Engineering Multilayered Hepatic Cell Sheet Model Using Oxygen-Supplying MeHA/CPO Hydrogel
Abstract
1. Introduction
2. Materials and Methods
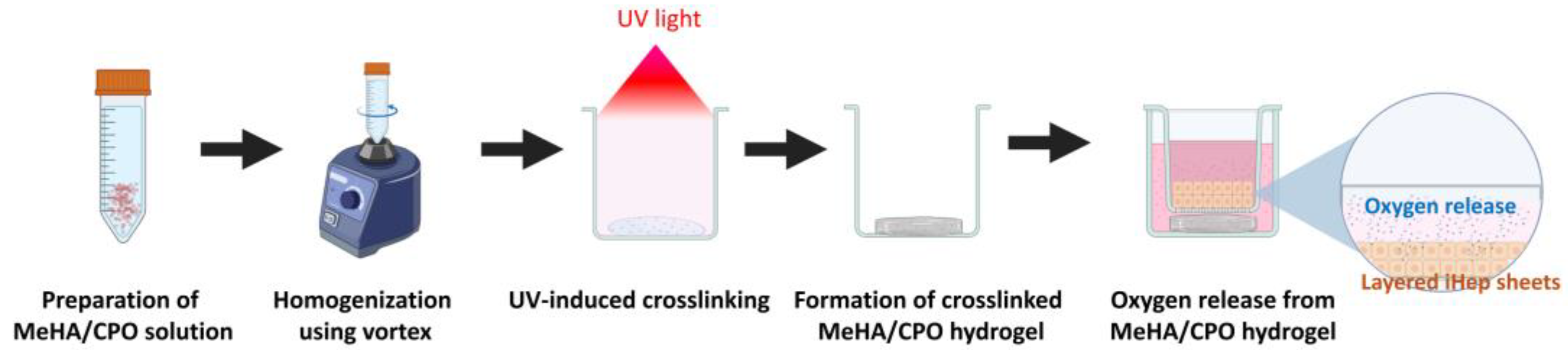
2.1. Preparation of Calcium Peroxide-Crosslinked Meha Hydrogel
2.2. Rheological Characterization of Crosslinked Meha Hydrogel
2.3. Oxygen Release Profile of Crosslinked Meha Hydrogel
2.4. Scanning Electron Microscopy (SEM) Analysis
2.5. In Vitro Cytotoxicity Assessment
2.6. Fabrication of Single- and Double-Layered Cell Sheets
2.7. Gene Expression Analysis
2.8. Albumin Secretion Assessment
2.9. Histological Analysis
3. Results
3.1. Meha-COP Hydrogel Formation Behavior Varies Depending on Meha and CPO Concentrations
3.2. Crosslinking of Meha-CPO Hydrogel Induces Gel-like Rheological Behavior
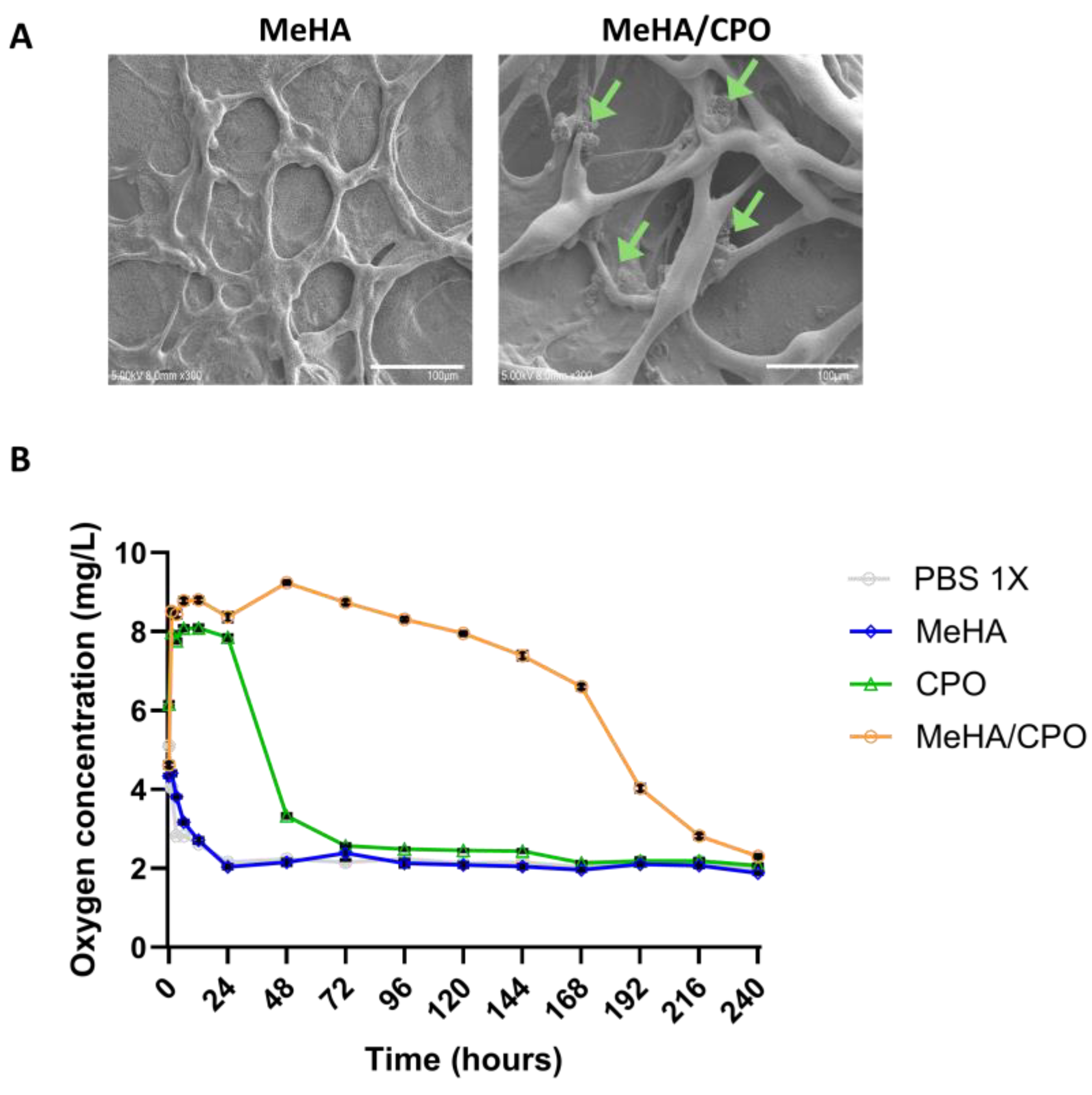
3.3. Oxygen-Releasing Performance of Meha/CPO Hydrogel
3.4. The Meha/CPO Hydrogel Layer Did Not Adversely Affect the Morphology or Viability of Ipsc-Derived Hepatocytes
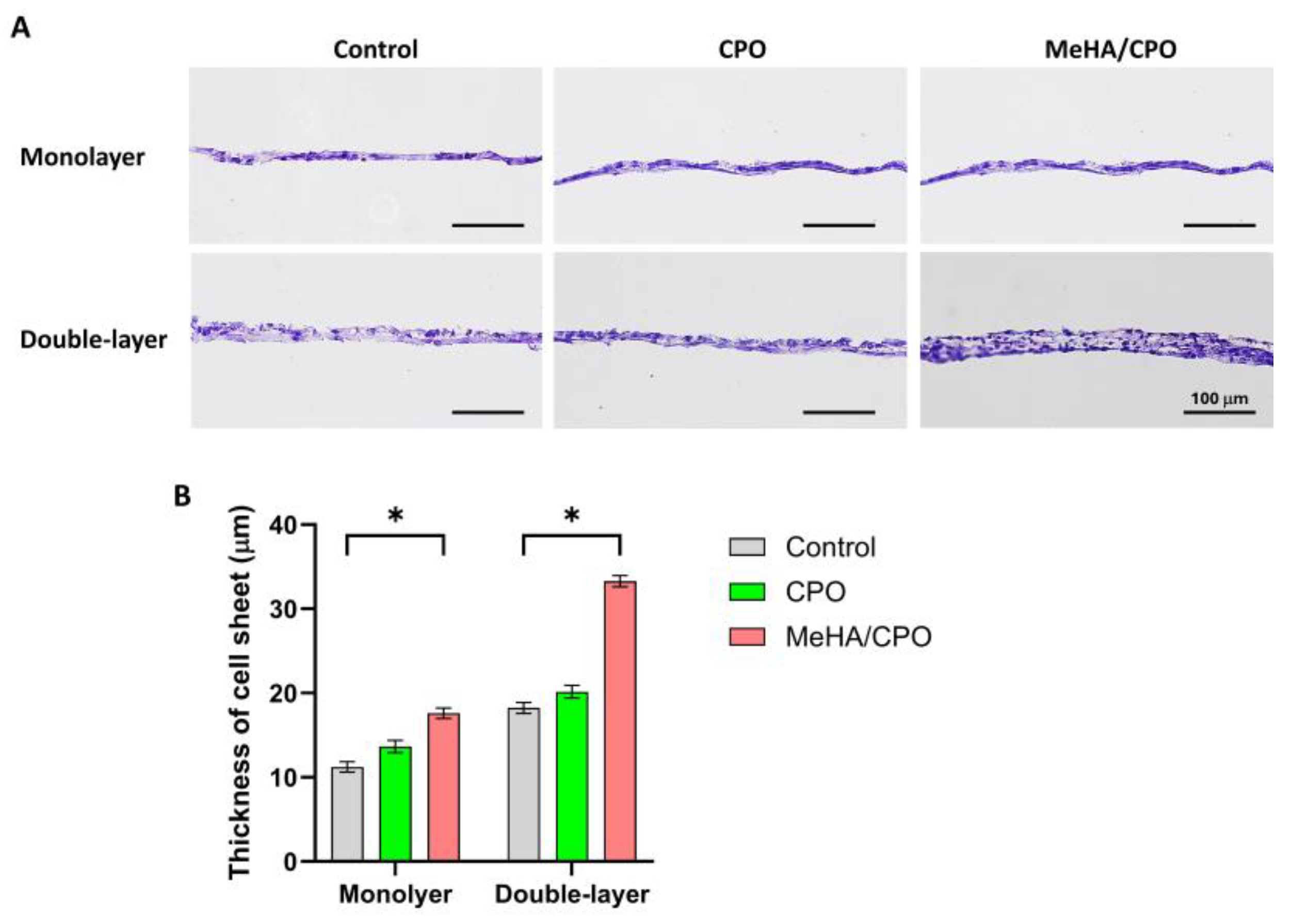
3.5. Meha/CPO Hydrogel Enhances the Structural Integrity of Multilayered Ihep Sheets
3.6. MeHA/CPO Hydrogel Enhances Functional Activity of Multilayered iHep Sheets
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| MeHA | Methacrylated hyaluronic acid |
| CPO | Calcium peroxide |
| iHep | iPSC-derived hepatocyte |
| PBS | Phosphate-buffered saline |
| HGF | Hepatocyte growth factor |
| VEGF | Vascular endothelial growth factor |
| Alb | Albumin |
| HNF4α | Hepatocyte nuclear factor 4 alpha |
| AFP | Alpha-fetoprotein |
| UV | Ultraviolet |
| FBS | Fetal bovine serum |
| EC | Endothelial cell |
| DE | Definitive endoderm |
| HE | Hepatic endoderm |
| IMH | Immature hepatocyte |
| MH | Maure hepatocyte |
| TRCD | Temperature-responsive culture dishes |
References
- Bhatia, S.N.; Underhill, G.H.; Zaret, K.S.; Fox, I.J. Cell and tissue engineering for liver disease. Sci. Transl. Med. 2014, 6, 245sr2. [Google Scholar] [CrossRef]
- Bosch, F.X.; Ribes, J.; Díaz, M.; Cléries, R. Primary liver cancer: Worldwide incidence and trends. Gastroenterology 2004, 127, S5–S16. [Google Scholar] [CrossRef]
- Kim, W.R.; Brown, R.S., Jr.; Terrault, N.A.; El-Serag, H. Burden of liver disease in the United States: Summary of a workshop. Hepatology 2002, 36, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, D.; Brouwers, J.F.; Hamer, K.; Geurts, M.H.; Luciana, L.; Massalini, S.; López-Iglesias, C.; Peters, P.J.; Rodríguez-Colman, M.J.; Chuva de Sousa Lopes, S.; et al. Engineered human hepatocyte organoids enable CRISPR-based target discovery and drug screening for steatosis. Nat. Biotechnol. 2023, 41, 1567–1581. [Google Scholar] [CrossRef] [PubMed]
- Yanagi, Y.; Nakayama, K.; Taguchi, T.; Enosawa, S.; Tamura, T.; Yoshimaru, K.; Matsuura, T.; Hayashida, M.; Kohashi, K.; Oda, Y.; et al. In vivo and ex vivo methods of growing a liver bud through tissue connection. Sci. Rep. 2017, 7, 14085. [Google Scholar] [CrossRef] [PubMed]
- Olgasi, C.; Cucci, A.; Follenzi, A. iPSC-Derived Liver Organoids: A Journey from Drug Screening, to Disease Modeling, Arriving to Regenerative Medicine. Int. J. Mol. Sci. 2020, 21, 6215. [Google Scholar] [CrossRef]
- Goldring, C.; Antoine, D.J.; Bonner, F.; Crozier, J.; Denning, C.; Fontana, R.J.; Hanley, N.A.; Hay, D.C.; Ingelman-Sundberg, M.; Juhila, S.; et al. Stem cell-derived models to improve mechanistic understanding and prediction of human drug-induced liver injury. Hepatology 2017, 65, 710–721. [Google Scholar] [CrossRef]
- Maepa, S.W.; Ndlovu, H. Advances in generating liver cells from pluripotent stem cells as a tool for modeling liver diseases. Stem Cells 2020, 38, 606–612. [Google Scholar] [CrossRef]
- Rashid, S.T.; Corbineau, S.; Hannan, N.; Marciniak, S.J.; Miranda, E.; Alexander, G.; Huang-Doran, I.; Griffin, J.; Ahrlund-Richter, L.; Skepper, J.; et al. Modeling inherited metabolic disorders of the liver using human induced pluripotent stem cells. J. Clin. Investig. 2010, 120, 3127–3136. [Google Scholar] [CrossRef]
- Yamato, M.; Okano, T. Cell sheet engineering. Mater. Today 2004, 7, 42–47. [Google Scholar] [CrossRef]
- Yang, J.; Yamato, M.; Nishida, K.; Ohki, T.; Kanzaki, M.; Sekine, H.; Shimizu, T.; Okano, T. Cell delivery in regenerative medicine: The cell sheet engineering approach. J. Control Release 2006, 116, 193–203. [Google Scholar] [CrossRef]
- Okano, T.; Yamada, N.; Okuhara, M.; Sakai, H.; Sakurai, Y. Mechanism of cell detachment from temperature-modulated, hydrophilic-hydrophobic polymer surfaces. Biomaterials 1995, 16, 297–303. [Google Scholar] [CrossRef]
- Yang, J.; Yamato, M.; Kohno, C.; Nishimoto, A.; Sekine, H.; Fukai, F.; Okano, T. Cell sheet engineering: Recreating tissues without biodegradable scaffolds. Biomaterials 2005, 26, 6415–6422. [Google Scholar] [CrossRef]
- Bou-Ghannam, S.; Kim, K.; Grainger, D.W.; Okano, T. 3D cell sheet structure augments mesenchymal stem cell cytokine production. Sci. Rep. 2021, 11, 8170. [Google Scholar] [CrossRef]
- Bou-Ghannam, S.; Kim, K.; Kondo, M.; Grainger, D.W.; Okano, T. Mesenchymal Stem Cell Sheet Centrifuge-Assisted Layering Augments Pro-Regenerative Cytokine Production. Cells 2022, 11, 2840. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, Y.; Shimizu, T.; Yamato, M.; Okano, T. Scaffold-free tissue engineering using cell sheet technology. RSC Adv. 2012, 2, 2184–2190. [Google Scholar] [CrossRef]
- Kim, K.; Bou-Ghannam, S.; Okano, T. Cell sheet tissue engineering for scaffold-free three-dimensional (3D) tissue reconstruction. Methods Cell Biol. 2020, 157, 143–167. [Google Scholar] [CrossRef]
- Sekine, H.; Shimizu, T.; Sakaguchi, K.; Dobashi, I.; Wada, M.; Yamato, M.; Kobayashi, E.; Umezu, M.; Okano, T. In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nat. Commun. 2013, 4, 1399. [Google Scholar] [CrossRef] [PubMed]
- Thorp, H.; Kim, K.; Kondo, M.; Grainger, D.W.; Okano, T. Fabrication of hyaline-like cartilage constructs using mesenchymal stem cell sheets. Sci. Rep. 2020, 10, 20869. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Ohashi, K.; Utoh, R.; Kano, K.; Okano, T. Preserved liver-specific functions of hepatocytes in 3D co-culture with endothelial cell sheets. Biomaterials 2012, 33, 1406–1413. [Google Scholar] [CrossRef]
- Kim, K.; Utoh, R.; Ohashi, K.; Kikuchi, T.; Okano, T. Fabrication of functional 3D hepatic tissues with polarized hepatocytes by stacking endothelial cell sheets in vitro. J. Tissue Eng. Regen. Med. 2017, 11, 2071–2080. [Google Scholar] [CrossRef]
- Balis, U.J.; Behnia, K.; Dwarakanath, B.; Bhatia, S.N.; Sullivan, S.J.; Yarmush, M.L.; Toner, M. Oxygen consumption characteristics of porcine hepatocytes. Metab. Eng. 1999, 1, 49–62. [Google Scholar] [CrossRef]
- Gholipourmalekabadi, M.; Zhao, S.; Harrison, B.S.; Mozafari, M.; Seifalian, A.M. Oxygen-Generating Biomaterials: A New, Viable Paradigm for Tissue Engineering? Trends. Biotechnol. 2016, 34, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Fu, Z.; Wang, H.; Liu, Z.; Gao, M.; Luo, Y.; Zhang, M.; Wang, J.; Ni, D. Calcium Peroxide-Based Hydrogels Enable Biphasic Release of Hydrogen Peroxide for Infected Wound Healing. Adv. Sci. 2024, 11, e2404813. [Google Scholar] [CrossRef]
- Oh, S.H.; Ward, C.L.; Atala, A.; Yoo, J.J.; Harrison, B.S. Oxygen generating scaffolds for enhancing engineered tissue survival. Biomaterials 2009, 30, 757–762. [Google Scholar] [CrossRef]
- Toftdal, M.S.; Taebnia, N.; Kadumudi, F.B.; Andresen, T.L.; Frogne, T.; Winkel, L.; Grunnet, L.G.; Dolatshahi-Pirouz, A. Oxygen releasing hydrogels for beta cell assisted therapy. Int. J. Pharm. 2021, 602, 120595. [Google Scholar] [CrossRef] [PubMed]
- Willemen, N.G.A.; Hassan, S.; Gurian, M.; Li, J.; Allijn, I.E.; Shin, S.R.; Leijten, J. Oxygen-Releasing Biomaterials: Current Challenges and Future Applications. Trends Biotechnol. 2012, 39, 1144–1159. [Google Scholar] [CrossRef]
- Xie, X.; Sun, X.; Lin, W.; Yang, X.; Wang, R. Preparation and Properties of Calcium Peroxide/Poly(ethylene glycol)@Silica Nanoparticles with Controlled Oxygen-Generating Behaviors. Materials 2025, 18, 2568. [Google Scholar] [CrossRef]
- Ursini, O.; Grieco, M.; Sappino, C.; Capodilupo, A.L.; Giannitelli, S.M.; Mauri, E.; Bucciarelli, A.; Coricciati, C.; de Turris, V.; Gigli, G.; et al. Modulation of Methacrylated Hyaluronic Acid Hydrogels Enables Their Use as 3D Cultured Model. Gels 2023, 9, 801. [Google Scholar] [CrossRef]
- Park, S.H.; Park, J.Y.; Ji, Y.B.; Ju, H.J.; Min, B.H.; Kim, M.S. An injectable click-crosslinked hyaluronic acid hydrogel modified with a BMP-2 mimetic peptide as a bone tissue engineering scaffold. Acta Biomater. 2020, 117, 108–120. [Google Scholar] [CrossRef]
- Schuurmans, C.C.L.; Mihajlovic, M.; Hiemstra, C.; Ito, K.; Hennink, W.E.; Vermonden, T. Hyaluronic acid and chondroitin sulfate (meth)acrylate-based hydrogels for tissue engineering: Synthesis, characteristics and pre-clinical evaluation. Biomaterials 2021, 268, 120602. [Google Scholar] [CrossRef]
- Dovedytis, M.; Liu, Z.J.; Bartlett, S. Hyaluronic acid and its biomedical applications: A review. Eng. Regen. 2020, 1, 102–113. [Google Scholar] [CrossRef]
- Bayarsaikhan, D.; Bayarsaikhan, G.; Kang, H.A.; Lee, S.B.; Han, S.H.; Okano, T.; Kim, K.; Lee, B. A Study on iPSC-Associated Factors in the Generation of Hepatocytes. Tissue Eng. Regen. Med. 2024, 21, 1245–1254. [Google Scholar] [CrossRef]
- Bayarsaikhan, D.; Bayarsaikhan, G.; Lee, J.; Okano, T.; Kim, K.; Lee, B. Development of iPSC-derived FIX-secreting hepatocyte sheet as a novel treatment tool for hemophilia B treatment. Stem Cell Res. Ther. 2025, 16, 88. [Google Scholar] [CrossRef]
- Bell, C.C.; Chouhan, B.; Andersson, L.C.; Andersson, H.; Dear, J.W.; Williams, D.P.; Söderberg, M. Functionality of primary hepatic non-parenchymal cells in a 3D spheroid model and contribution to acetaminophen hepatotoxicity. Arch. Toxicol. 2020, 94, 1251–1263. [Google Scholar] [CrossRef]
- Kang, H.K.; Sarsenova, M.; Kim, D.H.; Kim, M.S.; Lee, J.Y.; Sung, E.A.; Kook, M.G.; Kim, N.G.; Choi, S.W.; Ogay, V.; et al. Establishing a 3D In Vitro Hepatic Model Mimicking Physiologically Relevant to In Vivo State. Cells 2021, 10, 1268. [Google Scholar] [CrossRef]
- Lauschke, V.M.; Hendriks, D.F.; Bell, C.C.; Andersson, T.B.; Ingelman-Sundberg, M. Novel 3D Culture Systems for Studies of Human Liver Function and Assessments of the Hepatotoxicity of Drugs and Drug Candidates. Chem. Res. Toxicol. 2016, 29, 1936–1955. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Ooka, M.; Margolis, R.J.; Xia, M. Liver three-dimensional cellular models for high-throughput chemical testing. Cell Rep. Methods 2023, 3, 100432. [Google Scholar] [CrossRef] [PubMed]
- Wagner, B.A.; Venkataraman, S.; Buettner, G.R. The rate of oxygen utilization by cells. Free Radic. Biol. Med. 2011, 51, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Treyer, A.; Müsch, A. Hepatocyte polarity. Compr. Physiol. 2013, 3, 243–287. [Google Scholar] [CrossRef]
- Ohashi, K.; Yokoyama, T.; Yamato, M.; Kuge, H.; Kanehiro, H.; Tsutsumi, M.; Amanuma, T.; Iwata, H.; Yang, J.; Okano, T.; et al. Engineering functional two- and three-dimensional liver systems in vivo using hepatic tissue sheets. Nat. Med. 2007, 13, 880–885. [Google Scholar] [CrossRef]
- Ma, X.; Huang, T.; Chen, X.; Li, Q.; Liao, M.; Fu, L.; Huang, J.; Yuan, K.; Wang, Z.; Zeng, Y. Molecular mechanisms in liver repair and regeneration: From physiology to therapeutics. Signal Transduct. Target. Ther. 2025, 10, 63. [Google Scholar] [CrossRef]
- Stanger, B.Z.; Greenbaum, L. The role of paracrine signals during liver regeneration. Hepatology. 2012, 56, 1577–1579. [Google Scholar] [CrossRef] [PubMed]
- Drixler, T.A.; Vogten, M.J.; Ritchie, E.D.; van Vroonhoven, T.J.; Gebbink, M.F.; Voest, E.E.; Borel Rinkes, I.H. Liver regeneration is an angiogenesis- associated phenomenon. Ann. Surg. 2002, 236, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Nakamura, T. Hepatocyte growth factor: Renotropic role and potential therapeutics for renal diseases. Kidney Int. 2001, 59, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- Michalopoulos, G.K. Liver regeneration. J. Cell Physiol. 2007, 213, 286–300. [Google Scholar] [CrossRef]




| Target | Forward/Reverse (5′-3′) | Annealing Temp (°C) |
|---|---|---|
| HGF | F: 5′-GAG AGT TGG GTT CTT ACT GCA CG-3′ | 60.2 |
| R: 5′-CTC ATC TCC TCT TCC GTG GAC A-3′ | 60 | |
| VEGF | F: 5′-TTG CCT TGC TGC TCT ACC TCC A-3′ | 60 |
| R: 5′-GAT GGC AGT AGC TGC GCT GAT-3′ | 60 | |
| Alb | F: 5′-TGC CAA ACA GAG ACT CAA GT-3′ | 53.4 |
| R: 5′-TCA GCA GGC ATC TCA TCA TT-3′ | 53.4 | |
| HNF4a | F: 5′CAT GGC CAA GAT TGA CAA CCT-3′ | 56.2 |
| R: 5′-TTC CCA TATGTT CCT GCA TCA G-3′ | 56.4 | |
| AFP | F: 5′-ACA ATT CTT CTT TGG GCT GC-3′ | 53.4 |
| R: 5′-GCC ACA TCC AGG ACT AGT TT-3′ | 55.4 | |
| ITGB1 | F: 5′-GGA TTC TCC AGA AGG TGG TTT CG-3′ | 60.2 |
| R: 5′-TGC CAC CAA GTT TCC CAT CTC C-3′ | 60 | |
| β-catenin | F: 5′-TGA GGA CAA GCC ACA AGA TTA C-3′ | 56.4 |
| R: 5′-TCC ACC AGA GTG AAA AGA ACG-3′ | 56.2 | |
| Fibronectin | F: 5′-ACA ACA CCG AGG TGA CTG AGA C-3′ | 60 |
| R: 5′-GGA CAC AACGAT GCT TCC TGA G-3′ | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.; Han, S.H.; Oh, J.; Bayarsaikhan, D.; Kim, M.S.; Kim, D.; Okano, T.; Lee, B. Engineering Multilayered Hepatic Cell Sheet Model Using Oxygen-Supplying MeHA/CPO Hydrogel. Bioengineering 2025, 12, 1132. https://doi.org/10.3390/bioengineering12101132
Kim K, Han SH, Oh J, Bayarsaikhan D, Kim MS, Kim D, Okano T, Lee B. Engineering Multilayered Hepatic Cell Sheet Model Using Oxygen-Supplying MeHA/CPO Hydrogel. Bioengineering. 2025; 12(10):1132. https://doi.org/10.3390/bioengineering12101132
Chicago/Turabian StyleKim, Kyungsook, So Hee Han, Jiyoen Oh, Delger Bayarsaikhan, Moon Suk Kim, Dayoen Kim, Teruo Okano, and Bonghee Lee. 2025. "Engineering Multilayered Hepatic Cell Sheet Model Using Oxygen-Supplying MeHA/CPO Hydrogel" Bioengineering 12, no. 10: 1132. https://doi.org/10.3390/bioengineering12101132
APA StyleKim, K., Han, S. H., Oh, J., Bayarsaikhan, D., Kim, M. S., Kim, D., Okano, T., & Lee, B. (2025). Engineering Multilayered Hepatic Cell Sheet Model Using Oxygen-Supplying MeHA/CPO Hydrogel. Bioengineering, 12(10), 1132. https://doi.org/10.3390/bioengineering12101132






