Detecting Event-Related Spectral Perturbations in Right-Handed Sensorimotor Cortical Responses Using OPM-MEG
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Subjects
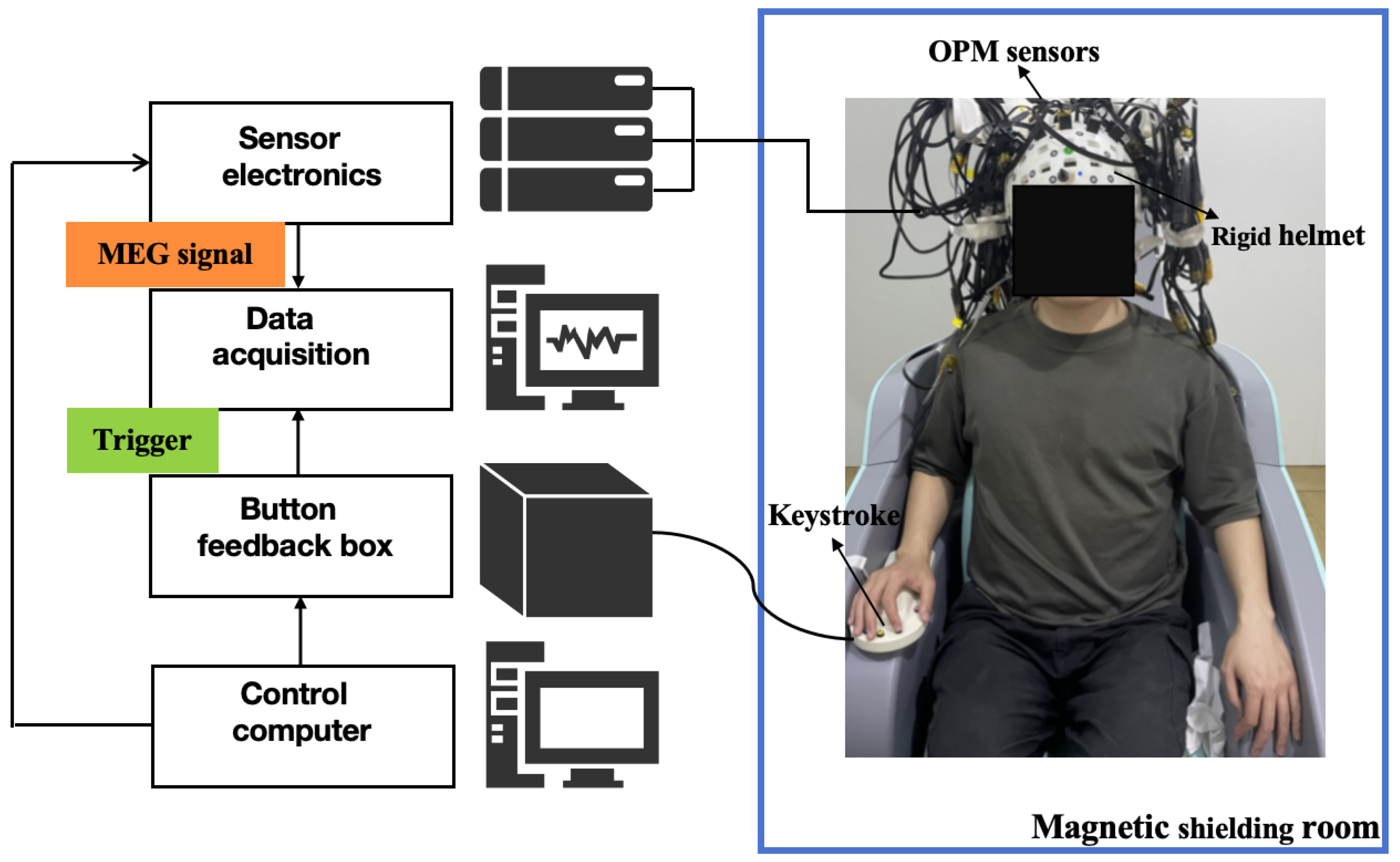
2.2. OPM-MEG System
2.3. Experimental Design and Preprocessing
2.4. Registration and Source Localization
2.5. ERSP
2.6. ERD/ERS Quantification
2.7. Statistical Analysis
3. Results
3.1. Analysis of Oscillation Activity of Contralateral Brain
3.2. Analysis of Oscillation Activity of Ipsilateral Brain
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boto, E.; Holmes, N.; Leggett, J.; Roberts, G.; Shah, V.; Meyer, S.S.; Muñoz, L.D.; Mullinger, K.J.; Tierney, T.M.; Bestmann, S.; et al. Moving magnetoencephalography towards real-world applications with a wearable system. Nature 2018, 555, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Kim, K. Instrumentation for measuring MEG signals. In Magnetoencephalography: From Signals to Dynamic Cortical Networks; Springer: Berlin/Heidelberg, Germany, 2019; pp. 41–71. [Google Scholar]
- Alem, O.; Hughes, K.J.; Buard, I.; Cheung, T.P.; Maydew, T.; Griesshammer, A.; Holloway, K.; Park, A.; Lechuga, V.; Coolidge, C.; et al. An integrated full-head OPM-MEG system based on 128 zero-field sensors. Front. Neurosci. 2023, 17, 1190310. [Google Scholar] [CrossRef]
- An, K.m.; Shim, J.H.; Kwon, H.; Lee, Y.H.; Yu, K.K.; Kwon, M.; Chun, W.Y.; Hirosawa, T.; Hasegawa, C.; Iwasaki, S.; et al. Detection of the 40 Hz auditory steady-state response with optically pumped magnetometers. Sci. Rep. 2022, 12, 17993. [Google Scholar] [CrossRef]
- Gialopsou, A.; Abel, C.; James, T.M.; Coussens, T.; Bason, M.G.; Puddy, R.; Di Lorenzo, F.; Rolfs, K.; Voigt, J.; Sander, T.; et al. Improved spatio-temporal measurements of visually evoked fields using optically-pumped magnetometers. Sci. Rep. 2021, 11, 22412. [Google Scholar] [CrossRef]
- Godfrey, M.; Singh, K.D. Measuring robust functional connectivity from resting-state MEG using amplitude and entropy correlation across frequency bands and temporal scales. NeuroImage 2021, 226, 117551. [Google Scholar] [CrossRef] [PubMed]
- Iivanainen, J.; Carter, T.R.; Trumbo, M.C.; McKay, J.; Taulu, S.; Wang, J.; Stephen, J.M.; Schwindt, P.D.; Borna, A. Single-trial classification of evoked responses to auditory tones using OPM-and SQUID-MEG. J. Neural Eng. 2023, 20, 056032. [Google Scholar] [CrossRef]
- Corvilain, P.; Wens, V.; Bourguignon, M.; Capparini, C.; Fourdin, L.; Ferez, M.; Feys, O.; De Tiège, X.; Bertels, J. Pushing the boundaries of MEG based on optically pumped magnetometers towards early human life. Imaging Neurosci. 2025, 3, imag_a_00489. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; van Es, M.; Woolrich, M.; Gohil, C. Comparison between EEG and MEG of static and dynamic resting-state networks. Hum. Brain Mapp. 2024, 45, e70018. [Google Scholar] [CrossRef]
- Xu, W.; Liao, P.; Cao, M.; White, D.J.; Lyu, B.; Gao, J.H. Facilitating cognitive neuroscience research with 80-sensor optically pumped magnetometer magnetoencephalography (OPM-MEG). NeuroImage 2025, 311, 121182. [Google Scholar] [CrossRef]
- Lu, H.; Li, Y.; Gao, Y.; Liu, Y.; Ning, X. Investigating the Coherence Between Motor Cortex During Rhythmic Finger Tapping Using OPM-MEG. Photonics 2025, 12, 766. [Google Scholar] [CrossRef]
- Rea, M.; Boto, E.; Holmes, N.; Hill, R.; Osborne, J.; Rhodes, N.; Leggett, J.; Rier, L.; Bowtell, R.; Shah, V.; et al. A 90-channel triaxial magnetoencephalography system using optically pumped magnetometers. Ann. N. Y. Acad. Sci. 2022, 1517, 107–124. [Google Scholar] [CrossRef]
- Feys, O.; Wens, V.; Depondt, C.; Rikir, E.; Gaspard, N.; Van Paesschen, W.; Aeby, A.; Bodart, O.; Carrette, E.; Holmes, N.; et al. On-scalp magnetoencephalography based on optically pumped magnetometers to investigate temporal lobe epilepsy. Epilepsia 2025, 66, e142–e151. [Google Scholar] [CrossRef] [PubMed]
- An, N.; Gao, Z.; Li, W.; Cao, F.; Wang, W.; Xu, W.; Wang, C.; Xiang, M.; Gao, Y.; Wang, D.; et al. Source localization comparison and combination of OPM-MEG and fMRI to detect sensorimotor cortex responses. Comput. Methods Programs Biomed. 2024, 254, 108292. [Google Scholar] [CrossRef] [PubMed]
- Winterer, G.; Carver, F.W.; Musso, F.; Mattay, V.; Weinberger, D.R.; Coppola, R. Complex relationship between BOLD signal and synchronization/desynchronization of human brain MEG oscillations. Hum. Brain Mapp. 2007, 28, 805–816. [Google Scholar] [CrossRef]
- Stevenson, C.M.; Wang, F.; Brookes, M.J.; Zumer, J.M.; Francis, S.T.; Morris, P.G. Paired pulse depression in the somatosensory cortex: Associations between MEG and BOLD fMRI. Neuroimage 2012, 59, 2722–2732. [Google Scholar] [CrossRef]
- Xiang, J.; DeGrauw, X.; Korman, A.M.; Allen, J.R.; O’Brien, H.L.; Kabbouche, M.A.; Powers, S.W.; Hershey, A.D. Neuromagnetic abnormality of motor cortical activation and phases of headache attacks in childhood migraine. PLoS ONE 2013, 8, e83669. [Google Scholar] [CrossRef]
- Mostofsky, S.H.; Rimrodt, S.L.; Schafer, J.G.; Boyce, A.; Goldberg, M.C.; Pekar, J.J.; Denckla, M.B. Atypical motor and sensory cortex activation in attention-deficit/hyperactivity disorder: A functional magnetic resonance imaging study of simple sequential finger tapping. Biol. Psychiatry 2006, 59, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Rier, L.; Zamyadi, R.; Zhang, J.; Emami, Z.; Seedat, Z.A.; Mocanu, S.; Gascoyne, L.E.; Allen, C.M.; Scadding, J.W.; Furlong, P.L.; et al. Mild traumatic brain injury impairs the coordination of intrinsic and motor-related neural dynamics. Neuroimage Clin. 2021, 32, 102841. [Google Scholar] [CrossRef]
- Meng, L.F.; Lu, C.P.; Li, Y.W. Hemispheric lateralization of event-related brain potentials in different processing phases during unimanual finger movements. Sensors 2008, 8, 2900–2912. [Google Scholar] [CrossRef]
- Zapała, D.; Iwanowicz, P.; Francuz, P.; Augustynowicz, P. Handedness effects on motor imagery during kinesthetic and visual-motor conditions. Sci. Rep. 2021, 11, 13112. [Google Scholar] [CrossRef]
- Feige, B.; Kristeva-Feige, R.; Rossi, S.; Pizzella, V.; Rossini, P.M. Neuromagnetic study of movement-related changes in rhythmic brain activity. Brain Res. 1996, 734, 252–260. [Google Scholar] [CrossRef]
- Gyulai, A.; Körmendi, J.; Issa, M.F.; Juhasz, Z.; Nagy, Z. Event-Related Spectral Perturbation, Inter Trial Coherence, and Functional Connectivity in motor execution: A comparative EEG study of old and young subjects. Brain Behav. 2023, 13, e3176. [Google Scholar] [CrossRef]
- Onishi, H.; Sugawara, K.; Yamashiro, K.; Sato, D.; Suzuki, M.; Kirimoto, H.; Tamaki, H.; Murakami, H.; Kameyama, S. Neuromagnetic activation following active and passive finger movements. Brain Behav. 2013, 3, 178–192. [Google Scholar] [CrossRef]
- Tzourio-Mazoyer, N.; Petit, L.; Zago, L.; Crivello, F.; Vinuesa, N.; Joliot, M.; Jobard, G.; Mellet, E.; Mazoyer, B. Between-hand difference in ipsilateral deactivation is associated with hand lateralization: fMRI mapping of 284 volunteers balanced for handedness. Front. Hum. Neurosci. 2015, 9, 5. [Google Scholar] [CrossRef]
- Fukuda, H.; Odagaki, M.; Hiwaki, O.; Kodabashi, A.; Fujimoto, T. Brain activity during bilateral rapid alternate finger tapping measured with magnetoencephalography. J. Appl. Phys. 2009, 105, 07B313. [Google Scholar] [CrossRef]
- Zhang, Z.; Koike, Y. Clustered event related spectral perturbation (ERSP) feature in right hand motor imagery classification. Front. Neurosci. 2022, 16, 867480. [Google Scholar] [CrossRef]
- Rivero, G.R.; Tanner, Z.; Rier, L.; Hill, R.M.; Shah, V.; Rea, M.; Doyle, C.; Osborne, J.; Bobela, D.; Morris, P.G.; et al. OPM-MEG reveals dynamics of beta bursts underlying attentional processes in sensory cortex. Sci. Rep. 2025, 15, 30471. [Google Scholar] [CrossRef]
- Bourguignon, M.; De Tiège, X.; de Beeck, M.O.; Pirotte, B.; Van Bogaert, P.; Goldman, S.; Hari, R.; Jousmäki, V. Functional motor-cortex mapping using corticokinematic coherence. Neuroimage 2011, 55, 1475–1479. [Google Scholar] [CrossRef]
- Zhu, F.F.; Maxwell, J.P.; Hu, Y.; Zhang, Z.G.; Lam, W.; Poolton, J.M.; Masters, R.S. EEG activity during the verbal-cognitive stage of motor skill acquisition. Biol. Psychol. 2010, 84, 221–227. [Google Scholar] [CrossRef]
- Nakayashiki, K.; Saeki, M.; Takata, Y.; Hayashi, Y.; Kondo, T. Modulation of event-related desynchronization during kinematic and kinetic hand movements. J. Neuroeng. Rehabil. 2014, 11, 90. [Google Scholar] [CrossRef]
- Jia, L.; Li, J.; Song, X.; Ning, X.; Yang, J.; Qi, S.; Long, T.; Wu, Z.; Wang, R. Hardware-Based Interference Suppression Techniques for OPM-MEG: A Review. IEEE Sens. J. 2024, 25, 2090–2102. [Google Scholar] [CrossRef]
- Jia, L.; Song, X.; Suo, Y.; Li, J.; Long, T.; Ning, X. Magnetic field interference suppression for minimized SERF atomic magnetometer. Sens. Actuators A Phys. 2023, 351, 114188. [Google Scholar] [CrossRef]
- Yuan, S.; Cui, P.; Shi, M.; Zhang, X.; Yang, J.; Zhang, L.; Ma, Y. Biplanar coils design for spatial nonlinear magnetic fields using an enhanced target field method. J. Phys. D Appl. Phys. 2024, 57, 405002. [Google Scholar] [CrossRef]
- Yuan, S.; Shi, M.; Zhang, L.; Yang, J.; Li, T.; Ma, Y. A Design Method of Biplanar Uniform Field Coils Based on Magnetic Shielding Rooms. IEEE Trans. Magn. 2024, 60, 7201904. [Google Scholar] [CrossRef]
- Stavrinou, M.L.; Moraru, L.; Cimponeriu, L.; Della Penna, S.; Bezerianos, A. Evaluation of cortical connectivity during real and imagined rhythmic finger tapping. Brain Topogr. 2007, 19, 137–145. [Google Scholar] [CrossRef]
- Guo, X.; Xiang, J.; Wang, Y.; O’Brien, H.; Kabbouche, M.; Horn, P.; Powers, S.W.; Hershey, A.D. Aberrant neuromagnetic activation in the motor cortex in children with acute migraine: A magnetoencephalography study. PLoS ONE 2012, 7, e50095. [Google Scholar] [CrossRef]
- Tierney, T.M.; Alexander, N.; Mellor, S.; Holmes, N.; Seymour, R.; O’Neill, G.C.; Maguire, E.A.; Barnes, G.R. Modelling optically pumped magnetometer interference in MEG as a spatially homogeneous magnetic field. NeuroImage 2021, 244, 118484. [Google Scholar] [CrossRef]
- Fischl, B. FreeSurfer. Neuroimage 2012, 62, 774–781. [Google Scholar] [CrossRef]
- Hämäläinen, M.S.; Ilmoniemi, R.J. Interpreting magnetic fields of the brain: Minimum norm estimates. Med. Biol. Eng. Comput. 1994, 32, 35–42. [Google Scholar] [CrossRef]
- Lin, F.H.; Witzel, T.; Ahlfors, S.P.; Stufflebeam, S.M.; Belliveau, J.W.; Hämäläinen, M.S. Assessing and improving the spatial accuracy in MEG source localization by depth-weighted minimum-norm estimates. Neuroimage 2006, 31, 160–171. [Google Scholar] [CrossRef]
- Fischl, B.; Sereno, M.I.; Tootell, R.B.; Dale, A.M. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum. Brain Mapp. 1999, 8, 272–284. [Google Scholar] [CrossRef]
- Gramfort, A.; Luessi, M.; Larson, E.; Engemann, D.A.; Strohmeier, D.; Brodbeck, C.; Parkkonen, L.; Hämäläinen, M.S. MNE software for processing MEG and EEG data. Neuroimage 2014, 86, 446–460. [Google Scholar] [CrossRef]
- Gramfort, A.; Luessi, M.; Larson, E.; Engemann, D.A.; Strohmeier, D.; Brodbeck, C.; Goj, R.; Jas, M.; Brooks, T.; Parkkonen, L.; et al. MEG and EEG data analysis with MNE-Python. Front. Neuroinf. 2013, 7, 267. [Google Scholar] [CrossRef]
- Cao, F.; An, N.; Xu, W.; Wang, W.; Li, W.; Wang, C.; Yang, Y.; Xiang, M.; Gao, Y.; Ning, X. OMMR: Co-registration toolbox of OPM-MEG and MRI. Front. Neurosci. 2022, 16, 984036. [Google Scholar] [CrossRef]
- Durka, P.J.; Zygierewicz, J.; Klekowicz, H.; Ginter, J.; Blinowska, K.J. On the statistical significance of event-related EEG desynchronization and synchronization in the time-frequency plane. IEEE Trans. Biomed. Eng. 2004, 51, 1167–1175. [Google Scholar] [CrossRef]
- Benjamini, Y.; Yekutieli, D. The control of the false discovery rate in multiple testing under dependency. Ann. Stat. 2001, 29, 1165–1188. [Google Scholar] [CrossRef]
- Rier, L.; Rhodes, N.; Pakenham, D.O.; Boto, E.; Holmes, N.; Hill, R.M.; Rivero, G.R.; Shah, V.; Doyle, C.; Osborne, J.; et al. Tracking the neurodevelopmental trajectory of beta band oscillations with optically pumped magnetometer-based magnetoencephalography. eLife 2024, 13, RP94561. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, Y.; Xu, B.; Zhang, D. The developmental trajectory of task-related frontal EEG theta/beta ratio in childhood. Dev. Cogn. Neurosci. 2023, 60, 101233. [Google Scholar] [CrossRef]
- Peng, J.; Zikereya, T.; Shao, Z.; Shi, K. The neuromechanical of Beta-band corticomuscular coupling within the human motor system. Front. Neurosci. 2024, 18, 1441002. [Google Scholar] [CrossRef]
- Rayson, H.; Debnath, R.; Alavizadeh, S.; Fox, N.; Ferrari, P.F.; Bonaiuto, J.J. Detection and analysis of cortical beta bursts in developmental EEG data. Dev. Cogn. Neurosci. 2022, 54, 101069. [Google Scholar] [CrossRef]
- Ji, D.; Xiao, X.; Wu, J.; He, X.; Zhang, G.; Guo, R.; Liu, M.; Xu, M.; Lin, Q.; Jung, T.P.; et al. A user-friendly visual brain-computer interface based on high-frequency steady-state visual evoked fields recorded by OPM-MEG. J. Neural Eng. 2024, 21, 036024. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Ma, Y.; Wu, H.; Wang, R.; Wang, R.; Liu, C.; Gao, Y.; Ning, X. The Gradient of Spontaneous Oscillations Across Cortical Hierarchies Measured by Wearable Magnetoencephalography. Technologies 2024, 12, 254. [Google Scholar] [CrossRef]
- Feys, O.; Corvilain, P.; Van Hecke, A.; Sculier, C.; Rikir, E.; Legros, B.; Gaspard, N.; Leurquin-Sterk, G.; Holmes, N.; Brookes, M.; et al. Recording of ictal epileptic activity using on-scalp magnetoencephalography. Ann. Neurol. 2023, 93, 419–421. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Li, Y.; Xiang, M.; Ma, Y.; Gao, Y.; Ning, X. Detecting Event-Related Spectral Perturbations in Right-Handed Sensorimotor Cortical Responses Using OPM-MEG. Bioengineering 2025, 12, 1022. https://doi.org/10.3390/bioengineering12101022
Lu H, Li Y, Xiang M, Ma Y, Gao Y, Ning X. Detecting Event-Related Spectral Perturbations in Right-Handed Sensorimotor Cortical Responses Using OPM-MEG. Bioengineering. 2025; 12(10):1022. https://doi.org/10.3390/bioengineering12101022
Chicago/Turabian StyleLu, Hao, Yong Li, Min Xiang, Yuyu Ma, Yang Gao, and Xiaolin Ning. 2025. "Detecting Event-Related Spectral Perturbations in Right-Handed Sensorimotor Cortical Responses Using OPM-MEG" Bioengineering 12, no. 10: 1022. https://doi.org/10.3390/bioengineering12101022
APA StyleLu, H., Li, Y., Xiang, M., Ma, Y., Gao, Y., & Ning, X. (2025). Detecting Event-Related Spectral Perturbations in Right-Handed Sensorimotor Cortical Responses Using OPM-MEG. Bioengineering, 12(10), 1022. https://doi.org/10.3390/bioengineering12101022







