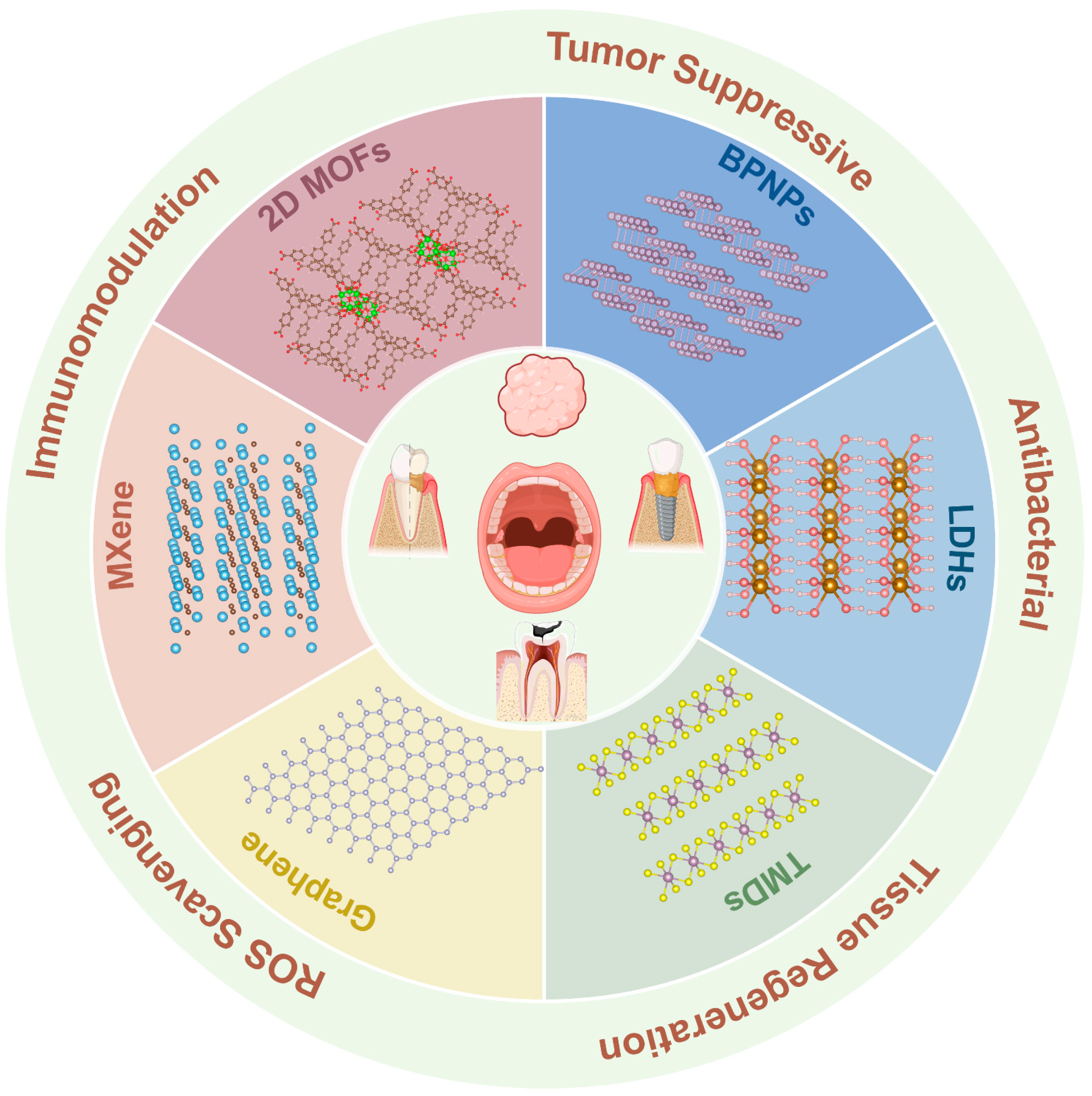
Advances of Functional Two-Dimensional Nanomaterials in the Treatment of Oral Diseases
Abstract
1. Introduction
2. Unique Advantages Beneficial for Oral Treatment
2.1. High Specific Surface Area
2.2. Surface Modifiability and Functionalization
2.3. Photothermal and Photodynamic Activity
2.4. High Mechanical Stability
2.5. Endogenous Bioactivity
2.5.1. Antibacterial Effects and Mechanisms
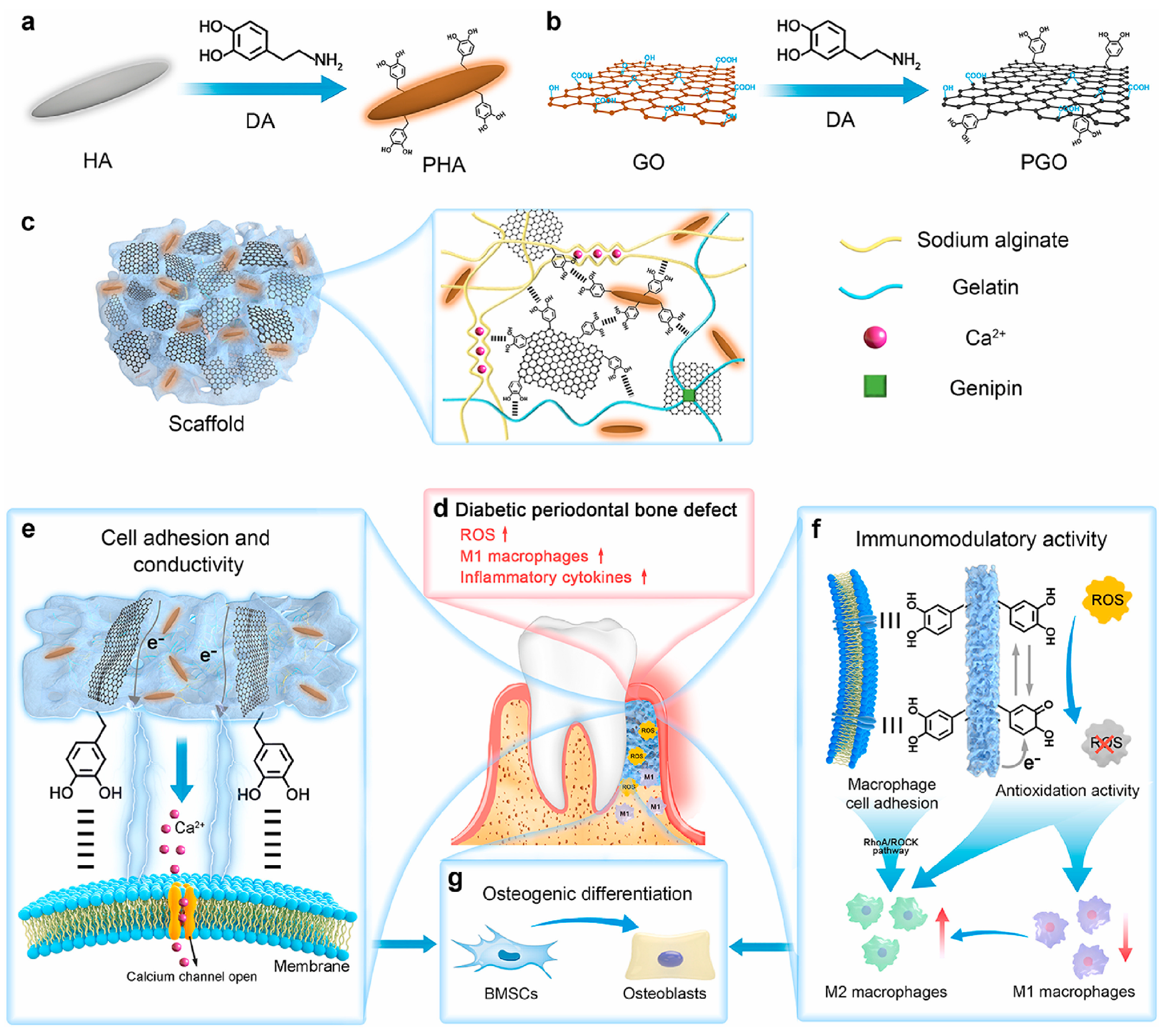
2.5.2. Immunomodulatory Effects and Mechanisms
2.5.3. Osteogenic Promotion and Underlying Mechanisms
2.5.4. Promotion of Tissue Repair
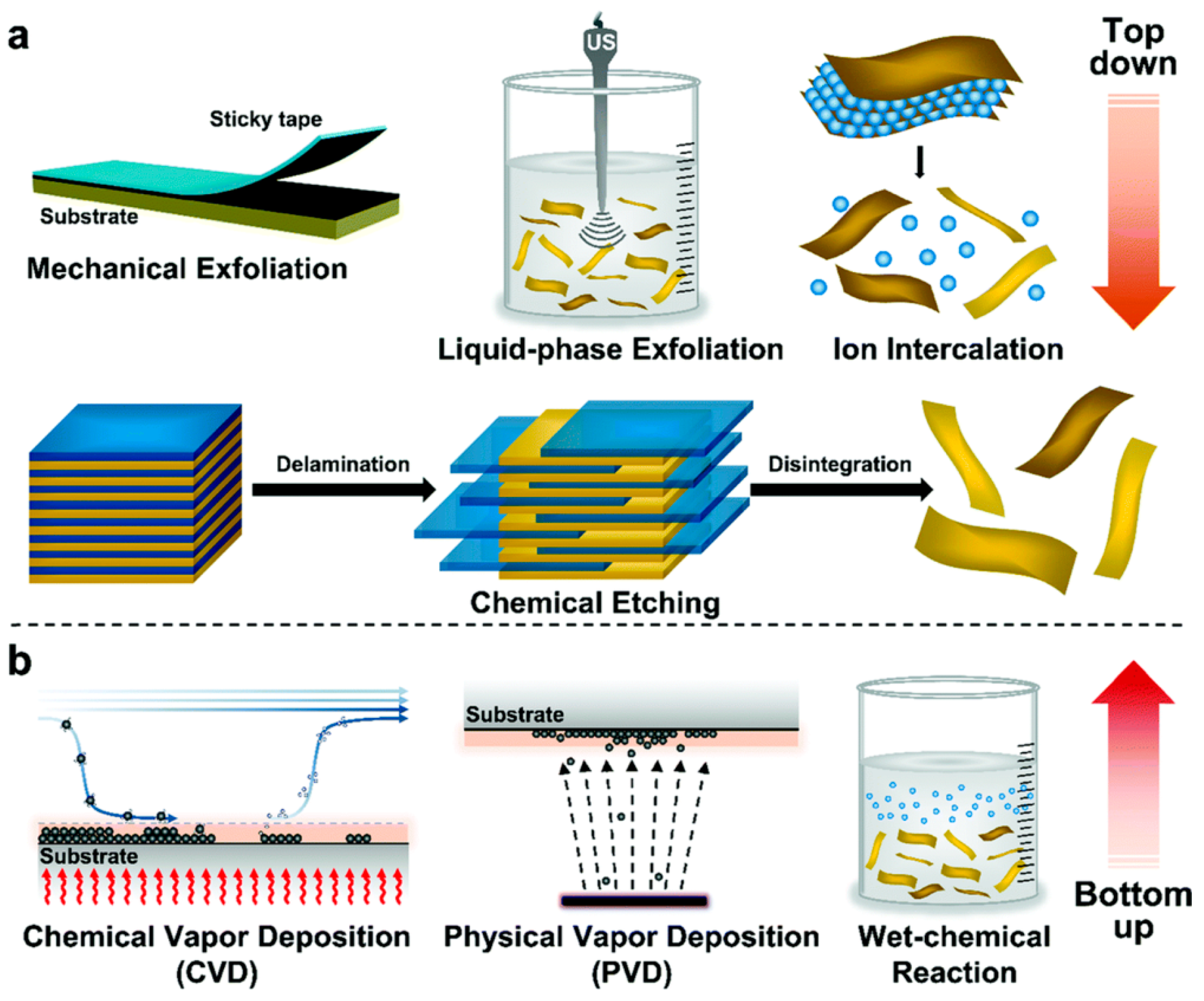
3. Synthesis of Two-Dimensional Nanomaterials
3.1. Top-Down Methods
3.1.1. Mechanical Exfoliation
3.1.2. Liquid-Phase Exfoliation
3.1.3. Chemical Intercalation and Exfoliation Method
3.2. Bottom-Up Methods
3.2.1. Chemical Vapor Deposition (CVD)
3.2.2. Physical Vapor Deposition (PVD)
3.2.3. Hydrothermal/Solvothermal Synthesis
4. Application in Oral Diseases
4.1. Graphene and Its Derivatives
4.1.1. Dental Caries
Inhibition of Cariogenic Bacteria
Promoting Remineralization
4.1.2. Oral Squamous Cell Carcinoma
Drug Delivery Platforms
Photothermal Therapy (PTT)
Photodynamic Therapy (PDT)
4.1.3. Periodontitis
Inhibition of Periodontal Pathogens
Promotion of Periodontal Bone Regeneration
4.1.4. Peri-Implantitis
Inhibiting Bacterial Adhesion
Promoting Osseointegration and Soft Tissue Sealing
4.2. Black Phosphorus Nanosheets (BPNSs)
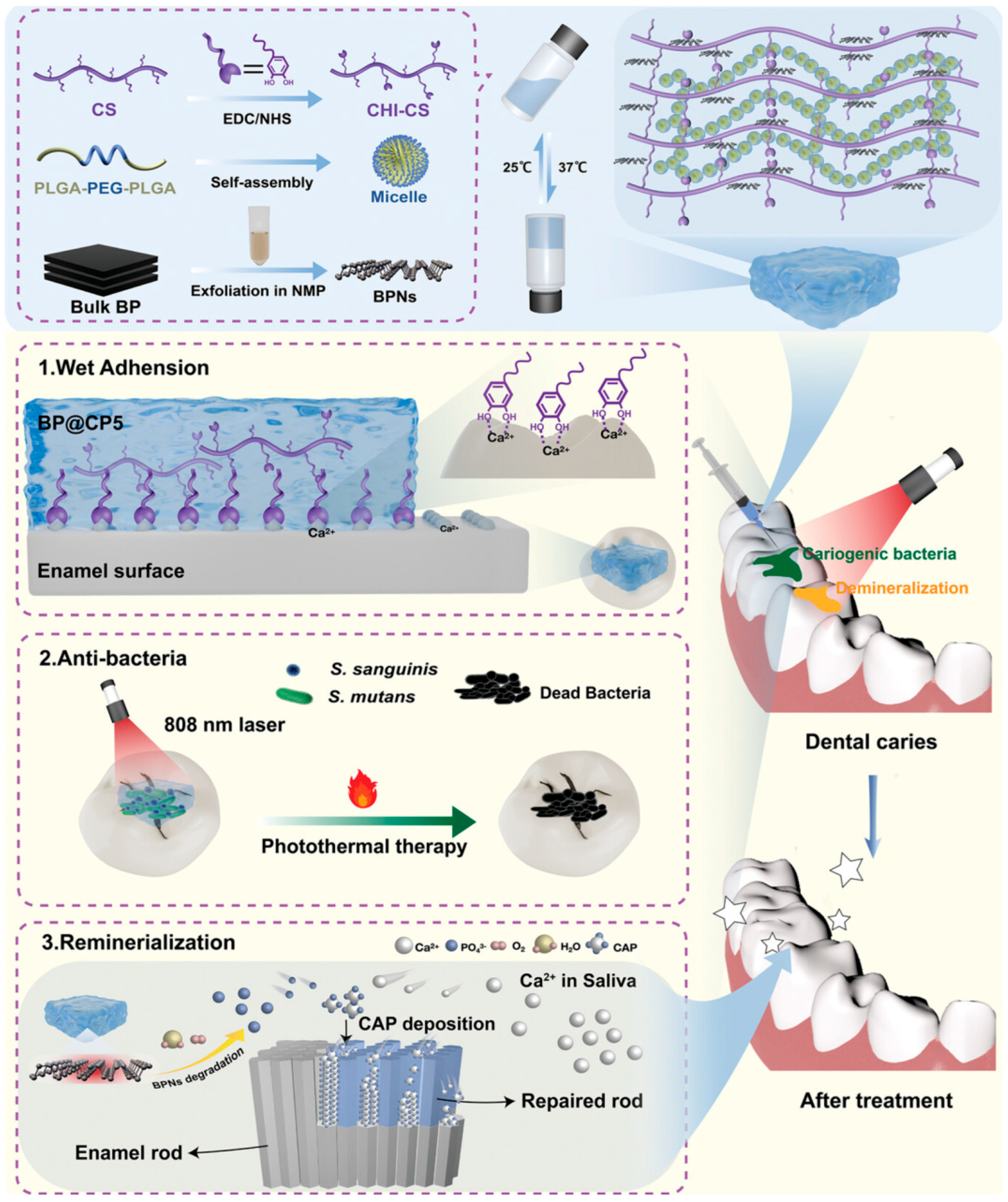
4.2.1. Dental Caries
Inhibition of Cariogenic Bacteria
Promotion of Remineralization
4.2.2. Oral Squamous Cell Carcinoma
4.2.3. Periodontitis
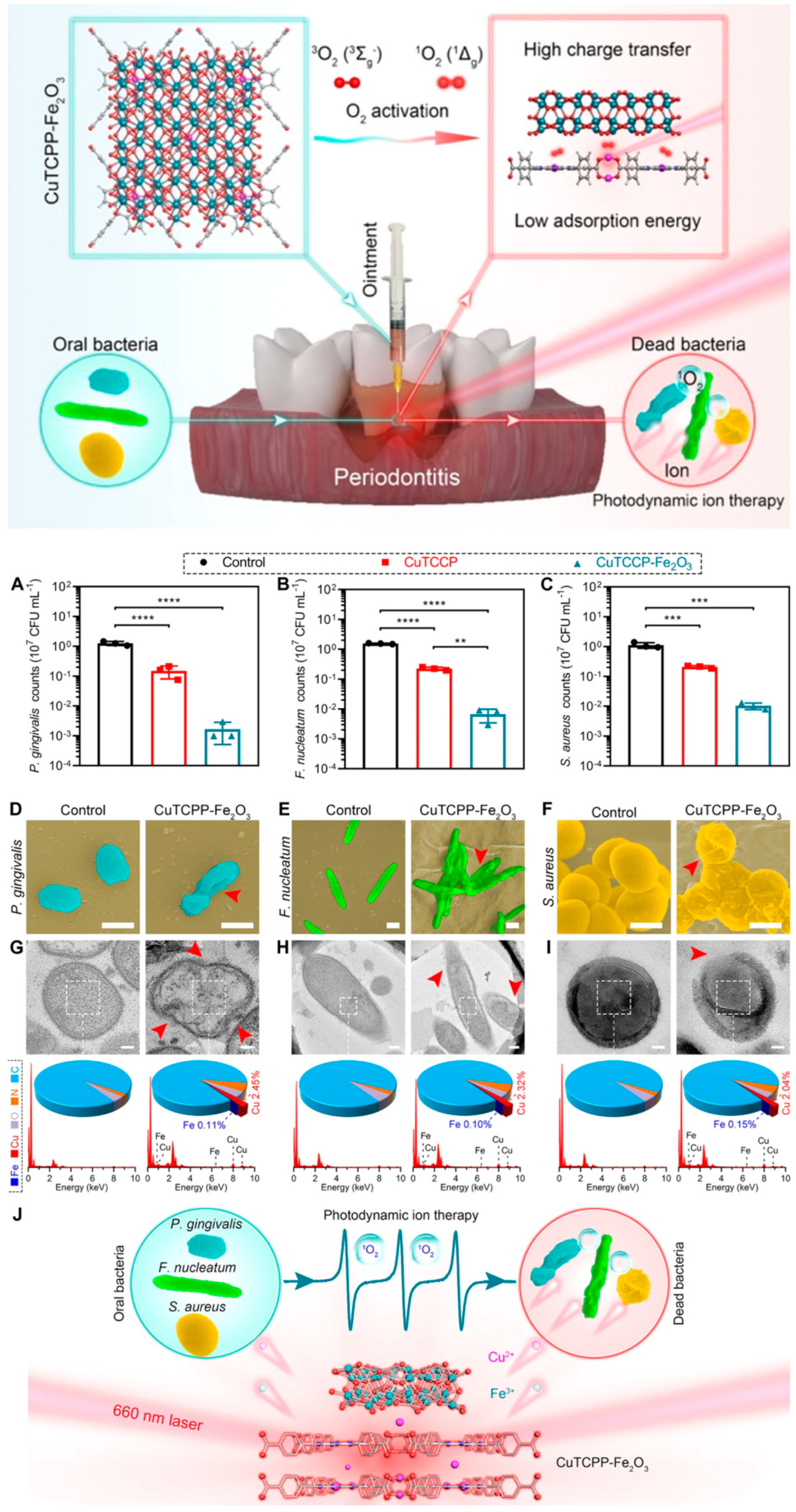
4.3. 2D Metal–Organic Frameworks (2D MOFs)
Periodontitis
4.4. MXene
4.4.1. Periodontitis
Inhibition of Periodontal Pathogens
Promotion of Periodontal Bone Regeneration
4.4.2. Peri-Implantitis
4.5. Transition Metal Dichalcogenides (TMDs)
Periodontitis
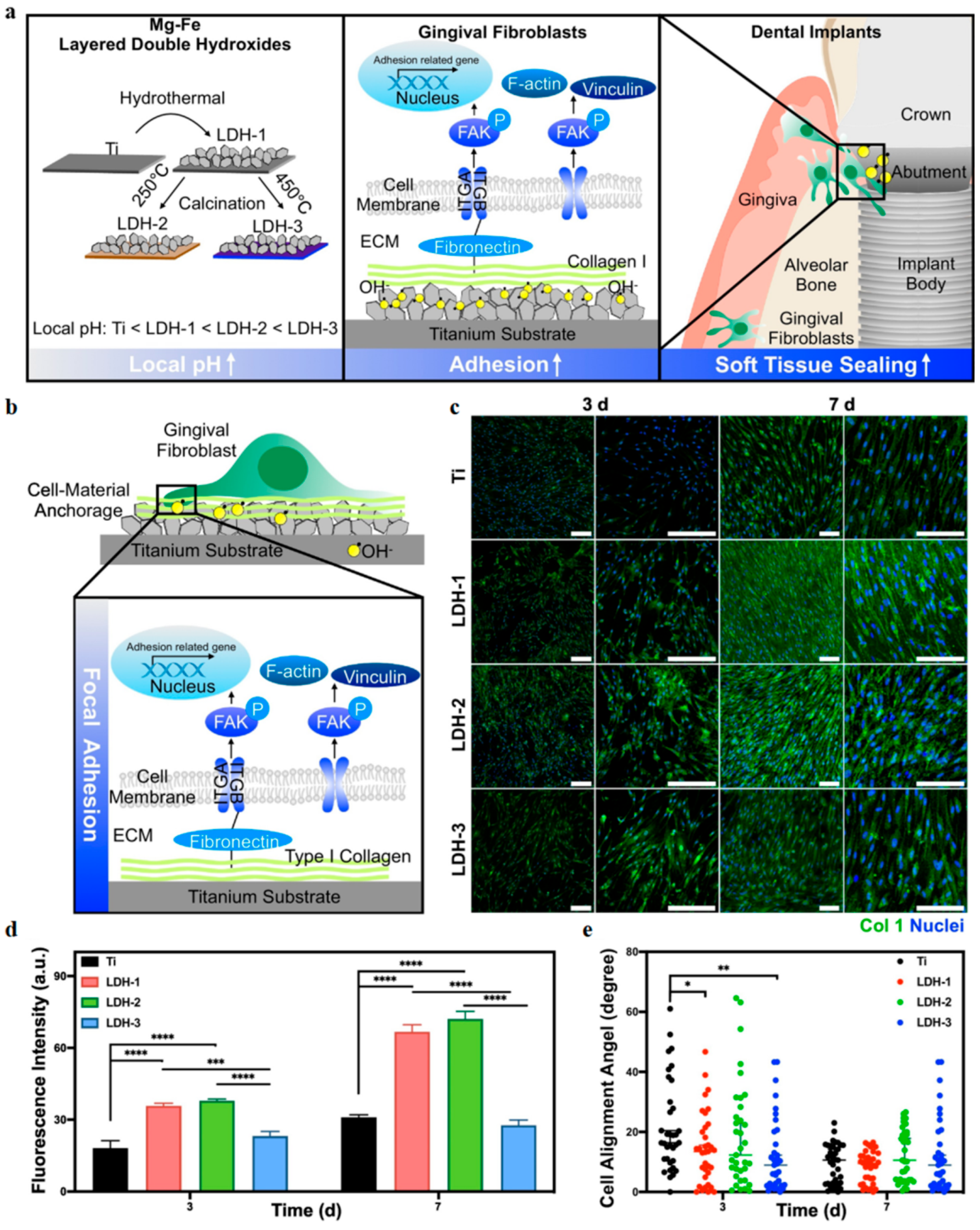
4.6. Layered Double Hydroxides (LDHs)
4.6.1. Periodontitis
4.6.2. Dental Implants
5. Advantages and Improvement
5.1. Multifunctional Integration Advantage for Adapting to Complex Oral Pathology
5.2. Biological Safety Still Need Comprehensive Evaluation
5.3. Structural Stability and Functional Maintenance Are Still Limited
5.4. Clinical Translation Challenges Remain to Be Overcome
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wu, J.; Chen, J.; Lv, C.; Zhou, L. Global, regional, and National levels and trends in burden of dental caries and periodontal disease from 1990 to 2035: Result from the global burden of disease study 2021. BMC Oral Health 2025, 25, 844. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Dutt, U.; Radenkov, I.; Jain, S. WHO’s global oral health status report 2022: Actions, discussion and implementation. Oral Dis. 2024, 30, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, H.; Chang, L.; Xu, J.; Zhou, X.; Zhang, C.; Peng, Q. Efficient Removal of Dental Plaque Biofilm from Training Typodont Teeth via Water Flosser. Bioengineering 2023, 10, 1061. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Y.; Li, S.; Li, Y.; Chang, L.; Yao, Y.; Peng, Q. Advances of functional nanomaterials as either therapeutic agents or delivery systems in the treatment of periodontitis. Biomater. Adv. 2025, 175, 214326. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chang, L.; Gao, H.; Yu, C.; Gao, Y.; Peng, Q. Nanomaterials-based advanced systems for photothermal / photodynamic therapy of oral cancer. Eur. J. Med. Chem. 2024, 272, 116508. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dong, J.; Zhang, T.; Peng, Q. Graphene-based nanomaterials and their potentials in advanced drug delivery and cancer therapy. J. Control. Release 2018, 286, 64–73. [Google Scholar] [CrossRef]
- Li, H.; Yin, D.; Li, W.; Tang, Q.; Zou, L.; Peng, Q. Polydopamine-based nanomaterials and their potentials in advanced drug delivery and therapy. Colloids Surf. B Biointerfaces 2021, 199, 111502. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, M.; Zhang, T.; Peng, Q. 2D nanomaterials-based delivery systems and their potentials in anticancer synergistic photo-immunotherapy. Colloids Surf. B Biointerfaces 2024, 242, 114074. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, X.; Gong, F.; Liu, T.; Liu, Z. 2D Nanomaterials for Cancer Theranostic Applications. Adv. Mater. 2020, 32, 1902333. [Google Scholar] [CrossRef]
- Amara, U.; Hussain, I.; Ahmad, M.; Mahmood, K.; Zhang, K. 2D MXene-Based Biosensing: A Review. Small 2023, 19, 2205249. [Google Scholar] [CrossRef]
- Gao, Y.; Dong, Y.; Yang, S.; Mo, A.; Zeng, X.; Chen, Q.; Peng, Q. Size-dependent photothermal antibacterial activity of Ti3C2Tx MXene nanosheets against methicillin-resistant Staphylococcus aureus. J. Colloid Interface Sci. 2022, 617, 533–541. [Google Scholar] [CrossRef]
- Yu, C.-H.; Chen, G.-Y.; Xia, M.-Y.; Xie, Y.; Chi, Y.-Q.; He, Z.-Y.; Zhang, C.-L.; Zhang, T.; Chen, Q.-M.; Peng, Q. Understanding the sheet size-antibacterial activity relationship of graphene oxide and the nano-bio interaction-based physical mechanisms. Colloids Surf. B Biointerfaces 2020, 191, 111009. [Google Scholar] [CrossRef]
- Xu, G.; Li, J.; Zhang, S.; Cai, J.; Deng, X.; Wang, Y.; Pei, P. Two-dimensional nano-biomaterials in regulating the tumor microenvironment for immunotherapy. Nano TransMed 2024, 3, 100045. [Google Scholar] [CrossRef]
- Li, J.; Song, S.; Meng, J.; Tan, L.; Liu, X.; Zheng, Y.; Li, Z.; Yeung, K.W.K.; Cui, Z.; Liang, Y.; et al. 2D MOF Periodontitis Photodynamic Ion Therapy. J. Am. Chem. Soc. 2021, 143, 15427–15439. [Google Scholar] [CrossRef]
- Luo, D.; Liu, X.; Dai, S.; Yi, J.; Tang, N.; Cai, Y.; Bao, X.; Hu, M.; Liu, Z. Highly Crystalline Copper Aluminum-Layered Double Hydroxides with Intrinsic Fenton-Like Catalytic Activity for Robust Oral Health Management. Inorg. Chem. 2024, 63, 10691–10704. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Q.; Zhang, Y.; Yang, Y.; Zhou, X.; Peng, W.; Liang, Z.; Zeng, X.; Wang, Q.; Gao, N. Charge-reversal nanomedicine based on black phosphorus for the development of A Novel photothermal therapy of oral cancer. Drug Deliv. 2021, 28, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Lee, J.H.; Hong, S.W.; Lee, J.H.; Han, D.-W. Nanocomposites for Enhanced Osseointegration of Dental and Orthopedic Implants Revisited: Surface Functionalization by Carbon Nanomaterial Coatings. J. Compos. Sci. 2021, 5, 23. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, Z.; Liu, S.; Li, P.; Yu, S.; Ling, D.; Li, F. Nano-bio interactions between 2D nanomaterials and mononuclear phagocyte system cells. BMEMat 2024, 2, e12066. [Google Scholar] [CrossRef]
- Mei, X.; Xu, S.; Hu, T.; Peng, L.; Gao, R.; Liang, R.; Wei, M.; Evans, D.G.; Duan, X. Layered double hydroxide monolayers for controlled loading and targeted delivery of anticancer drugs. Nano Res. 2018, 11, 195–205. [Google Scholar] [CrossRef]
- Kriplani, S.; Sedani, S. Comparative evaluation of the effect of 2% graphene oxide and 5% hydroxyapatite nanoparticles in isolation and in combination on cicro tensile bond strength of 5th generation adhesive. F1000Research 2023, 12, 514. [Google Scholar] [CrossRef]
- Mondal, B.; Mahendranath, A.; Som, A.; Bose, S.; Ahuja, T.; Kumar, A.A.; Ghosh, J.; Pradeep, T. Rapid reaction of MoS2 nanosheets with Pb2+ and Pb4+ ions in solution. Nanoscale 2018, 10, 1807–1814. [Google Scholar] [CrossRef]
- Wang, S.; Weng, J.; Fu, X.; Lin, J.; Fan, W.; Lu, N.; Qu, J.; Chen, S.; Wang, T.; Huang, P. Black Phosphorus Nanosheets for Mild Hyperthermia-Enhanced Chemotherapy and Chemo-Photothermal Combination Therapy. Nanotheranostics 2017, 1, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpour, S.; Nanda, A.; Walsh, L.J.; Xu, C. Microbial Decontamination and Antibacterial Activity of Nanostructured Titanium Dental Implants: A Narrative Review. Nanomaterials 2021, 11, 2336. [Google Scholar] [CrossRef]
- Das, T.N.; Moyra, S.; Sharafudheen, R.A.; Ghosh, A.; Ramesh, A.; Maji, T.K.; Ghosh, G. Organic two-dimensional nanostructures: Harnessing soft matter for multifunctional applications. J. Mol. Liq. 2024, 416, 126506. [Google Scholar] [CrossRef]
- Dash, B.S.; Lu, Y.-J.; Chen, J.-P. Enhancing Photothermal/Photodynamic Therapy for Glioblastoma by Tumor Hypoxia Alleviation and Heat Shock Protein Inhibition Using IR820-Conjugated Reduced Graphene Oxide Quantum Dots. ACS Appl. Mater. Interfaces 2024, 16, 13543–13562. [Google Scholar] [CrossRef]
- Yao, W.; Xu, P.; Zhao, J.; Ling, L.; Li, X.; Zhang, B.; Cheng, N.; Pang, Z. RGD functionalized polymeric nanoparticles targeting periodontitis epithelial cells for the enhanced treatment of periodontitis in dogs. J. Colloid Interface Sci. 2015, 458, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Anitasari, S.; Wu, C.-Z.; Shen, Y.-K. PCL/Graphene Scaffolds for the Osteogenesis Process. Bioengineering 2023, 10, 305. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Y.; Chen, Y.; Liu, L.; Mo, A.; Peng, Q. Nanomaterials-based photothermal therapy and its potentials in antibacterial treatment. J. Control. Release 2020, 328, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Takemura, K. Surface Plasmon Resonance (SPR)- and Localized SPR (LSPR)-Based Virus Sensing Systems: Optical Vibration of Nano- and Micro-Metallic Materials for the Development of Next-Generation Virus Detection Technology. Biosensors 2021, 11, 250. [Google Scholar] [CrossRef]
- Li, C.; Cheng, Y.; Li, D.; An, Q.; Zhang, W.; Zhang, Y.; Fu, Y. Antitumor Applications of Photothermal Agents and Photothermal Synergistic Therapies. Int. J. Mol. Sci. 2022, 23, 7909. [Google Scholar] [CrossRef]
- Zhong, D.; Wu, H.; Wu, Y.; Li, Y.; Yang, J.; Gong, Q.; Luo, K.; Gu, Z. Redox dual-responsive dendrimeric nanoparticles for mutually synergistic chemo-photodynamic therapy to overcome drug resistance. J. Control. Release 2021, 329, 1210–1221. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.-Y.; Zhu, Z.-L.; Zhang, W.-L.; Yin, Y.-J.; Tang, Y.-L.; Liang, X.-H.; Zhang, L. Light stimulus responsive nanomedicine in the treatment of oral squamous cell carcinoma. Eur. J. Med. Chem. 2020, 199, 112394. [Google Scholar] [CrossRef]
- Ye, C.; Peng, Q. Mechanical Stabilities and Properties of Graphene-like 2D III-Nitrides: A Review. Crystals 2023, 13, 12. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Apostu, A.M.; Sufaru, I.-G.; Tanculescu, O.; Stoleriu, S.; Doloca, A.; Ciocan Pendefunda, A.A.; Solomon, S.M. Can Graphene Pave the Way to Successful Periodontal and Dental Prosthetic Treatments? A Narrative Review. Biomedicines 2023, 11, 2354. [Google Scholar] [CrossRef]
- Li, Y.; Yan, J.; Liu, Y.; Xie, X.-M. Super Tough and Intelligent Multibond Network Physical Hydrogels Facilitated by Ti3C2Tx MXene Nanosheets. ACS Nano 2022, 16, 1567–1577. [Google Scholar] [CrossRef]
- Phakatkar, A.; Firlar, E.; Alzate, L.; Song, B.; Narayanan, S.; Rojaee, R.; Foroozan, T.; Deivanayagam, R.; Banner, D.; Shahbazian-Yassar, R.; et al. TEM Studies on Antibacterial Mechanisms of Black Phosphorous Nanosheets. Int. J. Nanomed. 2020, 15, 3071–3085. [Google Scholar] [CrossRef]
- Park, D.; Kim, N.K.; Shin, W.-R.; Osuji, C.O. Persistent Photoinduced Antibacterial Activity of MoS2 Nanosheets Immobilized in Porous Polymer Beads. ACS Appl. Mater. Interfaces 2025, 17, 342–350. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Zhao, L.; Qi, Z.; Gou, J.; Zhang, S.; Zhang, J.Z. Recent advances in ultrathin two-dimensional materials and biomedical applications for reactive oxygen species generation and scavenging. Nanoscale 2020, 12, 19516–19535. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Sun, T.; Zeng, J.; He, T.; He, Y.; Xu, D.; Liu, R.; Tan, W.; Zang, X.; Yan, J.; et al. Enhanced Immune Modulation and Bone Tissue Regeneration through an Intelligent Magnetic Scaffold Targeting Macrophage Mitochondria. Adv. Healthc. Mater. 2025, 14, 2500163. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zou, L.; Jiang, L.; Zhao, Z.; Liu, J. Osteoinductive and antimicrobial mechanisms of graphene-based materials for enhancing bone tissue engineering. J. Tissue Eng. Regen. Med. 2021, 15, 915–935. [Google Scholar] [CrossRef]
- Huang, K.; Wu, J.; Gu, Z. Black Phosphorus Hydrogel Scaffolds Enhance Bone Regeneration via a Sustained Supply of Calcium-Free Phosphorus. ACS Appl. Mater. Interfaces 2019, 11, 2908–2916. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, X.; Ouyang, J.; Chu, D.; Han, F.; Shi, L.; Liu, R.; Guo, Z.; Gu, G.X.; Tao, W.; et al. Ca2+-supplying black phosphorus-based scaffolds fabricated with microfluidic technology for osteogenesis. Bioact. Mater. 2021, 6, 4053–4064. [Google Scholar] [CrossRef]
- Jiang, X.; Ma, J.; Xue, K.; Chen, J.; Zhang, Y.; Zhang, G.; Wang, K.; Yao, Z.; Hu, Q.; Lin, C.; et al. Highly Bioactive MXene-M2-Exosome Nanocomposites Promote Angiogenic Diabetic Wound Repair through Reconstructing High Glucose-Derived Immune Inhibition. ACS Nano 2024, 18, 4269–4286. [Google Scholar] [CrossRef]
- Bai, X.; Wang, R.; Hu, X.; Dai, Q.; Guo, J.; Cao, T.; Du, W.; Cheng, Y.; Xia, S.; Wang, D.; et al. Two-Dimensional Biodegradable Black Phosphorus Nanosheets Promote Large Full-Thickness Wound Healing through In Situ Regeneration Therapy. ACS Nano 2024, 18, 3553–3574. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Zhang, H.; You, J.; Zhou, J.; Ren, S.; Feng, J.; Han, Y.; Zhang, Y.; Zhou, Y. 3D-printed intelligent photothermal conversion Nb2C MXene composite scaffolds facilitate the regulation of angiogenesis-osteogenesis coupling for vascularized bone regeneration. Mater. Today Bio 2025, 31, 101647. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Feng, W.; Chen, Y. Two-dimensional biomaterials: Material science, biological effect and biomedical engineering applications. Chem. Soc. Rev. 2021, 50, 11381–11485. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Huang, Y.; Pan, Y.-H.; Yang, R.; Bao, L.-H.; Meng, L.; Luo, H.-L.; Cai, Y.-Q.; Liu, G.-D.; Zhao, W.-J.; Zhou, Z.; et al. Universal mechanical exfoliation of large-area 2D crystals. Nat. Commun. 2020, 11, 2453. [Google Scholar] [CrossRef]
- Raza, A.; Hassan, J.Z.; Ikram, M.; Ali, S.; Farooq, U.; Khan, Q.; Maqbool, M. Advances in Liquid-Phase and Intercalation Exfoliations of Transition Metal Dichalcogenides to Produce 2D Framework. Adv. Mater. Interfaces 2021, 8, 2002205. [Google Scholar] [CrossRef]
- Wu, P.; Thenuwara, H.N.; Senevirathna, H.L. Entropy-driven liquid-phase exfoliation of non-Van-Der-Waals crystals into nanoplatelets. FlatChem 2023, 41, 100540. [Google Scholar] [CrossRef]
- Shen, J.; He, Y.; Wu, J.; Gao, C.; Keyshar, K.; Zhang, X.; Yang, Y.; Ye, M.; Vajtai, R.; Lou, J.; et al. Liquid Phase Exfoliation of Two-Dimensional Materials by Directly Probing and Matching Surface Tension Components. Nano Lett. 2015, 15, 5449–5454. [Google Scholar] [CrossRef]
- Nandihalli, N. Microwave-driven synthesis and modification of nanocarbons and hybrids in liquid and solid phases. J. Energy Storage 2025, 111, 115315. [Google Scholar] [CrossRef]
- Xu, Y.; Cao, H.; Xue, Y.; Li, B.; Cai, W. Liquid-Phase Exfoliation of Graphene: An Overview on Exfoliation Media, Techniques, and Challenges. Nanomaterials 2018, 8, 942. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Young, R.J.; Backes, C.; Zhao, W.; Zhang, X.; Zhukov, A.A.; Tillotson, E.; Conlan, A.P.; Ding, F.; Haigh, S.J.; et al. Mechanisms of Liquid-Phase Exfoliation for the Production of Graphene. ACS Nano 2020, 14, 10976–10985. [Google Scholar] [CrossRef]
- Zhao, Y.; Su, Y.; Guo, Y.; Wu, C. Intercalation-assisted Exfoliation Strategy for Two-dimensional Materials Preparation. Chem. Res. Chin. Univ. 2020, 36, 518–524. [Google Scholar] [CrossRef]
- Yang, R.; Fan, Y.; Mei, L.; Shin, H.S.; Voiry, D.; Lu, Q.; Li, J.; Zeng, Z. Synthesis of atomically thin sheets by the intercalation-based exfoliation of layered materials. Nat. Synth. 2023, 2, 101–118. [Google Scholar] [CrossRef]
- Liu, M.; Fernandes, D.C.C.; Saleeba, Z.S.S.L.; Hurt, R.H. Controlled Release of Molecular Intercalants from Two-Dimensional Nanosheet Films. ACS Nano 2021, 15, 20105–20115. [Google Scholar] [CrossRef]
- Kotsakidis, J.C.; Stephen, G.M.; DeJarld, M.; Myers-Ward, R.L.; Daniels, K.M.; Gaskill, D.K.; Fuhrer, M.S.; Butera, R.E.; Hanbicki, A.T.; Friedman, A.L. Charged Impurity Scattering and Electron–Electron Interactions in Large-Area Hydrogen Intercalated Bilayer Graphene. ACS Appl. Mater. Interfaces 2024, 16, 61194–61203. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, Z.; Zhang, Z.; Niu, W.; Li, C.; Yang, N.; Chen, B.; Zhang, H. Two-Dimensional Metal Nanomaterials: Synthesis, Properties, and Applications. Chem. Rev. 2018, 118, 6409–6455. [Google Scholar] [CrossRef]
- Arafat, A.; Islam, M.S.; Ferdous, N.; Islam, A.S.M.J.; Sarkar, M.M.H.; Stampfl, C.; Park, J. Atomistic reaction mechanism of CVD grown MoS2 through MoO3 and H2S precursors. Sci. Rep. 2022, 12, 16085. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Wang, P.; Ke, X.; Xie, J.; Yue, M.; Zhao, M.; Zhang, K.; Dong, Y.; Xu, Q.; Zou, C.; et al. Low-temperature chemical vapor deposition growth of 2D materials. Electron 2025, 3, e43. [Google Scholar] [CrossRef]
- Wan, Y.; Li, E.; Yu, Z.; Huang, J.-K.; Li, M.-Y.; Chou, A.-S.; Lee, Y.-T.; Lee, C.-J.; Hsu, H.-C.; Zhan, Q.; et al. Low-defect-density WS2 by hydroxide vapor phase deposition. Nat. Commun. 2022, 13, 4149. [Google Scholar] [CrossRef]
- Saba, T.; Saad, K.S.K.; Rashid, A.B. Precise surface engineering: Leveraging chemical vapor deposition for enhanced biocompatibility and durability in biomedical implants. Heliyon 2024, 10, e37976. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsson, J.T.; Anders, A.; von Keudell, A. Foundations of physical vapor deposition with plasma assistance. Plasma Sources Sci. Technol. 2022, 31, 83001. [Google Scholar] [CrossRef]
- Safin Kaosar Saad, K.; Saba, T.; Bin Rashid, A. Application of PVD coatings in medical implantology for enhanced performance, biocompatibility, and quality of life. Heliyon 2024, 10, e35541. [Google Scholar] [CrossRef]
- Li, Y.; Yin, X.; Guo, Y.; Liu, D.; Zhong, Y.-l.; Xu, M.; Li, Y.; Deng, A.; Tang, F.; Shi, Z.; et al. Physical Vapor Deposition Growth of Ultrathin Molybdenum Dioxide Nanosheets with Excellent Conductivity. Adv. Eng. Mater. 2022, 24, 2101358. [Google Scholar] [CrossRef]
- Txintxurreta, J.; G-Berasategui, E.; Ortiz, R.; Hernández, O.; Mendizábal, L.; Barriga, J. Indium Tin Oxide Thin Film Deposition by Magnetron Sputtering at Room Temperature for the Manufacturing of Efficient Transparent Heaters. Coatings 2021, 11, 92. [Google Scholar] [CrossRef]
- Böke, F.; Giner, I.; Keller, A.; Grundmeier, G.; Fischer, H. Plasma-Enhanced Chemical Vapor Deposition (PE-CVD) yields better Hydrolytical Stability of Biocompatible SiOx Thin Films on Implant Alumina Ceramics compared to Rapid Thermal Evaporation Physical Vapor Deposition (PVD). ACS Appl. Mater. Interfaces 2016, 8, 17805–17816. [Google Scholar] [CrossRef] [PubMed]
- Narula, U.; Tan, C.M. Engineering a PVD-Based Graphene Synthesis Method. IEEE Trans. Nanotechnol. 2017, 16, 784–789. [Google Scholar] [CrossRef]
- Byrappa, K.; Adschiri, T. Hydrothermal technology for nanotechnology. Prog. Cryst. Growth Charact. Mater. 2007, 53, 117–166. [Google Scholar] [CrossRef]
- Nandihalli, N.; Liang, R.; Wijethunge, D.; Zhou, N.; Kleinke, H. Thermoelectric properties of Ni0.05Mo3Sb5.4Te1.6 composites with NiSb nanocoating. AIP Adv. 2018, 8, 125304. [Google Scholar] [CrossRef]
- Chen, J.; Song, Y.; Gan, Y.; Cai, W.; Wang, H.; Liu, H.; Dai, B.; Peng, Y.; Wang, C. Time-resolved Solvothermal Synthesis for Controlling Lateral Size of 2D Metal–Organic Layers. Small Methods 2025, 9, 2402078. [Google Scholar] [CrossRef] [PubMed]
- Jiu, J.; Suganuma, K.; Nogi, M. Effect of additives on the morphology of single-crystal Au nanosheet synthesized using the polyol process. J. Mater. Sci. 2011, 46, 4964–4970. [Google Scholar] [CrossRef]
- Arslan, S.; Ekrikaya, S.; Ildiz, N.; Yusufbeyoglu, S.; Ocsoy, İ. Evaluation of the antibacterial activity of dental adhesive containing biogenic silver nanoparticles decorated nanographene oxide nanocomposites (Ag@nGO NCs) and effect on bond strength to dentine. Odontology 2024, 112, 341–354. [Google Scholar] [CrossRef]
- Mao, M.; Zhang, W.; Huang, Z.-W.; Huang, J.; Wang, J.; Li, W.; Gu, S. Graphene Oxide-Copper Nanocomposites Suppress Cariogenic Streptococcus mutans Biofilm Formation. Int. J. Nanomed. 2021, 16, 7727–7739. [Google Scholar] [CrossRef]
- Lu, B.-Y.; Zhu, G.-Y.; Yu, C.-H.; Chen, G.-Y.; Zhang, C.-L.; Zeng, X.; Chen, Q.-M.; Peng, Q. Functionalized graphene oxide nanosheets with unique three-in-one properties for efficient and tunable antibacterial applications. Nano Res. 2021, 14, 185–190. [Google Scholar] [CrossRef]
- Lee, S.-M.; Yoo, K.-H.; Yoon, S.-Y.; Kim, I.-R.; Park, B.-S.; Son, W.-S.; Ko, C.-C.; Son, S.-A.; Kim, Y.-I. Enamel Anti-Demineralization Effect of Orthodontic Adhesive Containing Bioactive Glass and Graphene Oxide: An In-Vitro Study. Materials 2018, 11, 1728. [Google Scholar] [CrossRef]
- Son, S.-A.; Kim, D.-H.; Yoo, K.-H.; Yoon, S.-Y.; Kim, Y.-I. Mesoporous Bioactive Glass Combined with Graphene Oxide Quantum Dot as a New Material for a New Treatment Option for Dentin Hypersensitivity. Nanomaterials 2020, 10, 621. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.D.; Aboelwafa, M.R. The impact of fluorinated graphene versus nanohydroxyapatite crystals on artificial white spot lesion microhardness and color change. BMC Oral Health 2025, 25, 1041. [Google Scholar] [CrossRef]
- Li, X.; Liang, X.; Wang, Y.; Wang, D.; Teng, M.; Xu, H.; Zhao, B.; Han, L. Graphene-Based Nanomaterials for Dental Applications: Principles, Current Advances, and Future Outlook. Front. Bioeng. Biotechnol. 2022, 10, 804201. [Google Scholar] [CrossRef]
- Tang, B.; Ma, W.; Lin, Y. Emerging applications of anti-angiogenic nanomaterials in oncotherapy. J. Control. Release 2023, 364, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Xia, X.; Qi, J.; Hu, X.; Chen, Q.; Liu, J.; Ji, N.; Zhao, H. Silmitasertib-induced macropinocytosis promoting DDP intracellular uptake to enhance cell apoptosis in oral squamous cell carcinoma. Drug Deliv. 2021, 28, 2480–2494. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Jiang, H.; Wang, Z.; Cai, L.-Y.; Jiang, Y.-C.; Xie, L.; Zhou, Y.; Zeng, X.; Ji, N.; Shen, Y.-Q.; et al. Adrenergic Blockade by Nebivolol to Suppress Oral Squamous Cell Carcinoma Growth via Endoplasmic Reticulum Stress and Mitochondria Dysfunction. Front. Pharmacol. 2021, 12, 691998. [Google Scholar] [CrossRef]
- Trikkaliotis, D.G.; Christoforidis, A.K.; Mitropoulos, A.C.; Kyzas, G.Z. Graphene Oxide Synthesis, Properties and Characterization Techniques: A Comprehensive Review. ChemEngineering 2021, 5, 64. [Google Scholar] [CrossRef]
- Yura, Y.; Hamada, M. Oral Immune-Related Adverse Events Caused by Immune Checkpoint Inhibitors: Salivary Gland Dysfunction and Mucosal Diseases. Cancers 2022, 14, 792. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Du, J.; Wang, X.; Duan, A.; Gao, R.; Liu, J.; Li, B. Graphene oxide loaded with tumor-targeted peptide and anti-cancer drugs for cancer target therapy. Sci. Rep. 2021, 11, 1725. [Google Scholar] [CrossRef]
- Andreeva, T.; Stoichev, S.; Taneva, S.; Krastev, R. Hybrid graphene oxide/polysaccharide nanocomposites with controllable surface properties and biocompatibility. Carbohydr. Polym. 2018, 181, 78–85. [Google Scholar] [CrossRef]
- Ou, L.; Sun, T.; Liu, M.; Zhang, Y.; Zhou, Z.; Zhan, X.; Lu, L.; Zhao, Q.; Lai, R.; Shao, L. Efficient miRNA Inhibitor Delivery with Graphene Oxide-Polyethylenimine to Inhibit Oral Squamous Cell Carcinoma. Int. J. Nanomed. 2020, 15, 1569–1583. [Google Scholar] [CrossRef]
- Wei, Z.; Yin, X.; Cai, Y.; Xu, W.; Song, C.; Wang, Y.; Zhang, J.; Kang, A.; Wang, Z.; Han, W. Antitumor effect of a Pt-loaded nanocomposite based on graphene quantum dots combats hypoxia-induced chemoresistance of oral squamous cell carcinoma. Int. J. Nanomed. 2018, 13, 1505–1524. [Google Scholar] [CrossRef]
- Li, R.; Gao, R.; Zhao, Y.; Zhang, F.; Wang, X.; Li, B.; Wang, L.; Ma, L.; Du, J. pH-responsive graphene oxide loaded with targeted peptide and anticancer drug for OSCC therapy. Front. Oncol. 2022, 12, 930920. [Google Scholar] [CrossRef]
- Chen, G.; Yang, Z.; Yu, X.; Yu, C.; Sui, S.; Zhang, C.; Bao, C.; Zeng, X.; Chen, Q.; Peng, Q. Intratumor delivery of amino-modified graphene oxide as a multifunctional photothermal agent for efficient antitumor phototherapy. J. Colloid Interface Sci. 2023, 652, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wan, C.; Li, Y.; Jiao, X.; Liu, T.; Gu, Y.; Gao, R.; Liu, J.; Li, B. Nanocarrier-based drug delivery system with dual targeting and NIR/pH response for synergistic treatment of oral squamous cell carcinoma. Colloids Surf. B Biointerfaces 2024, 244, 114179. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, Y.; Song, Z.; Gu, Y.; Jiao, X.; Wan, C.; Liu, T.; Zhang, R.; Gao, R.; Wang, X. A Graphene-Based Lipid Modulation Nanoplatform for Synergetic Lipid Starvation/ Chemo/Photothermal Therapy of Oral Squamous Cell Carcinoma. Int. J. Nanomed. 2024, 19, 11235–11255. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-L.; Lin, K.; Yang, L. Progress in Nanocarriers Codelivery System to Enhance the Anticancer Effect of Photodynamic Therapy. Pharmaceutics 2021, 13, 1951. [Google Scholar] [CrossRef]
- Karagianni, A.; Tsierkezos, N.G.; Prato, M.; Terrones, M.; Kordatos, K.V. Application of carbon-based quantum dots in photodynamic therapy. Carbon 2023, 203, 273–310. [Google Scholar] [CrossRef]
- Gierlich, P.; Mata, A.I.; Donohoe, C.; Brito, R.M.M.; Senge, M.O.; Gomes-da-Silva, L.C. Ligand-Targeted Delivery of Photosensitizers for Cancer Treatment. Molecules 2020, 25, 5317. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Yi, C.; Chen, G.; Li, Y.; Zhou, Y.; Chen, G.; Li, Y.; He, Y.; Yu, D. Host Immune Response Triggered by Graphene Quantum-Dot-Mediated Photodynamic Therapy for Oral Squamous Cell Carcinoma. Int. J. Nanomed. 2020, 15, 9627–9638. [Google Scholar] [CrossRef]
- Shih, C.-Y.; Huang, W.-L.; Chiang, I.T.; Su, W.-C.; Teng, H. Biocompatible hole scavenger–assisted graphene oxide dots for photodynamic cancer therapy. Nanoscale 2021, 13, 8431–8441. [Google Scholar] [CrossRef]
- How, K.Y.; Song, K.P.; Chan, K.G. Porphyromonas gingivalis: An Overview of Periodontopathic Pathogen below the Gum Line. Front. Microbiol. 2016, 7, 53. [Google Scholar] [CrossRef]
- Mysak, J.; Podzimek, S.; Sommerova, P.; Lyuya-Mi, Y.; Bartova, J.; Janatova, T.; Prochazkova, J.; Duskova, J. Porphyromonas gingivalis: Major Periodontopathic Pathogen Overview. J. Immunol. Res. 2014, 2014, 476068. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Han, N.; Du, J.; Guo, L.; Luo, Z.; Liu, Y. Pathogenesis of Important Virulence Factors of Porphyromonas gingivalis via Toll-Like Receptors. Front. Cell. Infect. Microbiol. 2019, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhu, X.; Qi, Z.; Wang, C.; Mao, X.; Zhu, C.; He, Z.; Li, M.; Tang, Z. Killing Dental Pathogens Using Antibacterial Graphene Oxide. ACS Appl. Mater. Interfaces 2015, 7, 5605–5611. [Google Scholar] [CrossRef]
- Xia, M.-Y.; Xie, Y.; Yu, C.-H.; Chen, G.-Y.; Li, Y.-H.; Zhang, T.; Peng, Q. Graphene-based nanomaterials: The promising active agents for antibiotics-independent antibacterial applications. J. Control. Release 2019, 307, 16–31. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Etemad-Moghadam, S.; Alaeddini, M.; Miri Mousavi, R.s.; Bahador, A. DNA-aptamer-nanographene oxide as a targeted bio-theragnostic system in antimicrobial photodynamic therapy against Porphyromonas gingivalis. Sci. Rep. 2022, 12, 12161. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Feng, X.; Zhang, G.; Li, H.; Zhou, F.; Xie, Y.; Li, T.; Zhao, C.; Luo, W.; Xiong, Y.; et al. Artesunate ameliorates ligature-induced periodontitis by attenuating NLRP3 inflammasome-mediated osteoclastogenesis and enhancing osteogenic differentiation. Int. Immunopharmacol. 2023, 123, 110749. [Google Scholar] [CrossRef]
- Park, J.; Park, S.; Kim, J.E.; Jang, K.-J.; Seonwoo, H.; Chung, J.H. Enhanced Osteogenic Differentiation of Periodontal Ligament Stem Cells Using a Graphene Oxide-Coated Poly(ε-caprolactone) Scaffold. Polymers 2021, 13, 797. [Google Scholar] [CrossRef]
- Kawamoto, K.; Miyaji, H.; Nishida, E.; Miyata, S.; Kato, A.; Tateyama, A.; Furihata, T.; Shitomi, K.; Iwanaga, T.; Sugaya, T. Characterization and evaluation of graphene oxide scaffold for periodontal wound healing of class II furcation defects in dog. Int. J. Nanomed. 2018, 13, 2365–2376. [Google Scholar] [CrossRef]
- Li, Y.; Yang, L.; Hou, Y.; Zhang, Z.; Chen, M.; Wang, M.; Liu, J.; Wang, J.; Zhao, Z.; Xie, C.; et al. Polydopamine-mediated graphene oxide and nanohydroxyapatite-incorporated conductive scaffold with an immunomodulatory ability accelerates periodontal bone regeneration in diabetes. Bioact. Mater. 2022, 18, 213–227. [Google Scholar] [CrossRef]
- Agarwalla, S.V.; Ellepola, K.; Silikas, N.; Castro Neto, A.H.; Seneviratne, C.J.; Rosa, V. Persistent inhibition of Candida albicans biofilm and hyphae growth on titanium by graphene nanocoating. Dent. Mater. 2021, 37, 370–377. [Google Scholar] [CrossRef]
- Wei, J.; Qiao, S.; Zhang, X.; Li, Y.; Zhang, Y.; Wei, S.; Shi, J.; Lai, H. Graphene-Reinforced Titanium Enhances Soft Tissue Seal. Front. Bioeng. Biotechnol. 2021, 9, 665305. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Niu, C.; Chu, M.; Liu, M.; Chi, Y. The preparation and characterization of graphene oxide-multiwalled minocycline coatings on ultrafine-grained titanium implants for enhanced performance studies. Front. Oral Health 2025, 6, 1565325. [Google Scholar] [CrossRef] [PubMed]
- Rosa, V.; Malhotra, R.; Agarwalla, S.V.; Morin, J.L.P.; Luong-Van, E.K.; Han, Y.M.; Chew, R.J.J.; Seneviratne, C.J.; Silikas, N.; Tan, K.S.; et al. Graphene Nanocoating: High Quality and Stability upon Several Stressors. J. Dent. Res. 2021, 100, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, Q.; Zhao, W.; Qiao, B.; Cui, H.; Fan, J.; Li, H.; Tu, X.; Jiang, D. In vitro and in vivo biocompatibility and osteogenesis of graphene-reinforced nanohydroxyapatite polyamide66 ternary biocomposite as orthopedic implant material. Int. J. Nanomed. 2016, 11, 3179–3189. [Google Scholar] [CrossRef]
- Su, Z.; Zhang, J.; Tan, P.; Zhu, S.; Jiang, N. Selective Polyetheretherketone Implants Combined with Graphene Cause Definitive Cell Adhesion and Osteogenic Differentiation. Int. J. Nanomed. 2022, 17, 5327–5338. [Google Scholar] [CrossRef]
- Lu, J.; Sun, J.; Zou, D.; Song, J.; Yang, S. Graphene-Modified Titanium Surface Enhances Local Growth Factor Adsorption and Promotes Osteogenic Differentiation of Bone Marrow Stromal Cells. Front. Bioeng. Biotechnol. 2021, 8, 621788. [Google Scholar] [CrossRef]
- Kang, M.S.; Jeong, S.J.; Lee, S.H.; Kim, B.; Hong, S.W.; Lee, J.H.; Han, D.-W. Reduced graphene oxide coating enhances osteogenic differentiation of human mesenchymal stem cells on Ti surfaces. Biomater. Res. 2025, 25, 4. [Google Scholar] [CrossRef]
- Shin, Y.C.; Bae, J.-H.; Lee, J.H.; Raja, I.S.; Kang, M.S.; Kim, B.; Hong, S.W.; Huh, J.-B.; Han, D.-W. Enhanced osseointegration of dental implants with reduced graphene oxide coating. Biomater. Res. 2022, 26, 11. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Y.; Hou, Z.; Wu, J.; Liu, F.; Wu, J.; Yeung, K.W.K.; Qian, W.; Liu, X.; Kong, L.; et al. NIR-I Light-Activated Antibiotic Delivery & PDT via TiO2/Graphene Metastructure for Enhanced Antibacterial Activity and Osseointegration of Ti Implants. Adv. Healthc. Mater. 2025, 14, 2500743. [Google Scholar] [CrossRef]
- Gao, Y.; Kang, K.; Luo, B.; Sun, X.; Lan, F.; He, J.; Wu, Y. Graphene oxide and mineralized collagen-functionalized dental implant abutment with effective soft tissue seal and romotely repeatable photodisinfection. Regen. Biomater. 2022, 9, rbac024. [Google Scholar] [CrossRef]
- Pandey, A.; Nikam, A.N.; Fernandes, G.; Kulkarni, S.; Padya, B.S.; Prassl, R.; Das, S.; Joseph, A.; Deshmukh, P.K.; Patil, P.O.; et al. Black Phosphorus as Multifaceted Advanced Material Nanoplatforms for Potential Biomedical Applications. Nanomaterials 2021, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Thurakkal, S.; Zhang, X. Recent Advances in Chemical Functionalization of 2D Black Phosphorous Nanosheets. Adv. Sci. 2020, 7, 1902359. [Google Scholar] [CrossRef]
- Peng, L.; Abbasi, N.; Xiao, Y.; Xie, Z. Black Phosphorus: Degradation Mechanism, Passivation Method, and Application for In Situ Tissue Regeneration. Adv. Mater. Interfaces 2020, 7, 2001538. [Google Scholar] [CrossRef]
- Naskar, A.; Kim, K.-s. Black phosphorus nanomaterials as multi-potent and emerging platforms against bacterial infections. Microb. Pathog. 2019, 137, 103800. [Google Scholar] [CrossRef]
- Ouyang, J.; Liu, R.-Y.; Chen, W.; Liu, Z.; Xu, Q.; Zeng, K.; Deng, L.; Shen, L.; Liu, Y.-N. A black phosphorus based synergistic antibacterial platform against drug resistant bacteria. J. Mater. Chem. B 2018, 6, 6302–6310. [Google Scholar] [CrossRef]
- Acosta, S.; Quintana, M. Chemically Functionalized 2D Transition Metal Dichalcogenides for Sensors. Sensors 2024, 24, 1817. [Google Scholar] [CrossRef]
- Sun, Z.; Xie, H.; Tang, S.; Yu, X.-F.; Guo, Z.; Shao, J.; Zhang, H.; Huang, H.; Wang, H.; Chu, P.K. Ultrasmall Black Phosphorus Quantum Dots: Synthesis and Use as Photothermal Agents. Angew. Chem. Int. Ed. 2015, 54, 11526–11530. [Google Scholar] [CrossRef]
- Aksoy, İ.; Küçükkeçeci, H.; Sevgi, F.; Metin, Ö.; Hatay Patir, I. Photothermal Antibacterial and Antibiofilm Activity of Black Phosphorus/Gold Nanocomposites against Pathogenic Bacteria. ACS Appl. Mater. Interfaces 2020, 12, 26822–26831. [Google Scholar] [CrossRef]
- Ran, Y.; Shi, J.; Ding, Y.; Li, L.; Lu, D.; Zeng, Y.; Qiu, D.; Yu, J.; Cai, X.; Pan, Y. Black Phosphorus Nanosheets-Loaded Mussel-Inspired Hydrogel with Wet Adhesion, Photothermal Antimicrobial, and In Situ Remineralization Capabilities for Caries Prevention. Adv. Sci. 2024, 11, 2409155. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Pan, T.; Cui, H.; Zhao, Z.; Chu, P.K.; Yu, X.-F. Black Phosphorus: Bioactive Nanomaterials with Inherent and Selective Chemotherapeutic Effects. Angew. Chem. Int. Ed. 2019, 58, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Schröder, H.C.; Wang, X. Inorganic Polyphosphates As Storage for and Generator of Metabolic Energy in the Extracellular Matrix. Chem. Rev. 2019, 119, 12337–12374. [Google Scholar] [CrossRef] [PubMed]
- Kong, N.; Ji, X.; Wang, J.; Sun, X.; Chen, G.; Fan, T.; Liang, W.; Zhang, H.; Xie, A.; Farokhzad, O.C.; et al. ROS-Mediated Selective Killing Effect of Black Phosphorus: Mechanistic Understanding and Its Guidance for Safe Biomedical Applications. Nano Lett. 2020, 20, 3943–3955. [Google Scholar] [CrossRef]
- Li, Z.; Yang, L.; Zhang, D.; Wang, W.; Huang, Q.; Liu, Q.; Shi, K.; Yu, Y.; Gao, N.; Chen, H.; et al. Mussel-inspired “plug-and-play” hydrogel glue for postoperative tumor recurrence and wound infection inhibition. J. Colloid Interface Sci. 2023, 650, 1907–1917. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Yin, J.; Chen, Y.; Pan, S.; Yao, H.; Gao, Y.; Shi, J. 2D-Black-Phosphorus-Reinforced 3D-Printed Scaffolds: A Stepwise Countermeasure for Osteosarcoma. Adv. Mater. 2018, 30, 1705611. [Google Scholar] [CrossRef]
- Dong, W.; Wang, H.; Liu, H.; Zhou, C.; Zhang, X.; Wang, S.; He, L. Potential of Black Phosphorus in Immune-Based Therapeutic Strategies. Bioinorg. Chem. Appl. 2022, 2022, 3790097. [Google Scholar] [CrossRef]
- Qin, R.; Cao, J.; Li, J.; Qiu, D.; Lin, H.; Wang, Y.; Bian, Y.; Wang, Y.; Du, Y.; Yuan, H. Targeting iron overload and macrophage polarization to treat diabetic periodontitis: Mechanisms and therapeutic strategies. Life Sci. 2025, 377, 123805. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, Y.; Wang, R.; Wang, T.; Guo, F.; Bian, Y.; Wang, T.; Ma, Q.; Yuan, H.; Du, Y.; et al. Injectable Thermosensitive Gel CH-BPNs-NBP for Effective Periodontitis Treatment through ROS-Scavenging and Jaw Vascular Unit Protection. Adv. Healthc. Mater. 2024, 13, 2400533. [Google Scholar] [CrossRef]
- Li, X.; Ren, S.; Song, L.; Gu, D.; Peng, H.; Zhao, Y.; Liu, C.; Yang, J.; Miao, L. Combined Black Phosphorus Nanosheets with ICG/aPDT is an Effective. Int. J. Nanomed. 2023, 18, 813–827. [Google Scholar] [CrossRef]
- Tao, S.; Yang, Y.; Wu, C.; Yang, J.; Wang, Z.; Zhou, F.; Liang, K.; Deng, Y.; Li, J.; Li, J. Nanocapsuled Neutrophil Extracellular Trap Scavenger Combating Chronic Infectious Bone Destruction Diseases. Adv. Sci. 2025, 12, 2411274. [Google Scholar] [CrossRef]
- Gunathilaka, T.M.; Shimomura, M. Nanoscale Evaluation of the Degradation Stability of Black Phosphorus Nanosheets Functionalized with PEG and Glutathione-Stabilized Doxorubicin Drug-Loaded Gold Nanoparticles in Real Functionalized System. Molecules 2024, 29, 1746. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Huang, D.; Zhao, C.; Zhao, Y. Abalone-Inspired Adhesive and Photo-Responsive Microparticle Delivery Systems for Periodontal Drug Therapy. Adv. Sci. 2022, 9, 2202829. [Google Scholar] [CrossRef]
- He, Y.; Tang, Y.; Zeng, B.; Chen, X.; Yuan, L.; Lu, Y.; Du, W.; Li, R.; Han, Y.; Deng, F.; et al. Black phosphorus quantum dot-modified ADSCs as a novel therapeutic for periodontitis bone loss coupling of osteogenesis and osteoimmunomodulation. Mater. Today Bio 2024, 27, 101122. [Google Scholar] [CrossRef]
- Zhong, X.; Zhang, Y.; Tan, L.; Zheng, T.; Hou, Y.; Hong, X.; Du, G.; Chen, X.; Zhang, Y.; Sun, X. An aluminum adjuvant-integrated nano-MOF as antigen delivery system to induce strong humoral and cellular immune responses. J. Control. Release 2019, 300, 81–92. [Google Scholar] [CrossRef]
- Xue, B.; Geng, X.; Cui, H.; Chen, H.; Wu, Z.; Chen, H.; Li, H.; Zhou, Z.; Zhao, M.; Tan, C.; et al. Size engineering of 2D MOF nanosheets for enhanced photodynamic antimicrobial therapy. Chin. Chem. Lett. 2023, 34, 108140. [Google Scholar] [CrossRef]
- Xu, X.; Ding, M.; Liu, K.; Lv, F.; Miao, Y.; Liu, Y.; Gong, Y.; Huo, Y.; Li, H. The synthesis and highly effective antibacterial properties of Cu-3, 5-dimethy l-1, 2, 4-triazole metal organic frameworks. Front. Chem. 2023, 11, 1124303. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, Y.; Liu, J.; Lin, Y.; Jiao, J.; Chen, B.; Wang, W.; Wu, S.; Li, C. Photothermal therapy with regulated Nrf2/NF-κB signaling pathway for treating bacteria-induced periodontitis. Bioact. Mater. 2022, 9, 428–445. [Google Scholar] [CrossRef]
- Adegoke, K.A.; Adegoke, O.R.; Adigun, R.A.; Maxakato, N.W.; Bello, O.S. Two-dimensional metal-organic frameworks: From synthesis to biomedical, environmental, and energy conversion applications. Coord. Chem. Rev. 2022, 473, 214817. [Google Scholar] [CrossRef]
- Farasati Far, B.; Rabiee, N.; Iravani, S. Environmental implications of metal–organic frameworks and MXenes in biomedical applications: A perspective. RSC Adv. 2023, 13, 34562–34575. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, L.; Li, M.; Shu, H.; Jia, N.; Gao, Y.; Shi, R.; Yang, X.; Zhang, Z.; Zhang, L. Anti-osteosarcoma trimodal synergistic therapy using NiFe-LDH and MXene nanocomposite for enhanced biocompatibility and efficacy. ACTA Pharm. Sin. B 2024, 14, 1329–1344. [Google Scholar] [CrossRef]
- Yu, Y.; You, Z.; Li, X.; Lou, F.; Xiong, D.; Ye, L.; Wang, Z. Injectable Nanocomposite Hydrogels with Strong Antibacterial, Osteoinductive, and ROS-Scavenging Capabilities for Periodontitis Treatment. ACS Appl. Mater. Interfaces 2024, 16, 14421–14433. [Google Scholar] [CrossRef]
- Yu, C.; Sui, S.; Yu, X.; Huang, W.; Wu, Y.; Zeng, X.; Chen, Q.; Wang, J.; Peng, Q. Ti3C2Tx MXene loaded with indocyanine green for synergistic photothermal and photodynamic therapy for drug-resistant bacterium. Colloids Surf. B Biointerfaces 2022, 217, 112663. [Google Scholar] [CrossRef]
- Afolabi, M.A.; Xiao, D.; Chen, Y. The Impact of Surface Chemistry and Synthesis Conditions on the Adsorption of Antibiotics onto MXene Membranes. Molecules 2024, 29, 148. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Li, F.; Wang, Y.; Zou, H.; Shang, J.; Fan, Y.; Liu, H.; Xu, Z.; Li, R.; Liu, H. MXene-modified 3D printed scaffold for photothermal therapy and facilitation of oral mucosal wound reconstruction. Mater. Des. 2023, 227, 111731. [Google Scholar] [CrossRef]
- Cui, D.; Kong, N.; Ding, L.; Guo, Y.; Yang, W.; Yan, F. Ultrathin 2D Titanium Carbide MXene (Ti3C2Tx) Nanoflakes Activate WNT/HIF-1α-Mediated Metabolism Reprogramming for Periodontal Regeneration. Adv. Healthc. Mater. 2021, 10, 2101215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, S.; Ding, N.; Ma, P.; Zhang, Z. Two-dimensional MXene/nano-hydroxyapatite nanocomposite promotes osteogenesis by photothermal conversion. Ceram. Int. 2023, 49, 29001–29009. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, F.; Cao, L.; Wang, H.; Hu, J.; Qiu, S.; Liu, Y.; Zhang, Y.; Wang, J.; Jiang, X. ROS-balancing-engineered bio-heterojunction hydrogel accelerated the infected bone regeneration based on Sono-chemo dynamic therapy. Bioact. Mater. 2025, 52, 440–459. [Google Scholar] [CrossRef]
- Kang, M.S.; Yu, Y.; Park, R.; Heo, H.J.; Lee, S.H.; Hong, S.W.; Kim, Y.H.; Han, D.-W. Highly Aligned Ternary Nanofiber Matrices Loaded with MXene Expedite Regeneration of Volumetric Muscle Loss. Nano-Micro Lett. 2024, 16, 73. [Google Scholar] [CrossRef]
- Jo, H.J.; Kang, M.S.; Heo, H.J.; Jang, H.J.; Park, R.; Hong, S.W.; Kim, Y.H.; Han, D.-W. Skeletal muscle regeneration with 3D bioprinted hyaluronate/gelatin hydrogels incorporating MXene nanoparticles. Int. J. Biol. Macromol. 2024, 265, 130696. [Google Scholar] [CrossRef]
- Yang, C.; Luo, Y.; Lin, H.; Ge, M.; Shi, J.; Zhang, X. Niobium Carbide MXene Augmented Medical Implant Elicits Bacterial Infection Elimination and Tissue Regeneration. ACS Nano 2021, 15, 1086–1099. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Zhao, S.; Dong, W.; Ma, W.; Zhou, X.; Wang, Y.; Zhang, M. Surface Modification of Carbon Fiber-Reinforced Polyetheretherketone with MXene Nanosheets for Enhanced Photothermal Antibacterial Activity and Osteogenicity. ACS Biomater. Sci. Eng. 2022, 8, 2375–2389. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, W.; Yang, C.; Zhang, X.; Lyu, C. Sulfonated polyetheretherketone enriched with MXene V2C promotes bone formation via WNT/β-catenin signaling in bone marrow mesenchymal stem cells. Med. Eng. Phys. 2025, 141, 104361. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Wang, X.; Tian, Z.; Jia, F.; Wang, J.; Xie, L.; Lan, J.; Han, P.; Lin, H.; Huang, X.; et al. Controlled-release of cinnamaldehyde from MXene/ZIF8/gelatin composite coatings: An integrated strategy to combat implant-associated infection. Colloids Surf. B Biointerfaces 2025, 251, 114615. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, Y.; Mo, A. Multilayered Titanium Carbide MXene Film for Guided Bone Regeneration. Int. J. Nanomed. 2019, 14, 10091–10103. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lu, X.; Chai, Q.; Huang, B.; Dai, S.; Wang, P.; Liu, J.; Zhao, Z.; Li, X.; Liu, B.; et al. Engineered Niobium Carbide MXenzyme-Integrated Self-Adaptive Coatings Inhibiting Periprosthetic Osteolysis by Orchestrating Osteogenesis–Osteoclastogenesis Balance. ACS Appl. Mater. Interfaces 2024, 16, 29805–29822. [Google Scholar] [CrossRef]
- Asadi Tokmedash, M.; Min, J. Designer Micro-/Nanocrumpled MXene Multilayer Coatings Accelerate Osteogenesis and Regulate Macrophage Polarization. ACS Appl. Mater. Interfaces 2024, 16, 21415–21426. [Google Scholar] [CrossRef]
- Rothammer, B.; Feile, K.; Werner, S.; Frank, R.; Bartz, M.; Wartzack, S.; Schubert, D.W.; Drummer, D.; Detsch, R.; Wang, B.; et al. Ti3C2T x -UHMWPE Nanocomposites—Towards an Enhanced Wear-Resistance of Biomedical Implants. J. Biomed. Mater. Res. Part A 2025, 113, e37819. [Google Scholar] [CrossRef]
- Bagheri, S.; Farokhnezhad, M.; Esmaeilzadeh, M. Transition metal dichalcogenide coated gold nanoshells for highly effective photothermal therapy. Phys. Chem. Chem. Phys. 2023, 25, 33038–33047. [Google Scholar] [CrossRef]
- Tyagi, N.; Arya, R.K.K.; Bisht, D.; Wadhwa, P.; Kumar Upadhyay, T.; Kumar Sethiya, N.; Jindal, D.K.; Pandey, S.; Kumar, D. Mechanism and potentialities of photothermal and photodynamic therapy of transition metal dichalcogenides (TMDCs) against cancer. Luminescence 2024, 39, e4770. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J. Hybrid nanomaterials of WS2 or MoS2 nanosheets with liposomes: Biointerfaces and multiplexed drug delivery. Nanoscale 2017, 9, 13187–13194. [Google Scholar] [CrossRef]
- Tang, M.; Ma, R.; Ding, Y.; Fei, Y.; Wang, Y.; Wang, G.; Zhang, C.; Chen, Y.; Dong, X.; Chen, P.; et al. Tailored regulation of ferroptosis in periodontitis using thermosensitive ionic liquid hydrogel based on galangin/L-cysteine-decorated MoS2 nanoflowers. J. Control. Release 2025, 384, 113928. [Google Scholar] [CrossRef]
- Yu, P.; Li, Y.; Sun, H.; Zhang, H.; Kang, H.; Wang, P.; Xin, Q.; Ding, C.; Xie, J.; Li, J. Mimicking Antioxidases and Hyaluronan Synthase: A Zwitterionic Nanozyme for Photothermal Therapy of Osteoarthritis. Adv. Mater. 2023, 35, 2303299. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, C.; Chen, X.; Liu, J.; Yu, Q.; Liu, Y.; Liu, J. Drug Delivery System Based on Near-Infrared Light-Responsive Molybdenum Disulfide Nanosheets Controls the High-Efficiency Release of Dexamethasone to Inhibit Inflammation and Treat Osteoarthritis. ACS Appl. Mater. Interfaces 2019, 11, 11587–11601. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zou, H.; Wu, X.; Chen, Y.; Situ, B.; Zheng, L.; Yang, G. Fullerene-like MoS2 Nanoparticles as Cascade Catalysts Improving Lubricant and Antioxidant Abilities of Artificial Synovial Fluid. ACS Biomater. Sci. Eng. 2019, 5, 3079–3088. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Soyhan, I.; Warszawik, E.; van Rijn, P. Layered Double Hydroxides: Recent Progress and Promising Perspectives Toward Biomedical Applications. Adv. Sci. 2024, 11, 2306035. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, B.; Xu, X.; Su, M.; Xi, M.; Yin, Z. Dextran sulfate–modified pH-sensitive layered double hydroxide nanocomposites for treatment of rheumatoid arthritis. Drug Deliv. Transl. Res. 2021, 11, 1096–1106. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Kolmas, J.; Przekora, A. Biological Response to Macroporous Chitosan-Agarose Bone Scaffolds Comprising Mg- and Zn-Doped Nano-Hydroxyapatite. Int. J. Mol. Sci. 2019, 20, 3835. [Google Scholar] [CrossRef]
- Bian, Y.; Cai, X.; Lv, Z.; Xu, Y.; Wang, H.; Tan, C.; Liang, R.; Weng, X. Layered Double Hydroxides: A Novel Promising 2D Nanomaterial for Bone Diseases Treatment. Adv. Sci. 2023, 10, 2301806. [Google Scholar] [CrossRef]
- Chen, J.; Guan, X.; Chen, L.; Zheng, B.; Li, F.; Fang, C.; Fu, Y.; Li, X.; Wang, H.; Zhou, Y. Customized Hydrogel System for the Spatiotemporal Sequential Treatment of Periodontitis Propelled by ZEB1. Adv. Sci. 2025, 12, 2503338. [Google Scholar] [CrossRef]
- Niu, J.; Guo, Y.; Jing, G.; Wang, H.; Yang, L.; Li, Y.; Gao, Y.; Wang, H.; Li, A.; Xu, X.; et al. Anion-Dependent Layered Double Hydroxide Nanoparticles Regulate Differentiation of CD206+ CX3CR1+ Macrophages by Inhibiting the IL-17 Signaling Pathway Contributing to Inflammatory Bowel Disease. Adv. Funct. Mater. 2024, 34, 2305042. [Google Scholar] [CrossRef]
- Gu, Z.; Wu, A.; Li, L.; Xu, Z.P. Influence of Hydrothermal Treatment on Physicochemical Properties and Drug Release of Anti-Inflammatory Drugs of Intercalated Layered Double Hydroxide Nanoparticles. Pharmaceutics 2014, 6, 235–248. [Google Scholar] [CrossRef]
- Yang, H.-y.; van Ee, R.J.; Timmer, K.; Craenmehr, E.G.M.; Huang, J.H.; Öner, F.C.; Dhert, W.J.A.; Kragten, A.H.M.; Willems, N.; Grinwis, G.C.M.; et al. A novel injectable thermoresponsive and cytocompatible gel of poly(N-isopropylacrylamide) with layered double hydroxides facilitates siRNA delivery into chondrocytes in 3D culture. Acta Biomater. 2015, 23, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gianfreda, F.; Danesi, C.; Bollero, P.; Ermini, A.; Pizzoferrato, R.; Nicolai, E. In Situ Growth of Mg-Fe Layered Double Hydroxides (LDH) Film on Titanium Dental Implant Substrates for pH Regulation in Oral Environments. Crystals 2023, 13, 1636. [Google Scholar] [CrossRef]
- Yin, Y.; Jian, L.; Li, B.; Liang, C.; Han, X.; Zhao, X.; Wang, D. Mg-Fe layered double hydroxides modified titanium enhanced the adhesion of human gingival fibroblasts through regulation of local pH level. Mater. Sci. Eng. C 2021, 131, 112485. [Google Scholar] [CrossRef]
- Liang, L.; Yin, Y.; Guo, Z.; Liu, T.; Ouyang, Z.; Zhou, J.; Xiao, J.; Zhao, L.; Wu, H. Sequentially activating macrophages M1 and M2 phenotypes by lipopolysaccharide-containing Mg-Fe layered double hydroxides coating on the Ti substrate. Colloids Surf. B Biointerfaces 2023, 222, 113066. [Google Scholar] [CrossRef]
- Chu, M.; Sun, Z.; Fan, Z.; Yu, D.; Mao, Y.; Guo, Y. Bi-directional regulation functions of lanthanum-substituted layered double hydroxide nanohybrid scaffolds via activating osteogenesis and inhibiting osteoclastogenesis for osteoporotic bone regeneration. Theranostics 2021, 11, 6717–6734. [Google Scholar] [CrossRef]
- Wu, W.; Sun, X.; Zhu, C.; Zhang, F.; Zeng, R.; Zou, Y.-H.; Li, S. Biocorrosion resistance and biocompatibility of Mg–Al layered double hydroxide/poly-L-glutamic acid hybrid coating on magnesium alloy AZ31. Prog. Org. Coat. 2020, 147, 105746. [Google Scholar] [CrossRef]
- Cheng, S.; Lan, L.; Li, M.; Chu, X.; Zhong, H.; Yao, M.; Peng, F.; Zhang, Y. Pure Mg–Al Layered Double Hydroxide Film on Magnesium Alloys for Orthopedic Applications. ACS Omega 2021, 6, 24575–24584. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, V.; Reina, G.; Orecchioni, M.; Furesi, G.; Thiele, S.; Gardin, C.; Zavan, B.; Cuniberti, G.; Bianco, A.; Rauner, M.; et al. Stimulation of bone formation by monocyte-activator functionalized graphene oxide in vivo. Nanoscale 2019, 11, 19408–19421. [Google Scholar] [CrossRef]
- Liu, X.; George, M.N.; Li, L.; Gamble, D.; Miller Ii, A.L.; Gaihre, B.; Waletzki, B.E.; Lu, L. Injectable Electrical Conductive and Phosphate Releasing Gel with Two-Dimensional Black Phosphorus and Carbon Nanotubes for Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2020, 6, 4653–4665. [Google Scholar] [CrossRef]
- Li, B.L.; Li, R.; Zou, H.L.; Ariga, K.; Li, N.B.; Leong, D.T. Engineered functionalized 2D nanoarchitectures for stimuli-responsive drug delivery. Mater. Horiz. 2020, 7, 455–469. [Google Scholar] [CrossRef]
- Xiong, J.; Gao, H. Matrix metalloproteases-responsive nanomaterials for tumor targeting diagnosis and treatment. J. Microencapsul. 2017, 34, 440–453. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, Z.; Zhang, M.; Lang, P.; Li, J.; Liu, Z.; Zhang, Z.; Li, L.; Zhang, L. Cuproptosis-immunotherapy using PD-1 overexpressing T cell membrane-coated nanosheets efficiently treats tumor. J. Control. Release 2023, 362, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, X.; Zhao, N.; Chen, H.; Guo, G. Advancements in pH-responsive nanocarriers: Enhancing drug delivery for tumor therapy. Expert Opin. Drug Deliv. 2023, 20, 1623–1642. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, G.; Lu, B.; Qian, Z.; Peng, Q. Protein corona formed in the gastrointestinal tract and its impacts on oral delivery of nanoparticles. Med. Res. Rev. 2021, 41, 1835–1850. [Google Scholar] [CrossRef]
- Xu, W.; Xu, M.; Xiao, Y.; Yu, L.; Xie, H.; Jiang, X.; Chen, M.; Gao, H.; Wang, L. Changes in target ability of nanoparticles due to protein corona composition and disease state. Asian J. Pharm. Sci. 2022, 17, 401–411. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, X.; Sun, L.; Wei, Y.; Wei, X. Cellular Toxicity and Immunological Effects of Carbon-based Nanomaterials. Part. Fibre Toxicol. 2019, 16, 18. [Google Scholar] [CrossRef]
- Khalid, Z.; Hadi, F.; Xie, J.; Chandrabose, V.; Oh, J.-M. The Future of MXenes: Exploring Oxidative Degradation Pathways and Coping with Surface/Edge Passivation Approach. Small 2025, 21, 2407856. [Google Scholar] [CrossRef]
- Guan, G.; Han, M.-Y. Functionalized Hybridization of 2D Nanomaterials. Adv. Sci. 2019, 6, 1901837. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Liang, M.; Li, C.; Ji, X.; Zhang, J.; Xie, W.; Reis, R.L.; Li, F.-R.; Gu, S.; Wang, Y. Design Strategies of Tumor-Targeted Delivery Systems Based on 2D Nanomaterials. Small Methods 2022, 6, 2200853. [Google Scholar] [CrossRef]


| Diseases | Materials | Author | Methods | Mechanism |
|---|---|---|---|---|
| Dental Caries | Ag@nGO | Arslan et al. [75] | Silver nanoparticles synthesized by chamomile extract (biogenic) or chemical methods were immobilized on nano-graphene oxide (nGO) to form Ag@nGO NCs, which were then added (0.05% w/w) into dental adhesives (Clearfil SE Bond). | Ag+ release combined with graphene-induced membrane damage and ROS generation provided strong antibacterial and antibiofilm effects against oral pathogens. |
| GO-Cu NCs | Mao et al. [76] | Copper nanoparticles are anchored onto graphene oxide to form stable nanocomposites with sustained Cu2+ release. | Disrupting S. mutans biofilm formation by impairing EPS matrix synthesis, downregulating gtfB/C, gbpB, upregulating rnc, and interfering with carbohydrate metabolism, while maintaining good biocompatibility. | |
| AGONSs | Lu et al. [77] | Ethylenediamine-modified GO nanosheets with enhanced photothermal activity | Kills S. mutans via positive charge binding, membrane cutting, and NIR-induced photothermal/ROS effects. | |
| BAG@GO | Lee et al. [78] | BAG synthesized by sol–gel method and combined with GO, then incorporated at 1%, 3%, 5% into orthodontic adhesives. Mechanical, antibacterial, and anti-demineralization properties were tested. | BAG released Ca2+/PO43− ions to buffer acidity and promote remineralization, while GO provided antibacterial effects via oxidative stress and bacterial membrane damage. Together, BAG@GO adhesives showed improved microhardness, anti-demineralization, and antibacterial efficacy. | |
| MBN@GOQD | Son et al. [79] | MBN synthesized by modified sol–gel method and subsequently coated with GOQD using colloidal processing. | GOQD promotes rapid nucleation and deposition of hydroxyapatite while maintaining Ca, Si, and P ion release, thereby enhancing remineralization and effectively sealing dentinal tubules for desensitization. | |
| FG | Shaheen et al. [80] | FG nanosheets were synthesized by hydrothermal method and prepared as a gel | FG significantly increased the microhardness of enamel (especially at depths of 100–150 μm) and improved the Ca/P ratio, as well as the color (ΔE00); its mechanism is related to fluoride doping promoting the formation of fluorapatite, enhancing acid resistance and penetration. n-HAp primarily promotes surface remineralization by providing Ca2+ and PO43− ions. | |
| Oral Squamous Cell Carcinoma (OSCC) | GO-PEI-miR-214 | Ou et al. [89] | Functionalized GO with PEI used for miRNA inhibitor delivery | The complex prevents the progression of OSCC by suppressing miR-214 levels, leading to increased expression of PTEN and p53, and inhibiting the PI3K/Akt pathway. It shows strong anticancer effects both in vitro and in vivo without affecting organ tissues. |
| PEG-GQDs-Pt (GPt) | Wei et al. [90] | Graphene quantum dots synthesized by chemical oxidation, covalently bound with cisplatin (Pt) and PEGylated | Overcomes hypoxia-induced chemoresistance by enhancing Pt accumulation, inducing S-phase arrest and apoptosis, and increasing tumor targeting via EPR effect, thereby inhibiting OSCC growth with reduced systemic toxicity. | |
| DOX@NGO-BBN-AF750 | Li et al. [91] | Carboxylated nano-graphene oxide (NGO) non-covalently coupled with bombesin antagonist peptide (BBN-AF750) and doxorubicin (DOX) through π–π stacking and hydrogen bonding | GRPR-targeted and pH-responsive nanocarrier enabling imaging-guided therapy; promotes tumor-specific DOX release, enhances uptake by HSC-3 cells, shows dose- and pH-dependent cytotoxicity, improves stability, prolongs drug half-life, and achieves effective OSCC inhibition. | |
| AGO | Chen et al. [92] | Graphene oxide (GO) modified with amino groups through chemical reaction with ethylenediamine | AGO demonstrates significantly enhanced photothermal effects under near-infrared (NIR) irradiation, with improved cell uptake and retention in tumor tissues. It induces cell apoptosis and effectively inhibits tumor growth both in vitro (HSC-3 cells) and in vivo (tumor-bearing mice), without affecting normal tissues. AGO is promising for photothermal therapy (PTT) of oral squamous cell carcinoma (OSCC). | |
| DOX@GO-HA-HN-1 | Li et al. [93] | Graphene oxide (GO) functionalized with hyaluronic acid (HA) and HN-1 peptide for dual-targeted drug delivery | The system synergistically enhances drug delivery via HA/CD44 and HN-1 targeting, while NIR irradiation promotes localized DOX release and photothermal therapy (PTT), improving OSCC treatment efficacy. In vitro and in vivo results demonstrate high targeting efficiency, reduced toxicity, and enhanced therapeutic outcomes. | |
| Graphene oxide(GO)+SB-204990+DOX | Li et al. [94] | SB-204990 and DOX loaded onto carboxylated graphene oxide nanoplatform (NSD) through hydrogen bonding and π-π stacking | Triple therapy (lipid starvation, chemotherapy, and photothermal therapy) synergistically increases intracellular drug concentration, significantly inhibiting tumor growth. Photothermal therapy enhances drug effectiveness and reduces chemotherapy resistance. | |
| GQD-PEG | Zhang et al. [98] | Graphene Quantum Dots (GQDs) covalently bonded with Polyethylene Glycol (PEG) through chemical reactions | The composite material generates singlet oxygen (1O2) under light activation, inducing strong phototoxicity and leading to OSCC cell apoptosis. In vivo, it enhances tumor accumulation via the EPR effect, provides targeted therapy, reduces systemic toxicity, and triggers significant host immune responses with increased CD8 T cells and pro-inflammatory cytokines (such as IFN-γ, TNF-α), showing excellent anti-tumor effects. | |
| NGOD-AA | Shi et al. [99] | NGODs synthesized from natural graphite via modified Hummers’ method, NH3 annealing, nitric acid oxidation, and hydrothermal treatment in NH4OH; combined with AA as a hole scavenger | NGODs (∼4.4 nm, highly crystalline) act as photosensitizers for PDT; AA scavenges photogenerated holes, shifting mechanism from Type-II (1O2) to Type-I, efficiently producing H2O2 under white light. This selectively killed oral cancer (OECM-1), lung (PC-9), head & neck (HONE-1), and colon (HCT-116) cancer cells via apoptosis and necrosis, while sparing normal fibroblasts and keratinocytes, showing high biocompatibility and tumor selectivity. | |
| Periodontitis | DNA-aptamer-NGO | Pourhajibagher et al. [105] | NGO synthesized via modified Hummer’s method, then conjugated with FAM-labeled DNA aptamer specific to P. gingivalis | Targeted aPDT under 980 nm diode laser irradiation induced ROS generation, reduced P. gingivalis viability by 4.33 Log10, disrupted biofilms, suppressed virulence gene expression (fimA, rgpA), upregulated oxidative stress gene (oxyR), and decreased metabolic activity, while showing low cytotoxicity and hemocompatibility. |
| PCL-GO | Park et al. [107] | 3D printed PCL scaffold fabricated by melt-extrusion, surface treated with oxygen plasma for 5 min, then dip-coated with GO solution (0.125–0.5 mg/mL) prepared via modified Hummer’s method | GO coating with plasma treatment enhanced scaffold hydrophilicity, protein adsorption, and PDLSC adhesion, significantly promoted osteogenic differentiation (increased ALP activity, calcium deposition, and osteopontin expression), improved osteoconductivity while reducing GO consumption compared to polymer blending. | |
| GO Scaffold | Kawamoto et al. [108] | Graphene oxide (GO) was dispersed onto a 3D collagen scaffold using a GO dispersion method | The GO scaffold enhances periodontal tissue healing in dog class II furcation defects. It promotes bone regeneration, formation of periodontal ligament-like and cementum-like tissues, and enhances cell migration and proliferation. The GO scaffold shows improved tissue formation compared to untreated scaffolds with minimal cytotoxicity. | |
| PGO-PHA-AG Scaffold | Li et al. [109] | Reduced graphene oxide (GO) and hydroxyapatite (PHA) were co-functionalized with polydopamine (PDA) to form a conductive scaffold. | The scaffold possesses multiple functions including antioxidative properties, immunomodulation, and conductivity. It regulates the diabetic periodontal microenvironment, promotes osteogenic differentiation of BMSCs, reduces M1 macrophage polarization, activates M2 macrophages to secrete osteogenesis-related cytokines, and consequently promotes periodontal bone regeneration. | |
| Peri-implantitis | TiGD/TiGV | Agarwalla et al. [110] | Graphene coating was deposited on titanium surfaces using chemical vapor deposition with a vacuum-assisted technique. The coating process was repeated 2 times (TiGD) or 5 times (TiGV). | The graphene coating significantly inhibited Candida albicans biofilm formation and hyphal growth. The study found that, regardless of the number of layers, the graphene nanocoating effectively prevented mature biofilm formation, reduced microbial attachment, and hindered biofilm maturation. This coating strategy offers long-lasting effects in preventing microbial attachment without relying on antibiotics. |
| Ti-0.125G | Wei et al. [111] | Graphene powder was mixed with titanium powder, followed by ultrasonic dispersion and ball milling. The mixture was sintered under vacuum at 900 °C and 50 MPa, resulting in a graphene-reinforced titanium composite. | Combines graphene and titanium to enhance antibacterial properties and soft tissue integration for dental implants. It significantly reduces bacterial biofilm formation (e.g., S. mutans, F. nucleatum, P. gingivalis), promotes gingival fibroblast (HGF) adhesion, proliferation, and migration, and improves soft tissue sealing without compromising bioactivity. The mechanism involves electron transfer disrupting bacterial respiration and decreasing microbial vitality. | |
| Graphene Oxide-Minocycline Composite Coating | Liu et al. [112] | Electrochemical deposition and liquid-phase deposition techniques | The coating is synthesized by depositing graphene oxide (GO) on the surface of ultrafine-grained titanium and loading minocycline (MC) on it. The coating demonstrates antibacterial, osteogenic, and anti-inflammatory effects. The experiments show that the coating exhibits significant antibacterial properties against Staphylococcus aureus and effectively inhibits microbial adhesion and biofilm formation, with no significant toxicity to osteoblasts. It enhances the long-term stability and antibacterial capacity of the implant. | |
| Graphene-Coated Titanium Sheets | Lu et al. [116] | The titanium sheets, treated with SLA, were coated with graphene (rGO) through chemical reduction and modified chemically to enhance their ability to adsorb growth factors | The graphene coating enhanced the osteogenic capability of the titanium sheets by adsorbing and sustainedly releasing concentrated growth factors, promoting osteogenic differentiation of bone marrow stromal cells (BMSCs), and activating the RhoA/ROCK1/ERK1/2 signaling pathway, significantly accelerating bone formation. It exhibited excellent biocompatibility and bone repair potential. | |
| rGO-Ti | Kang et al. [117] | Graphene oxide (GO) is sonicated in water, then reduced with hydrazine hydrate to obtain reduced graphene oxide (rGO). rGO is uniformly coated on titanium (Ti) substrates using the MDD technique | The rGO coating enhances the hydrophilicity of the Ti substrate, promoting the proliferation and osteogenic differentiation of human mesenchymal stem cells (hMSCs). The material improves bone integration and has potential applications in orthopedics and dental implants. | |
| rGO-ST | Shin et al. [118] | Reduced graphene oxide (rGO) coating on SLA-treated titanium surfaces | rGO coating enhances osteogenic differentiation and osteointegration by improving surface wettability and protein adsorption. It promotes cell attachment, proliferation, and mineralization, leading to significantly increased bone formation and higher bone-to-implant contact (BIC) in vivo. |
| Material Category | Representative Materials | Main Advantages | Main Disadvantages |
|---|---|---|---|
| Graphene and Derivatives | Graphene (GO, rGO, GQDs, AGO, FG) | 1. Extremely high specific surface area, enhancing drug loading capacity and surface reactivity; 2. High conductivity and thermal conductivity, suitable for photothermal therapy (PTT); 3. Good biocompatibility, can be functionalized for improved targeting; 4. Excellent antibacterial properties, particularly effective against S. mutans and other oral pathogens. | 1. High production cost, especially for derivatives (e.g., GO), which tend to aggregate, affecting stability; 2. Long-term biodegradability issues, may cause immune response or toxicity; 3. Limited application in complex oral environments over long periods. |
| BP | Black Phosphorus Nanosheets (BPNSs), Nitrogen-Doped BP Quantum Dots (BPQDs) | 1. Good biodegradability, degradation products are non-toxic phosphates that aid bone regeneration; 2. Strong photothermal conversion ability, suitable for photothermal therapy (PTT); 3. Photodynamic therapy (PDT) efficacy; 4. Strong antioxidant capacity, regulates immune response. | 1. Poor photothermal stability, easy to degrade in air; 2. Excessive degradation may lead to short-lived therapeutic effects; 3. Synthesis process may introduce impurities, affecting biological safety. |
| MXene | Ti3C2Tx, Nb2C, etc. | 1. High conductivity, good hydrophilicity, suitable for drug delivery; 2. Strong photothermal properties, suitable for photothermal therapy (PTT); 3. Effectively scavenges ROS, has antioxidant and immunomodulatory functions; 4. High surface reactivity, suitable for surface modification and functionalization. | 1. Insufficient biodegradability and long-term stability, may release metal ions leading to toxicity; 2. May cause immune response in certain environments; 3. Difficult to control high-efficiency preparation and stability. |
| LDHs | ZnAl-LDH, CuAl-LDH, MgFe-LDH, etc. | 1. Releases various metal ions, regulates immune response; 2. Good biocompatibility, suitable for long-term drug delivery; 3. High surface area and ion exchange capacity, enhancing drug loading and release. | 1. Ion release may cause accumulation in the body; 2. Poor stability in aqueous environments, may lead to degradation issues; 3. Long-term safety and stability need further validation. |
| TMDs | MoS2, WS2, etc. | 1. Efficient photothermal conversion, suitable for photothermal therapy (PTT); 2. Can be surface-modified to enhance drug delivery functions; 3. Synergizes with other therapies (e.g., PDT) to enhance efficacy; 4. Has antioxidant properties, scavenges ROS. | 1. May have structural defects during preparation, affecting performance; 2. Photothermal efficiency is influenced by material size and surface conditions; 3. Photostability and biocompatibility need further research. |
| 2D MOFs | Fe2O3-Porphyrin MOF, etc. | 1. Porous structure with high drug loading capacity; 2. Drug release can be controlled through functionalization and metal node modulation; 3. Strong photothermal and photodynamic therapy capabilities, suitable for targeted treatment; 4. Tunable surface chemistry, easy for surface modification. | 1. Complex preparation processes, difficult for large-scale production; 2. Poor water stability, may degrade in the oral environment; 3. Biocompatibility is not fully validated, posing challenges for clinical translation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Meng, R.; Wang, Y.; Sun, Y.; Qiao, J.; Yao, Y.; Peng, Q. Advances of Functional Two-Dimensional Nanomaterials in the Treatment of Oral Diseases. Bioengineering 2025, 12, 1021. https://doi.org/10.3390/bioengineering12101021
Xu Z, Meng R, Wang Y, Sun Y, Qiao J, Yao Y, Peng Q. Advances of Functional Two-Dimensional Nanomaterials in the Treatment of Oral Diseases. Bioengineering. 2025; 12(10):1021. https://doi.org/10.3390/bioengineering12101021
Chicago/Turabian StyleXu, Ziyi, Rong Meng, Yue Wang, Yuxuan Sun, Jiao Qiao, Yang Yao, and Qiang Peng. 2025. "Advances of Functional Two-Dimensional Nanomaterials in the Treatment of Oral Diseases" Bioengineering 12, no. 10: 1021. https://doi.org/10.3390/bioengineering12101021
APA StyleXu, Z., Meng, R., Wang, Y., Sun, Y., Qiao, J., Yao, Y., & Peng, Q. (2025). Advances of Functional Two-Dimensional Nanomaterials in the Treatment of Oral Diseases. Bioengineering, 12(10), 1021. https://doi.org/10.3390/bioengineering12101021






