Microwave Breast Imaging System Modules, Enhancing Scan Quality and Reliability of Diagnostic Outputs During Clinical Testing
Abstract
1. Introduction
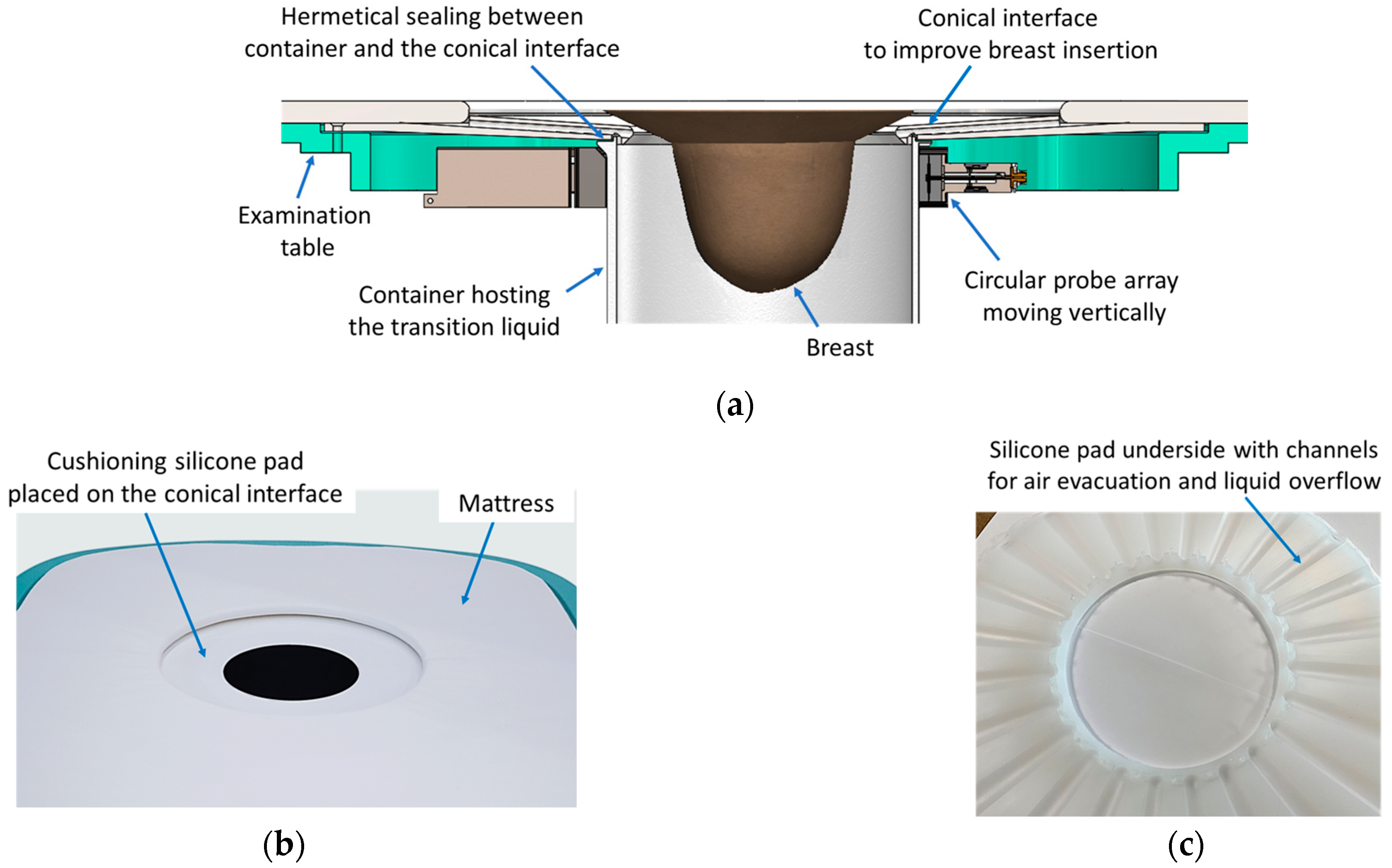
- Ergonomic accessories, which contribute to improved immersion of the breast in the transition liquid and deeper ultimate insertion in the scanning zone.
- An optical system embedded in the MWBI scanner and contributing to: (a) significant improvement in breast positioning in the scanner, (b) better management of the achieved quality of scan, and (c) rationalization of the ROI localization in the MWBI images and its association to pre-diagnosed lesions in reference imaging data, in cases of misaligned, non-vertical and/or twisted insertion of the breast in the MWBI scanner. This new optical system was introduced in the pilot clinical investigation with Wavelia#2, while the Optical Breast Contour Detection (OBCD) subsystem that was implemented in a separate examination table and formed part of the Wavelia#1 scanner, as presented in detail in [39] and employed during the FiH clinical investigation, was rendered obsolete for Wavelia#2.
- A new data processing module that was designed and implemented during the pilot clinical investigation with Wavelia#2, to assess the quality of the MWBI scan at the uppermost coronal section being scanned, close to the examination table, and reject portions of it, resulting in MWBI image formation and analysis based on a partial MWBI scan, if justified. The algorithm and set of criteria for scan quality assessment are based on geometrical analysis of the reconstructed contour of the breast. A dedicated module for MWBI scan-based breast contour reconstruction was part of the Wavelia data processing methodology since its early development [40], albeit significantly upgraded recently, in the scope of the Wavelia#2 development, as introduced in [41]. The importance of the breast contour reconstruction to the MWBI achieved imaging quality was earlier investigated in the MWBI research community, and various distinct methodologies have been considered and accordingly developed [42,43,44,45,46,47]. A breast contour assessment-based estimation tool, employed to define a partial MWBI scan of sufficient quality to deliver reliable images and image analysis outputs, i.e., ROIs and associated features, for meaningful clinical analysis, was, for the first time, conceived and implemented for Wavelia#2.
2. Materials and Methods
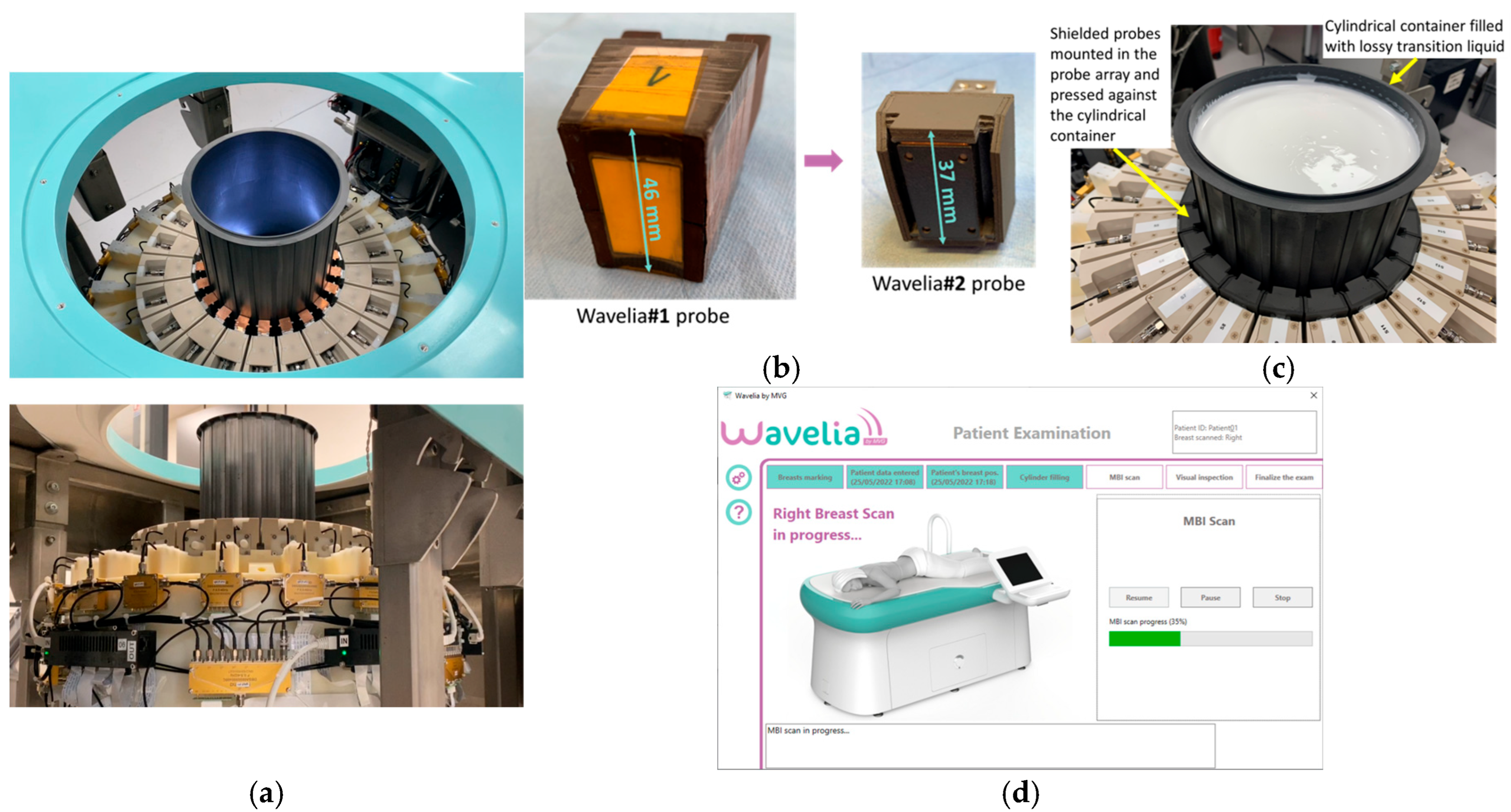
2.1. The Wavelia#2 Microwave Breast Imaging (MWBI) Scanner Prototype
2.2. Wavelia#2 Pilot Clinical Investigation
2.2.1. The Study Dataset
2.2.2. The Main Study Outcomes: Diagnostic Performance and Usability of the Wavelia#2 MWBI Scanner
- Stratified lesion detection results:
- MWBI-based lesion sizing: concordance with ultrasound:
- Lesion characterization: malignant-to-benign lesion discriminating features:
- Phenomenological qualitative assessment of specificity in healthy contralateral breasts of the enrolled patients:
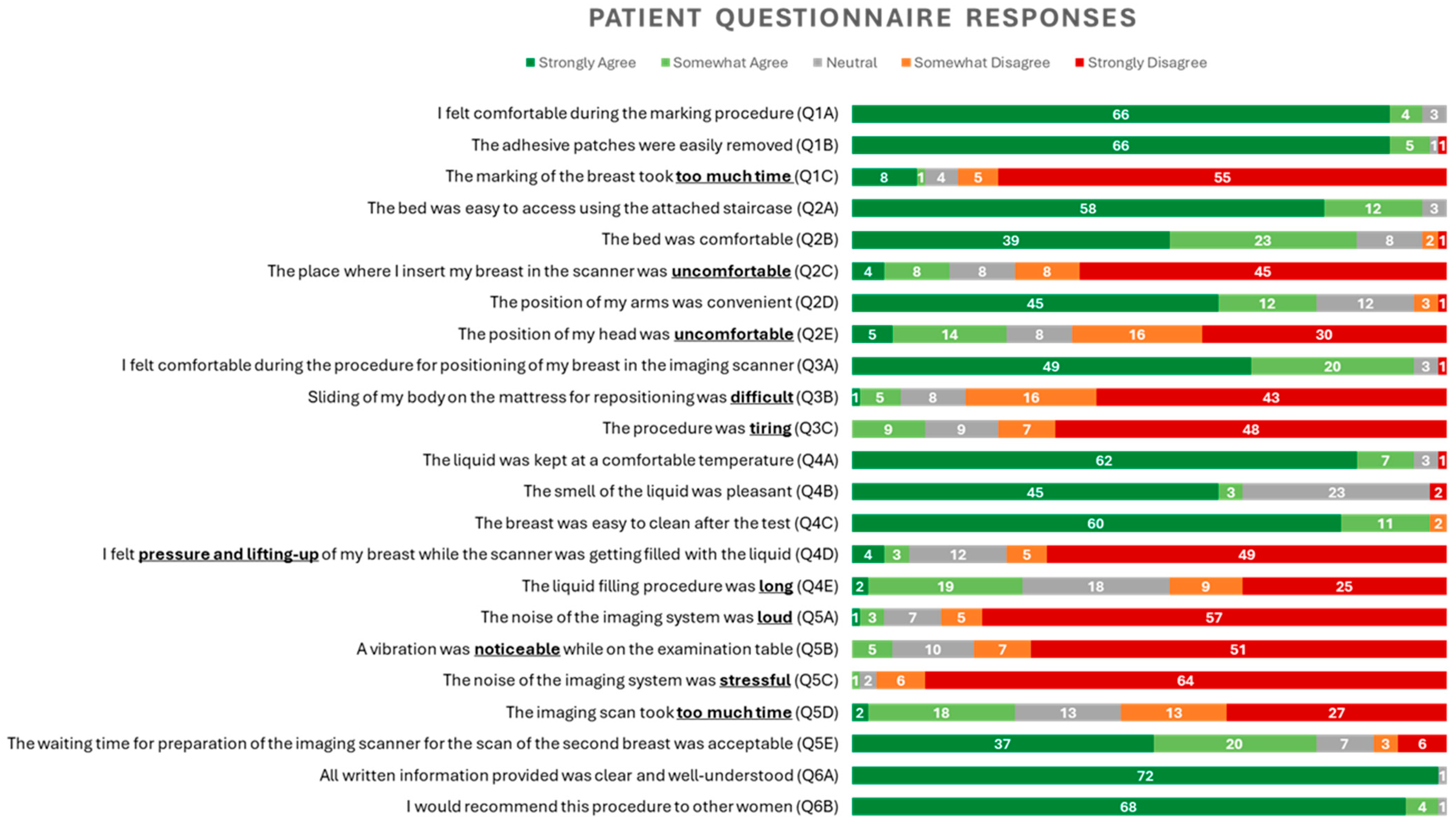
- Patient-reported outcomes: usability and comfort of the Wavelia#2 MWBI scanner:
2.3. The Ergonomic Interface of the Wavelia#2 Examination Table with the Breast
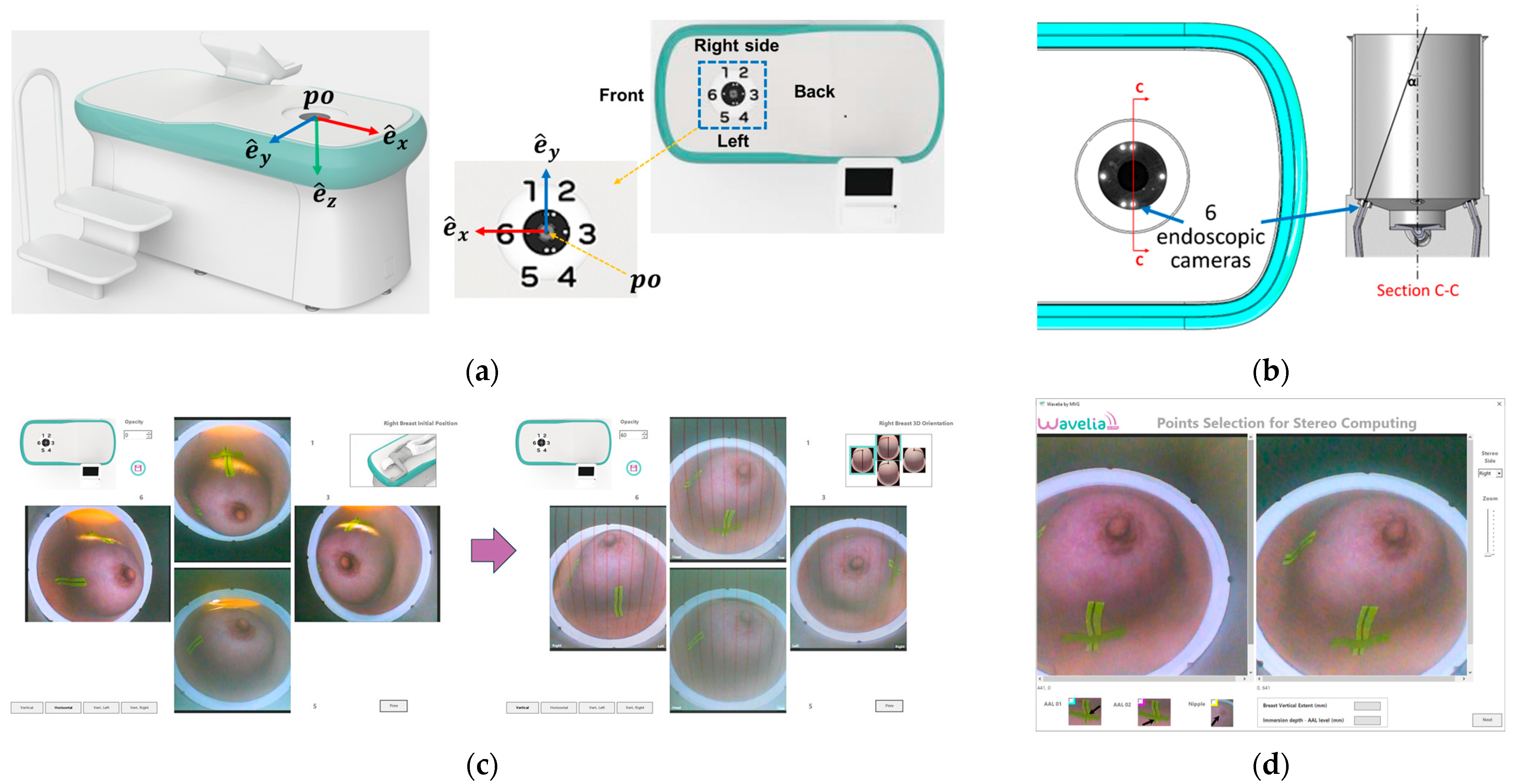
2.4. The Aid-to-Breast Positioing Module: Endoscopic Cameras Employment for Inspection and Partial Control of the Breast Position in the Scanner
- The C-N line, connecting the midpoint of the clavicle (C) to the nipple (N), is used to control the azimuthal orientation of the breast and ensure valid quadrant definition in the MWBI images.
- The AAL line (Anterior Axillary Line), which is used to control the immersion depth of the breast in the scanner, provides a means to better quantify the ratio of distance-to-nipple over distance-to-chest wall for the identified ROIs.
- The BP line. This is the line perpendicular to the AAL line and passing through the nipple of the breast. The purpose of marking the BP line is to control the verticality of the breast in the scanner, for valid breast quadrant definition on the MWBI images.
2.5. The MWBI Breast Contour Extraction Module: Semi-Automated Definition of Partial Scan Extent
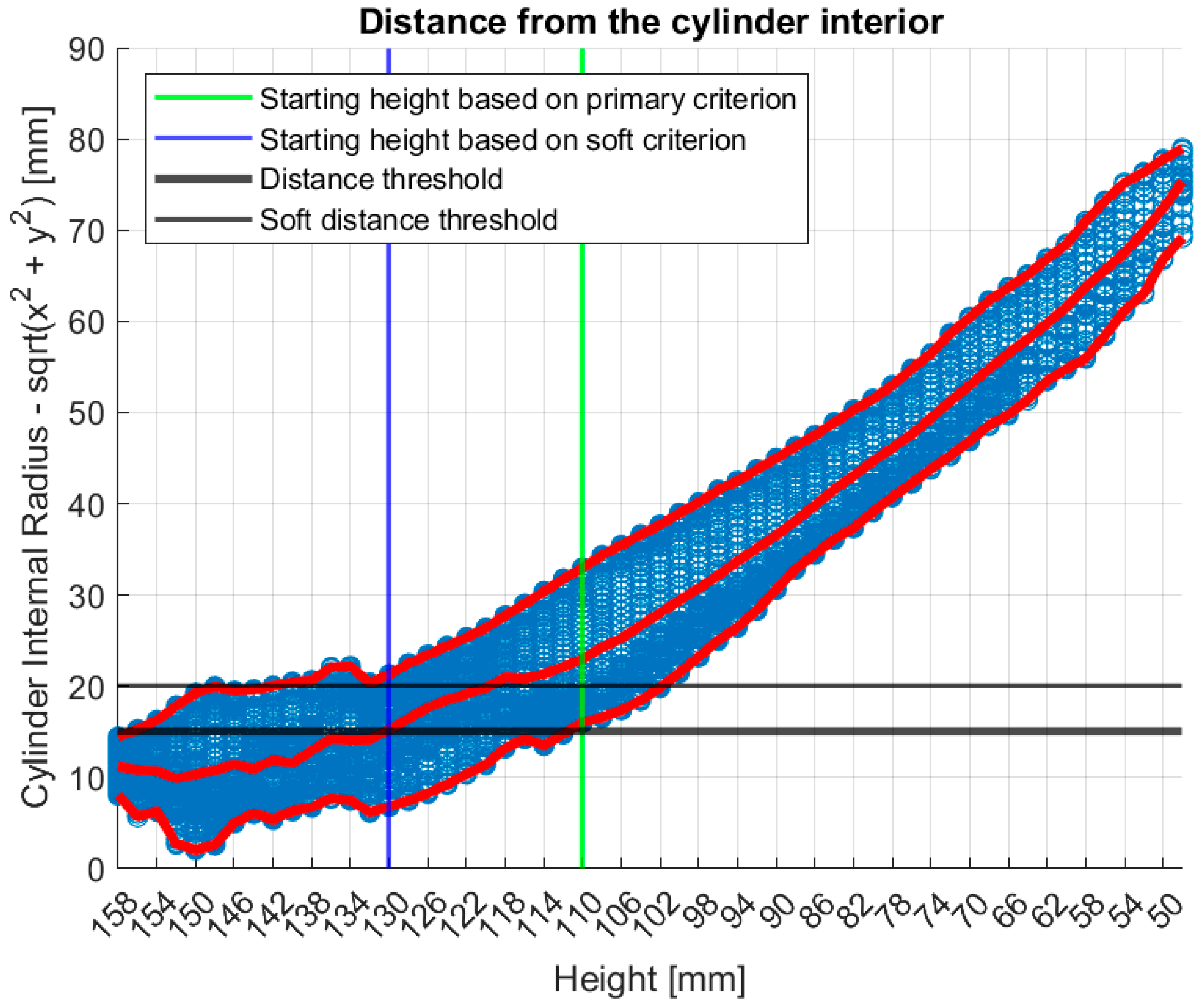
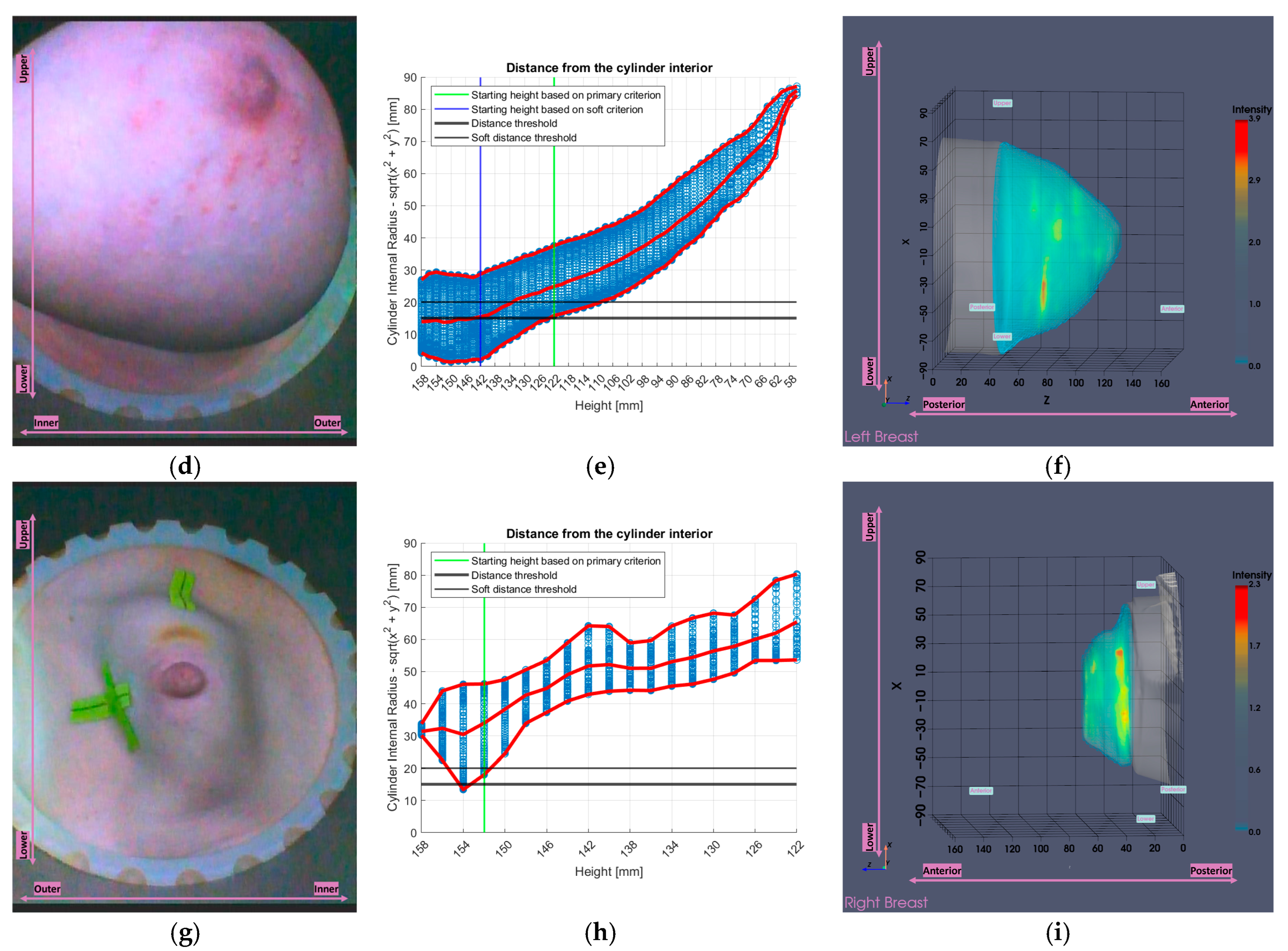
2.5.1. Partial Scan Generation for Enhanced Imaging Fidelity—Large Breasts
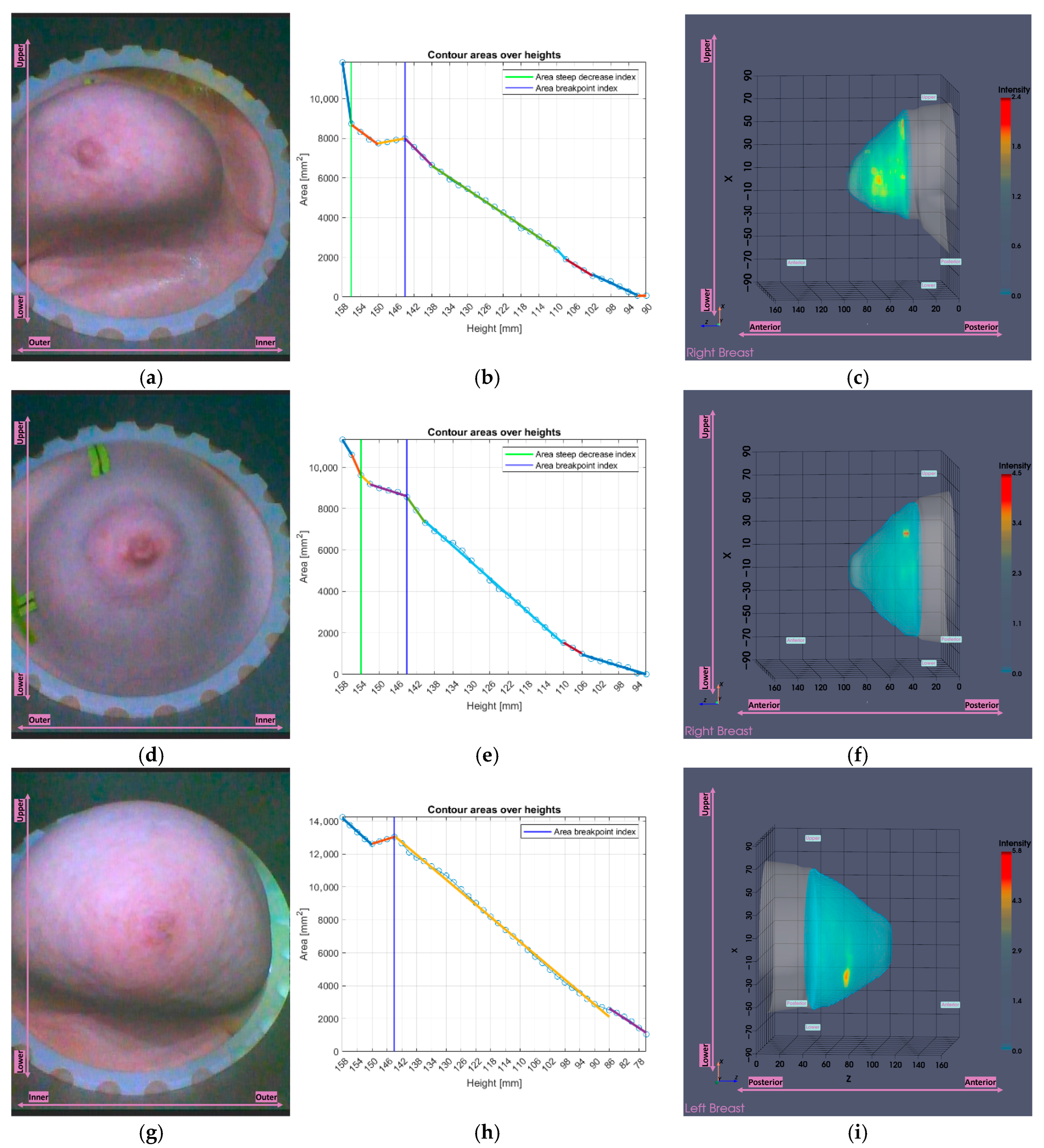
2.5.2. Partial Scan Generation for Enhanced Imaging Fidelity—Small Breasts
3. Results
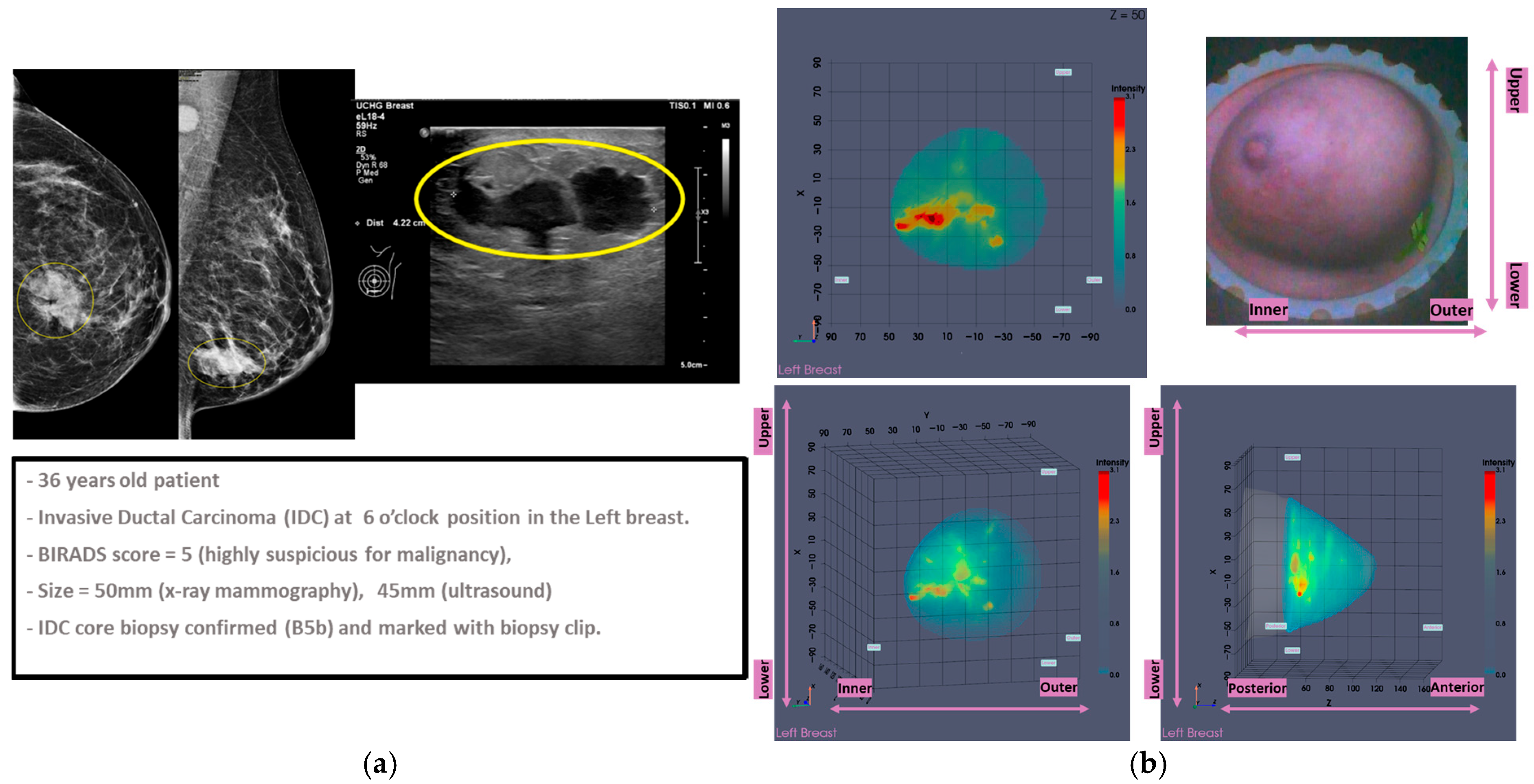
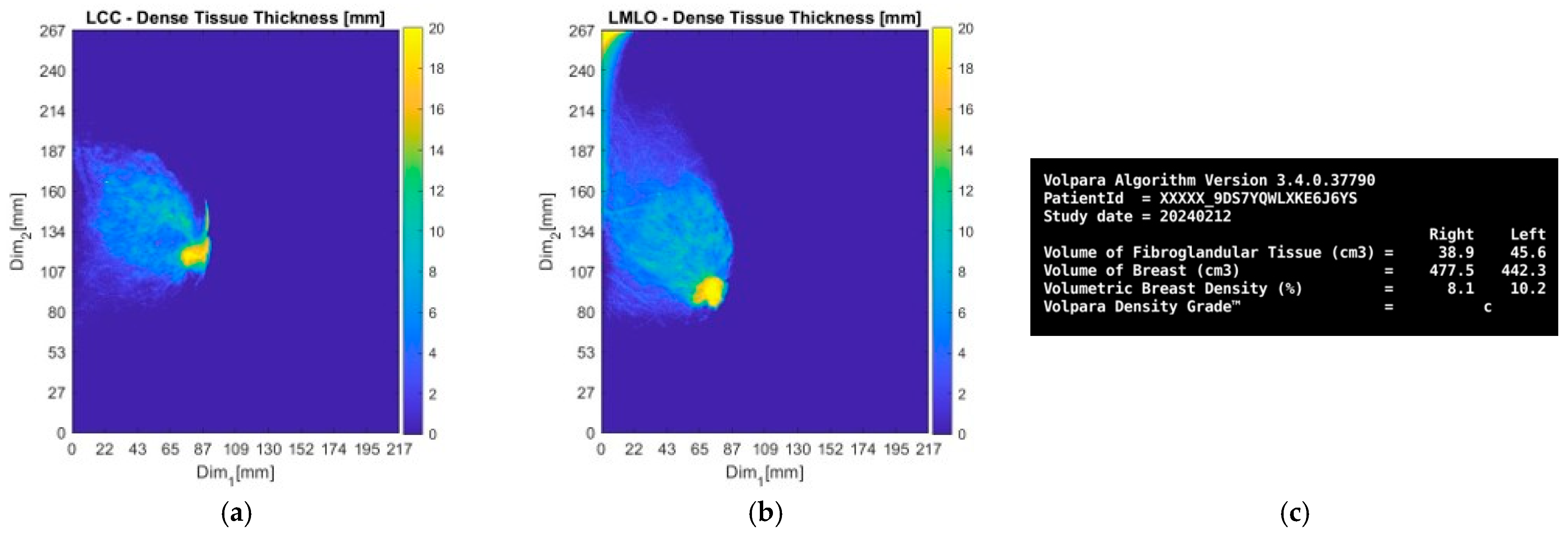
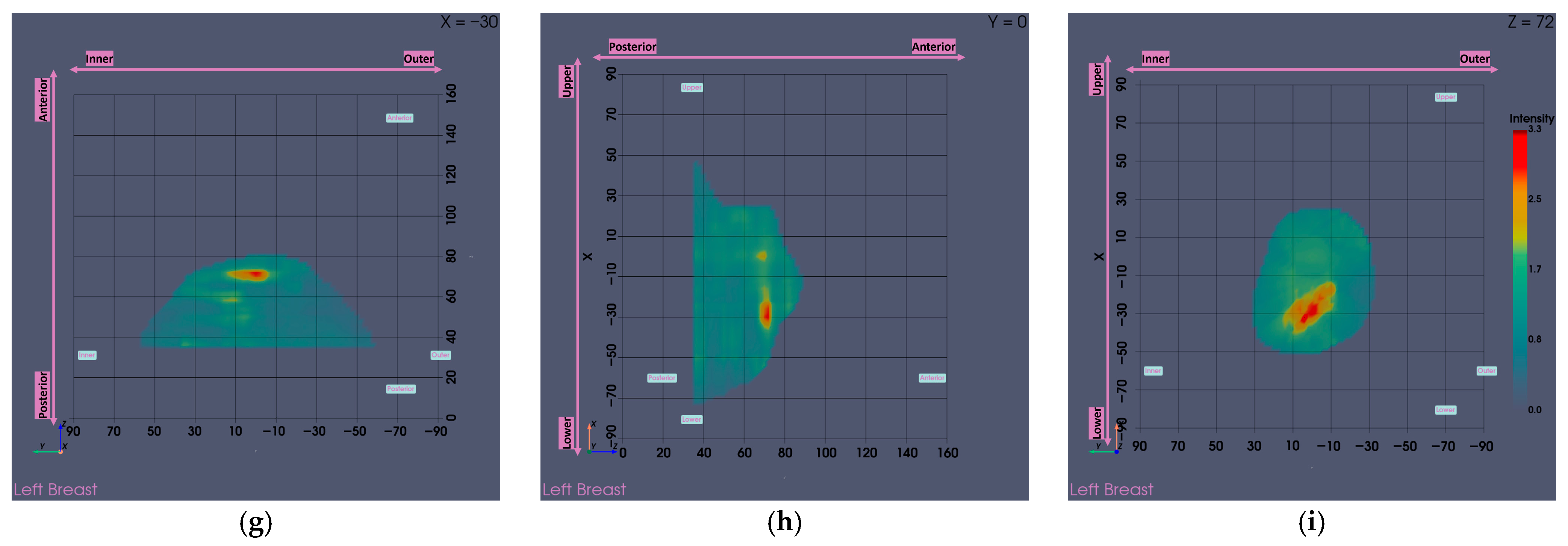
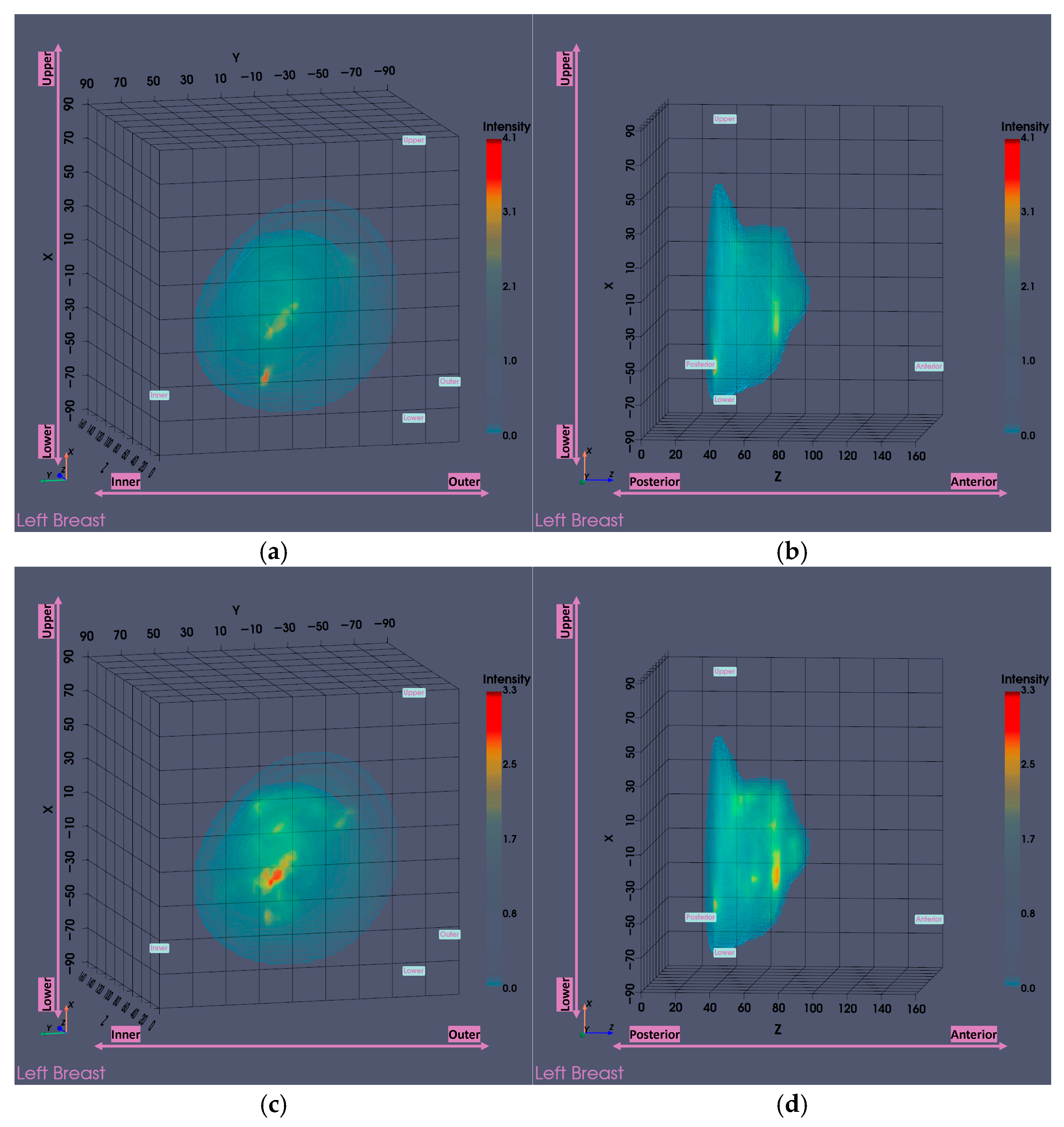
3.1. MWBI Partial Scan of Acceptable Quality and Relevance: Full Example of MWBI Patient Scan Imaging and Image Analysis Outputs for Radiological and Clinical Analysis
3.2. Functional Analysis of the Partial Scan Generation Tools
3.2.1. Large Breasts Tool
3.2.2. Small Breasts Tool
3.3. Breast Volume-Based Evaluation of the Joint Impact of the Partial Scan Generation Tools
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Large Breasts Tool: Technical Specifications
| Symbol | Value (Units) | Description |
|---|---|---|
| — (—) | Set of all measured heights along z-axis | |
| — (—) | ||
| — (mm) | ||
| — (—) | ||
| — (mm) | ||
| — (—) | ||
| 90 (mm) | Probe array internal radius | |
| — (—) | Variable for referring to contour points | |
| — (mm) | to probe the array | |
| 5/360 (—) | Primary indicator percentage threshold of contour points close to the probe array | |
| 15 (mm) | Primary indicator distance threshold | |
| — (—) | Set of heights marked for filtering by primary indicator | |
| — (mm) | Maximum preserved height by primary indicator | |
| — (—) | Candidate sets of heights marked for filtering by soft indicator | |
| 20 (mm) | Soft indicator supplementary distance threshold | |
| — (mm) | Candidate maximum preserved heights by soft indicator | |
| — (mm) | Selected maximum preserved height by soft indicator | |
| 158 (mm) | Maximum scanning position of the MWBI scanner |
Appendix B. Small Breasts Tool: Technical Specifications
- Initialization: Treat the full areas vector as a single segment.
- Select segment: Identify the segment seg (containing n elements) with the largest deviation from linearity, quantified by its Mean Squared Error (MSE).
- Evaluate candidate splits: For each potential breakpoint b within seg:
- Divide seg into two sub-segments:and .
- Fit a linear model to each sub-segment and compute the corresponding MSEs:
- Compute the joint MSE (MSEjoint) for the split: .
- Select optimal split: Choose the candidate breakpoint that minimizes MSEjoint and update the segments list.
- Iterate: Repeat steps 2–4, always selecting the segment with the highest MSE, until the longest segment remains unchanged between two consecutive iterations. This indicates that further splits would not meaningfully reduce the total MSE (MSEtotal) of the approximation, which is defined as the sum of the MSEs of all current segments.
- Identify candidates: Locate all two-point segments in the current segments list.
- Evaluate potential merges: Merge each candidate individually with each of its neighboring segments. Fit a new linear model to the merged data and compute its error MSEmerged. The error increase is quantified as , where MSE1, MSE2 are the errors of the two original segments prior to merging.
- Select merge: Apply the merge with the smallest ΔMSE, provided that the updated MSEtotal after merging remains below a reference threshold:
- Iterate: Repeat steps 2,3 until no two-point segments remain or no candidate merge satisfies the MSE constraint.
- Compute segment slopes: For each segment i, compute the slope si of the linear fit.
- Compute slope ratios: For each pair of adjacent segments [i − 1, i], calculate the slope ratio:
- Flag potential breakpoints: A breakpoint is flagged if all the following conditions are met:
- Current slope is negative (si < 0).
- Either the preceding slope is non-negative (si-1 ≥ 0) or r ≤ 0.5.
- The three-segment region [i − 1, i + 1] contains at least minEvaluableLength points (empirically set to 5).
- The region length is maximal among all evaluated regions.
- Refine breakpoint location: Apply a two-segment linear approximation over the three-segments region [i − 1, i + 1] to determine the precise breakpoint.
- Select final breakpoint: Accept the breakpoint only if it lies within the first third of the areas vector, ensuring it corresponds to the near-chest wall region.
- Fit global slope: Fit a linear model to the entire areas vector to obtain the global slope sglobal (always negative).
- Identify steep segments: Mark segments with slope si ≤ 2·sglobal located within the first half of the areas vector, ensuring their relevance to the near-chest wall region.
- Group adjacent steep segments: Combine consecutive steep segments into regions.
- Select final region: Choose the longest region among all steep regions. The last index of this region defines the boundary of the non-breast tissue.
| Symbol | Value (Units) | Description |
|---|---|---|
| — (mm2) | Vector of breast contour areas at descending height order | |
| — (mm4) | Sum of 2 segments MSEs for evaluating possible split | |
| — (mm4) | Sum of all current segments MSEs | |
| — (mm4) | Segment MSE for evaluating possible merge | |
| — (mm4) | MSE difference for quantifying merge impact | |
| — (mm4) | Total MSE before post-processing merging | |
| 1.2 (—) | Maximum allowed increase in total MSE during post-processing merge | |
| — (mm2/mm) | ||
| — (—) | slope | |
| 5 (points) | ||
| — (mm2/mm) | vector |
References
- Fear, E.C.; Meaney, P.M.; Stuchly, M.A. Microwaves for Breast Cancer Detection? IEEE Potentials 2003, 22, 12–18. [Google Scholar] [CrossRef]
- Fear, E.C.; Hagness, S.C.; Meaney, P.M.; Okoniewski, M.; Stuchly, M.A. Enhancing breast tumor detection with near-field imaging. IEEE Microw. Mag. 2002, 3, 48–56. [Google Scholar] [CrossRef]
- Nikolova, N.K. Microwave imaging for breast cancer. IEEE Microw. Mag. 2011, 12, 78–94. [Google Scholar] [CrossRef]
- Conceição, R.C.; Mohr, J.J.; O’Halloran, M. An Introduction to Microwave Imaging for Breast Cancer Detection; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Kwon, S.; Lee, S. Recent Advances in Microwave Imaging for Breast Cancer Detection. Int. J. Biomed. Imaging 2016, 2016, 5054912. [Google Scholar] [CrossRef]
- O’Loughlin, D.; O’Halloran, M.; Moloney, B.M.; Glavin, M.; Jones, E.; Elahi, M.A. Microwave breast imaging: Clinical advances and remaining challenges. IEEE Trans. Biomed. Eng. 2018, 65, 2580–2590. [Google Scholar] [CrossRef]
- Aldhaeebi, M.A.; Alzoubi, K.; Almoneef, T.S.; Bamatra, S.M.; Attia, H.; Ramahi, O.M. Review of microwaves techniques for breast cancer detection. Sensors 2020, 20, 2390. [Google Scholar] [CrossRef] [PubMed]
- Benny, R.; Anjit, T.A.; Mythili, P. An overview of microwave imaging for breast tumor detection. Prog. Electromagn. Res. B 2020, 87, 61–91. [Google Scholar] [CrossRef]
- Origlia, C.; Rodriguez-Duarte, D.O.; Vasquez, J.A.T.; Bolomey, J.-C.; Vipiana, F. Review of Microwave Near-Field Sensing and Imaging Devices in Medical Applications. Sensors 2024, 24, 4515. [Google Scholar] [CrossRef] [PubMed]
- Lazebnik, M.; McCartney, L.; Popovic, D.; Watkins, C.B.; Lindstrom, M.J.; Harter, J.; Sewall, S.; Magliocco, A.; Booske, J.H.; Okoniewski, M.; et al. A large-scale study of the ultrawideband microwave dielectric properties of normal breast tissue obtained from reduction surgeries. Phys. Med. Biol. 2007, 52, 2637–2656. [Google Scholar] [CrossRef]
- Lazebnik, M.; Popovic, D.; McCartney, L.; Watkins, C.B.; Lindstrom, M.J.; Harter, J.; Sewall, S.; Ogilvie, T.; Magliocco, A.; Breslin, T.M.; et al. A large-scale study of the ultrawideband microwave dielectric properties of normal, benign and malignant breast tissues obtained from cancer surgeries. Phys. Med. Biol. 2007, 52, 6093–6115. [Google Scholar] [CrossRef]
- Sugitani, T.; Kubota, S.I.; Kuroki, S.I.; Sogo, K.; Arihiro, K.; Okada, M.; Kadoya, T.; Hide, M.; Oda, M.; Kikkawa, T. Complex permittivities of breast tumor tissues obtained from cancer surgeries. Appl. Phys. Lett. 2014, 104, 253702. [Google Scholar] [CrossRef]
- Martellosio, A.; Pasian, M.; Bozzi, M.; Perregrini, L.; Mazzanti, A.; Svelto, F.; Summers, P.E.; Renne, G.; Preda, L.; Bellomi, M. Dielectric properties characterization from 0.5 to 50 GHz of breast cancer tissues. IEEE Trans. Microw. Theory Tech. 2017, 65, 998–1011. [Google Scholar] [CrossRef]
- Campbell, A.M.; Land, D.V. Dielectric properties of female human breast tissue measured in vitro at 3.2 GHz. Phys. Med. Biol. 1992, 37, 193–210. [Google Scholar] [CrossRef]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The dielectric properties of biological tissues: III. Parametric models for the dielectric spectrum of tissues. Phys. Med. Biol. 1996; 41, 2271–2293. [Google Scholar]
- Moloney, B.M.; O’Loughlin, D.; Elwahab, S.A.; Kerin, M.J. Breast cancer detection—A synopsis of conventional modalities and the potential role of microwave imaging. Diagnostics 2020, 10, 103. [Google Scholar] [CrossRef]
- Meaney, P.M.; Fanning, M.W.; Li, D.; Poplack, S.P.; Paulsen, K.D. A clinical prototype for active microwave imaging of the breast. IEEE Trans. Microw. Theory Tech. 2000, 48, 1841–1853. [Google Scholar] [CrossRef]
- Meaney, P.M.; Kaufman, P.A.; Muffly, L.S.; Click, M.; Poplack, S.P.; Wells, W.A.; Schwartz, G.N.; di Florio-Alexander, R.M.; Tosteson, T.D.; Li, Z.; et al. Microwave imaging for neoadjuvant chemotherapy monitoring: Initial clinical experience. Breast Cancer Res. 2013, 15, R35. [Google Scholar] [CrossRef] [PubMed]
- Shere, M.; Preece, A.; Craddock, I.; Leendertz, J.; Klemm, M. Multistatic radar: First trials of a new breast imaging modality. Breast Cancer Res. 2009, 11, O5. [Google Scholar] [CrossRef]
- Shere, M.; Lyburn, I.; Sidebottom, R.; Massey, H.; Gillett, C.; Jones, L. MARIA®M5: A multicentre clinical study to evaluate the ability of the Micrima radio-wave radar breast imaging system (MARIA®) to detect lesions in the symptomatic breast. Eur. J. Radiol. 2019, 116, 61–67. [Google Scholar] [CrossRef]
- Sidebottom, R.; Webb, D.; Bishop, B.; Mohammed, K.; Allen, S. Results for the London investigation into dielectric scanning of lesions study of the MARIA® M6 breast imaging system. Br. J. Radiol. 2024, 97, 549–552. [Google Scholar] [CrossRef]
- Khalid, B.; Khalesi, B.; Ghavami, N.; Sani, L.; Vispa, A.; Badia, M.; Dudley, S.; Ghavami, M.; Tiberi, G. 3D Huygens Principle Based Microwave Imaging Through MammoWave Device: Validation Through Phantoms. IEEE Access 2022, 10, 106770–106780. [Google Scholar] [CrossRef]
- Sani, L.; Vispa, A.; Loretoni, R.; Duranti, M.; Ghavami, N.; Sánchez-Bayuela, D.A.; Caschera, S.; Paoli, M.; Bigotti, A.; Badia, M.; et al. Breast lesion detection through MammoWave device: Empirical detection capability assessment of microwave images’ parameters. PLoS ONE 2021, 16, e0250005. [Google Scholar] [CrossRef]
- Sánchez-Bayuela, D.Á.; Ghavami, N.; Tiberi, G.; Sani, L.; Vispa, A.; Bigotti, A.; Raspa, G.; Badia, M.; Papini, L.; Ghavami, M.; et al. A multicentric, single arm, prospective, stratified clinical investigation to evaluate MammoWave’s ability in breast lesions detection. PLoS ONE 2023, 18, e0288312. [Google Scholar] [CrossRef]
- Janjic, A.; Akduman, I.; Cayoren, M.; Bugdayci, O.; Aribal, M.E. Microwave Breast Lesion Classification–Results from Clinical Investigation of the SAFE Microwave Breast Cancer System. Acad. Radiol. 2022, 30, S1–S8. [Google Scholar] [CrossRef]
- Yurtseven, A.; Janjic, A.; Cayoren, M.; Bugdayci, O.; Aribal, M.E.; Akduman, I. XGBoost Enhances the Performance of SAFE: A Novel Microwave Imaging System for Early Detection of Malignant Breast Cancer. Cancers 2025, 17, 214. [Google Scholar] [CrossRef]
- Porter, E.; Coates, M.; Popovic, M. An Early Clinical Study of Time-Domain Microwave Radar for Breast Health Monitoring. IEEE Trans. Biomed. Eng. 2016, 63, 530–539. [Google Scholar] [CrossRef]
- Fear, E.C.; Bourqui, J.; Curtis, C.; Mew, D.; Docktor, B.; Romano, C. Microwave breast imaging with a monostatic radar-based system: A study of application to patients. IEEE Trans. Microw. Theory Tech. 2013, 61, 2119–2128. [Google Scholar] [CrossRef]
- Fasoula, A.; Moloney, B.M.; Duchesne, L.; Cano, J.D.G.; Oliveira, B.L.; Bernard, J.; Kerin, M.J. Super-resolution radar imaging for breast cancer detection with microwaves: The integrated information selection criteria. In Proceedings of the 41st Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Berlin, Germany, 23–27 July 2019. [Google Scholar]
- Fasoula, A.; Duchesne, L.; Abdoush, Y.; Baracco, J.M. Frequency-dependent, configurable, sensor fidelity zone for microwave breast imaging: System dimensioning and image quality enhancement. In Proceedings of the IEEE Conference on Antenna Measurements & Applications (CAMA), Antibes Juan-les-Pins, France, 15–17 November 2021; pp. 487–492. [Google Scholar]
- Fasoula, A.; Duchesne, L.; Cano, J.G.; Lawrence, P.; Robin, G.; Bernard, J.-G. On-Site Validation of a Microwave Breast Imaging System, before First Patient Study. Diagnostics 2018, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Fasoula, A.; Arvanitis, P.; Duchesne, L. Repeatability assessement of the Wavelia#2 Microwave Breast Imaging scan: Experimental performance analysis prior to clinical investigation. In Microwave Technologies-Recent Advances and New Trends and Applications; Savci, H.Ş., Arvas, E., Eds.; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar] [CrossRef]
- Fasoula, A.; Duchesne, L.; Cano, J.D.G.; Moloney, B.M.; Elwahab, S.M.A.; Kerin, M.J. Automated breast lesion detection and characterization with the wavelia microwave breast imaging system: Methodological proof-of-concept on first-in-human patient data. Appl. Sci. 2021, 11, 9998. [Google Scholar] [CrossRef]
- MVG Industries, TN.32.1.17.SATF, First-In-Human Clinical Investigation Protocol. 2018. Available online: https://www.clinicaltrials.gov/study/NCT03475992 (accessed on 1 October 2025).
- Moloney, B.M.; McAnena, P.F.; Elwahab, S.M.A.; Fasoula, A.; Duchesne, L.; Cano, J.D.G.; Glynn, C.; O’Connell, A.; Ennis, R.; Lowery, A.J.; et al. Microwave Imaging in Breast Cancer–Results from the First-in-Human Clinical Investigation of the Wavelia System. Acad. Radiol. 2021, 29, S211–S222. [Google Scholar] [CrossRef]
- Moloney, B.M.; McAnena, P.F.; Elwahab, S.M.; Fasoula, A.; Duchesne, L.; Cano, J.D.G.; Glynn, C.; O’Connell, A.M.; Ennis, R.; Lowery, A.J.; et al. The Wavelia Microwave Breast Imaging System–Tumour discriminating features and their clinical usefulness. Br. J. Radiol. 2021, 94, 20210907. [Google Scholar] [CrossRef]
- MVG Industries, TP.102.17.22.PAR, Wavelia#2, Pilot#1 Clinical Investigation Protocol. 2023. Available online: https://www.clinicaltrials.gov/study/NCT05757427 (accessed on 1 October 2025).
- Fasoula, A.; Bernard, J.-G.; Robin, G.; Duchesne, L. Elaborated breast phantoms and experimental benchmarking of a microwave breast imaging system before first clinical study. In Proceedings of the 12th European Conference on Antennas and Propagation (EuCAP 2018), London, UK, 9–13 April 2018. [Google Scholar]
- Cano, J.D.G.; Fasoula, A.; Duchesne, L.; Bernard, J.G. Wavelia breast imaging: The optical breast contour detection subsystem. Appl. Sci. 2020, 10, 1234. [Google Scholar] [CrossRef]
- Lawrence, P.; Fasoula, A.; Duchesne, L. RF-based Breast Surface Estimation–Registration with Reference Imaging Modality. In Proceedings of the IEEE International Symposium on Antennas and Propagation & USNC/URSI National Radio Science Meeting, Boston, MA, USA, 8–13 July 2018. [Google Scholar]
- Papatrechas, G.; Fasoula, A.; Duchesne, L. Wavelia #2: The Microwave Breast Surface Estimation Module. In Proceedings of the 2024 IEEE International Symposium on Biomedical Imaging (ISBI), Athens, Greece, 27–30 May 2024; pp. 1–5. [Google Scholar] [CrossRef]
- Sarafianou, M.; Preece, A.W.; Craddock, I.J.; Klemm, M.; Leendertz, J.A. Evaluation of Two Approaches for Breast Surface Measurement Applied to a Radar-Based Imaging System. IEEE Trans. Antennas Propag. 2016, 64, 609–617. [Google Scholar] [CrossRef]
- Kurrant, D.; Bourqui, J.; Fear, E. Surface estimation for microwave imaging. Sensors 2017, 17, 1658. [Google Scholar] [CrossRef]
- Williams, T.C.; Bourqui, J.; Cameron, T.R.; Okoniewski, M.; Fear, E.C. Laser surface estimation for microwave breast imaging systems. IEEE Trans. Biomed. Eng. 2011, 58, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Winters, D.W.; Shea, J.D.; Madsen, E.L.; Frank, G.R.; Van Veen, B.D.; Hagness, S.C. Estimating the breast surface using UWB microwave monostatic backscatter measurements. IEEE Trans. Biomed. Eng. 2008, 55, 247–256. [Google Scholar] [CrossRef]
- Endo, F.; Kidera, S. Accuracy enhanced beamforming method based on envelope surface extraction for non-contact UWB breast cancer radar. In Proceedings of the 2016 International Symposium on Antennas and Propagation (ISAP), Okinawa, Japan, 24–28 October 2016. [Google Scholar]
- Helbig, M.; Geyer, C.; Hein, M.; Hilger, I.; Schwarz, U.; Sachs, J. A breast surface estimation algorithm for UWB microwave imaging. In Proceedings of the 4th European Conference of the International Federation for Medical and Biological Engineering, Antwerp, Belgium, 23–27 November 2008. [Google Scholar] [CrossRef]
- Highnam, R.; Brady, M.; Yaffe, M.J.; Karssemeijer, N.; Harvey, J. Robust breast composition measurement-VolparaTM. In Lecture Notes in Computer Science (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics); Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Teo, I.; Whelehan, P.; MacAskill, J.E.; Vinnicombe, S.; Munnoch, D.A.; Evans, A. VolparaTM as a measurement tool for breast volume. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, 581–582. [Google Scholar] [CrossRef]
- Sickles, E.A.; D’Orsi, C.J.; Bassett, L.W.; Appleton, C.M.; Berg, W.A.; Burnside, E.S. Acr Bi-Rads®Mammography, ACR BI-RADS®Atlas, Breast Imaging Reporting and Data System; American College of Radiology: Reston, VA, USA, 2013; Volume 5. [Google Scholar]
- Fasoula, A.; Papatrechas, G.; Arvanitis, P.; Duchesne, L.; Cano, J.D.G.; Donnell, J.O.; Elwahab, S.A.; Kerin, M. Wavelia Microwave Breast Imaging Phase#2 clinical investigation: Methodological Evolutions and Multidimensional Radiomics Analysis towards controlled Specificity. Cancers 2025, 17, 2973. [Google Scholar] [CrossRef]
- Fasoula, A.; Duchesne, L.; Moloney, B.M.; Cano, J.D.G.; Chenot, C.; Oliveira, B.L.; Bernard, J.G.; Elwahab, S.M.A.; Kerin, M.J. Pilot patient study with the Wavelia Microwave Breast Imaging system for breast cancer detection: Clinical feasibility and identified technical challenges. In Proceedings of the 2020 14th European Conference on Antennas and Propagation (EuCAP), Copenhagen, Denmark, 15–20 March 2020. [Google Scholar] [CrossRef]
- Yeh, E.D.; Georgian-Smith, D.; Raza, S.; Bussolari, L.; Pawlisz-Hoff, J.; Birdwell, R.L. Positioning in breast MR imaging to optimize image quality. RadioGraphics 2014, 34, E1–E17. [Google Scholar] [CrossRef]
- Huang, N.S.; Quan, C.L.; Mo, M.; Chen, J.J.; Yang, B.L.; Huang, X.; Wu, J. A prospective study of breast anthropomorphic measurements, volume & ptosis in 605 Asian patients with breast cancer or benign breast disease. PLoS ONE 2017, 12, e0172122. [Google Scholar] [CrossRef]












Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papatrechas, G.; Fasoula, A.; Arvanitis, P.; Duchesne, L.; Raveneau, A.; Gil Cano, J.D.; O’ Donnell, J.; Abd Elwahab, S.; Kerin, M. Microwave Breast Imaging System Modules, Enhancing Scan Quality and Reliability of Diagnostic Outputs During Clinical Testing. Bioengineering 2025, 12, 1079. https://doi.org/10.3390/bioengineering12101079
Papatrechas G, Fasoula A, Arvanitis P, Duchesne L, Raveneau A, Gil Cano JD, O’ Donnell J, Abd Elwahab S, Kerin M. Microwave Breast Imaging System Modules, Enhancing Scan Quality and Reliability of Diagnostic Outputs During Clinical Testing. Bioengineering. 2025; 12(10):1079. https://doi.org/10.3390/bioengineering12101079
Chicago/Turabian StylePapatrechas, Giannis, Angie Fasoula, Petros Arvanitis, Luc Duchesne, Alexis Raveneau, Julio Daniel Gil Cano, John O’ Donnell, Sami Abd Elwahab, and Michael Kerin. 2025. "Microwave Breast Imaging System Modules, Enhancing Scan Quality and Reliability of Diagnostic Outputs During Clinical Testing" Bioengineering 12, no. 10: 1079. https://doi.org/10.3390/bioengineering12101079
APA StylePapatrechas, G., Fasoula, A., Arvanitis, P., Duchesne, L., Raveneau, A., Gil Cano, J. D., O’ Donnell, J., Abd Elwahab, S., & Kerin, M. (2025). Microwave Breast Imaging System Modules, Enhancing Scan Quality and Reliability of Diagnostic Outputs During Clinical Testing. Bioengineering, 12(10), 1079. https://doi.org/10.3390/bioengineering12101079







