Integrating Spatial Omics and Deep Learning: Toward Predictive Models of Cardiomyocyte Differentiation Efficiency
Abstract
1. Introduction
2. Research Methods and Materials
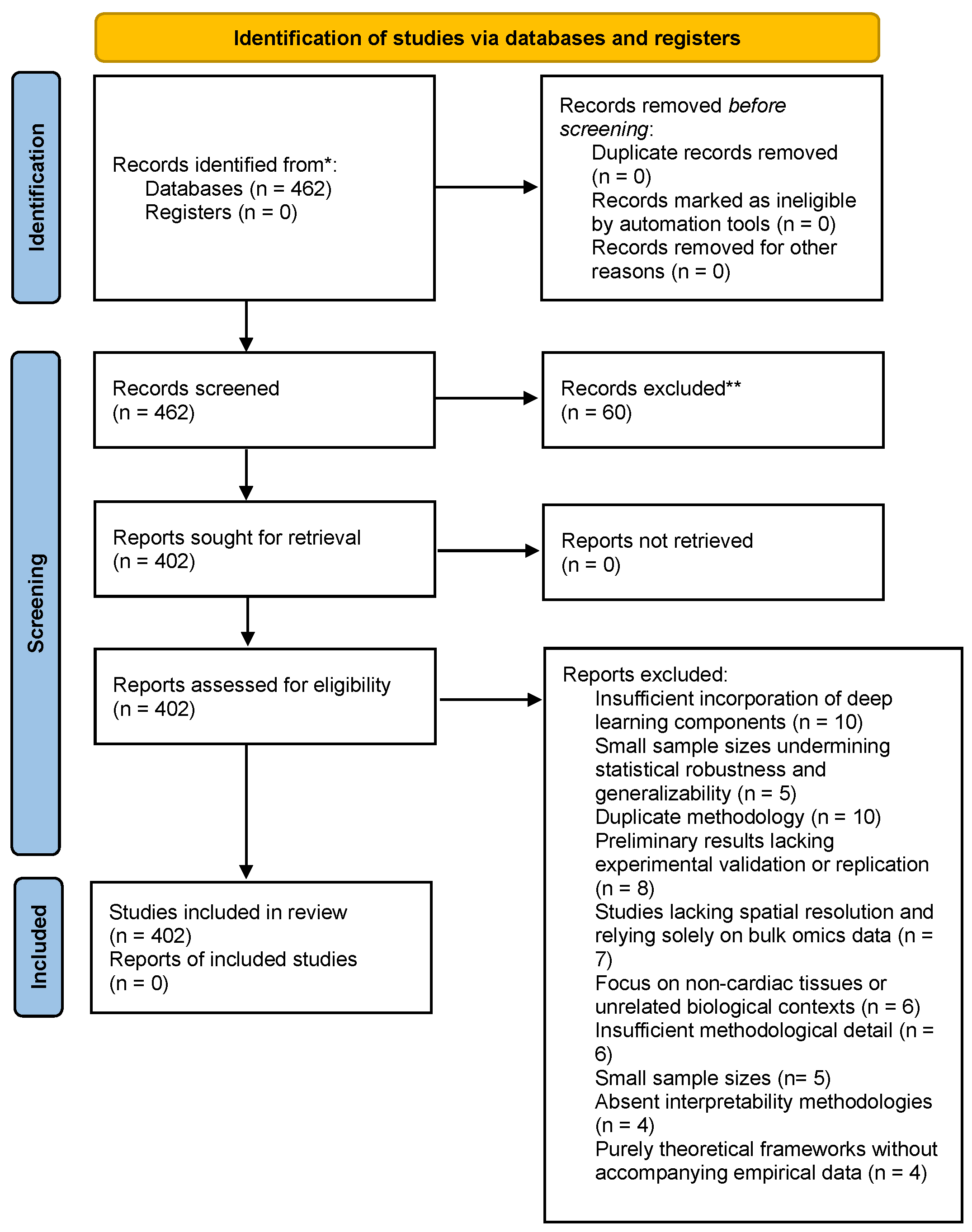
2.1. Study Selection and Screening
- Duplicate methodologies (n = 10)
- Insufficient incorporation of deep learning components, such as minimal or no use of GNNs/RNNs/attention networks (n = 10)
- Preliminary results lacking experimental validation or replication (n = 8)
- Studies lacking spatial resolution and relying solely on bulk omics data (n = 7)
- Focus on non-cardiac tissues or unrelated biological contexts (n = 6)
- Insufficient methodological detail impeding reproducibility (n = 6)
- Small sample sizes interpretability methodologies for AI models (n = 4)
- Purely theoretical frameworks without accompanying empirical data (n = 4).
2.2. Eligibility Assessment and Synthesis
3. Cardiomyocyte Differentiation and Regenerative Medicine
3.1. Cardiomyocyte Biology and Development
3.2. Current Challenges in iPSC-CM Technology
4. Spatial Multi-Omics in Cardiac Research
4.1. Human Developmental Cardiac Datasets
4.2. Regenerative Model Systems
| Subcategory | Dataset | Modality | Tissue | Temporal Info | Integration Considerations | Citation |
|---|---|---|---|---|---|---|
| Developmental | Human Cell Atlas Heart Development | scRNA-seq + Spatial Transcriptomics | Human embryonic heart | Yes (5.5–14 Weeks Post Conception (WPC)) | Different platforms (10x Visium vs. scRNA-seq) require cross-modal anchoring (e.g., Seurat v4 WNN, Harmony) to align spatial and transcriptomic resolution. Challenges include batch effects from donors or technologies, potential loss of spatial details during integration, computational scalability for large datasets (e.g., >500,000 cells), and ensuring accurate cell type deconvolution without over-smoothing heterogeneous populations. These issues impact the reliability of organ-wide atlases by introducing artefacts in cellular interaction mapping. | [29] |
| Spatial dynamics of developing human heart | scRNA-seq + MERFISH | Human embryonic heart | Yes (9–16 WPC) | MERFISH has higher spatial resolution but limited gene coverage vs. scRNA-seq; integration often requires feature selection (e.g., shared genes) + imputation methods using methods like Tangram (probabilistic mapping to minimise divergence), gimVI (deep generative joint modelling), or Spatialscope (score-based diffusion models with Potts spatial smoothness). Limitations include resolution mismatch (MERFISH vs. Visium-like spot-based aggregation, leading to potential loss of fine details), over-smoothing of spatial heterogeneity, high computational costs, dependency on scRNA-seq reference quality (poor quality introduces artefacts), and batch effects requiring correction. | [30] | |
| Developing human heart (EGA) | snRNA-seq + Spatial Transcriptomics + ISS | Human embryonic heart | Yes (4.5, 6.5, 9 WPC) | ISS vs snRNA-seq differ in throughput and detection sensitivity; anchor-based correction recommended (e.g., Seurat anchors for label transfer). Challenges include varying detection rates leading to incomplete gene profiles, batch effects from multi-modal data sources, normalisation difficulties for low-abundance transcripts, and integration of imaging-based ISS with sequencing data without losing spatial precision. These issues can hinder accurate reconstruction of early cardiac development trajectories, potentially introducing biases in cell state identification. | [31] | |
| Mouse Heart Spatiotemporal Atlas (Stereo-seq) | Spatial Transcriptomics (Stereo-seq) | Mouse heart | Yes (embryonic day 20 (E20), postnatal day 1 (P01), postnatal day 4 (P04), postnatal day 14 (P14)) | Stereo-seq has ultra-high resolution; batch alignment needed for cross-species inference (human vs. mouse) using ortholog mapping and methods like Harmony or MNN. Challenges involve handling large data volumes (>500,000 spots), accuracy of ortholog mapping across species, potential over-smoothing in dimensionality reduction, and temporal batch effects from multiple developmental stages. These limitations impact comparative analyses with human data, risking misinterpretation of conserved cardiac organogenesis mechanisms. | [32] | |
| Mouse heart spatial transcriptomics (Visium) | scRNA-seq | iPSC-derived cardiomyocytes | Yes (pluripotency (day 0), germ layer specification (day 2), progenitor cardiac cell state (day 5), committed cardiac cell state (day 15), definitive cell state (day 30)) | Integration across iPSC protocols requires batch-effect correction (MNN, LIGER) due to lab-specific variability. Challenges include variability in differentiation efficiency leading to heterogeneous cell states, data sparsity in scRNA-seq, ensuring accurate cell type mapping without reference overfitting, and handling temporal trajectories with potential dropout events. These issues affect modelling of cardiomyocyte maturation, potentially leading to biassed predictions of protocol outcomes. | [33] | |
| Human heart organoids spatial atlas | scRNA-seq + Spatial transcriptomics | Human heart organoids | Yes (approximately 3 days after seeding (day 0)–day 20 of differentiation) | Organoids differ from in vivo tissues in cellular composition; transfer learning-based integration may be required (e.g., using pre-trained models from in vivo data). Challenges encompass discrepancies in cell maturity and states between organoids and native tissues, limited spatial resolution in miniaturised models, batch effects from culture conditions, and validation against human samples to avoid artefactual networks. These limitations influence insights into morphogenesis, risking overgeneralisation from in vitro to in vivo contexts. | [34] | |
| Human SAN Cell Atlas | scRNA-seq + scATAC-seq | Human sinoatrial node (iPSC) | Yes (Differentiation) | Requires multi-modal alignment (RNA + ATAC); weighted nearest neighbour (WNN) or MOFA+ commonly applied for joint embedding. Challenges include differing data sparsity (ATAC more sparse than RNA), accuracy of peak-to-gene linking, computational demands for integrating epigenetic and transcriptomic layers, and handling differentiation-induced variability. These issues impact pacemaker cell identification, potentially introducing errors in regulatory network inference. | [35] | |
| Adult (Physiological) | Adult human heart cell atlas | scRNA-seq + snRNA-seq | Adult human heart | No (Adult) | snRNA-seq vs scRNA-seq differ in transcript detection bias (nuclear vs. cytoplasmic); normalisation across modalities essential (e.g., using SCTransform). Challenges involve lower gene detection in snRNA-seq, integration without losing rare cell types, batch effects from anatomical regions, and ensuring comparability in large-scale atlases (>500,000 cells). These limitations affect cellular heterogeneity mapping, risking underrepresentation of dynamic states in healthy hearts. | [36] |
| Spatially resolved multiomics of human cardiac niches | scRNA-seq + snATAC-seq + Spatial Transcriptomics | Adult human heart | No (Adult) | Multi-omics alignment requires matrix factorisation or LIGER for shared latent space inference. Challenges include integrating three modalities with varying resolutions (spatial vs. single-cell), batch variations from donors or regions, preserving spatial context in multi-omic inference, and handling sparsity in ATAC data. These issues influence niche discovery, potentially leading to incomplete cellular interaction models | [37] | |
| Human cardiac conduction system | scRNA-seq + Spatial Transcriptomics | Human cardiac conduction system | No (Adult) | Cell type resolution differs across datasets; label transfer + cross-modal anchoring recommended. Challenges encompass aligning conduction-specific markers, handling low-abundance pacemaker cells, spatial deconvolution accuracy in heterogeneous tissues, and batch effects from sample preparation. These limitations affect understanding of electrical signalling, risking misattribution of cell roles. | [6,37,38] | |
| Pathological/Regenerative | Spatial multi-omic map of human MI (Myocardial Infarction) | snRNA-seq + snATAC-seq + Spatial Transcriptomics | Human infarcted heart | Yes (Post-MI timepoints) | Post-MI inflammatory environments induce batch-specific effects; regression-based correction (e.g., Harmony) improves comparability. Challenges include disease-induced heterogeneity complicating alignment, integrating chromatin/epigenetic with spatial data, temporal variability across MI stages, and sparsity in infarct zones. These issues impact remodelling maps, potentially biassing therapeutic target identification. | [33] |
| Human heart spatial transcriptomics (Disease) | Spatial Transcriptomics | Human heart (disease) | No (Disease states) | Differences in sample preparation (frozen vs. FFPE) require careful normalisation (e.g., using sctransform or DESeq2). Challenges involve tissue quality variations in diseased samples, artefact removal from pathology-induced noise, ensuring comparability across disease states, and handling low-resolution spots in heterogeneous lesions. These limitations affect disease progression modelling, risking inaccurate spatial gene expression profiles. | [31,39] | |
| Zebrafish heart regeneration atlas | scRNA-seq + Spatial Transcriptomics (Stereo-seq) | Zebrafish heart | Yes (8 timepoints of zebrafish heart regeneration stages) | Cross-species integration requires ortholog mapping + dimensionality reduction alignment. Challenges include species-specific gene expression differences, handling high-resolution Stereo-seq data volumes, temporal alignment across regeneration stages, and batch effects from injury timepoints. These issues influence comparative regenerative studies, potentially leading to translational gaps with human models | [40] | |
| iPSC-CM scRNA-seq + scATAC-seq | scRNA-seq + scATAC-seq | iPSC-derived cardiomyocytes | Yes (Day 0–30) | Multi-modal integration (RNA + ATAC) typically handled via Seurat v4 WNN or scGLUE for joint analysis. Challenges encompass sparsity in ATAC-seq data, linking enhancers to genes accurately, variability in iPSC differentiation trajectories, and computational scaling for time-series data. These limitations affect maturation dynamics insights, risking biassed regulatory network reconstructions. | [41] |
5. Deep Learning Architectures for Spatial Cardiac Data
5.1. GNNs in Cardiac Applications
| Model Architecture | Dataset | Accuracy (%) | AUROC | F1 Score | Precision | Recall | Baseline Comparison | References |
|---|---|---|---|---|---|---|---|---|
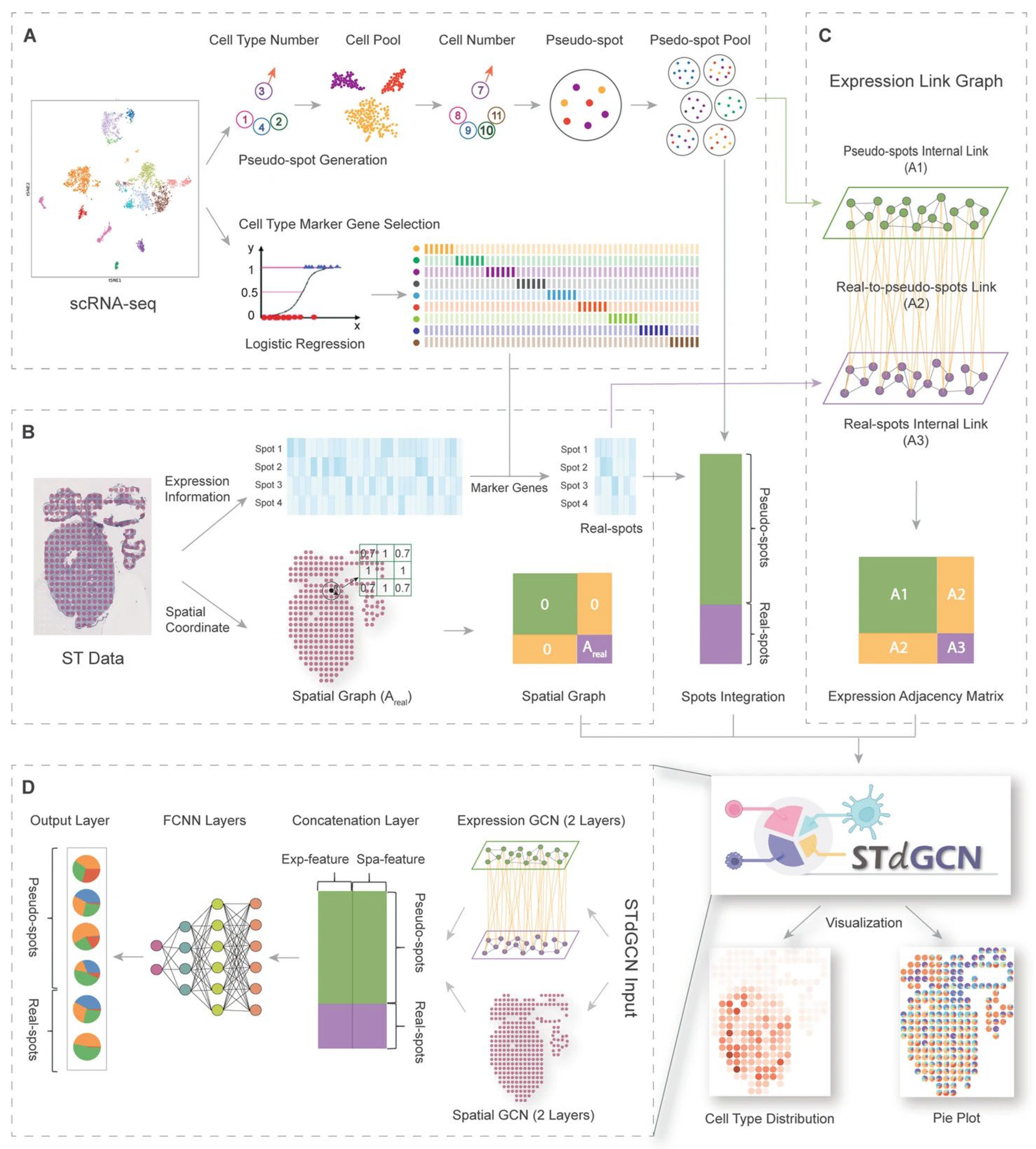
| STdGCN | Human breast cancer & heart development | - | 0.92 | 0.85 | - | - | RCTD, SPOTlight, Cell2location, DSTG, CARD | [54] |
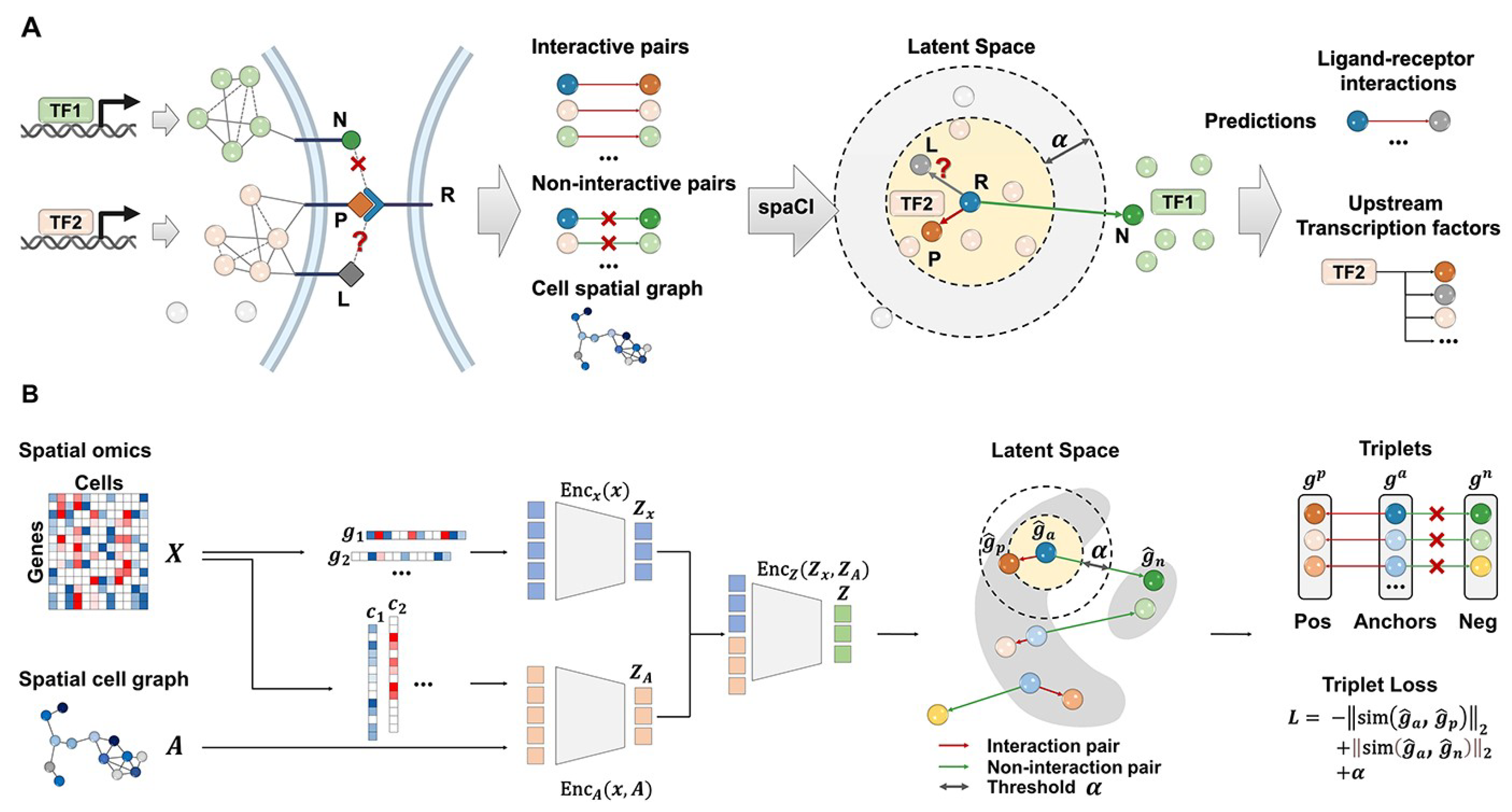
| spaCI (GNN+attention) | Spatial transcriptomics | - | 0.82 | Accurately identified true interaction pairs across 4 cohorts Cohort 1: mean ± SE: 0.852 ± 0.014 Cohort 2: 0.817 ± 0.05 Cohort 3: 0.859 ± 0.06 Cohort 4: 0.853 ± 0.03 | - | - | iTALK, CellPhoneDB, CellChat, Connectome | [50] |
| LSTM (cardiac prediction) | EHR cardiac data | - | 0.76 | - | - | - | Logistic Regression, Naïve Bayes | [55,56,57] |
| Graph Transformer (GT) | Heart failure prediction | - | 0.7925 | 0.5361 | - | - | Random Forest, GraphSAGE, GAT | [58] |
| Random Forest (baseline) | Heart failure prediction | 0.91 | 0.90 | 0.91 | 0.92 | 0.90 | None (serves as baseline, compared to XGBoost, KNN, logistic regression) | [59] |
| GNN-LSTM (hybrid) | Heart failure prediction | 98.90 | - | - | - | - | Random Forest, LSTM | [60,61,62] |
5.2. RNNs for Temporal Modelling
5.3. Integrated Spatiotemporal Architectures
5.4. Explainability Challenges
5.5. Real-World Validation Limitations
6. Predictive Modelling for Differentiation Efficiency
6.1. AI Approaches for Predicting Differentiation Outcomes
6.2. Model Evaluation and Validation Strategies
7. Limitations and Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A. Search Strategies
References
- Roshanravan, N.; Ghaffari, S.; Bastani, S.; Pahlavan, S.; Asghari, S.; Doustvandi, M.A.; Jalilzadeh-Razin, S.; Dastouri, M. Human cardiac organoids: A recent revolution in disease modeling and regenerative medicine. J. Cardiovasc. Thorac. Res. 2023, 15, 68–72. [Google Scholar] [CrossRef]
- Palmer, J.A.; Rosenthal, N.; Teichmann, S.A.; Litvinukova, M. Revisiting Cardiac Biology in the Era of Single Cell and Spatial Omics. Circ. Res. 2024, 134, 1681–1702. [Google Scholar] [CrossRef] [PubMed]
- Vickovic, S.; Lötstedt, B.; Klughammer, J.; Mages, S.; Segerstolpe, A.; Rozenblatt-Rosen, O.; Regev, A. SM-Omics is an automated platform for high-throughput spatial multi-omics. Nat. Commun. 2022, 13, 795. [Google Scholar] [CrossRef]
- Misra, A.; Baker, C.D.; Pritchett, E.M.; Villar, K.N.B.; Ashton, J.M.; Small, E.M. Characterizing neonatal heart maturation, regeneration, and scar resolution using spatial transcriptomics. J. Cardiovasc. Dev. Dis. 2022, 9, 1. [Google Scholar] [CrossRef]
- Song, Q.; Su, J. DSTG: Deconvoluting spatial transcriptomics data through graph-based artificial intelligence. Brief. Bioinform. 2021, 22, bbaa414. [Google Scholar] [CrossRef]
- Nguyen, Q.; Tung, L.W.; Lin, B.; Sivakumar, R.; Sar, F.; Singhera, G.; Wang, Y.; Parker, J.; Le Bihan, S.; Singh, A.; et al. Spatial Transcriptomics in Human Cardiac Tissue. Int. J. Mol. Sci. 2025, 26, 995. [Google Scholar] [CrossRef]
- See, K.; Tan, W.L.W.; Lim, E.H.; Tiang, Z.; Lee, L.T.; Li, P.Y.Q.; Luu, T.D.A.; Ackers-Johnson, M.; Foo, R.S. Single cardiomyocyte nuclear transcriptomes reveal a lincRNA-regulated de-differentiation and cell cycle stress-response in vivo. Nat. Commun. 2017, 8, 225. [Google Scholar] [CrossRef] [PubMed]
- Zebrowski, D.C.; Jensen, C.H.; Becker, R.; Ferrazzi, F.; Baun, C.; Hvidsten, S.; Sheikh, S.P.; Polizzotti, B.D.; Andersen, D.C.; Engel, F.B. Cardiac injury of the newborn mammalian heart accelerates cardiomyocyte terminal differentiation. Sci. Rep. 2017, 7, 8362. [Google Scholar] [CrossRef] [PubMed]
- Lázár, E.; Sadek, H.A.; Bergmann, O. Cardiomyocyte renewal in the human heart: Insights from the fall-out. Eur. Hear. J. 2017, 38, 2333–2342. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, C.; Xu, J.; Zhao, M.T.; Van Bortle, K.; Cheng, X.; Wang, G.; Chang, H.Y.; Wu, J.C.; Snyder, M.P. Genome-Wide Temporal Profiling of Transcriptome and Open-Chromatin of Early Cardiomyocyte Differentiation Derived From hiPSCs and hESCs. Circ. Res. 2017, 121, 376–391. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Liu, Z.; Ren, Z.H.; Chen, H.X.; Zhang, Y.; Zhang, Z.; Cao, N.; Luo, G.Z. Co-effects of m6A and chromatin accessibility dynamics in the regulation of cardiomyocyte differentiation. Epigenetics Chromatin 2023, 16, 32. [Google Scholar] [CrossRef]
- Paige, S.L.; Plonowska, K.; Xu, A.; Wu, S.M. Molecular Regulation of Cardiomyocyte Differentiation. Circ. Res. 2015, 116, 341–353. [Google Scholar] [CrossRef]
- Parikh, A.; Wu, J.; Blanton, R.M.; Tzanakakis, E.S. Signaling Pathways and Gene Regulatory Networks in Cardiomyocyte Differentiation. Tissue Eng. Part B Rev. 2015, 21, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Rowton, M.; Guzzetta, A.; Rydeen, A.B.; Moskowitz, I.P. Control of cardiomyocyte differentiation timing by intercellular signaling pathways. Semin. Cell Dev. Biol. 2021, 118, 94–106. [Google Scholar] [CrossRef]
- Cianflone, E.; Scalise, M.; Marino, F.; Salerno, L.; Salerno, N.; Urbanek, K.; Torella, D. The negative regulation of gene expression by microRNAs as key driver of inducers and repressors of cardiomyocyte differentiation. Clin. Sci. 2022, 136, 1179–1203. [Google Scholar] [CrossRef]
- Elorbany, R.; Popp, J.M.; Rhodes, K.; Strober, B.J.; Barr, K.; Qi, G.; Gilad, Y.; Battle, A. Single-cell sequencing reveals lineage-specific dynamic genetic regulation of gene expression during human cardiomyocyte differentiation. PLoS Genet. 2022, 18, e1009666. [Google Scholar] [CrossRef]
- Buonaiuto, G.; Desideri, F.; Setti, A.; Palma, A.; D’Angelo, A.; Storari, G.; Santini, T.; Laneve, P.; Trisciuoglio, D.; Ballarino, M. Long noncoding RNA HSCHARME is altered in human cardiomyopathies and promotes stem cell-derived cardiomyocyte differentiation by splicing regulation. bioRxiv 2025. [Google Scholar] [CrossRef]
- Guan, T.; Dominguez, C.X.; Amezquita, R.A.; Laidlaw, B.J.; Cheng, J.; Henao-Mejia, J.; Williams, A.; Flavell, R.A.; Lu, J.; Kaech, S.M. ZEB1, ZEB2, and the miR-200 family form a counterregulatory network to regulate CD8+ T cell fates. J. Exp. Med. 2018, 215, 1153–1168. [Google Scholar] [CrossRef]
- Bak, S.T.; Harvald, E.B.; Ellman, D.G.; Mathiesen, S.B.; Chen, T.; Fang, S.; Andersen, K.S.; Fenger, C.D.; Burton, M.; Thomassen, M.; et al. Ploidy-stratified single cardiomyocyte transcriptomics map Zinc Finger E-Box Binding Homeobox 1 to underly cardiomyocyte proliferation before birth. Basic. Res. Cardiol. 2023, 118, 8. [Google Scholar] [CrossRef] [PubMed]
- Minakawa, T.; Yamashita, J.K. Extracellular vesicles and microRNAs in the regulation of cardiomyocyte differentiation and proliferation. Arch. Biochem. Biophys. 2023, 749, 109791. [Google Scholar] [CrossRef] [PubMed]
- Haniffa, M.; Taylor, D.; Linnarsson, S.; Aronow, B.J.; Bader, G.D.; Barker, R.A.; Camara, P.G.; Camp, J.G.; Chédotal, A.; Copp, A.; et al. A roadmap for the Human Developmental Cell Atlas. Nature 2021, 597, 196. [Google Scholar] [CrossRef] [PubMed]
- Holman, A.R.; Tran, S.; Destici, E.; Farah, E.N.; Li, T.; Nelson, A.C.; Engler, A.J.; Chi, N.C. Single-cell multi-modal integrative analyses highlight functional dynamic gene regulatory networks directing human cardiac development. Cell Genom. 2024, 4, 100680. [Google Scholar] [CrossRef]
- Cranley, J.; Bayraktar, S.; Kanemaru, K.; Knight-Schrijver, V.; Pett, J.P.; Davaapil, H.; Gambardella, L.; Sinha, S.; Teichmann, S. A spatially-resolved multiomic cell atlas reveals gene regulatory networks underlying cell specification in the developing human heart. Eur. Heart J. 2023, 44 (Suppl. S2), ehad655-3042. [Google Scholar] [CrossRef]
- Regev, A.; Teichmann, S.A.; Lander, E.S.; Amit, I.; Benoist, C.; Birney, E.; Bodenmiller, B.; Campbell, P.; Carninci, P.; Clatworthy, M.; et al. The human cell atlas. Elife 2017, 6, e27041. [Google Scholar] [CrossRef]
- Cao, J.; O’Day, D.R.; Pliner, H.A.; Kingsley, P.D.; Deng, M.; Daza, R.M.; Zager, M.A.; Aldinger, K.A.; Blecher-Gonen, R.; Zhang, F.; et al. A human cell atlas of fetal gene expression. Science (1979) 2020, 370, 808. [Google Scholar] [CrossRef]
- Suryawanshi, H.; Clancy, R.; Morozov, P.; Halushka, M.K.; Buyon, J.P.; Tuschl, T. Cell atlas of the foetal human heart and implications for autoimmune-mediated congenital heart block. Cardiovasc. Res. 2020, 116, 1446–1457. [Google Scholar] [CrossRef]
- Jiang, T.; Jin, X.; Gao, Y.; Zhou, W.; Yu, J.; Li, Y.; Xu, J.; Cai, B. CardioAtlas: Deciphering the single-cell transcriptome landscape in cardiovascular tissues and diseases. Biomark. Res. 2024, 12, 149. [Google Scholar] [CrossRef]
- Lázár, E.; Mauron, R.; Andrusivová, Ž.; Foyer, J.; He, M.; Larsson, L.; Shakari, N.; Salas, S.M.; Avenel, C.; Sariyar, S.; et al. Spatial Dynamics of the Developing Human Heart. bioRxiv 2024. [Google Scholar] [CrossRef]
- Farah, E.N.; Hu, R.K.; Kern, C.; Zhang, Q.; Lu, T.Y.; Ma, Q.; Tran, S.; Zhang, B.; Carlin, D.; Monell, A.; et al. Spatially organized cellular communities form the developing human heart. Nature 2024, 627, 854–864. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, W.; Yang, T.; Li, L.; Ma, X.; Chen, J.; Wang, J.; Huang, Y.; Gould, J.; Lu, H.; et al. STOmicsDB: A comprehensive database for spatial transcriptomics data sharing, analysis and visualization. Nucleic Acids Res. 2024, 52, D1053–D1061. [Google Scholar] [CrossRef]
- Kang, J.; Li, Q.; Liu, J.; Du, L.; Liu, P.; Liu, F.; Wang, Y.; Shen, X.; Luo, X.; Wang, N.; et al. Exploring the cellular and molecular basis of murine cardiac development through spatiotemporal transcriptome sequencing. Gigascience 2025, 14, giaf012. [Google Scholar] [CrossRef]
- Kuppe, C.; Ramirez Flores, R.O.; Li, Z.; Hayat, S.; Levinson, R.T.; Liao, X.; Hannani, M.T.; Tanevski, J.; Wünnemann, F.; Nagai, J.S.; et al. Spatial multi-omic map of human myocardial infarction. Nature 2022, 608, 766–777. [Google Scholar] [CrossRef]
- Hoang, P.; Kowalczewski, A.; Sun, S.; Winston, T.S.; Archilla, A.M.; Lemus, S.M.; Ercan-Sencicek, A.G.; Gupta, A.R.; Liu, W.; Kontaridis, M.I.; et al. Engineering spatial-organized cardiac organoids for developmental toxicity testing. Stem Cell Rep. 2021, 16, 1228–1244. [Google Scholar] [CrossRef]
- Engel, J.L.; Zhang, X.; Lu, D.R.; Vila, O.F.; Arias, V.; Lee, J.; Hale, C.; Hsu, Y.-H.; Li, C.-M.; Wu, R.S.; et al. Single Cell Multi-Omics of an iPSC Model of Human Sinoatrial Node Development Reveals Genetic Determinants of Heart Rate and Arrhythmia Susceptibility. bioRxiv 2023. [Google Scholar] [CrossRef]
- Litviňuková, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Worth, C.L.; Lindberg, E.L.; Kanda, M.; Polanski, K.; Heinig, M.; Lee, M.; et al. Cells of the adult human heart. Nature 2020, 588, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Kanemaru, K.; Cranley, J.; Muraro, D.; Miranda, A.M.A.; Ho, S.Y.; Wilbrey-Clark, A.; Patrick Pett, J.; Polanski, K.; Richardson, L.; Litvinukova, M.; et al. Spatially resolved multiomics of human cardiac niches. Nature 2025, 619, 801–810, Correction in Nature 2025, 640, E4. [Google Scholar] [CrossRef] [PubMed]
- van Eif, V.W.W.; Devalla, H.D.; Boink, G.J.J.; Christoffels, V.M. Transcriptional regulation of the cardiac conduction system. Nat. Rev. Cardiol. 2018, 15, 617–630. [Google Scholar] [CrossRef]
- Friedman, C.E.; Nguyen, Q.; Lukowski, S.W.; Helfer, A.; Chiu, H.S.; Miklas, J.; Levy, S.; Suo, S.; Han, J.D.J.; Osteil, P.; et al. Single-Cell Transcriptomic Analysis of Cardiac Differentiation from Human PSCs Reveals HOPX-Dependent Cardiomyocyte Maturation. Cell Stem Cell 2018, 23, 586–598.e8. [Google Scholar] [CrossRef]
- Li, L.; Lu, M.; Guo, L.; Zhang, X.; Liu, Q.; Zhang, M.; Gao, J.; Xu, M.; Lu, Y.; Zhang, F.; et al. An organ-wide spatiotemporal transcriptomic and cellular atlas of the regenerating zebrafish heart. Nat. Commun. 2025, 16, 3716. [Google Scholar] [CrossRef]
- Cyganek, L.; Tiburcy, M.; Sekeres, K.; Gerstenberg, K.; Bohnenberger, H.; Lenz, C.; Henze, S.; Stauske, M.; Salinas, G.; Zimmermann, W.H.; et al. Deep phenotyping of human induced pluripotent stem cell-derived atrial and ventricular cardiomyocytes. JCI Insight 2018, 3, e99941. [Google Scholar] [CrossRef]
- Zafar, I.; Anwar, S.; kanwal, F.; Yousaf, W.; Nisa, F.U.; Kausar, T.; ul Ain, Q.; Unar, A.; Kamal, M.A.; Rashid, S.; et al. Reviewing methods of deep learning for intelligent healthcare systems in genomics and biomedicine. Biomed. Signal Process. Control 2023, 86, 105263. [Google Scholar] [CrossRef]
- John, C.; Sahoo, J.; Madhavan, M.; Mathew, O.K. Convolutional Neural Networks: A Promising Deep Learning Architecture for Biological Sequence Analysis. Curr. Bioinform. 2023, 18, 537–558. [Google Scholar] [CrossRef]
- Naseer, S.; Ali, R.F.; Khan, Y.D.; Dominic, D.D. iGluK-Deep: Computational identification of lysine glutarylation sites using deep neural networks with general pseudo amino acid compositions. J. Biomol. Struct. Dyn. 2022, 40, 11691–11704. [Google Scholar] [CrossRef] [PubMed]
- Pimpalkar, A.; Gandhewar, N.; Shelke, N.; Patil, S.; Chhabria, S. An Efficient Deep Convolutional Neural Networks Model for Genomic Sequence Classification. In Genomics at the Nexus of AI, Computer Vision, and Machine Learning; Wiley: Hoboken, NJ, USA, 2024; pp. 345–375. [Google Scholar] [CrossRef]
- Khandelwal, R.; Nayarisseri, A.; Khandelwal, R.; Nayarisseri, A. A Machine learning approach for the prediction of efficient iPSC modeling. In MOL2NET’19, Conference on Molecular, Biomed., Comput. & Network Science and Engineering, 5th ed.; MDPI: Basel, Switzerland, 2019; p. 6376. [Google Scholar] [CrossRef]
- Matray, V.; Amlani, F.; Feyel, F.; Néron, D. A hybrid numerical methodology coupling Reduced Order Modeling and Graph Neural Networks for non-parametric geometries: Applications to structural dynamics problems. arXiv 2024. [Google Scholar] [CrossRef]
- Zhou, J.; Cui, G.; Hu, S.; Zhang, Z.; Yang, C.; Liu, Z.; Wang, L.; Li, C.; Sun, M. Graph neural networks: A review of methods and applications. AI Open 2020, 1, 57–81. [Google Scholar] [CrossRef]
- Wu, Z.; Pan, S.; Chen, F.; Long, G.; Zhang, C.; Yu, P.S. A Comprehensive Survey on Graph Neural Networks. IEEE Trans. Neural Netw. Learn. Syst. 2021, 32, 4–24. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Zhang, T.; Yang, B.; Su, J.; Song, Q. spaCI: Deciphering spatial cellular communications through adaptive graph model. Brief. Bioinform. 2023, 24, bbac563. [Google Scholar] [CrossRef]
- Li, S.; Hua, H.; Chen, S. Graph neural networks for single-cell omics data: A review of approaches and applications. Brief. Bioinform. 2025, 26, bbaf109. [Google Scholar] [CrossRef]
- Song, T.; Cosatto, E.; Wang, G.; Kuang, R.; Gerstein, M.; Min, M.R.; Warrell, J. Predicting spatially resolved gene expression via tissue morphology using adaptive spatial GNNs. Bioinformatics 2024, 40, ii111–ii119. [Google Scholar] [CrossRef]
- Yang, Y.; Hossain, M.Z.; Stone, E.; Rahman, S. Spatial transcriptomics analysis of gene expression prediction using exemplar guided graph neural network. Pattern Recognit. 2023, 145, 109966. [Google Scholar] [CrossRef]
- Li, Y.; Luo, Y. STdGCN: Spatial transcriptomic cell-type deconvolution using graph convolutional networks. Genome Biol. 2024, 25, 206. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-Y.; van Baar, J.; Wittenburg, K.; Sullivan, A. Analysis of the contribution and temporal dependency of LSTM layers for reinforcement learning tasks. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR) Explanable AI Workshop, Long Beach, CA, USA, 16–20 June 2019; pp. 99–102. [Google Scholar]
- Gao, Y.; Lewis, N.; Calhoun, V.D.; Miller, R.L. Interpretable LSTM model reveals transiently-realized patterns of dynamic brain connectivity that predict patient deterioration or recovery from very mild cognitive impairment. Comput. Biol. Med. 2023, 161, 107005. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.; Smith, S.; Khan, Y.M.; Langabeer, J.R.; Foraker, R.E. Application of a time-series deep learning model to predict cardiac dysrhythmias in electronic health records. PLoS ONE 2021, 16, e0239007. [Google Scholar] [CrossRef]
- Boll, H.O.; Amirahmadi, A.; Soliman, A.; Byttner, S.; Recamonde-Mendoza, M. Graph Neural Networks for Heart Failure Prediction on an EHR-Based Patient Similarity Graph. In Proceedings of the Anais Estendidos do XXV Simpósio Brasileiro de Computação Aplicada à Saúde, Porto Alegre, Brazil, 9–13 June 2025; pp. 121–126. [Google Scholar] [CrossRef]
- Teja, M.D.; Rayalu, G.M. Optimizing heart disease diagnosis with advanced machine learning models: A comparison of predictive performance. BMC Cardiovasc. Disord. 2025, 25, 212. [Google Scholar] [CrossRef]
- Wani, S.A.; Khan, S.A.; Quadri, S.M.K. Application of Deep Learning for Single Cell Multi-Omics: A State-of-the-Art Review. Arch. Comput. Methods Eng. 2025, 32, 2987–3029. [Google Scholar] [CrossRef]
- Molho, D.; Ding, J.; Tang, W.; Li, Z.; Wen, H.; Wang, Y.; Venegas, J.; Jin, W.; Liu, R.; Su, R.; et al. Deep Learning in Single-cell Analysis. ACM Trans. Intell. Syst. Technol. 2024, 15, 1–62. [Google Scholar] [CrossRef]
- Alrashdi, I.; Taloba, A.I. Integration of graph neural networks and long short-term memory models for advancing heart failure prediction. Alex. Eng. J. 2025, 127, 143–163. [Google Scholar] [CrossRef]
- Babichev, S.; Liakh, I.; Kalinina, I. Applying a Recurrent Neural Network-Based Deep Learning Model for Gene Expression Data Classification. Appl. Sci. 2023, 13, 11823. [Google Scholar] [CrossRef]
- Monti, M.; Fiorentino, J.; Milanetti, E.; Gosti, G.; Tartaglia, G.G. Prediction of Time Series Gene Expression and Structural Analysis of Gene Regulatory Networks Using Recurrent Neural Networks. Entropy 2022, 24, 141. [Google Scholar] [CrossRef]
- Ma, T.; Wang, J. GraphPath: A graph attention model for molecular stratification with interpretability based on the pathway-pathway interaction network. Bioinformatics 2024, 40, btae165. [Google Scholar] [CrossRef]
- Prakash, A.; Banerjee, M. An interpretable block-attention network for identifying regulatory feature interactions. Brief. Bioinform. 2023, 24, bbad250. [Google Scholar] [CrossRef]
- Choi, S.R.; Lee, M. Transformer Architecture and Attention Mechanisms in Genome Data Analysis: A Comprehensive Review. Biology 2023, 12, 1033. [Google Scholar] [CrossRef]
- Bao, L.-L.; Zhang, C.-X.; Zhang, J.-S.; Guo, R. A two-stage spatial prediction modeling approach based on graph neural networks and neural processes. Expert. Syst. Appl. 2024, 258, 125173. [Google Scholar] [CrossRef]
- Kaadoud, I.C.; Rougier, N.P.; Alexandre, F. Knowledge extraction from the learning of sequences in a long short term memory (LSTM) architecture. Knowl. Based Syst. 2022, 235, 107657. [Google Scholar] [CrossRef]
- Rajabi, E.; Etminani, K. Knowledge-graph-based explainable AI: A systematic review. J. Inf. Sci. 2024, 50, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.T.; Kuintzle, R.; Teegarden, A.; Merrill, E.; Danaee, P.; Hendrix, D.A. A deep recurrent neural network discovers complex biological rules to decipher RNA protein-coding potential. Nucleic Acids Res. 2018, 46, 8105–8113. [Google Scholar] [CrossRef]
- Gillani, M.; Pollastri, G. Protein subcellular localization prediction tools. Comput. Struct. Biotechnol. J. 2024, 23, 1796–1807. [Google Scholar] [CrossRef] [PubMed]
- Waikhom, L.; Patgiri, R. A survey of graph neural networks in various learning paradigms: Methods, applications, and challenges. Artif. Intell. Rev. 2023, 56, 6295–6364. [Google Scholar] [CrossRef]
- Min, S.; Gao, Z.; Peng, J.; Wang, L.; Qin, K.; Fang, B. STGSN—A Spatial–Temporal Graph Neural Network framework for time-evolving social networks. Knowl. Based Syst. 2021, 214, 106746. [Google Scholar] [CrossRef]
- Sathyan, A.; Weinberg, A.I.; Cohen, K. Interpretable AI for bio-medical applications. Complex Eng. Syst. 2022, 2, 18. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.M.; Rajesh, A.; Asaad, M.; Nelson, J.A.; Coert, J.H.; Mehrara, B.J.; Butler, C.E. A Surgeon’s Guide to Artificial Intelligence-Driven Predictive Models. Am. Surg. 2023, 89, 11–19. [Google Scholar] [CrossRef]
- Vujović, Ž. Classification Model Evaluation Metrics. Int. J. Adv. Comput. Sci. Appl. 2021, 12, 599–606. [Google Scholar] [CrossRef]
- Rajkomar, A.; Dai, A.M.; Sun, M.; Hardt, M.; Chen, K.; Rough, K.; Dean, J. Reply: Metrics to assess machine learning models. npj Digit. Med. 2018, 1, 57. [Google Scholar] [CrossRef]
- Long, Y.; Ang, K.S.; Sethi, R.; Liao, S.; Heng, Y.; van Olst, L.; Ye, S.; Zhong, C.; Xu, H.; Zhang, D.; et al. Deciphering spatial domains from spatial multi-omics with SpatialGlue. Nat. Methods 2024, 21, 1658–1667. [Google Scholar] [CrossRef]
- Xu, N.; Kosma, C.; Vazirgiannis, M. TimeGNN: Temporal Dynamic Graph Learning for Time Series Forecasting. In Complex Networks & Their Applications XII; Cherifi, H., Rocha, L.M., Cherifi, C., Donduran, M., Eds.; Springer Nature Switzerland: Cham, Switzerland, 2024; pp. 87–99. [Google Scholar]
- Rossi, E.; Chamberlain, B.; Frasca, F.; Eynard, D.; Monti, F.; Bronstein, M. Temporal Graph Networks for Deep Learning on Dynamic Graphs. arXiv 2020, arXiv:2006.10637. [Google Scholar] [CrossRef]
- Pan, Q.; Hu, W.; Zhu, J. Series saliency: Temporal interpretation for multivariate time series forecasting. arXiv 2020, arXiv:2012.09324. [Google Scholar] [CrossRef]
- Katrompas, A.; Metsis, V. Temporal Attention Signatures for Interpretable Time-Series Prediction. In Artificial Neural Networks and Machine Learning—ICANN 2023; Iliadis, L., Papaleonidas, A., Angelov, Jayne, C., Eds.; Springer Nature Switzerland: Cham, Switzerland, 2023; pp. 268–280. [Google Scholar]
- Tian, T.; Wan, J.; Song, Q.; Wei, Z. Clustering single-cell RNA-seq data with a model-based deep learning approach. Nat. Mach. Intell. 2019, 1, 191–198. [Google Scholar] [CrossRef]
- Collin, C.B.; Gebhardt, T.; Golebiewski, M.; Karaderi, T.; Hillemanns, M.; Khan, F.M.; Salehzadeh-Yazdi, A.; Kirschner, M.; Krobitsch, S.; Kuepfer, L. Computational Models for Clinical Applications in Personalized Medicine—Guidelines and Recommendations for Data Integration and Model Validation. J. Pers. Med. 2022, 12, 166. [Google Scholar] [CrossRef]
- Overbey, E.G.; Das, S.; Cope, H.; Madrigal, P.; Andrusivova, Z.; Frapard, S.; Klotz, R.; Bezdan, D.; Gupta, A.; Scott, R.T.; et al. Challenges and considerations for single-cell and spatially resolved transcriptomics sample collection during spaceflight. Cell Rep. Methods 2022, 2, 100325. [Google Scholar] [CrossRef] [PubMed]
- Baghdassarian, H.M.; Dimitrov, D.; Armingol, E.; Saez-Rodriguez, J.; Lewis, N.E. Combining LIANA and Tensor-cell2cell to decipher cell-cell communication across multiple samples. Cell Rep. Methods 2024, 4, 100758. [Google Scholar] [CrossRef]
- Ge, Z.; Hou, J.; Nayak, A. GNN-based End-to-end Delay Prediction in Software Defined Networking. In Proceedings of the 18th Annual International Conference on Distributed Computing in Sensor Systems, DCOSS 2022, Los Angeles, CA, USA, 30 May–1 June 2022; pp. 372–378. [Google Scholar] [CrossRef]
- Ying, R.; Bourgeois, D.; You, J.; Zitnik, M.; Leskovec, J. GNNExplainer: Generating Explanations for Graph Neural Networks. Adv. Neural Inf. Process Syst. 2019, 32, 9240–9251. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7138248/ (accessed on 11 August 2025).
- Cho, K.; Van Merriënboer, B.; Gulcehre, C.; Bahdanau, D.; Bougares, F.; Schwenk, H.; Bengio, Y. Learning phrase representations using RNN encoder-decoder for statistical machine translation. In Proceedings of the EMNLP 2014—2014 Conference on Empirical Methods in Natural Language Processing, Proceedings of the Conference, Doha, Qatar, 25–29 October 2014; Association for Computational Linguistics (ACL): Baltimore, MD, USA, 2014; pp. 1724–1734. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Gravier, G.; Sebillot, P. A Study of the Plausibility of Attention between RNN Encoders in Natural Language Inference. In Proceedings of the 20th IEEE International Conference on Machine Learning and Applications, ICMLA 2021, Pasadena, CA, USA, 13–16 December 2021; pp. 1623–1629. [Google Scholar] [CrossRef]
- Zhao, M.; He, W.; Tang, J.; Zou, Q.; Guo, F. A hybrid deep learning framework for gene regulatory network inference from single-cell transcriptomic data. Brief. Bioinform. 2022, 23, bbab568. [Google Scholar] [CrossRef]
- Liu, S.; Li, T.; Ding, H.; Tang, B.; Wang, X.; Chen, Q.; Yan, J.; Zhou, Y. A hybrid method of recurrent neural network and graph neural network for next-period prescription prediction. Int. J. Mach. Learn. Cybern. 2020, 11, 2849–2856. [Google Scholar] [CrossRef]
- Dobner, J.; Diecke, S.; Krutmann, J.; Prigione, A.; Rossi, A. Reassessment of marker genes in human induced pluripotent stem cells for enhanced quality control. Nat. Commun. 2024, 15, 8547. [Google Scholar] [CrossRef]
- Bargaje, R.; Trachana, K.; Shelton, M.N.; McGinnis, C.S.; Zhou, J.X.; Chadick, C.; Cook, S.; Cavanaugh, C.; Huang, S.; Hood, L. Cell population structure prior to bifurcation predicts efficiency of directed differentiation in human induced pluripotent cells. Proc. Natl. Acad. Sci. USA 2017, 114, 2271–2276. [Google Scholar] [CrossRef]
- Shang, Y.; Jiang, K.; Wang, L.; Zhang, Z.; Zhou, S.; Liu, Y.; Dong, J.; Wu, H. The 30-days hospital readmission risk in diabetic patients: Predictive modeling with machine learning classifiers. BMC Med. Inform. Decis. Mak. 2021, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Löbel, W.; Finklea, F.; Halloin, C.; Ritzenhoff, K.; Manstein, F.; Mohammadi, S.; Hashemi, M.; Zweigerdt, R.; Lipke, E.; et al. Prediction of Human Induced Pluripotent Stem Cell Cardiac Differentiation Outcome by Multifactorial Process Modeling. Front. Bioeng. Biotechnol. 2020, 8, 557784. [Google Scholar] [CrossRef]
- Dimitriadis, T.; Gneiting, T.; Jordan, A.I. Stable reliability diagrams for probabilistic classifiers. Proc. Natl. Acad. Sci. USA 2021, 118, e2016191118. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Hashemi, M.; Finklea, F.B.; Lipke, E.A.; Cremaschi, S. Differentiating Engineered Tissue Images and Experimental Factors to Classify Cardiomyocyte Content. Tissue Eng. Part A 2023, 29, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Park, H.J.; Kim, M.S. Spatial Transcriptomics in Thyroid Cancer: Applications, Limitations, and Future Perspectives. Cells 2025, 14, 936. [Google Scholar] [CrossRef]
- Choe, K.; Pak, U.; Pang, Y.; Hao, W.; Yang, X. Advances and Challenges in Spatial Transcriptomics for Developmental Biology. Biomolecules 2023, 13, 156. [Google Scholar] [CrossRef] [PubMed]
- Park, H.E.; Jo, S.H.; Lee, R.H.; Macks, C.P.; Ku, T.; Park, J.; Lee, C.W.; Hur, J.K.; Sohn, C.H. Spatial Transcriptomics: Technical Aspects of Recent Developments and Their Applications in Neuroscience and Cancer Research. Adv. Sci. 2023, 10, 2206939. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Jin, K.; Yao, Y.; Jin, L.; Shao, X.; Li, C.; Lu, X.; Fan, X. Spatial integration of multi-omics single-cell data with SIMO. Nat. Commun. 2025, 16, 1265. [Google Scholar] [CrossRef]
- Vo, T.; Prakrithi, P.; Jones, K.; Yoon, S.; Lam, P.Y.; Kao, Y.C.; Ma, N.; Tan, S.X.; Jin, X.; Zhou, C.; et al. Assessing spatial sequencing and imaging approaches to capture the molecular and pathological heterogeneity of archived cancer tissues. J. Pathol. 2025, 265, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhou, M.; Gao, B.; Wang, F.; Jin, S.; Liu, Q.; Wang, G. stGRL: Spatial domain identification, denoising, and imputation algorithm for spatial transcriptome data based on multi-task graph contrastive representation learning. BMC Biol. 2025, 23, 177. [Google Scholar] [CrossRef]
- Mulvey, J.F.; Meyer, E.L.; Svenningsen, M.S.; Lundby, A. Integrating -Omic Technologies across Modality, Space, and Time to Decipher Remodeling in Cardiac Disease. Curr. Cardiol. Rep. 2025, 27, 74. [Google Scholar] [CrossRef]
- Patkar, S.; Rosean, T.R.; Patel, P.; Harmon, S.; Choyke, P.; Jamaspishvili, T.; & Turkbey, B. Towards interpretable molecular and spatial analysis of the tumor microenvironment from digital histopathology images with HistoTME-v2. bioRxiv 2025. [Google Scholar] [CrossRef]
- Dixit, S.; Suryawanshi, S.J.; Arora, S.; Sharma, N.; Shukla, V.K. A Comprehensive Review on the Good Manufacturing Practices Standards: Directive 91/356 of the European Commission. In Understanding Pharmaceutical Standards and Regulations; Routledge: Oxfordshire, UK, 2025; pp. 20–39. [Google Scholar] [CrossRef]
- Miyagawa, S.; Kawamura, T.; Ito, E.; Takeda, M.; Iseoka, H.; Yokoyama, J.; Harada, A.; Mochizuki-Oda, N.; Imanishi-Ochi, Y.; Li, J.; et al. Pre-clinical evaluation of the efficacy and safety of human induced pluripotent stem cell-derived cardiomyocyte patch. Stem Cell Res. Ther. 2024, 15, 1–15. [Google Scholar] [CrossRef]
- Rasekh, M.; Arshad, M.S.; Ahmad, Z. Advances in Drug Delivery Integrated with Regenerative Medicine: Innovations, Challenges, and Future Frontiers. Pharmaceutics 2025, 17, 456. [Google Scholar] [CrossRef]
- Sabatino, G.; Mazzucchi, E.; Toader, C.; Covache-Busuioc, R.-A. Precision Neuro-Oncology in Glioblastoma: AI-Guided CRISPR Editing and Real-Time Multi-Omics for Genomic Brain Surgery. Int. J. Mol. Sci. 2025, 26, 7364. [Google Scholar] [CrossRef]
- Keeling, P.; Clark, J.; Finucane, S. Challenges in the clinical implementation of precision medicine companion diagnostics. Expert. Rev. Mol. Diagn. 2020, 20, 593–599. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kgabeng, T.; Wang, L.; Ngwangwa, H.M.; Pandelani, T. Integrating Spatial Omics and Deep Learning: Toward Predictive Models of Cardiomyocyte Differentiation Efficiency. Bioengineering 2025, 12, 1037. https://doi.org/10.3390/bioengineering12101037
Kgabeng T, Wang L, Ngwangwa HM, Pandelani T. Integrating Spatial Omics and Deep Learning: Toward Predictive Models of Cardiomyocyte Differentiation Efficiency. Bioengineering. 2025; 12(10):1037. https://doi.org/10.3390/bioengineering12101037
Chicago/Turabian StyleKgabeng, Tumo, Lulu Wang, Harry M. Ngwangwa, and Thanyani Pandelani. 2025. "Integrating Spatial Omics and Deep Learning: Toward Predictive Models of Cardiomyocyte Differentiation Efficiency" Bioengineering 12, no. 10: 1037. https://doi.org/10.3390/bioengineering12101037
APA StyleKgabeng, T., Wang, L., Ngwangwa, H. M., & Pandelani, T. (2025). Integrating Spatial Omics and Deep Learning: Toward Predictive Models of Cardiomyocyte Differentiation Efficiency. Bioengineering, 12(10), 1037. https://doi.org/10.3390/bioengineering12101037









