A Dual-Thread Lag–Locking Screw Enhances Single Lateral Plate Fixation in Bicondylar Tibial Plateau Fractures: A Biomechanical Study
Abstract
1. Introduction
2. Materials and Methods
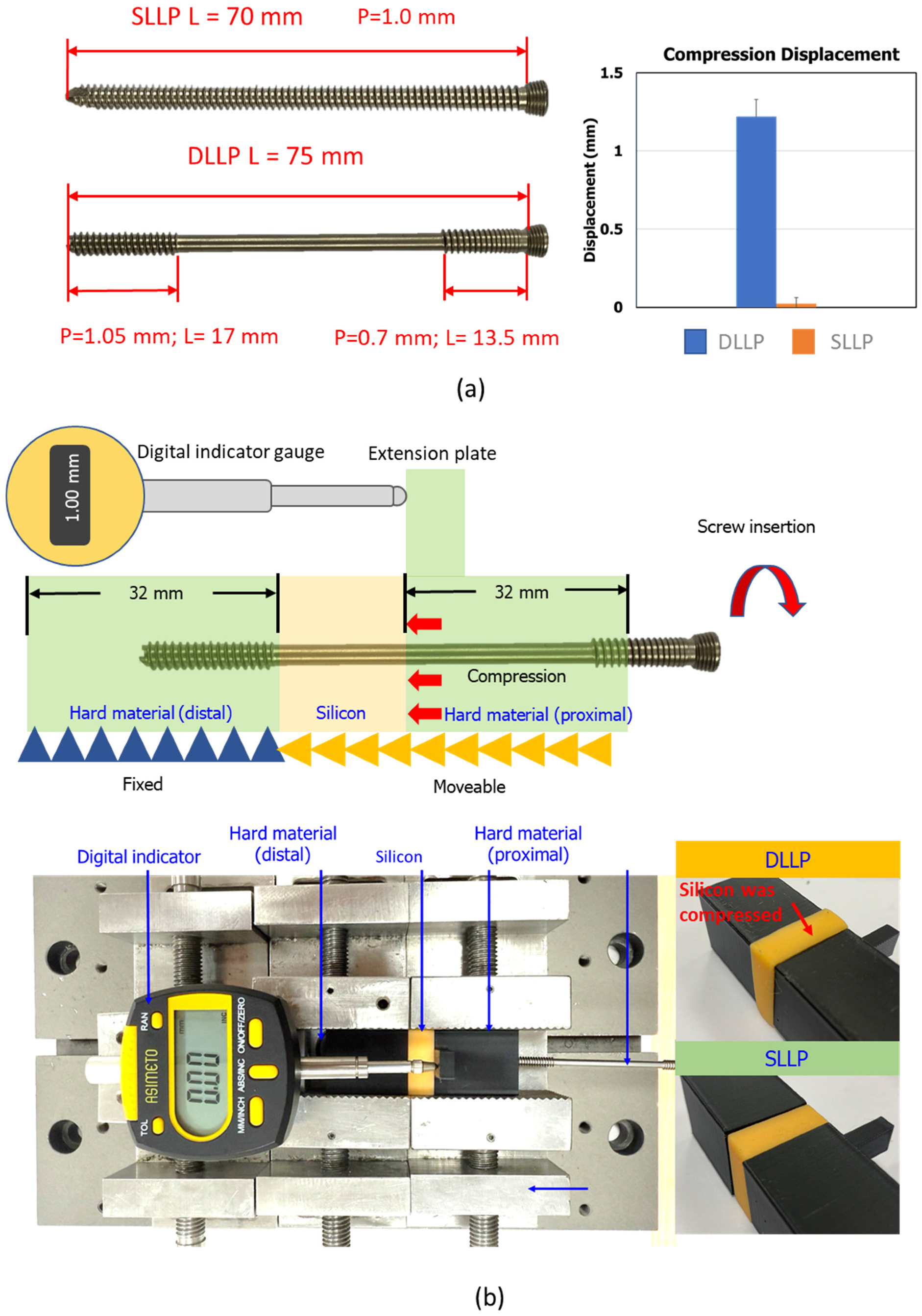
2.1. Compression Capability Evaluation of Screw Insertion
2.2. Fabrication of Biomechanical Test Specimens for Tibial Plateau Fracture Reduction
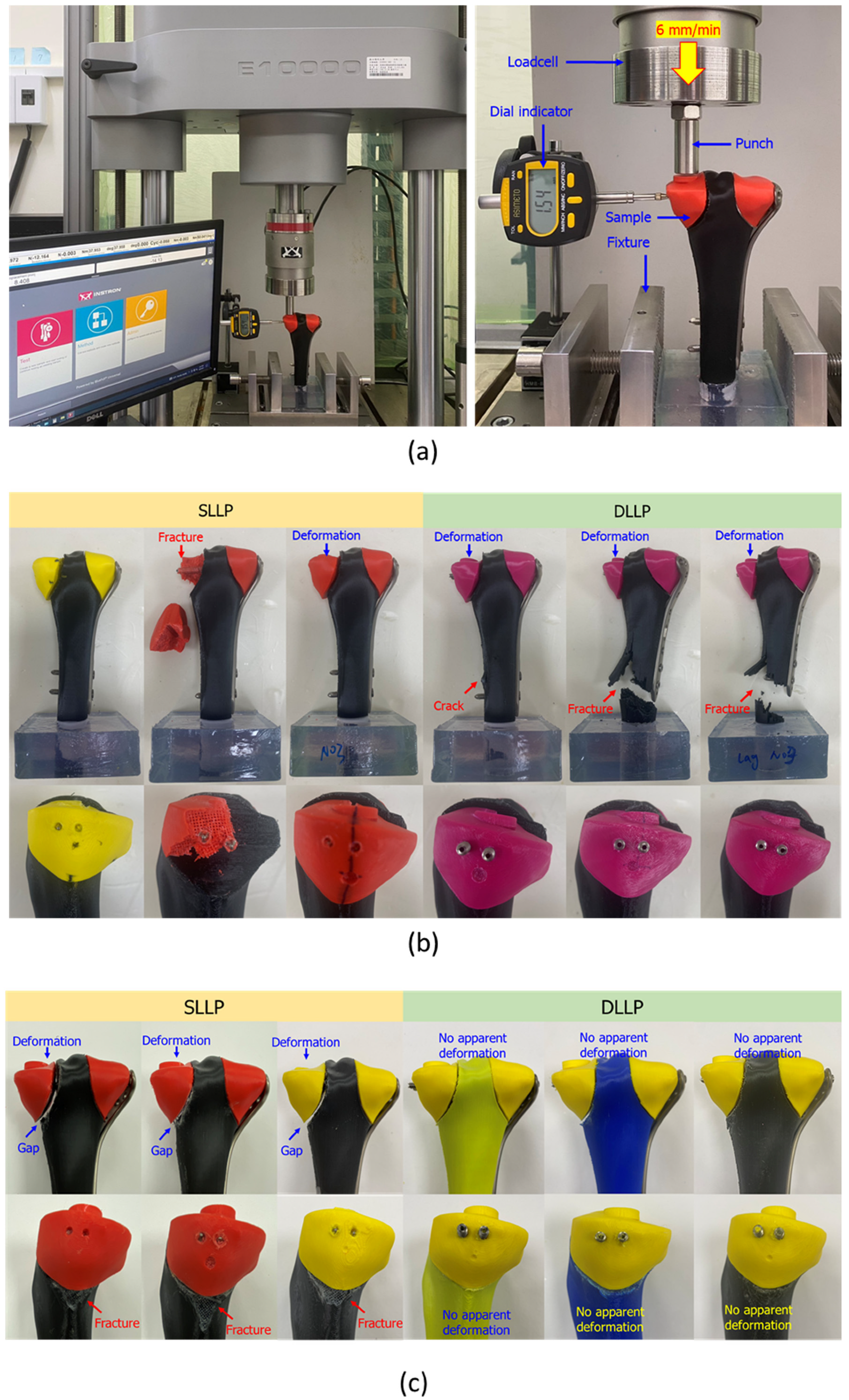
2.3. Static and Dynamic Biomechanical Tests of Tibial Plateau Fracture Reduction
3. Results
4. Discussion
5. Conclusions
- Immediate compression: DLLP generated measurable interfragmentary compression during screw insertion (mean displacement: 1.22 ± 0.11 mm) compared with negligible compression for SLLP (0.02 ± 0.04 mm, p < 0.05).
- Static strength: DLLP exhibited a significantly greater maximum failure force (7801.51 ± 358.95 N) than SLLP (6224.84 ± 411.20 N, p < 0.05) and higher resistance to lateral displacement at 2 mm (3394.85 ± 392.81 N vs. 2766.36 ± 64.51 N, p = 0.03).
- Fatigue resistance: All DLLP constructs withstood 1,000,000 cycles, with displacement maintained below the clinical threshold, while all SLLP constructs failed prematurely, with a mean fatigue life of 408,679 ± 128,286 cycles.
- Failure modes: SLLP tended to cause medial fragment subsidence or fragmentation, whereas DLLP, due to its greater stability, transferred failure to the distal tibial shaft, reflecting improved medial support.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Millar, S.C.; Arnold, J.B.; Thewlis, D.; Fraysse, F.; Solomon, L.B. A systematic literature review of tibial plateau fractures: What classifications are used and how reliable and useful are they? Injury 2018, 49, 473–490. [Google Scholar] [CrossRef] [PubMed]
- Mthethwa, J.; Chikate, A. A review of the management of tibial plateau fractures. Musculoskelet. Surg. 2018, 102, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.K.; Cooper, S.A.; Collinge, C. Bicondylar tibial plateau fractures: A critical analysis review. JBJS Rev. 2018, 6, e4. [Google Scholar] [CrossRef] [PubMed]
- Samsami, S.; Pätzold, R.; Neuy, T.; Greinwald, M.; Müller, P.E.; Chevalier, Y.; Püschel, K.; Augat, P. Biomechanical Comparison of 2 Double Plating Methods in a Coronal Fracture Model of Bicondylar Tibial Plateau Fractures. J. Orthop. Trauma 2022, 36, e129–e135. [Google Scholar] [CrossRef]
- Wei, G.; Niu, X.; Li, Y.; Chang, T.; Zhang, J.; Wang, H.; Li, X.; He, Y.; Wang, R.; Tian, F.; et al. Biomechanical analysis of internal fixation system stability for tibial plateau fractures. Front. Bioeng. Biotechnol. 2023, 11, 1199944. [Google Scholar] [CrossRef]
- Çağlar, C.; Akcaalan, S.; Özaslan, H.; Bozer, M.; Emre, F.; Uğurlu, M. Comparative Analysis of Single Lateral Locked Plate and Double Locked Plate Application in the Treatment of Bicondylar Tibial Plateau Fractures. Cureus 2021, 13, e19298. [Google Scholar] [CrossRef]
- Raj, M.; Singh, S.K.; Rajput, A.K.; Gill, S.P.; Verma, S.K.; Sonarkar, S.S. The Comparative Analysis of Single Plating Versus Double Plating in the Treatment of Unstable Bicondylar Proximal Tibial Plateau Fractures. Cureus 2023, 15, e46840. [Google Scholar] [CrossRef]
- Chang, H.; Zhu, Y.; Zheng, Z.; Chen, W.; Zhao, S.; Zhang, Y.; Zhang, Y. Meta-analysis shows that highly comminuted bicondylar tibial plateau fractures treated by single lateral locking plate give similar outcomes as dual plate fixation. Int. Orthop. 2016, 40, 2129–2141. [Google Scholar] [CrossRef]
- Hung, C.Y.; Shen, P.Y.; Chen, J.C.; Su, Y.D.; Lee, T.C. Comparison of functional outcomes of unilateral periarticular locking plate, hybrid dual plates, and classic dual buttress plates in the treatment of bicondylar tibial plateau fractures. FJMD 2024, 15, 125–130. [Google Scholar] [CrossRef]
- Kumar, V.; Singhroha, M.; Arora, K.; Sahu, A.; Beniwal, R.; Kundu, A. A clinico-radiological study of bicondylar tibial plateau fractures managed with dual locking plates. J. Clin. Orthop. Trauma 2021, 21, 101563. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Tian, S.; Tan, Z.; Deng, X.; Zhao, K.; Zheng, Z.; Chen, W.; Hou, Z.; Zhang, Y. Dual plating or dual plating combined with compression bolts for bicondylar tibial plateau fractures: A retrospective comparative study. Sci. Rep. 2021, 11, 7768. [Google Scholar] [CrossRef] [PubMed]
- Vélez, D.A.G.; Headford, M.; Suresh, K.V.; Liberatos, P.M.; Bledsoe, G.; Revak, T. Biomechanical analysis of dual versus lateral locked plating in elderly bicondylar tibial plateau fractures: Does medial comminution matter? Injury 2022, 53, 3109–3114. [Google Scholar] [CrossRef] [PubMed]
- Citak, C.; Kayali, C.; Ozan, F.; Altay, T.; Karahan, H.G.; Yamak, K. Lateral locked plating or dual plating: A comparison of two methods in simple bicondylar tibial plateau fractures. Clin. Orthop. Surg. 2019, 11, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Misaghi, A.; Doan, J.; Bastrom, T.; Pennock, A.T. Biomechanical Evaluation of Plate Versus Lag Screw Only Fixation of Distal Fibula Fractures. J. Foot Ankle Surg. 2015, 54, 896–899. [Google Scholar] [CrossRef]
- Chatain, G.P.; Oldham, A.; Uribe, J.; Duhon, B.; Gardner, M.J.; Witt, J.P.; Yerby, S.; Kelly, B.P. Biomechanics of sacroiliac joint fixation using lag screws: A cadaveric study. J. Orthop. Surg. Res. 2023, 18, 807. [Google Scholar] [CrossRef]
- Maher, M.; Baldini, T.H.; Parry, J.A.; Mauffrey, C. The potential biomechanical advantage of lag by technique screw fixation of the posterior pelvic ring. Eur. J. Orthop. Surg. Traumatol. 2020, 30, 1045–1048. [Google Scholar] [CrossRef]
- Sevencan, A.; Şenol, M.S.; Mısır, A.; Aycan, O.E.; Albayrak, A.; Uçpunar, H. Comparison of cannulated lag screws and lateral locking plate in the treatment of Schatzker type II tibial plateau fractures. Jt. Dis. Relat. Surg. 2020, 31, 130–136. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J.; Wang, H.; He, L.; Huang, B.; Dadbakhsh, S.; Bartolo, P. Fabrication and development of mechanical metamaterials via additive manufacturing for biomedical applications: A review. Int. J. Extrem. Manuf. 2025, 7, 012001. [Google Scholar] [CrossRef]
- Bernadi-Forteza, A.; Mallon, M.; Velasco-Gallego, C.; Cubo-Mateo, N. A Systematic Review on the Generation of Organic Structures through Additive Manufacturing Techniques. Polymers 2024, 16, 2027. [Google Scholar] [CrossRef]
- Shi, Q.; Chen, J.; Chen, J.; Liu, Y.; Wang, H. Application of additively manufactured bone scaffold: A systematic review. Biofabrication 2024, 16, 022007. [Google Scholar] [CrossRef]
- Wu, P.K.; Tsai, W.C.; Wang, H.W.; Lin, C.L. Establishment of design guidelines for patient-specific 3D-printed reconstruction implants for large distal femoral defects. Mater. Des. 2025, 251, 113668. [Google Scholar]
- Huang, T.H.; Huang, S.F.; Yu, L.Y.; Lo, C.L.; Chang, Y.P.; Lin, C.L. Novel patient-specific gingival soft-tissue expander development for large bone defects using silicone 3D-printing technology. Int. J. Bioprint. 2024, 10, 2918. [Google Scholar]
- Wang, H.W.; Chen, C.H.; Chen, K.H.; Zeng, Y.H.; Lin, C.L. Designing a 3D-printed medical implant with mechanically macrostructural topology and microbionic lattices: A novel wedge-shaped spacer for high tibial osteotomy and biomechanical study. Int. J. Bioprint. 2024, 10, 1584. [Google Scholar]
- ISO 527-1:2019; Plastics—Determination of Tensile Properties. Part 1: General Principles. ISO: Geneva, Switzerland, 2019.
- Jiang, J.; Xu, D.; Ji, Z.; Wang, F.; Jia, R.; Wang, J.; Hong, H.; Zhang, H.; Li, J. Application of a combined cancellous lag screw enhances the stability of locking plate fixation of osteoporotic lateral tibial plateau fracture by providing interfragmentary compression force. J. Orthop. Surg. Res. 2024, 19, 139. [Google Scholar] [CrossRef] [PubMed]
- Wehner, T.; Claes, L.; Simon, U. Internal loads in the human tibia during gait. Clin. Biomech. 2009, 24, 299–302. [Google Scholar] [CrossRef]
- Griffin, L.V.; Harris, R.M.; Zubak, J.J. Fatigue strength of common tibial intramedullary nail distal locking screws. J. Orthop. Surg. Res. 2009, 4, 11. [Google Scholar] [CrossRef]
- Diffo, K.; Maas, S.; Waldmann, D.; Zilian, A.; Dueck, K.; Pape, D. Biomechanical properties of five different currently used implants for open-wedge high tibial osteotomy. J. Exp. Orthop. 2015, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Nägl, K.; Reisinger, A.; Pahr, D.H. The biomechanical behavior of 3D printed human femoral bones based on generic and patient-specific geometries. 3D Print Med. 2022, 8, 35. [Google Scholar] [PubMed]
- Ciklaçandır, S.; Mihçin, S.; İsler, Y. Detailed Investigation of Three-Dimensional Modeling and Printing Technologies from Medical Images to Analyze Femoral Head Fractures Using Finite Element Analysis. IRBM 2022, 43, 604–613. [Google Scholar] [CrossRef]
- Liao, C.Y.; Huang, S.F.; Tsai, W.C.; Chang, C.Y.; Lin, C.L. Biomechanical comparison of using traditional and 3D-printed titanium alloy anatomical assembly thin anterior plating under three patellar fracture conditions. Virtual Phys. Prototyp. 2024, 19, e2404982. [Google Scholar] [CrossRef]


| Static Test | Max. Force (N) | Axial 2 mm Disp. Force (N) | Lateral 2 mm Disp. Force (N) |
|---|---|---|---|
| SLLP-No1 | 6674.59 | 1404.80 | 2828.10 |
| SLLP-No2 | 6131.82 | 967.47 | 2699.40 |
| SLLP-No3 | 5868.12 | 1615.24 | 2771.57 |
| Average (Std.) | 6224.84 (411.20) | 1329.17 (330.44) | 2766.36 (64.51) |
| DLLP-No1 | 8161.21 | 1549.31 | 3083.02 |
| DLLP-No2 | 7800.00 | 1426.13 | 3265.50 |
| DLLP-No3 | 7443.31 | 1572.36 | 3836.02 |
| Average (Std.) | 7801.51 (358.95) | 1515.93 (78.62) | 3394.85 (392.81) |
| t-test | p = 0.00 * | p = 0.20 | p = 0.03 * |
| Dynamic Test | Total Cycle | Max. Axial Disp. (mm) | Max. Lateral Disp. (mm) |
|---|---|---|---|
| SLLP-No1 | 453,349 | 2.021 | 1.27 |
| SLLP-No2 | 264,029 | 2.002 | 1.42 |
| SLLP-No3 | 508,658 | 2.028 | 1.44 |
| Average (Std.) | 408,679 (128,286) | 2.017 (0.013) | 1.377 (0.093) |
| DLLP-No1 | 1,000,000 | 1.601 | 1.11 |
| DLLP-No2 | 1,000,000 | 1.054 | 0.92 |
| DLLP-No3 | 1,000,000 | 1.334 | 0.89 |
| Average (Std.) | 1,000,000 | 1.330 (0.274) | 0.973 (0.119) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, Y.-H.; Wang, H.-W.; Tsai, W.-C.; Lin, C.-L. A Dual-Thread Lag–Locking Screw Enhances Single Lateral Plate Fixation in Bicondylar Tibial Plateau Fractures: A Biomechanical Study. Bioengineering 2025, 12, 1023. https://doi.org/10.3390/bioengineering12101023
Chan Y-H, Wang H-W, Tsai W-C, Lin C-L. A Dual-Thread Lag–Locking Screw Enhances Single Lateral Plate Fixation in Bicondylar Tibial Plateau Fractures: A Biomechanical Study. Bioengineering. 2025; 12(10):1023. https://doi.org/10.3390/bioengineering12101023
Chicago/Turabian StyleChan, Ya-Han, Hsuan-Wen Wang, Wei-Che Tsai, and Chun-Li Lin. 2025. "A Dual-Thread Lag–Locking Screw Enhances Single Lateral Plate Fixation in Bicondylar Tibial Plateau Fractures: A Biomechanical Study" Bioengineering 12, no. 10: 1023. https://doi.org/10.3390/bioengineering12101023
APA StyleChan, Y.-H., Wang, H.-W., Tsai, W.-C., & Lin, C.-L. (2025). A Dual-Thread Lag–Locking Screw Enhances Single Lateral Plate Fixation in Bicondylar Tibial Plateau Fractures: A Biomechanical Study. Bioengineering, 12(10), 1023. https://doi.org/10.3390/bioengineering12101023







