Surgical Approaches to Retinal Gene Therapy: 2025 Update
Abstract
1. Introduction
2. Methods
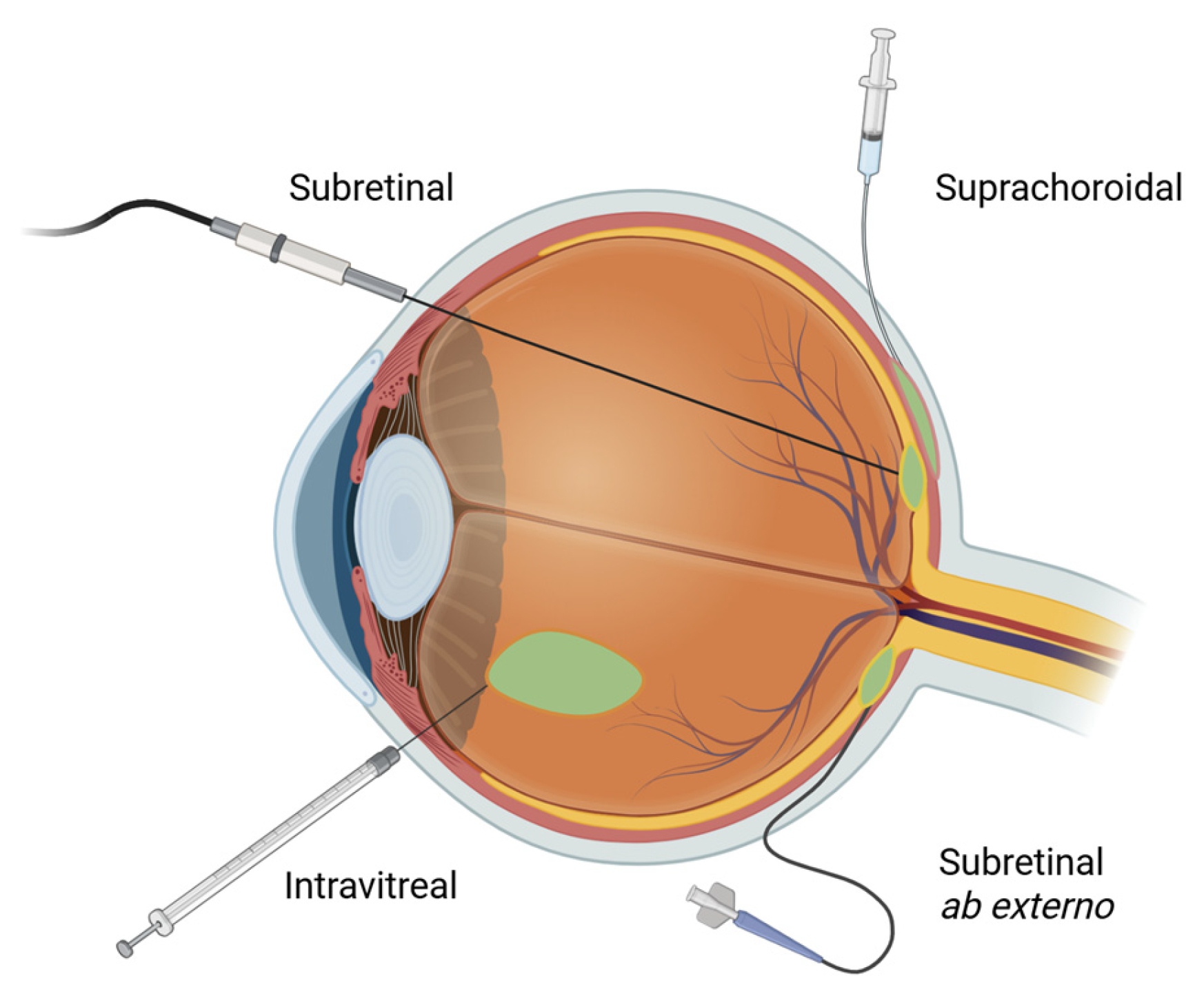
3. Subretinal Delivery
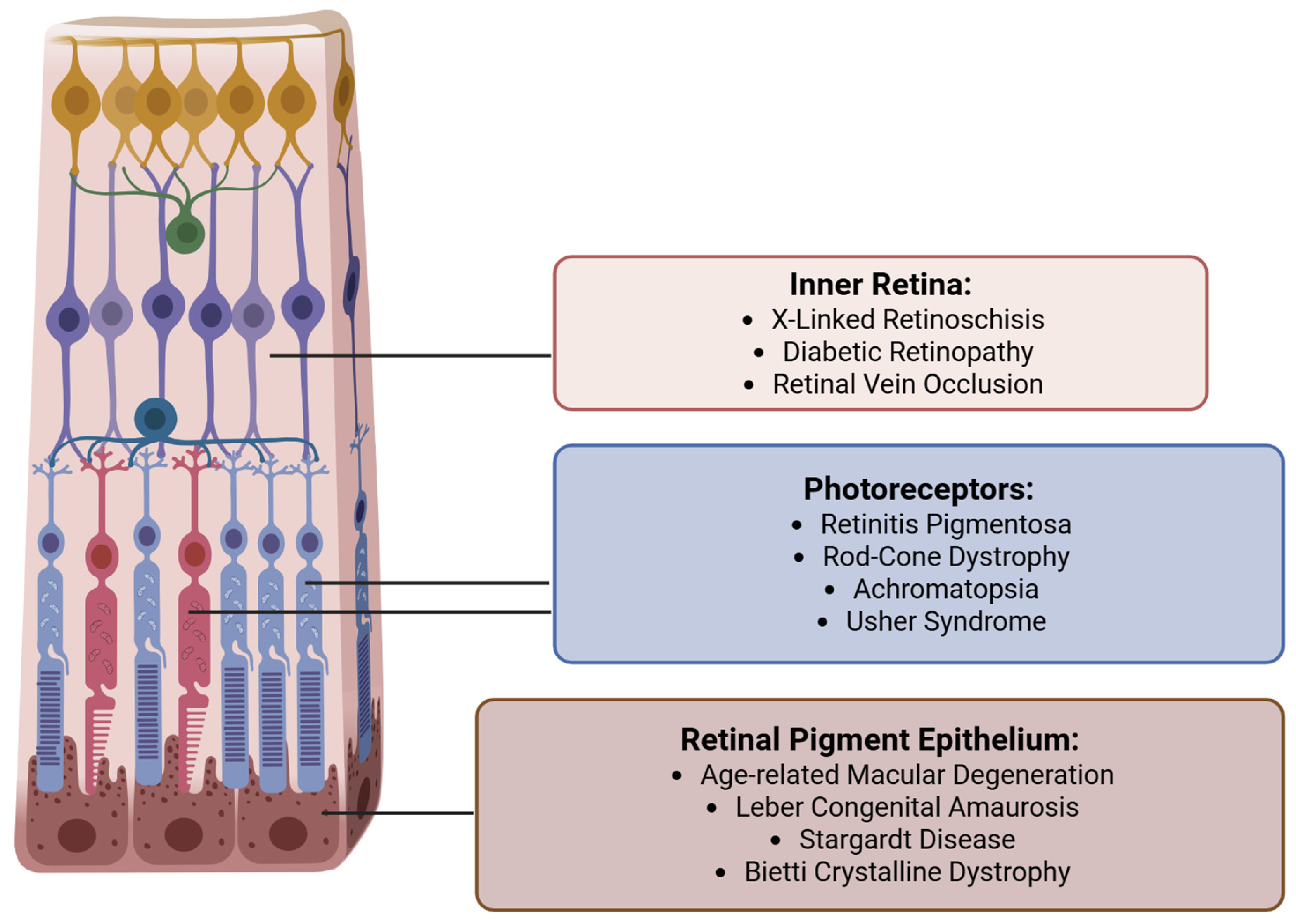
3.1. Background
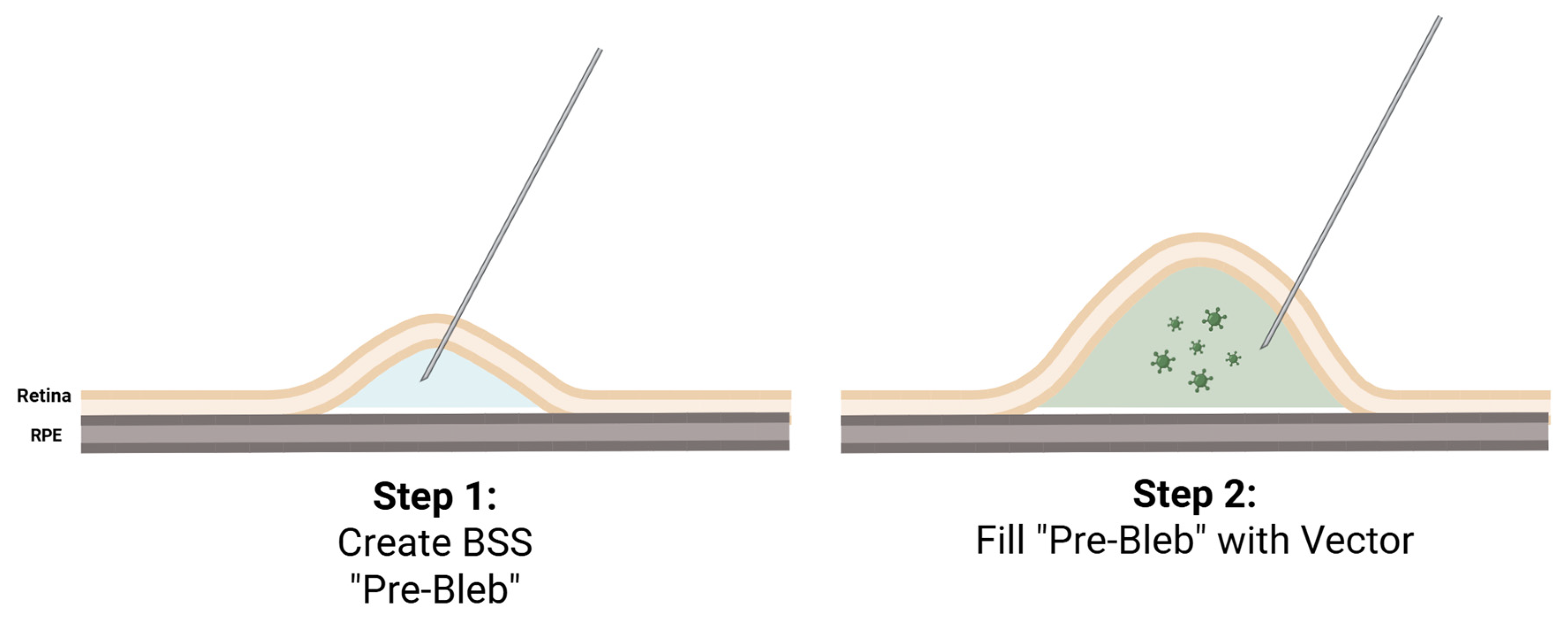
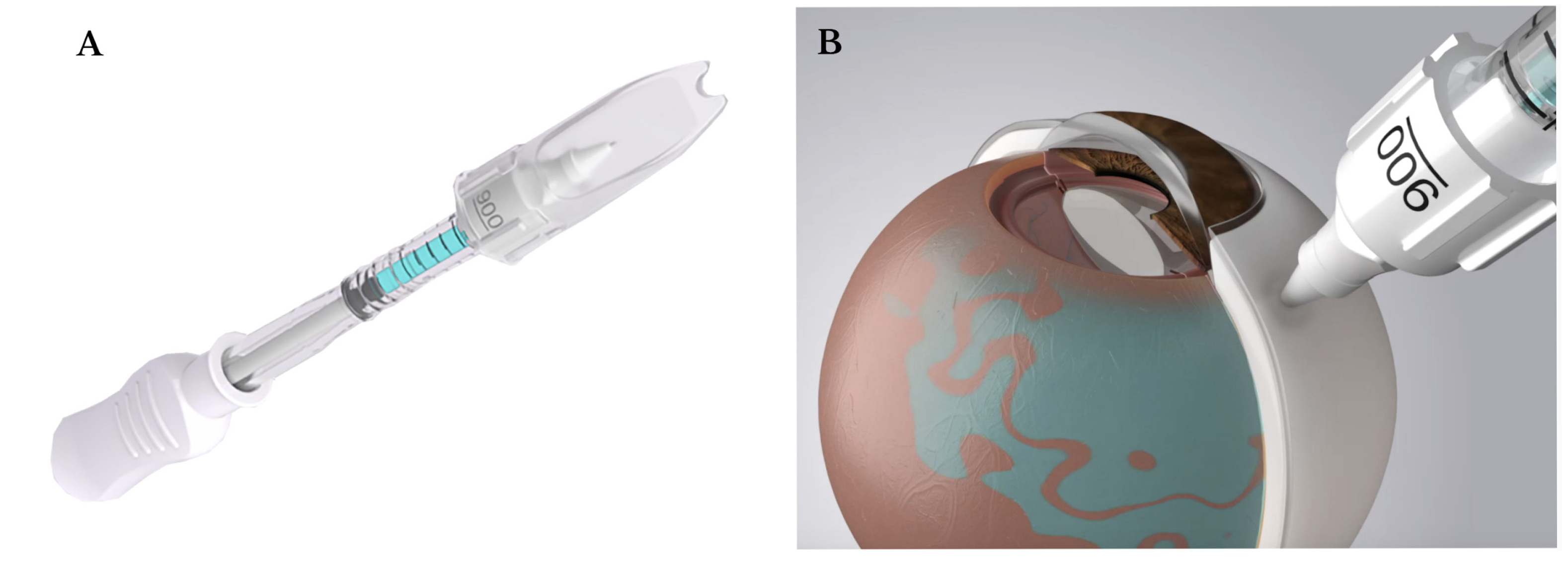
3.2. Current Subretinal Gene Therapy Delivery Technique
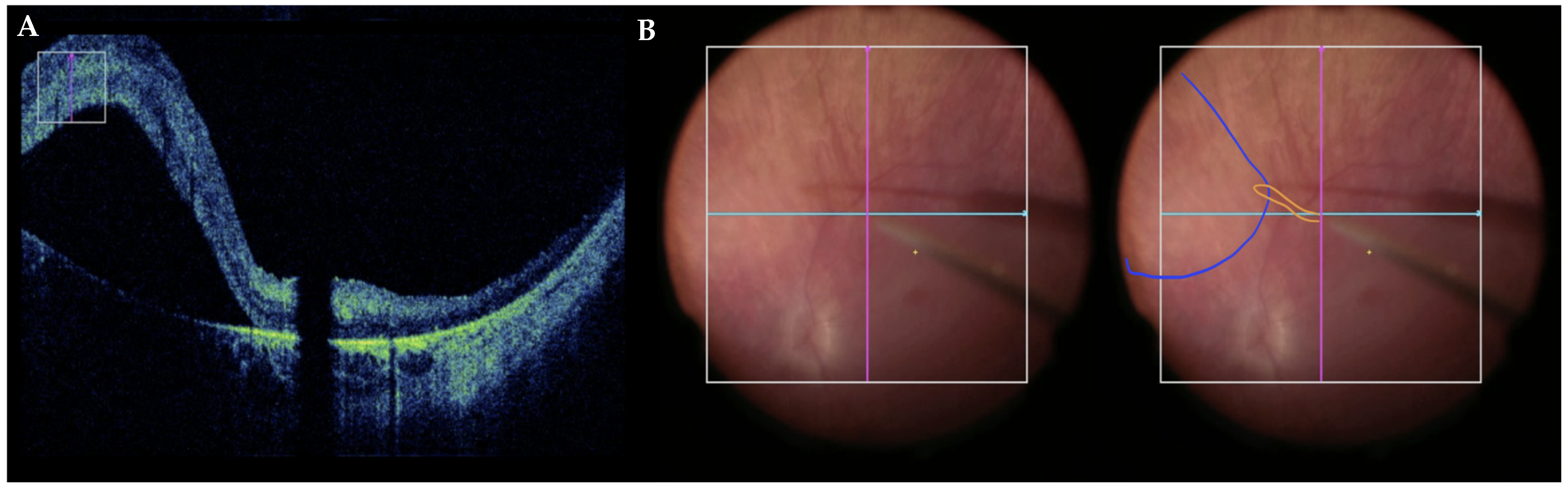
3.3. Optimizing the Pars Plana Vitrectomy Approach
3.4. Robot-Assisted Subretinal Delivery
| Study | Subject | Robot | Automated | Results | Limitations |
|---|---|---|---|---|---|
| Maierhofer et al. (2023) [35] | Ex vivo porcine eyes | Custom robot | No |
|
|
| |||||
| Yang et al. (2022) [36] | Ex vivo porcine eyes | Custom robot | No |
|
|
| Huang et al. (2023) [37] | Retinal model | iORBIS robotic manipulator | Yes |
|
|
| Arikan et al. (2025) [41] | Ex vivo porcine eyes | Steady Hand Eye Robot | Yes |
|
|
| Zhang et al. (2024) [38] | Silicone eye phantom; ex vivo porcine eyes | Steady Hand Eye Robot | Yes |
|
|
| Dehghani et al. (2023) [40] | Ex vivo porcine eyes | Steady Hand Eye Robot | Yes |
|
|
| Abid et al. (2022) [39] | Ex vivo porcine eyes | Steady Hand Eye Robot | Yes |
|
|
3.5. Novel Non-Vitrectomy Subretinal Approaches
4. Suprachoroidal Delivery
4.1. Background
4.2. Preclinical Developments
4.3. Clinical Trials
5. Intravitreal Delivery
5.1. Background
5.2. Preclinical Developments
5.3. Clinical Trials
6. Discussion
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aghajanpour, S.; Amiriara, H.; Ebrahimnejad, P.; Slavcev, R.A. Advancing Ocular Gene Therapy: A Machine Learning Approach to Enhance Delivery, Uptake and Gene Expression. Drug Discov. Today 2025, 30, 104359. [Google Scholar] [CrossRef]
- Li, Q.; Miller, R.; Han, P.-Y.; Pang, J.; Dinculescu, A.; Chiodo, V.; Hauswirth, W.W. Intraocular Route of AAV2 Vector Administration Defines Humoral Immune Response and Therapeutic Potential. Mol. Vis. 2008, 14, 1760–1769. [Google Scholar]
- Wu, H.; Dong, L.; Jin, S.; Zhao, Y.; Zhu, L. Innovative Gene Delivery Systems for Retinal Disease Therapy. Neural Regen. Res. 2026, 21, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.; Wilson, J.; Sun, D.; Forbes, B.; Maguire, A. Adenovirus Vector-Mediated in Vivo Gene Transfer into Adult Murine Retina. Investig. Ophthalmol. Vis. Sci. 1994, 35, 2535–2542. [Google Scholar] [PubMed]
- Drag, S.; Dotiwala, F.; Upadhyay, A.K. Gene Therapy for Retinal Degenerative Diseases: Progress, Challenges, and Future Directions. Investig. Ophthalmol. Vis. Sci. 2023, 64, 39. [Google Scholar] [CrossRef]
- Bennett, J.; Maguire, A.M. SECTION 4 Translational Basic Science 36 Gene Therapy for Retinal Disease. In Ryan’s Retina; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Ameri, H.; Kesavamoorthy, N.; Bruce, D.N. Frequency and Pattern of Worldwide Ocular Gene Therapy Clinical Trials up to 2022. Biomedicines 2023, 11, 3124. [Google Scholar] [CrossRef]
- Dhurandhar, D.; Sahoo, N.K.; Mariappan, I.; Narayanan, R. Gene Therapy in Retinal Diseases: A Review. Indian J. Ophthalmol. 2021, 69, 2257–2265. [Google Scholar] [CrossRef]
- Arnold, C. Gene Therapy Targets the Retina to Treat Eye Disease. Nat. Med. 2025, 31, 2–3. [Google Scholar] [CrossRef]
- Dalkara, D.; Kolstad, K.D.; Caporale, N.; Visel, M.; Klimczak, R.R.; Schaffer, D.V.; Flannery, J.G. Inner Limiting Membrane Barriers to AAV-Mediated Retinal Transduction from the Vitreous. Mol. Ther. 2009, 17, 2096–2102. [Google Scholar] [CrossRef]
- Sørensen, N.B. Subretinal Surgery: Functional and Histological Consequences of Entry into the Subretinal Space. Acta Ophthalmol. 2019, 97, 1–23. [Google Scholar] [CrossRef]
- Xue, K.; Groppe, M.; Salvetti, A.P.; MacLaren, R.E. Technique of Retinal Gene Therapy: Delivery of Viral Vector into the Subretinal Space. Eye 2017, 31, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Varin, J.; Morival, C.; Maillard, N.; Adjali, O.; Cronin, T. Risk Mitigation of Immunogenicity: A Key to Personalized Retinal Gene Therapy. Int. J. Mol. Sci. 2021, 22, 12818. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.W.; Hsu, S.; Ng, T.F. The Role of Retinal Pigment Epithelial Cells in Regulation of Macrophages/Microglial Cells in Retinal Immunobiology. Front. Immunol. 2021, 12, 724601. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Yan, B. Ocular Immune Privilege and Retinal Pigment Epithelial Cells. J. Leukoc. Biol. 2023, 113, 288–304. [Google Scholar] [CrossRef]
- Davis, J.L.; Gregori, N.Z.; MacLaren, R.E.; Lam, B.L. Surgical Technique for Subretinal Gene Therapy in Humans with Inherited Retinal Degeneration. Retina 2019, 39, S2–S8. [Google Scholar] [CrossRef]
- Kay, C. SECTION 4 Investigational Surgery and Techniques Subretinal Gene Therapy Delivery. In Operative Techniques in Vitreoretinal Surgery; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Vrellaku, B.; Sethw Hassan, I.; Howitt, R.; Webster, C.P.; Harriss, E.; McBlane, F.; Betts, C.; Schettini, J.; Lion, M.; Mindur, J.E.; et al. A Systematic Review of Immunosuppressive Protocols Used in AAV Gene Therapy for Monogenic Disorders. Mol. Ther. 2024, 32, 3220–3259. [Google Scholar] [CrossRef]
- Fan, K.C.; Yannuzzi, N.A.; Patel, N.A.; Negron, C.I.; Sisk, R.A.; Nagiel, A.; Berrocal, A.M. Surgical Techniques for the Subretinal Delivery of Pediatric Gene Therapy. Ophthalmol. Retin. 2020, 4, 644–645. [Google Scholar] [CrossRef]
- Gregori, N.Z.; Lam, B.L.; Davis, J.L. Intraoperative Use of Microscope-Integrated Optical Coherence Tomography for Subretinal Gene Therapy Delivery. Retina 2019, 39, S9–S12. [Google Scholar] [CrossRef]
- Ding, K.; Shen, J.; Hafiz, Z.; Hackett, S.F.; Lima e Silva, R.; Khan, M.; Lorenc, V.E.; Chen, D.; Chadha, R.; Zhang, M.; et al. AAV8-Vectored Suprachoroidal Gene Transfer Produces Widespread Ocular Transgene Expression. J. Clin. Investig. 2019, 129, 4901–4911. [Google Scholar] [CrossRef]
- Ladha, R.; Caspers, L.E.; Willermain, F.; de Smet, M.D. Subretinal Therapy: Technological Solutions to Surgical and Immunological Challenges. Front. Med. 2022, 9, 846782. [Google Scholar] [CrossRef]
- Gange, W.S.; Sisk, R.A.; Besirli, C.G.; Lee, T.C.; Havunjian, M.; Schwartz, H.; Borchert, M.; Sengillo, J.D.; Mendoza, C.; Berrocal, A.M.; et al. Perifoveal Chorioretinal Atrophy after Subretinal Voretigene Neparvovec-Rzyl for RPE65-Mediated Leber Congenital Amaurosis. Ophthalmol. Retina 2022, 6, 58–64. [Google Scholar] [CrossRef]
- Reichel, F.F.; Seitz, I.; Wozar, F.; Dimopoulos, S.; Jung, R.; Kempf, M.; Kohl, S.; Kortüm, F.C.; Ott, S.; Pohl, L.; et al. Development of Retinal Atrophy after Subretinal Gene Therapy with Voretigene Neparvovec. Br. J. Ophthalmol. 2023, 107, 1331–1335. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.; Borchert, M.; Lee, T.C.; Nagiel, A. Subretinal Deposits in Young Patients Treated with Voretigene Neparvovec-Rzyl for RPE65-Mediated Retinal Dystrophy. Br. J. Ophthalmol. 2023, 107, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Price, K.W.; Pennesi, M.E.; Lauer, A.K.; Bailey, S.T. Iatrogenic Choroidal Neovascularization Associated with Subretinal Gene Therapy Surgery. Am. J. Ophthalmol. Case Rep. 2022, 27, 101677. [Google Scholar] [CrossRef] [PubMed]
- Olufsen, M.E.; Spindler, L.; Sørensen, N.B.; Christiansen, A.T.; Alberti, M.; Heegaard, S.; Kiilgaard, J.F. Controlled Subretinal Injection Pressure Prevents Damage in Pigs. Ophthalmologica 2022, 245, 285–294. [Google Scholar] [CrossRef]
- Dormegny, L.; Studer, F.; Sauer, A.; Ballonzoli, L.; Speeg-Schatz, C.; Bourcier, T.; Dollfus, H.; Gaucher, D. Could Internal Limiting Membrane Peeling before Voretigen Neparvovec-Ryzl Subretinal Injection Prevent Focal Chorioretinal Atrophy? Heliyon 2024, 10, e25154. [Google Scholar] [CrossRef]
- Takahashi, K.; Morizane, Y.; Hisatomi, T.; Tachibana, T.; Kimura, S.; Hosokawa, M.M.; Shiode, Y.; Hirano, M.; Doi, S.; Toshima, S.; et al. The Influence of Subretinal Injection Pressure on the Microstructure of the Monkey Retina. PLoS ONE 2018, 13, e0209996. [Google Scholar] [CrossRef]
- Scruggs, B.A.; Vasconcelos, H.M.; Matioli da Palma, M.; Kogachi, K.; Pennesi, M.E.; Yang, P.; Bailey, S.T.; Lauer, A.K. Injection Pressure Levels for Creating Blebs during Subretinal Gene Therapy. Gene Ther. 2022, 29, 601–607. [Google Scholar] [CrossRef]
- Li, Y.; Wolf, M.D.; Kulkarni, A.D.; Bell, J.; Chang, J.S.; Nimunkar, A.; Radwin, R.G. In Situ Tremor in Vitreoretinal Surgery. Hum. Factors J. Hum. Factors Ergon. Soc. 2021, 63, 1169–1181. [Google Scholar] [CrossRef]
- Sisk, R.A.; Berger, T.A.; Williams, E.R.; Riemann, C.D. Intraoperative Bleb Behavior in Subretinal Gene Augmentation Therapy for Inherited Retinal Diseases. Retina 2023, 43, 1763–1772. [Google Scholar] [CrossRef]
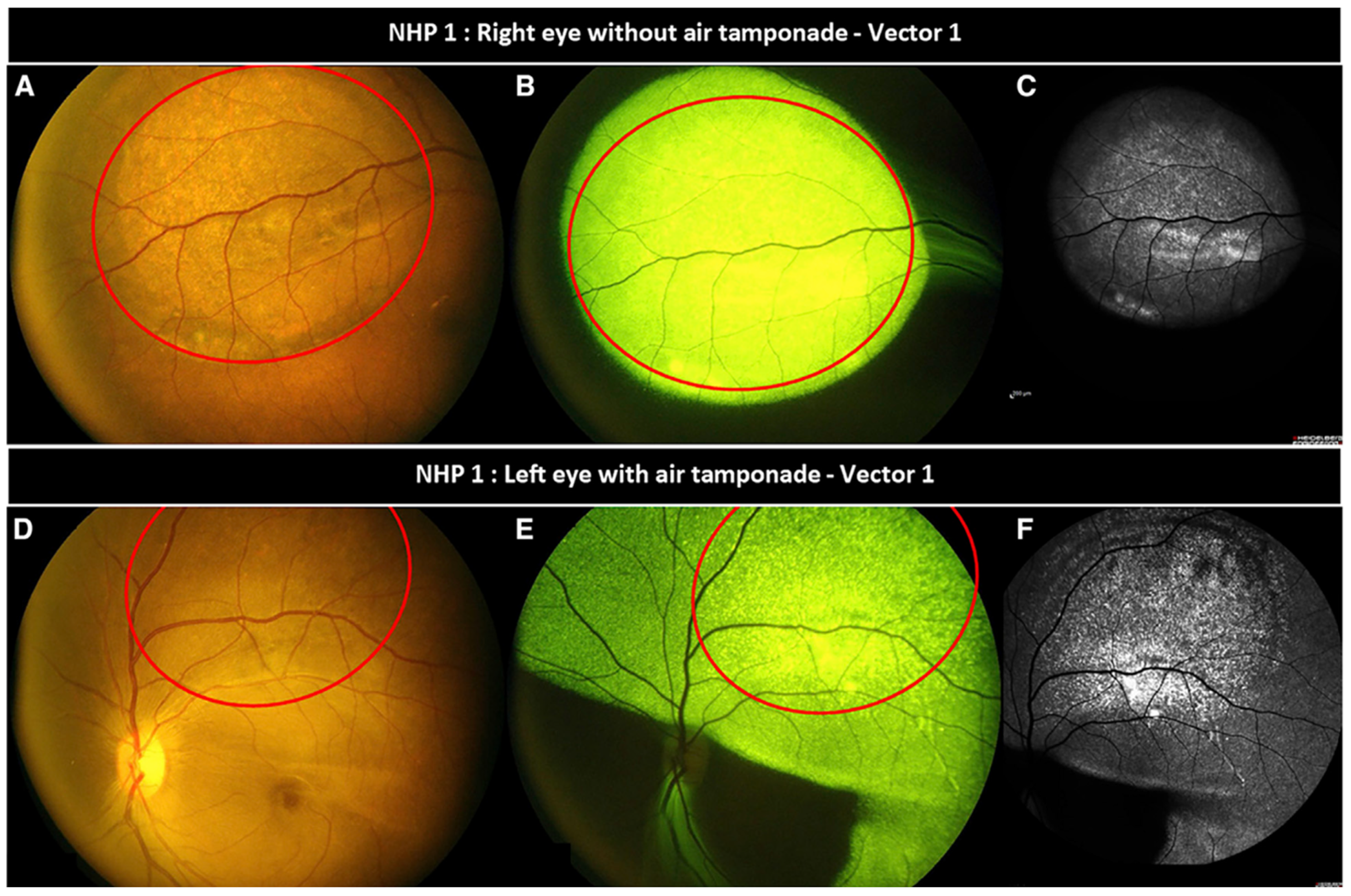
- Ducloyer, J.-B.; Pichard, V.; Mevel, M.; Galy, A.; Lefevre, G.M.; Brument, N.; Alvarez-Dorta, D.; Deniaud, D.; Mendes-Madeira, A.; Libeau, L.; et al. Intravitreal Air Tamponade after AAV2 Subretinal Injection Modifies Retinal EGFP Distribution. Mol. Ther. Methods Clin. Dev. 2023, 28, 387–393. [Google Scholar] [CrossRef]
- Garg, S.J.; Gekeler, F. Subretinal air used as a ball valve to stabilize a retinal bleb. Retin. Cases Brief Rep. 2023, 17, 189–190. [Google Scholar] [CrossRef]
- Maierhofer, N.A.; Jablonka, A.-M.; Roodaki, H.; Nasseri, M.A.; Eslami, A.; Klaas, J.; Lohmann, C.P.; Maier, M.; Zapp, D. IOCT-Guided Simulated Subretinal Injections: A Comparison between Manual and Robot-Assisted Techniques in an Ex-Vivo Porcine Model. J. Robot Surg. 2023, 17, 2735–2742. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Jin, X.; Wang, Z.; Fang, Y.; Li, Z.; Yang, Z.; Cong, J.; Yang, Y.; Huang, Y.; Wang, L. Robot-Assisted Subretinal Injection System: Development and Preliminary Verification. BMC Ophthalmol. 2022, 22, 484. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Yang, H.; Chen, T.; Chen, C. Analysis on Key Parameters in Subretinal Injection Facilitating a Predictable and Automated Robot-assisted Treatment in Gene Therapy. Int. J. Med. Robot. Comput. Assist. Surg. 2023, 19, e2560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Kim, J.W.; Gehlbach, P.; Iordachita, I.; Kobilarov, M. Autonomous Needle Navigation in Subretinal Injections via IOCT. IEEE Robot Autom. Lett. 2024, 9, 4154–4161. [Google Scholar] [CrossRef]
- Abid, A.; Duval, R.; Boutopoulos, C. Development and Ex-Vivo Validation of 36G Polyimide Cannulas Integrating a Guiding Miniaturized OCT Probe for Robotic Assisted Subretinal Injections. Biomed. Opt. Express 2022, 13, 850. [Google Scholar] [CrossRef]
- Dehghani, S.; Sommersperger, M.; Zhang, P.; Martin-Gomez, A.; Busam, B.; Gehlbach, P.; Navab, N.; Nasseri, M.A.; Iordachita, I. Robotic Navigation Autonomy for Subretinal Injection via Intelligent Real-Time Virtual IOCT Volume Slicing. In Proceedings of the 2023 IEEE International Conference on Robotics and Automation (ICRA), London, UK, 29 May–2 June 2023; IEEE: New York, NY, USA, 2023; pp. 4724–4731. [Google Scholar]
- Arikan, D.; Zhang, P.; Sommersperger, M.; Dehghani, S.; Esfandiari, M.; Taylor, R.H.; Nasseri, M.A.; Gehlbach, P.; Navab, N.; Iordachita, I. Real-Time Deformation-Aware Control for Autonomous Robotic Subretinal Injection under IOCT Guidance. arXiv 2024, arXiv:2411.06557. [Google Scholar]
- Łajczak, P.M.; Nawrat, Z. Sharper Vision, Steady Hands: Can Robots Improve Subretinal Drug Delivery? Systematic Review. J. Robot Surg. 2024, 18, 235. [Google Scholar] [CrossRef]
- Hejri, A.; Chrenek, M.A.; Goehring, N.T.; Bowland, I.I.; Noel, R.; Yan, J.; Nickerson, J.M.; Prausnitz, M.R. A Non-Surgical Method for Subretinal Delivery by Trans-Scleral Microneedle Injection. Bioeng. Transl. Med. 2025, 10, e10755. [Google Scholar] [CrossRef]
- Kasetty, V.M.; Monsalve, P.F.; Sethi, D.; Yousif, C.; Hessburg, T.; Kumar, N.; Hamad, A.E.; Desai, U.R. Cataract Progression after Primary Pars Plana Vitrectomy for Uncomplicated Rhegmatogenous Retinal Detachments in Young Adults. Int. J. Retina Vitreous 2024, 10, 19. [Google Scholar] [CrossRef]
- Luo, X.; Chen, X.; Gong, S.; Li, X. Two-Port Subretinal Injection without Vitrectomy for the Treatment of Bietti Crystalline Dystrophy. Chin. J. Ocul. Fundus Dis. 2024, 40, 429–433. [Google Scholar]
- Wood, E.H.; Rao, P.; Mahmoud, T.H. Nanovitreoretinal Subretinal Gateway Device to Displace Submacular Hemorrhage: Access to the Subretinal Space Without Vitrectomy. Retina 2022, 42, 2225–2228. [Google Scholar] [CrossRef]
- Gyroscope Therapeutics Announces FDA Clearance for Orbit Subretinal Delivery System. Available online: https://www.synconaltd.com/news-insights/news/gyroscope-therapeutics-announces-fda-clearance-for-orbit-subretinal-delivery-system (accessed on 21 June 2025).
- Hancock, S.E.; Wan, C.-R.; Fisher, N.E.; Andino, R.V.; Ciulla, T.A. Biomechanics of Suprachoroidal Drug Delivery: From Benchtop to Clinical Investigation in Ocular Therapies. Expert Opin. Drug Deliv. 2021, 18, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Chae, J.J.; Prausnitz, M.R. Targeting Drug Delivery within the Suprachoroidal Space. Drug Discov. Today 2019, 24, 1654–1659. [Google Scholar] [CrossRef] [PubMed]
- Kansara, V.; Muya, L.; Wan, C.; Ciulla, T.A. Suprachoroidal Delivery of Viral and Nonviral Gene Therapy for Retinal Diseases. J. Ocul. Pharmacol. Ther. 2020, 36, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Lampen, S.I.R.; Khurana, R.N.; Noronha, G.; Brown, D.M.; Wykoff, C.C. Suprachoroidal Space Alterations Following Delivery of Triamcinolone Acetonide: Post-Hoc Analysis of the Phase 1/2 HULK Study of Patients with Diabetic Macular Edema. Ophthalmic Surg. Lasers Imaging Retin 2018, 49, 692–697. [Google Scholar] [CrossRef]
- Chung, S.H.; Mollhoff, I.N.; Mishra, A.; Sin, T.-N.; Ngo, T.; Ciulla, T.; Sieving, P.; Thomasy, S.M.; Yiu, G. Host Immune Responses after Suprachoroidal Delivery of AAV8 in Nonhuman Primate Eyes. Hum. Gene Ther. 2021, 32, 682–693. [Google Scholar] [CrossRef]
- Shen, J.; Kim, J.; Tzeng, S.Y.; Ding, K.; Hafiz, Z.; Long, D.; Wang, J.; Green, J.J.; Campochiaro, P.A. Suprachoroidal Gene Transfer with Nonviral Nanoparticles. Sci. Adv. 2020, 6, eaba1606. [Google Scholar] [CrossRef]
- Kicińska, A.K.; Rękas, M. Alternative Application of an ITrack Microcatheter and Canaloplasty: Case Report and Literature Review. Expert Opin. Drug Deliv. 2023, 20, 1201–1208. [Google Scholar] [CrossRef]
- Naftali Ben Haim, L.; Moisseiev, E. Drug Delivery via the Suprachoroidal Space for the Treatment of Retinal Diseases. Pharmaceutics 2021, 13, 967. [Google Scholar] [CrossRef]
- Yeh, S.; Ciulla, T. Suprachoroidal Triamcinolone Acetonide Injectable Suspension for Macular Edema Associated with Noninfectious Uveitis: An in-Depth Look at Efficacy and Safety. Am. J. Manag. Care 2023, 29, S19–S28. [Google Scholar] [PubMed]
- SCS Microinjector. Available online: https://clearsidebio.com/technology/scs-microinjector/ (accessed on 21 June 2025).
- Shen, J.; Lima e Silva, R.; Zhang, M.; Luly, K.M.; Hackett, S.F.; Tzeng, S.Y.; Lowmaster, S.M.; Shannon, S.R.; Wilson, D.R.; Green, J.J.; et al. Suprachoroidal Gene Transfer with Nonviral Nanoparticles in Large Animal Eyes. Sci. Adv. 2024, 10, eadl3576. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.; Khurana, R.N.; Shah, M.; Henry, C.R.; Wang, R.C.; Kissner, J.M.; Ciulla, T.A.; Noronha, G. Efficacy and Safety of Suprachoroidal CLS-TA for Macular Edema Secondary to Noninfectious Uveitis. Ophthalmology 2020, 127, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.R.; Shah, M.; Barakat, M.R.; Dayani, P.; Wang, R.C.; Khurana, R.N.; Rifkin, L.; Yeh, S.; Hall, C.; Ciulla, T. Suprachoroidal CLS-TA for Non-Infectious Uveitis: An Open-Label, Safety Trial (AZALEA). Br. J. Ophthalmol. 2022, 106, 802–806. [Google Scholar] [CrossRef]
- Khurana, R.N.; Merrill, P.; Yeh, S.; Suhler, E.; Barakat, M.R.; Uchiyama, E.; Henry, C.R.; Shah, M.; Wang, R.C.; Kapik, B.; et al. Extension Study of the Safety and Efficacy of CLS-TA for Treatment of Macular Oedema Associated with Non-Infectious Uveitis (MAGNOLIA). Br. J. Ophthalmol. 2021, 106, 1139–1144. [Google Scholar] [CrossRef]
- Pitcher, J.D.; Eye, H. Suprachoroidal Delivery of Investigational ABBV-RGX-314 for Neovascular AMD: Results from the Phase II AAVIATE® Study; RegenxBio: Rockville, MD, USA, 2024. [Google Scholar]
- Barakat, M.R. Suprachoroidal Delivery of Investigational ABBV-RGX-314 for Diabetic Retinopathy: The Phase II ALTITUDE® Study Dose Levels 1 and 2: One Year Results; RegenxBio: Rockville, MD, USA, 2023. [Google Scholar]
- Lam, L.A.; Mehta, S.; Lad, E.M.; Emerson, G.G.; Jumper, J.M.; Awh, C.C. Task Force on Intravitreal Injection Supplemental Services Intravitreal Injection Therapy: Current Techniques and Supplemental Services. J. Vitreoretin. Dis. 2021, 5, 438–447. [Google Scholar] [CrossRef]
- Battu, R.; Ratra, D.; Gopal, L. Newer Therapeutic Options for Inherited Retinal Diseases: Gene and Cell Replacement Therapy. Indian J. Ophthalmol. 2022, 70, 2316–2325. [Google Scholar] [CrossRef]
- Feuer, W.J.; Schiffman, J.C.; Davis, J.L.; Porciatti, V.; Gonzalez, P.; Koilkonda, R.D.; Yuan, H.; Lalwani, A.; Lam, B.L.; Guy, J. Gene Therapy for Leber Hereditary Optic Neuropathy. Ophthalmology 2016, 123, 558–570. [Google Scholar] [CrossRef]
- Tan, T.-E.; Fenner, B.J.; Barathi, V.A.; Tun, S.B.B.; Wey, Y.S.; Tsai, A.S.H.; Su, X.; Lee, S.Y.; Cheung, C.M.G.; Wong, T.Y.; et al. Gene-Based Therapeutics for Acquired Retinal Disease: Opportunities and Progress. Front. Genet. 2021, 12, 795010. [Google Scholar] [CrossRef]
- Ross, M.; Ofri, R. The Future of Retinal Gene Therapy: Evolving from Subretinal to Intravitreal Vector Delivery. Neural Regen. Res. 2021, 16, 1751–1759. [Google Scholar] [CrossRef]
- Nuzbrokh, Y.; Ragi, S.D.; Tsang, S.H. Gene Therapy for Inherited Retinal Diseases. Ann. Transl. Med. 2021, 9, 1278. [Google Scholar] [CrossRef]
- Ahn, S.; Siontas, O.; Koester, J.; Krol, J.; Fauser, S.; Müller, D.J. Magnetically Guided Adeno-Associated Virus Delivery for the Spatially Targeted Transduction of Retina in Eyes. Adv. Healthc. Mater. 2024, 13, 2401577. [Google Scholar] [CrossRef]
- Wang, J.-H.; Cui, M.; Liu, H.; Guo, P.; McGowan, J.; Cheng, S.-Y.; Gessler, D.J.; Xie, J.; Punzo, C.; Tai, P.W.L.; et al. Cell-Penetrating Peptide-Grafted AAV2 Capsids for Improved Retinal Delivery via Intravitreal Injection. Mol. Ther. Methods Clin. Dev. 2025, 33, 101426. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, Y.; Zhao, L.; Li, X.; Zhang, B.; Tang, Y.; Zhou, T.; Zheng, Z.; Li, A.; Wei, J.; et al. Novel Intravitreal Retina-Targeting and Immune-Evading Adeno-Associated Virus Capsid Variant. Preprints 2024, 2024092179. [Google Scholar] [CrossRef]
- Chiu, W.; Lin, T.-Y.; Chang, Y.-C.; Isahwan-Ahmad Mulyadi Lai, H.; Lin, S.-C.; Ma, C.; Yarmishyn, A.A.; Lin, S.-C.; Chang, K.-J.; Chou, Y.-B.; et al. An Update on Gene Therapy for Inherited Retinal Dystrophy: Experience in Leber Congenital Amaurosis Clinical Trials. Int. J. Mol. Sci. 2021, 22, 4534. [Google Scholar] [CrossRef] [PubMed]
- Pennesi, M.E.; Yang, P.; Birch, D.G.; Weng, C.Y.; Moore, A.T.; Iannaccone, A.; Comander, J.I.; Jayasundera, T.; Chulay, J.; Chulay, J.; et al. Intravitreal Delivery of RAAV2tYF-CB-HRS1 Vector for Gene Augmentation Therapy in Patients with X-Linked Retinoschisis. Ophthalmol. Retin. 2022, 6, 1130–1144. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P. Biogen Bails on AGTC after Ocular Gene Therapy Flunks Trial; Fierce Biotech: New York, NY, USA, 2018. [Google Scholar]
- Khanani, A.M.; Boyer, D.S.; Wykoff, C.C.; Regillo, C.D.; Busbee, B.G.; Pieramici, D.; Danzig, C.J.; Joondeph, B.C.; Major, J.C.; Turpcu, A.; et al. Safety and Efficacy of Ixoberogene Soroparvovec in Neovascular Age-Related Macular Degeneration in the United States (OPTIC): A Prospective, Two-Year, Multicentre Phase 1 Study. eClinicalMedicine 2024, 67, 102394. [Google Scholar] [CrossRef]
- Smith, K. 4DMT Highlights Robust and Durable Clinical Activity for 4D-150 and Design of 4FRONT Phase 3 Program at 4D-150 Wet AMD Development Day; 4DMT: Emeryville, CA, USA, 2024. [Google Scholar]
- AlEissa, M.M.; Alhawsawi, A.A.; Alonazi, R.; Magharbil, E.; Aljahdali, A.; AlBalawi, H.B.; Alali, N.M.; Hameed, S.; Abu-Amero, K.K.; Magliyah, M.S. Advances in Precision Therapeutics and Gene Therapy Applications for Retinal Diseases: Impact and Future Directions. Genes 2025, 16, 847. [Google Scholar] [CrossRef]
- Zimmermann, M.; Lubinga, S.J.; Banken, R.; Rind, D.; Cramer, G.; Synnott, P.G.; Chapman, R.H.; Khan, S.; Carlson, J. Cost Utility of Voretigene Neparvovec for Biallelic RPE65-Mediated Inherited Retinal Disease. Value Health 2019, 22, 161–167. [Google Scholar] [CrossRef]
- Jayasundera, K.T.; Abuzaitoun, R.O.; Lacy, G.D.; Abalem, M.F.; Saltzman, G.M.; Ciulla, T.A.; Johnson, M.W. Challenges of Cost-Effectiveness Analyses of Novel Therapeutics for Inherited Retinal Diseases. Am. J. Ophthalmol. 2022, 235, 90–97. [Google Scholar] [CrossRef]


| Vectors | Advantages | Disadvantages | References |
|---|---|---|---|
| Adenoviral (~1–3%) |
|
| |
|
| [3,5,6,7] | |
| |||
| Adeno-associated (~80–90%) |
|
| [3,7] |
|
| ||
| Lentiviral/Retroviral (~5–10%) |
|
| [3,7] |
| |||
| |||
| |||
| Non-Viral (Liposomes, Polymers, Oligonucleotide, Nanoparticles) (1–2%) |
|
| [5,7,8] |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, M.J.; Sheth, S.; Mar, J.; Gregori, N.Z.; Sengillo, J.D. Surgical Approaches to Retinal Gene Therapy: 2025 Update. Bioengineering 2025, 12, 1122. https://doi.org/10.3390/bioengineering12101122
Patel MJ, Sheth S, Mar J, Gregori NZ, Sengillo JD. Surgical Approaches to Retinal Gene Therapy: 2025 Update. Bioengineering. 2025; 12(10):1122. https://doi.org/10.3390/bioengineering12101122
Chicago/Turabian StylePatel, Milin J., Sohum Sheth, Jessica Mar, Ninel Z. Gregori, and Jesse D. Sengillo. 2025. "Surgical Approaches to Retinal Gene Therapy: 2025 Update" Bioengineering 12, no. 10: 1122. https://doi.org/10.3390/bioengineering12101122
APA StylePatel, M. J., Sheth, S., Mar, J., Gregori, N. Z., & Sengillo, J. D. (2025). Surgical Approaches to Retinal Gene Therapy: 2025 Update. Bioengineering, 12(10), 1122. https://doi.org/10.3390/bioengineering12101122






