Preliminary Evidence of Exogenous Hydrogen Peroxide Formation via Plant Transpiration: Toward a Nature-Based Solution for Air Quality and Climate Mitigation
Abstract
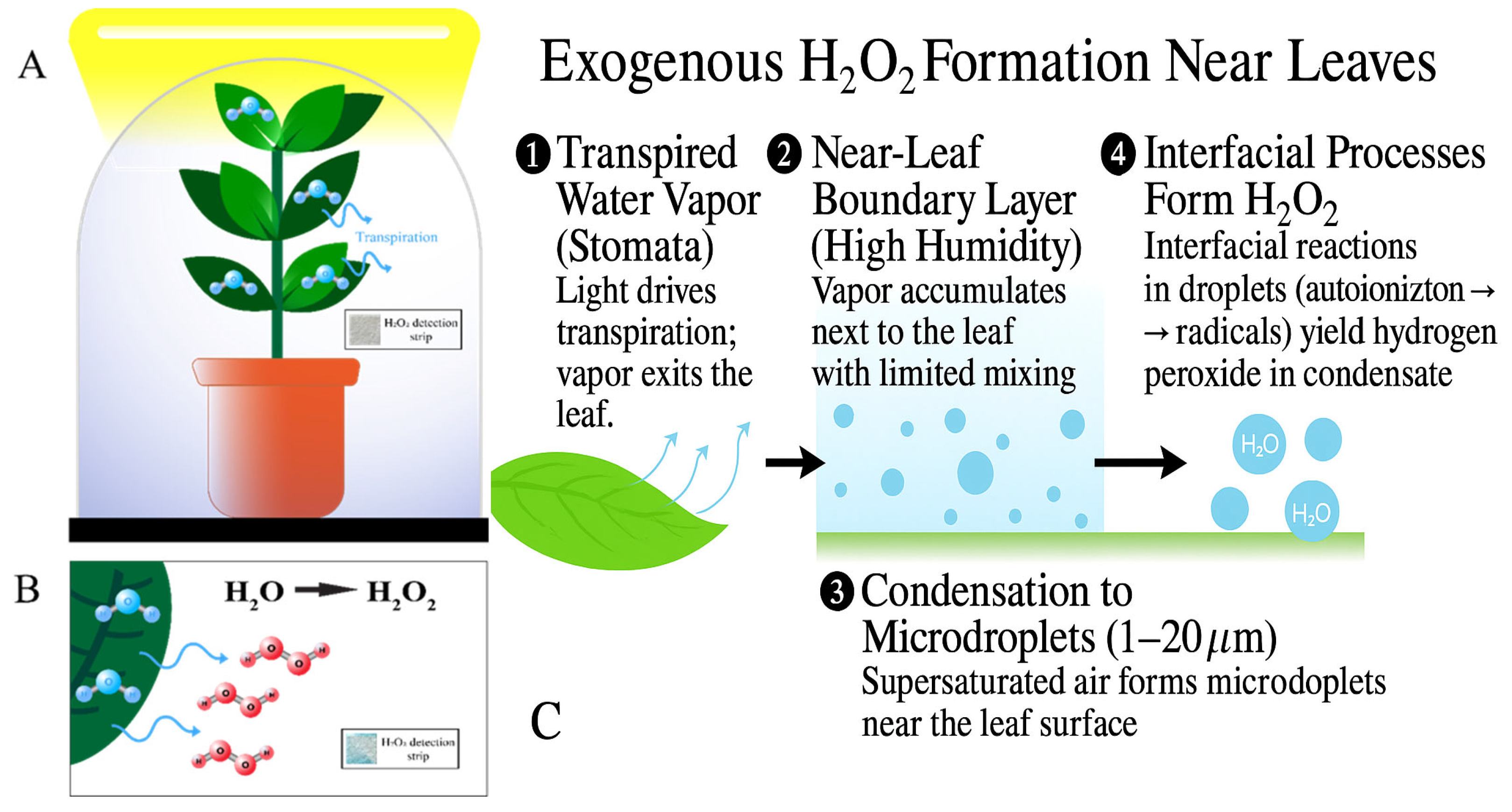
1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up
2.2. Quantification of H2O2 Production
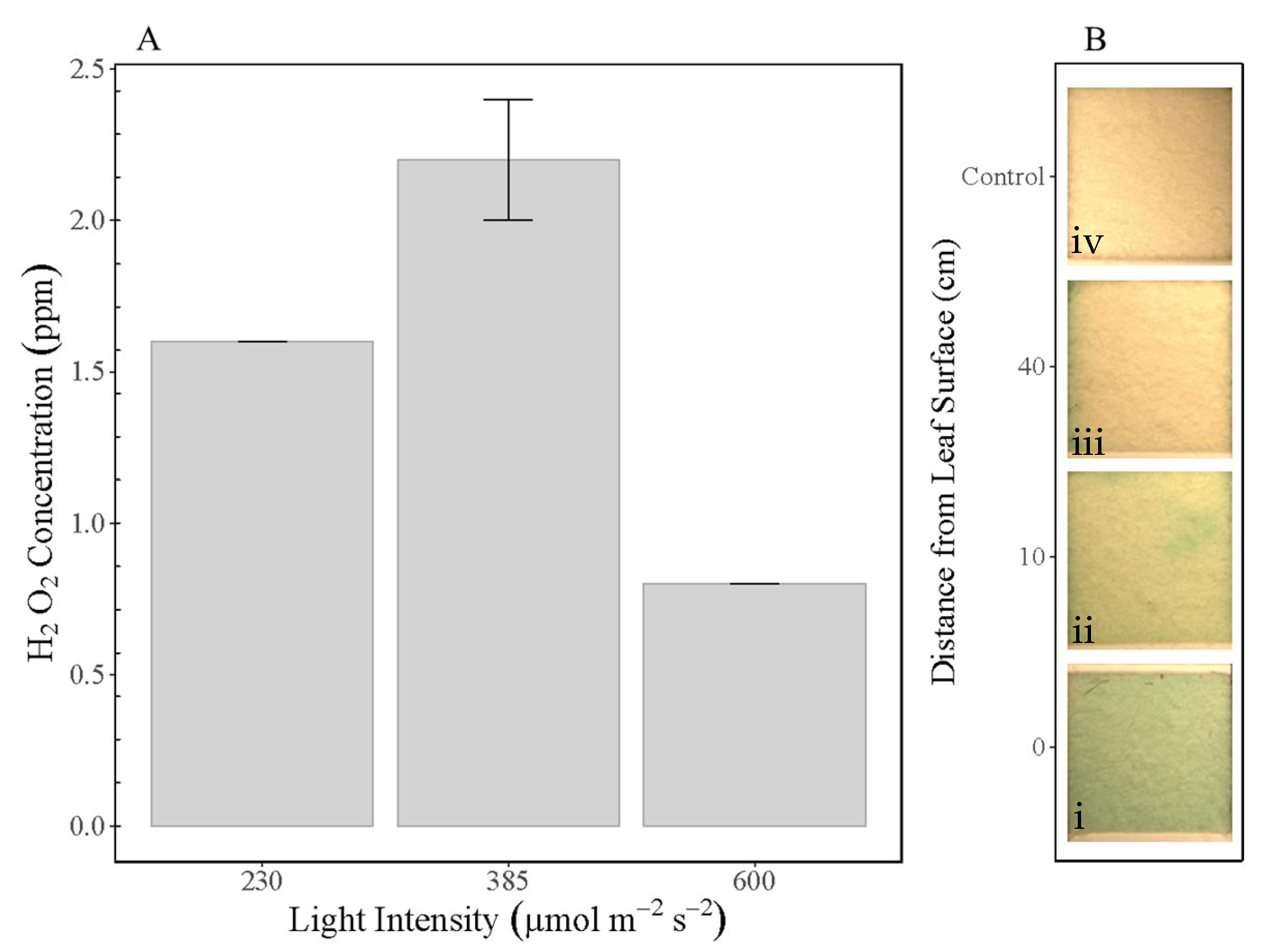
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diener, A.; Mudu, P. How can vegetation protect us from air pollution? A critical review on green spaces’ mitigation abilities for air-borne particles from a public health perspective-with implications for urban planning. Sci. Total Environ. 2021, 796, 148605. [Google Scholar] [CrossRef] [PubMed]
- Austin, A.T.; Zanne, A.E.; Bardgett, R. Whether in life or in death: Fresh perspectives on how plants affect biogeochemical cycling. J. Ecol. 2015, 103, 1367–1371. [Google Scholar] [CrossRef]
- Gligorovski, S.; Strekowski, R.; Barbati, S. Environmental Implications of Hydroxyl Radicals (•OH). Chem. Rev. 2015, 115, 13051–13092. [Google Scholar] [CrossRef] [PubMed]
- Travis, K.R.; Heald, C.L.; Allen, H.M.; Apel, E.C. Constraining Remote Oxidation Capacity with ATom Observations. Atmos. Chem. Phys. 2020, 20, 7753–7772. [Google Scholar] [CrossRef]
- Lelieveld, J.; Dentener, F.; Peters, W.; Krol, M. On the role of hydroxyl radicals in the self-cleansing capacity of the troposphere. Atmos. Chem. Phys. 2004, 4, 2337–2344. [Google Scholar] [CrossRef]
- Rao, M.J.; Duan, M.; Ikram, M.; Zheng, B. ROS Regulation and Antioxidant Responses in Plants under Air Pollution: Molecular Signaling, Metabolic Adaptation, and Biotechnological Solutions. Antioxidants 2025, 14, 907. [Google Scholar] [CrossRef]
- Cheeseman, J.M. Hydrogen peroxide concentrations in leaves under natural conditions. J. Exp. Bot. 2006, 57, 2435–2444. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Kroumova, A.B.M.; Sahoo, D.K.; Raha, S.; Goodin, M.; Maiti, I.B.; Wagner, G.J. Expression of an apoplast-directed, T-phylloplanin-GFP fusion gene confers resistance against Peronospora tabacina in susceptible tobacco. Plant Cell Rep. 2013, 32, 1771–1782. [Google Scholar] [CrossRef]
- Lee, J.K.; Walker, K.L.; Han, H.S.; Kang, J.; Prinz, F.B.; Waymouth, R.M.; Nam, H.G.; Zare, R.N. Spontaneous Generation of Hydrogen Peroxide from Aqueous Microdroplets. Proc. Natl. Acad. Sci. USA 2019, 116, 19294–19298. [Google Scholar] [CrossRef]
- Musskopf, N.H.; Gallo, A., Jr.; Zhang, P.; Petry, J.; Mishra, H. The Air–Water Interface of Water Microdroplets Formed by Ultrasonication or Condensation Does Not Produce H2O2. J. Phys. Chem. Lett. 2021, 12, 11422–11429. [Google Scholar] [CrossRef]
- Lee, J.K.; Han, H.S.; Chaikasetsin, S.; Marron, D.P.; Waymouth, R.M.; Prinz, F.B.; Zare, R.N. Condensing Water Vapor to Droplets Generates Hydrogen Peroxide. Proc. Natl. Acad. Sci. USA 2020, 117, 30934–30941. [Google Scholar] [CrossRef]
- Janhäll, S. Review on Urban Vegetation and Particle Air Pollution-Deposition and Dispersion. Atmos. Environ. 2015, 105, 130–137. [Google Scholar] [CrossRef]
- Rios-Castillo, A.G.; Gonzalez-Rivas, F.; Rodriguez-Jerez, J.J. Bactericidal Efficacy of Hydrogen Peroxide-Based Disinfectants Against Gram-Positive and Gram-Negative Bacteria on Stainless Steel Surfaces. J. Food Sci. 2017, 82, 2351–2356. [Google Scholar] [CrossRef]
- Sakugawa, H.; Kaplan, I.R.; Tsai, W.; Cohen, Y. Atmospheric hydrogen peroxide. Environ. Sci. Technol. 1990, 24, 1452–1462. [Google Scholar] [CrossRef]
- Wuebbles, D.J.; Hayhoe, K. Atmospheric methane and global change. Earth-Sci. Rev. 2002, 57, 177–210. [Google Scholar] [CrossRef]
- Quillet, A.; Peng, C.; Garneau, M. Toward Dynamic Global Vegetation Models for Simulating Vegetation-Climate Interactions and Feedbacks: Recent Developments, Limitations, and Future Challenges. Can. J. For. Res. 2010, 40, 1234–1250. [Google Scholar] [CrossRef]
- Sellers, R.M. Spectrophotometric Determination of Hydrogen Peroxide Using Potassium Titanium (IV) Oxalate. Analyst 1990, 105, 950–954. [Google Scholar] [CrossRef]
- Colussi, A.J. Mechanism of Hydrogen Peroxide Formation on Sprayed Water Microdroplets. J. Am. Chem. Soc. 2023, 145, 22306–22315. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Yu, H.; Wang, X.; Sun, Z.; Lu, D. Accelerated Photochemical Generation of Reactive Species in Microdroplets by Humic-Like Substances: Role of the Air-Water Interface and Molecular Mechanism. Environ. Sci. Technol. 2025, 59, 1432–1441. [Google Scholar] [CrossRef] [PubMed]
- Zotarelli, L.; Dukes, M.D.; Romero, C.C.; Migliaccio, K.W.; Morgan, K.T. Step by step calculation of the Penman-Monteith Evapotranspiration (FAO-56 Method): AE459, 2/2010. EDIS 2010, 2010. [Google Scholar] [CrossRef]
- Carpenter, W.; Nautiyal, J. Light Intensity and Air Movement Effects on Leaf Temperatures and Growth of Shade-requiring Greenhouse Crops. J. Am. Soc. Hortic. Sci. 1969, 94, 212–214. [Google Scholar] [CrossRef]
- Slesak, I.; Libik, M.; Karpinska, B. The Role of Hydrogen Peroxide in Regulation of Plant Metabolism and Cellular Signalling in Response to Environmental Stresses. Acta Biochim. Pol. 2007, 54, 39–50. [Google Scholar] [CrossRef]
- Becker, K.H.; Brockmann, K.J.; Bechara, J. Production of hydrogen peroxide in forest air by reaction of ozone with terpenes. Nature 1990, 346, 256–258. [Google Scholar] [CrossRef]
- Kardiman, R.; Ræbild, A. Relationship between stomatal density, size and speed of opening in Sumatran rainforest species. Tree Physiol. 2018, 38, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Shahpoury, P.; Lelieveld, S.; Srivastava, D.; Baccarini, A.; Mastin, J.; Berkemeier, T.; Celo, V.; Dabek-Zlotorzynska, E.; Harner, T.; Lammel, G.; et al. Seasonal Changes in the Oxidative Potential of Urban Air Pollutants: The Influence of Emission Sources and Proton- and Ligand-Mediated Dissolution of Transition Metals. ACS ES&T Air 2024, 1, 1262–1275. [Google Scholar] [CrossRef] [PubMed]
- García, J.; Dominguez, C.; Gonzalez, R.; Ramirez, P.; Torres, M. A review of emerging organic contaminants (EOCs), antibiotic resistant bacteria (ARB), and antibiotic resistance genes (ARGs) in the environment: Increasing removal with wetlands and reducing environmental impacts. Bioresour. Technol. 2020, 307, 123228. [Google Scholar] [CrossRef]
- Han, Y.; Li, J.; Wang, Q.; Zhang, H.; Chen, X. Plant-based remediation of air pollution: A review. J. Environ. Manag. 2022, 301, 113860. [Google Scholar] [CrossRef]
- El-Tanbouly, R.; Hassan, Z.; El-Messeiry, S. The Role of Indoor Plants in Air Purification and Human Health in the Context of COVID-19 Pandemic: A Proposal for a Novel Line of Inquiry. Front. Mol. Biosci. 2021, 8, 709395. [Google Scholar] [CrossRef]
- Fitzky, A.C.; Sandén, H.; Karl, T.; Fares, S.; Calfapietra, C.; Grote, R.; Saunier, A.; Rewald, B. The Interplay Between Ozone and Urban Vegetation. Front. For. Glob. Chang. 2019, 2, 50. [Google Scholar] [CrossRef]
- McFiggans, G.; Mentel, T.F.; Wildt, J.; Pullinen, I.; Kang, S. Secondary organic aerosol reduced by mixture of atmospheric vapours. Nature 2019, 565, 587–593. [Google Scholar] [CrossRef]
- Hamryszczak, Z.; Dienhart, D.; Brendel, B.; Rohloff, R.; Marno, D.; Martinez, M.; Harder, H.; Pozzer, A.; Bohn, B.; Zöger, M.; et al. Hydrogen Peroxide in the Upper Tropical Troposphere over the Atlantic Ocean and Western Africa during the CAFE-Africa Campaign. Atmos. Chem. Phys. 2023, 23, 5929–5948. [Google Scholar] [CrossRef]
- Finlayson-Pitts, B.J.; Pitts, J.N., Jr. Atmospheric Chemistry: Fundamentals and Experimental Techniques; Wiley: Hoboken, NJ, USA, 1986. [Google Scholar]
- Allen, H.M.; Crounse, J.D.; Kim, M.J.; Teng, A.P. H2O2 and CH3OOH (MHP) in the Remote Atmosphere: 1. Global Distribution and Regional Influences. J. Geophys. Res. Atmos. 2022, 127, e2021JD035701. [Google Scholar] [CrossRef]
- Wedow, J.M.; Ainsworth, E.A.; Li, S. Plant biochemistry influences tropospheric ozone formation, destruction, deposition, and response. Trends Biochem. Sci. 2021, 46, 992–1002. [Google Scholar] [CrossRef]
- Wolfe, G.M.; Nicely, J.M.; Clair, J.M.; Hanisco, T.F.; Liao, J.; Oman, L.D.; Brune, W.H.; Miller, D.O.; Thames, A.B.; González Abad, G.; et al. Mapping Hydroxyl Variability throughout the Global Remote Troposphere via Synthesis of Airborne and Satellite Formaldehyde Observations. Proc. Natl. Acad. Sci. USA 2019, 116, 11171–11180. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samadi, S.; Sharifyazd, S.; Cabling, L.P.B.; Dekker, I.; Hawkins, B.J.; Buckley, H.L.; Dubrawski, K.L. Preliminary Evidence of Exogenous Hydrogen Peroxide Formation via Plant Transpiration: Toward a Nature-Based Solution for Air Quality and Climate Mitigation. Bioengineering 2025, 12, 1201. https://doi.org/10.3390/bioengineering12111201
Samadi S, Sharifyazd S, Cabling LPB, Dekker I, Hawkins BJ, Buckley HL, Dubrawski KL. Preliminary Evidence of Exogenous Hydrogen Peroxide Formation via Plant Transpiration: Toward a Nature-Based Solution for Air Quality and Climate Mitigation. Bioengineering. 2025; 12(11):1201. https://doi.org/10.3390/bioengineering12111201
Chicago/Turabian StyleSamadi, Saman, Shabnam Sharifyazd, Ludwig Paul B. Cabling, Isaac Dekker, Barbara J. Hawkins, Heather L. Buckley, and Kristian L. Dubrawski. 2025. "Preliminary Evidence of Exogenous Hydrogen Peroxide Formation via Plant Transpiration: Toward a Nature-Based Solution for Air Quality and Climate Mitigation" Bioengineering 12, no. 11: 1201. https://doi.org/10.3390/bioengineering12111201
APA StyleSamadi, S., Sharifyazd, S., Cabling, L. P. B., Dekker, I., Hawkins, B. J., Buckley, H. L., & Dubrawski, K. L. (2025). Preliminary Evidence of Exogenous Hydrogen Peroxide Formation via Plant Transpiration: Toward a Nature-Based Solution for Air Quality and Climate Mitigation. Bioengineering, 12(11), 1201. https://doi.org/10.3390/bioengineering12111201






