Synthesis of the Supramolecular Structure of Vanadium Pentoxide Nanoparticles with Native and Modified β-Cyclodextrins for Antimicrobial Performance
Abstract
1. Introduction
2. Results and Discussion
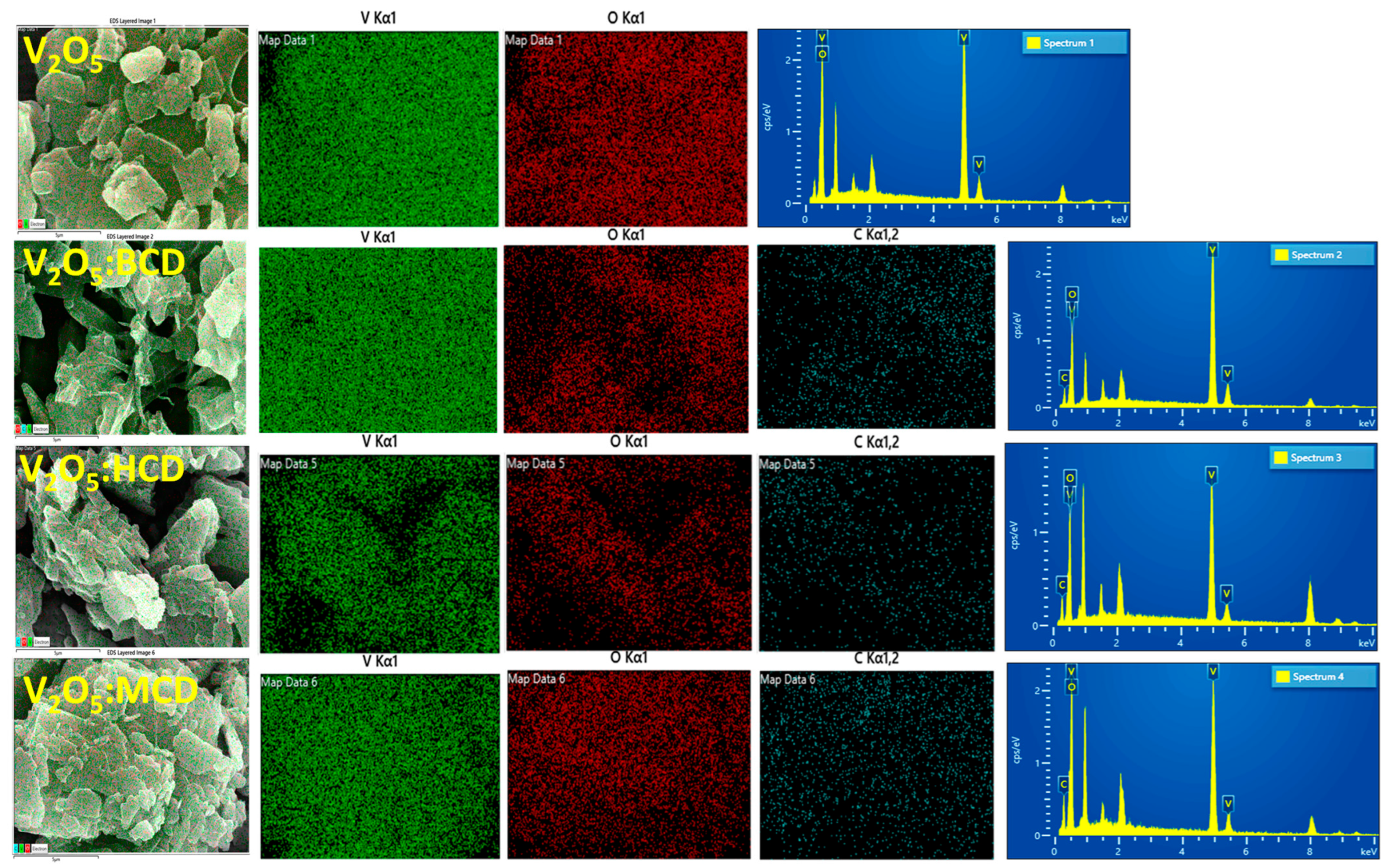
2.1. Characterization of V2O5 NPs, Including Loaded with CDs
2.1.1. Powder XRD Analysis
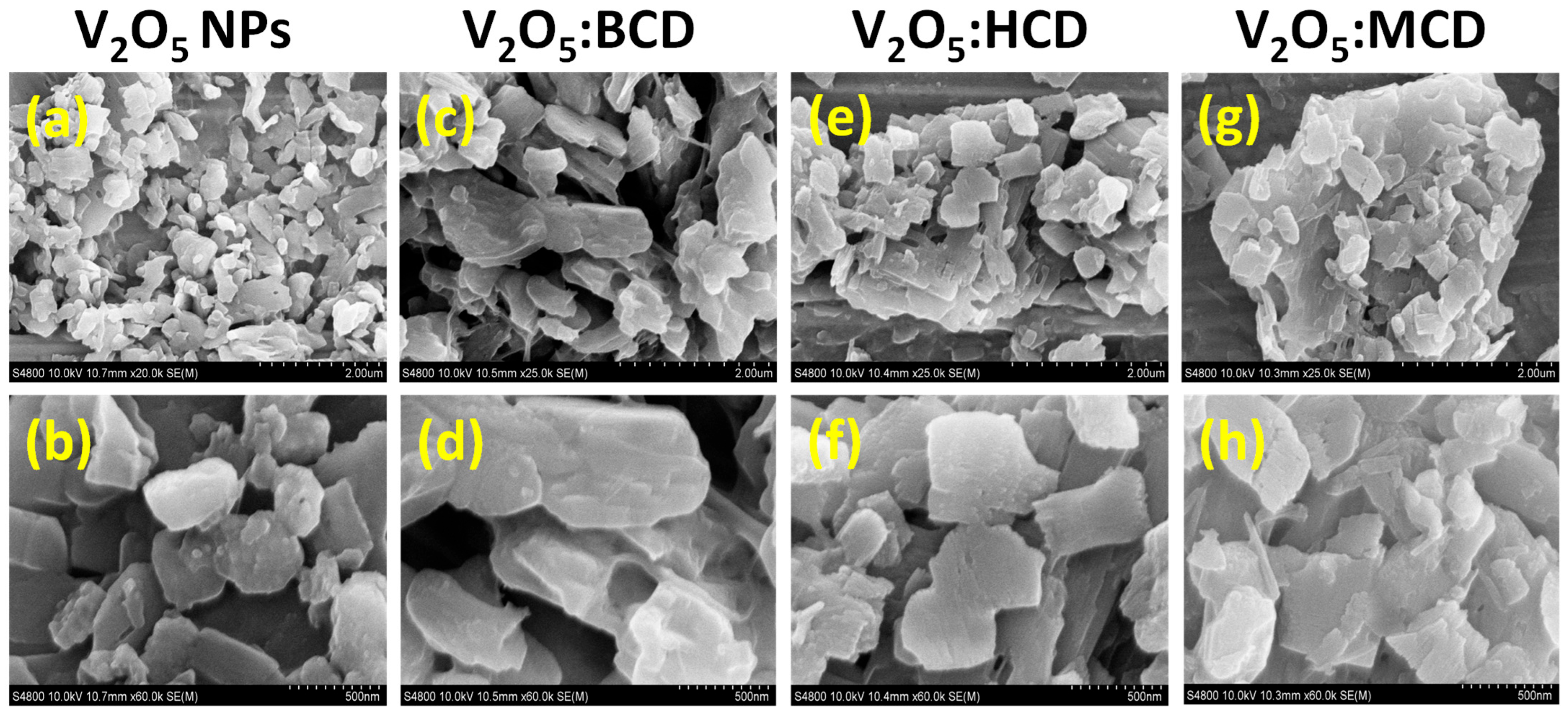
2.1.2. FE-SEM Analysis
2.1.3. FT-IR Spectral Analysis
2.1.4. 1H NMR Spectral Analysis
1H NMR Spectral Analysis of BCD
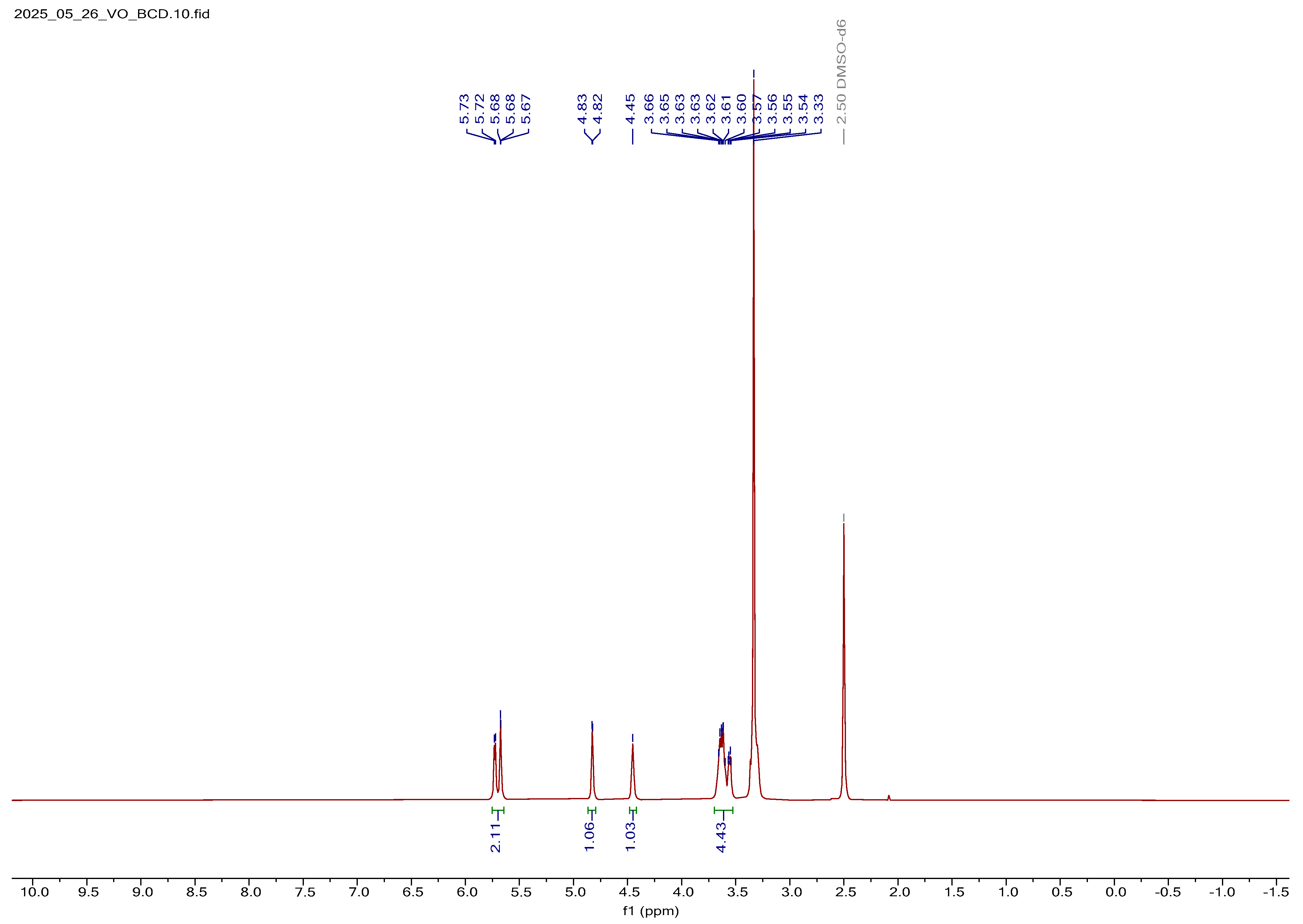
1H NMR Spectral Analysis of V2O5-Loaded BCD
1H NMR Spectral Analysis of HCD
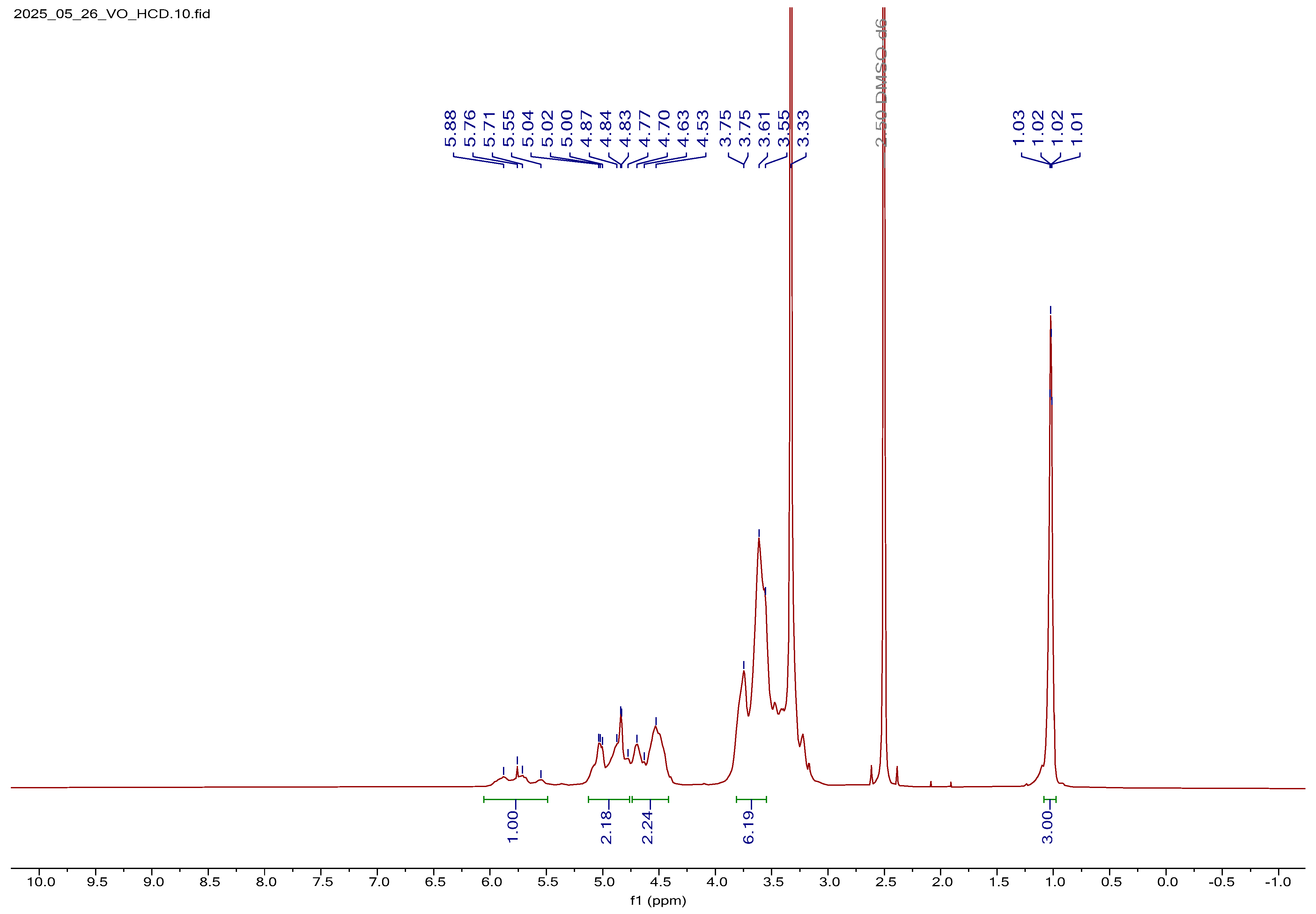
1H NMR Spectral Analysis of V2O5:HCD
Analysis of MCD 1H NMR Spectrum
1H NMR Spectral Analysis of V2O5:MCD
2.2. Antifungal and Antibacterial Efficacy of NPs
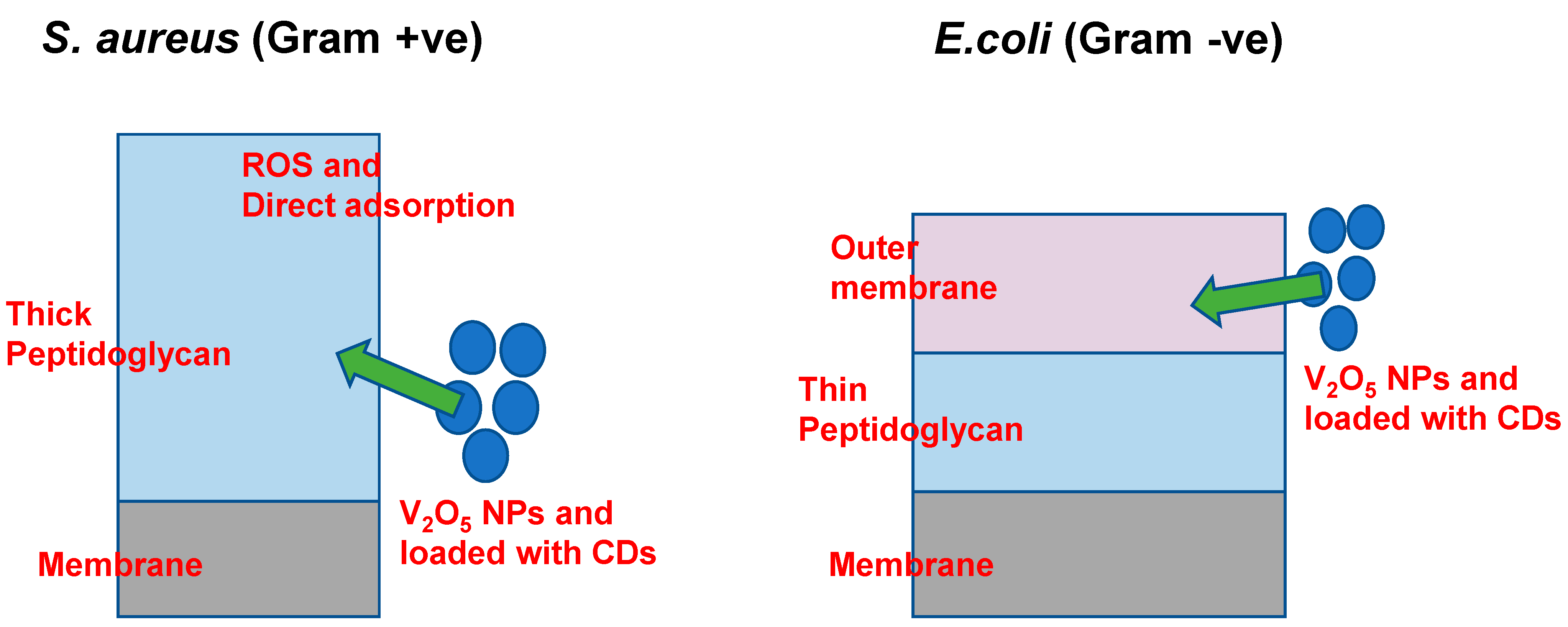
2.3. Proposed Antimicrobial Mechanism of V2O5 NPs
2.4. Limitations of the Current Study
2.5. Future Scope of the Current Study
3. Materials and Methods
3.1. Materials
3.2. Synthesis of V2O5 NPs
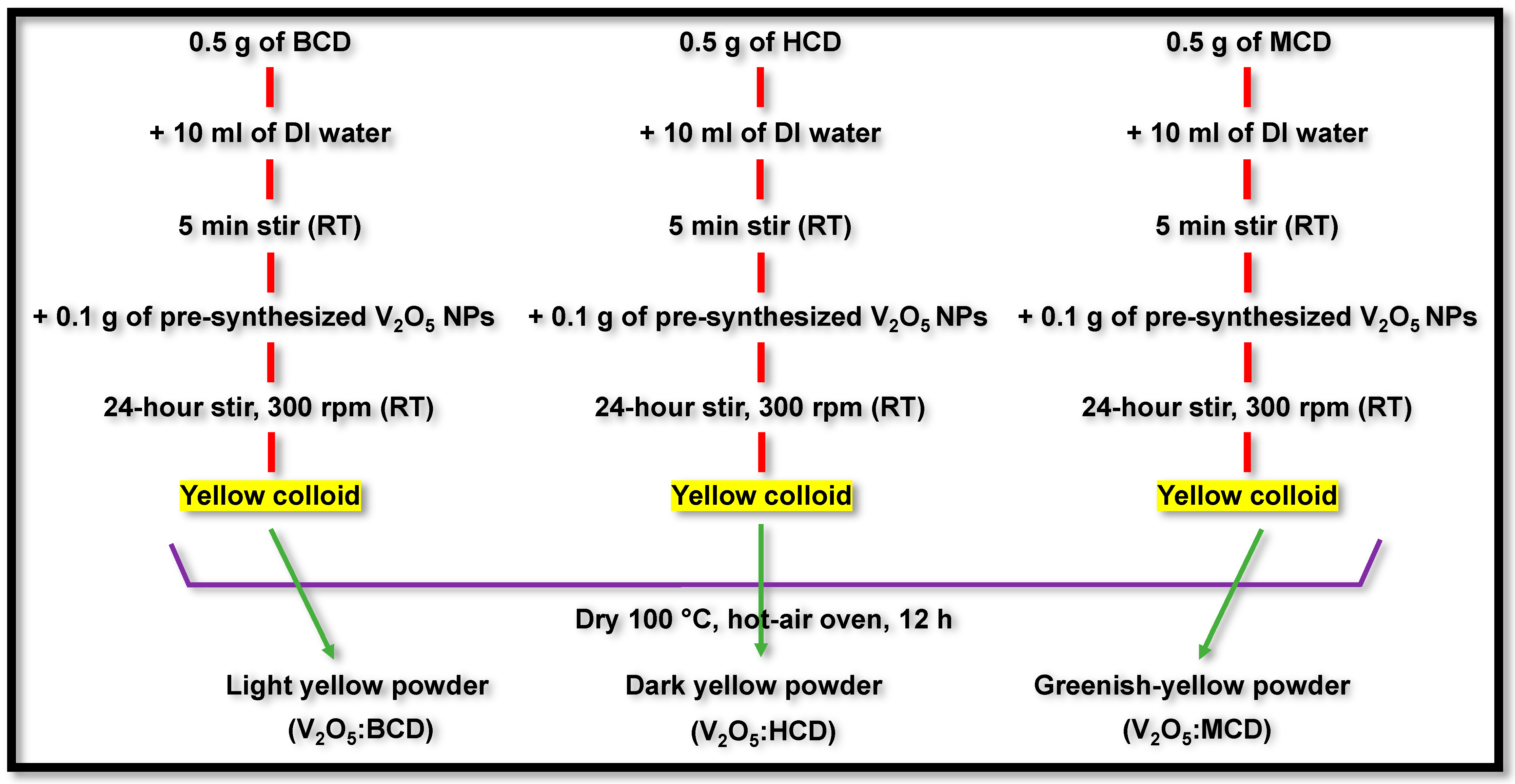
3.3. Synthesis of V2O5:BCD, V2O5:HCD, and V2O5:MCD
3.4. Antibacterial and Antifungal Activity (Agar Well Diffusion Method)
3.5. Instruments Used
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, W.; Rhim, J.W. Titanium dioxide (TiO2) for the manufacture of multifunctional active food packaging films. Food Packag. Shelf Life 2022, 31, 100806. [Google Scholar] [CrossRef]
- Nikolic, M.V.; Vasiljevic, Z.Z.; Auger, S.; Vidic, J. Metal oxide nanoparticles for safe active and intelligent food packaging. Trends Food Sci. Technol. 2021, 116, 655–668. [Google Scholar] [CrossRef]
- Stuparu-Cretu, M.; Braniste, G.; Necula, G.-A.; Stanciu, S.; Stoica, D.; Stoica, M. Metal Oxide Nanoparticles in Food Packaging and Their Influence on Human Health. Foods 2023, 12, 1882. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, G.; Indira Priyadharsini, C.; Ranjith, R.; Thammasak, R.; Orawan, R.; Pazhanivel, T.; Sanya, S. Synergistic Enhancement for Photocatalytic and Antibacterial Properties of Vanadium Pentoxide (V2O5) Nanocomposite Supported on Multi-Walled Carbon Nanotubes. J. Clust. Sci. 2025, 36, 75. [Google Scholar]
- Nguyen, H.H.; Truong, T.V.A.; Nguyen, M.T.; Nguyen, H.S.; Le Dao, H.Y.; Nguyen, M.D.; Nguyen, T.H.N.; Tran, D.D.; Dang, T.C.M.; Nguyen, T.H.; et al. Characterization and biological prospects of various calcined temperature-V2O5 nanoparticles synthesized by Citrus hystrix fruit extract. Biochem. Biophys. Res. Commun. 2024, 719, 150043. [Google Scholar]
- Ali, J.H.; Uday, M.N.; Majid, S.J.; Falah, A.-H. Mutlak, Synthesis of vanadium pentoxide nanoparticles via laser ablation for biomedical applications. Int. J. Mod. Phys. B 2024, 38, 2450230. [Google Scholar]
- Bajpai, V.K.; Kamle, M.; Shukla, S.; Mahato, D.K.; Chandra, P.; Hwang, S.K.; Kumar, P.; Huh, Y.S.; Han, Y.K. Prospects of using nanotechnology for food preservation, safety, and security. J. Food. Drug. Anal. 2018, 26, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, H.M.; Youssef, A.M.; Abdel-Aziz, M.S. Development of antimicrobial nanocomposite films based on V2O5 nanoparticles embedded into biopolymer matrices for food packaging applications. Food Packag. Shelf Life 2020, 26, 100567. [Google Scholar]
- Khan, M.S.; Zaidi, A.; Wani, P.A. Role of nanomaterials in biological systems. In Nanotechnology in Soil Science and Plant Nutrition; Springer Publications: Berlin/Heidelberg, Germany, 2009; pp. 129–143. [Google Scholar]
- Emamifar, A.; Kadivar, M.; Shahedi, M.; Soleimanian-Zad, S. Evaluation of nanocomposite packaging containing Ag and ZnO on shelf life of fresh orange juice. Inn. Food. Sci. Emerg. Tech. 2011, 11, 742–748. [Google Scholar] [CrossRef]
- Acosta, A.A.D.; Camarillo, E.; Cano, F.J.; Reyes-Vallejo, O.; Olvera, M.D.L.L. Sustainable design on manufacturing V2O5 nanoparticles and analysis of their material properties for CO gas sensors. Adv. Nat. Sci. Nanosci. Nanotechnol. 2025, 16, 025019. [Google Scholar]
- Negrescu, A.M.; Killian, M.S.; Raghu, S.N.V.; Schmuki, P.; Mazare, A.; Cimpean, A. Metal Oxide Nanoparticles: Review of Synthesis, Characterization and Biological Effects. J. Funct. Biomater. 2022, 13, 274. [Google Scholar] [CrossRef]
- Alameen, A.S.; Undre, S.B.; Undre, P.B. Synthesis, dispersion, functionalization, biological and antioxidant activity of metal oxide nanoparticles: Review. Nano-Struct. Nano-Objects 2024, 39, 101298. [Google Scholar] [CrossRef]
- Peng, H.; Ping, H.; Tuan, D.V.; Ming, L.; Shancheng, W.; Yujie, K.; Xianting, Z.; Liqiang, M.; Yi, L. Vanadium Oxide: Phase Diagrams, Structures, Synthesis, and Applications. Chem. Rev. 2023, 123, 4353–4415. [Google Scholar] [CrossRef] [PubMed]
- Goni-Ciaurriz, L.; Gonz’alez-Gaitano, G.; V’elaz, I. Cyclodextrin-grafted nanoparticles as food preservative carriers. Int. J. Pharm. 2020, 588, 119664. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gaitano, G.; Isasi, J.; Velaz, I.; Zornoza, A. Drug carrier systems based on cyclodextrin supramolecular assemblies and polymers: Present and perspectives. Curr. Pharm. Des. 2017, 23, 411–432. [Google Scholar] [CrossRef]
- Yaowen, L.; Dur, E.S.; Saeed, A.; Yue, W.; Rui, L.; Jianwu, D.; Suqing, L.; Wen, Q. Recent advances in cyclodextrin-based films for food packaging. Food. Chem. 2022, 370, 131026. [Google Scholar]
- Velázquez-Contreras, F.; Zamora-Ledezma, C.; López-González, I.; Meseguer-Olmo, L.; Núñez-Delicado, E.; Gabaldón, J.A. Cyclodextrins in Polymer-Based Active Food Packaging: A Fresh Look at Nontoxic, Biodegradable, and Sustainable Technology Trends. Polymers 2022, 14, 104. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, T.M.; Al-Rasheed, H.H.; Alaqil, Z.M.; Hajri, A.K.; Elsayed, N.H. Green Synthesis of Magnetic Supramolecules β-Cyclodextrin/Iron Oxide Na-noparticles for Photocatalytic and Antibacterial Applications. ACS Omega 2023, 8, 32067–32077. [Google Scholar] [CrossRef]
- Sherin, P.; Sunny, K. Studies on the antibacterial activity of water-soluble iron oxide nanoparticle-cyclodextrin aggregates against selected human pathogenic bacteria. Nano-Struct. Nano-Objects 2018, 16, 347–353. [Google Scholar]
- Leire, G.-C.; Pablo, R.-V.; Carlos, G.; Itziar, V. Photocatalytic and antibacterial performance of β-cyclodextrin-TiO2 nanoparticles loading sorbic and benzoic acids. Colloids Interface Sci. Commun. 2023, 57, 100747. [Google Scholar]
- Arti, H.; Vishal, R.P.; Serhii, T. Investigation of Aloe vera mediated ZnO-β-CD nanocomposite for photocatalysis and antimicrobial applications. Sci. Rep. 2025, 15, 22871. [Google Scholar]
- Anakha, D.R.; Vyshnavi, T.V.; Sabarinathan, P.; Ramasamy, Y.; Vishal, B. Beta cyclodextrin stabilized cupric oxide nanoparticles assisted thermal therapy for lung tumor and its effective in vitro anticancer activity. Sci. Rep. 2025, 15, 28983. [Google Scholar] [CrossRef]
- Maya, I.; Madona, L.; Anne, P.; Jean-Marc, G.; Olivier, G.; Jean-François, L. Beneficial effect of β-cyclodextrin assisted synthesis of CuO/Hydroxyapatite catalyst in toluene oxidation. ChemCatChem 2023, 15, e202200943. [Google Scholar]
- Subramanian, R.; Sakthivel, P.; Nagarathinam, K.; Ponnusamy, V. Photocatalytic effect of β-cyclodextrin on semiconductors for the removal of Acid Violet dye under UV light irradiation. Desalination Water Treat. 2014, 52, 3432–3444. [Google Scholar] [CrossRef]
- Sakthivel, P.; Ponnusamy, V. Modification of the photocatalytic performance of various metal oxides by the addition of β-cyclodextrin under visible light irradiation. J. Water Process Eng. 2017, 16, 329–337. [Google Scholar] [CrossRef]
- Peter, S.; Harald, K. Residual stress determination in thin films by X-ray diffraction and the widespread analytical practice applying a biaxial stress model: An outdated oversimplification? Appl. Surf. Sci. 2021, 541, 148531. [Google Scholar] [CrossRef]
- Subramanian, R.; Sakthivel, P.; Nagarathinam, K.; Ponnusamy, V. Enhanced photocatalytic activity of metal oxides/β-cyclodextrin nanocomposites for decoloration of Rhodamine B dye under solar light irradiation. Appl. Water Sci. 2017, 7, 115–127. [Google Scholar]
- Lalithamba, H.S.; Akshitha, D.N.; Aisha, S.; Prashanth, G.K.; Nagendra, G. Green synthesis of V2O5 nanoparticles: Anticancer, antioxidant activity, application in biodiesel production and amino acid derived thioacids. Results Chem. 2025, 15, 102260. [Google Scholar] [CrossRef]
- Yew, Y.P.; Shameli, K.; Mohamad, S.E.; Lee, K.X.; Teow, S.-Y. Green synthesized montmorillonite/carrageenan/Fe3O4 nanocomposites for pH-responsive release of protocatechuic acid and its anticancer activity. Int. J. Mol. Sci. 2020, 21, 4851. [Google Scholar] [CrossRef]
- Rajaram, R.; Sonaimuthu, M.; Sekar, A.; Eun, H.C.; Fatiha, M.; Neour, L.; Yong, R.L. Wa-ter-soluble inclusion complexes for a novel anti-viral agent with low toxicity; Oseltamivir with the β-cyclodextrins. J. Mol. Liq. 2022, 366, 120297. [Google Scholar]
- Rajaram, R.; Sonaimuthu, M.; Sekar, A.; Eun, H.C.; Fatiha, M.; Neour, L.; Kuppusamy, M.; Yong, R.L. A novel and water-soluble material for coronavirus inactivation from oseltamivir in the cavity of methyl and sulfated-β-cyclodextrins through inclusion complexation. J. Pharm. Biomed. Anal. 2022, 221, 115057. [Google Scholar]
- Loftsson, T.; Duchêne, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Jha, E.; Panda, P.K.; Thirumurugan, A. Antibacterial activity of vanadium pentoxide nanostructures: Role of ROS-mediated DNA damage and lipid peroxidation. J. Inorg. Biochem. 2020, 203, 110909. [Google Scholar]
- Declan, A.G.; Biwen, W.; Margareth, S.; Fabián, A.C.; Jurian, W.; Rupa, R.; Pamela, G.; Kürşad, T.; Michaela, W.; Henrik, S.; et al. Membrane depolarization kills dormant Bacillus subtilis cells by generating a lethal dose of ROS. Nat. Commun. 2024, 15, 6877. [Google Scholar] [CrossRef]
- Subramanian, P.; David, S.A. Antibacterial Activity of V2O5 Nanoparticles. Uttar Pradesh J. Zool. 2024, 45, 446–450. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Thian, E.S.; Wang, M.; Wang, Z. Antibacterial activity of nanomaterials against Gram-positive and Gram-negative bacteria: A review. J. Mat. Sci. Tech. 2018, 34, 1825–1834. [Google Scholar]
- Natan, M.; Banin, E. From nano to micro: Using nanotechnology to combat micro-organisms and their multidrug resistance. FEMS Microbiol. Rev. 2017, 41, 302–322. [Google Scholar] [CrossRef]
- Jinhua, L.; Huaijuan, Z.; Jiaxing, W.; Donghui, W.; Ruxiang, S.; Xianlong, Z.; Ping, J.; Xuanyong, L. Oxidative stress-mediated selective antimicrobial ability of nano-VO2 against Gram-positive bacteria for environmental and biomedical applications. Nanoscale 2016, 8, 11907–11923. [Google Scholar]
- Yael, N.S.; Jason, A.; Urs, O.H.; Horacio, B. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar]
- Chakradhar, S.B.; Nagesh, G.Y.; Ambika Prasad, M.V.N. Synthesis, Spectral Characterization, and Antibacterial and Antifungal Studies of PANI/V2O5 Nanocomposites. Int. J. Chem. Eng. 2016, 6, 3479248. [Google Scholar]
- Ali, A.T.; Abdul Karem, L.K. Biosynthesis, Characterization, Adsorption and Antimicrobial studies of Vanadium Oxide Nanoparticles Using Punica Granatum Extract. Baghdad. Sci. J. 2024, 21, 17. [Google Scholar] [CrossRef]



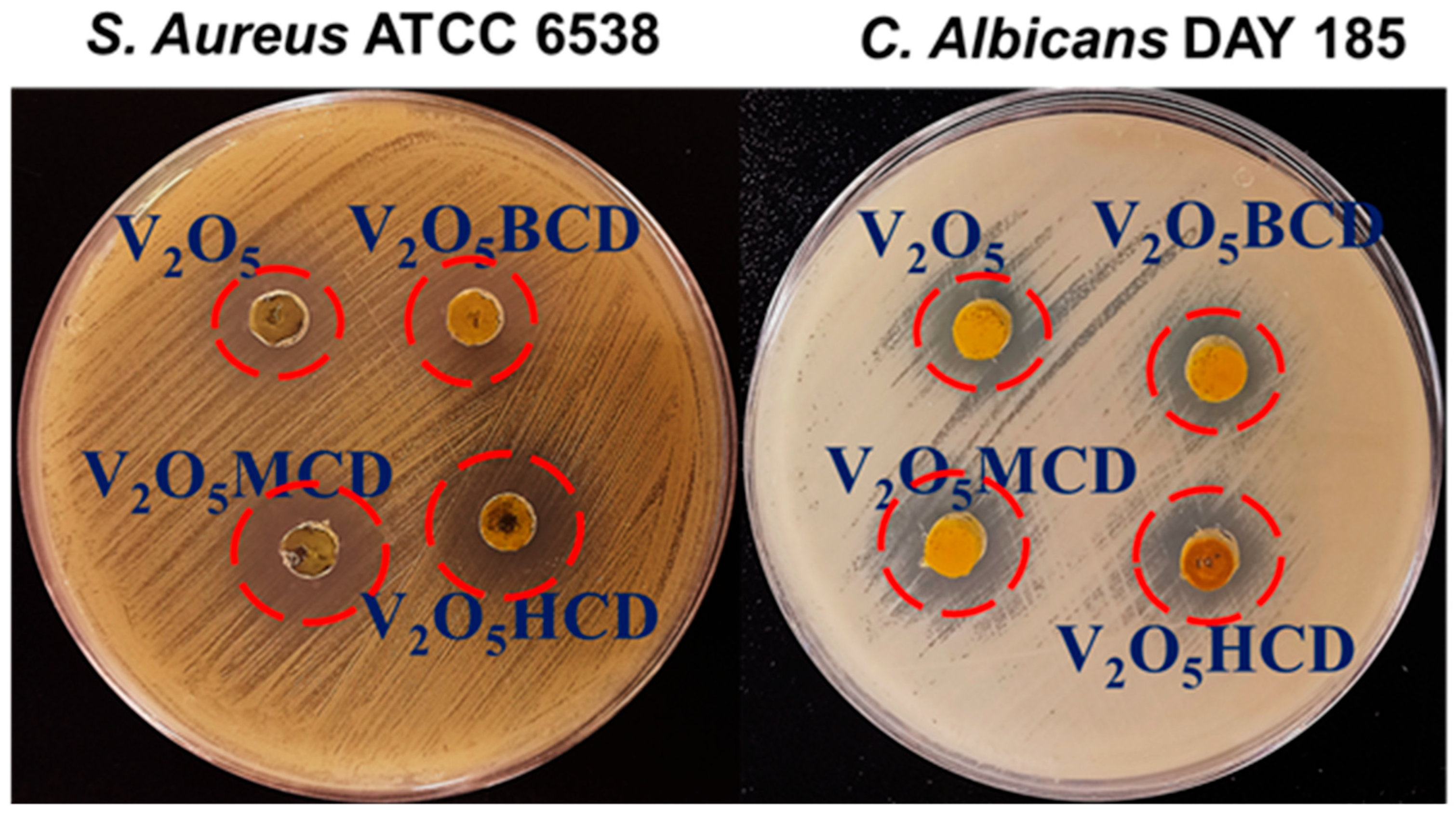
| Bacterial Strains | Zone of Inhibition (mm) | |||
|---|---|---|---|---|
| Control (V2O5) | (V2O5:BCD) | (V2O5:HCD) | (V2O5:MCD) | |
| S. aureus | 7.3 ± 0.2 | 8.5 ± 0.3 | 9.3 ± 0.9 | 11.4 ± 0.9 |
| E. coli | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| C. albicans | 6.2 ± 0.3 | 6.4 ± 0.1 | 7.8 ± 0.4 | 10.2 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajamohan, R.; Thamaraiselvi, K.; Raorane, C.J.; Murugavel, K.; Govindasamy, C.; Kim, S.-C.; Sun, S. Synthesis of the Supramolecular Structure of Vanadium Pentoxide Nanoparticles with Native and Modified β-Cyclodextrins for Antimicrobial Performance. Bioengineering 2025, 12, 1010. https://doi.org/10.3390/bioengineering12101010
Rajamohan R, Thamaraiselvi K, Raorane CJ, Murugavel K, Govindasamy C, Kim S-C, Sun S. Synthesis of the Supramolecular Structure of Vanadium Pentoxide Nanoparticles with Native and Modified β-Cyclodextrins for Antimicrobial Performance. Bioengineering. 2025; 12(10):1010. https://doi.org/10.3390/bioengineering12101010
Chicago/Turabian StyleRajamohan, Rajaram, Kanagaraj Thamaraiselvi, Chaitany Jayprakash Raorane, Kuppusamy Murugavel, Chandramohan Govindasamy, Seong-Cheol Kim, and Seho Sun. 2025. "Synthesis of the Supramolecular Structure of Vanadium Pentoxide Nanoparticles with Native and Modified β-Cyclodextrins for Antimicrobial Performance" Bioengineering 12, no. 10: 1010. https://doi.org/10.3390/bioengineering12101010
APA StyleRajamohan, R., Thamaraiselvi, K., Raorane, C. J., Murugavel, K., Govindasamy, C., Kim, S.-C., & Sun, S. (2025). Synthesis of the Supramolecular Structure of Vanadium Pentoxide Nanoparticles with Native and Modified β-Cyclodextrins for Antimicrobial Performance. Bioengineering, 12(10), 1010. https://doi.org/10.3390/bioengineering12101010











