Label-Free Cancer Detection Methods Based on Biophysical Cell Phenotypes
Abstract
1. Introduction
2. Microfluidics Systems for Cell Separation and Enrichment
2.1. Passive Cell Separation Microfluidics
2.1.1. Deterministic Lateral Displacement
2.1.2. Inertial Focusing and Centrifugal Microfluidics
2.1.3. Microfiltration
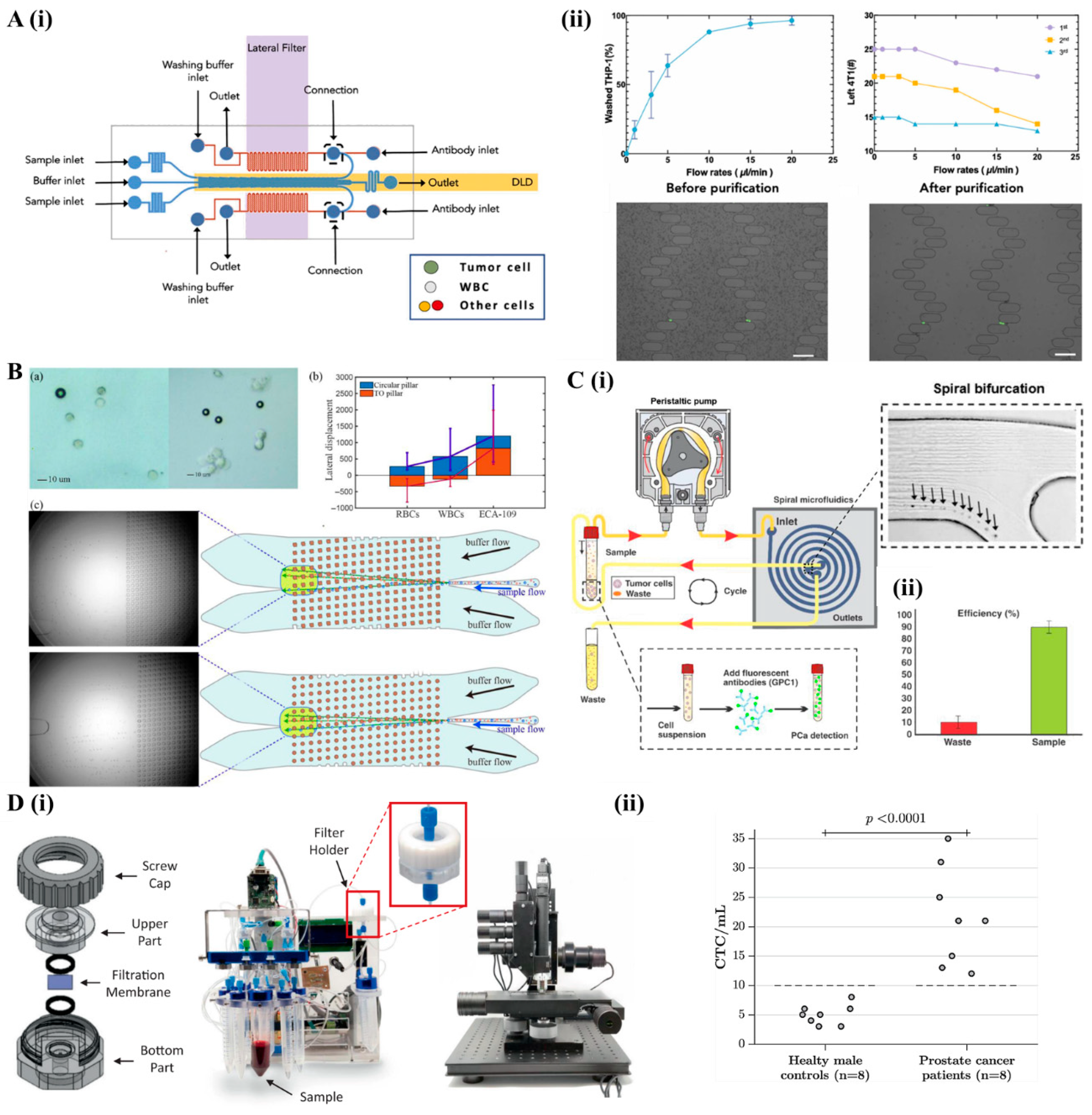
2.2. Active Cell Separation Microfluidic
2.2.1. Acoustofluidics
2.2.2. Magnetofluidics
2.2.3. Dielectrophoresis Microfluidics

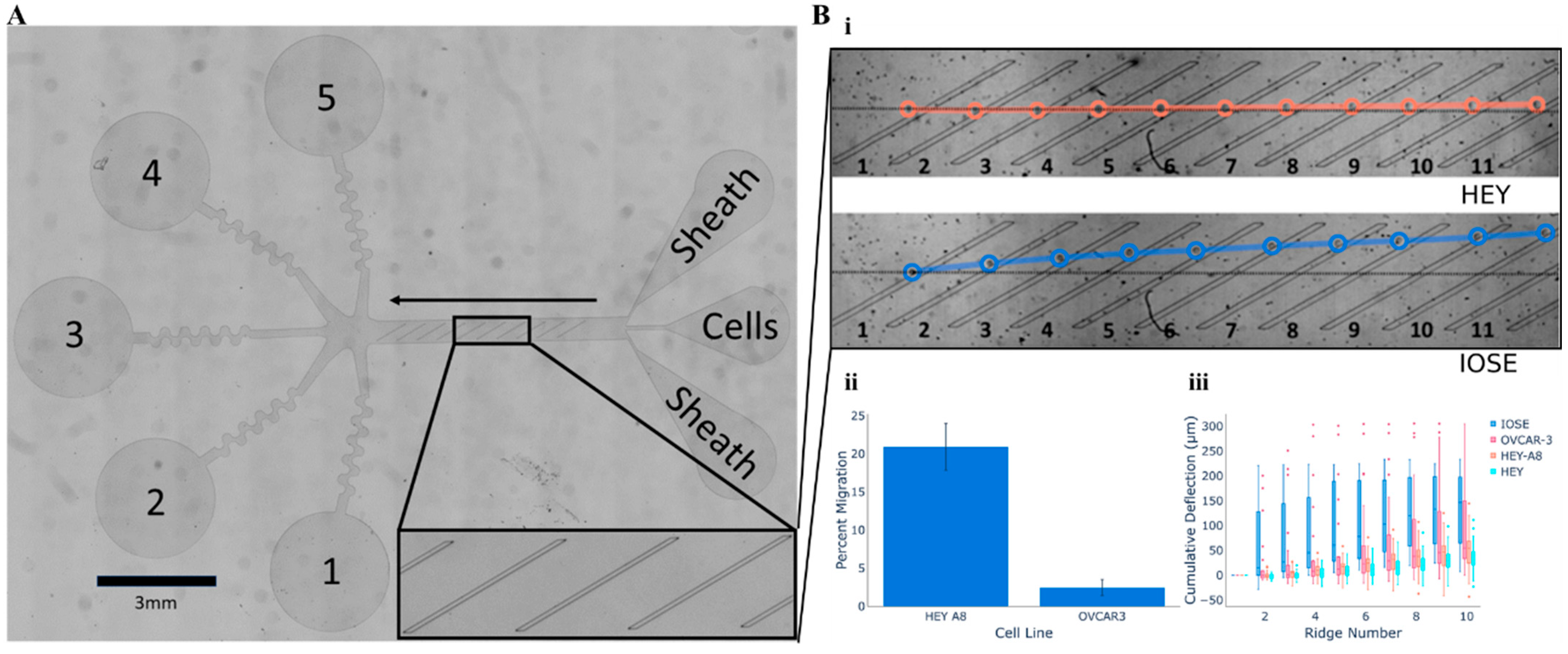
2.3. Cellular Mobility Within Microfluidic Arrays
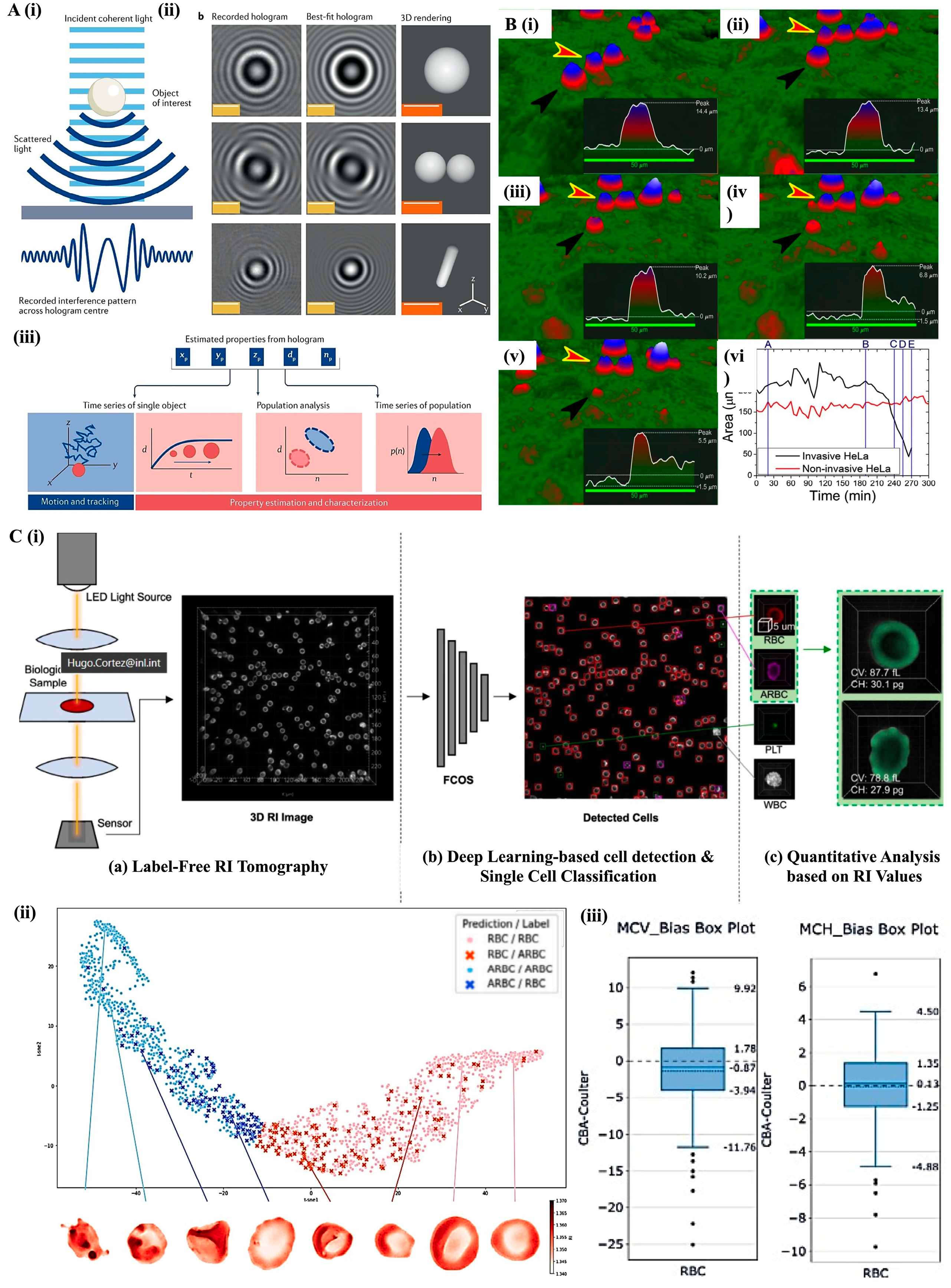
3. Label-Free Microscopy Techniques for Cellular Analysis
3.1. Phase-Contrast Microscopy—Morphological Profiling and Segmentation

3.2. Holographic Microscopy: Quantitative Phase Imaging for 3D Characterization
4. Cytometric Techniques
4.1. Deformability Cytometry: Mechanical Fingerprinting of Cells
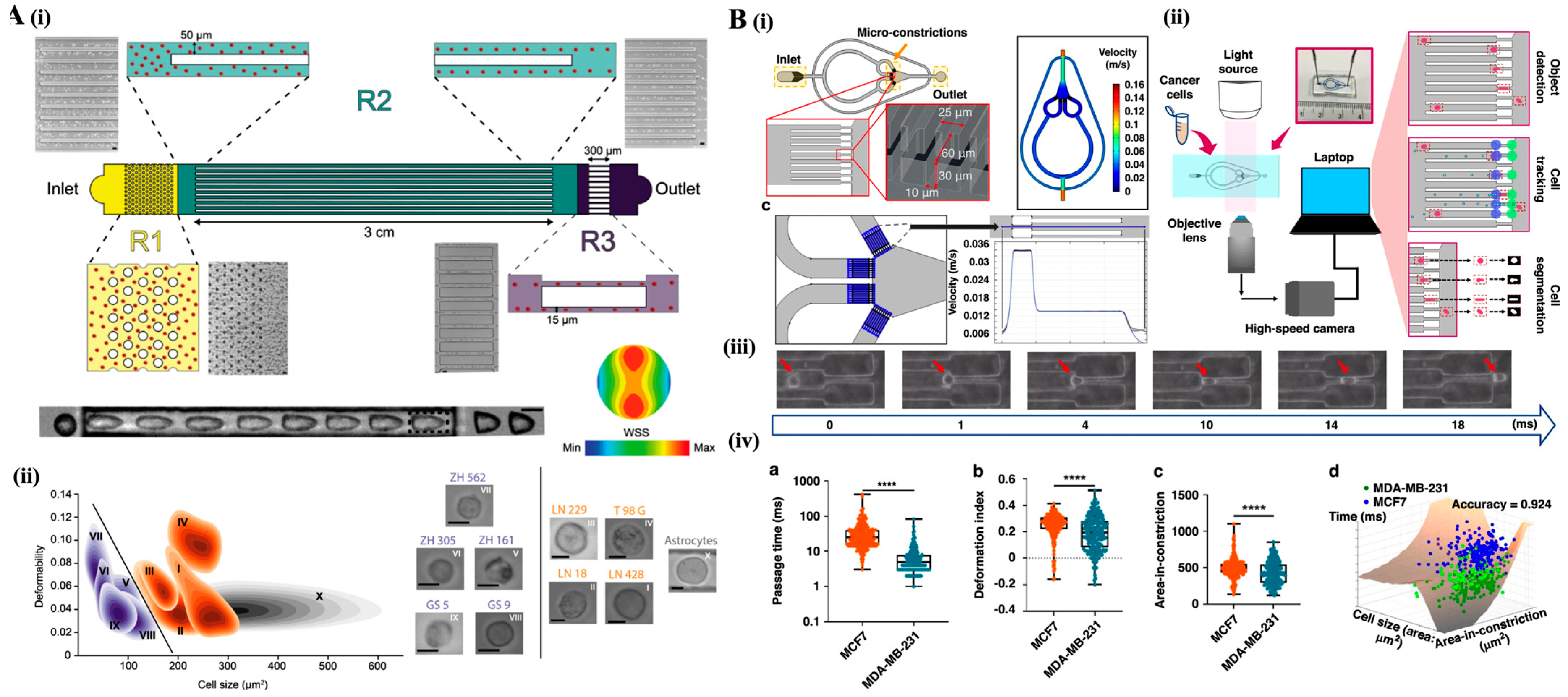
4.2. Impedance Cytometry: Electrical Cell Characterization
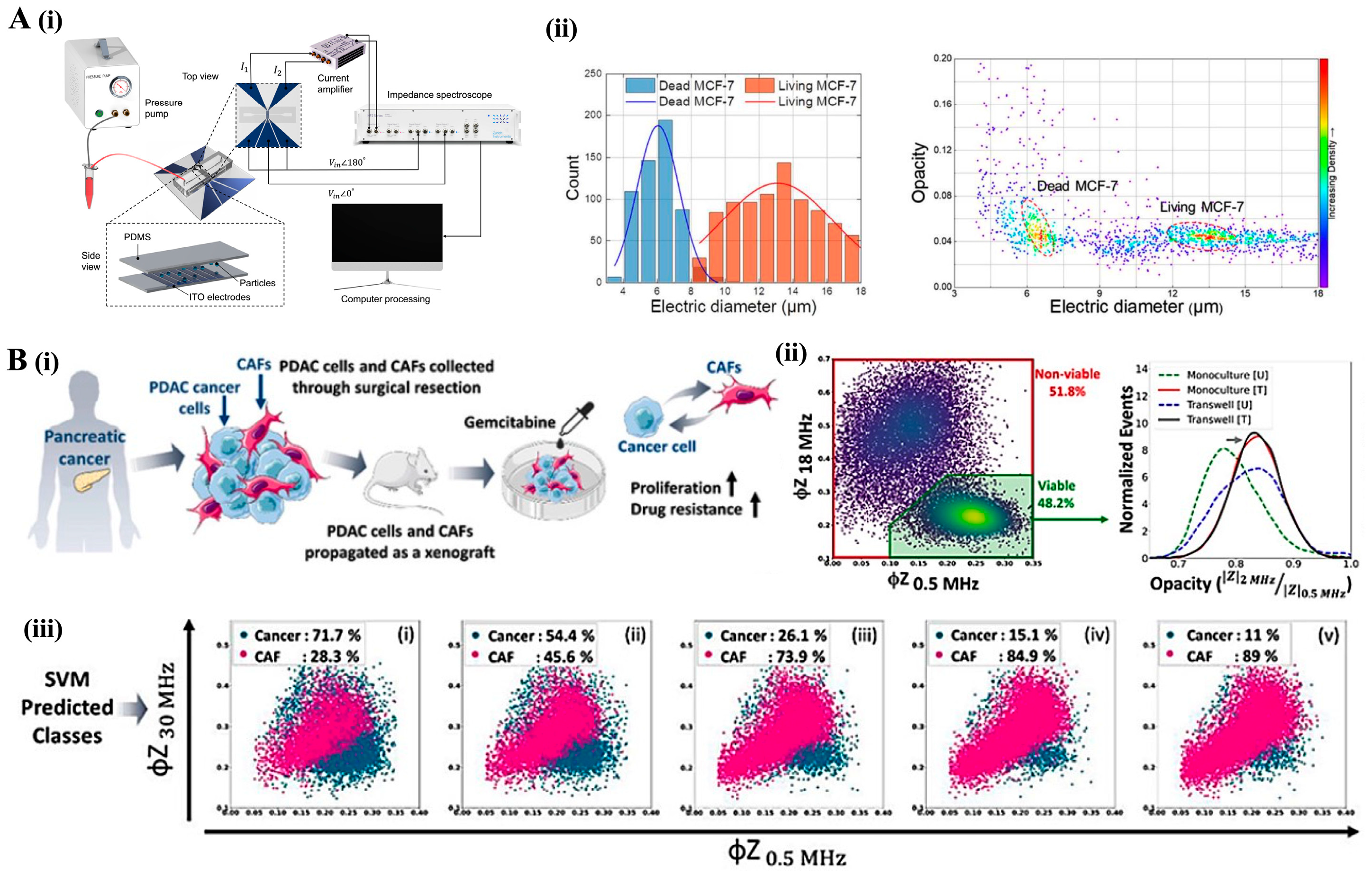
4.3. Imaging Flow Cytometry: High-Throughput Morphological and Functional Analysis
Multimodal Integration and AI-Enhanced Applications in IFC
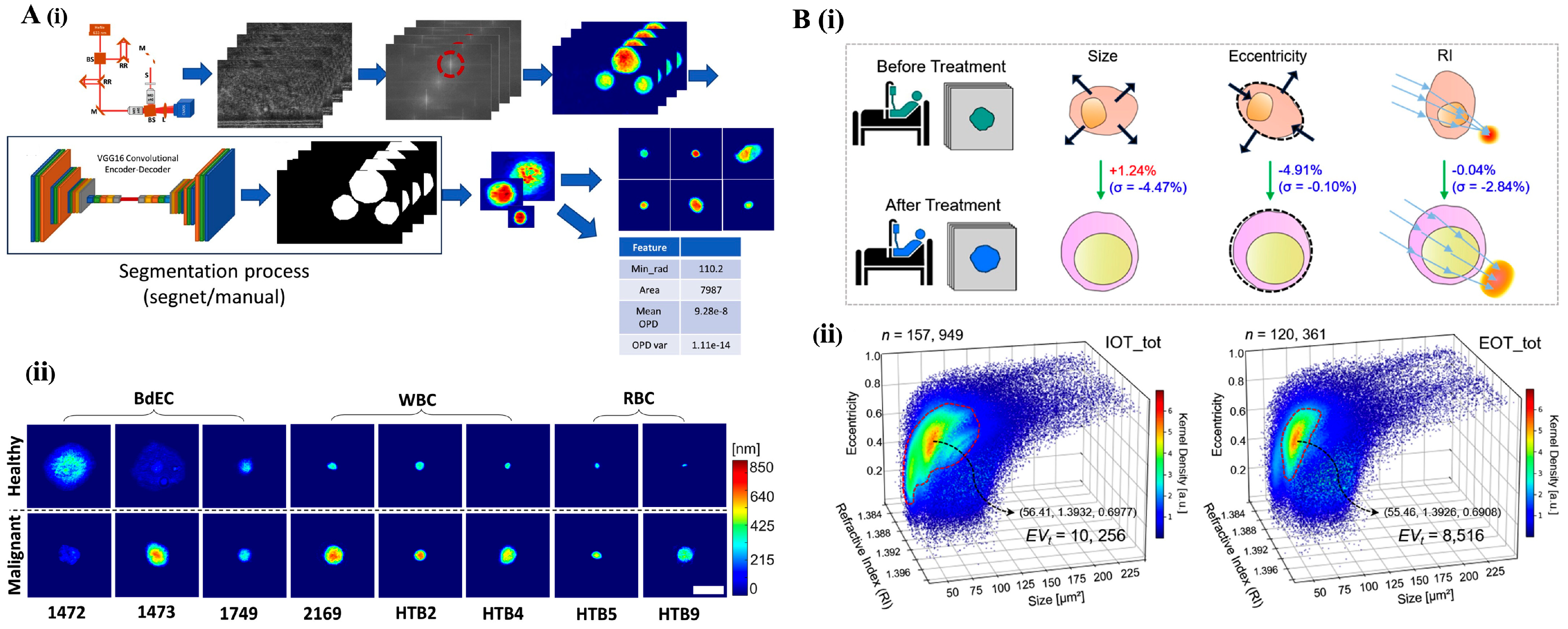
5. Cell and Particle Scattering Techniques
5.1. Dynamic Light Scattering and Zeta Potential

5.2. Surface-Enhanced Raman Spectroscopy
6. Electro-Mechanical and Surface Characterization
6.1. Atomic Force Microscopy
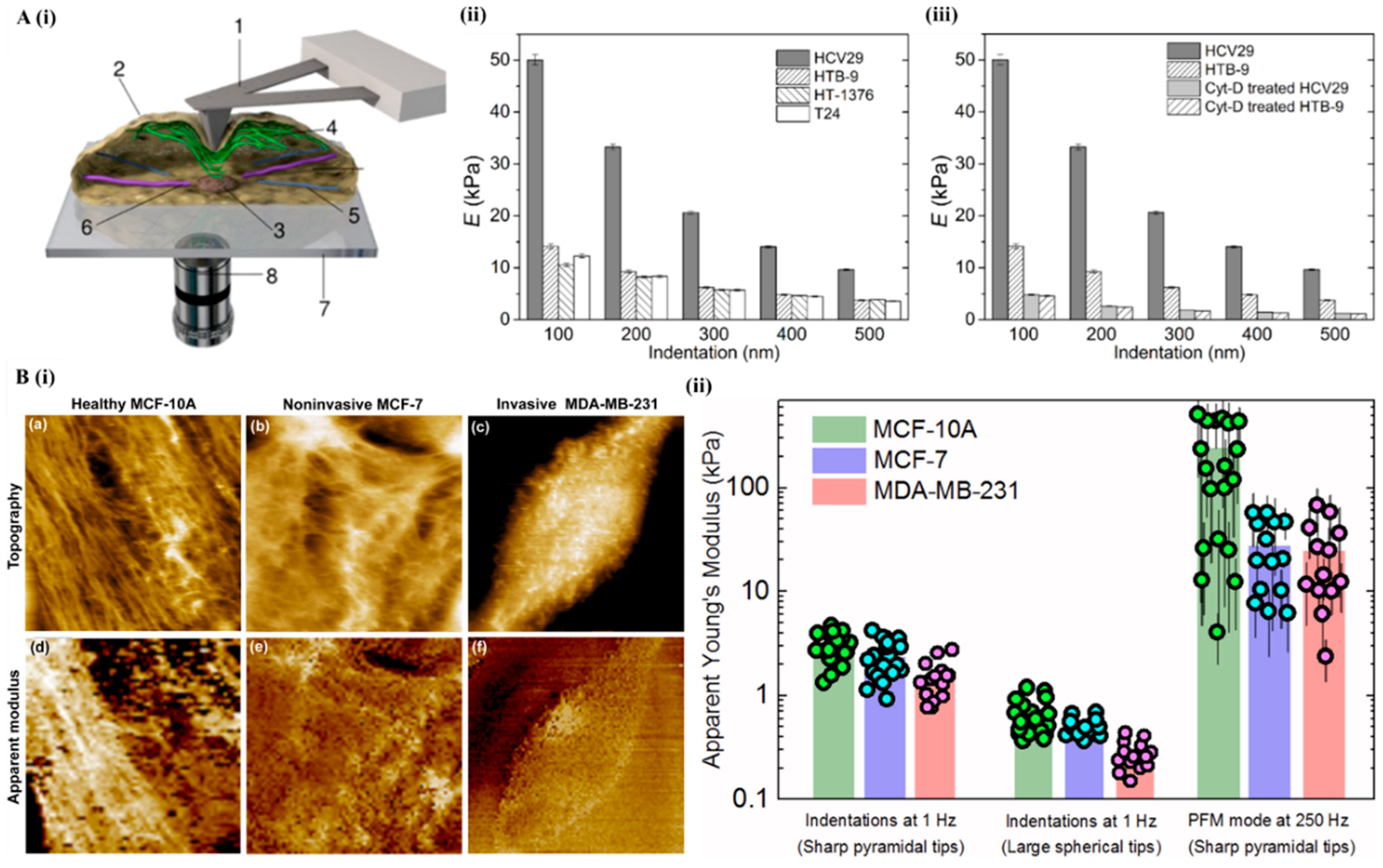
6.2. Electrical Impedance Spectroscopy
7. Clinical Translation

8. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Available online: https://www.who.int/ (accessed on 13 August 2024).
- Jayanthi, V.S.P.K.S.A.; Das, A.B.; Saxena, U. Recent Advances in Biosensor Development for the Detection of Cancer Biomarkers. Biosens. Bioelectron. 2017, 91, 15–23. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Available online: https://www.cancer.gov/ (accessed on 23 August 2024).
- Pulumati, A.; Pulumati, A.; Dwarakanath, B.S.; Verma, A.; Papineni, R.V.L. Technological Advancements in Cancer Diagnostics: Improvements and Limitations. Cancer Rep. 2023, 6, e1764. [Google Scholar] [CrossRef]
- Syedmoradi, L.; Norton, M.L.; Omidfar, K. Point-of-Care Cancer Diagnostic Devices: From Academic Research to Clinical Translation. Talanta 2021, 225, 122002. [Google Scholar] [CrossRef]
- Zhang, S.X.; Liu, L.; Zhao, W. Targeting Biophysical Cues: A Niche Approach to Study, Diagnose, and Treat Cancer. Trends Cancer 2018, 4, 268–271. [Google Scholar] [CrossRef]
- Yadav, S.; Barton, M.J.; Nguyen, N.-T. Biophysical Properties of Cells for Cancer Diagnosis. J. Biomech. 2019, 86, 1–7. [Google Scholar] [CrossRef]
- Huang, Z.; Cao, L. Quantitative Phase Imaging Based on Holography: Trends and New Perspectives. Light Sci. Appl. 2024, 13, 145. [Google Scholar] [CrossRef]
- Zangle, T.A.; Teitell, M.A. Live-Cell Mass Profiling: An Emerging Approach in Quantitative Biophysics. Nat. Methods 2014, 11, 1221–1228. [Google Scholar] [CrossRef]
- Ker, D.F.E.; Eom, S.; Sanami, S.; Bise, R.; Pascale, C.; Yin, Z.; Huh, S.; Osuna-Highley, E.; Junkers, S.N.; Helfrich, C.J.; et al. Phase Contrast Time-Lapse Microscopy Datasets with Automated and Manual Cell Tracking Annotations. Sci. Data 2018, 5, 180237. [Google Scholar] [CrossRef] [PubMed]
- Hejna, M.; Jorapur, A.; Song, J.S.; Judson, R.L. High Accuracy Label-Free Classification of Single-Cell Kinetic States from Holographic Cytometry of Human Melanoma Cells. Sci. Rep. 2017, 7, 11943. [Google Scholar] [CrossRef]
- Boya, M.; Ozkaya-Ahmadov, T.; Swain, B.E.; Chu, C.-H.; Asmare, N.; Civelekoglu, O.; Liu, R.; Lee, D.; Tobia, S.; Biliya, S.; et al. High Throughput, Label-Free Isolation of Circulating Tumor Cell Clusters in Meshed Microwells. Nat. Commun. 2022, 13, 3385. [Google Scholar] [CrossRef] [PubMed]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic Light Scattering: A Practical Guide and Applications in Biomedical Sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef]
- Farshchi, F.; Hasanzadeh, M. Microfluidic Biosensing of Circulating Tumor Cells (CTCs): Recent Progress and Challenges in Efficient Diagnosis of Cancer. Biomed. Pharmacother. 2021, 134, 111153. [Google Scholar] [CrossRef]
- Vasseur, A.; Kiavue, N.; Bidard, F.; Pierga, J.; Cabel, L. Clinical Utility of Circulating Tumor Cells: An Update. Mol. Oncol. 2021, 15, 1647–1666. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M. The Origins and the Future of Microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Akgönüllü, S.; Bakhshpour, M.; Pişkin, A.K.; Denizli, A. Microfluidic Systems for Cancer Diagnosis and Applications. Micromachines 2021, 12, 1349. [Google Scholar] [CrossRef] [PubMed]
- Gurkan, U.A.; Wood, D.K.; Carranza, D.; Herbertson, L.H.; Diamond, S.L.; Du, E.; Guha, S.; Di Paola, J.; Hines, P.C.; Papautsky, I.; et al. Next Generation Microfluidics: Fulfilling the Promise of Lab-on-a-Chip Technologies. Lab Chip 2024, 24, 1867–1874. [Google Scholar] [CrossRef]
- Chiang, C.-C.; Anne, R.; Chawla, P.; Shaw, R.M.; He, S.; Rock, E.C.; Zhou, M.; Cheng, J.; Gong, Y.-N.; Chen, Y.-C. Deep Learning Unlocks Label-Free Viability Assessment of Cancer Spheroids in Microfluidics. Lab Chip 2024, 24, 3169–3182. [Google Scholar] [CrossRef]
- Tang, H.; Niu, J.; Pan, X.; Jin, H.; Lin, S.; Cui, D. Topology Optimization Based Deterministic Lateral Displacement Array Design for Cell Separation. J. Chromatogr. A 2022, 1679, 463384. [Google Scholar] [CrossRef]
- Zhang, T.; Di Carlo, D.; Lim, C.T.; Zhou, T.; Tian, G.; Tang, T.; Shen, A.Q.; Li, W.; Li, M.; Yang, Y.; et al. Passive Microfluidic Devices for Cell Separation. Biotechnol. Adv. 2024, 71, 108317. [Google Scholar] [CrossRef]
- Mendelaar, P.A.J.; Kraan, J.; Van, M.; Zeune, L.L.; Terstappen, L.W.M.M.; Oomen-de Hoop, E.; Martens, J.W.M.; Sleijfer, S. Defining the Dimensions of Circulating Tumor Cells in a Large Series of Breast, Prostate, Colon, and Bladder Cancer Patients. Mol. Oncol. 2021, 15, 116–125. [Google Scholar] [CrossRef]
- Huang, S.-J.; Chang, C.-M.; Lu, Y.-T.; Liu, C.-H. Microfluidic Biochip for Target Tumor Cell and Cell-Cluster Sorting. Sens. Actuators B Chem. 2023, 394, 134369. [Google Scholar] [CrossRef]
- Burger, R.; Kirby, D.; Glynn, M.; Nwankire, C.; O’Sullivan, M.; Siegrist, J.; Kinahan, D.; Aguirre, G.; Kijanka, G.; Gorkin, R.A.; et al. Centrifugal Microfluidics for Cell Analysis. Curr. Opin. Chem. Biol. 2012, 16, 409–414. [Google Scholar] [CrossRef]
- Rzhevskiy, A.S.; Razavi Bazaz, S.; Ding, L.; Kapitannikova, A.; Sayyadi, N.; Campbell, D.; Walsh, B.; Gillatt, D.; Ebrahimi Warkiani, M.; Zvyagin, A.V. Rapid and Label-Free Isolation of Tumour Cells from the Urine of Patients with Localised Prostate Cancer Using Inertial Microfluidics. Cancers 2019, 12, 81. [Google Scholar] [CrossRef]
- Lin, C.-C.; Tsai, J.-C.; Liu, Y.-Z.; Kuo, J.-N. Label-Free Cancer Cell Separation from Whole Blood on Centrifugal Microfluidic Platform Using Hydrodynamic Technique. Microfluid. Nanofluid. 2024, 28, 10. [Google Scholar] [CrossRef]
- Ozkumur, E.; Shah, A.M.; Ciciliano, J.C.; Emmink, B.L.; Miyamoto, D.T.; Brachtel, E.; Yu, M.; Chen, P.; Morgan, B.; Trautwein, J.; et al. Inertial Focusing for Tumor Antigen–Dependent and –Independent Sorting of Rare Circulating Tumor Cells. Sci. Transl. Med. 2013, 5, 179ra47. [Google Scholar] [CrossRef]
- Yee-de León, J.F.; Soto-García, B.; Aráiz-Hernández, D.; Delgado-Balderas, J.R.; Esparza, M.; Aguilar-Avelar, C.; Wong-Campos, J.D.; Chacón, F.; López-Hernández, J.Y.; González-Treviño, A.M.; et al. Characterization of a Novel Automated Microfiltration Device for the Efficient Isolation and Analysis of Circulating Tumor Cells from Clinical Blood Samples. Sci. Rep. 2020, 10, 7543. [Google Scholar] [CrossRef]
- Chu, C.-H.; Liu, R.; Ozkaya-Ahmadov, T.; Swain, B.E.; Boya, M.; El-Rayes, B.; Akce, M.; Bilen, M.A.; Kucuk, O.; Sarioglu, A.F. Negative Enrichment of Circulating Tumor Cells from Unmanipulated Whole Blood with a 3D Printed Device. Sci. Rep. 2021, 11, 20583. [Google Scholar] [CrossRef]
- Mansor, M.A.; Jamrus, M.A.; Lok, C.K.; Ahmad, M.R.; Petrů, M.; Koloor, S.S.R. Microfluidic Device for Both Active and Passive Cell Separation Techniques: A Review. Sens. Actuators Rep. 2025, 9, 100277. [Google Scholar] [CrossRef]
- Zhang, P.; Bachman, H.; Ozcelik, A.; Huang, T.J. Acoustic Microfluidics. Annu. Rev. Anal. Chem. 2020, 13, 17–43. [Google Scholar] [CrossRef]
- Wu, M.; Ozcelik, A.; Rufo, J.; Wang, Z.; Fang, R.; Jun Huang, T. Acoustofluidic Separation of Cells and Particles. Microsyst. Nanoeng. 2019, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Mao, Z.; Peng, Z.; Zhou, L.; Chen, Y.; Huang, P.-H.; Truica, C.I.; Drabick, J.J.; El-Deiry, W.S.; Dao, M.; et al. Acoustic Separation of Circulating Tumor Cells. Proc. Natl. Acad. Sci. USA 2015, 112, 4970–4975. [Google Scholar] [CrossRef] [PubMed]
- Augustsson, P.; Magnusson, C.; Nordin, M.; Lilja, H.; Laurell, T. Microfluidic, Label-Free Enrichment of Prostate Cancer Cells in Blood Based on Acoustophoresis. Anal. Chem. 2012, 84, 7954–7962. [Google Scholar] [CrossRef]
- Antfolk, M.; Kim, S.H.; Koizumi, S.; Fujii, T.; Laurell, T. Label-Free Single-Cell Separation and Imaging of Cancer Cells Using an Integrated Microfluidic System. Sci. Rep. 2017, 7, 46507. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, A.; Chen, S.; Lum, G.Z.; Zhang, X. A Perspective on Magnetic Microfluidics: Towards an Intelligent Future. Biomicrofluidics 2022, 16, 011301. [Google Scholar] [CrossRef] [PubMed]
- Durmus, N.G.; Tekin, H.C.; Guven, S.; Sridhar, K.; Arslan Yildiz, A.; Calibasi, G.; Ghiran, I.; Davis, R.W.; Steinmetz, L.M.; Demirci, U. Magnetic Levitation of Single Cells. Proc. Natl. Acad. Sci. USA 2015, 112, E3661–E3668. [Google Scholar] [CrossRef]
- Zhao, W.; Cheng, R.; Lim, S.H.; Miller, J.R.; Zhang, W.; Tang, W.; Xie, J.; Mao, L. Biocompatible and Label-Free Separation of Cancer Cells from Cell Culture Lines from White Blood Cells in Ferrofluids. Lab Chip 2017, 17, 2243–2255. [Google Scholar] [CrossRef] [PubMed]
- Kecili, S.; Yilmaz, E.; Ozcelik, O.S.; Anil-Inevi, M.; Gunyuz, Z.E.; Yalcin-Ozuysal, O.; Ozcivici, E.; Tekin, H.C. ΜDACS Platform: A Hybrid Microfluidic Platform Using Magnetic Levitation Technique and Integrating Magnetic, Gravitational, and Drag Forces for Density-Based Rare Cancer Cell Sorting. Biosens. Bioelectron. X 2023, 15, 100392. [Google Scholar] [CrossRef]
- Liang, K.; Yaman, S.; Patel, R.K.; Parappilly, M.S.; Walker, B.S.; Wong, M.H.; Durmus, N.G. Magnetic Levitation and Sorting of Neoplastic Circulating Cell Hybrids. bioRxiv 2022. bioRxiv:11.03.515127. [Google Scholar] [CrossRef]
- Dietz, M.S.; Sutton, T.L.; Walker, B.S.; Gast, C.E.; Zarour, L.; Sengupta, S.K.; Swain, J.R.; Eng, J.; Parappilly, M.; Limbach, K.; et al. Relevance of Circulating Hybrid Cells as a Non-Invasive Biomarker for Myriad Solid Tumors. Sci. Rep. 2021, 11, 13630. [Google Scholar] [CrossRef]
- Munaz, A.; Shiddiky, M.J.A.; Nguyen, N.-T. Recent Advances and Current Challenges in Magnetophoresis Based Micro Magnetofluidics. Biomicrofluidics 2018, 12, 031501. [Google Scholar] [CrossRef]
- Chan, J.Y.; Ahmad Kayani, A.B.; Md Ali, M.A.; Kok, C.K.; Majlis, B.Y.; Hoe, S.L.L.; Marzuki, M.; Khoo, A.S.-B.; Ostrikov, K.K.; Rahman, M.A.; et al. Dielectrophoresis-Based Microfluidic Platforms for Cancer Diagnostics. Biomicrofluidics 2018, 12, 011503. [Google Scholar] [CrossRef]
- Kwizera, E.A.; Sun, M.; White, A.M.; Li, J.; He, X. Methods of Generating Dielectrophoretic Force for Microfluidic Manipulation of Bioparticles. ACS Biomater. Sci. Eng. 2021, 7, 2043–2063. [Google Scholar] [CrossRef] [PubMed]
- Varmazyari, V.; Habibiyan, H.; Ghafoorifard, H.; Ebrahimi, M.; Ghafouri-Fard, S. A Dielectrophoresis-Based Microfluidic System Having Double-Sided Optimized 3D Electrodes for Label-Free Cancer Cell Separation with Preserving Cell Viability. Sci. Rep. 2022, 12, 12100. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Nguyen, H.T.; Ngo, N.A.; Nguyen, M.C.; Bui Thu, H.; Ducrée, J.; Chu Duc, T.; Bui, T.T.; Do Quang, L. Numerical Study on a Facing Electrode Configuration Dielectrophoresis Microfluidic System for Efficient Biological Cell Separation. Sci. Rep. 2024, 14, 27627. [Google Scholar] [CrossRef]
- Lan, M.; Ren, Z.; Cheng, C.; Li, G.; Yang, F. Small Extracellular Vesicles Detection Using Dielectrophoresis-Based Microfluidic Chip for Diagnosis of Breast Cancer. Biosens. Bioelectron. 2024, 259, 116382. [Google Scholar] [CrossRef]
- Stone, N.E.; Raj, A.; Young, K.M.; DeLuca, A.P.; Chrit, F.E.; Tucker, B.A.; Alexeev, A.; McDonald, J.; Benigno, B.B.; Sulchek, T. Label-Free Microfluidic Enrichment of Cancer Cells from Non-Cancer Cells in Ascites. Sci. Rep. 2021, 11, 18032. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Rahman, Z.; ten Dijke, P.; Boukany, P.E. Microfluidics Meets 3D Cancer Cell Migration. Trends Cancer 2022, 8, 683–697. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Yin, T.-I.; Reyes, D.; Urban, G.A. Microfluidic Chip with Integrated Electrical Cell-Impedance Sensing for Monitoring Single Cancer Cell Migration in Three-Dimensional Matrixes. Anal. Chem. 2013, 85, 11068–11076. [Google Scholar] [CrossRef]
- Lovitt, R.W.; Wright, C.J. MICROSCOPY | Light Microscopy. In Encyclopedia of Food Microbiology; Elsevier: Amsterdam, The Netherlands, 1999; pp. 1379–1388. [Google Scholar]
- Douglas, B.M.; Ron, O.; Stanley, S.; Michael, W. Davidson MicroscopyU—The Source for Microscopy Education. Available online: https://www.microscopyu.com/techniques/phase-contrast/introduction-to-phase-contrast-microscopy (accessed on 8 April 2025).
- Pastorek, L.; Venit, T.; Hozák, P. Holography Microscopy as an Artifact-Free Alternative to Phase-Contrast. Histochem. Cell Biol. 2018, 149, 179–186. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, H.; Chen, S.; Huang, Y.; Guan, Q. Cancer Cells Detection in Phase-Contrast Microscopy Images Based on Faster R-CNN. In Proceedings of the 2016 9th International Symposium on Computational Intelligence and Design (ISCID), Hangzhou, China, 10–11 December 2016; IEEE: New York, NY, USA, 2016; pp. 363–367. [Google Scholar]
- Chan, T.J.; Zhang, X.; Mak, M. Biophysical Informatics Reveals Distinctive Phenotypic Signatures and Functional Diversity of Single-Cell Lineages. Bioinformatics 2023, 39, btac833. [Google Scholar] [CrossRef] [PubMed]
- Edlund, C.; Jackson, T.R.; Khalid, N.; Bevan, N.; Dale, T.; Dengel, A.; Ahmed, S.; Trygg, J.; Sjögren, R. LIVECell—A Large-Scale Dataset for Label-Free Live Cell Segmentation. Nat. Methods 2021, 18, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Han, J.; Kim, Y.S.; Lee, Y.; Yang, S. A Novel Method for Effective Cell Segmentation and Tracking in Phase Contrast Microscopic Images. Sensors 2021, 21, 3516. [Google Scholar] [CrossRef] [PubMed]
- Beres, B.; Kovacs, K.D.; Kanyo, N.; Peter, B.; Szekacs, I.; Horvath, R. Label-Free Single-Cell Cancer Classification from the Spatial Distribution of Adhesion Contact Kinetics. ACS Sens. 2024, 9, 5815–5827. [Google Scholar] [CrossRef] [PubMed]
- Calin, V.L.; Mihailescu, M.; Petrescu, G.E.D.; Lisievici, M.G.; Tarba, N.; Calin, D.; Ungureanu, V.G.; Pasov, D.; Brehar, F.M.; Gorgan, R.M.; et al. Grading of Glioma Tumors Using Digital Holographic Microscopy. Heliyon 2024, 10, e29897. [Google Scholar] [CrossRef]
- El-Schich, Z. Digital Holographic Microscopy: A Noninvasive Method to Analyze the Formation of Spheroids. Biotechniques 2021, 71, 598–603. [Google Scholar] [CrossRef]
- Pirone, D.; Bianco, V.; Miccio, L.; Memmolo, P.; Psaltis, D.; Ferraro, P. Beyond Fluorescence: Advances in Computational Label-Free Full Specificity in 3D Quantitative Phase Microscopy. Curr. Opin. Biotechnol. 2024, 85, 103054. [Google Scholar] [CrossRef]
- Kelly, D.P. Resolution Limits in Practical Digital Holographic Systems. Opt. Eng. 2009, 48, 095801. [Google Scholar] [CrossRef]
- El-Schich, Z.; Leida Mölder, A.; Gjörloff Wingren, A. Quantitative Phase Imaging for Label-Free Analysis of Cancer Cells—Focus on Digital Holographic Microscopy. Appl. Sci. 2018, 8, 1027. [Google Scholar] [CrossRef]
- Dubois, F.; Yourassowsky, C.; Monnom, O.; Legros, J.-C.; Debeir, O.; Van Ham, P.; Kiss, R.; Decaestecker, C. Digital Holographic Microscopy for the Three-Dimensional Dynamic Analysis of in Vitro Cancer Cell Migration. J. Biomed. Opt. 2006, 11, 054032. [Google Scholar] [CrossRef]
- Nagy, Á.G.; Székács, I.; Bonyár, A.; Horvath, R. Simple and Automatic Monitoring of Cancer Cell Invasion into an Epithelial Monolayer Using Label-Free Holographic Microscopy. Sci. Rep. 2022, 12, 10111. [Google Scholar] [CrossRef]
- El-Schich, Z.; Mölder, A.; Tassidis, H.; Härkönen, P.; Falck Miniotis, M.; Gjörloff Wingren, A. Induction of Morphological Changes in Death-Induced Cancer Cells Monitored by Holographic Microscopy. J. Struct. Biol. 2015, 189, 207–212. [Google Scholar] [CrossRef]
- Roitshtain, D.; Wolbromsky, L.; Bal, E.; Greenspan, H.; Satterwhite, L.L.; Shaked, N.T. Quantitative Phase Microscopy Spatial Signatures of Cancer Cells. Cytom. Part A 2017, 91, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-K.; Lee, B.-W.; Fujii, F.; Kim, J.K.; Pack, C.-G. Physicochemical Properties of Nucleoli in Live Cells Analyzed by Label-Free Optical Diffraction Tomography. Cells 2019, 8, 699. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.V.; Pantanowitz, L.; Liu, Y. Quantitative Phase Imaging to Improve the Diagnostic Accuracy of Urine Cytology. Cancer Cytopathol. 2016, 124, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Calin, V.L.; Mihailescu, M.; Scarlat, E.I.; Baluta, A.V.; Calin, D.; Kovacs, E.; Savopol, T.; Moisescu, M.G. Evaluation of the Metastatic Potential of Malignant Cells by Image Processing of Digital Holographic Microscopy Data. FEBS Open Bio 2017, 7, 1527–1538. [Google Scholar] [CrossRef]
- Kemper, B.; Carl, D.; Schnekenburger, J.; Bredebusch, I.; Schäfer, M.; Domschke, W.; von Bally, G. Investigation of Living Pancreas Tumor Cells by Digital Holographic Microscopy. J. Biomed. Opt. 2006, 11, 034005. [Google Scholar] [CrossRef]
- Rehman, A.; An, H.; Park, S.; Moon, I. Automated Classification of Elliptical Cancer Cells with Stain-Free Holographic Imaging and Self-Supervised Learning. Opt. Laser Technol. 2024, 174, 110646. [Google Scholar] [CrossRef]
- Raub, C.B.; Nehmetallah, G. Holography, Machine Learning, and Cancer Cells. Cytom. Part A 2017, 91, 754–756. [Google Scholar] [CrossRef]
- Paidi, S.K.; Shah, V.; Raj, P.; Glunde, K.; Pandey, R.; Barman, I. Coarse Raman and Optical Diffraction Tomographic Imaging Enable Label-Free Phenotyping of Isogenic Breast Cancer Cells of Varying Metastatic Potential. Biosens. Bioelectron. 2021, 175, 112863. [Google Scholar] [CrossRef]
- Ryu, D.; Bak, T.; Ahn, D.; Kang, H.; Oh, S.; Min, H.; Lee, S.; Lee, J. Deep Learning-Based Label-Free Hematology Analysis Framework Using Optical Diffraction Tomography. Heliyon 2023, 9, e18297. [Google Scholar] [CrossRef]
- Martin, C.; Altman, L.E.; Rawat, S.; Wang, A.; Grier, D.G.; Manoharan, V.N. In-Line Holographic Microscopy with Model-Based Analysis. Nat. Rev. Methods Primers 2022, 2, 83. [Google Scholar] [CrossRef]
- Pierzchalski, A.; Mittag, A.; Tárnok, A. Introduction A: Recent advances in cytometry instrumentation, probes, and methods—Review. Methods Cell Biol. 2011, 102, 1–21. [Google Scholar]
- Vasdekis, A.E.; Stephanopoulos, G. Review of Methods to Probe Single Cell Metabolism and Bioenergetics. Metab. Eng. 2015, 27, 115–135. [Google Scholar] [CrossRef]
- Lee, K.C.M.; Guck, J.; Goda, K.; Tsia, K.K. Toward Deep Biophysical Cytometry: Prospects and Challenges. Trends Biotechnol. 2021, 39, 1249–1262. [Google Scholar] [CrossRef]
- Urbanska, M.; Muñoz, H.E.; Shaw Bagnall, J.; Otto, O.; Manalis, S.R.; Di Carlo, D.; Guck, J. A Comparison of Microfluidic Methods for High-Throughput Cell Deformability Measurements. Nat. Methods 2020, 17, 587–593. [Google Scholar] [CrossRef]
- Asghari, M.; Ivetich, S.D.; Aslan, M.K.; Aramesh, M.; Melkonyan, O.; Meng, Y.; Xu, R.; Colombo, M.; Weiss, T.; Balabanov, S.; et al. Real-Time Viscoelastic Deformability Cytometry: High-Throughput Mechanical Phenotyping of Liquid and Solid Biopsies. Sci. Adv. 2024, 10, eabj1133. [Google Scholar] [CrossRef]
- Hua, H.; Zou, S.; Ma, Z.; Guo, W.; Fong, C.Y.; Khoo, B.L. A Deformability-Based Biochip for Precise Label-Free Stratification of Metastatic Subtypes Using Deep Learning. Microsyst. Nanoeng. 2023, 9, 120. [Google Scholar] [CrossRef]
- Tang, T.; Julian, T.; Ma, D.; Yang, Y.; Li, M.; Hosokawa, Y.; Yalikun, Y. A Review on Intelligent Impedance Cytometry Systems: Development, Applications and Advances. Anal. Chim. Acta 2023, 1269, 341424. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wang, C.; Liang, X.; Li, J.; Li, S.; Wu, J.J.; Su, T.; Li, J. Label-Free Sensing of Cell Viability Using a Low-Cost Impedance Cytometry Device. Micromachines 2023, 14, 407. [Google Scholar] [CrossRef] [PubMed]
- Salahi, A.; Honrado, C.; Moore, J.; Adair, S.; Bauer, T.W.; Swami, N.S. Supervised Learning on Impedance Cytometry Data for Label-Free Biophysical Distinction of Pancreatic Cancer Cells versus Their Associated Fibroblasts under Gemcitabine Treatment. Biosens. Bioelectron. 2023, 231, 115262. [Google Scholar] [CrossRef] [PubMed]
- Kokabi, M.; Rather, G.M.; Javanmard, M. Ionic Cell Microscopy: A New Modality for Visualizing Cells Using Microfluidic Impedance Cytometry and Generative Artificial Intelligence. Biosens. Bioelectron. X 2025, 24, 100619. [Google Scholar] [CrossRef]
- Zuba-Surma, E.K.; Kucia, M.; Abdel-Latif, A.; Lillard, J.W.; Ratajczak, M.Z. The ImageStream System: A Key Step to a New Era in Imaging. Folia Histochem. Cytobiol. 2007, 45, 279–290. [Google Scholar]
- Dudaie, M.; Dotan, E.; Barnea, I.; Haifler, M.; Shaked, N.T. Detection of Bladder Cancer Cells Using Quantitative Interferometric Label-Free Imaging Flow Cytometry. Cytom. Part A 2024, 105, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Barteneva, N.S.; Fasler-Kan, E.; Vorobjev, I.A. Imaging Flow Cytometry. J. Histochem. Cytochem. 2012, 60, 723–733. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Q.; Chu, R.; Song, K.; Su, X. Single-Detector Dual-Modality Imaging Flow Cytometry for Label-Free Cell Analysis with Machine Learning. Opt. Lasers Eng. 2023, 168, 107665. [Google Scholar] [CrossRef]
- Lee, K.C.M.; Wang, M.; Cheah, K.S.E.; Chan, G.C.F.; So, H.K.H.; Wong, K.K.Y.; Tsia, K.K. Quantitative Phase Imaging Flow Cytometry for Ultra-Large-Scale Single-Cell Biophysical Phenotyping. Cytom. Part A 2019, 95, 510–520. [Google Scholar] [CrossRef]
- Park, S.; Yoon, S.E.; Song, Y.; Tian, C.; Baek, C.; Cho, H.; Kim, W.S.; Kim, S.J.; Cho, S.-Y. A Simple Approach to Biophysical Profiling of Blood Cells in Extranodal NK/T Cell Lymphoma Patients Using Deep Learning-Integrated Image Cytometry. BMEMat 2025, 3, e12128. [Google Scholar] [CrossRef]
- Blasi, T.; Hennig, H.; Summers, H.D.; Theis, F.J.; Cerveira, J.; Patterson, J.O.; Davies, D.; Filby, A.; Carpenter, A.E.; Rees, P. Label-Free Cell Cycle Analysis for High-Throughput Imaging Flow Cytometry. Nat. Commun. 2016, 7, 10256. [Google Scholar] [CrossRef]
- Ross Hallett, F. Scattering and Particle Sizing Applications. In Encyclopedia of Spectroscopy and Spectrometry; Elsevier: Amsterdam, The Netherlands, 1999; pp. 2488–2494. [Google Scholar]
- Lin, M.; Liu, T.; Zheng, Y.; Ma, X. Dynamic Light Scattering Microscopy Sensing Mitochondria Dynamics for Label-Free Detection of Triple-Negative Breast Cancer Enhanced by Deep Learning. Biomed. Opt. Express 2023, 14, 5048. [Google Scholar] [CrossRef]
- Akagi, T.; Kato, K.; Hanamura, N.; Kobayashi, M.; Ichiki, T. Evaluation of Desialylation Effect on Zeta Potential of Extracellular Vesicles Secreted from Human Prostate Cancer Cells by On-Chip Microcapillary Electrophoresis. Jpn. J. Appl. Phys. 2014, 53, 06JL01. [Google Scholar] [CrossRef]
- Jia, Z.; Li, J.; Gao, L.; Yang, D.; Kanaev, A. Dynamic Light Scattering: A Powerful Tool for In Situ Nanoparticle Sizing. Colloids Interfaces 2023, 7, 15. [Google Scholar] [CrossRef]
- Moleón Baca, J.A.; Ontiveros Ortega, A.; Aránega Jiménez, A.; Granados Principal, S. Cells Electric Charge Analyses Define Specific Properties for Cancer Cells Activity. Bioelectrochemistry 2022, 144, 108028. [Google Scholar] [CrossRef]
- Kanyo, N.; Kovacs, K.D.; Saftics, A.; Szekacs, I.; Peter, B.; Santa-Maria, A.R.; Walter, F.R.; Dér, A.; Deli, M.A.; Horvath, R. Glycocalyx Regulates the Strength and Kinetics of Cancer Cell Adhesion Revealed by Biophysical Models Based on High Resolution Label-Free Optical Data. Sci. Rep. 2020, 10, 22422. [Google Scholar] [CrossRef]
- Hughes, M.P. The Cellular Zeta Potential: Cell Electrophysiology beyond the Membrane. Integr. Biol. 2024, 16, zyae003. [Google Scholar] [CrossRef]
- Avci, E.; Yilmaz, H.; Sahiner, N.; Tuna, B.G.; Cicekdal, M.B.; Eser, M.; Basak, K.; Altıntoprak, F.; Zengin, I.; Dogan, S.; et al. Label-Free Surface Enhanced Raman Spectroscopy for Cancer Detection. Cancers 2022, 14, 5021. [Google Scholar] [CrossRef]
- Wang, X.; Qian, X.; Beitler, J.J.; Chen, Z.G.; Khuri, F.R.; Lewis, M.M.; Shin, H.J.C.; Nie, S.; Shin, D.M. Detection of Circulating Tumor Cells in Human Peripheral Blood Using Surface-Enhanced Raman Scattering Nanoparticles. Cancer Res. 2011, 71, 1526–1532. [Google Scholar] [CrossRef]
- Guerrini, L.; Alvarez-Puebla, R.A. Surface-Enhanced Raman Spectroscopy in Cancer Diagnosis, Prognosis and Monitoring. Cancers 2019, 11, 748. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Li, Y.; Li, Z. Early Cancer Detection by SERS Spectroscopy and Machine Learning. Light. Sci. Appl. 2023, 12, 234. [Google Scholar] [CrossRef]
- Heinisch, J.J.; Lipke, P.N.; Beaussart, A.; Chatel, S.E.K.; Dupres, V.; Alsteens, D.; Dufrêne, Y.F. Atomic Force Microscopy—Looking at Mechanosensors on the Cell Surface. J. Cell Sci. 2012, 125, 4189–4195. [Google Scholar] [CrossRef]
- Haeri, Z.; Shokoufi, M.; Jenab, M.; Janzen, R.; Golnaraghi, F. Electrical Impedance Spectroscopy for Breast Cancer Diagnosis: Clinical Study. Integr. Cancer Sci. Ther. 2016, 3. [Google Scholar] [CrossRef]
- Nalwa, H.S.; Webster, T.J. Cancer Nanotechnology: Nanomaterials for Cancer Diagnosis and Therapy; American Scientific Publishers: Valencia, CA, USA, 2007; ISBN 1588830713. [Google Scholar]
- Payton, O.D.; Picco, L.; Scott, T.B. High-Speed Atomic Force Microscopy for Materials Science. Int. Mater. Rev. 2016, 61, 473–494. [Google Scholar] [CrossRef]
- Zemła, J.; Danilkiewicz, J.; Orzechowska, B.; Pabijan, J.; Seweryn, S.; Lekka, M. Atomic Force Microscopy as a Tool for Assessing the Cellular Elasticity and Adhesiveness to Identify Cancer Cells and Tissues. Semin. Cell Dev. Biol. 2018, 73, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Mezencev, R.; Kim, B.; Wang, L.; McDonald, J.; Sulchek, T. Cell Stiffness Is a Biomarker of the Metastatic Potential of Ovarian Cancer Cells. PLoS ONE 2012, 7, e46609. [Google Scholar] [CrossRef]
- Ramos, J.R.; Pabijan, J.; Garcia, R.; Lekka, M. The Softening of Human Bladder Cancer Cells Happens at an Early Stage of the Malignancy Process. Beilstein J. Nanotechnol. 2014, 5, 447–457. [Google Scholar] [CrossRef]
- Calzado-Martín, A.; Encinar, M.; Tamayo, J.; Calleja, M.; San Paulo, A. Effect of Actin Organization on the Stiffness of Living Breast Cancer Cells Revealed by Peak-Force Modulation Atomic Force Microscopy. ACS Nano 2016, 10, 3365–3374. [Google Scholar] [CrossRef]
- Najera, J.; Rosenberger, M.R.; Datta, M. Atomic Force Microscopy Methods to Measure Tumor Mechanical Properties. Cancers 2023, 15, 3285. [Google Scholar] [CrossRef]
- Li, M.; Xi, N.; Wang, Y.; Liu, L. Atomic Force Microscopy for Revealing Micro/Nanoscale Mechanics in Tumor Metastasis: From Single Cells to Microenvironmental Cues. Acta Pharmacol. Sin. 2021, 42, 323–339. [Google Scholar] [CrossRef]
- Ojarand, J.; Min, M.; Koel, A. Multichannel Electrical Impedance Spectroscopy Analyzer with Microfluidic Sensors. Sensors 2019, 19, 1891. [Google Scholar] [CrossRef]
- Buscaglia, L.A.; Oliveira, O.N.; Carmo, J.P. Roadmap for Electrical Impedance Spectroscopy for Sensing: A Tutorial. IEEE Sens. J. 2021, 21, 22246–22257. [Google Scholar] [CrossRef]
- Han, A.; Yang, L.; Frazier, A.B. Quantification of the Heterogeneity in Breast Cancer Cell Lines Using Whole-Cell Impedance Spectroscopy. Clin. Cancer Res. 2007, 13, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Kim, H.S.; Frazier, A.B.; Chen, Z.G.; Shin, D.M.; Han, A. Whole-Cell Impedance Analysis for Highly and Poorly Metastatic Cancer Cells. J. Microelectromech. Syst. 2009, 18, 808–817. [Google Scholar] [CrossRef]
- Crowell, L.; Yakisich, J.; Aufderheide, B.; Adams, T. Electrical Impedance Spectroscopy for Monitoring Chemoresistance of Cancer Cells. Micromachines 2020, 11, 832. [Google Scholar] [CrossRef]
- Kalina, T. Reproducibility of Flow Cytometry Through Standardization: Opportunities and Challenges. Cytom. Part A 2020, 97, 137–147. [Google Scholar] [CrossRef]
- Pillay, T.S.; Topcu, D.İ.; Yenice, S. Harnessing AI for Enhanced Evidence-Based Laboratory Medicine (EBLM). Clin. Chim. Acta 2025, 569, 120181. [Google Scholar] [CrossRef]
- Ma, L.; Guo, H.; Zhao, Y.; Liu, Z.; Wang, C.; Bu, J.; Sun, T.; Wei, J. Liquid Biopsy in Cancer: Current Status, Challenges and Future Prospects. Signal Transduct. Target. Ther. 2024, 9, 336. [Google Scholar] [CrossRef]
| BIOPHYSICAL LABEL-FREE METHOD | TECHNIQUE | CHALLENGES | REQUIREMENTS FOR CLINICAL TRANSLATION |
|---|---|---|---|
| MICROFLUIDIC SYSTEMS FOR CELL SEPARATION AND ENRICHMEMT | Passive Cell Separation Microfluidics | DLD: Limited to size-dependent discrimination; Not sensitive to cellular shape or deformability. IF: Does not separate cells with similar biophysical features. MF: Shear stress damage; Not indicated for small volumes of samples |
|
| Active Cell Separation Microfluidics | AC: Cell misalignment upon entry; potential for clogging; loss of rare cells post-separation processing MG: More expensive micromachining DC: Low single-cell resolution |
| |
| Cellular Mobility within Microfluidic Arrays | EICS: Maintaining high cell viability; Only for metastatic potential. |
| |
| MICROSCOPY FOR CELLULAR ANALYSIS | Phase-Contrast Microscopy | Limited to 2D imaging; Does not quantify subtle changes in depth; Segmentation errors in overlapping cells. |
|
| Holographic Microscopy | Requires high computational power for 3D reconstruction; sensitivity to noise |
| |
| CYTOMETRY | Deformability Cytometry | vDC: Only assess cell deformability depending on size. cDC: Lower throughput than vDC; |
|
| Impedance Cytometry | Sensitivity issues with small volumes and rare populations; Only relies on impedance feature |
| |
| Imaging Flow Cytometry | High cost and complexity of systems; Requires expert handling of multimodal data; software limitations: |
| |
| CELL AND PARTICLE SCATTERING ANALYSIS | Dynamic Light Scattering | Sensitive to temperature, pH …; Difficulty in differentiating between similar particles; requires high-quality and volume samples: |
|
| Surface-Enhanced Raman Spectroscopy | Requires high signal enhancement; Sample damage with intense laser use.; Low throughput. |
| |
| ELECTRO-MECHANICAL AND SURFACE CHARACTERIZATION | Atomic Force Microscopy | Slow data acquisition time; Difficulty in analyzing complex samples |
|
| Electrical Impedance Spectroscopy | Sensitivity challenges in microfluidic devices with small sample volumes; Need of advanced statistical modules. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calejo, I.; Azevedo, A.C.; Monteiro, R.L.; Cruz, F.; Canadas, R.F. Label-Free Cancer Detection Methods Based on Biophysical Cell Phenotypes. Bioengineering 2025, 12, 1045. https://doi.org/10.3390/bioengineering12101045
Calejo I, Azevedo AC, Monteiro RL, Cruz F, Canadas RF. Label-Free Cancer Detection Methods Based on Biophysical Cell Phenotypes. Bioengineering. 2025; 12(10):1045. https://doi.org/10.3390/bioengineering12101045
Chicago/Turabian StyleCalejo, Isabel, Ana Catarina Azevedo, Raquel L. Monteiro, Francisco Cruz, and Raphaël F. Canadas. 2025. "Label-Free Cancer Detection Methods Based on Biophysical Cell Phenotypes" Bioengineering 12, no. 10: 1045. https://doi.org/10.3390/bioengineering12101045
APA StyleCalejo, I., Azevedo, A. C., Monteiro, R. L., Cruz, F., & Canadas, R. F. (2025). Label-Free Cancer Detection Methods Based on Biophysical Cell Phenotypes. Bioengineering, 12(10), 1045. https://doi.org/10.3390/bioengineering12101045







