From Echocardiography to CT/MRI: Lessons for AI Implementation in Cardiovascular Imaging in LMICs—A Systematic Review and Narrative Synthesis
Abstract
1. Introduction
2. Methods
2.1. Protocol Registration and Study Design
2.2. Search Strategy
2.3. Study Selection
2.4. Definition of AI
- Supervised ML (e.g., linear classifiers/regressors, decision trees, support vector machines);
- Unsupervised ML (e.g., clustering, dimensionality reduction);
- Hybrid/ensemble methods (e.g., bagging, boosting, stacking);
- Deep learning (e.g., convolutional neural networks, recurrent neural networks, transformers).
2.5. Data Extraction
2.6. Data Synthesis
3. Results
3.1. Summary of Included Studies
- Study characteristics (Table 2):
| First Author/Year | Population | Country | Imaging Modality |
|---|---|---|---|
| Alizadehsani (2013a) [25] | 303 patients (Z-Alizadeh Sani dataset) | Iran | Echocardiography |
| Alizadehsani (2018) [26] | 500 patients (extended Z-Alizadeh Sani dataset) | Iran | Echocardiography |
| Alizadehsani (2013b) [27] | 303 patients (Z-Alizadeh Sani dataset) | Iran | Echocardiography + Lab features |
| Hoodbhoy (2018) [28] | 525 pregnant women | Pakistan | Doppler echocardiography |
| Asmare (2021) [29] | 124 RHD cases + 127 controls | Ethiopia | Phonocardiogram |
| Sayadi (2022) [30] | 303 patients (Z-Alizadeh Sani dataset) | Iran | Echocardiography + Lab features |
| Ekambaram (2023) [31] | Patients with suspected valve emergencies | South Africa | Handheld POCUS (HoPE) |
| Nguyen (2023) [32] | 109 public + 60 local patients | Vietnam | Echocardiography |
| Brown (2024) [20] | 511 echocardiograms (282 RHD, 229 controls) | Uganda | Echocardiography + Doppler |
| Firima (2024) [33] | 756 participants | Lesotho | Focused echocardiography |
| Soh (2024) [34] | 157 participants | Australia (rural) | AI-guided TTE |
| Tromp (2024) [35] | 94 patients | Tunisia | Nurse-led AI-POCUS |
- AI methods and objectives (Table 3):
| First Author/Year | AI/ML Model | Objective |
|---|---|---|
| Alizadehsani (2013a) [25] | Bagging with SMO, neural networks, naïve Bayes | Non-invasive CAD diagnosis |
| Alizadehsani (2018) [26] | SVM, naïve Bayes, decision trees | Stenosis prediction (LAD, LCX, RCA) |
| Alizadehsani (2013b) [27] | Decision trees, bagging | Non-invasive coronary stenosis diagnosis |
| Hoodbhoy (2018) [28] | Multiple kernel learning | Predict adverse perinatal outcomes |
| Asmare (2021) [29] | SVM (RBF kernel) | Automated RHD screening via heart sounds |
| Sayadi (2022) [30] | Logistic regression, SVM | Early CAD detection with minimal features |
| Ekambaram (2023) [31] | Bayesian ML framework | Valve emergency diagnosis |
| Nguyen (2023) [32] | Ensemble learning (SVM, LR, DT, KNN) | MI detection |
| Brown (2024) [20] | SVM, CNNs, transformers | Automated RHD detection (MR analysis) |
| Firima (2024) [33] | Deep learning | LVH diagnosis with focused echo |
| Soh (2024) [34] | Deep learning | Valve disease and HF diagnosis in rural settings |
| Tromp (2024) [35] | Deep learning (AI-TRIO) | Nurse-led HF detection with AI-POCUS |
- Outcomes and challenges (Table 4):
| First Author/Year | Outcome | Challenges |
|---|---|---|
| Alizadehsani (2013a) [25] | 94.1% accuracy (SMO) | Limited dataset, reliance on manual features |
| Alizadehsani (2018) [26] | 96.4% accuracy, 100% sensitivity, 88.1% specificity | Lack of clinical implementation, generalizability |
| Alizadehsani (2013b) [27] | 79.5% LAD, 61.5% LCX, 69% RCA accuracy | Small dataset, no real-world testing |
| Hoodbhoy (2018) [28] | Protocol; results awaited | Synthetic data, need for external validation |
| Asmare (2021) [29] | 96% sensitivity, 96% specificity, F1 = 96% | Imbalanced data, open-access quality concerns |
| Sayadi (2022) [30] | 95.5% accuracy, 95.9% sensitivity, 91.7% specificity | Small dataset, no external validation |
| Ekambaram (2023) [31] | Improved time to diagnosis in valve emergencies | Handheld device limitations, operator dependency |
| Nguyen (2023) [32] | 91.4% accuracy (public), 76.7% (local) | High computational needs, dataset localization issues |
| Brown (2024) [20] | AUC 0.93 (SVM), 0.84 (DL ensemble) | Limited aortic inclusion, device generalization issues |
| Firima (2024) [33] | 87.5% evaluable images, 81.9% confirmed | Manual intervention required, quality maintenance |
| Soh (2024) [34] | 42.3% with abnormalities detected | Variable image quality, novice dependency |
| Tromp (2024) [35] | 92% sensitivity, 81% specificity | Small sample, nurse variability, limited generalization |
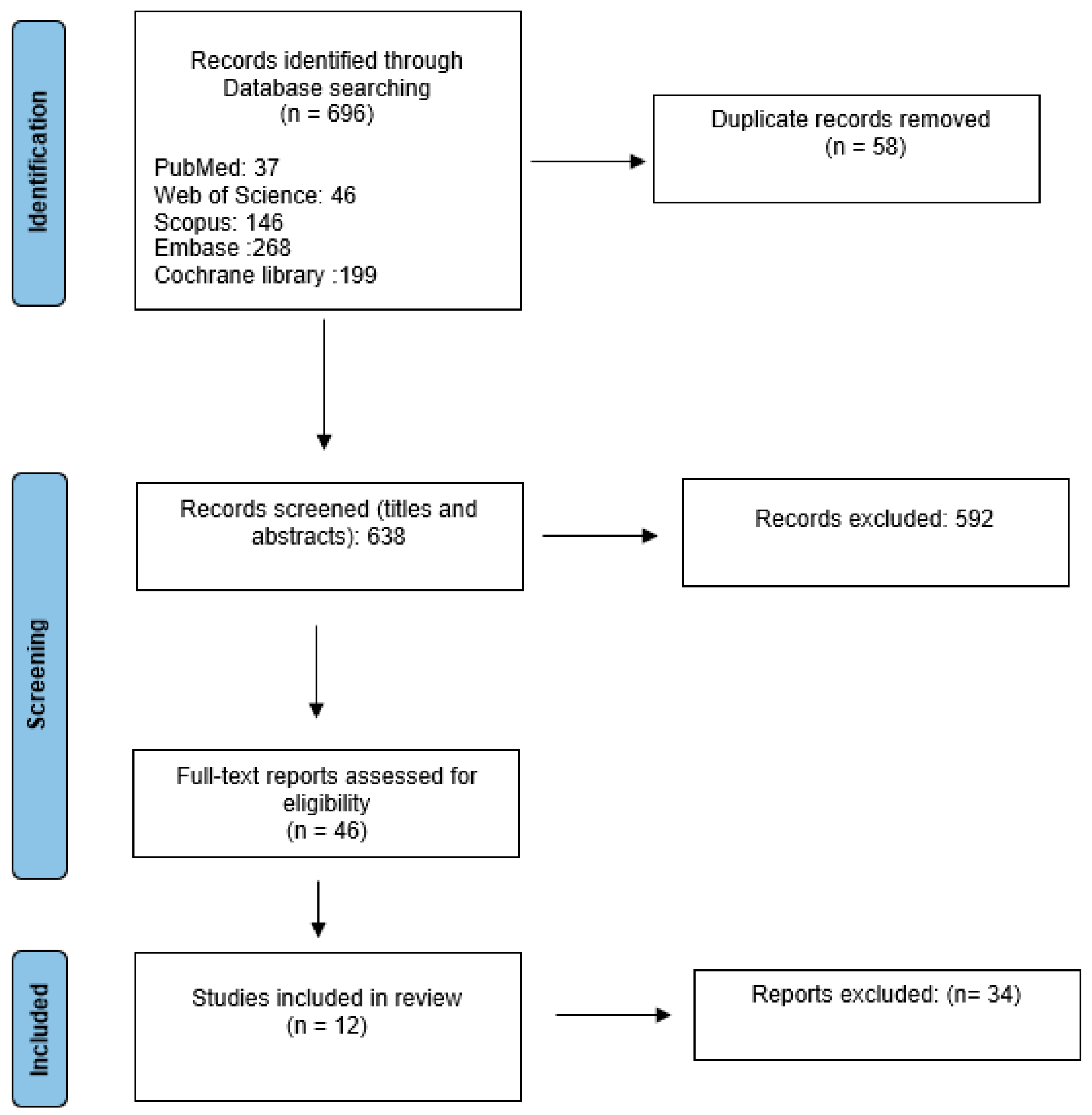
3.2. Study Selection Process
3.3. Types of Low-Resource Settings
3.4. Application of AI in Cardiovascular Imaging
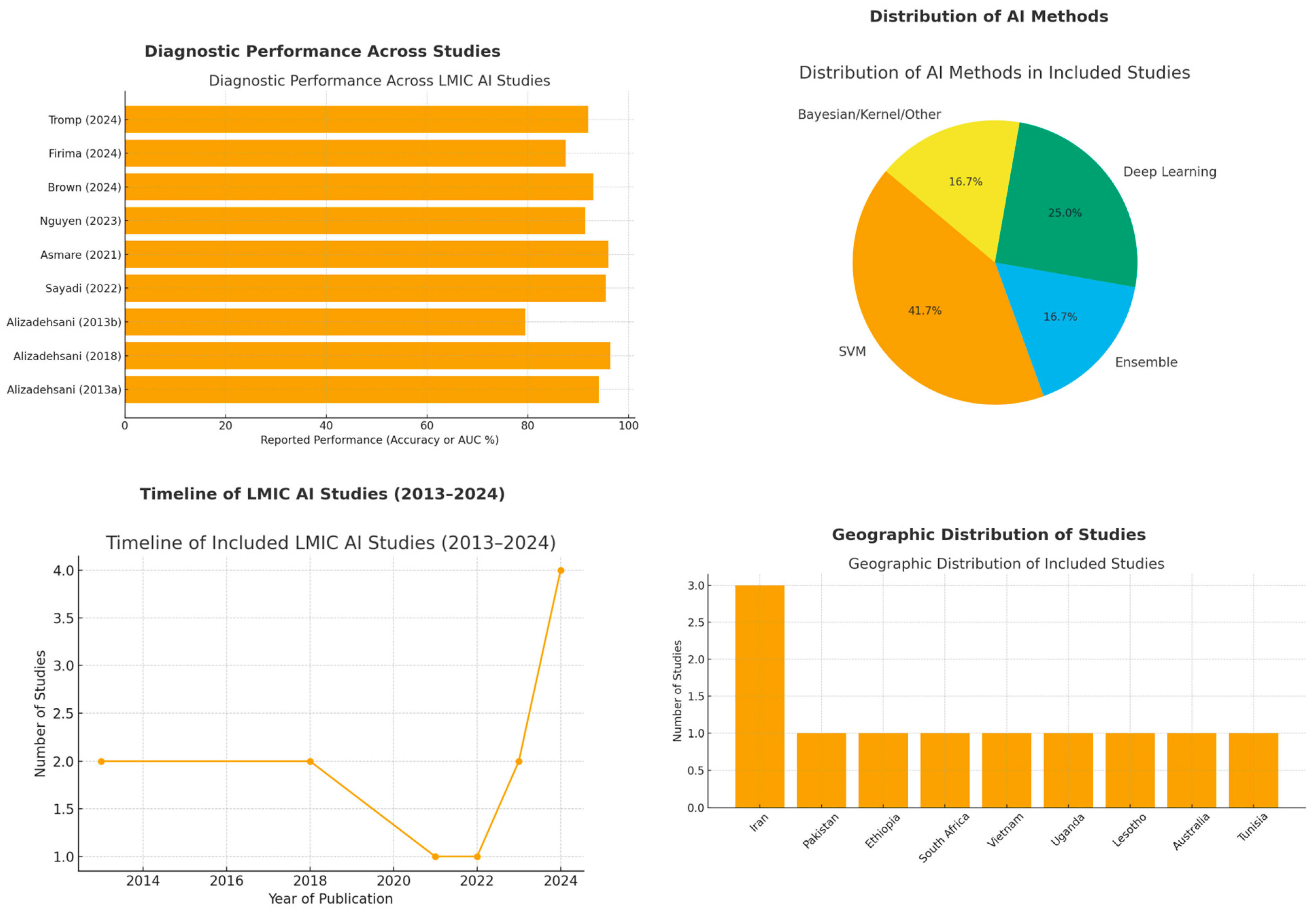
3.4.1. Diagnostic Performance Across Studies
- Coronary Artery Disease (CAD)
- Alizadehsani et al. (2013, 2018) [25,26,27] achieved up to 96.4% accuracy and 100% sensitivity on echocardiography using SVMs with advanced feature engineering, whereas their earlier methods had a lower accuracy (79.5% LAD, 61.5% LCX, 69.0% RCA), underscoring the impact of feature selection and algorithm choice. Overall, Sequential Minimal Optimization, SVMs, and ensemble models consistently outperformed conventional techniques.
- Sayadi et al. (2022) [30] similarly achieved 95.4% accuracy and 95.9% sensitivity for early CAD detection with a diminished feature set on echocardiography, highlighting the feasibility of streamlined models in resource-limited settings.
- Myocardial Infarction (MI) and Left Ventricular Hypertrophy (LVH)
- Nguyen et al. (2023) [32] used ensemble learning for myocardial infarction (MI) detection and reported an F1 score of 0.942 on a public dataset, although external validation on local patients saw a performance drop to 76.7% accuracy.
- Firima et al. (2024) [33] and Soh et al. (2024) [34] evaluated AI-assisted echocardiography for LVH and valve disease diagnoses. Firima et al. found 87.5% of images met the quality criteria for reliable interpretation, while Soh et al. reported diagnostic quality in over 70% of scans by non-expert operators, demonstrating that AI guidance can maintain accuracy in low-resource settings.
- Rheumatic Heart Disease (RHD)
- Asmare et al. (2021) [29] developed a phonocardiogram-based screening model with 96% sensitivity and specificity, providing an inexpensive, non-invasive tool suited for large-scale RHD screening programs in endemic regions.
- Brown et al. (2024) [20] reported an AUC of 0.93 using SVM and 0.84 for a DL ensemble, focusing on automated mitral regurgitation detection form Doppler echocardiography in RHD. This approach further reduced reliance on specialist echocardiographers.
- Valvular Emergencies and Other Conditions
- Ekambaram et al. (2023) [31] introduced a Bayesian-inspired diagnostic framework for acute left-sided valve emergencies, leveraging handheld point-of-care echocardiography (HoPE). Despite limited Doppler capabilities, the method significantly improved the time to diagnosis in resource-constrained emergency settings.
3.4.2. Clinical Implications in LMICs
- Task Shifting
- Early Detection and Screening
- Maternal and Neonatal Health
- Improving Accessibility and Scalability
- Potential for Cost Effectiveness
3.4.3. Challenges and Barriers
- Limited Dataset Diversity and Small Sample Sizes
- ○
- Nguyen et al. [32] reported reduced accuracy when moving from a public dataset to local patient data, indicating that algorithms trained on narrowly representative datasets may underperform in external validation.
- ○
- Operator Dependence
- ○
- ○
- Soh et al. [34] similarly reported that non-expert operators sometimes struggled to obtain complete echocardiographic views, resulting in inconclusive scans in a minority of cases.
- Infrastructure Constraints
- ○
- ○
- Hoodbhoy et al. [28] noted that data collection and storage require stable networks, which can be challenging in LMIC settings.
- Algorithm Generalizability
- ○
- ○
- Nguyen et al. [32] specifically mentioned high computational demands that could limit algorithm deployment on lower-end hardware available in LMIC settings.
- Reliance on Protocol or Synthetic Data
- Miscellaneous Factors
- ○
- While some studies, such as Asmare et al. [29], addressed financial and policy considerations in passing, direct cost analyses or detailed funding barriers were not comprehensively reported.
- ○
3.4.4. Future Directions
- Expanding and Diversifying Datasets
- Multi-Center Validation and Prospective Trials
- Improving Algorithm Adaptability
- ○
- Firima et al. [33] noted that ML models should be optimized to handle variable imaging quality and operator experience. They proposed iterative algorithm training using feedback loops from actual clinical deployment to ensure sustained performance in resource-limited environments.
- Capacity Building and Workforce Training
- Infrastructure and Policy Support
- ○
- Asmare et al. [29] and Hoodbhoy et al. [28] acknowledged the importance of infrastructure development, including reliable power, internet connectivity, and maintenance of portable devices. They also noted the need for supportive policies and regulatory frameworks to facilitate funding, data governance, and ethical implementation of AI technologies.
4. Discussion
- Diagnostic Performance and Clinical Impact
- Decentralization and Task Shifting
- Time and Workforce Savings
- Barriers to Implementation
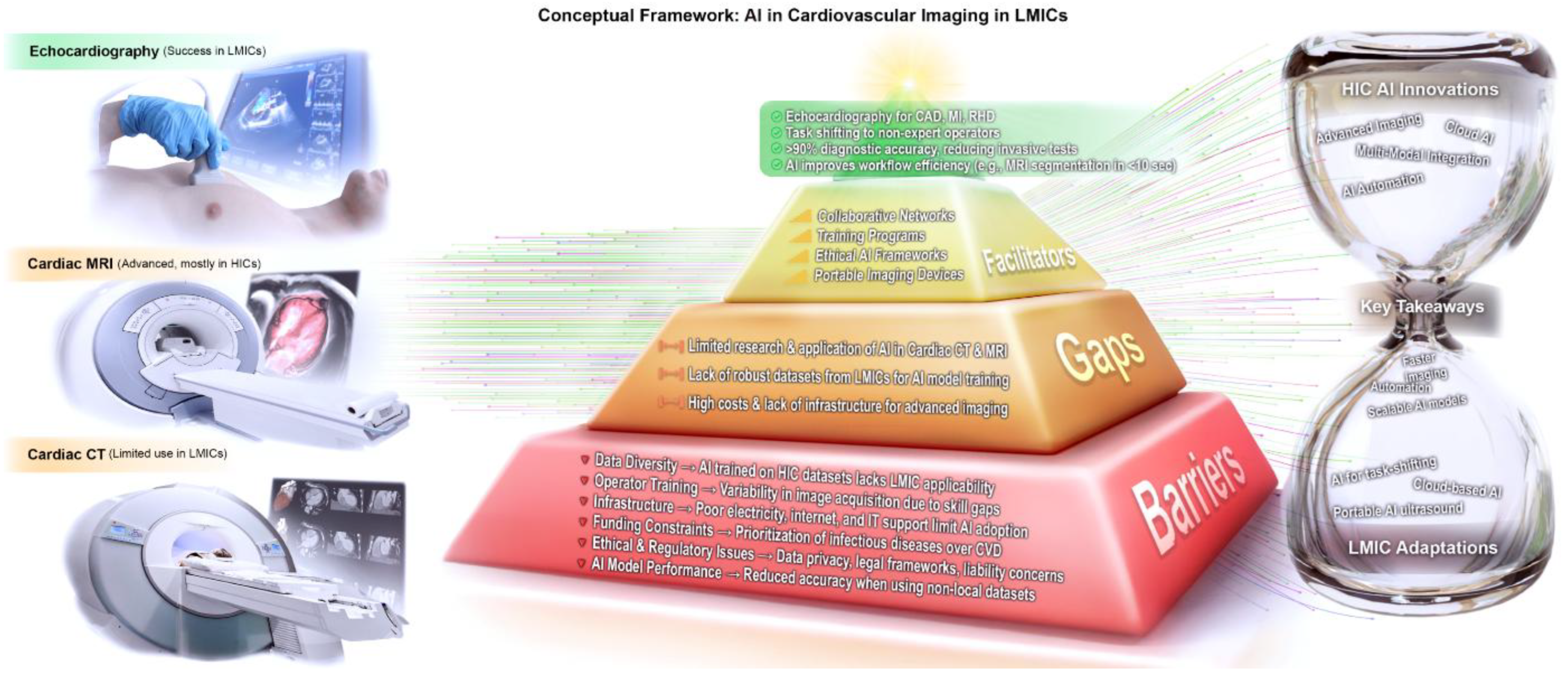
- Lessons from AI Applications in Cardiac MRI and CT (HIC Experience)
- Cardiac Magnetic Resonance (CMR)
- Risk Stratification and Prognostication: AI models integrate CMR data with clinical parameters for personalized CAD and cardiomyopathy risk predictions [63]. Emerging frameworks can automate scan parameters to lower operator dependency [64]. Effective LMIC deployment will require diverse data, local validation, and robust infrastructure and policy support.
- Cardiac Computed Tomography (CCT)
- Acquisition, Reconstruction, and Radiation Reduction: AI optimizes low-dose CCT protocols, enhancing image quality while cutting radiation exposure [65]. LMICs could partially adopt these techniques with infrastructure or cloud-based solutions.
- CAD Detection and Plaque Characterization: AI-augmented CCTA improves sensitivity and specificity for stenosis and enables quantitative plaque assessment [66]. The CLARIFY study showed strong overall performance but noted variability in high-risk plaque feature detection [66,67,68]. Such tools could expand non-invasive CAD diagnostics in LMICs if software and data capabilities permit.
- Structural Heart Segmentation: Automated segmentation of chambers and vessels guides procedures like TAVI, accelerating planning and reducing variability [69]. LMIC tertiary centers could streamline interventions with these tools.
- Challenges and Future Directions: Limited LMIC data, unclear regulations, and “black box” concerns hinder CCT AI adoption. Future efforts should focus on local validation, data governance, and development of models that predict plaque instability to prevent acute events.
- Overarching Lessons for LMIC Adoption (Figure 4)
- Gradual Implementation
- ○
- Real-time CMR or advanced plaque quantification may be too resource intensive for a broad LMIC rollout. Smaller trials or simple AI segmentation can still yield benefits.
- Local Data and Validation
- ○
- HIC algorithms require retraining or validation on LMIC cohorts, using global pre-trained models with local fine tuning.
- ○
- Infrastructure and Cost Effectiveness
- ○
- Strategies that reduce scan time and operator dependence address LMIC needs but start-up hardware and software costs must be balanced against long-term labor savings and improved diagnostics.
- ○
- Ethical and Regulatory Frameworks
- ○
- Transparent, explainable AI is essential for trust in low-technology medical settings; clear rules on data ownership, privacy, and liability are needed to safeguard patients.
- ○
- Governments must add AI education to medical training and promote collaboration between HICs and LMICs to build capacity in resource-limited settings.
- ○
- Resource-limited health institutions have low participation in AI development; increasing their involvement is critical to produce tools validated for diverse global populations [8].
- Collaborations and Funding
- ○
- Partnerships between local institutions, industry, academia, and NGOs can channel resources toward pilot programs that demonstrate cost effectiveness and scalability, eventually guiding policy decisions to invest in advanced imaging solutions for the wider populace [69].
- Comparing AI Models (Figure 5)
- Data Volume and QualityIn LMICs often constrained by small or non-diverse datasets, simpler algorithms like Naïve Bayes, decision trees, or linear SVM may be more practical but risk underfitting. More-complex architectures (e.g., CNNs, transformers) can excel with large, high-quality datasets prevalent in HIC research but may falter in low-resource environments without sufficient data or computing power.
- Computational InfrastructureDeploying advanced DL models for CMR or CCT typically demands GPU acceleration, stable electricity, and robust IT support, and such resources may be limited in LMIC contexts. Hence, moderate-complexity algorithms (e.g., ensemble trees) might strike a balance between accuracy and feasibility.
- Clinical ApplicationAutomated segmentation in MRI or CT often relies on U-Net-based frameworks, while echocardiography in LMICs frequently uses SVM or random forests for classification tasks (RHD detection, LVH identification). The task at hand, be it segmenting structures, identifying stenosis, or stratifying risk, should guide which model best balances interpretability, speed, and accuracy.
- Limitations Encountered
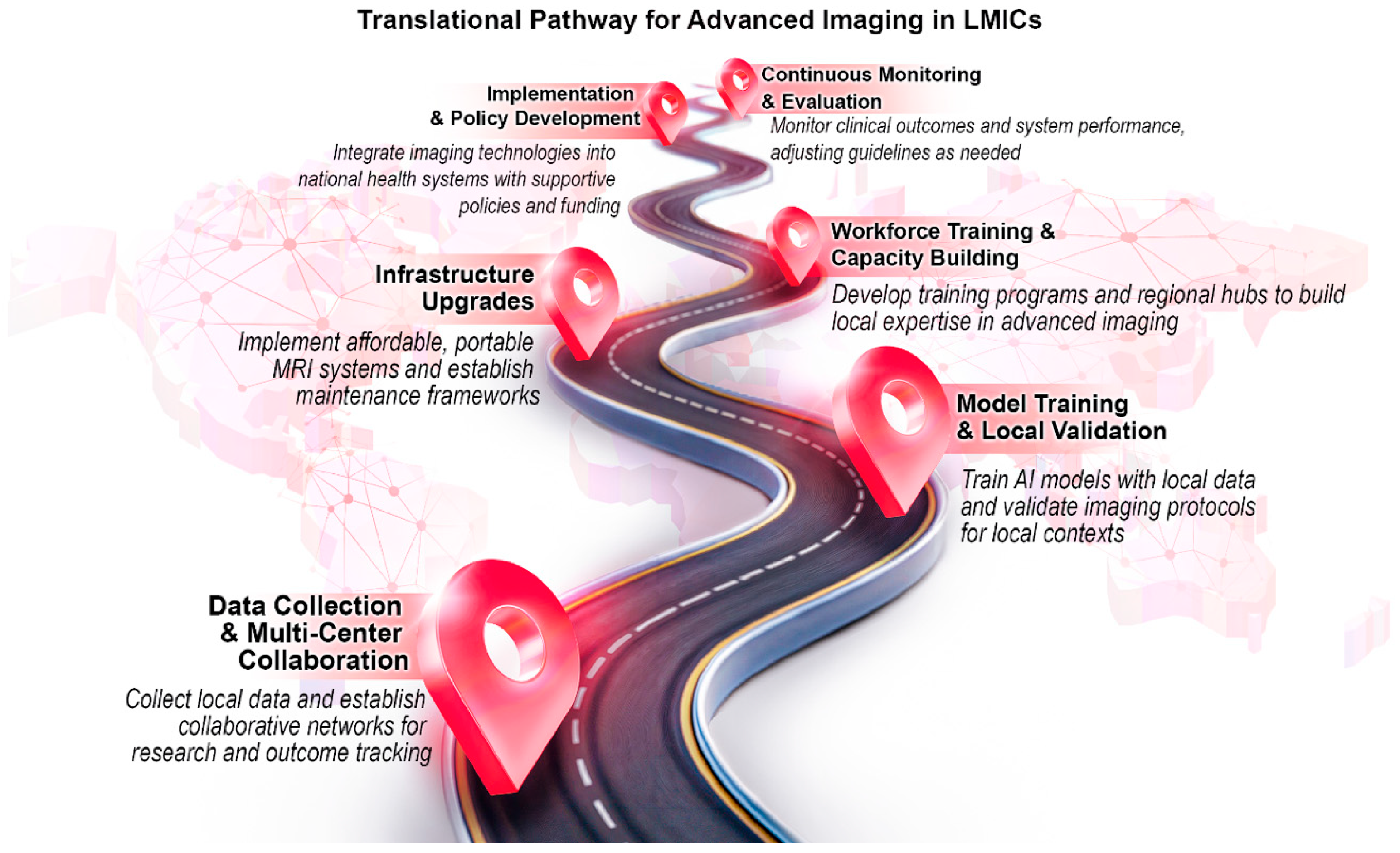
- Future Directions and Recommendations
- Strengthening Data Repositories
- Pool anonymized imaging across LMIC centers through regional hubs.
- Establish standardized imaging protocols and metadata collection to facilitate cross-site harmonization.
- Fine-tune HIC-trained models on smaller local samples using federated learning or transfer learning to reduce reliance on large, centralized datasets.
- Building Workforce Capacity
- Develop modular AI curricula integrated into medical and allied health training programs, with tiered certifications for nurses, technicians, and general practitioners.
- Train a cadre of “super-users”—clinicians or technicians at regional hospitals who receive advanced training in AI imaging tools. These individuals serve as local experts who provide mentorship, ensure quality control, and support surrounding facilities with troubleshooting and guidance.
- Deploy AI systems with built-in quality control dashboards that provide immediate feedback to novice operators, further reducing variability.
- Scalable Infrastructure and Technology
- Prioritize portable and battery-operated imaging devices with integrated AI, particularly for rural outreach.
- Explore hybrid cloud–edge computing solutions to overcome intermittent internet access, where data are pre-processed locally and synced centrally when connectivity allows.
- Develop cost-effectiveness frameworks that balance upfront hardware costs against long-term reductions in invasive testing and workforce strain.
- Ethical, Legal, and Regulatory Frameworks
- Establish regional regulatory sandboxes in LMICs to allow pilot testing of AI tools under controlled conditions while governance frameworks are developed.
- Incorporate explainability modules (e.g., heatmaps, decision trees) into AI outputs to build clinician trust.
- Create open-source template policies for data governance, privacy, and algorithmic accountability that can be adapted by LMIC ministries of health.
- Cross-Sector Collaborations
- Incentivize joint programs between local universities, NGOs, and private companies to run small-scale pilots (e.g., AI-assisted RHD screening in schools, nurse-led CMR segmentation in tertiary centers).
- Secure donor and government funding specifically tied to measurable outcomes such as reduction in time to diagnosis or an increase in number of patients screened rather than generic “AI capacity building.”
- Launch multi-center prospective trials evaluating diagnostic accuracy, cost effectiveness, and patient outcomes across diverse LMIC populations to guide scale-up.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- WHO. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 2 January 2025).
- Yuyun, M.F.; Sliwa, K.; Kengne, A.P.; Mocumbi, A.O.; Bukhman, G. Cardiovascular Diseases in Sub-Saharan Africa Compared to High-Income Countries: An Epidemiological Perspective. Glob. Heart 2020, 15, 15. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, J.W.; Zang, G.Y.; Pu, J. The primary use of artificial intelligence in cardiovascular diseases: What kind of potential role does artificial intelligence play in future medicine? J. Geriatr. Cardiol. 2019, 16, 585–591. [Google Scholar]
- Moosavi, A.; Huang, S.; Vahabi, M.; Motamedivafa, B.; Tian, N.; Mahmood, R.; Liu, P.; Sun, C.L.F. Prospective Human Validation of Artificial Intelligence Interventions in Cardiology. JACC Adv. 2024, 3 Pt 2, 101202. [Google Scholar] [CrossRef]
- Qin, C.; Murali, S.; Lee, E.; Supramaniam, V.; Hausenloy, D.J.; Obungoloch, J.; Brecher, J.; Lin, R.; Ding, H.; Akudjedu, T.N.; et al. Sustainable low-field cardiovascular magnetic resonance in changing healthcare systems. Eur. Heart J.-Cardiovasc. Imaging 2022, 23, e246–e260. [Google Scholar] [CrossRef]
- Lakshmanan, S.; Mbanze, I. A comparison of cardiovascular imaging practices in Africa, North America, and Europe: Two faces of the same coin. Eur. Heart J.-Imaging Methods Pract. 2023, 1, qyad005. [Google Scholar] [CrossRef]
- The Citizen. Tanzania Faces Acute Shortage of Radiology, Imaging Experts. 2021. Available online: https://www.thecitizen.co.tz/tanzania/news/national/tanzania-faces-acute-shortage-of-radiology-imaging-experts-2581012 (accessed on 2 January 2025).
- Mollura, D.J.; Culp, M.P.; Pollack, E.; Battino, G.; Scheel, J.R.; Mango, V.L.; Elahi, A.; Schweitzer, A.; Dako, F. Artificial Intelligence in Low- and Middle-Income Countries: Innovating Global Health Radiology. Radiology 2020, 297, 513–520. [Google Scholar] [CrossRef]
- Hanneman, K.; Playford, D.; Dey, D.; van Assen, M.; Mastrodicasa, D.; Cook, T.S.; Gichoya, J.W.; Williamson, E.E.; Rubin, G.D.; American Heart Association Council on Cardiovascular Radiology and Intervention. Value Creation Through Artificial Intelligence and Cardiovascular Imaging: A Scientific Statement from the American Heart Association. Circulation 2024, 149, e296–e311. [Google Scholar] [CrossRef]
- Lim, L.J.; Tison, G.H.; Delling, F.N. Artificial Intelligence in Cardiovascular Imaging. Methodist DeBakey Cardiovasc. J. 2020, 16, 138–145. [Google Scholar] [CrossRef]
- Hosny, A.; Parmar, C.; Quackenbush, J.; Schwartz, L.H.; Aerts, H.J.W.L. Artificial intelligence in radiology. Nat. Rev. Cancer 2018, 18, 500–510. [Google Scholar] [CrossRef]
- Khalifa, M.; Albadawy, M. Artificial Intelligence for Clinical Prediction: Exploring Key Domains and Essential Functions. Comput. Methods Programs Biomed. Update 2024, 5, 100148. [Google Scholar] [CrossRef]
- Alami, H.; Rivard, L.; Lehoux, P.; Hoffman, S.J.; Cadeddu, S.B.M.; Savoldelli, M.; Samsri, M.A.; Ahmed, M.A.A.; Fleet, R.; Fortin, J.-P. Artificial intelligence in health care: Laying the Foundation for Responsible, sustainable, and inclusive innovation in low-and middle-income countries. Glob. Health 2020, 16, 52. [Google Scholar] [CrossRef]
- The Lancet Public Health. Next generation public health: Towards precision and fairness. Lancet Public Health 2019, 4, e209. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Umer, H.; Faruqe, F. Artificial intelligence for low income countries. Humanit. Soc. Sci. Commun. 2024, 11, 1422. [Google Scholar] [CrossRef]
- Ahmed, Z.; Bhinder, K.K.; Tariq, A.; Tahir, M.J.; Mehmood, Q.; Tabassum, M.S.; Malik, M.; Aslam, S.; Asghar, M.S.; Yousaf, Z. Knowledge, attitude, and practice of artificial intelligence among doctors and medical students in Pakistan: A cross-sectional online survey. Ann. Med. Surg. 2022, 76, 103493. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, R.R.; Nakeshimana, A.; Olubeko, O. Addressing Fairness, Bias, and Appropriate Use of Artificial Intelligence and Machine Learning in Global Health. Front. Artif. Intell. 2020, 3, 561802. [Google Scholar] [CrossRef]
- Hanna, M.; Pantanowitz, L.; Jackson, B.; Palmer, O.; Visweswaran, S.; Pantanowitz, J.; Deebajah, M.; Rashidi, H.H. Ethical and Bias Considerations in Artificial Intelligence (AI)/Machine Learning. Mod. Pathol. 2025, 38, 100686. [Google Scholar] [CrossRef]
- Brown, K.; Roshanitabrizi, P.; Rwebembera, J.; Okello, E.; Beaton, A.; Linguraru, M.G.; Sable, C.A. Using Artificial Intelligence for Rheumatic Heart Disease Detection by Echocardiography: Focus on Mitral Regurgitation. J. Am. Heart Assoc. 2024, 13, e031257. [Google Scholar] [CrossRef]
- Providência, R.; Aali, G.; Zhu, F.; Katairo, T.; Ahmad, M.; Bray, J.J.H.; Pelone, F.; Khanji, M.Y.; Marijon, E.; Cassandra, M.; et al. Handheld echocardiography for the screening and diagnosis of rheumatic heart disease: A systematic review to inform WHO guidelines. Lancet Glob. Health 2024, 12, e983–e994. [Google Scholar] [CrossRef]
- Farina, J.M.; Pereyra, M.; Mahmoud, A.K.; Scalia, I.G.; Abbas, M.T.; Chao, C.J.; Barry, T.; Ayoub, C.; Banerjee, I.; Arsanjani, R. Artificial Intelligence-Based Prediction of Cardiovascular Diseases from Chest Radiography. J. Imaging 2023, 9, 236. [Google Scholar] [CrossRef]
- Pal, B.; Kamran, S.A.; Lutnick, B.; Lucas, M.; Parmar, C.; Shah, A.P.; Apfel, D.; Fakharzadeh, S.; Miller, L.; Cula, G.; et al. GRASP-PsONet: Gradient-based Removal of Spurious Patterns for PsOriasis Severity Classification. arXiv 2025, arXiv:2506.21883. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Aki, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Alizadehsani, R.; Habibi, J.; Alizadeh Sani, Z.; Mashayekhi, H.; Boghrati, R.; Ghandeharioun, A.; Khozeimeh, F.; Alizadeh-Sani, F. Diagnosing Coronary Artery Disease via Data Mining Algorithms by Considering Laboratory and Echocardiography Features. Res. Cardiovasc. Med. 2013, 2, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Alizadehsani, R.; Hosseini, M.J.; Khosravi, A.; Khozeimeh, F.; Roshanzamir, M.; Sarrafzadegan, N.; Nahavandi, S. Non-invasive detection of coronary artery disease in high-risk patients based on the stenosis prediction of separate coronary arteries. Comput. Methods Programs Biomed. 2018, 162, 119–127. [Google Scholar] [CrossRef]
- Alizadehsani, R.; Habibi, J.; Hosseini, M.J.; Mashayekhi, H.; Boghrati, R.; Ghandeharioun, A.; Bahadorian, B.; Sani, Z.A. A data mining approach for diagnosis of coronary artery disease. Comput. Methods Programs Biomed. 2013, 111, 52–61. [Google Scholar] [CrossRef]
- Hoodbhoy, Z.; Hasan, B.; Jehan, F.; Bijnens, B.; Chowdhury, D. Machine learning from fetal flow waveforms to predict adverse perinatal outcomes: A study protocol. Gates Open Res. 2018, 2. [Google Scholar] [CrossRef]
- Asmare, M.H.; Filtjens, B.; Woldehanna, F.; Janssens, L.; Vanrumste, B. Rheumatic Heart Disease Screening Based on Phonocardiogram. Sensors 2021, 21, 6558. [Google Scholar] [CrossRef]
- Sayadi, M.; Varadarajan, V.; Sadoughi, F.; Chopannejad, S.; Langarizadeh, M. A Machine Learning Model for Detection of Coronary Artery Disease Using Noninvasive Clinical Parameters. Life 2022, 12, 1933. [Google Scholar] [CrossRef]
- Ekambaram, K.; Hassan, K. Establishing a Novel Diagnostic Framework Using Handheld Point-of-Care Focused-Echocardiography (HoPE) for Acute Left-Sided Cardiac Valve Emergencies: A Bayesian Approach for Emergency Physicians in Resource-Limited Settings. Diagnostics 2023, 13, 2581. [Google Scholar] [CrossRef]
- Nguyen, T.; Nguyen, P.; Tran, D.; Pham, H.; Nguyen, Q.; Le, T.; Van, H.; Do, B.; Tran, P.; Le, V.; et al. Ensemble learning of myocardial displacements for myocardial infarction detection in echocardiography. Front. Cardiovasc. Med. 2023, 10, 1185172. [Google Scholar] [CrossRef]
- Firima, E.; Gonzalez, L.; Manthabiseng, M.; Bane, M.; Lukau, B.; Leigh, B.; Kaufmann, B.A.; Weisser, M.; Amstutz, A.; Tromp, J.; et al. Implementing focused echocardiography and AI-supported analysis in a population-based survey in Lesotho: Implications for community-based cardiovascular disease care models. Hypertens. Res. 2024, 47, 708–713. [Google Scholar] [CrossRef]
- Soh, C.H.; Wright, L.; Baumann, A.; Seidel, B.; Yu, C.; Nolan, M.; Mylius, T.; Marwick, T.H.; Territory, N. Use of artificial intelligence-guided echocardiography to detect cardiac dysfunction and heart valve disease in rural and remote areas: Rationale and design of the AGILE-echo trial. Am. Heart J. 2024, 277, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Tromp, J.; Sarra, C.; Nidhal, B.; Mejdi, B.M.; Zouari, F.; Hummel, Y.; Mzoughi, K.; Kraiem, S.; Fehri, W.; Gamra, H.; et al. Nurse-led home-based detection of cardiac dysfunction by ultrasound: Results of the CUMIN pilot study. Eur. Heart J. Digit. Health 2024, 5, 163–169. [Google Scholar] [CrossRef] [PubMed]
- World Bank Country and Lending Groups—World Bank Data Help Desk. Available online: https://blogs.worldbank.org/en/opendata/new-world-bank-country-classifications-income-level-2022-2023 (accessed on 2 January 2025).
- Nair, M.; Svedberg, P.; Larsson, I.; Nygren, J.M. A comprehensive overview of barriers and strategies for AI implementation in healthcare: Mixed-method design. PLoS ONE 2024, 19, e0305949. [Google Scholar] [CrossRef] [PubMed]
- Haddiya, I.; Ramdani, S. Artificial intelligence in healthcare: A focus on the best practices. In ITM Web of Conferences, Marrakech, Morocco, 13 December 2024; EDP Sciences: Les Ulis, France, 2024; Volume 69. [Google Scholar]
- Maleki Varnosfaderani, S.; Forouzanfar, M. The Role of AI in Hospitals and Clinics: Transforming Healthcare in the 21st Century. Bioengineering 2024, 11, 337. [Google Scholar] [CrossRef]
- Jamthikar, A.; Gupta, D.; Khanna, N.N.; Araki, T.; Saba, L.; Nicolaides, A.; Sharma, A.; Omerzu, T.; Suri, H.S.; Gupta, A.; et al. A Special Report on Changing Trends in Preventive Stroke/Cardiovascular Risk Assessment Via B-Mode Ultrasonography. Curr. Atheroscler. Rep. 2019, 21, 25. [Google Scholar] [CrossRef]
- Baldassarre Lauren, A.; Ganatra, S.; Lopez-Mattei, J.; Yang Eric, H.; Zaha Vlad, G.; Wong Timothy, C.; Ayoub, C.; DeCara, J.M.; Dent, S. Advances in Multimodality Imaging in Cardio-Oncology. J. Am. Coll. Cardiol. 2022, 80, 1560–1578. [Google Scholar] [CrossRef]
- Yilgwan, C.S.; Gurumdimma, N.; Sulague, R.M.; Kpodonu, J. Gaps, Obstacles, and Opportunities in Rheumatic Heart Disease Research: Where Are We Now? JACC Adv. 2023, 2, 100293. [Google Scholar] [CrossRef]
- Lugossy, A.-M.; Anton, K.; Dako, F.; Dixon, R.G.; DuCharme, P.A.; Duggan, C.; Durand, M.A.; Einstein, S.A.; Elahi, A.; Kesselman, A.; et al. Building Radiology Equity: Themes from the 2023 RAD-AID Conference on International Radiology and Global Health. J. Am. Coll. Radiol. 2024, 21, 1194–1200. [Google Scholar] [CrossRef]
- Yang, J.; Dung, N.T.; Thach, P.N.; Phong, N.T.; Phu, V.D.; Phu, K.D.; Yen, L.M.; Thy, D.B.X.; Soltan, A.A.S.; Thwaites, L.; et al. Generalizability assessment of AI models across hospitals in a low-middle and high income country. Nat. Commun. 2024, 15, 8270. [Google Scholar] [CrossRef]
- Ramwala, O.A.; Lowry, K.P.; Cross, N.M.; Hsu, W.; Austin, C.C.; Mooney, S.D.; Lee, C.I. Establishing a Validation Infrastructure for Imaging-Based Artificial Intelligence Algorithms Before Clinical Implementation. J. Am. Coll. Radiol. 2024, 21, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Al Kuwaiti, A.; Nazer, K.; Al-Reedy, A.; Al-Shehri, S.; Al-Muhanna, A.; Subbarayalu, A.V.; Muhanna, D.A.; Al-Muhana, F.A. A Review of the Role of Artificial Intelligence in Healthcare. J. Pers. Med. 2023, 13, 951. [Google Scholar] [CrossRef] [PubMed]
- Lämmermann, L.; Hofmann, P.; Urbach, N. Managing artificial intelligence applications in healthcare: Promoting information processing among stakeholders. Int. J. Inf. Manag. 2024, 75, 102728. [Google Scholar] [CrossRef]
- Alizadehsani, R.; Abdar, M.; Roshanzamir, M.; Khosravi, A.; Kebria, P.M.; Khozeimeh, F.; Nahavandi, S.; Sarrafzadegan, N.; Archarya, U.R. Machine learning-based coronary artery disease diagnosis: A comprehensive review. Comput. Biol. Med. 2019, 111, 103346. [Google Scholar] [CrossRef]
- Lones, M.A. Avoiding common machine learning pitfalls. Patterns 2024, 5, 101046. [Google Scholar] [CrossRef]
- Norori, N.; Hu, Q.; Aellen, F.M.; Faraci, F.D.; Tzovara, A. Addressing bias in big data and AI for health care: A call for open science. Patterns 2021, 2, 100347. [Google Scholar] [CrossRef]
- Thomford, N.E.; Bope, C.D.; Agamah, F.E.; Dzobo, K.; Owusu Ateko, R.; Chimusa, E.; Mazandu, G.K.; Ntumba, S.B.; Dandara, C.; Wonkam, A. Implementing Artificial Intelligence and Digital Health in Resource-Limited Settings? Top 10 Lessons We Learned in Congenital Heart Defects and Cardiology. OMICS A J. Integr. Biol. 2019, 24, 264–277. [Google Scholar] [CrossRef]
- Ashinze, P.; Akande, E.; Bethrand, C.; Obafemi, E.; David, O.O.O.; Akobe, S.N.; Joyce, N.O.; Izuchukwu, O.J.; Okoro, N.P. Artificial intelligence: Transforming cardiovascular healthcare in Africa. Egypt. Heart J. 2024, 76, 120. [Google Scholar] [CrossRef]
- Becker, D.M.; Tafoya, C.A.; Becker, S.L.; Kruger, G.H.; Tafoya, M.J.; Becker, T.K. The use of portable ultrasound devices in low- and middle-income countries: A systematic review of the literature. Trop. Med. Int. Health 2016, 21, 294–311. [Google Scholar] [CrossRef]
- Wanjiku, G.; Dreizler, L.; Wu, S.; Baird, J.; Wachira, B. Utility of hand-held ultrasound for image acquisition and interpretation by trained Kenyan providers. Ultrasound J. 2023, 15, 12. [Google Scholar] [CrossRef]
- Bulto, L.N.; Hendriks, J.M. The burden of cardiovascular disease in Africa: Prevention challenges and opportunities for mitigation. Eur. J. Cardiovasc. Nurs. 2024, 23, e88–e90. [Google Scholar] [CrossRef] [PubMed]
- Ueda, D.; Kakinuma, T.; Fujita, S.; Kamagata, K.; Fushimi, Y.; Ito, R.; Matsui, Y.; Nozaki, T.; Nakaura, T.; Fujima, N.; et al. Fairness of artificial intelligence in healthcare: Review and recommendations. Jpn. J. Radiol. 2024, 42, 3–15. [Google Scholar] [CrossRef] [PubMed]
- El-Rewaidy, H.; Fahmy, A.S.; Pashakhanloo, F.; Cai, X.; Kucukseymen, S.; Csecs, I.; Neisius, U.; Haji-Valizadeh, H.; Menze, B.; Nezafat, R. Multi-domain convolutional neural network (MD-CNN) for radial reconstruction of dynamic cardiac MRI. Magn. Reson. Med. 2021, 85, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Ghosh, S.; Haji-Valizadeh, H.; Pathrose, A.; Schiffers, F.; Lee, D.C.; Freed, B.H.; Markl, M.; Cossairt, O.S.; Katsaggelos, A.K.; et al. Rapid reconstruction of highly undersampled, non-Cartesian real-time cine k-space data using a per ceptual complex neural network (PCNN). NMR Biomed. 2021, 34, e4405. [Google Scholar] [CrossRef]
- Campello, V.M.; Gkontra, P.; Izquierdo, C.; Martin-Isla, C.; Sojoudi, A.; Full, P.M.; Maier-Hein, K.; Zhang, Y.; He, Z.; Ma, J.; et al. Multi-centre, multi-vendor and multi-disease cardiac segmentation: The M&Ms challenge. IEEE Trans. Med. Imaging 2021, 40, 3543–3554. [Google Scholar]
- Bernard, O.; Lalande, A.; Zotti, C.; Cervenansky, F.; Yang, X.; Heng, P.-A.; Cetin, I.; Lekadir, K.; Camara, O.; Ballester, M.A.G.; et al. Deep learning techniques for automatic MRI cardiac multi-structures segmentation and diagnosis: Is the problem solved? IEEE Trans. Med. Imaging 2018, 37, 2514–2525. [Google Scholar] [CrossRef]
- Papetti, D.M.; Abeelen, K.; Davies, R.; Menè, R.; Heilbron, F.; Perelli, F.P.; Artico, J.; Seraphim, A.; Moon, J.C.; Parati, G.; et al. An accurate and time-efficient deep learning-based system for automated segmentation and reporting of cardiac magnetic resonance detected ischemic scar. Comput. Methods Programs Biomed. 2023, 229, 107321. [Google Scholar] [CrossRef]
- Wang, Y.-R.; Yang, K.; Wen, Y.; Wang, P.; Hu, Y.; Lai, Y.; Wang, Y.; Zhao, K.; Tang, S.; Zhang, A.; et al. Screening and diagnosis of cardiovascular disease using artificial intelligence-enabled cardiac magnetic resonance imaging. Nat. Med. 2024, 30, 1471–1480. [Google Scholar] [CrossRef]
- Martini, N.; Aimo, A.; Barison, A.; Della Latta, D.; Vergaro, G.; Aquaro, G.D.; Ripoli, A.; Emdin, M.; Chiappino, D. Deep learning to diagnose cardiac amyloidosis from cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2020, 22, 8. [Google Scholar] [CrossRef]
- Ohta, Y.; Tateishi, E.; Morita, Y.; Nishii, T.; Kotoku, A.; Horinouchi, H.H. Optimization of null point in Look-locker images for myocardial late gadolinium enhancement imaging using deep learning and a smartphone. Eur. Radiol. 2023, 33, 4688–4697. [Google Scholar] [CrossRef]
- Wolterink, J.M.; Leiner, T.; Viergever, M.A.; Isgum, I. Generative adversarial networks for noise reduction in low-dose CT. IEEE Trans. Med. Imaging 2017, 36, 2536–2545. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.D.; Marques, H.; Kumar, V.; Griffin, W.F.; Rahban, H.; Karlsberg, R.P.; Zeman, R.K.; Katz, R.J.; Earls, J.P. CT evaluation by artificial intelligence for atherosclerosis, stenosis and vascular morphology (CLARIFY): A multi-center, international study. J. Cardiovasc. Comput. Tomogr. 2021, 15, 470–476. [Google Scholar] [CrossRef]
- Jonas, R.A.; Weerakoon, S.; Fisher, R.; Griffin, W.F.; Kumar, V.; Rahban, H.; Marques, H.; Karlsberg, R.P.; Jennings, R.S.; Crabtree, T.R.; et al. Interobserver variability among expert readers quantifying plaque volume and plaque characteristics on coronary CT angiography: A CLARIFY trial sub-study. Clin. Imaging 2022, 91, 19–25. [Google Scholar] [CrossRef]
- Conte, E.; Mushtaq, S.; Pontone, G.; Li Piani, L.; Ravagnani, P.; Galli, S.; Collet, C.; Sonck, J.; Odoardo, L.D.; Guglielmo, M.; et al. Plaque quantification by coronary computed tomography angiography using intravascular ultrasound as a reference standard: A comparison between standard and last generation computed tomography scanners. Eur. Heart J.-Cardiovasc. Imaging 2020, 21, 191–201. [Google Scholar]
- Blanke, P.; Weir-McCall, J.R.; Achenbach, S.; Delgado, V.; Hausleiter, J.; Jilaihawi, H.; Marwan, M.; Nørgaard, B.L.; Piazza, N.; Schoenhagen, P.; et al. Computed tomography imaging in the context of transcatheter aortic valve implant ation (TAVI)/transcatheter aortic valve replacement (TAVR): An expert consensus document of the society of cardiovascular computed tomography. JACC Cardiovasc. Imaging 2019, 12, 1–24. [Google Scholar] [CrossRef]
- Chaban, Y.V.; Vosshenrich, J.; McKee, H.; Gunasekaran, S.; Brown, M.J.; Atalay, M.K.; Heye, T.; Markl, M.; Woolen, S.A.; Simonetti, O.P.; et al. Environmental Sustainability and MRI: Challenges, Opportunities, and a Call for Action. J. Magn. Reson. Imaging 2024, 59, 1149–1167. [Google Scholar] [CrossRef]
- Pal, B.; Thalengala, A.; Chawla, A. Renal calculus composition assessment from radiographic images using machine learning technique. Res. Biomed. Eng. 2025, 41, 28. [Google Scholar] [CrossRef]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marey, A.; Mehrtabar, S.; Afify, A.; Pal, B.; Trvalik, A.; Adeleke, S.; Umair, M. From Echocardiography to CT/MRI: Lessons for AI Implementation in Cardiovascular Imaging in LMICs—A Systematic Review and Narrative Synthesis. Bioengineering 2025, 12, 1038. https://doi.org/10.3390/bioengineering12101038
Marey A, Mehrtabar S, Afify A, Pal B, Trvalik A, Adeleke S, Umair M. From Echocardiography to CT/MRI: Lessons for AI Implementation in Cardiovascular Imaging in LMICs—A Systematic Review and Narrative Synthesis. Bioengineering. 2025; 12(10):1038. https://doi.org/10.3390/bioengineering12101038
Chicago/Turabian StyleMarey, Ahmed, Saba Mehrtabar, Ahmed Afify, Basudha Pal, Arcadia Trvalik, Sola Adeleke, and Muhammad Umair. 2025. "From Echocardiography to CT/MRI: Lessons for AI Implementation in Cardiovascular Imaging in LMICs—A Systematic Review and Narrative Synthesis" Bioengineering 12, no. 10: 1038. https://doi.org/10.3390/bioengineering12101038
APA StyleMarey, A., Mehrtabar, S., Afify, A., Pal, B., Trvalik, A., Adeleke, S., & Umair, M. (2025). From Echocardiography to CT/MRI: Lessons for AI Implementation in Cardiovascular Imaging in LMICs—A Systematic Review and Narrative Synthesis. Bioengineering, 12(10), 1038. https://doi.org/10.3390/bioengineering12101038