induced Amplification of TGFB1-Induced Mesenchymal Stem Cell–Mediated Sodium Iodide Symporter (NIS) Gene 131I Therapy. Clin. Cancer Res. 2019, 25, 5997–6008. [Google Scholar] [CrossRef]
- Seon, J.K.; Kuppa, S.S.; Kang, J.Y.; Lee, J.S.; Park, S.A.; Yoon, T.R.; Park, K.S.; Kim, H.K. Peptide derived from stromal cell-derived factor 1δ enhances the in vitro expression of osteogenic proteins via bone marrow stromal cell differentiation and promotes bone formation in in vivo models. Biomater. Sci. 2023, 11, 6587–6599. [Google Scholar] [CrossRef]
- Xu, S.; Liu, B.; Fan, J.; Xue, C.; Lu, Y.; Li, C.; Cui, D. Engineered mesenchymal stem cell-derived exosomes with high CXCR4 levels for targeted siRNA gene therapy against cancer. Nanoscale 2022, 14, 4098–4113. [Google Scholar] [CrossRef]
- Liu, A.; Liu, Y.; Ye, R.D. Structural basis of CXCR4 assembly and regulation. Cell Rep. 2025, 44, 115255. [Google Scholar] [CrossRef]
- Prabha, S.; Merali, C.; Sehgal, D.; Nicolas, E.; Bhaskar, N.; Flores, M.; Bhatnagar, S.; Nethi, S.K.; Barrero, C.A.; Merali, S.; et al. Incorporation of paclitaxel in mesenchymal stem cells using nanoengineering upregulates antioxidant response, CXCR4 expression and enhances tumor homing. Mater. Today Bio 2023, 19, 100567. [Google Scholar] [CrossRef]
- Yang, N.; Chen, T.; Wang, L.; Liu, R.; Niu, Y.; Sun, L.; Yao, B.; Wang, Y.; Yang, W.; Liu, Q.; et al. CXCR4 mediates matrix stiffness-induced downregulation of UBTD1 driving hepatocellular carcinoma progression via YAP signaling pathway. Theranostics 2020, 10, 5790–5801. [Google Scholar] [CrossRef]
- Kuang, S.; He, F.; Liu, G.; Sun, X.; Dai, J.; Chi, A.; Tang, Y.; Li, Z.; Gao, Y.; Deng, C.; et al. CCR2-engineered mesenchymal stromal cells accelerate diabetic wound healing by restoring immunological homeostasis. Biomaterials 2021, 275, 120963. [Google Scholar] [CrossRef]
- Tang, J.; Chen, Y.; Wang, C.; Xia, Y.; Yu, T.; Tang, M.; Meng, K.; Yin, L.; Yang, Y.; Shen, L.; et al. The role of mesenchymal stem cells in cancer and prospects for their use in cancer therapeutics. Medcomm 2024, 5, e663. [Google Scholar] [CrossRef]
- Ho, Y.K.; Woo, J.Y.; Loke, K.M.; Deng, L.-W.; Too, H.-P. Enhanced anti-tumor efficacy with multi-transgene armed mesenchymal stem cells for treating peritoneal carcinomatosis. J. Transl. Med. 2024, 22, 463. [Google Scholar] [CrossRef] [PubMed]
- Hervás-Salcedo, R.; Fernández-García, M.; Hernando-Rodríguez, M.; Suárez-Cabrera, C.; Bueren, J.A.; Yáñez, R.M. Improved efficacy of mesenchymal stromal cells stably expressing CXCR4 and IL-10 in a xenogeneic graft versus host disease mouse model. Front. Immunol. 2023, 14, 1062086. [Google Scholar] [CrossRef] [PubMed]
- Audouard, E.; Michel, F.; Pierroz, V.; Kim, T.; Rousselot, L.; Gillet-Legrand, B.; Dufayet-Chauffaut, G.; Buchmann, P.; Florea, M.; Khel, A.; et al. Bioelectronic cell-based device provides a strategy for the treatment of the experimental model of multiple sclerosis. J. Control. Release 2022, 352, 994–1008. [Google Scholar] [CrossRef]
- Hou, X.; Shou, C.; He, M.; Xu, J.; Cheng, Y.; Yuan, Z.; Lan, M.; Zhao, Y.; Yang, Y.; Chen, X.; et al. A combination of LightOn gene expression system and tumor microenvironment-responsive nanoparticle delivery system for targeted breast cancer therapy. Acta Pharm. Sin. B 2020, 10, 1741–1753. [Google Scholar] [CrossRef]
- Lee, E.A.; Kim, S.; Jin, Y.; Cho, S.-W.; Yang, K.; Hwang, N.S.; Kim, H.D. In situ microenvironment remodeling using a dual-responsive system: Photodegradable hydrogels and gene activation by visible light. Biomater. Sci. 2022, 10, 3981–3992. [Google Scholar] [CrossRef] [PubMed]
- Trinh, T.; Thompson, I.A.P.; Clark, F.; Remington, J.M.; Eisenstein, M.; Li, J.; Soh, H.T. A Photoresponsive Intramolecular Triplex Motif That Enables Rapid and Reversible Control of Aptamer Binding Activity. ACS Nano 2022, 16, 14549–14557. [Google Scholar] [CrossRef]
- Dong, J.; Du, X.; Zhang, Y.; Zhuang, T.; Cui, X.; Li, Z. Thermo/glutathione-sensitive release kinetics of heterogeneous magnetic micro-organogel prepared by sono-catalysis. Colloids Surf. B Biointerfaces 2021, 208, 112109. [Google Scholar] [CrossRef]
- Bauman, L.; Zhao, B. Multi-thermo responsive double network composite hydrogel for 3D printing medical hydrogel mask. J. Colloid Interface Sci. 2023, 638, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.H.; Zhu, C.Y.; Yu, P.H.; Ma, Y.; Hussain, L.; Naranmandura, H.; Wang, Q.Q. The landscape of novel strategies for acute myeloid leukemia treatment: Therapeutic trends, challenges, and future directions. Toxicol. Appl. Pharmacol. 2023, 473, 116585. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wu, C.; Liu, Y.; Hu, L.; Shang, W.; Gao, Z.; Xia, N. Folate functionalized pH-sensitive photothermal therapy traceable hollow mesoporous silica nanoparticles as a targeted drug carrier to improve the antitumor effect of doxorubicin in the hepatoma cell line SMMC-7721. Drug Deliv. 2020, 27, 258–268. [Google Scholar] [CrossRef]
- Luo, L.; Zang, G.; Liu, B.; Qin, X.; Zhang, Y.; Chen, Y.; Zhang, H.; Wu, W.; Wang, G. Bioengineering CXCR4-overexpressing cell membrane functionalized ROS-responsive nanotherapeutics for targeting cerebral ischemia-reperfusion injury. Theranostics 2021, 11, 8043–8056. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Chen, Z. Emerging role of exosomes in cancer therapy: Progress and challenges. Mol. Cancer 2025, 24, 13. [Google Scholar] [CrossRef]
- Taheri, M.; Tehrani, H.A.; Dehghani, S.; Alibolandi, M.; Arefian, E.; Ramezani, M. Nanotechnology and bioengineering approaches to improve the potency of mesenchymal stem cell as an off-the-shelf versatile tumor delivery vehicle. Med. Res. Rev. 2024, 44, 1596–1661. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Zhang, H.; Tian, R.; Liu, H.; Wang, Z.; Wang, Z.; Tian, J.; Cui, Y.; Ren, S.; Zuo, X.; et al. Exosomal EPHA2 derived from highly metastatic breast cancer cells promotes angiogenesis by activating the AMPK signaling pathway through Ephrin A1-EPHA2 forward signaling. Theranostics 2022, 12, 4127–4146. [Google Scholar] [CrossRef]
- Yu, P.; Han, Y.; Meng, L.; Tian, Y.; Jin, Z.; Luo, J.; Han, C.; Xu, W.; Kong, L.; Zhang, C. Exosomes derived from pulmonary metastatic sites enhance osteosarcoma lung metastasis by transferring the miR-194/215 cluster targeting MARCKS. Acta Pharm. Sin. B 2024, 14, 2039–2056. [Google Scholar] [CrossRef]
- An, F.; Hou, Z.; Wang, X.; Wang, Z.; Jing, C.; Sui, R.; Wu, Y.; Ma, Y.; Chang, C.; Liu, S.; et al. A microfluidic demonstration of “cluster-sprout-infiltrating” mode for hypoxic mesenchymal stem cell guided cancer cell migration. Biomaterials 2022, 290, 121848. [Google Scholar] [CrossRef]
- Wu, J.; Wu, J.; Xiang, W.; Gong, Y.; Feng, D.; Fang, S.; Wu, Y.; Liu, Z.; Li, Y.; Chen, R.; et al. Engineering exosomes derived from TNF-α preconditioned IPFP-MSCs enhance both yield and therapeutic efficacy for osteoarthritis. J. Nanobiotechnol. 2024, 22, 555. [Google Scholar] [CrossRef] [PubMed]
- Zong, C.; Meng, Y.; Ye, F.; Yang, X.; Li, R.; Jiang, J.; Zhao, Q.; Gao, L.; Han, Z.; Wei, L. AIF1+CSF1R+ MSCs, induced by TNF-α, act to generate an inflammatory microenvironment and promote hepatocarcinogenesis. Hepatology 2023, 78, 434–451. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, C.; Shin, Y.; Wang, S.; Han, J.; Kim, M.; Kim, J.M.; Shin, S.-C.; Lee, B.-J.; Kim, T.-J.; et al. sEVs from tonsil-derived mesenchymal stromal cells alleviate activation of hepatic stellate cells and liver fibrosis through miR-486-5p. Mol. Ther. 2021, 29, 1471–1486. [Google Scholar] [CrossRef] [PubMed]
- McKenna, M.K.; Englisch, A.; Brenner, B.; Smith, T.; Hoyos, V.; Suzuki, M.; Brenner, M.K. Mesenchymal stromal cell delivery of oncolytic immunotherapy improves CAR-T cell antitumor activity. Mol. Ther. 2021, 29, 1808–1820. [Google Scholar] [CrossRef]
- Qu, C.; Zhang, H.; Cao, H.; Tang, L.; Mo, H.; Liu, F.; Zhang, L.; Yi, Z.; Long, L.; Yan, L.; et al. Tumor buster—Where will the CAR-T cell therapy ‘missile’ go? Mol. Cancer 2022, 21, 201. [Google Scholar] [CrossRef]


| Source | Therapeutic Strategy | Synergistic Drug/Method | Results | Reference |
|---|---|---|---|---|
| Adipose tissue-derived MSCs (AT-MSCs) | Exosome-mediated miR-122 delivery | Sorafenib | Exosomes regulated target gene expression via miR-122, enhancing hepatocellular carcinoma (HCC) sensitivity to chemotherapy. | [98] |
| Exosome-mediated miR-199a delivery | Doxorubicin | Significantly improved HCC chemosensitivity by inhibiting the mTOR pathway and reduced tumor volume in vivo. | [99] | |
| Cell-based therapy | Radiotherapy | Inhibited HCC cell growth, migration, and invasion. | [16] | |
| Bone marrow-derived MSCs (BMSCs) | Measles virus infection (oncolytic viral vector) | None | Measles virus-infected MSCs evaded immune clearance, reduced tumor volume, and prolonged survival in HCC models without systemic immune reactions. | [63] |
| Exosome-encapsulated norcantharidin (NCTD) | None | Exosomes acted as drug carriers, enhancing antitumor effects against HCC. | [100] | |
| Exosome-delivered GRP78-targeting siRNA | Sorafenib | Reversed sorafenib resistance in HCC and significantly reduced tumor volume while prolonging survival in murine models. | [101] | |
| T cell-activating MSCs | CAR-T cell therapy | Enhanced specific cytotoxicity against HCC via Glypican-3-targeted CAR-T cells. | [102] | |
| Cell-based therapy | None | Exosomes modulated cancer stemness in HCC, suppressing tumor growth. | [103] | |
| Human umbilical cord MSCs (hUC-MSCs) | Conditionally replicative adenovirus (CRAd) delivery | None | Liver differentiation-dependent viral release enabled specific HCC cell elimination with minimal hepatotoxicity. | [104] |
| AFP promoter-driven sTRAIL expression | 5-FU | Suppressed orthotopic HCC tumor growth, extended median survival, and caused no significant hepatic/renal toxicity. | [105] | |
| Exosome-mediated miR-499a-5p delivery | None | Attenuated liver fibrosis by targeting ETS1/GPX4-mediated ferroptosis in hepatic stellate cells, potentially preventing HCC progression. | [66] | |
| MSCs (unspecified source) | Cell-based therapy | Sorafenib | Combined therapy suppressed HCC proliferation, reduced angiogenesis, and induced apoptosis while maintaining MSC-mediated anti-inflammatory effects. | [106] |
| Oncolytic adenovirus delivery | Oncolytic virotherapy | Enhanced antitumor efficacy against HCC while preventing hepatotoxicity. | [62] | |
| IL-12 genetic modification | Prophylactic monotherapy | Demonstrated significant cancer prevention efficacy in three unestablished tumor models, including HCC. | [107] | |
| Tissue-specific suicide gene system | Ganciclovir | Targeted HCC stromal microenvironment selectively, suppressing tumor growth. | [74] | |
| HNF4α overexpression | None | Suppressed HCC development via downregulation of Wnt/β-catenin signaling. | [108] | |
| TRAIL genetic modification | Cisplatin | MSCs as TRAIL delivery vehicles enhanced apoptosis induction in HCC cells and synergized with cisplatin to inhibit tumor growth. | [109] | |
| Apoptin genetic modification | None | Induced HCC apoptosis significantly, reduced tumor weight in animal models without systemic toxicity. | [110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Wang, J.-P.; Hong, D.-F.; Yang, C.; Naranmandura, H. Mesenchymal Stem Cell-Mediated Targeted Drug Delivery Systems for Hepatocellular Carcinoma: Current Advances and Future Directions. Bioengineering 2025, 12, 1206. https://doi.org/10.3390/bioengineering12111206
Gao Y, Wang J-P, Hong D-F, Yang C, Naranmandura H. Mesenchymal Stem Cell-Mediated Targeted Drug Delivery Systems for Hepatocellular Carcinoma: Current Advances and Future Directions. Bioengineering. 2025; 12(11):1206. https://doi.org/10.3390/bioengineering12111206
Chicago/Turabian StyleGao, Yang, Jian-Ping Wang, De-Fei Hong, Chang Yang, and Hua Naranmandura. 2025. "Mesenchymal Stem Cell-Mediated Targeted Drug Delivery Systems for Hepatocellular Carcinoma: Current Advances and Future Directions" Bioengineering 12, no. 11: 1206. https://doi.org/10.3390/bioengineering12111206
APA StyleGao, Y., Wang, J.-P., Hong, D.-F., Yang, C., & Naranmandura, H. (2025). Mesenchymal Stem Cell-Mediated Targeted Drug Delivery Systems for Hepatocellular Carcinoma: Current Advances and Future Directions. Bioengineering, 12(11), 1206. https://doi.org/10.3390/bioengineering12111206








