3D Printing of Shape Memory Resin for Orthodontic Aligners with Green Synthesized Antimicrobial ZnO Nanoparticles Coatings: Toward Bioactive Devices
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
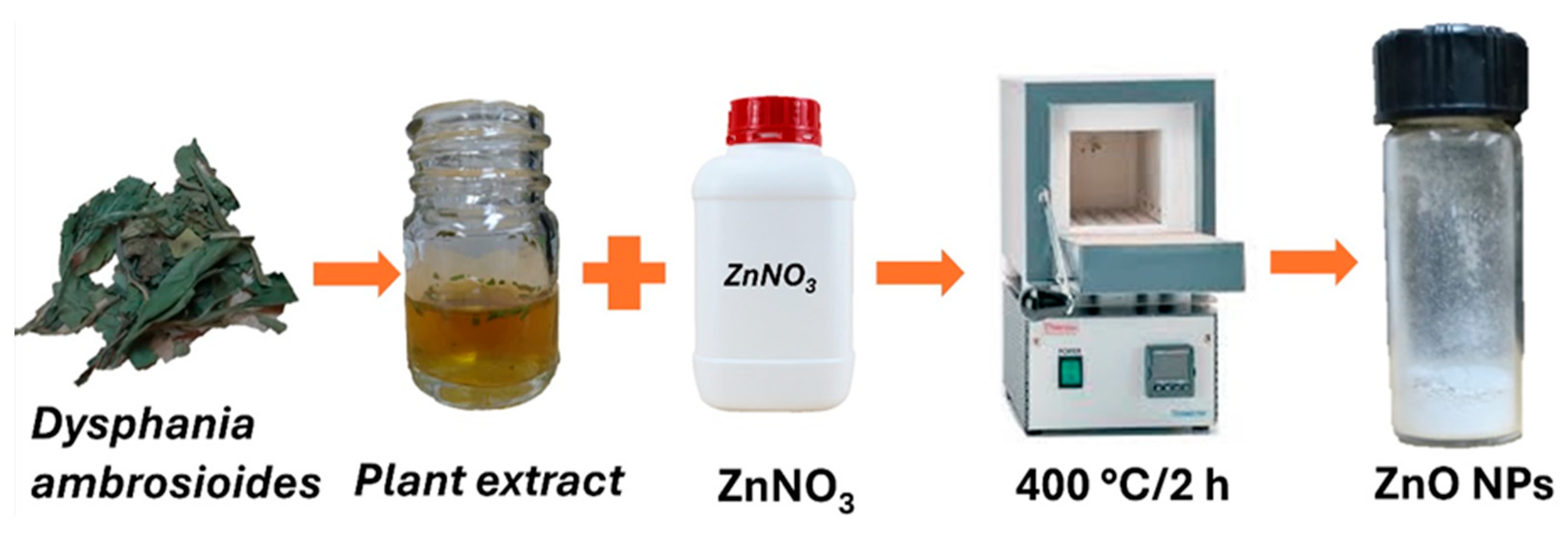
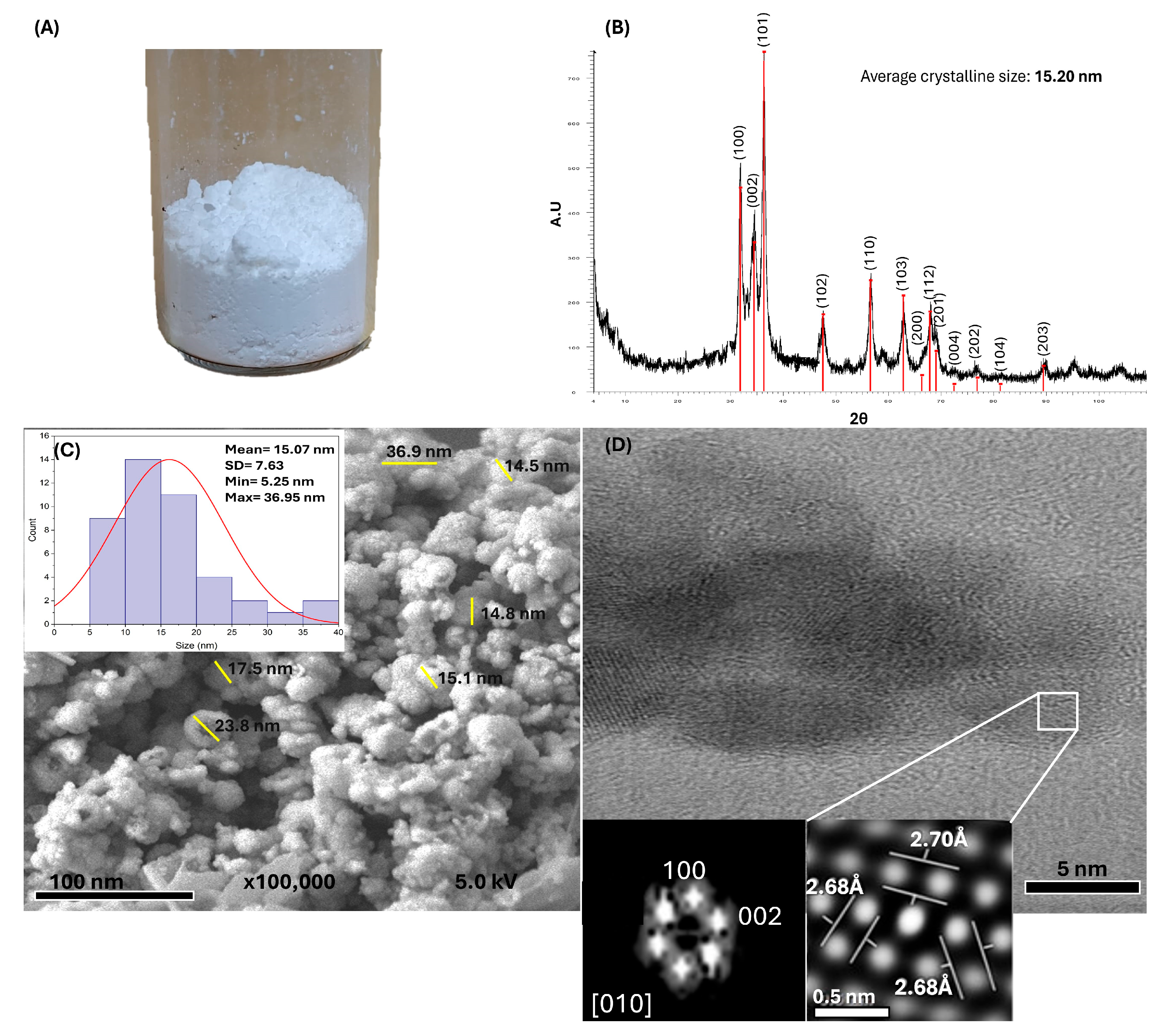
2.2. Synthesis and Characterization of ZnO NPs
2.3. Design and 3D Printing of the Resin Disc for Biocompatibility and Antibacterial Evaluations
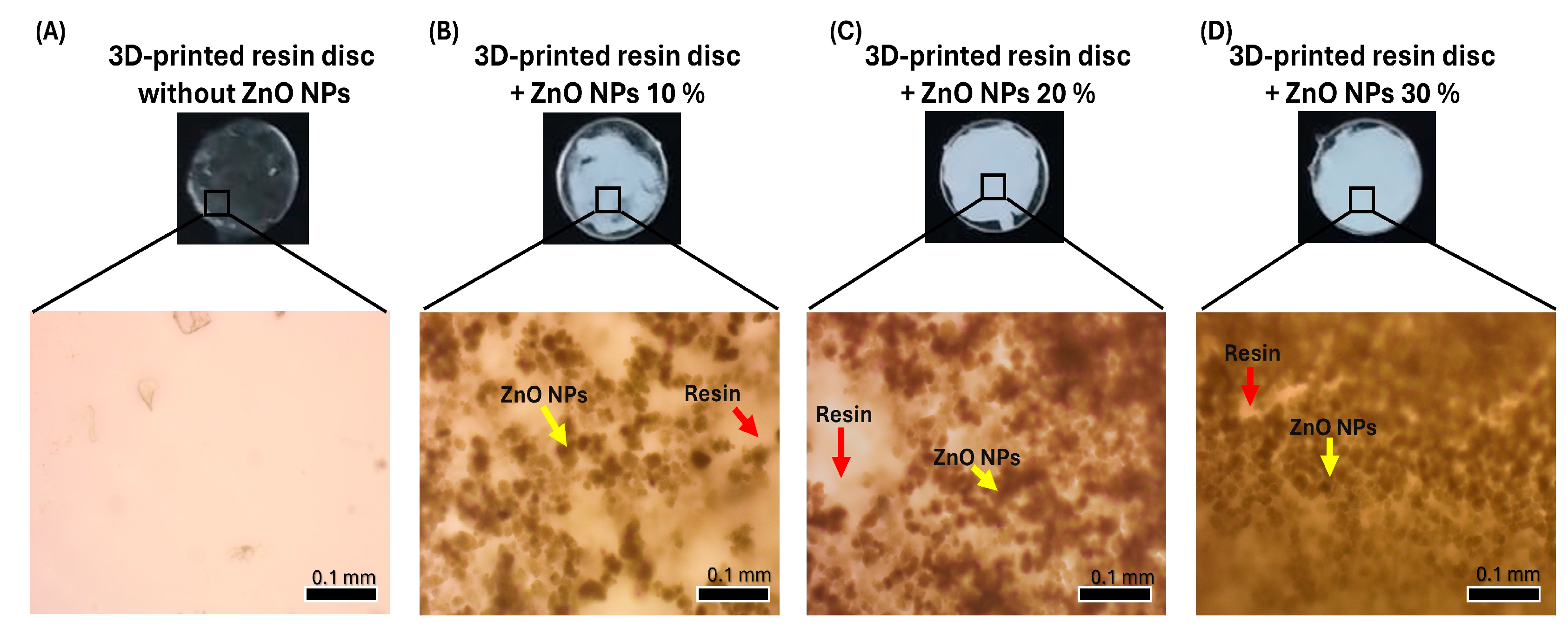
2.4. Coating the 3D-Printed Resin Disc with ZnO NPs
2.5. Surface Roughness Test
2.6. Biocompatibility Evaluation
2.6.1. Cell Culture
2.6.2. WST-1 Assay
2.7. Antibacterial Evaluation
2.8. Statistical Analysis
3. Results
3.1. Green Synthesis and Characterization of ZnO NPs
3.2. Design, 3D Printing of the Resin Disc, and Coatings with ZnO NPs
3.3. Surface Roughness Test
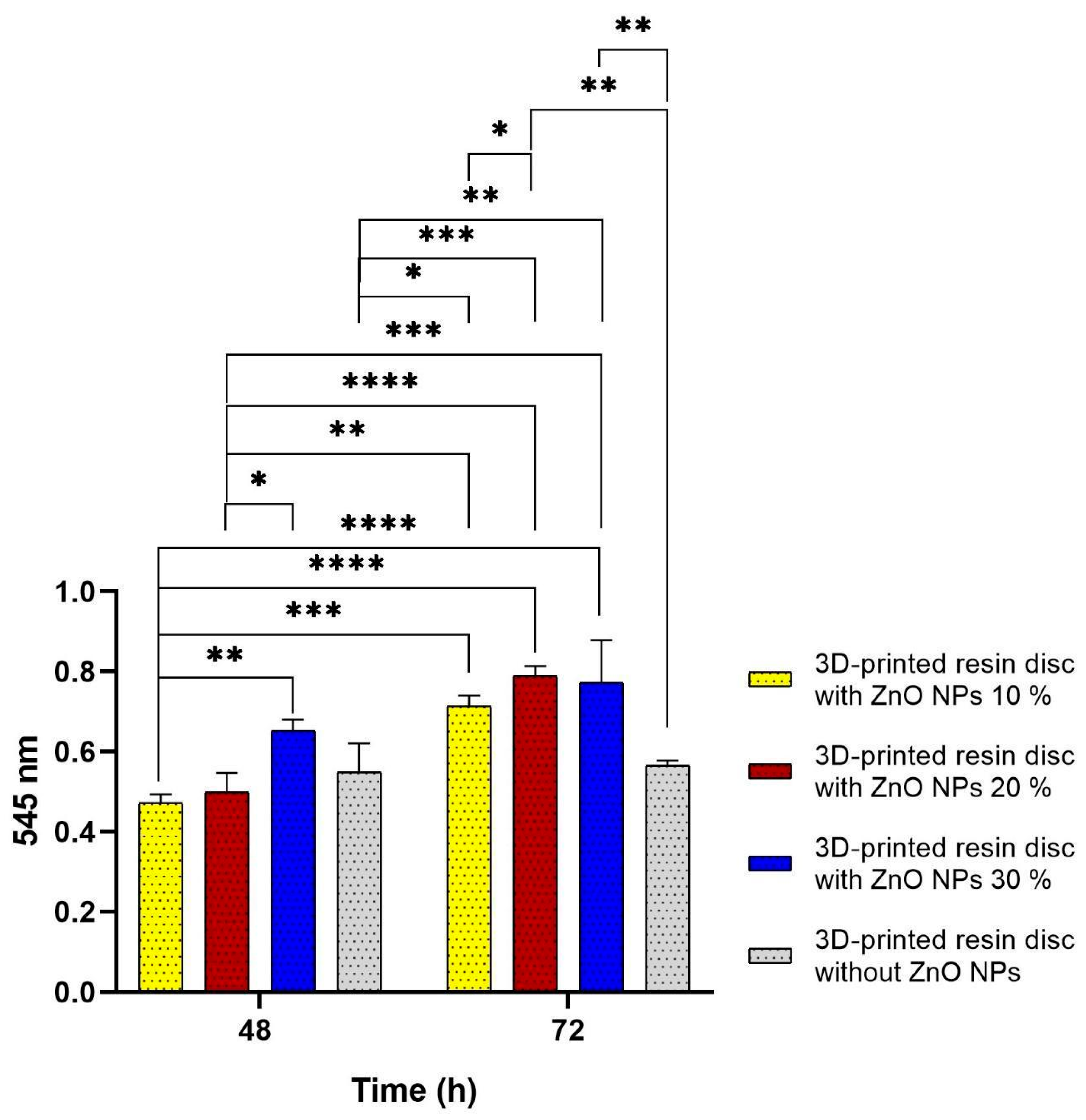
3.4. Biocompatibility Evaluation
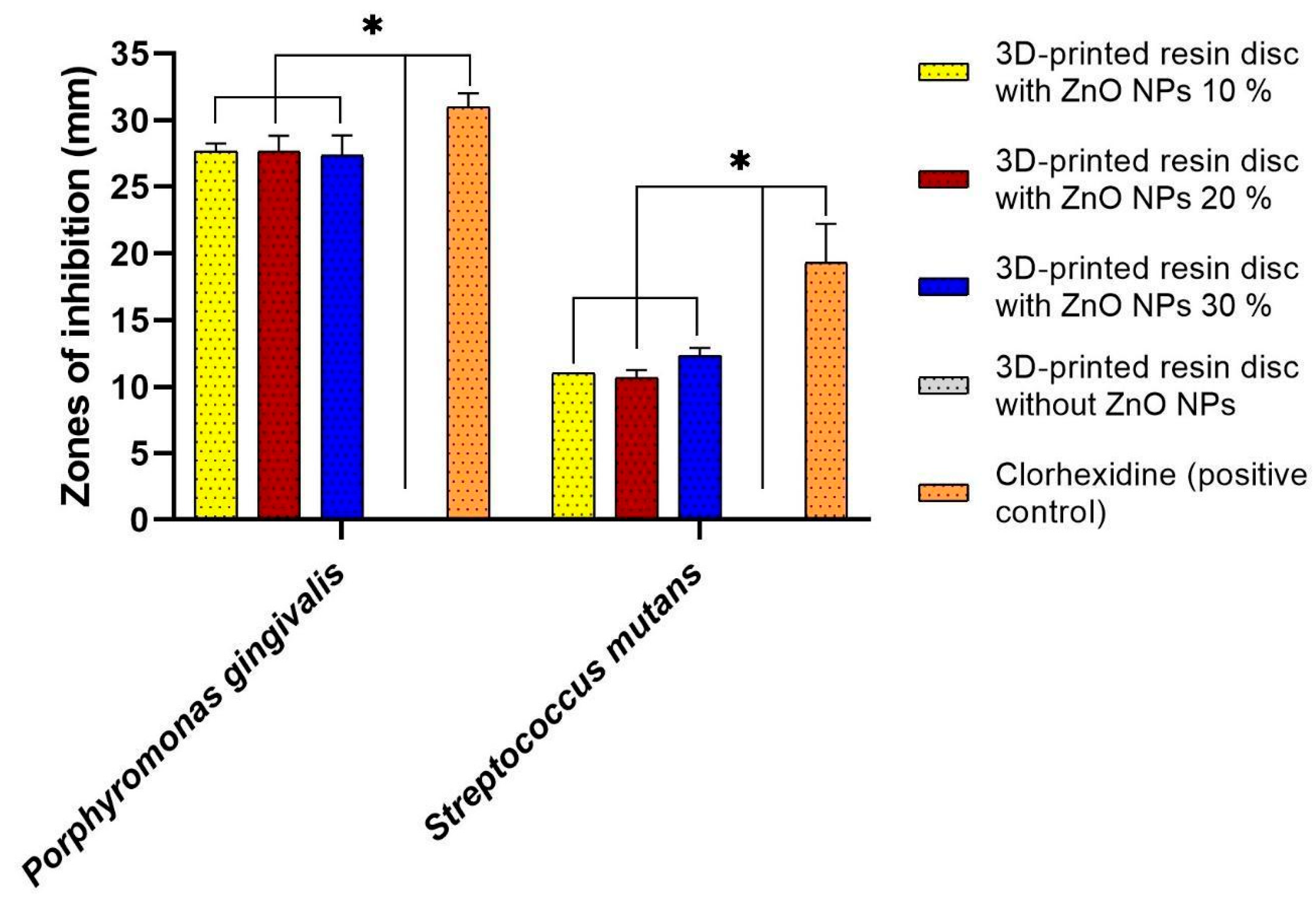
3.5. Antibacterial Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ZnO NPs | Zinc oxide nanoparticles |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| FBS | Fetal bovine serum |
| TSA | Trypticase soy agar |
| XRD | X-ray diffraction |
| FESEM | Field-emission scanning electron microscopy |
| TEM | Transmission electron microscopy |
| hGFs | Human gingival fibroblasts |
References
- Alwafi, A.; Bichu, Y.M.; Avanessian, A.; Adel, S.M.; Vaid, N.R.; Zou, B. Overview of Systematic Reviews and Meta-Analyses Assessing the Predictability and Clinical Effectiveness of Clear Aligner Therapy. Dent. Rev. 2023, 3, 100074. [Google Scholar] [CrossRef]
- Tamer, I.; Oztas, E.; Marsan, G. Orthodontic Treatment with Clear Aligners and The Scientific Reality Behind Their Marketing: A Literature Review. Turk. J. Orthod. 2019, 32, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Belanche Monterde, A.; Flores-Fraile, J.; Pérez Pevida, E.; Zubizarreta-Macho, Á. Biofilm Composition Changes During Orthodontic Clear Aligners Compared to Multibracket Appliances: A Systematic Review. Microorganisms 2025, 13, 1039. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Mangal, U.; Seo, J.-Y.; Kim, H.; Bai, N.; Cha, J.-Y.; Lee, K.-J.; Kwon, J.-S.; Choi, S.-H. Enhancing Biofilm Resistance and Preserving Optical Translucency of 3D Printed Clear Aligners through Carboxybetaine-Copolymer Surface Treatment. Dent. Mater. 2024, 40, 1575–1583. [Google Scholar] [CrossRef]
- Sfondrini, M.F.; Butera, A.; Di Michele, P.; Luccisano, C.; Ottini, B.; Sangalli, E.; Gallo, S.; Pascadopoli, M.; Gandini, P.; Scribante, A. Microbiological Changes during Orthodontic Aligner Therapy: A Prospective Clinical Trial. Appl. Sci. 2021, 11, 6758. [Google Scholar] [CrossRef]
- Hamad, T.; Hamasaeed, N. Effect Of Fixed Brackets and Invisalign on Oral Total Bacterial Load and Profiles of Porphyromonas Gingivalis, Streptococcus mutans, and Streptococcus sobrinus: A Qpcr Study. J. Med. Health Stud. 2025, 6, 110–121. [Google Scholar] [CrossRef]
- Rouzi, M.; Zhang, X.; Jiang, Q.; Long, H.; Lai, W.; Li, X. Impact of Clear Aligners on Oral Health and Oral Microbiome During Orthodontic Treatment. Int. Dent. J. 2023, 73, 603–611. [Google Scholar] [CrossRef]
- Moghanian, A.; Asadi, P.; Akbari, M.; Mohammad Aliha, M.R.; Kizilkurtlu, A.A.; Akpek, A.; Safaee, S. New Trends in 3D and 4D Printed Dental and Orthopedic Implants: Methods, Applications and Future Directions. Bioprinting 2025, 48, e00406. [Google Scholar] [CrossRef]
- Ehrmann, G.; Ehrmann, A. 3D Printing of Shape Memory Polymers. J. Appl. Polym. Sci. 2021, 138, 50847. [Google Scholar] [CrossRef]
- Tang, Z.; Gong, J.; Cao, P.; Tao, L.; Pei, X.; Wang, T.; Zhang, Y.; Wang, Q.; Zhang, J. 3D Printing of a Versatile Applicability Shape Memory Polymer with High Strength and High Transition Temperature. Chem. Eng. J. 2022, 431, 134211. [Google Scholar] [CrossRef]
- Alkhamees, A. The New Additive Era of Orthodontics: 3D-Printed Aligners and Shape Memory Polymers—The Latest Trend—And Their Environmental Implications. J. Orthod. Sci. 2024, 13, 55. [Google Scholar] [CrossRef]
- Bichu, Y.M.; Alwafi, A.; Liu, X.; Andrews, J.; Ludwig, B.; Bichu, A.Y.; Zou, B. Advances in Orthodontic Clear Aligner Materials. Bioact. Mater. 2023, 22, 384–403. [Google Scholar] [CrossRef]
- Atta, I.; Bourauel, C.; Alkabani, Y.; Mohamed, N.; Kim, H.; Alhotan, A.; Ghoneima, A.; Elshazly, T. Physiochemical and Mechanical Characterisation of Orthodontic 3D Printed Aligner Material Made of Shape Memory Polymers (4D Aligner Material). J. Mech. Behav. Biomed. Mater. 2024, 150, 106337. [Google Scholar] [CrossRef] [PubMed]
- Chistyakov, E.M.; Kolpinskaya, N.; Posokhova, V.; Chuev, V. Dental Composition Modified with Aryloxyphosphazene Containing Carboxyl Groups. Polymers 2020, 12, 1176. [Google Scholar] [CrossRef] [PubMed]
- Kireev, V.V.; Chistyakov, E.M.; Filatov, S.N.; Tupikov, A.S.; Panfilova, D.V.; Chetverikova, A.I. Polymeric Dental Composites Modified with Carboxy Phosphazene Methacrylates. Russ. J. Appl. Chem. 2015, 88, 866–870. [Google Scholar] [CrossRef]
- Kostić, M.; Igić, M.; Gligorijević, N.; Nikolić, V.; Stošić, N.; Nikolić, L. The Use of Acrylate Polymers in Dentistry. Polymers 2022, 14, 4511. [Google Scholar] [CrossRef]
- Gupta, D.; Boora, A.; Thakur, A.; Gupta, T.K. Green and Sustainable Synthesis of Nanomaterials: Recent Advancements and Limitations. Environ. Res. 2023, 231, 116316. [Google Scholar] [CrossRef]
- Bonilla-Represa, V.; Abalos-Labruzzi, C.; Herrera-Martinez, M.; Guerrero-Pérez, M.O. Nanomaterials in Dentistry: State of the Art and Future Challenges. Nanomaterials 2020, 10, 1770. [Google Scholar] [CrossRef]
- Reyes-Carmona, L.; Camps, E.; Campos-González, E.; Mercado-Celis, G.; Cervantes-Garduño, A.; Pérez-Ibarra, E.A.; Álvarez-Chimal, R.; Rodil, S.E.; Almaguer-Flores, A. Antimicrobial Evaluation of Bismuth Subsalicylate Nanoparticles Synthesized by Laser Ablation against Clinical Oral Microorganisms. Opt. Laser Technol. 2023, 158, 108930. [Google Scholar] [CrossRef]
- Schmalz, G.; Hickel, R.; van Landuyt, K.L.; Reichl, F.-X. Scientific Update on Nanoparticles in Dentistry. Int. Dent. J. 2018, 68, 299–305. [Google Scholar] [CrossRef]
- Bourgi, R.; Doumandji, Z.; Cuevas-Suárez, C.E.; Ben Ammar, T.; Laporte, C.; Kharouf, N.; Haikel, Y. Exploring the Role of Nanoparticles in Dental Materials: A Comprehensive Review. Coatings 2025, 15, 33. [Google Scholar] [CrossRef]
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and Its Importance for Human Health: An Integrative Review. J. Res. Med. Sci. 2013, 18, 144–157. [Google Scholar] [PubMed]
- Chiriac, V.; Stratulat, D.N.; Calin, G.; Nichitus, S.; Burlui, V.; Stadoleanu, C.; Popa, M.; Popa, I.M. Antimicrobial Property of Zinc Based Nanoparticles. IOP Conf. Ser. Mater. Sci. Eng. 2016, 133, 012055. [Google Scholar] [CrossRef]
- Pushpalatha, C.; Suresh, J.; Gayathri, V.; Sowmya, S.; Augustine, D.; Alamoudi, A.; Zidane, B.; Mohammad Albar, N.H.; Patil, S. Zinc Oxide Nanoparticles: A Review on Its Applications in Dentistry. Front. Bioeng. Biotechnol. 2022, 10, 917990. [Google Scholar] [CrossRef] [PubMed]
- Gharibnavaz, M.; Arash, V.; Pournajaf, A.; Najafi, F.; Rahmati Kamel, M.; Seyedmajidi, S. Study on the Antibacterial Properties and Optical Characteristics of Clear Orthodontic Aligners Coated with Zinc Oxide and Magnesium Oxide Nanoparticles. Orthod. Craniofacial Res. 2025, 28, 496–506. [Google Scholar] [CrossRef]
- Alshameri, A.W.; Owais, M. Antibacterial and Cytotoxic Potency of the Plant-Mediated Synthesis of Metallic Nanoparticles Ag NPs and ZnO NPs: A Review. OpenNano 2022, 8, 100077. [Google Scholar] [CrossRef]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Scale-up Polymeric-Based Nanoparticles Drug Delivery Systems: Development and Challenges. OpenNano 2022, 7, 100048. [Google Scholar] [CrossRef]
- Ke, C.-L.; Deng, F.-S.; Chuang, C.-Y.; Lin, C.-H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential Oils as Antimicrobial Agents—Myth or Real Alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef]
- Álvarez-Chimal, R.; García-Pérez, V.I.; Álvarez-Pérez, M.A.; Arenas-Alatorre, J.Á. Green Synthesis of ZnO Nanoparticles Using a Dysphania Ambrosioides Extract. Structural Characterization and Antibacterial Properties. Mater. Sci. Eng. C 2021, 118, 111540. [Google Scholar] [CrossRef]
- Aydin Sevinç, B.; Hanley, L. Antibacterial Activity of Dental Composites Containing Zinc Oxide Nanoparticles. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94B, 22–31. [Google Scholar] [CrossRef]
- Puspitasari, R.; Irnawati, D.; Widjijono. The Effect of Zinc Oxide (ZnO) Nanoparticle Concentration on the Adhesion of Mucin and Streptococcus mutans to Heat-Cured Acrylic Resin. Dent. Mater. J. 2023, 42, 791–799. [Google Scholar] [CrossRef]
- Carbajal-Casique, M.N.; Prez-Snchez, L.; Alvarez-Perez, M.A.; Rayo-Lpez, G.D.; Reyes-Esqueda, J.-A.; Marichi-Rodrguez, F.; Bello, J.S. Influence of White Pigment on the Properties of Polylactic Acid Scaffolds for 3D Printing: In Vitro and in Vivo Analysis. AIMS Bioeng. 2025, 12, 225–248. [Google Scholar] [CrossRef]
- Meraat, R.; Ziabari, A.A.; Issazadeh, K.; Shadan, N.; Jalali, K.M. Synthesis and Characterization of the Antibacterial Activity of Zinc Oxide Nanoparticles against Salmonella typhi. Acta Metall. Sin. Engl. Lett. 2016, 29, 601–608. [Google Scholar] [CrossRef]
- Álvarez-Chimal, R.; García-Pérez, V.I.; Álvarez-Pérez, M.A.; Tavera-Hernández, R.; Reyes-Carmona, L.; Martínez-Hernández, M.; Arenas-Alatorre, J.Á. Influence of the Particle Size on the Antibacterial Activity of Green Synthesized Zinc Oxide Nanoparticles Using Dysphania Ambrosioides Extract, Supported by Molecular Docking Analysis. Arab. J. Chem. 2022, 15, 103804. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kang, Y.; Yin, S.; Song, B.; Wei, L.; Chen, L.; Shao, L. Zinc Oxide Nanoparticles Induce Toxic Responses in Human Neuroblastoma SHSY5Y Cells in a Size-Dependent Manner. Int. J. Nanomed. 2017, 12, 8085–8099. [Google Scholar] [CrossRef]
- Shen, C.; James, S.A.; de Jonge, M.D.; Turney, T.W.; Wright, P.F.A.; Feltis, B.N. Relating Cytotoxicity, Zinc Ions, and Reactive Oxygen in ZnO Nanoparticle–Exposed Human Immune Cells. Toxicol. Sci. 2013, 136, 120–130. [Google Scholar] [CrossRef]
- Maheswaran, H.; Djearamane, S.; Tanislaus Antony Dhanapal, A.C.; Wong, L.S. Cytotoxicity of Green Synthesized Zinc Oxide Nanoparticles Using Musa Acuminata on Vero Cells. Heliyon 2024, 10, e31316. [Google Scholar] [CrossRef]
- Liao, C.; Jin, Y.; Li, Y.; Tjong, S.C. Interactions of Zinc Oxide Nanostructures with Mammalian Cells: Cytotoxicity and Photocatalytic Toxicity. Int. J. Mol. Sci. 2020, 21, 6305. [Google Scholar] [CrossRef]
- Tada-Oikawa, S.; Ichihara, G.; Suzuki, Y.; Izuoka, K.; Wu, W.; Yamada, Y.; Mishima, T.; Ichihara, S. Zn(II) Released from Zinc Oxide Nano/Micro Particles Suppresses Vasculogenesis in Human Endothelial Colony-Forming Cells. Toxicol. Rep. 2015, 2, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yang, H.; Archana, G.; Rakshit, M.; Ng, K.W.; Tay, C.Y. Human Keratinocytes Adapt to ZnO Nanoparticles Induced Toxicity via Complex Paracrine Crosstalk and Nrf2-Proteasomal Signal Transduction. Nanotoxicology 2018, 12, 1215–1229. [Google Scholar] [CrossRef] [PubMed]
- Moos, P.J.; Olszewski, K.; Honeggar, M.; Cassidy, P.; Leachman, S.; Woessner, D.; Cutler, N.S.; Veranth, J.M. Responses of Human Cells to ZnO Nanoparticles: A Gene Transcription Study. Metallomics 2011, 3, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.S.; Subramanian, A.S.; Su, P.-C. Zinc Oxide Nanoparticles as Additives for Improved Dimensional Accuracy in Vat Photopolymerization. Addit. Manuf. 2022, 59, 103118. [Google Scholar] [CrossRef]
- Quintero-Quiroz, C.; Acevedo, N.; Zapata-Giraldo, J.; Botero, L.E.; Quintero, J.; Zárate-Triviño, D.; Saldarriaga, J.; Pérez, V.Z. Optimization of Silver Nanoparticle Synthesis by Chemical Reduction and Evaluation of Its Antimicrobial and Toxic Activity. Biomater. Res. 2019, 23, 27. [Google Scholar] [CrossRef]
- Li, J.; Wu, Q.; Wu, J. Synthesis of Nanoparticles via Solvothermal and Hydrothermal Methods. In Handbook of Nanoparticles; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–28. [Google Scholar]
- Takai, T.; Shibatani, A.; Asakuma, Y.; Saptoro, A.; Phan, C. Microwave-Assisted Nanoparticle Synthesis Enhanced with Addition of Surfactant. Chem. Eng. Res. Des. 2022, 182, 714–718. [Google Scholar] [CrossRef]
- Mountrichas, G.; Pispas, S.; Kamitsos, E.I. Effect of Temperature on the Direct Synthesis of Gold Nanoparticles Mediated by Poly(Dimethylaminoethyl Methacrylate) Homopolymer. J. Phys. Chem. C 2014, 118, 22754–22759. [Google Scholar] [CrossRef]
- Nie, P.; Zhao, Y.; Xu, H. Synthesis, Applications, Toxicity and Toxicity Mechanisms of Silver Nanoparticles: A Review. Ecotoxicol. Environ. Saf. 2023, 253, 114636. [Google Scholar] [CrossRef]
- Anees, A.; Gupta, R.; Phanindra, P.V.; Fabiyi, O.A.; Thera, U.K.; Bello, T.T.; Ahmad, F. Green Synthesis of Nanoparticles and Applications. In Advanced Nanotechnology in Plants; CRC Press: Boca Raton, FL, USA, 2024; pp. 89–122. [Google Scholar]
- Álvarez-Chimal, R.; Ángel Arenas-Alatorre, J. Green Synthesis of Nanoparticles. A Biological Approach. In Advances in Green Chemistry [Working Title]; IntechOpen: London, UK, 2023. [Google Scholar]
- Altammar, K.A. A Review on Nanoparticles: Characteristics, Synthesis, Applications, and Challenges. Front. Microbiol. 2023, 14, 1155622. [Google Scholar] [CrossRef]
- Kumari, S.; Raturi, S.; Kulshrestha, S.; Chauhan, K.; Dhingra, S.; András, K.; Thu, K.; Khargotra, R.; Singh, T. A Comprehensive Review on Various Techniques Used for Synthesizing Nanoparticles. J. Mater. Res. Technol. 2023, 27, 1739–1763. [Google Scholar] [CrossRef]
- Vetchinkina, E.; Loshchinina, E.; Kupryashina, M.; Burov, A.; Pylaev, T.; Nikitina, V. Green Synthesis of Nanoparticles with Extracellular and Intracellular Extracts of Basidiomycetes. PeerJ 2018, 6, e5237. [Google Scholar] [CrossRef]
- kazemi, S.; Hosseingholian, A.; Gohari, S.D.; Feirahi, F.; Moammeri, F.; Mesbahian, G.; Moghaddam, Z.S.; Ren, Q. Recent Advances in Green Synthesized Nanoparticles: From Production to Application. Mater. Today Sustain. 2023, 24, 100500. [Google Scholar] [CrossRef]
- Król, A.; Railean-Plugaru, V.; Pomastowski, P.; Buszewski, B. Phytochemical Investigation of Medicago sativa L. Extract and Its Potential as a Safe Source for the Synthesis of ZnO Nanoparticles: The Proposed Mechanism of Formation and Antimicrobial Activity. Phytochem. Lett. 2019, 31, 170–180. [Google Scholar] [CrossRef]
- Patil, B.N.; Taranath, T.C. Limonia acidissima L. Leaf Mediated Synthesis of Zinc Oxide Nanoparticles: A Potent Tool against Mycobacterium tuberculosis. Int. J. Mycobacteriol. 2016, 5, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Ezealisiji, K.M.; Siwe-Noundou, X.; Maduelosi, B.; Nwachukwu, N.; Krause, R.W.M. Green Synthesis of Zinc Oxide Nanoparticles Using Solanum torvum (L) Leaf Extract and Evaluation of the Toxicological Profile of the ZnO Nanoparticles–Hydrogel Composite in Wistar Albino Rats. Int. Nano Lett. 2019, 9, 99–107. [Google Scholar] [CrossRef]
- Fahaduddin; Bal, T. Invitro- Invivo Evaluations of Green Synthesized Zinc Oxide (ZnO) Nanoparticles Using Ipomoea Aquatica Leaf Extract as Matric and Fillers. J. Mech. Behav. Biomed. Mater. 2024, 150, 106330. [Google Scholar] [CrossRef]
- Taher, B.B.; Rasheed, T.A. The Impact of Adding Chitosan Nanoparticles on Biofilm Formation, Cytotoxicity, and Certain Physical and Mechanical Aspects of Directly Printed Orthodontic Clear Aligners. Nanomaterials 2023, 13, 2649. [Google Scholar] [CrossRef]
- Vijayaram, S.; Razafindralambo, H.; Sun, Y.-Z.; Vasantharaj, S.; Ghafarifarsani, H.; Hoseinifar, S.H.; Raeeszadeh, M. Applications of Green Synthesized Metal Nanoparticles—A Review. Biol. Trace Elem. Res. 2024, 202, 360–386. [Google Scholar] [CrossRef]
- Ravi, I.; Kailasam, V. Assessment of Cytotoxicity of Clear Aligners Coated with Zinc Oxide Nanoparticles. J. Oral Biol. Craniofacial Res. 2025, 15, 262–265. [Google Scholar] [CrossRef]
- Anita, P.; Sathyanarayana, H.P.; Kumar, K.; Ramanathan, K.; Kailasam, V. Antimicrobial Efficacy of Zinc Oxide Nanoparticle-Coated Aligners on Streptococcus mutans and Candida albicans. Am. J. Orthod. Dentofac. Orthop. 2023, 163, 338–346. [Google Scholar] [CrossRef]
- Can, E.; Panayi, N.; Polychronis, G.; Papageorgiou, S.N.; Zinelis, S.; Eliades, G.; Eliades, T. In-House 3D-Printed Aligners: Effect of in Vivo Ageing on Mechanical Properties. Eur. J. Orthod. 2022, 44, 51–55. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, H.; Kim, H.-J.; Chung, C.J.; Choi, Y.J.; Kim, S.-J.; Cha, J.-Y. Thermo-Mechanical Properties of 3D Printed Photocurable Shape Memory Resin for Clear Aligners. Sci. Rep. 2022, 12, 6246. [Google Scholar] [CrossRef]
- Tadge, T.; Garje, S.; Saxena, V.; Raichur, A.M. Application of Shape Memory and Self-Healable Polymers/Composites in the Biomedical Field: A Review. ACS Omega 2023, 8, 32294–32310. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, R.O.; Chirani, N. Shape-Memory Polymers for Dental Applications. In Shape Memory Polymers for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2015; pp. 267–280. [Google Scholar]
- Aati, S.; Akram, Z.; Ngo, H.; Fawzy, A.S. Development of 3D Printed Resin Reinforced with Modified ZrO2 Nanoparticles for Long-Term Provisional Dental Restorations. Dent. Mater. 2021, 37, e360–e374. [Google Scholar] [CrossRef] [PubMed]
- Alshamrani, A.; Alhotan, A.; Kelly, E.; Ellakwa, A. Mechanical and Biocompatibility Properties of 3D-Printed Dental Resin Reinforced with Glass Silica and Zirconia Nanoparticles: In Vitro Study. Polymers 2023, 15, 2523. [Google Scholar] [CrossRef] [PubMed]
- Yüceer, Ö.M.; Kaynak Öztürk, E.; Çiçek, E.S.; Aktaş, N.; Bankoğlu Güngör, M. Three-Dimensional-Printed Photopolymer Resin Materials: A Narrative Review on Their Production Techniques and Applications in Dentistry. Polymers 2025, 17, 316. [Google Scholar] [CrossRef]
- Majeed, H.F.; Hamad, T.I.; Bairam, L.R. Enhancing 3D-Printed Denture Base Resins: A Review of Material Innovations. Sci. Prog. 2024, 107, 1–20. [Google Scholar] [CrossRef]
- Sardari, S.; Hheidari, A.; Ghodousi, M.; Rahi, A.; Pishbin, E. Nanotechnology in Tissue Engineering: Expanding Possibilities with Nanoparticles. Nanotechnology 2024, 35, 392002. [Google Scholar] [CrossRef]
- Alexander, R.; Liu, X. Soft Tissue Integration around Dental Implants: A Pressing Priority. Biomaterials 2025, 324, 123491. [Google Scholar] [CrossRef]
- Majhy, B.; Priyadarshini, P.; Sen, A.K. Effect of Surface Energy and Roughness on Cell Adhesion and Growth—Facile Surface Modification for Enhanced Cell Culture. RSC Adv. 2021, 11, 15467–15476. [Google Scholar] [CrossRef]
- Rachtanapun, C.; Sukunta, J.; Jantanasakulwong, K.; Thanakkasaranee, S.; Tantala, J.; Rachtanapun, P. Enhancing Antimicrobial Properties and Hydrophilicity of Environmentally Biodegradable Plastic Using Sparking-Deposited ZnO Nanoparticles. Food Biosci. 2025, 71, 107077. [Google Scholar] [CrossRef]
- Lam, W.T.; Babra, T.S.; Smith, J.H.D.; Bagley, M.C.; Spencer, J.; Wright, E.; Greenland, B.W. Synthesis and Evaluation of a Silver Nanoparticle/Polyurethane Composite That Exhibits Antiviral Activity against SARS-CoV-2. Polymers 2022, 14, 4172. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, J.; Li, Z.; Huang, J.; Wu, J.; Zhang, Y.; Ge, H.; Zhao, Y. Antibacterial Effect of Low-Concentration ZnO Nanoparticles on Sulfate-Reducing Bacteria under Visible Light. Nanomaterials 2023, 13, 2033. [Google Scholar] [CrossRef]
- Poppolo Deus, F.; Ouanounou, A. Chlorhexidine in Dentistry: Pharmacology, Uses, and Adverse Effects. Int. Dent. J. 2022, 72, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef] [PubMed]
- More, P.R.; Pandit, S.; Filippis, A.D.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver Nanoparticles: Bactericidal and Mechanistic Approach against Drug Resistant Pathogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef] [PubMed]
- Balderrama-González, A.-S.; Piñón-Castillo, H.-A.; Ramírez-Valdespino, C.-A.; Landeros-Martínez, L.-L.; Orrantia-Borunda, E.; Esparza-Ponce, H.-E. Antimicrobial Resistance and Inorganic Nanoparticles. Int. J. Mol. Sci. 2021, 22, 12890. [Google Scholar] [CrossRef]
- Girma, A.; Mebratie, G.; Mekuye, B.; Abera, B.; Bekele, T.; Alamnie, G. Antibacterial Capabilities of Metallic Nanoparticles and Influencing Factors. Nano Sel. 2024, 5, e202400049. [Google Scholar] [CrossRef]
- Anees Ahmad, S.; Sachi Das, S.; Khatoon, A.; Tahir Ansari, M.; Afzal, M.; Saquib Hasnain, M.; Kumar Nayak, A. Bactericidal Activity of Silver Nanoparticles: A Mechanistic Review. Mater. Sci. Energy Technol. 2020, 3, 756–769. [Google Scholar] [CrossRef]
- López de Dicastillo, C.; Guerrero Correa, M.; Martínez, F.B.; Streitt, C.; José Galotto, M. Antimicrobial Effect of Titanium Dioxide Nanoparticles. In Antimicrobial Resistance—A One Health Perspective; IntechOpen: London, UK, 2021. [Google Scholar]
- Derchi, G.; Vano, M.; Barone, A.; Covani, U.; Diaspro, A.; Salerno, M. Bacterial Adhesion on Direct and Indirect Dental Restorative Composite Resins: An in Vitro Study on a Natural Biofilm. J. Prosthet. Dent. 2017, 117, 669–676. [Google Scholar] [CrossRef]
- Gudmundsson, J.T. Physics and Technology of Magnetron Sputtering Discharges. Plasma Sources Sci. Technol. 2020, 29, 113001. [Google Scholar] [CrossRef]
- Garg, R.; Gonuguntla, S.; Sk, S.; Iqbal, M.S.; Dada, A.O.; Pal, U.; Ahmadipour, M. Sputtering Thin Films: Materials, Applications, Challenges and Future Directions. Adv. Colloid Interface Sci. 2024, 330, 103203. [Google Scholar] [CrossRef]
- Bahari, L.A.; Javadzadeh, Y.; Jalali, M.B.; Johari, P.; Nokhodchi, A.; Shokri, J. Nano-Suspension Coating as a Technique to Modulate the Drug Release from Controlled Porosity Osmotic Pumps for a Soluble Agent. Colloids Surf. B Biointerfaces 2017, 153, 27–33. [Google Scholar] [CrossRef]
- Pawlowski, L. Suspension and Solution Thermal Spray Coatings. Surf. Coat. Technol. 2009, 203, 2807–2829. [Google Scholar] [CrossRef]
- Yeadon, K.; Lai, E.P.C.; Huang, X.; Song, N. Influence of Surface Roughness and Metal Oxide Nanoparticles on Airframe with Icephobic Coatings. RSC Appl. Interfaces 2025, 2, 82–93. [Google Scholar] [CrossRef]
- Yoga, I.G.K.M.; Harsini, H.; Sunarintyas, S.; Handajani, J.; Nuryono, N.; Irnawati, D. Effect of Zinc Oxide Nanoparticle Concentration Coated on Acrylic Resin upon Surface Roughness and Abrasion Resistance. Maj. Kedokt. Gigi Indones. 2024, 10, 55. [Google Scholar] [CrossRef]

| Sample | (Ra, µm) |
|---|---|
| Uncoated resin disc | 0.65 ± 0.25 |
| Resin disc coated with 10% ZnO NPs | 1.98 ± 0.02 |
| Resin disc coated with 20% ZnO NPs | 5.73 ± 1.08 |
| Resin disc coated with 30% ZnO NPs | 8.10 ± 0.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teramoto-lida, A.; Álvarez-Chimal, R.; Reyes-Carmona, L.; Álvarez-Pérez, M.A.; Pozos-Guillen, A.; Vázquez-Vázquez, F.C. 3D Printing of Shape Memory Resin for Orthodontic Aligners with Green Synthesized Antimicrobial ZnO Nanoparticles Coatings: Toward Bioactive Devices. Bioengineering 2025, 12, 1193. https://doi.org/10.3390/bioengineering12111193
Teramoto-lida A, Álvarez-Chimal R, Reyes-Carmona L, Álvarez-Pérez MA, Pozos-Guillen A, Vázquez-Vázquez FC. 3D Printing of Shape Memory Resin for Orthodontic Aligners with Green Synthesized Antimicrobial ZnO Nanoparticles Coatings: Toward Bioactive Devices. Bioengineering. 2025; 12(11):1193. https://doi.org/10.3390/bioengineering12111193
Chicago/Turabian StyleTeramoto-lida, Airy, Rafael Álvarez-Chimal, Lorena Reyes-Carmona, Marco Antonio Álvarez-Pérez, Amaury Pozos-Guillen, and Febe Carolina Vázquez-Vázquez. 2025. "3D Printing of Shape Memory Resin for Orthodontic Aligners with Green Synthesized Antimicrobial ZnO Nanoparticles Coatings: Toward Bioactive Devices" Bioengineering 12, no. 11: 1193. https://doi.org/10.3390/bioengineering12111193
APA StyleTeramoto-lida, A., Álvarez-Chimal, R., Reyes-Carmona, L., Álvarez-Pérez, M. A., Pozos-Guillen, A., & Vázquez-Vázquez, F. C. (2025). 3D Printing of Shape Memory Resin for Orthodontic Aligners with Green Synthesized Antimicrobial ZnO Nanoparticles Coatings: Toward Bioactive Devices. Bioengineering, 12(11), 1193. https://doi.org/10.3390/bioengineering12111193










