Topical over Dermal Versus Transdermal Application of Cyanoacrylate in Wound Synthesis and Its Effects on Healing—Experimental Study
Abstract
1. Introduction
2. Material and Methods
2.1. Animal Experimentation
2.2. Wound Creation
2.3. Experimental Design
- Ethyl-cyanoacrylate (Three Bonder®—São Paulo, SP, Brazil);
- Butyl-cyanoacrylate (Histoacryl®, B. Braun—Melsungen, Germany);
- Octyl-cyanoacrylate (Liqui Band®—Winsford, UK).
- Topical over dermal (OD); approximation of the wound edges with anatomical forceps, followed by application of CA (right abdominal wound);
- Topical transdermal (TD); application to the wound edges, followed by approximation of the edges with anatomical forceps (left abdominal wound).
2.4. Sample Collection and Analysis
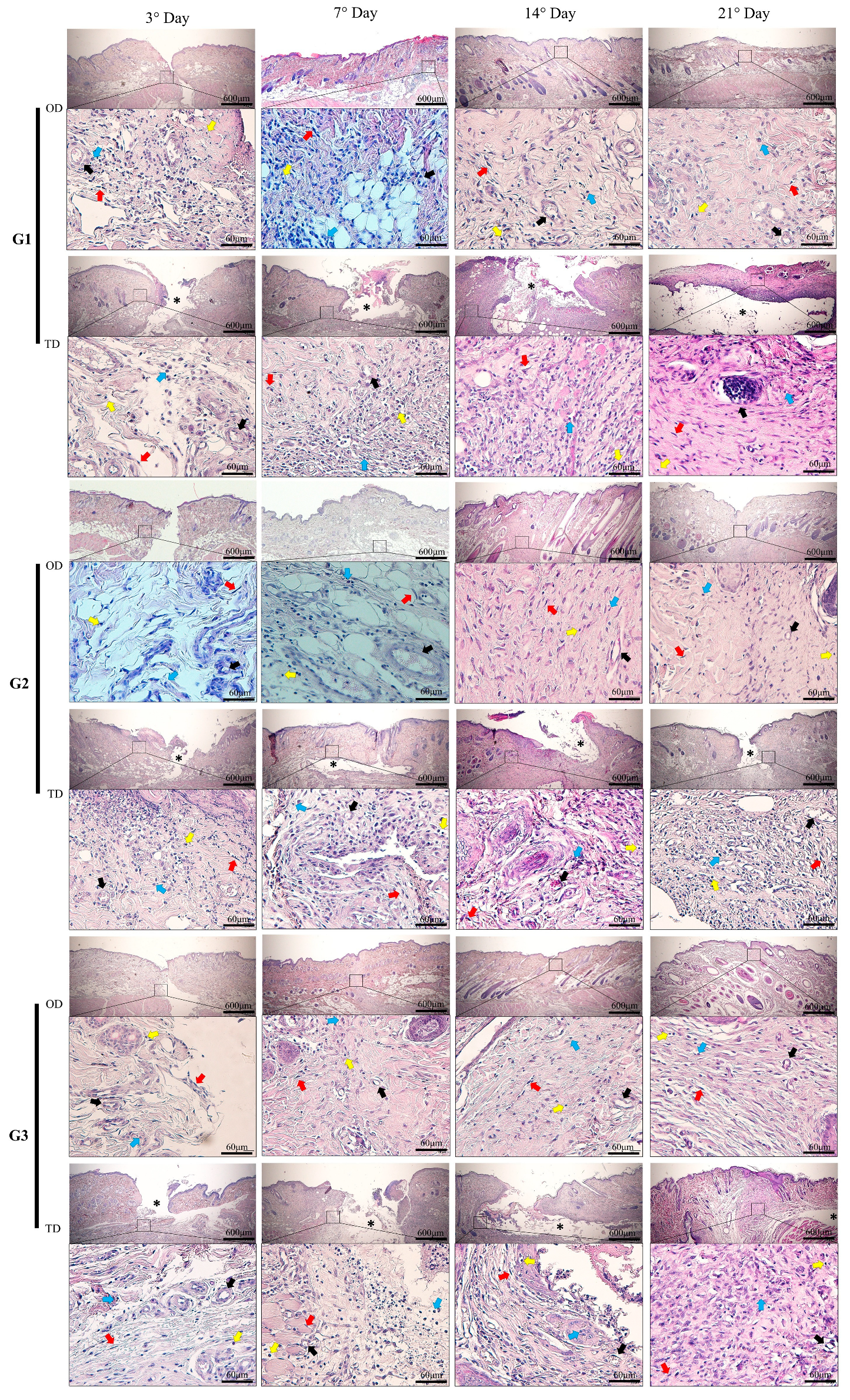
2.5. Histomorphometry
2.6. Immunohistochemistry
2.7. Oxidative Stress
2.8. Hydroxyproline (HO-Pro)
2.9. Glycosaminoglycans (GAG)
2.10. Statistical Analysis
3. Results
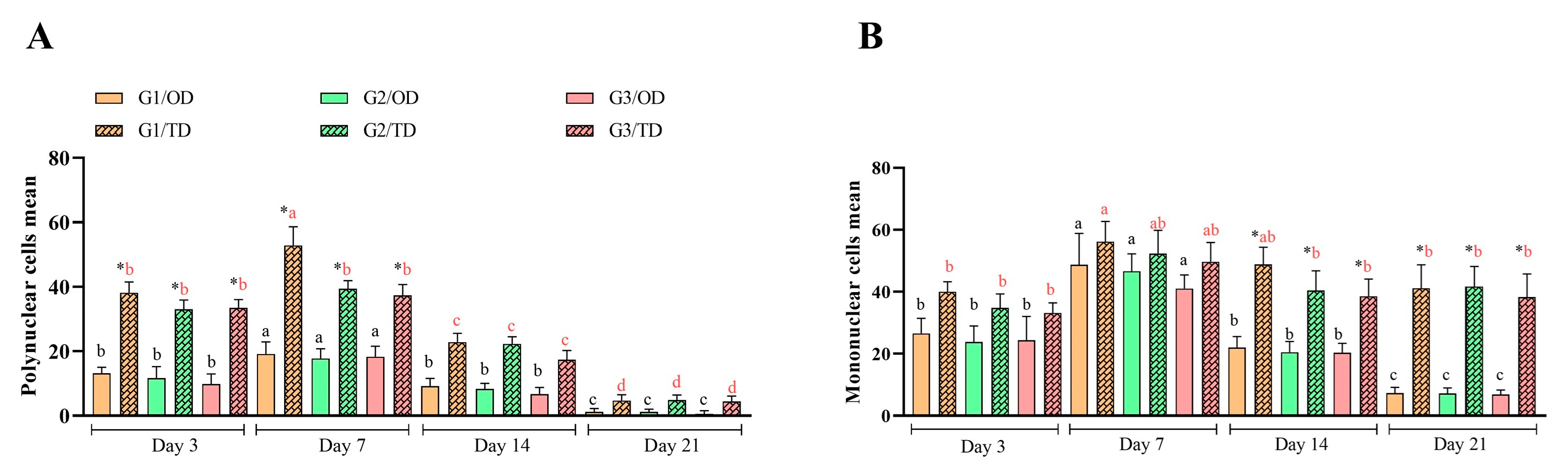
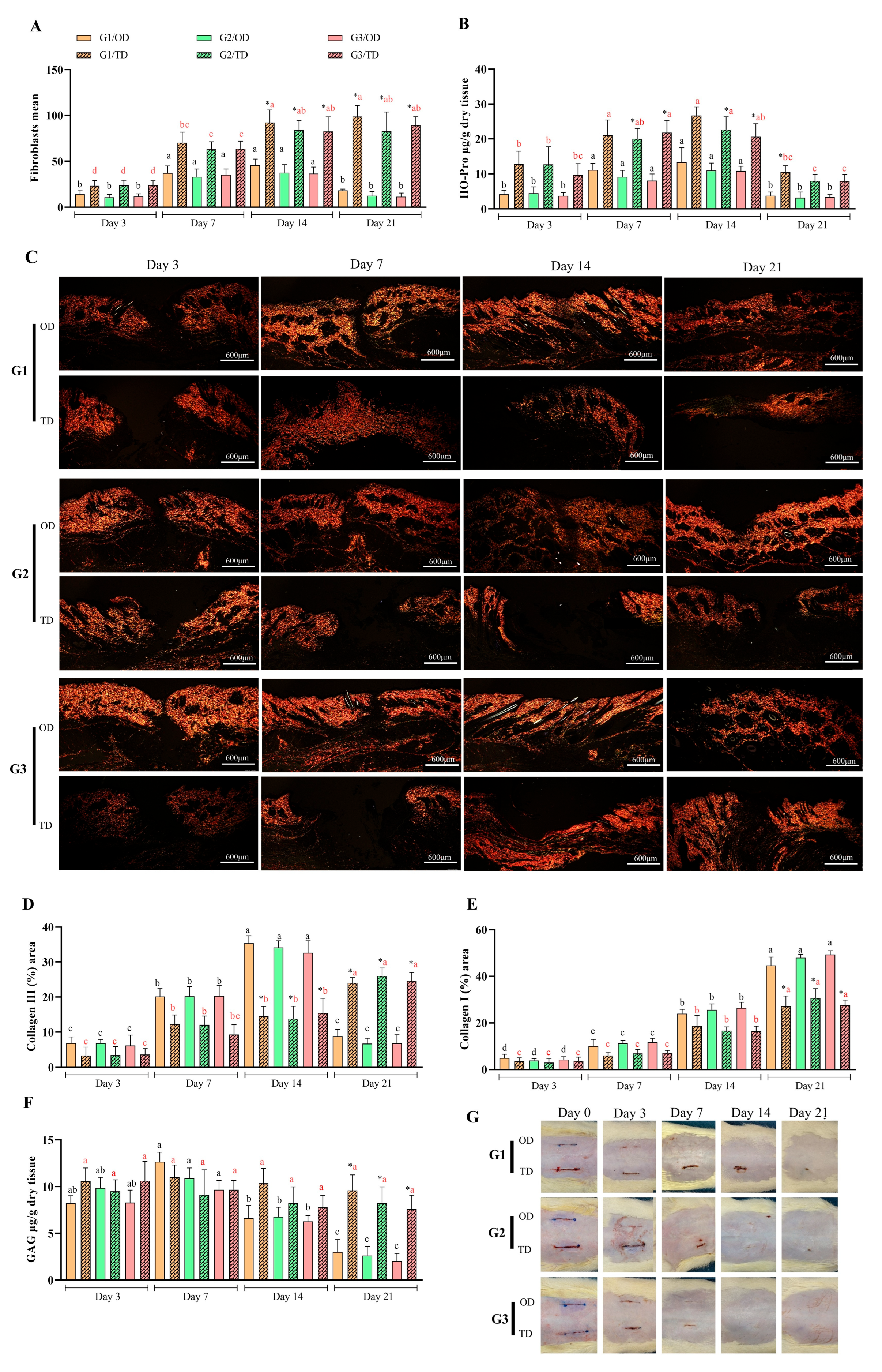
3.1. Inflammatory Infiltrate
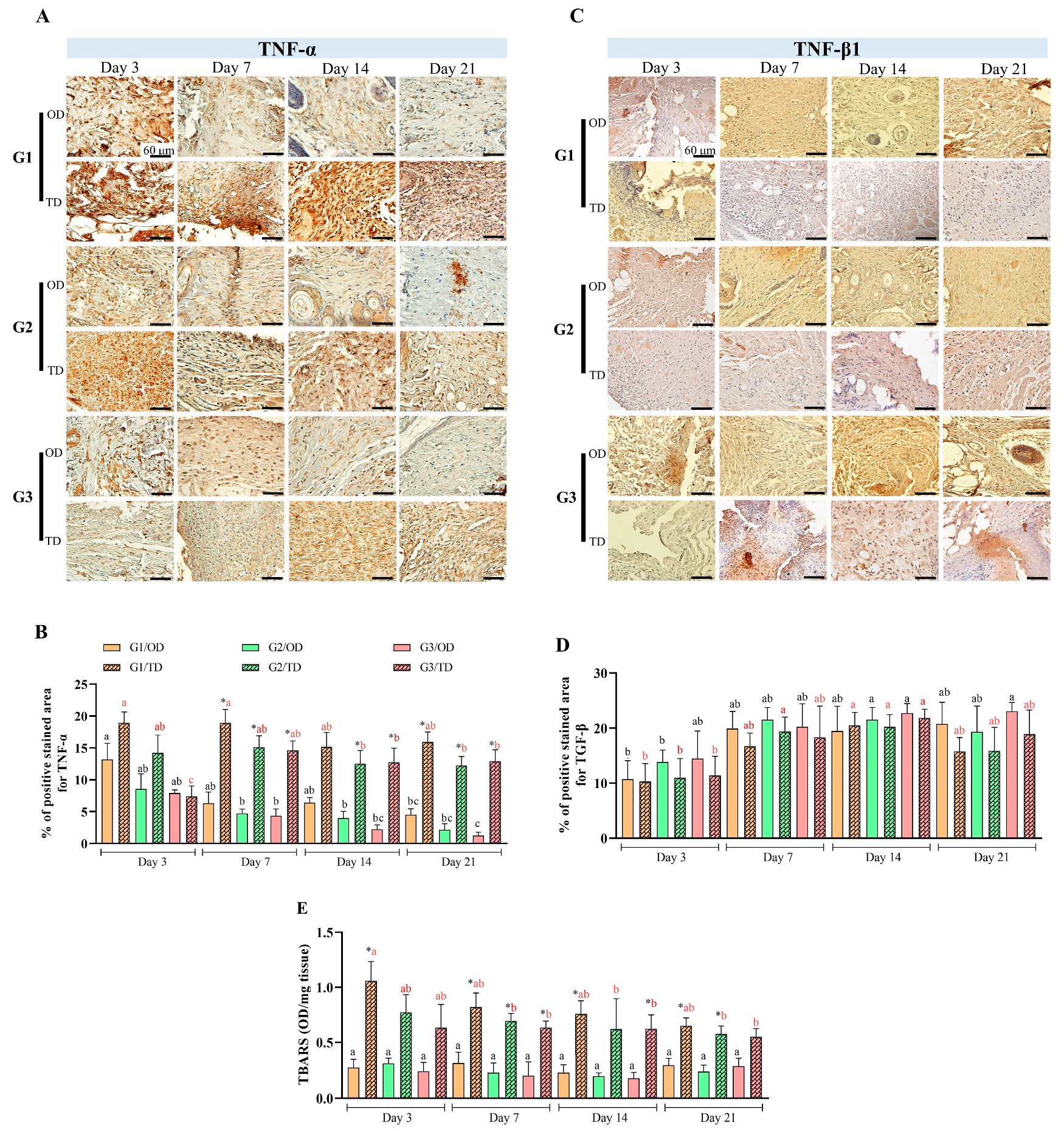
3.2. Inflammation and Oxidative Stress
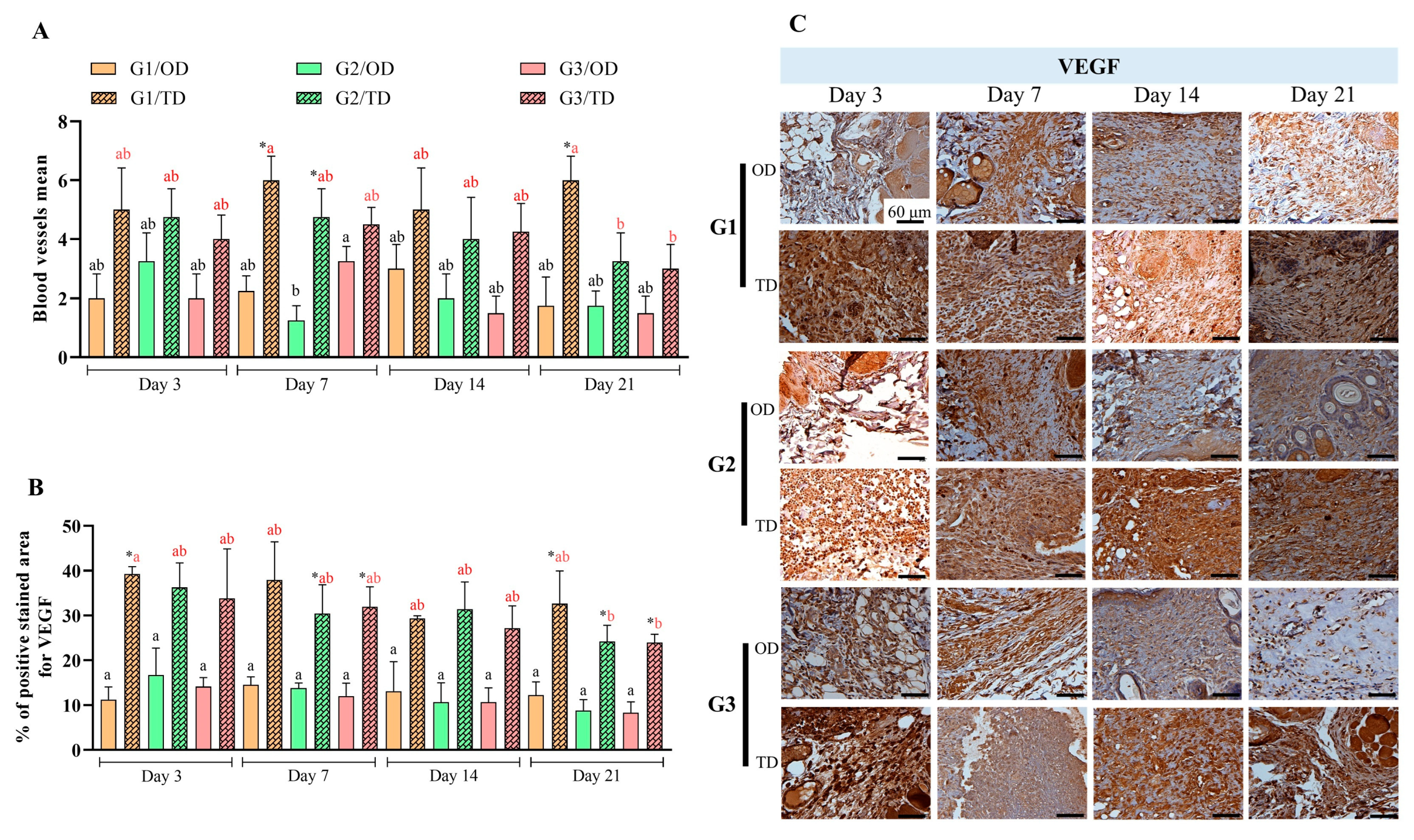
3.3. Neovascularization
3.4. Tissue Formation
3.5. Macroscopic Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dennis, C.; Sethu, S.; Nayak, S.; Mohan, L.; Morsi, Y.Y.; Manivasagam, G. Suture materials—Current and emerging trends. J. Biomed. Mater. Res. A 2016, 104, 1544–1559. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulou, C.; Atout, R.N.; Brownlee, M.; Schroth, R.J.; Kelekis-Cholakis, A. A randomized clinical trial of cyanoacrylate tissue adhesives in donor site of connective tissue grafts. J. Periodontol. 2019, 90, 608–615. [Google Scholar] [CrossRef]
- Bal-Ozturk, A.; Cecen, B.; Avci-Adali, M.; Topkaya, S.N.; Alarcin, E.; Yasayan, G.; Ethan, Y.C.; Bulkurcuoglu, B.; Akpek, A.; Avci, H.; et al. Tissue Adhesives: From Research to Clinical Translation. Nano Today 2021, 36, 101049. [Google Scholar] [CrossRef]
- Bhagat, V.; Becker, M.L. Degradable Adhesives for Surgery and Tissue Engineering. Biomacromolecules 2017, 18, 3009–3039. [Google Scholar] [CrossRef]
- Udaipuria, N.; Bhattacharya, S. Poly(alkyl cyanoacrylate) (PACA) nanoparticles as a versatile drug delivery system for cancer treatment. Int. J. Polym. Mater. 2025, 1–35. [Google Scholar] [CrossRef]
- Pascual, G.; Sotomayor, S.; Rodríguez, M.; Pérez-Köhler, B.; Kühnhardt, A.; Fernández-Gutiérrez, M.; San Román, J.; Bellón, J.M. Cytotoxicity of Cyanoacrylate-Based Tissue Adhesives and Short-Term Preclinical In Vivo Biocompatibility in Abdominal Hernia Repair. PLoS ONE 2016, 11, e0157920. [Google Scholar] [CrossRef]
- Singer, A.J.; Quinn, J.V.; Hollander, J.E. The Cyanoacrylate Topical Skin Adhesives. Am. J. Emerg. Med. 2008, 4, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Hahm, D.H.; Choi, Y.B. Antimicrobial Activity and Cytotoxicity of Prepolymer Allyl 2-cyanoacrylate and 2-Octyl Cyanoacrylate Mixture Adhesives for Topical Wound Closure. Materials 2023, 16, 3427. [Google Scholar] [CrossRef]
- Lee, Y.J.; Jung, G.B.; Choi, S.; Lee, G.; Kim, J.H.; Son, H.S.; Bae, H.; Park, H.K. Biocompatibility of a Novel Cyanoacrylate-Based Tissue Adhesive: Cytotoxicity and Biochemical Property Evaluation. PLoS ONE 2013, 8, e79761. [Google Scholar] [CrossRef]
- Mizrahi, B.; Stefanescu, C.F.; Yang, C.; Lawlor, M.W.; Ko, D.; Langer, R.; Kohane, D.S. Elasticity and Safety of Alkoxyethyl Cyanoacrylate Tissue Adhesives. Acta Biomater. 2011, 7, 3150–3157. [Google Scholar] [CrossRef] [PubMed]
- García Cerdá, D.; Ballester, A.M.; Aliena-Valero, A.; Carabén-Redaño, A.; Lloris, J.M. Use of Cyanoacrylate Adhesives in General Surgery. Surg. Today 2015, 45, 939–956. [Google Scholar] [CrossRef]
- Pascual, G.; Mesa-Ciller, C.; Rodríguez, M.; Pérez-Köhler, B.; Gómez-Gil, V.; Fernández-Gutiérrez, M.; San Román, J.; Bellón, J.M. Pre-clinical assay of the tissue integration and mechanical adhesion of several types of cyanoacrylate adhesives in the fixation of lightweight polypropylene meshes for abdominal hernia repair. PLoS ONE 2018, 13, e0206515. [Google Scholar] [CrossRef]
- Levin-Arama, M.; Abraham, L.; Waner, T.; Harmelin, A.; Steinberg, D.M.; Lahav, T.; Harlev, M. Subcutaneous Compared with Intraperitoneal KetamineXylazine for Anesthesia of Mice. J. Am. Assoc. Lab. Anim. Sci. 2016, 55, 794–800. [Google Scholar]
- Reimer, J.N.; Schuster, C.J.; Knight, C.G.; Pang, D.S.J.; Leung, V.S.Y. Intraperitoneal injection of sodium pentobarbital has the potential to elicit pain in adult rats (Rattus norvegicus). PLoS ONE 2020, 15, e0238123. [Google Scholar] [CrossRef]
- Viana, I.S.; Di Filippo, P.A.; Gobbi, F.P.; Ribeiro, R.B.; Carra, G.J.U.; Ribeiro, L.M.F.; Ribeiro, L.S.; Rocha, M.D.C.P.; Canola, P.A. Cyanoacrylate Adhesives for Cutaneous Wound Closure. Animals 2024, 14, 2678. [Google Scholar] [CrossRef] [PubMed]
- Aguilar Diaz De Leon, J.; Borges, C.R. Evaluation of Oxidative Stress in Biological Samples Using the Thiobarbituric Acid Reactive Substances Assay. J. Vis. Exp. 2020, 159, e61122. [Google Scholar] [CrossRef]
- Cissell, D.D.; Link, J.M.; Hu, J.C.; Athanasiou, K.A. A Modified Hydroxyproline Assay Based on Hydrochloric Acid in Ehrlich’s Solution Accurately Measures Tissue Collagen Content. Tissue Eng. Part C Methods 2017, 23, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Ladner, Y.D.; Alini, M.; Armiento, A.R. The Dimethylmethylene Blue Assay (DMMB) for the Quantification of Sulfated Glycosaminoglycans. Methods Mol. Biol. 2023, 2598, 115–121. [Google Scholar] [CrossRef]
- Poli, A.; Parisi, F.; Millanta, F.; Solfanelli, L.; García-Pastor, P.; Magliaro, C.; Miragliotta, V.; Burchielli, S. Fixation of polyvinylidene fluoride (PVDF) mesh with cyanoacrylate-derived glues in a rat experimental model: Histopathologic immunohistochemical and morphometric study. Hernia 2020, 24, 1263–1273. [Google Scholar] [CrossRef]
- Veríssimo, A.H.; Ribeiro, A.K.C.; Martins, A.R.L.A.; Gurgel, B.C.V.; Lins, R.D.A.U. Comparative analysis of the hemostatic, analgesic and healing effects of cyanoacrylate on free gingival graft surgical wounds in donor and recipient areas: A systematic review. J. Mater. Sci. Mater. Med. 2021, 32, 98. [Google Scholar] [CrossRef]
- Hassanshahi, A.; Moradzad, M.; Ghalamkari, S.; Fadaei, M.; Cowin, A.J.; Hassanshahi, M. Macrophage-Mediated Inflammation in Skin Wound Healing. Cells 2022, 11, 2953. [Google Scholar] [CrossRef] [PubMed]
- Dorantes, L.C.; Ayala, M.C. Skin Acute Wound Healing: A Comprehensive Review. Int. J. Inflamm. 2019, 2, 3706315. [Google Scholar] [CrossRef]
- Ilgenfritz Neto, J.; Aydos, R.D.; Silva, I.S.; Ramalho, R.T.; Ilgenfritz Júnior, J.; de Campos, G.G.O.; Nakamura, R.K.; Zanello Guerisoli, D.M. The Application of Cyanoacrilate Surgical Glue on Skin Suture in Rats. Acta Cirúrgica Bras. 2017, 32, 56–64. [Google Scholar] [CrossRef]
- Lämsä, T.; Jin, H.T.; Sand, J.; Nordback, I. Tissue adhesives and the pancreas: Biocompatibility and adhesive properties of 6 preparations. Pancreas 2008, 36, 261–266. [Google Scholar] [CrossRef]
- Suh, Y.J.; Yu, H.W.; Kim, S.J.; Choe, J.Y.; Park, H.J.; Choi, J.Y.; Lee, K.E. Biocompatibility of N-Butyl-2-Cyanoacrylate (Histoacryl) in Cervical Structures of Rats: Prospective in Vivo Study. Ann. Surg. Treat. Res. 2019, 96, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.E.; García, A.J. Macrophage Phenotypes in Tissue Repair and the Foreign Body Response: Implications for Biomaterial-Based Regenerative Medicine Strategies. Acta Biomater. 2021, 133, 4–16. [Google Scholar] [CrossRef]
- Novak, M.L.; Koh, T.J. Macrophage Phenotypes during Tissue Repair. J. Leukoc. Biol. 2013, 93, 875–881. [Google Scholar] [CrossRef]
- Jaganjac, M.; Cipak, A.; Schaur, R.J.; Zarkovic, N. Pathophysiology of neutrophil-mediated extracellular redox reactions. Front. Biosci. (Landmark Ed.) 2016, 21, 839–855. [Google Scholar] [CrossRef]
- Fernández-Gutiérrez, M.; Rodriguez-Mancheño, M.; Pérez-Köhler, B.; Pascual, G.; Bellón, J.M.; Román, J.S. Structural Analysis and Application of N-Alkyl Cyanoacrylate Surgical Adhesives to the Fixation of Meshes for Hernia Repair. Macromol. Biosci. 2016, 16, 1803–1814. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Hida, R.Y.; Silva, C.B.; Romero-Kusabara, I.L.; Mimica, L.M.J. Short-chain cyanoacrylates and long-chain cyanoacrylates (Dermabond) have different antimicrobial effects. BMJ Open Ophthalmol. 2021, 6, e000591. [Google Scholar] [CrossRef] [PubMed]
- Pascual, G.; García-Moreno, F.; Pérez-Köhler, B.; Rodríguez, M.; Benito-Martínez, S.; Bellón, J.M. Mesh Fixation Using a Cyanoacrylate Applied as a Spray Improves Abdominal Wall Tissue Repair. J. Surg. Res. 2020, 246, 26–33. [Google Scholar] [CrossRef]
- Shehab, A.A.; El-Batea, H.E.; El-Din, I.S.; Elfert, A.A. N-butyl cyanoacrylate- and alpha-cyanoacrylate induced hepatotoxicity and venous sclerosis in a rabbit model. Arab J. Gastroenterol. 2016, 17, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Adl, H.; Henkelman, E.; Goldman, R.D. Topical skin adhesives for laceration repair in children. Can. Fam. Physician 2021, 67, 260–262. [Google Scholar] [CrossRef]
- Johnson, K.E.; Wilgus, T.A. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv. Wound Care 2014, 3, 647–661. [Google Scholar] [CrossRef]
- Bala, M.M.; Şahin, A.A.; Boz, M.; Durukan, Y.; Fırat, T.; Pakdil, M.; Özturan, K.E. Effects of Cyanoacrylate in Rabbits with Induced Achilles Tendon Rupture. Med. Sci Monit. 2021, 27, e929709. [Google Scholar] [CrossRef]
- Shabestani Monfared, G.; Ertl, P.; Rothbauer, M. An on-chip wound healing assay fabricated by xurography for evaluation of dermal fibroblast cell migration and wound closure. Sci. Rep. 2020, 10, 16192. [Google Scholar] [CrossRef]
- Cialdai, F.; Risaliti, C.; Monici, M. Role of fibroblasts in wound healing and tissue remodeling on Earth and in space. Front. Bioeng. Biotechnol. 2022, 10, 958381. [Google Scholar] [CrossRef]
- Li, R.; Feng, D.; Han, S.; Zhai, X.; Yu, X.; Fu, Y.; Jin, F. Macrophages and fibroblasts in foreign body reactions: How mechanical cues drive cell functions? Mater. Today Bio 2023, 22, 100783. [Google Scholar] [CrossRef]
- Ayuk, S.M.; Abrahamse, H.; Houreld, N.N. The Role of Matrix Metalloproteinases in Diabetic Wound Healing in Relation to Photobiomodulation. J. Diabetes Res. 2016, 2016, 2897656. [Google Scholar] [CrossRef] [PubMed]
- Ghatak, S.; Maytin, E.V.; Mack, J.A.; Hascall, V.C.; Atanelishvili, I.; Moreno Rodriguez, R.; Markwald, R.R.; Misra, S. Roles of Proteoglycans and Glycosaminoglycans in Wound Healing and Fibrosis. Int. J. Cell Biol. 2015, 2015, 834893. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Dou, W.; Zeng, X.; Chen, X.; Gao, Y.; Liu, H.; Li, S. Recent Advances in the Degradability and Applications of Tissue Adhesives Based on Biodegradable Polymers. Int. J. Mol. Sci. 2024, 25, 5249. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viana, I.S.; Di Filippo, P.A.; Carra, G.J.U.; Gobbi, F.P.; Ribeiro, L.S.; Ribeiro, R.B.; Petri, F.A.M.; Favero, M.L.; Ribeiro, L.M.F.; Carvalho, E.C.Q.; et al. Topical over Dermal Versus Transdermal Application of Cyanoacrylate in Wound Synthesis and Its Effects on Healing—Experimental Study. Bioengineering 2025, 12, 1147. https://doi.org/10.3390/bioengineering12111147
Viana IS, Di Filippo PA, Carra GJU, Gobbi FP, Ribeiro LS, Ribeiro RB, Petri FAM, Favero ML, Ribeiro LMF, Carvalho ECQ, et al. Topical over Dermal Versus Transdermal Application of Cyanoacrylate in Wound Synthesis and Its Effects on Healing—Experimental Study. Bioengineering. 2025; 12(11):1147. https://doi.org/10.3390/bioengineering12111147
Chicago/Turabian StyleViana, Inácio Silva, Paula Alessandra Di Filippo, Gabriel João Unger Carra, Francielli Pereira Gobbi, Lara Souza Ribeiro, Rachel Bittencourt Ribeiro, Fernando Antônio M. Petri, Maria Luíza Favero, Luíza Maria Feitosa Ribeiro, Eulogio Carvalho Queiroz Carvalho, and et al. 2025. "Topical over Dermal Versus Transdermal Application of Cyanoacrylate in Wound Synthesis and Its Effects on Healing—Experimental Study" Bioengineering 12, no. 11: 1147. https://doi.org/10.3390/bioengineering12111147
APA StyleViana, I. S., Di Filippo, P. A., Carra, G. J. U., Gobbi, F. P., Ribeiro, L. S., Ribeiro, R. B., Petri, F. A. M., Favero, M. L., Ribeiro, L. M. F., Carvalho, E. C. Q., & Canola, P. A. (2025). Topical over Dermal Versus Transdermal Application of Cyanoacrylate in Wound Synthesis and Its Effects on Healing—Experimental Study. Bioengineering, 12(11), 1147. https://doi.org/10.3390/bioengineering12111147










