Classification of Hemiplegic Gait and Mimicked Hemiplegic Gait: A Treadmill Gait Analysis Study in Stroke Patients and Healthy Individuals
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Participant Selection
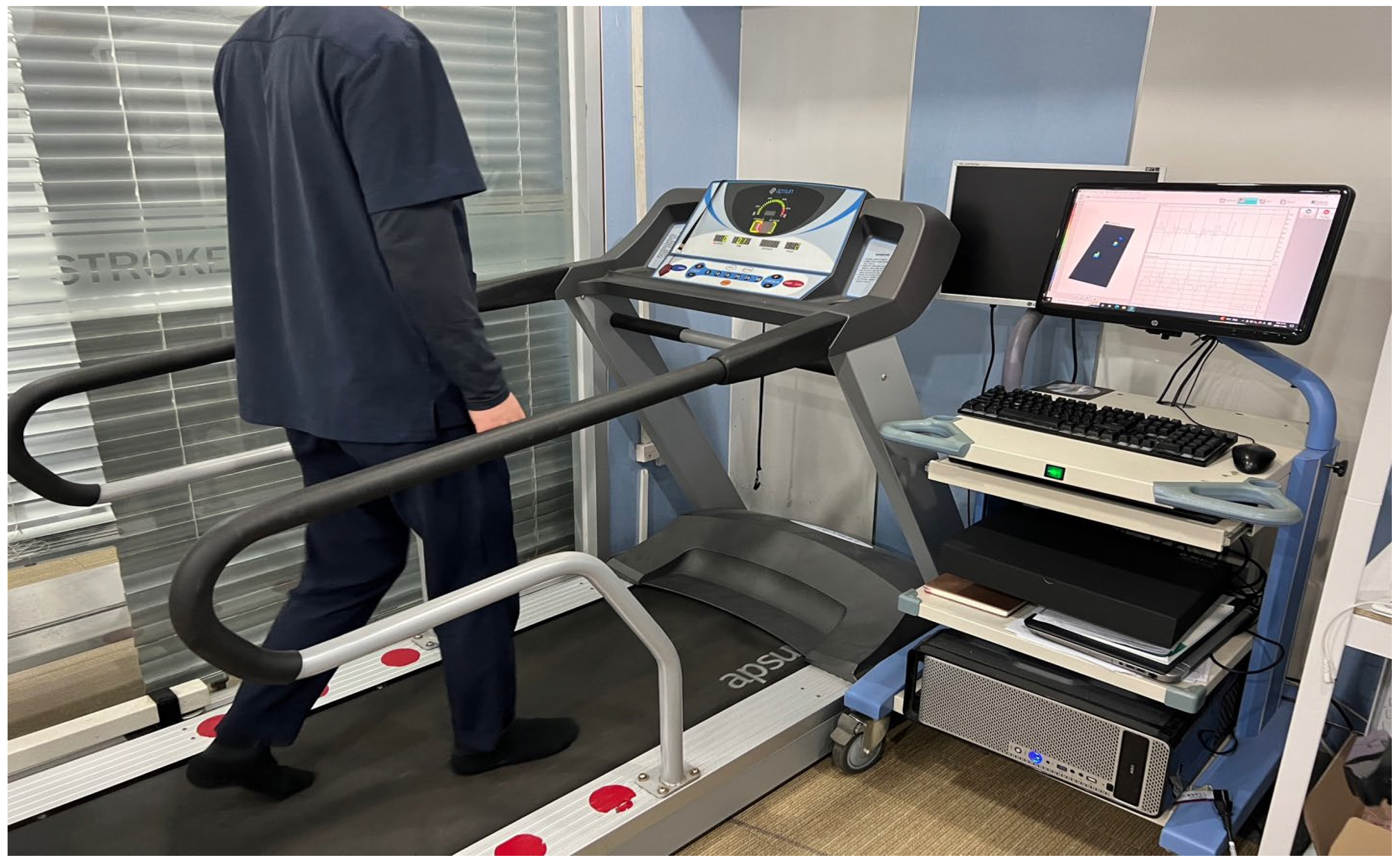
2.2. Experiment and Equipment
2.3. Gait Feature
2.4. Statistical Analysis
2.5. Machine Learning Model
3. Results
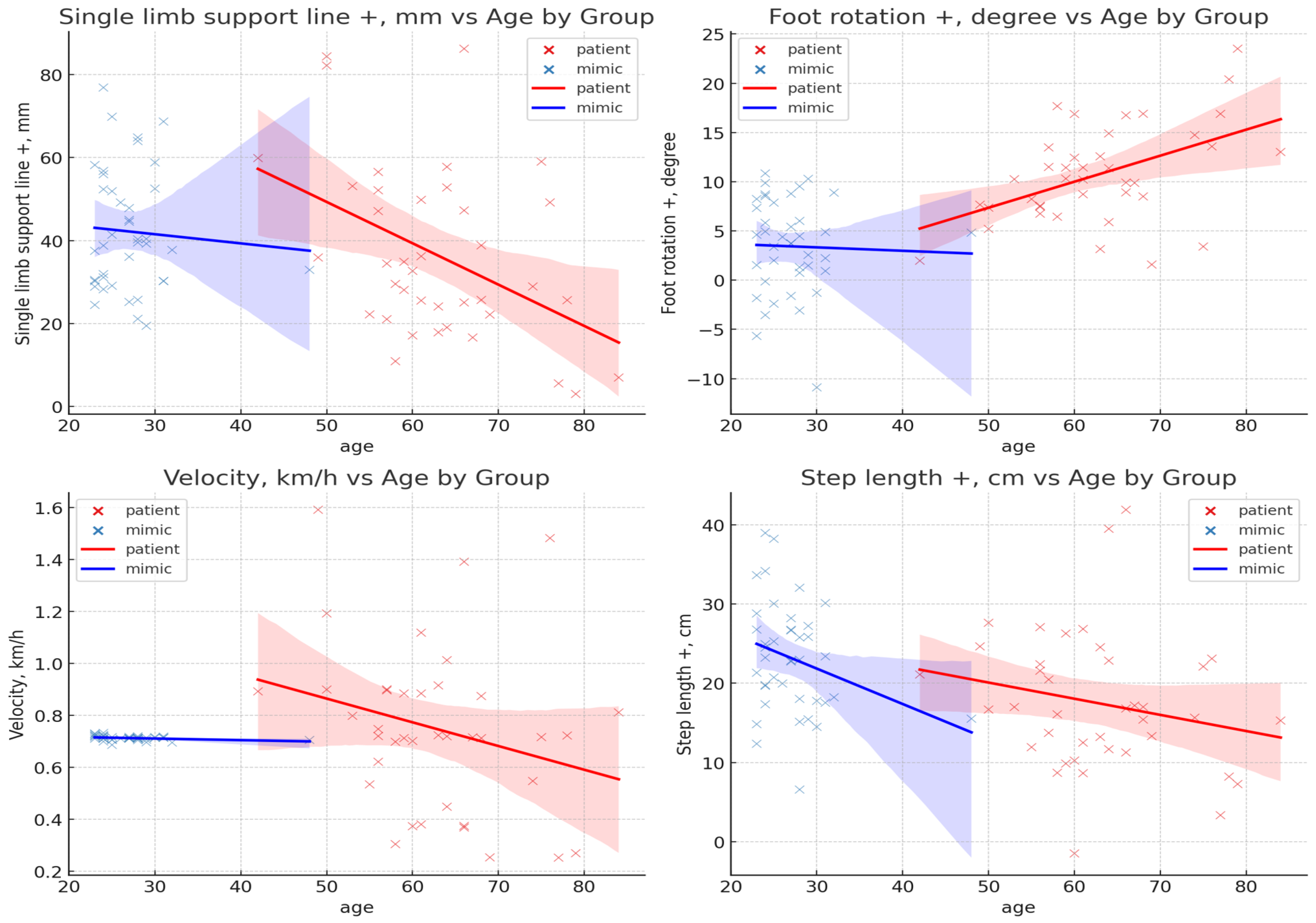
3.1. Result of Age Effect
3.2. Result of Gait Feature
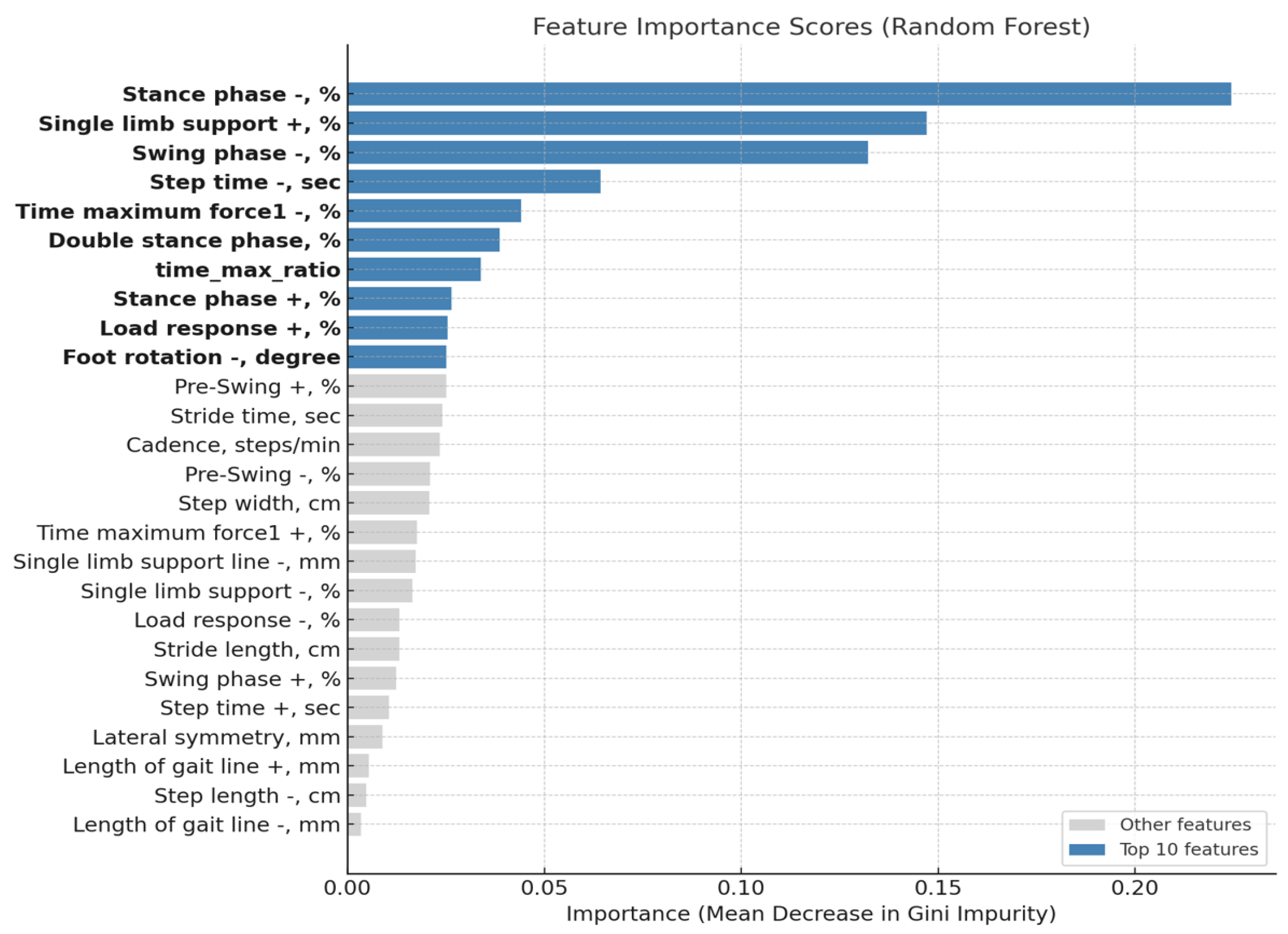
3.3. Result of Feature Importance Analysis
3.4. Result of Machine Learning Model
3.5. Result of Machine Learning Model Using Non-Significant Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Francisco, G.E.; Zhou, P. Post-Stroke Hemiplegic Gait: New Perspective and Insights. Front. Physiol. 2018, 9, 1021. [Google Scholar] [CrossRef]
- Balaban, B.; Tok, F. Gait Disturbances in Patients With Stroke. PM&R 2014, 6, 635–642. [Google Scholar] [CrossRef]
- Murphy, S.J.X.; Werring, D.J. Stroke: Causes and Clinical Features. Medicine 2020, 48, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Eby, S.; Zhao, H.; Song, P.; Vareberg, B.J.; Kinnick, R.; Greenleaf, J.F.; An, K.-N.; Chen, S.; Brown, A.W. Quantitative Evaluation of Passive Muscle Stiffness in Chronic Stroke. Am. J. Phys. Med. Rehabil. 2016, 95, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Awad, L.N.; Palmer, J.A.; Pohlig, R.T.; Binder-Macleod, S.A.; Reisman, D.S. Walking Speed and Step Length Asymmetry Modify the Energy Cost of Walking After Stroke. Neurorehabil. Neural Repair 2015, 29, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Saunders, D.H.; Sanderson, M.; Hayes, S.; Kilrane, M.; Greig, C.A.; Brazzelli, M.; Mead, G.E. Physical fitness training for stroke patients. Cochrane Database Syst. Rev. 2020, CD003316. [Google Scholar] [CrossRef]
- Felius, R.A.W.; Geerars, M.; Bruijn, S.M.; Van Dieën, J.H.; Wouda, N.C.; Punt, M. Reliability of IMU-Based Gait Assessment in Clinical Stroke Rehabilitation. Sensors 2022, 22, 908. [Google Scholar] [CrossRef] [PubMed]
- Kohnehshahri, F.S.; Merlo, A.; Mazzoli, D.; Bò, M.C.; Stagni, R. Machine Learning Applied to Gait Analysis Data in Cerebral Palsy and Stroke: A Systematic Review. Gait Posture 2024, 114, S39. [Google Scholar] [CrossRef]
- Dolan, V.F. Narcotic-Seeking Behavior and Self-Injury: A Report of Three Cases. J. Insur. Med. 2025, 52, 23–30. [Google Scholar] [CrossRef]
- Glaser, D. Fabricated or Induced Illness: From “Munchausen by Proxy” to Child and Family-Oriented Action. Child Abuse Negl. 2020, 108, 104649. [Google Scholar] [CrossRef]
- M Patel, H.; M, B. Reliability, Agreement, and Validity of FDM Zebris Pressure Platform to Measure Lower Limb Weight Distribution during Quiet Standing. Int. J. Curr. Res. Rev. 2023, 15, 1–7. [Google Scholar] [CrossRef]
- Esmaeilpour, F.; Letafatkar, A.; Karimi, M.T.; Khaleghi, M.; Rossettini, G.; Villafañe, J.H. Comparative Analysis of Ground Reaction Forces and Spatiotemporal Gait Parameters in Older Adults with Sway-Back Posture and Chronic Low Back Pain: A Cross-Sectional Study. BMC Sports Sci. Med. Rehabil. 2025, 17, 71. [Google Scholar] [CrossRef] [PubMed]
- Kalron, A.; Achiron, A. The Relationship between Fear of Falling to Spatiotemporal Gait Parameters Measured by an Instrumented Treadmill in People with Multiple Sclerosis. Gait Posture 2014, 39, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Lee, I.S.; Park, K.E.; Hong, H.J.; Sung, K.K.; Lee, S.K. The Change of Lateral Shift of Center of Pressure According to the Gait Improvement in Post-Stroke Hemiplegic Patients. J. Intern. Korean Med. 2014, 35, 448–454. [Google Scholar]
- Patterson, K.K.; Gage, W.H.; Brooks, D.; Black, S.E.; McIlroy, W.E. Evaluation of Gait Symmetry after Stroke: A Comparison of Current Methods and Recommendations for Standardization. Gait Posture 2010, 31, 241–246. [Google Scholar] [CrossRef]
- Green, S.B. How Many Subjects Does It Take to Do a Regression Analysis. Multivar. Behav. Res. 1991, 26, 499–510. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests; Springer Nature: Berlin/Heidelberg, Germany, 2001; Volume 45. [Google Scholar]
- Guyon, I.; Elisseeff, A. An Introduction to Variable and Feature Selection. J. Mach. Learn. Res. 2003, 3, 1157–1182. [Google Scholar]
- Chang, C.-C.; Lin, C.-J. LIBSVM: A Library for Support Vector Machines. ACM Trans. Intell. Syst. Technol. 2011, 2, 1–27. [Google Scholar] [CrossRef]
- Pedregosa, F.; Pedregosa, F.; Varoquaux, G.; Varoquaux, G.; Org, N.; Gramfort, A.; Gramfort, A.; Michel, V.; Michel, V.; Fr, L.; et al. Scikit-Learn: Machine Learning in Python. Mach. Learn. Python 2011, 12, 2825–2830. [Google Scholar]
- Kohavi, R. A Study of Cross-Validation and Bootstrap for Accuracy Estimation and Model Selection. Int. Jt. Conf. Artif. Intell. 1995, 14, 1137–1143. [Google Scholar]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction; Springer Series in Statistics; Springer: New York, NY, USA, 2009. [Google Scholar]
- Bohannon, R.W. Comfortable and Maximum Walking Speed of Adults Aged 20–79 Years: Reference Values and Determinants. Age Ageing 1997, 26, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S. Gait Speed and Survival in Older Adults. JAMA 2011, 305, 50. [Google Scholar] [CrossRef] [PubMed]
- Ruhe, A.; Fejer, R.; Walker, B. Center of Pressure Excursion as a Measure of Balance Performance in Patients with Non-Specific Low Back Pain Compared to Healthy Controls: A Systematic Review of the Literature. Eur. Spine J. 2011, 20, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Lafond, D.; Corriveau, H.; Hébert, R.; Prince, F. Intrasession reliability of center of pressure measures of postural steadiness in healthy elderly people. Arch. Phys. Med. Rehabil. 2004, 85, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Maki, B.E. Gait Changes in Older Adults: Predictors of Falls or Indicators of Fear? J. Am. Geriatr. Soc. 1997, 45, 313–320. [Google Scholar] [CrossRef]
- JudgeRoy, J.O.; Davis, B., III; Õunpuu, S. Step Length Reductions in Advanced Age: The Role of Ankle and Hip Kinetics. J. Gerontol. Ser. A 1996, 51A, M303–M312. [Google Scholar] [CrossRef]
- Prince, F.; Corriveau, H.; Hébert, R.; Winter, D.A. Gait in the Elderly. Gait Posture 1997, 5, 128–135. [Google Scholar] [CrossRef]
- Chen, G.; Patten, C.; Kothari, D.H.; Zajac, F.E. Gait Differences between Individuals with Post-Stroke Hemiparesis and Non-Disabled Controls at Matched Speeds. Gait Posture 2005, 22, 51–56. [Google Scholar] [CrossRef]
- Olney, S.J.; Richards, C. Hemiparetic Gait Following Stroke. Part I: Characteristics. Gait Posture 1996, 4, 136–148. [Google Scholar] [CrossRef]
- Patterson, K.K.; Parafianowicz, I.; Danells, C.J.; Closson, V.; Verrier, M.C.; Staines, W.R.; Black, S.E.; McIlroy, W.E. Gait Asymmetry in Community-Ambulating Stroke Survivors. Arch. Phys. Med. Rehabil. 2008, 89, 304–310. [Google Scholar] [CrossRef]
- Rezgui, T.; Megrot, F.; Fradet, L.; Marin, F. On the Imitation of CP Gait Patterns by Healthy Subjects. Gait Posture 2013, 38, 576–581. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| HG group | - Individuals diagnosed with stroke via CT or MRI and who have hemiparesis | - Individuals with clinical symptoms that could affect their walking ability (such as musculoskeletal diseases, acute sprain, etc.) |
| - Individuals capable of independent walking for more than 30 s on a treadmill (with Manual Muscle Testing (MMT) grades 3–5 for lower limbs, and Functional Ambulation Categories (FAC) 9 level 3–5). | ||
| MHG group | - Individuals who have not been diagnosed with stroke | |
| - Individuals who can mimic hemiplegic gait following the instructions of the medical staff |
| Characteristics | HG Group | MHG Group | |
|---|---|---|---|
| Subjects (number) | 39 | 40 | |
| Sex (number (%)) | Male | 20 (51.2%) | 28 (70%) |
| Female | 19 (48.8%) | 12 (30%) | |
| Age (Mean (SD)) | 62.8 (6.44) | 26.98 (2.27) | |
| Affected side (number (%)) | Left | 19 (47.5%) | 20 (50%) |
| Right | 21 (52.5%) | 20 (50%) | |
| MMT (median (IQR)) | Upper limb | 4 (1) | 5 (0) |
| Lower limb | 4 (0) | 5 (0) | |
| Feature (Unit) | Description | |
|---|---|---|
| Spatial feature | Foot rotation (degree) | positive: external rotation/negative: internal rotation |
| Step length (cm) | from heel contact of one foot to the other foot | |
| Stride length (cm) | initial contact of the same foot | |
| Step width (cm) | width between the feet | |
| Temporal feature | Step time (sec) | step time is the time taken between the heel contact of one foot and the heel contact of the other foot |
| Stride time (sec) | stride time is the elapsed time between the first contact of two consecutive footprints of the same foot | |
| Cadence (steps/min) | steps per minute | |
| Velocity (km/h) | walking speed during gait analysis | |
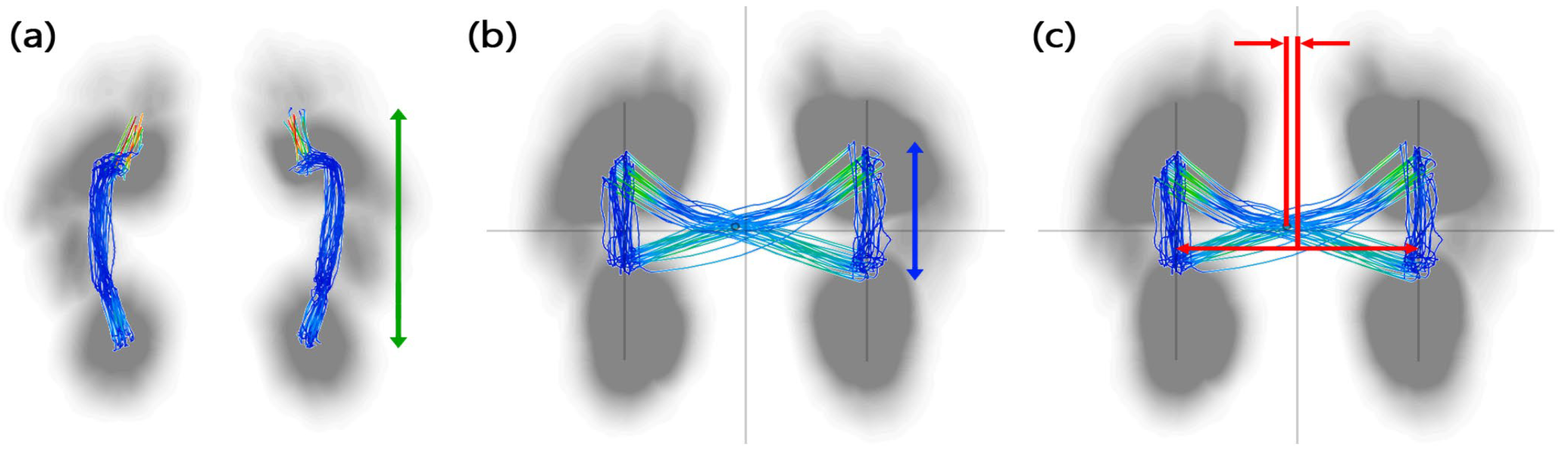
| CoP feature | Length of gait line (mm) | CoP movement on one foot during the entire stance phase |
| Single-limb support line (mm) | CoP movement during the single-leg support | |
| Lateral symmetry (mm) | horizontal distance from the center point of the horizontal line | |
| Gait event Feature | Stance phase (%) | from heel strike to toe off |
| Load response (%) | begins with initial contact, the instant the foot contacts the ground | |
| Single-limb support (%) | the swing phase where only one limb in in contact with the ground | |
| Pre-swing (%) | final phase of stance, starting with initial contact of the opposite limb | |
| Swing phase (%) | period during which the foot is in the air | |
| Double stance phase (%) | both feet are simultaneously in contact with the ground | |
| Force feature | Time maximum force 1 (%) | first maximum vertical force, which occurs at the end of loading response |
| Features (Test Type) | HG Group | MHG Group | p-Value | Cohen’s d | Gini |
|---|---|---|---|---|---|
| #Foot rotation (+) (b) | 10.74 ± 4.97 | 3.43 ± 4.82 | 0.000 ** | −1.492 | 0.051 |
| Foot rotation (−) (b) | 13.63 ± 8.14 | 3.96 ± 10.05 | 0.000 ** | −1.056 | 0.037 |
| #Step length (+) (b) | 17.48 ± 8.73 | 23.19 ± 7.02 | 0.002 * | 0.723 | 0.019 |
| Step length (−) (b) | 19.64 ± 8.48 | 20.32 ± 7.58 | 0.709 | −0.084 | 0.004 |
| Stride length (a) | 37.12 ± 15.64 | 43.51 ± 6.34 | 0.022 * | −0.538 | 0.011 |
| Step width (a) | 15.27 ± 3.51 | 16.37 ± 4.49 | 0.230 | −0.272 | 0.012 |
| Stance phase (+) (a) | 74.10 ± 5.54 | 78.32 ± 5.15 | 0.001 ** | −0.791 | 0.018 |
| Stance phase (−) (b) | 72.74 ± 5.37 | 59.00 ± 7.50 | 0.000 ** | 2.103 | 0.138 |
| Load Response (+) (b) | 23.59 ± 5.11 | 19.48 ± 6.31 | 0.002 * | 0.715 | 0.018 |
| Load Response (−) (a) | 23.72 ± 4.71 | 17.80 ± 4.71 | 0.000 ** | 1.256 | 0.038 |
| Single-limb support (+) (b) | 26.98 ± 5.61 | 41.06 ± 7.56 | 0.000 ** | −2.110 | 0.144 |
| Single-limb support (−) (b) | 25.26 ± 6.31 | 21.67 ± 5.13 | 0.007 * | 0.625 | 0.011 |
| Pre-Swing (+) (a) | 23.55 ± 4.93 | 17.79 ± 4.71 | 0.000 ** | 1.195 | 0.027 |
| Pre-Swing (−) (b) | 23.61 ± 5.29 | 19.50 ± 6.32 | 0.002 * | 0.704 | 0.019 |
| Swing phase (+) (a) | 25.90 ± 5.54 | 21.68 ± 5.15 | 0.001 ** | 0.791 | 0.014 |
| Swing phase (−) (b) | 27.26 ± 5.37 | 41.00 ± 7.50 | 0.000 ** | −2.103 | 0.155 |
| Double stance phase (a) | 47.10 ± 8.36 | 37.23 ± 8.09 | 0.000 ** | 1.200 | 0.037 |
| Step time (+) (b) | 0.98 ± 0.58 | 0.87 ± 0.22 | 0.274 | 0.252 | 0.008 |
| Step time (−) (b) | 1.02 ± 0.67 | 1.33 ± 0.22 | 0.010 * | −0.615 | 0.061 |
| Stride time (b) | 2.00 ± 1.24 | 2.20 ± 0.31 | 0.346 | −0.217 | 0.016 |
| Cadence (a) | 72.66 ± 25.57 | 55.78 ± 8.37 | 0.000 ** | 0.892 | 0.017 |
| #Velocity (a) | 0.75 ± 0.32 | 0.71 ± 0.01 | 0.501 | −0.155 | 0.025 |
| Length of gait line (+) (a) | 124.13 ± 22.62 | 134.88 ± 24.10 | 0.044 * | −0.460 | 0.007 |
| Length of gait line (−) (a) | 115.44 ± 27.07 | 101.73 ± 17.74 | 0.010 * | 0.601 | 0.007 |
| #Single-limb support line (+) (b) | 36.55 ± 20.92 | 42.19 ± 14.65 | 0.171 | 0.313 | 0.005 |
| Single-limb support line (−) (b) | 22.26 ± 15.07 | 24.47 ± 8.32 | 0.424 | −0.182 | 0.011 |
| Lateral symmetry (a) | −14.51 ± 24.26 | −21.60 ± 18.50 | 0.149 | 0.329 | 0.002 |
| Time maximum force 1 (+) (b) | 26.74 ± 5.44 | 28.50 ± 8.25 | 0.267 | −0.251 | 0.013 |
| Time maximum force 1 (−) (a) | 28.67 ± 5.25 | 20.52 ± 5.22 | 0.000 ** | 1.556 | 0.042 |
| Time maximum force ratio (b) | 0.95 ± 0.19 | 1.46 ± 0.57 | 0.000 ** | −1.196 | 0.035 |
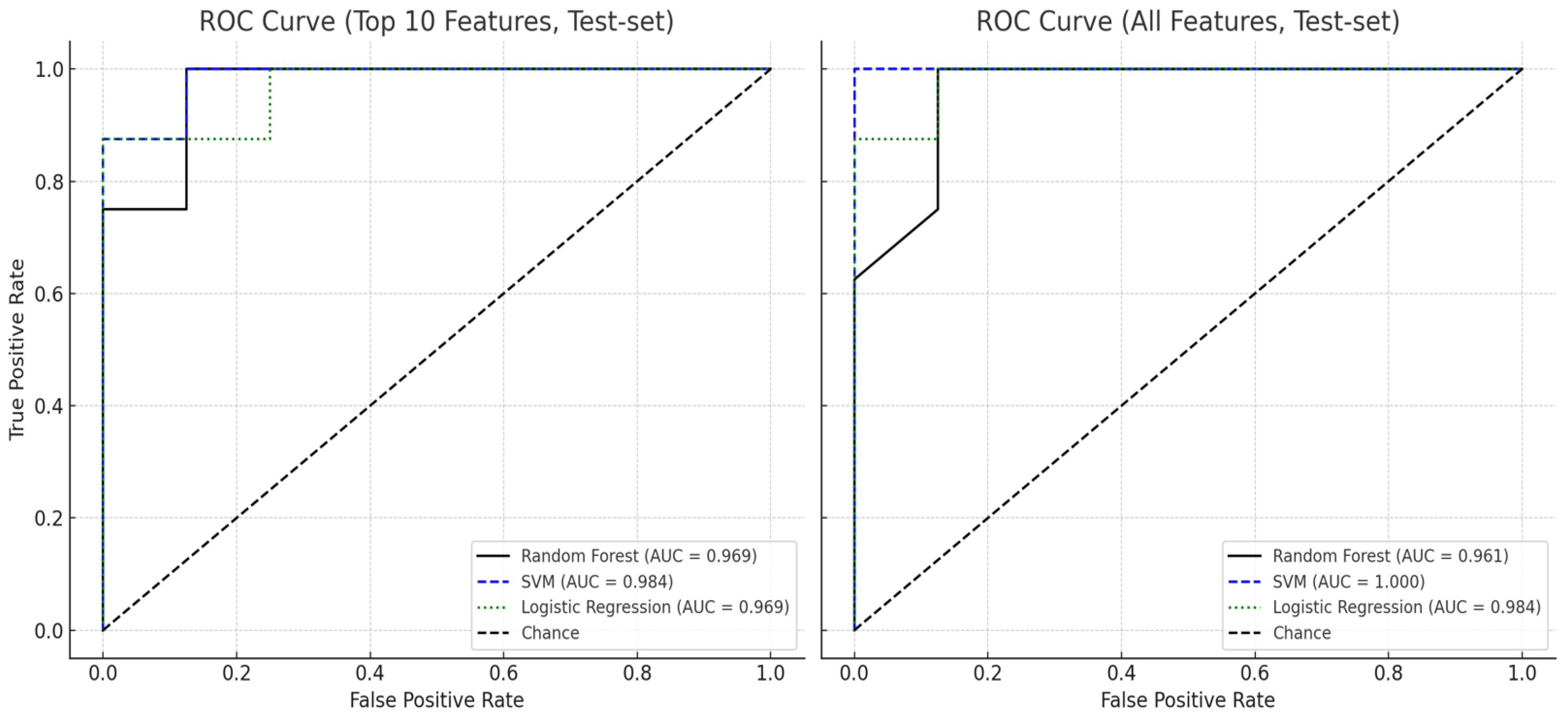
| Feature Set | Accuracy | F-1-Score | ROC AUC | |
|---|---|---|---|---|
| Random Forest | All features | 0.875 | 0.889 | 0.961 |
| Top 10 features | 0.923 | 0.924 | 0.949 | |
| SVM (RBF Kernel) | All features | 0.938 | 0.941 | 1.000 |
| Top 10 features | 0.911 | 0.913 | 0.961 | |
| Logistic Regression | All features | 0.911 | 0.901 | 0.932 |
| Top 10 features | 0.911 | 0.907 | 0.941 |
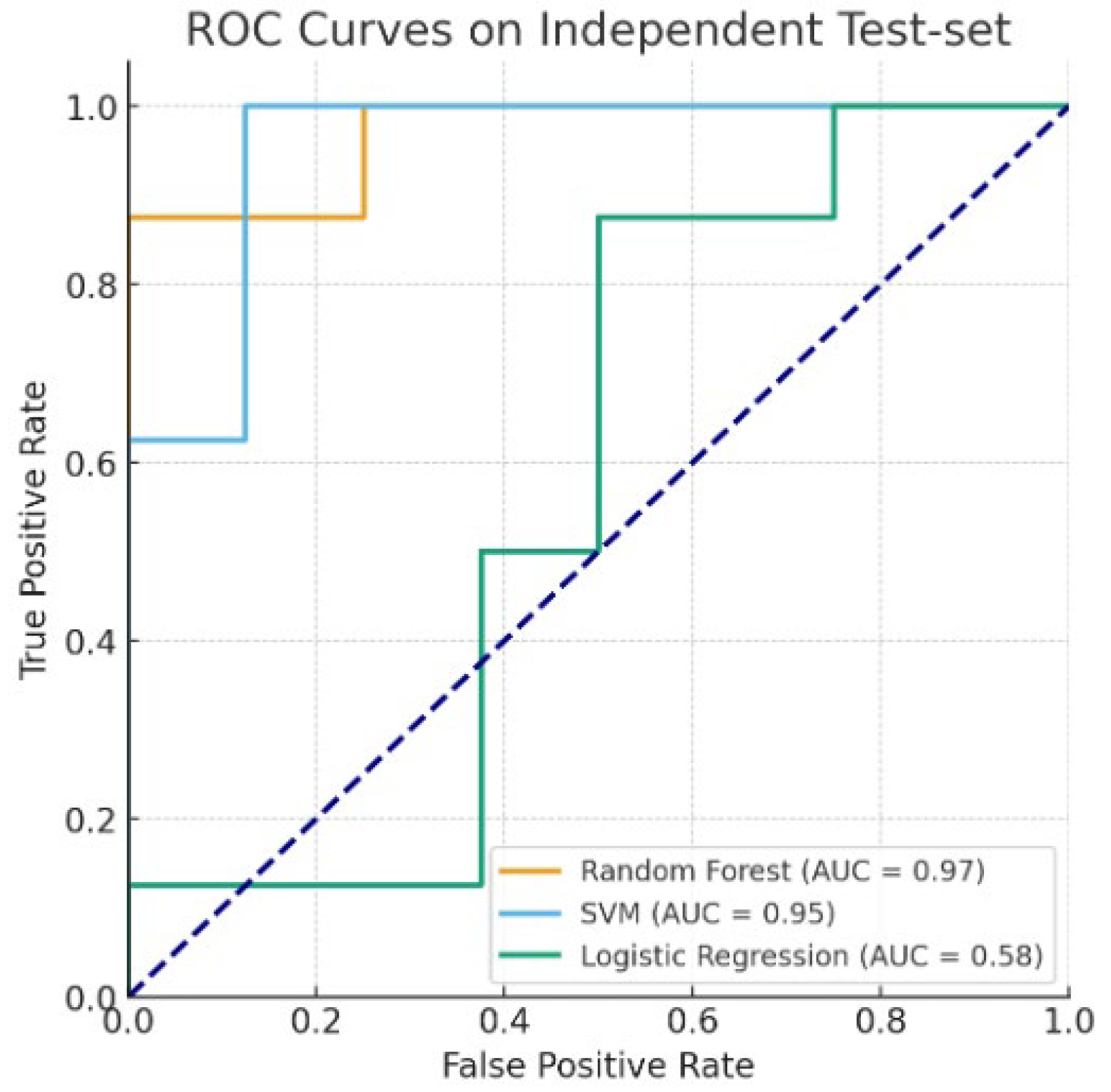
| Accuracy | F-1-Score | ROC AUC | |
|---|---|---|---|
| Random Forest | 0.875 | 0.875 | 0.969 |
| SVM (RBF Kernel) | 0.875 | 0.889 | 0.953 |
| Logistic Regression | 0.625 | 0.667 | 0.578 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-u.; Kwon, S.; Kim, C.-H.; Seo, J.-W.; Lee, S. Classification of Hemiplegic Gait and Mimicked Hemiplegic Gait: A Treadmill Gait Analysis Study in Stroke Patients and Healthy Individuals. Bioengineering 2025, 12, 1074. https://doi.org/10.3390/bioengineering12101074
Lee Y-u, Kwon S, Kim C-H, Seo J-W, Lee S. Classification of Hemiplegic Gait and Mimicked Hemiplegic Gait: A Treadmill Gait Analysis Study in Stroke Patients and Healthy Individuals. Bioengineering. 2025; 12(10):1074. https://doi.org/10.3390/bioengineering12101074
Chicago/Turabian StyleLee, Young-ung, Seungwon Kwon, Cheol-Hyun Kim, Jeong-Woo Seo, and Sangkwan Lee. 2025. "Classification of Hemiplegic Gait and Mimicked Hemiplegic Gait: A Treadmill Gait Analysis Study in Stroke Patients and Healthy Individuals" Bioengineering 12, no. 10: 1074. https://doi.org/10.3390/bioengineering12101074
APA StyleLee, Y.-u., Kwon, S., Kim, C.-H., Seo, J.-W., & Lee, S. (2025). Classification of Hemiplegic Gait and Mimicked Hemiplegic Gait: A Treadmill Gait Analysis Study in Stroke Patients and Healthy Individuals. Bioengineering, 12(10), 1074. https://doi.org/10.3390/bioengineering12101074










