The Effects of Virtual Reality Interventions on Motor Function Rehabilitation in Lower-Limb Amputees: A Systematic Review and Metanalysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection and Literature Data Extraction
2.4. Literature Quality Assessment
2.5. Risk of Bias Analysis
2.6. Meta-Analysis
3. Results
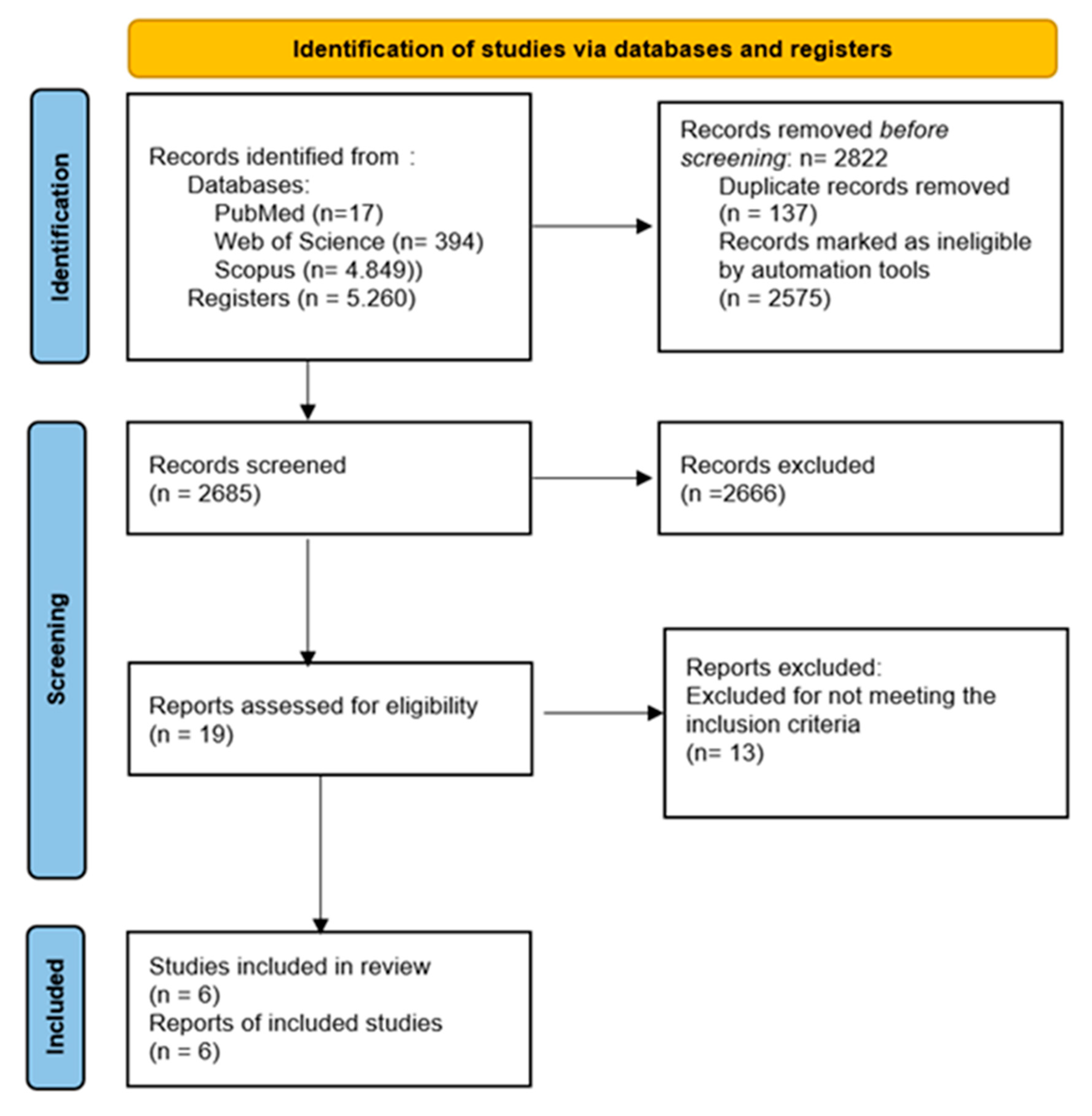
3.1. Study Selection
3.2. Study Characteristics
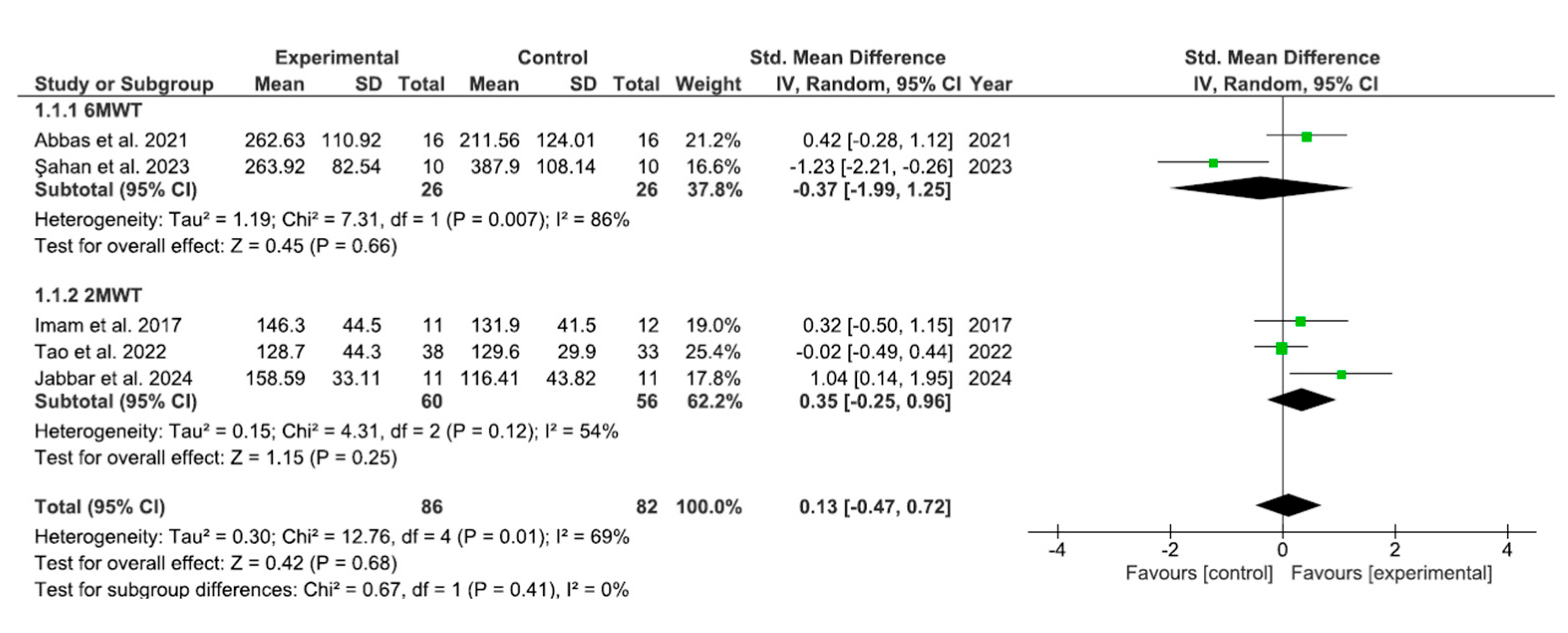
3.3. Results of Meta-Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nowygrod, R.; Egorova, N.; Greco, G.; Anderson, P.; Gelijns, A.; Moskowitz, A.; McKinsey, J.; Morrissey, N.; Kent, K.C. Trends, complications, and mortality in peripheral vascular surgery. J. Vasc. Surg. 2006, 43, 205–216. [Google Scholar] [CrossRef]
- Bernatchez, J.; Mayo, A.; Kayssi, A. The epidemiology of lower extremity amputations, strategies for amputation prevention, and the importance of patient-centered care. Semin. Vasc. Surg. 2021, 34, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Rivera, J.A.; Churovich, K.; Anderson, A.B.; Potter, B.K. Estimating recent US limb loss prevalence and updating future projections. Arch. Rehabil. Res. Clin. Transl. 2024, 6, 100376. [Google Scholar] [CrossRef] [PubMed]
- Chopra, A.; Azarbal, A.F.; Jung, E.; Abraham, C.Z.; Liem, T.K.; Landry, G.J.; Moneta, G.L.; Mitchell, E.L. Ambulation and functional outcome after major lower extremity amputation. J. Vasc. Surg. 2018, 67, 1521–1529. [Google Scholar] [CrossRef]
- Peters, E.J.; Childs, M.R.; Wunderlich, R.P.; Harkless, L.B.; Armstrong, D.G.; Lavery, L.A. Functional status of persons with diabetes-related lower-extremity amputations. Diabetes Care 2001, 24, 1799–1804. [Google Scholar] [CrossRef]
- Dillingham, T.R.; Pezzin, L.E.; MacKenzie, E.J. Discharge destination after dysvascular lower-limb amputations. Arch. Phys. Med. Rehabil. 2003, 84, 1662–1668. [Google Scholar] [CrossRef]
- Howard, M.C. A meta-analysis and systematic literature review of virtual reality rehabilitation programs. Comput. Hum. Behav. 2017, 70, 317–327. [Google Scholar] [CrossRef]
- Yoo, Y.J.; Lim, S.H. Assessment of Lower Limb Motor Function, Ambulation, and Balance After Stroke. Brain Neurorehabilit. 2022, 15, e17. [Google Scholar] [CrossRef]
- Asadzadeh, A.; Samad-Soltani, T.; Salahzadeh, Z.; Rezaei-Hachesu, P. Effectiveness of virtual reality-based exercise therapy in rehabilitation: A scoping review. Inform. Med. Unlocked 2021, 24, 100562. [Google Scholar] [CrossRef]
- Hao, J.; Chen, Z.; Remis, A.; He, Z. Virtual Reality-Based Rehabilitation to Restore Motor Function in People With Amputation: A Systematic Literature Review. Am. J. Phys. Med. Rehabil. 2023, 102, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Hali, K.; Manzo, M.A.; Koucheki, R.; Wunder, J.S.; Jenkinson, R.J.; Mayo, A.L.; Ferguson, P.C.; Lex, J.R. Use of virtual reality for the management of phantom limb pain: A systematic review. Disabil. Rehabil. 2024, 46, 629–636. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef]
- Schultheis, M.T.; Rizzo, A.A. The application of virtual reality technology in rehabilitation. Rehabil. Psychol. 2001, 46, 296–311. [Google Scholar] [CrossRef]
- Kalron, A.; Fonkatz, I.; Frid, L.; Baransi, H.; Achiron, A. The effect of balance training on postural control in people with multiple sclerosis using the CAREN virtual reality system: A pilot randomized controlled trial. J. Neuroeng. Rehabil. 2016, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Neir, C.; Sandin, D.; DeFanti, T. Surround-screen projection-based virtual reality: The design and implementation of the CAVE. In Proceedings of the SIGGRAPH’93: Proceedings of the 20th annual conference on Computer graphics and interactive techniques, Anaheim, CA, USA, 2–6 August 1993; pp. 135–142. [Google Scholar] [CrossRef]
- Ma, M.; Zheng, H. Virtual reality and serious games in healthcare. Stud. Comput. Intell. 2011, 337, 169–192. [Google Scholar]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Cochrane Training. Review Manager (RevMan) [Computer Program], Version 5.4; The Cochrane Collaboration: London, UK, 2020. Available online: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman (accessed on 21 July 2025).
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions version 6.2. 2021. Available online: https://www.training.cochrane.org/handbook (accessed on 21 July 2025).
- Imam, B.; Miller, W.C.; Finlayson, H.; Eng, J.J.; Jarus, T. A randomized controlled trial to evaluate the feasibility of the Wii Fit for improving walking in older adults with lower limb amputation. Clin. Rehabil. 2017, 31, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Abbas, R.L.; Cooreman, D.; Al Sultan, H.; El Nayal, M.; Saab, I.M.; El Khatib, A. The Effect of Adding Virtual Reality Training on Traditional Exercise Program on Balance and Gait in Unilateral, Traumatic Lower Limb Amputee. Games Health J. 2021, 10, 50–56, Erratum for: Games Health J. 2021, 10, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Tao, G.; Miller, W.C.; Eng, J.J.; Esfandiari, E.; Imam, B.; Lindstrom, H.; Payne, M.W. Group-based telerehabilitation intervention using Wii Fit to improve walking in older adults with lower limb amputation (WiiNWalk): A randomized control trial. Clin. Rehabil. 2022, 36, 331–341. [Google Scholar] [CrossRef]
- Şahan, T.Y.; Erbahçeci, F. Effects of Virtual Reality on Transtibial Amputation Rehabilitation Outcomes: A Randomized Study. Games Health J. 2023, 12, 459–467. [Google Scholar] [CrossRef]
- Jabbar, M.; Ashnagar, Z.; Hadian, M.R.; Jalaie, S.; Talebian Moghadam, S. Investigating the Effects of Exergame Training on Functional Activities Among Newly-Fitted Patients with Unilateral Transtibial Amputation: A Preliminary Study. J. Mod. Rehabil. 2024, 18, 371–382. [Google Scholar] [CrossRef]
- Steckel, B.M.; Schwertner, R.; Bücker, J.; Nazareth, A.C.P.; Bizarro, L.; Oliveira, A.A. Immersive virtual reality applied to the rehabilitation of patients with lower limb amputation: A small randomized controlled trial for feasibility study. Virtual Real. 2024, 28, 115. [Google Scholar] [CrossRef]
- Jones, W.S.; Patel, M.R.; Dai, D.; Subherwal, S.; Stafford, J.; Calhoun, S.; Peterson, E.D. Temporal trends and geographic variation of lower-extremity amputation in patients with peripheral artery disease: Results from U.S. Medicare 2000–2008. J Am Coll Cardiol 2012, 60, 2230–2236. [Google Scholar] [CrossRef] [PubMed]
- Dillingham, T.R.; Pezzin, L.E.; MacKenzie, E.J. Limb amputation and limb deficiency: Epidemiology and recent trends in the United States. South. Med. J. 2002, 95, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Ihmels, W.D.; Miller, R.H.; Russell Esposito, E. Residual limb strength and functional performance measures in individuals with unilateral transtibial amputation. Gait Posture 2022, 97, 159–164. [Google Scholar] [CrossRef]
- Montilla-Ibáñez, A.; Martínez-Amat, A.; Lomas-Vega, R.; Cruz-Díaz, D.; De la Torre-Cruz, M.J.; Casuso-Pérez, R.; Hita-Contreras, F. The Activities-specific Balance Confidence scale: Reliability and validity in Spanish patients with vestibular disorders. Disabil. Rehabil. 2017, 39, 697–703. [Google Scholar] [CrossRef]
- De Keersmaecker, E.; Van Bladel, A.; Zaccardi, S.; Lefeber, N.; Rodriguez-Guerrero, C.; Kerckhofs, E.; Jansen, B.; Swinnen, E. Virtual reality-enhanced walking in people post-stroke: Effect of optic flow speed and level of immersion on the gait biomechanics. J. Neuroeng. Rehabil. 2023, 20, 124. [Google Scholar] [CrossRef]
- Ghai, S.; Ghai, I.; Lamontagne, A. Virtual reality training enhances gait poststroke: A systematic review and meta-analysis. Ann. New York Acad. Sci. 2020, 1478, 18–42. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.; Kim, K. Gait Training with Virtual Reality-Based Real-Time Feedback for Chronic Post-Stroke Patients: A Pilot Study. Healthcare 2025, 13, 203. [Google Scholar] [CrossRef] [PubMed]
- Wezenberg, D.; van der Woude, L.H.; Faber, W.X.; de Haan, A.; Houdijk, H. Relation between aerobic capacity and walking ability in older adults with a lower-limb amputation. Arch. Phys. Med. Rehabil. 2013, 94, 1714–1720. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, F.; Martin Ginis, K.A.; MacKay, C.; Best, K.L.; Blanchette, V.; Cherif, A.; Robert, M.T.; Miller, W.C.; Gee, C.; Habra, N.; et al. Do Exercise Programs Improve Fitness, Mobility, and Functional Capacity in Adults With Lower Limb Amputation? A Systematic Review on the Type and Minimal Dose Needed. Arch. Phys. Med. Rehabil. 2024, 105, 1194–1211. [Google Scholar] [CrossRef]
- Chin, T.; Sawamura, S.; Fujita, H.; Nakajima, S.; Ojima, I.; Oyabu, H.; Nakagawa, A. Effect of endurance training program based on anaerobic threshold (AT) for lower limb amputees. J. Rehabil. Res. Dev. 2001, 38, 7–11. [Google Scholar]
- Ross, R.E.; Hart, E.; Williams, E.R.; Gregory, C.M.; Flume, P.A.; Mingora, C.M.; Woodbury, M.L. Combined Aerobic Exercise and Virtual Reality-Based Upper Extremity Rehabilitation Intervention for Chronic Stroke: Feasibility and Preliminary Effects on Physical Function and Quality of Life. Arch. Rehabil. Res. Clin. Transl. 2023, 5, 100244. [Google Scholar] [CrossRef]
- Chen, J.; Yan, S.; Yin, H.; Lin, D.; Mei, Z.; Ding, Z.; Wang, M.; Bai, Y.; Xu, G. Virtual reality technology improves the gait and balance function of the elderly: A meta-analysis of randomized controlled trials. Arch. Med Sci. 2024, 20, 1918–1929. [Google Scholar] [CrossRef]
- Abou, L.; Domohina Malala, V.; Yarnot, R.; Alluri, A.; Rice, L.A. Effects of Virtual Reality Therapy on Gait and Balance Among Individuals With Spinal Cord Injury: A Systematic Review and Meta-analysis. Neurorehabil. Neural Repair 2020, 34, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Rishi, P.; Sivach, P. The Effectiveness of Virtual Reality-Based Rehabilitation Versus Conventional Methods in Enhancing Functional Outcomes for Post-Operative Lower Limb Patients: A Systematic Review. Musculoskelet. Care 2025, 23, e70061. [Google Scholar] [CrossRef]
- Chen, L.; Ambrose Lo, W.L.; Mao, Y.R.; Ding, M.H.; Lin, Q.; Li, H.; Zhao, J.L.; Xu, Z.Q.; Bian, R.H.; Huang, D.F. Effect of Virtual Reality on Postural and Balance Control in Patients with Stroke: A Systematic Literature Review. Biomed. Res. Int. 2016, 2016, 7309272. [Google Scholar] [CrossRef]
- Paladugu, P.; Kumar, R.; Ong, J.; Waisberg, E.; Sporn, K. Virtual reality-enhanced rehabilitation for improving musculoskeletal function and recovery after trauma. J. Orthop. Surg. Res. 2025, 20, 404. [Google Scholar] [CrossRef]
- Farra, S.L.; Gneuhs, M.; Hodgson, E.; Kawosa, B.; Miller, E.T.; Simon, A.; Timm, N.; Hausfeld, J.M. Comparative Cost of Virtual Reality Training and Live Exercises for Training Hospital Workers for Evacuation. Comput. Inform. Nurs. 2019, 37, 446–454. [Google Scholar] [CrossRef]
- Aliprandi, M.; Pan, Y.; Mosley, C.; Gough, S. What is the cost of including virtual reality in neurological rehabilitation? A scoping review. Phys. Ther. Rev. 2022, 27, 329–345. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, M.; Yue, J.; Yang, J.; Xiao, Y.; Yang, J.; Cai, E. Effects of virtual reality with different modalities on upper limb recovery: A systematic review and network meta-analysis on optimizing stroke rehabilitation. Front. Neurol. 2025, 16, 1544135. [Google Scholar] [CrossRef]
- Yoo, Y.M.; Kim, K.H. Current understanding of nociplastic pain. Korean J. Pain 2024, 37, 107–118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, W.; Shi, M.; Liu, L.; Wang, S.; Deng, W.; Ma, Y.; Wang, Y. Effect of Virtual Reality–Based Therapies on Lower Limb Functional Recovery in Stroke Survivors: Systematic Review and Meta-Analysis. J. Med Internet Res. 2025, 27, e72364. [Google Scholar] [CrossRef] [PubMed]
- Arazpour, M.; Keshavarzi, F.; Gard, S.A. The effects of virtual reality environment simulations on balance and gait rehabilitation in persons with lower extremity amputation. Prosthet. Orthot. Int. 2025. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.; McLelland, C.; MacDonald, D.; Hamilton, D.F. Do digital interventions increase adherence to home exercise rehabilitation? A systematic review of randomised controlled trials. Arch. Physiother. 2022, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cheng, Z.; Wang, S.; Jia, Y. Effects of virtual reality-based intervention on depression in stroke patients: A meta-analysis. Sci. Rep. 2023, 13, 4381. [Google Scholar] [CrossRef]
| Study | Sample Size | Type of Amputation | Sample Age Mean (Male/Female Sex) | PEDro Scale |
|---|---|---|---|---|
| Imam et al., 2017 [22] | IG:11 CG: 12 | Unilateral transtibial amputation, unilateral transfemoral amputation, or knee disarticulation. | IG: 61.5 (12/2) CG: 62.5 (6/8) | 8/10 |
| Abbas et al., 2021 [23] | IG: 16 CG: 16 | Unilateral transtibial amputation or unilateral transfemoral amputation. | IG: 27.62 (15/1) CG: 27.62 (14/2) | 7/10 |
| Tao et al., 2022 [24] | IG: 38 CG: 33 | Unilateral transtibial amputation or unilateral transfemoral amputation. | IG: 66.6 (31/7) CG: 63.2 (30/3) | 7/10 |
| Şahan et al., 2023 [25] | IG: 10 CG: 10 | Unilateral transtibial amputation. | IG: 34 (10/0) CG: 32 (10/0) | 4/10 |
| Jabbar et al., 2024 [26] | IG: 11 CG: 11 | Unilateral transtibial amputation. | IG: 48.91 (NR) CG: 48.73 (NR) | 7/10 |
| Steckel et al., 2024 [27] | IG: 19 CG: 12 | Unilateral transtibial amputation, unilateral transfemoral amputation, or knee disarticulation. | IG: 52.7 (16/3) CG: 59.8 (8/4) | 5/10 |
| Study | Intervention Group | Control Group | Timing | Classification of Exercise Interventions | VR Level of Immersion | Outcomes of Lower-Limb Motor Function |
|---|---|---|---|---|---|---|
| Imam et al., 2017 [22] | Wii Fit training, balance, yoga, strength, and aerobics games | Big Brain Academy cognitive video games | IG: 3 times a week for 4 weeks CG: 3 times a week for 4 weeks | IG: Mixed modality CG: Cognitive | Exergame/WiiTM | 2MWT: IG > CG (p > 0.05); ABC: IG > CG (p > 0.05); SPPB: IG > CG (p > 0.05); SAM: IG > CG (p > 0.05); PASE: IG > CG (p > 0.05); LCI-5: IG < CG (p > 0.05); WWT-simple: IG > CG (p > 0.05); WWT-complex: IG > CG (p > 0.05) |
| Abbas et al., 2021 [23] | VR balance and gait training + conventional rehabilitation | Conventional rehabilitation only | IG: 3 times a week for 6 weeks CG: 3 times a week for 6 weeks | IG: Mixed modality CG: Biomechanical | Exergame/KinectTM | BBS: IG > CG (p < 0.05); TUG: IG > CG (p < 0.001); DGI: IG > CG (p < 0.001); 6MWT: IG > CG (p > 0.05) |
| Tao et al., 2022 [24] | WiiNWalk telerehabilitation | Big Brain Academy cognitive video games | IG: 4 weeks supervised + 4 weeks unsupervised CG: NR | IG: Biomechanical CG: Cognitive | Exergame/WiiTM | 2MWT: IG > CG (p < 0.05); SPPB: IG > CG (p > 0.05); FSST: IG > CG (p < 0.05); ABC: IG > CG (p < 0.05) |
| Şahan et al., 2023 [25] | Interactive VR exergames | Conventional prosthetic rehabilitation | IG: 3 times a week, 45 min sessions, 4 weeks CG: NR | IG: Mixed modality CG: Biomechanical and aerobic | Exergame/KinectTM | 6MWT: IG < CG (p < 0.05); single leg balance test (prosthesis limb): IG < CG (p < 0.05); single leg balance test (healthy limb): IG > CG (p > 0.05); 10MWT: IG > CG (p < 0.05); Cadence: IG > CG (p < 0.05) |
| Jabbar et al., 2024 [26] | Exergames + conventional rehabilitation | Conventional rehabilitation | IG: 3 times a week for 4 weeks CG: 3 times a week for 4 weeks | IG: Mixed modality CG: Biomechanical | Exergame/KinectTM | 2MWT: IG > CG (p > 0.05); TUG: IG > CG (p < 0.05); AMPRO: IG > CG (p > 0.05); PCI: IG < CG (p > 0.05). |
| Steckel et al., 2024 [27] | Immersive VR + standard rehabilitation | Standard rehabilitation | IG: 3 times a week, 30 min sessions for 4 weeks CG:NR | IG: Biomechanical CG: NR | Immersive/High | ABC: IG < CG (p > 0.05) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paillet, J.; del Valle Rodríguez, M.; Vázquez, J.H.; Ruiz-Matas Contreras, F.J.; Raya-Benítez, J.; Granados Santiago, M.; Valenza, M.C. The Effects of Virtual Reality Interventions on Motor Function Rehabilitation in Lower-Limb Amputees: A Systematic Review and Metanalysis. Bioengineering 2025, 12, 1170. https://doi.org/10.3390/bioengineering12111170
Paillet J, del Valle Rodríguez M, Vázquez JH, Ruiz-Matas Contreras FJ, Raya-Benítez J, Granados Santiago M, Valenza MC. The Effects of Virtual Reality Interventions on Motor Function Rehabilitation in Lower-Limb Amputees: A Systematic Review and Metanalysis. Bioengineering. 2025; 12(11):1170. https://doi.org/10.3390/bioengineering12111170
Chicago/Turabian StylePaillet, Jade, Manuel del Valle Rodríguez, Javier Herranz Vázquez, Francisco Javier Ruiz-Matas Contreras, Julia Raya-Benítez, María Granados Santiago, and Marie Carmen Valenza. 2025. "The Effects of Virtual Reality Interventions on Motor Function Rehabilitation in Lower-Limb Amputees: A Systematic Review and Metanalysis" Bioengineering 12, no. 11: 1170. https://doi.org/10.3390/bioengineering12111170
APA StylePaillet, J., del Valle Rodríguez, M., Vázquez, J. H., Ruiz-Matas Contreras, F. J., Raya-Benítez, J., Granados Santiago, M., & Valenza, M. C. (2025). The Effects of Virtual Reality Interventions on Motor Function Rehabilitation in Lower-Limb Amputees: A Systematic Review and Metanalysis. Bioengineering, 12(11), 1170. https://doi.org/10.3390/bioengineering12111170