Chondrogenic Maturation Governs hMSC Mechanoresponsiveness to Dynamic Compression
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Isolation and Expansion
2.2. Preparation and Culture of Cellular Fibrin Hydrogels
2.3. Application of Dynamic Compression (DC)
2.4. Mechanical Testing
2.5. Gene Expression Analysis
2.6. Quantitative Biochemical Analysis
2.7. Histological Analysis
2.8. Immunohistochemistry
2.9. Statistical Analysis
3. Results
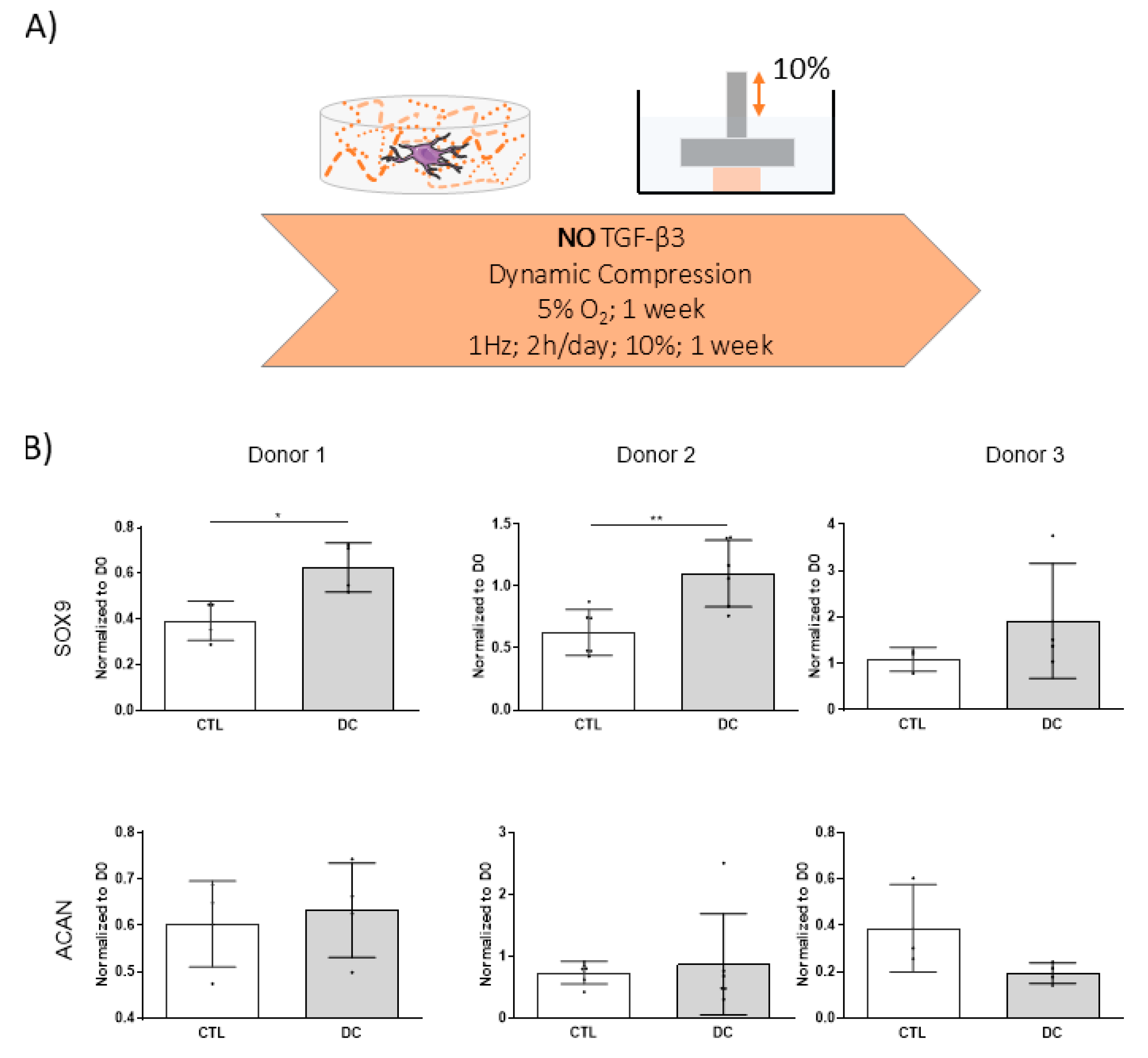
3.1. Dynamic Compression Alone Does Not Initiate Chondrogenic Differentiation of hMSCs
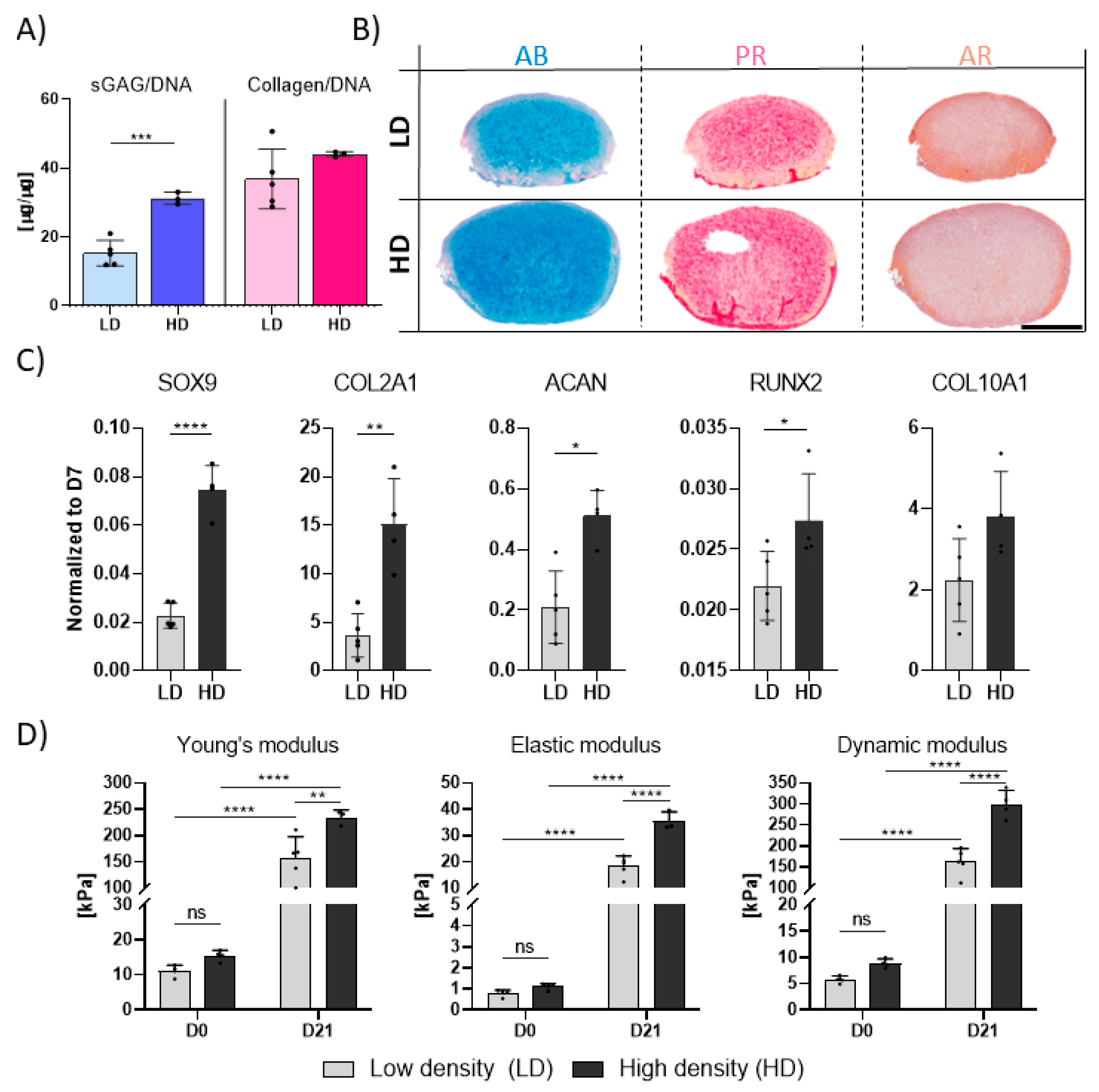
3.2. Higher Seeding Densities Support Superior TGF-β3-Mediated Chondrogenesis
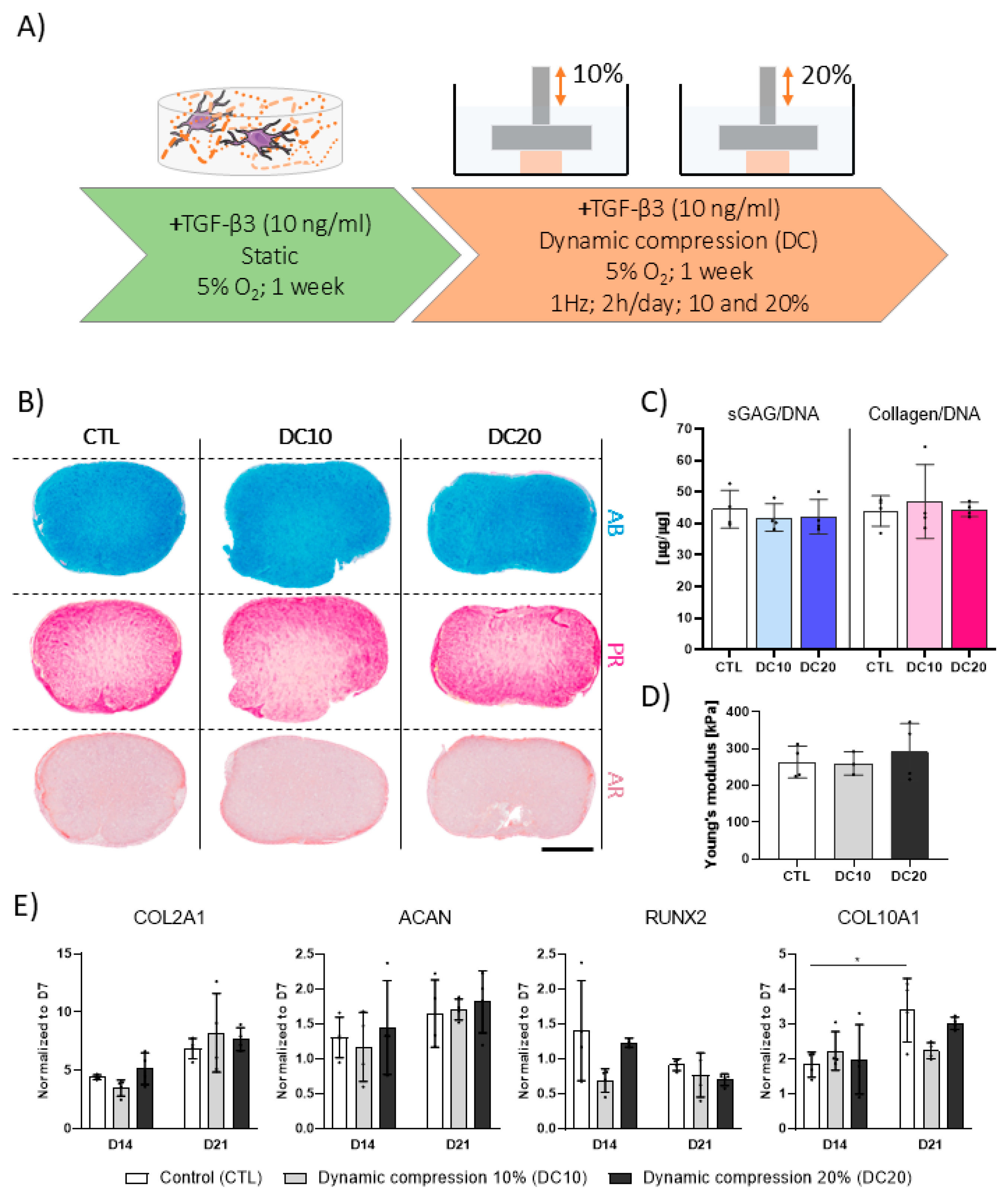
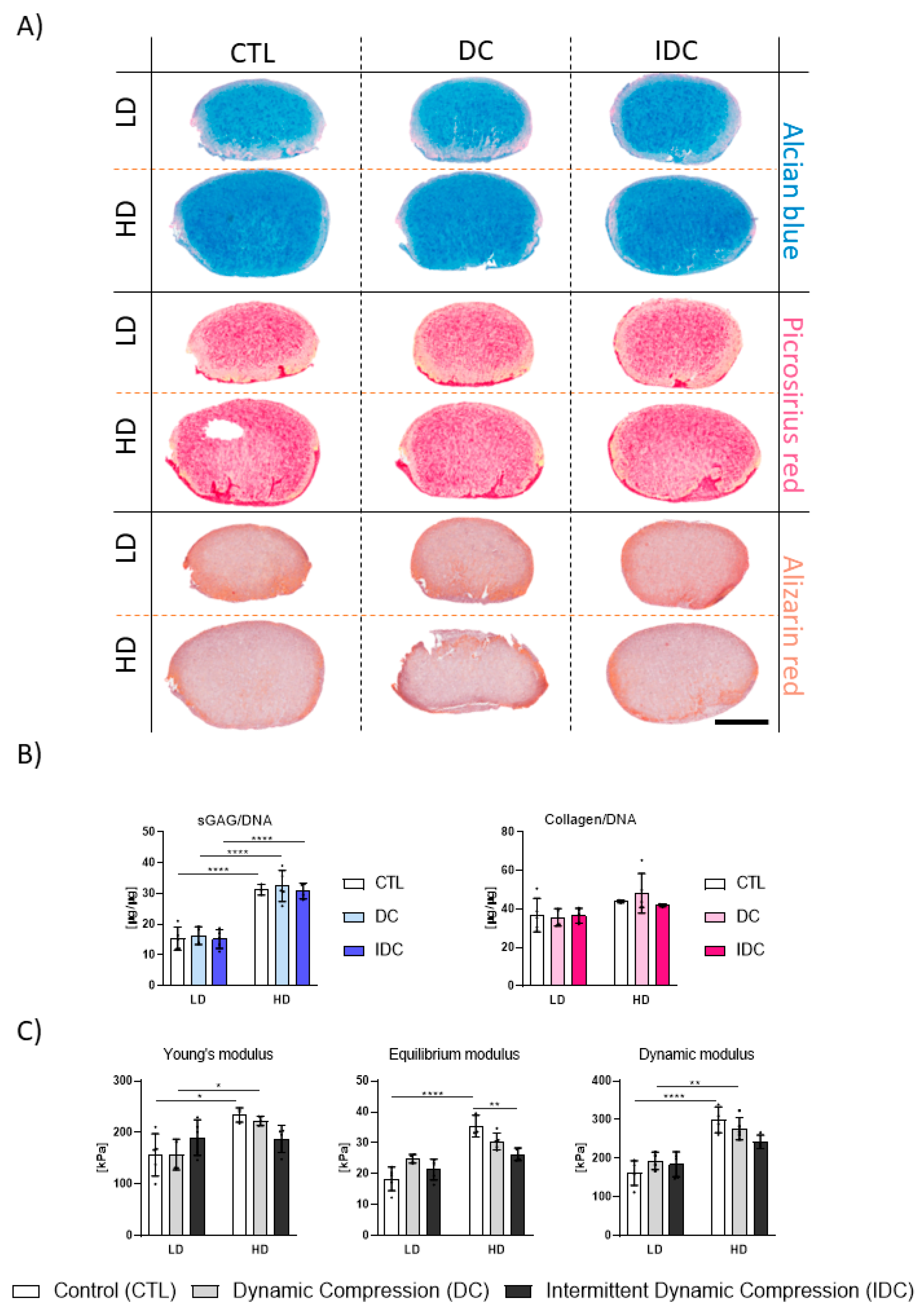
3.3. Higher Magnitudes of DC Are Not Beneficial to TGF-β3-Mediated Chondrogenesis
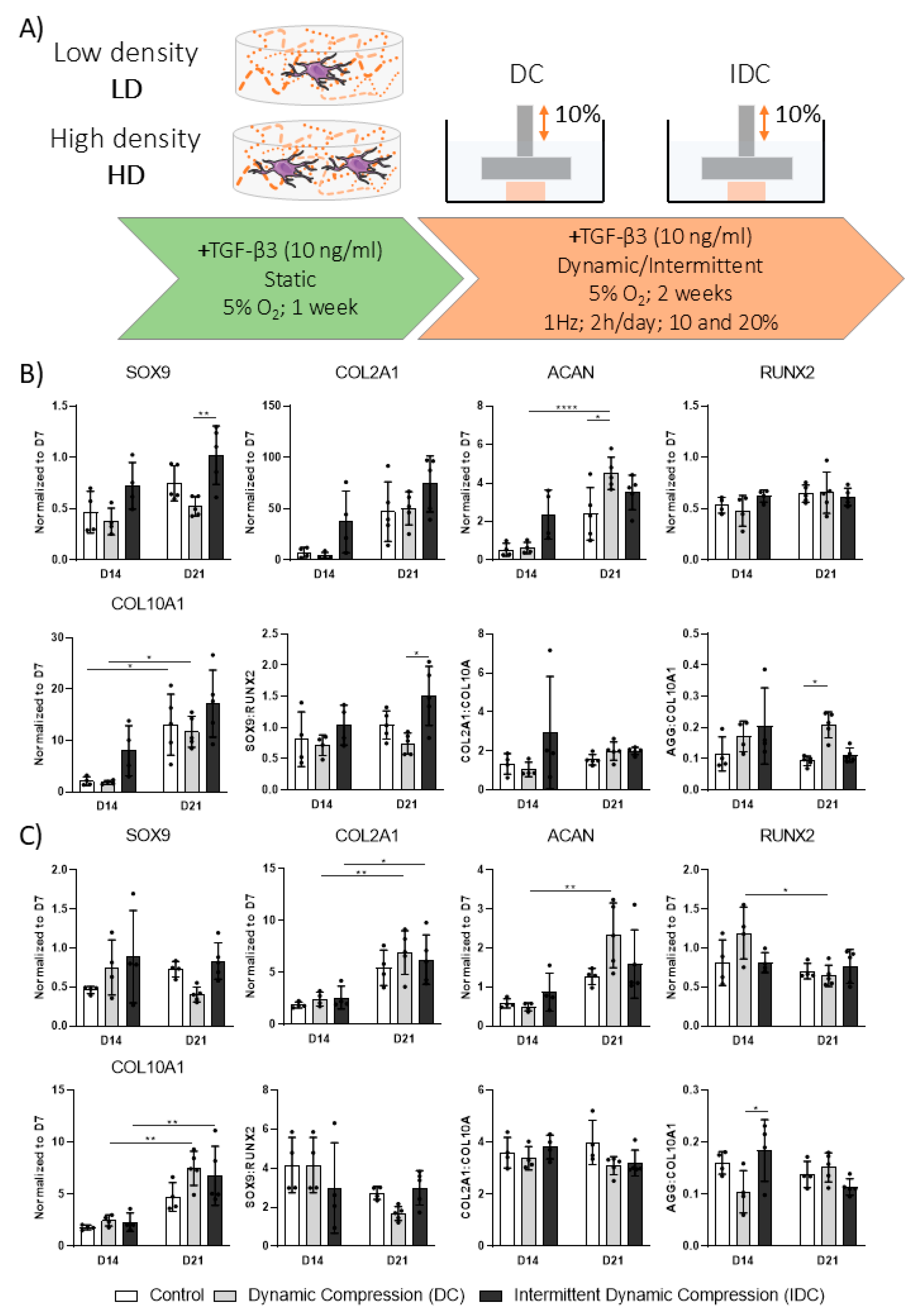
3.4. DC Regulates TGF-β3 Mediated Chondrogenesis of hMSCs in a Cell Density-Dependent Manner
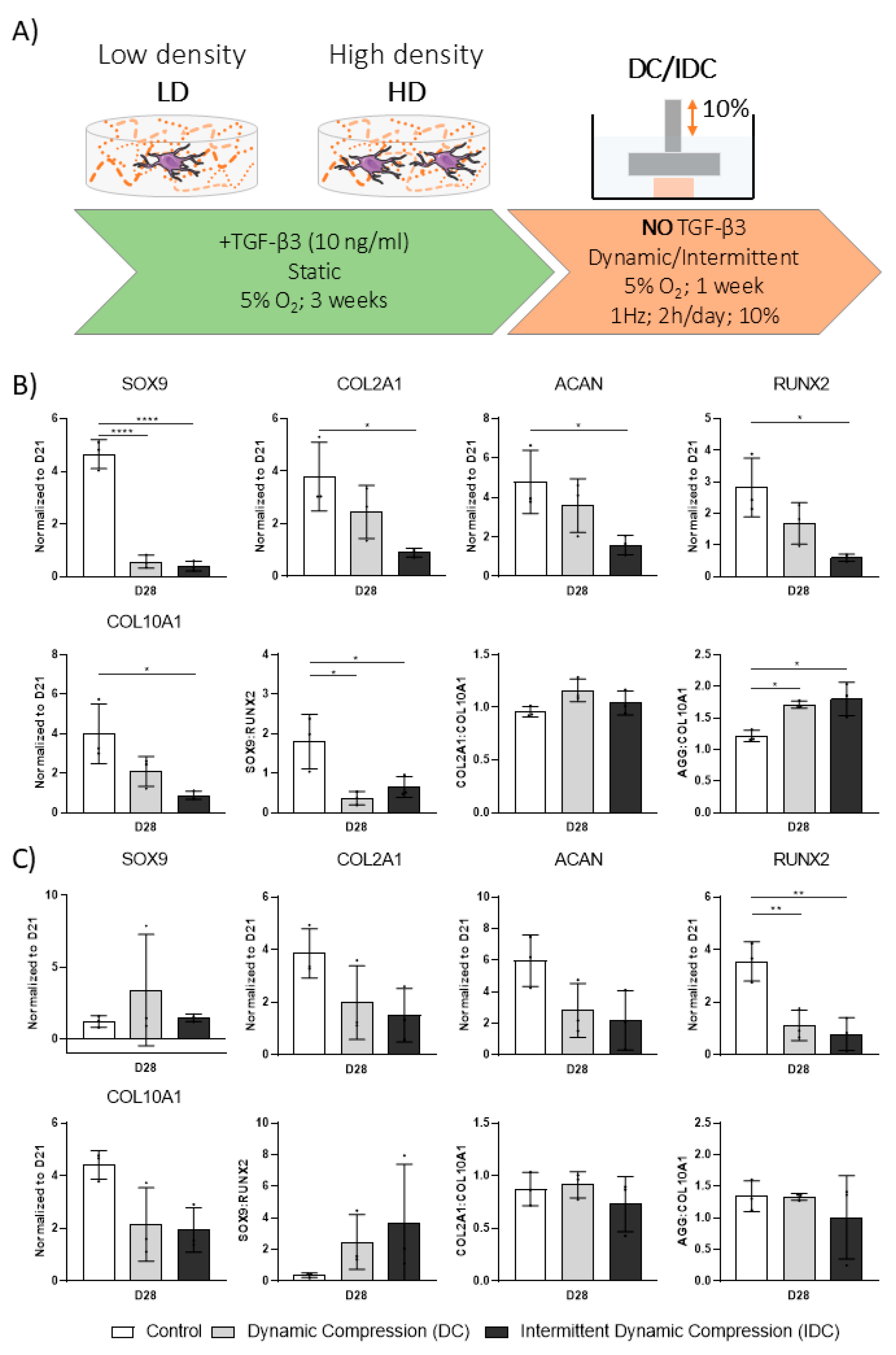
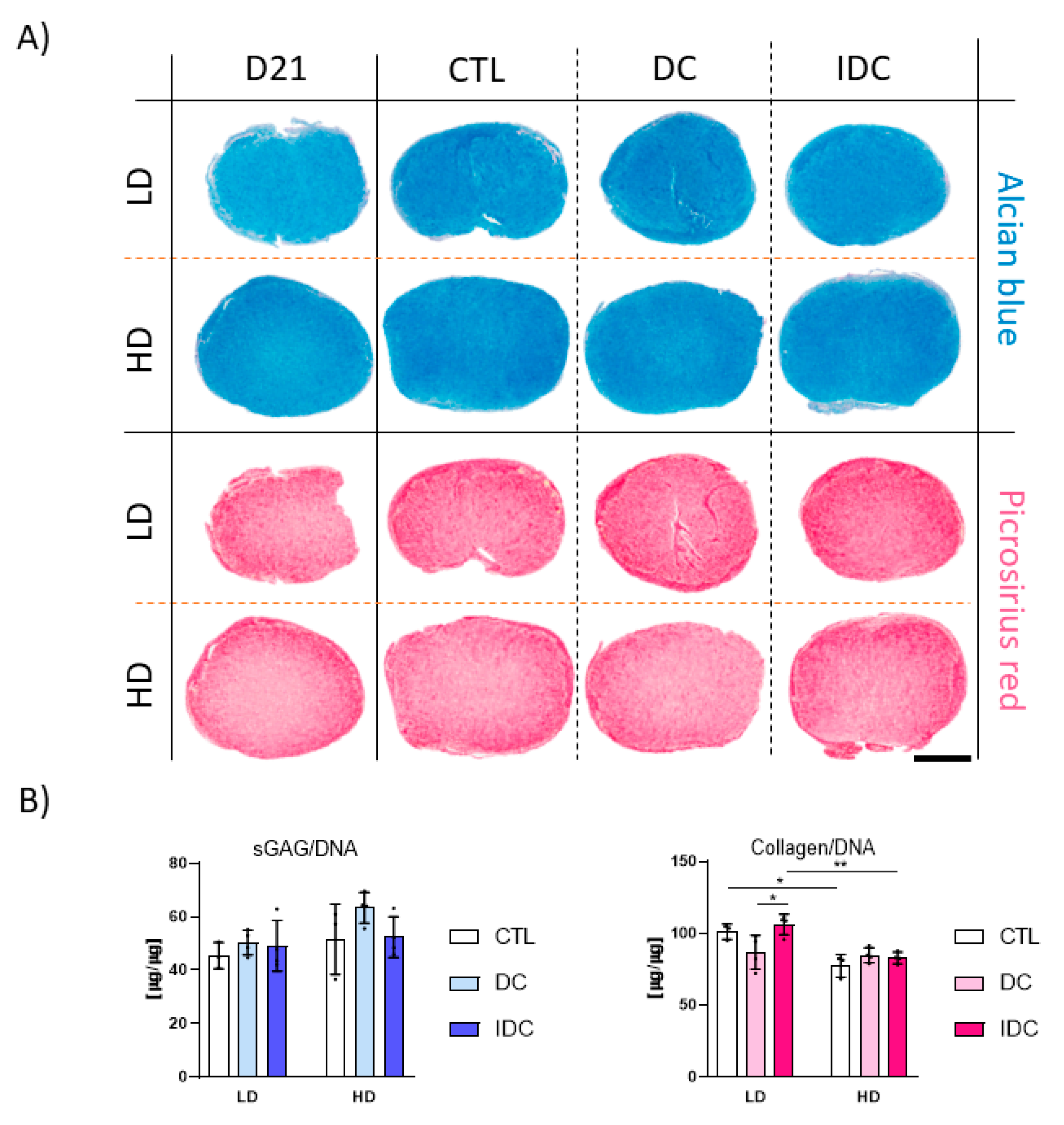
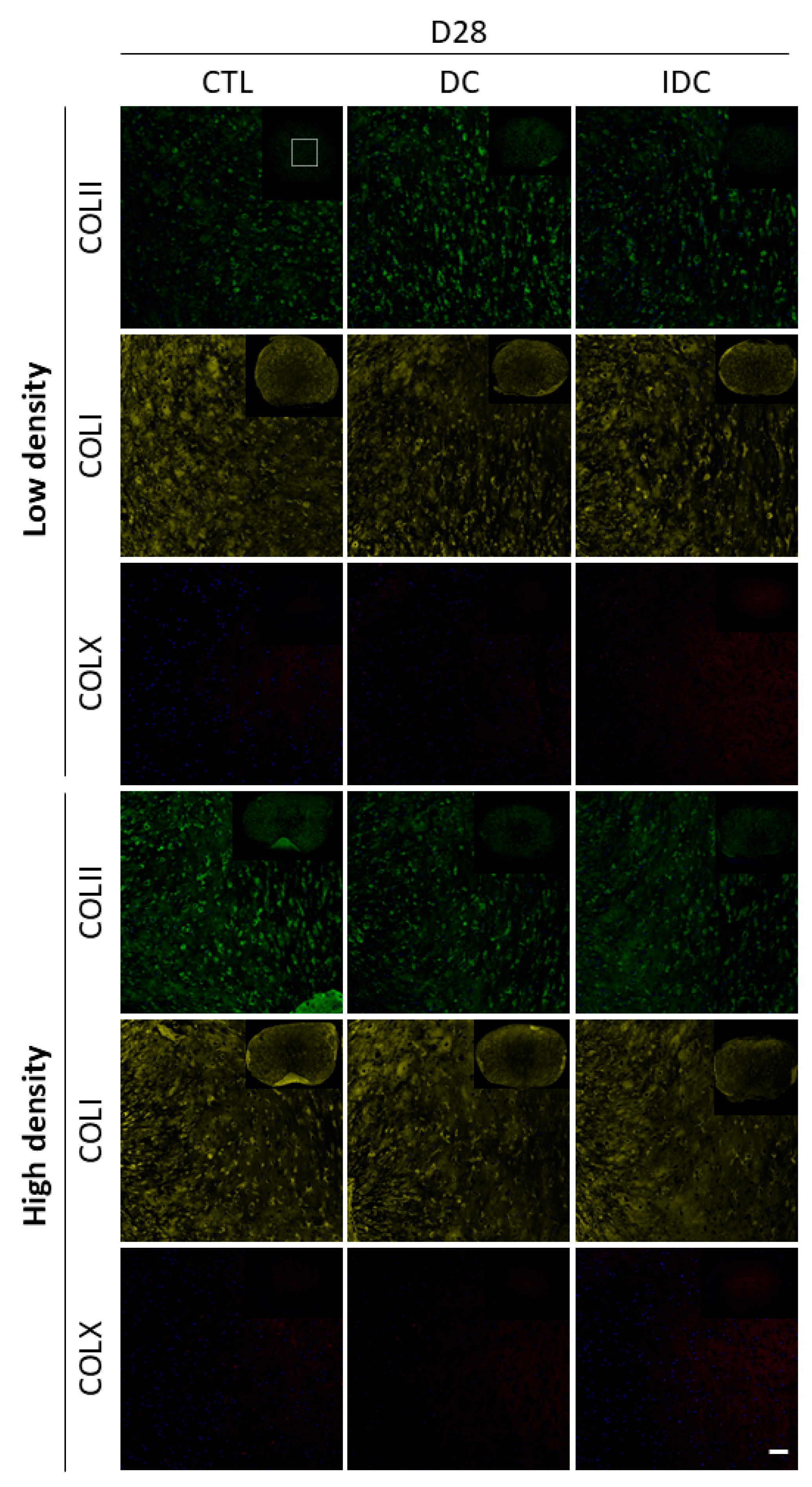
3.5. DC Can Be Detrimental to hMSC Chondrogenesis upon Removal of Exogenous TGF-β3
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Benya, P.D.; Shaffer, J.D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 1982, 30, 215–224. [Google Scholar] [CrossRef]
- Barbero, A.; Grogan, S.; Schäfer, D.; Heberer, M.; Mainil-Varlet, P.; Martin, I. Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrogenic capacity. Osteoarthr. Cartil. 2004, 12, 476–484. [Google Scholar] [CrossRef]
- Tuli, R.; Tuli, S.; Nandi, S.; Huang, X.; Manner, P.A.; Hozack, W.J.; Danielson, K.G.; Hall, D.J.; Tuan, R.S. Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J. Biol. Chem. 2003, 278, 41227–41236. [Google Scholar] [CrossRef]
- Johnstone, B.; Hering, T.M.; Caplan, A.I.; Goldberg, V.M.; Yoo, J.U. In Vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp. Cell Res. 1998, 238, 265–272. [Google Scholar] [CrossRef]
- Chariyev-Prinz, F.; Szojka, A.; Neto, N.; Burdis, R.; Monaghan, M.G.; Kelly, D.J. An assessment of the response of human MSCs to hydrostatic pressure in environments supportive of differential chondrogenesis. J. Biomech. 2023, 154, 111590. [Google Scholar] [CrossRef]
- Pelttari, K.; Winter, A.; Steck, E.; Goetzke, K.; Hennig, T.; Ochs, B.G.; Aigner, T.; Richter, W. Premature induction of hypertrophy during in Vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006, 54, 3254–3266. [Google Scholar] [CrossRef]
- Hellingman, C.A.; Koevoet, W.; Kops, N.; Farrell, E.; Jahr, H.; Liu, W.; de Jong, R.J.B.; Frenz, D.A.; van Osch, G.J.V.M. Fibroblast Growth Factor Receptors in In Vitro and In Vivo Chondrogenesis: Relating Tissue Engineering Using Adult Mesenchymal Stem Cells to Embryonic Development. Tissue Eng. Part A 2010, 16, 545–556. [Google Scholar] [CrossRef]
- Vinardell, T.; Sheehy, E.J.; Buckley, C.T.; Kelly, D.J. A Comparison of the Functionality and In Vivo Phenotypic Stability of Cartilaginous Tissues Engineered from Different Stem Cell Sources. Tissue Eng. Part A 2012, 18, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Zhai, D.Y.; Mauck, R.L.; Burdick, J.A. Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage. Tissue Eng. Part A 2011, 17, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wen, F.; Wu, Y.; Goh, G.S.H.; Ge, Z.; Tan, L.P.; Hui, J.H.P.; Yang, Z. Cross-talk between TGF-beta/SMAD and integrin signaling pathways in regulating hypertrophy of mesenchymal stem cell chondrogenesis under deferral dynamic compression. Biomaterials 2015, 38, 72–85. [Google Scholar] [CrossRef]
- Antunes, B.P.; Vainieri, M.L.; Alini, M.; Monsonego-Ornan, E.; Grad, S.; Yayon, A. Enhanced chondrogenic phenotype of primary bovine articular chondrocytes in Fibrin-Hyaluronan hydrogel by multi-axial mechanical loading and FGF18. Acta Biomater. 2020, 105, 170–179. [Google Scholar] [CrossRef]
- McDermott, A.M.; Eastburn, E.A.; Kelly, D.J.; Boerckel, J.D. Effects of chondrogenic priming duration on mechanoregulation of engineered cartilage. J. Biomech. 2021, 125, 110580. [Google Scholar] [CrossRef]
- Arvind, V.; Huang, A.H. Mechanobiology of limb musculoskeletal development. Ann. N. Y. Acad. Sci. 2017, 1409, 18–32. [Google Scholar] [CrossRef]
- Osborne, A.C.; Lamb, K.J.; Lewthwaite, J.C.; Dowthwaite, G.P.; Pitsillides, A.A. Short-term rigid and flaccid paralyses diminish growth of embryonic chick limbs and abrogate joint cavity formation but differentially preserve pre-cavitated joints. J. Musculoskelet. Neuronal Interact. 2002, 2, 448–456. [Google Scholar]
- Simon, M.R. The effect of dynamic loading on the growth of epiphyseal cartilage in the rat. Acta Anat. 1978, 102, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Shou, Y.; Teo, X.Y.; Wu, K.Z.; Bai, B.; Kumar, A.R.K.; Low, J.; Le, Z.; Tay, A. Dynamic Stimulations with Bioengineered Extracellular Matrix-Mimicking Hydrogels for Mechano Cell Reprogramming and Therapy. Adv. Sci. 2023, 10, 2300670. [Google Scholar] [CrossRef]
- Roos, E.M.; Dahlberg, L. Positive effects of moderate exercise on glycosaminoglycan content in knee cartilage: A four-month, randomized, controlled trial in patients at risk of osteoarthritis. Arthritis Rheum. 2005, 52, 3507–3514. [Google Scholar] [CrossRef] [PubMed]
- Boerckel, J.D.; Kolambkar, Y.M.; Stevens, H.Y.; Lin, A.S.P.; Dupont, K.M.; Guldberg, R.E. Effects of In Vivo mechanical loading on large bone defect regeneration. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2012, 30, 1067–1075. [Google Scholar] [CrossRef]
- Boerckel, J.D.; Uhrig, B.A.; Willett, N.J.; Huebsch, N.; Guldberg, R.E. Mechanical regulation of vascular growth and tissue regeneration In Vivo. Proc. Natl. Acad. Sci. USA 2011, 108, E674–E680. [Google Scholar] [CrossRef] [PubMed]
- Gardner, M.J.; van der Meulen, M.C.H.; Demetrakopoulos, D.; Wright, T.M.; Myers, E.R.; Bostrom, M.P. In Vivo cyclic axial compression affects bone healing in the mouse tibia. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2006, 24, 1679–1686. [Google Scholar] [CrossRef]
- He, A.; Liu, L.; Luo, X.; Liu, Y.; Liu, Y.; Liu, F.; Wang, X.; Zhang, Z.; Zhang, W.; Liu, W.; et al. Repair of osteochondral defects with in vitro engineered cartilage based on autologous bone marrow stromal cells in a swine model. Sci. Rep. 2017, 7, 40489. [Google Scholar] [CrossRef] [PubMed]
- Steck, E.; Fischer, J.; Lorenz, H.; Gotterbarm, T.; Jung, M.; Richter, W. Mesenchymal Stem Cell Differentiation in an Experimental Cartilage Defect: Restriction of Hypertrophy to Bone-Close Neocartilage. Stem Cells Dev. 2009, 18, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Frisbie, D.D.; Trotter, G.W.; Powers, B.E.; Rodkey, W.G.; Steadman, J.R.; Howard, R.D.; Park, R.D.; McIlwraith, C.W. Arthroscopic subchondral bone plate microfracture technique augments healing of large chondral defects in the radial carpal bone and medial femoral condyle of horses. Vet. Surg. VS 1999, 28, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Ladner, Y.D.; Kasper, H.; Armiento, A.R.; Stoddart, M.J. A multi-well bioreactor for cartilage tissue engineering experiments. iScience 2023, 26, 107092. [Google Scholar] [CrossRef]
- Fahy, N.; Alini, M.; Stoddart, M.J. Mechanical stimulation of mesenchymal stem cells: Implications for cartilage tissue engineering. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2018, 36, 52–63. [Google Scholar] [CrossRef]
- Anderson, D.E.; Johnstone, B. Dynamic Mechanical Compression of Chondrocytes for Tissue Engineering: A Critical Review. Front. Bioeng. Biotechnol. 2017, 5, 76. [Google Scholar] [CrossRef]
- Thorpe, S.D.; Buckley, C.T.; Vinardell, T.; O’Brien, F.J.; Campbell, V.A.; Kelly, D.J. The Response of Bone Marrow-Derived Mesenchymal Stem Cells to Dynamic Compression Following TGF-β3 Induced Chondrogenic Differentiation. Ann. Biomed. Eng. 2010 38 2896–2909. [CrossRef]
- Luo, L.; Thorpe, S.D.; Buckley, C.T.; Kelly, D.J. The effects of dynamic compression on the development of cartilage grafts engineered using bone marrow and infrapatellar fat pad derived stem cells. Biomed. Mater. 2015, 10, 55011. [Google Scholar] [CrossRef]
- Michalopoulos, E.; Knight, R.L.; Korossis, S.; Kearney, J.N.; Fisher, J.; Ingham, E. Development of Methods for Studying the Differentiation of Human Mesenchymal Stem Cells Under Cyclic Compressive Strain. Tissue Eng. Part C Methods 2012, 18, 252–262. [Google Scholar] [CrossRef]
- Li, Z.; Kupcsik, L.; Yao, S.-J.; Alini, M.; Stoddart, M.J. Mechanical load modulates chondrogenesis of human mesenchymal stem cells through the TGF-β pathway. J. Cell. Mol. Med. 2010, 14, 1338–1346. [Google Scholar] [CrossRef]
- Li, Z.; Yao, S.J.; Alini, M.; Stoddart, M.J. Chondrogenesis of human bone marrow mesenchymal stem cells in fibrin-polyurethane composites is modulated by frequency and amplitude of dynamic compression and shear stress. Tissue Eng. Part A 2010, 16, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.R.; Yong, K.W.; Choi, J.Y. Effects of mechanical loading on human mesenchymal stem cells for cartilage tissue engineering. J. Cell. Physiol. 2018, 233, 1913–1928. [Google Scholar] [CrossRef]
- Wang, T.; Dogru, S.; Dai, Z.; Kim, S.Y.; Vickers, N.A.; Albro, M.B. Physiologic Doses of Transforming Growth Factor-β Improve the Composition of Engineered Articular Cartilage. Tissue Eng. Part. A 2025, 31, 56–68. [Google Scholar] [CrossRef]
- Albro, M.B.; Nims, R.J.; Cigan, A.D.; Yeroushalmi, K.J.; Alliston, T.; Hung, C.T.; Ateshian, G.A. Accumulation of Exogenous Activated TGF-β in the Superficial Zone of Articular Cartilage. Biophys. J. 2013, 104, 1794–1804. [Google Scholar] [CrossRef]
- Kafienah, W.; Sims, T.J. Biochemical Methods for the Analysis of Tissue-Engineered Cartilage. In Cancer Cytogenetics: Methods and Protocols; Swansbury, J., Ed.; Humana Press: Totowa, NJ, USA, 2003; pp. 217–229. ISBN 978-1-59259-428-3. [Google Scholar]
- O’Conor, C.J.; Case, N.; Guilak, F. Mechanical regulation of chondrogenesis. Stem Cell Res. Ther. 2013, 4, 61. [Google Scholar] [CrossRef]
- Chariyev-Prinz, F.; Neto, N.; Monaghan, M.G.; Kelly, D.J. Time-Dependent Anabolic Response of hMSC-Derived Cartilage Grafts to Hydrostatic Pressure. J. Tissue Eng. Regen. Med. 2023, 2023, 9976121. [Google Scholar] [CrossRef] [PubMed]
- Gardner, O.F.W.; Fahy, N.; Alini, M.; Stoddart, M.J. Joint mimicking mechanical load activates TGFβ1 in fibrin-poly(ester-urethane) scaffolds seeded with mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2017, 11, 2663–2666. [Google Scholar] [CrossRef]
- Carroll, S.F.; Buckley, C.T.; Kelly, D.J. Measuring and Modeling Oxygen Transport and Consumption in 3D Hydrogels Containing Chondrocytes and Stem Cells of Different Tissue Origins. Front. Bioeng. Biotechnol. 2021, 9, 591126. [Google Scholar] [CrossRef]
- Mouw, J.K.; Connelly, J.T.; Wilson, C.G.; Michael, K.E.; Levenston, M.E. Dynamic Compression Regulates the Expression and Synthesis of Chondrocyte-Specific Matrix Molecules in Bone Marrow Stromal Cells. Stem Cells 2007, 25, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.H.; Baker, B.M.; Ateshian, G.A.; Mauck, R.L. Sliding contact loading enhances the tensile properties of mesenchymal stem cell-seeded hydrogels. Eur. Cells Mater. 2012, 24, 29–45. [Google Scholar] [CrossRef]
- Schatti, O.; Grad, S.; Goldhahn, J.; Salzmann, G.; Li, Z.; Alini, M.; Stoddart, M.J. A combination of shear and dynamic compression leads to mechanically induced chondrogenesis of human mesenchymal stem cells. Eur. Cells Mater. 2011, 22, 214–225. [Google Scholar] [CrossRef]
- Sanchez-Adams, J.; Leddy, H.A.; McNulty, A.L.; O’Conor, C.J.; Guilak, F. The mechanobiology of articular cartilage: Bearing the burden of osteoarthritis. Curr. Rheumatol. Rep. 2014, 16, 451. [Google Scholar] [CrossRef]
- Uzieliene, I.; Bironaite, D.; Bagdonas, E.; Pachaleva, J.; Sobolev, A.; Tsai, W.-B.; Kvederas, G.; Bernotiene, E. The Effects of Mechanical Load on Chondrogenic Responses of Bone Marrow Mesenchymal Stem Cells and Chondrocytes Encapsulated in Chondroitin Sulfate-Based Hydrogel. Int. J. Mol. Sci. 2023, 24, 2915. [Google Scholar] [CrossRef]
- Haugh, M.G.; Meyer, E.G.; Thorpe, S.D.; Vinardell, T.; Duffy, G.P.; Kelly, D.J. Temporal and spatial changes in cartilage-matrix-specific gene expression in mesenchymal stem cells in response to dynamic compression. Tissue Eng. Part A 2011, 17, 3085–3093. [Google Scholar] [CrossRef] [PubMed]
- Li, D.X.; Ma, Z.; Szojka, A.R.; Lan, X.; Kunze, M.; Mulet-Sierra, A.; Westover, L.; Adesida, A.B. Non-hypertrophic chondrogenesis of mesenchymal stem cells through mechano-hypoxia programing. J. Tissue Eng. 2023, 14, 20417314231172574. [Google Scholar] [CrossRef]
- Futrega, K.; Robey, P.G.; Klein, T.J.; Crawford, R.W.; Doran, M.R. A single day of TGF-β1 exposure activates chondrogenic and hypertrophic differentiation pathways in bone marrow-derived stromal cells. Commun. Biol. 2021, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Steward, A.J.; Wagner, D.R.; Kelly, D.J. The pericellular environment regulates cytoskeletal development and the differentiation of mesenchymal stem cells and determines their response to hydrostatic pressure. Eur. Cells Mater. 2013, 25, 167–178. [Google Scholar] [CrossRef] [PubMed]
- van Buul, G.M.; Villafuertes, E.; Bos, P.K.; Waarsing, J.H.; Kops, N.; Narcisi, R.; Weinans, H.; Verhaar, J.A.N.; Bernsen, M.R.; van Osch, G.J.V.M. Mesenchymal stem cells secrete factors that inhibit inflammatory processes in short-term osteoarthritic synovium and cartilage explant culture. Osteoarthr. Cartil. 2012, 20, 1186–1196. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Y.; Wang, Z.; Li, L.; Teng, H.; Jiang, Q. Effects of Mechanical Compression on Chondrogenesis of Human Synovium-Derived Mesenchymal Stem Cells in Agarose Hydrogel. Front. Bioeng. Biotechnol. 2021, 9, 697281. [Google Scholar] [CrossRef]
| Gene Name | Forward/Reverse |
|---|---|
| SOX9 | F: 5′-CTCTGGAGACTTCTGAACG R: 5′-AGATGTGCGTCTGCTC |
| RUNX2 | F: 5′-AAGCTTGATGACTCTAAACC R: 5′-TCTGTAATCTGACTCTGTCC |
| COL2A1 | F: 5′-GAAGAGTGGAGACTACTG R: 5′-CAGATGTGTTTCTTCTCCTG |
| ACAN | F: 5′-CACCCCATGCAATTTGAG R: 5′-AGATCATCACCACACAGTC |
| COL10A1 | F: 5′-GCTAGTATCCTTGAACTTGG R: 5′-CCTTTACTCTTTATGGTGTAGG |
| B2M | F: 5′-AAGGACTGGTCTTTCTATCTC R: 5′-GATCCCACTTAACTATCTTGG |
| RPL4 | F: 5′-GTAACTACAATCTTCCCATGC R: 5′-GGTCTTTGCATATGGGTTTAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chariyev-Prinz, F.; Burdis, R.; Kelly, D.J. Chondrogenic Maturation Governs hMSC Mechanoresponsiveness to Dynamic Compression. Bioengineering 2025, 12, 1075. https://doi.org/10.3390/bioengineering12101075
Chariyev-Prinz F, Burdis R, Kelly DJ. Chondrogenic Maturation Governs hMSC Mechanoresponsiveness to Dynamic Compression. Bioengineering. 2025; 12(10):1075. https://doi.org/10.3390/bioengineering12101075
Chicago/Turabian StyleChariyev-Prinz, Farhad, Ross Burdis, and Daniel J. Kelly. 2025. "Chondrogenic Maturation Governs hMSC Mechanoresponsiveness to Dynamic Compression" Bioengineering 12, no. 10: 1075. https://doi.org/10.3390/bioengineering12101075
APA StyleChariyev-Prinz, F., Burdis, R., & Kelly, D. J. (2025). Chondrogenic Maturation Governs hMSC Mechanoresponsiveness to Dynamic Compression. Bioengineering, 12(10), 1075. https://doi.org/10.3390/bioengineering12101075






