A 3D Epithelial–Mesenchymal Co-Culture Model of the Airway Wall Using Native Lung Extracellular Matrix
Abstract
1. Introduction
2. Materials and Methods
2.1. Processing of Lung Tissue and Primary Cells
2.2. Decellularization
2.3. ECM Hydrogel Preparation
2.4. ECM Hydrogel Rheology
2.5. Primary Human Fibroblast and Epithelial Cell Isolation and Expansion
2.6. Collagen IV Coating and Live/Dead Staining
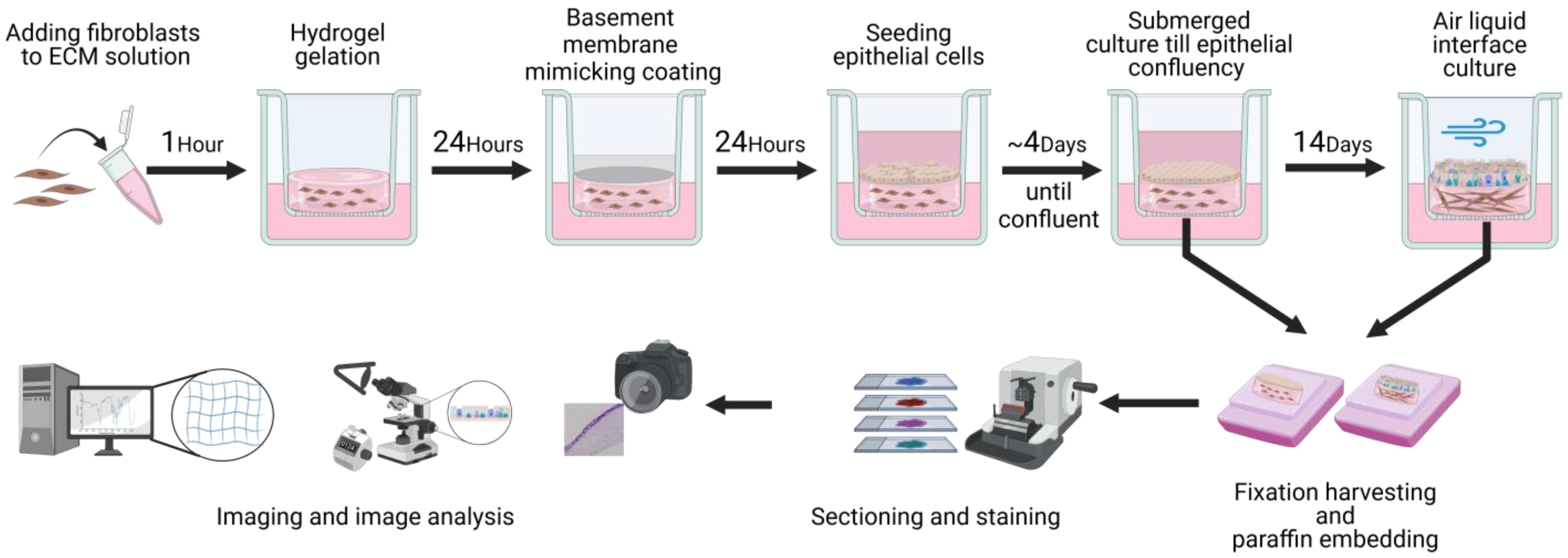
2.7. 3D Primary Fibroblast and Epithelial Cell Co-Culture
2.8. Histology and Immunohistochemistry
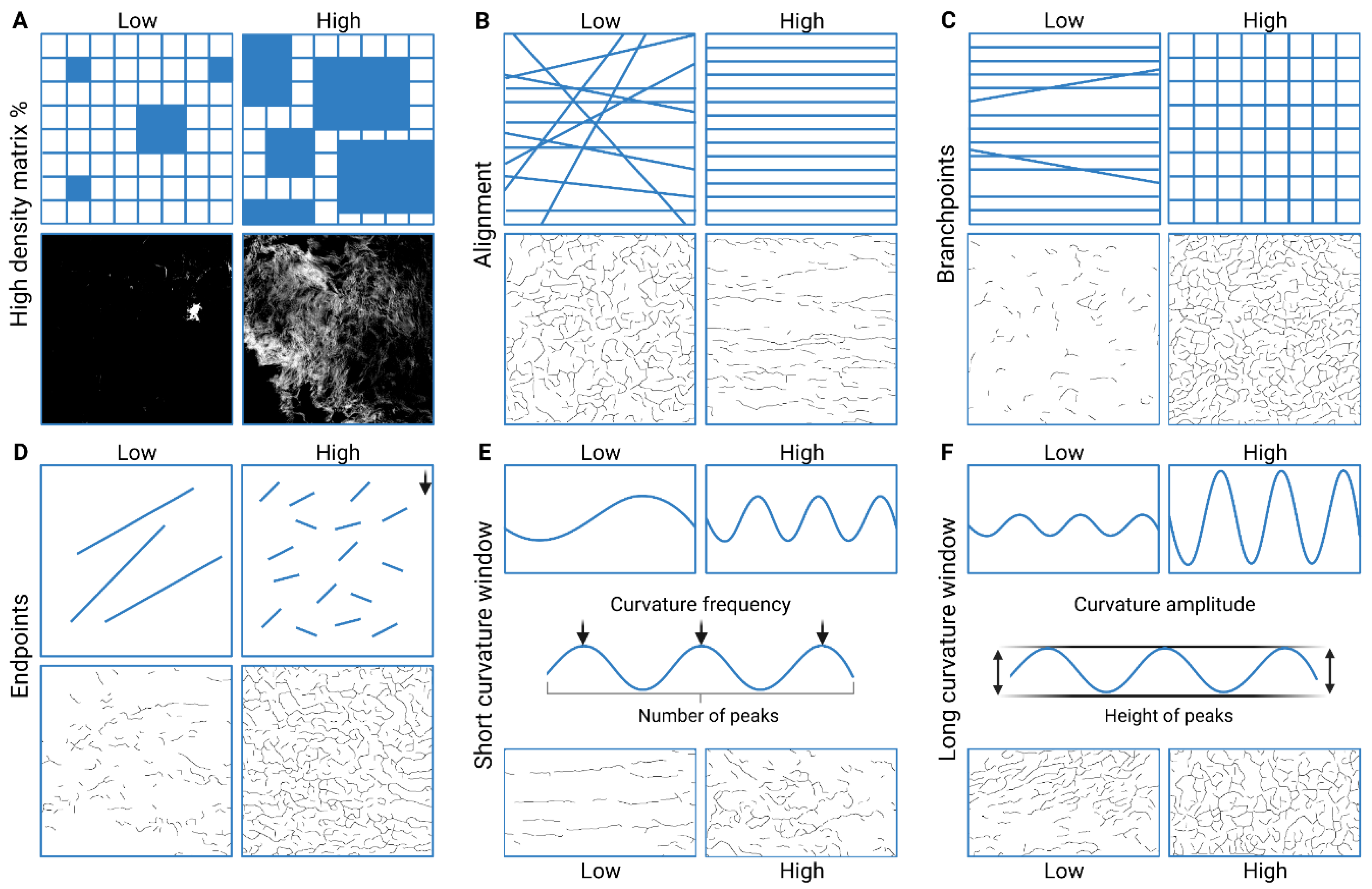
2.9. Imaging and Image Analysis
2.10. Statistical Analyses
3. Results
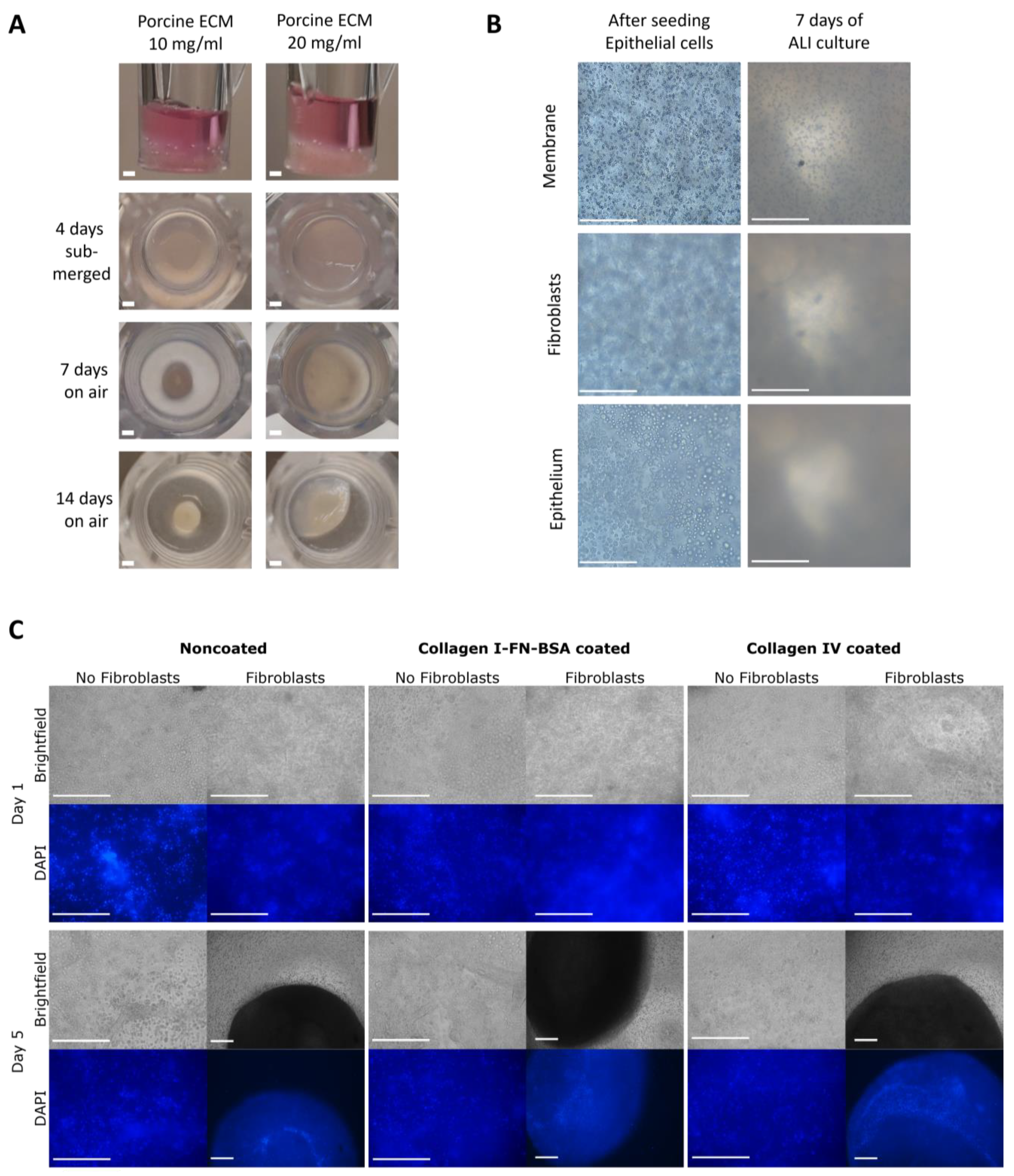
3.1. Collagen IV Coating of ECM Hydrogel Containing Human Lung Fibroblasts
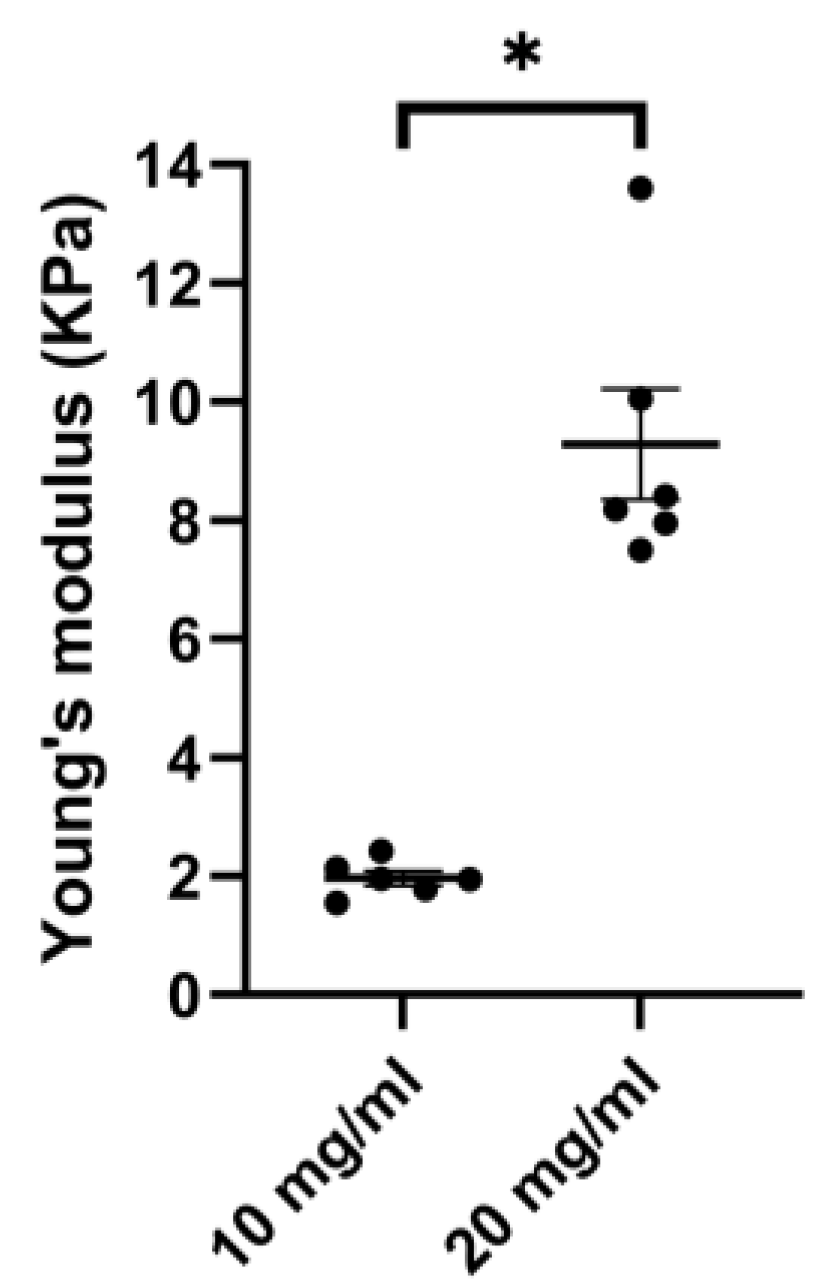
3.2. Porcine ECM Stiffness Depends on Concentration
3.3. Hydrogel Co-Culture Contraction Starts after Epithelial Cell Seeding
3.4. Variation of Hydrogel Co-Culture Contraction
3.5. Epithelial Cell Differentiation Irrespective of Coating Strategy
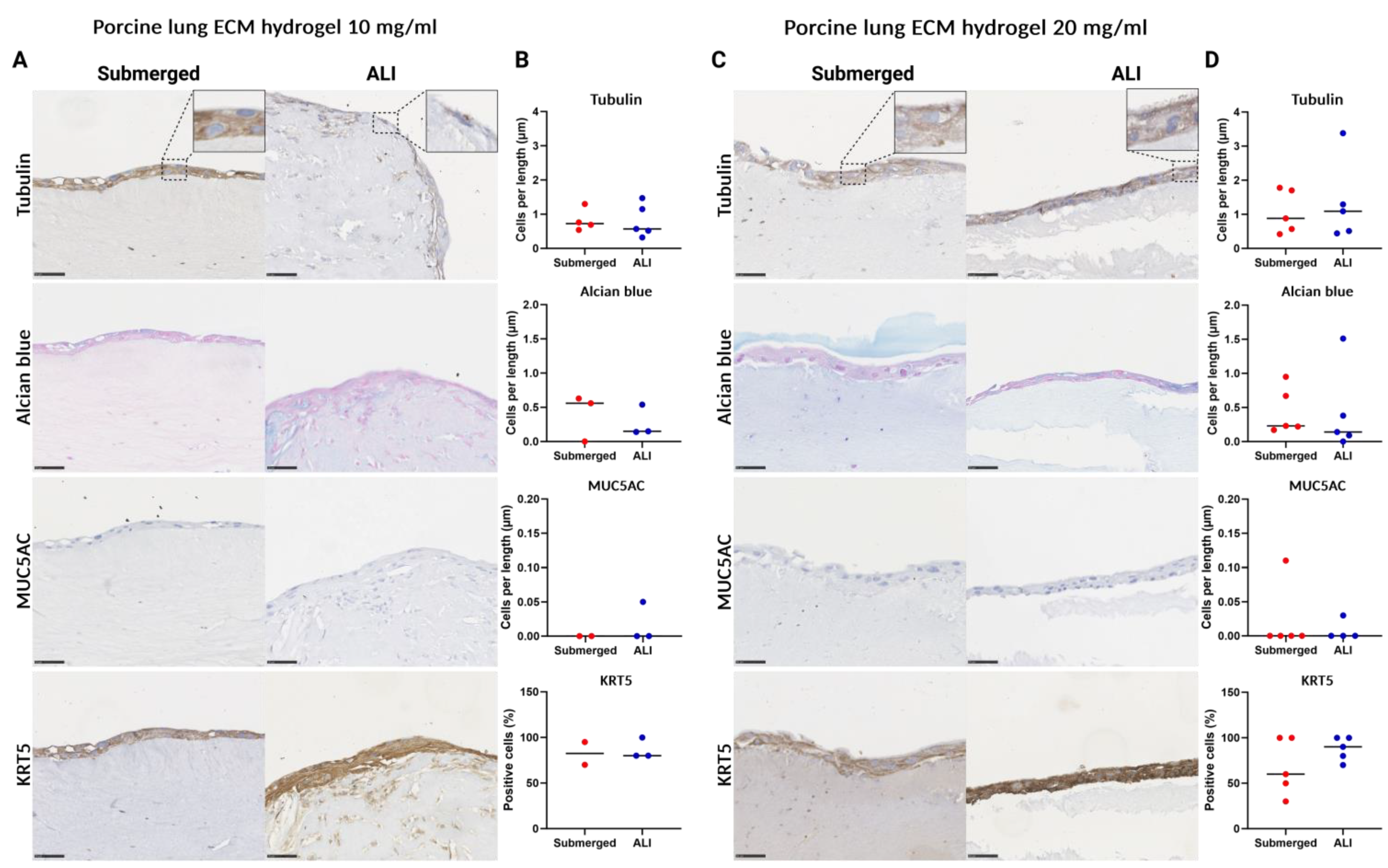
3.6. Porcine Lung ECM Hydrogel Concentration Does Not Affect Epithelial Differentiation
3.7. Human ECM Hydrogel Co-Culture Contraction
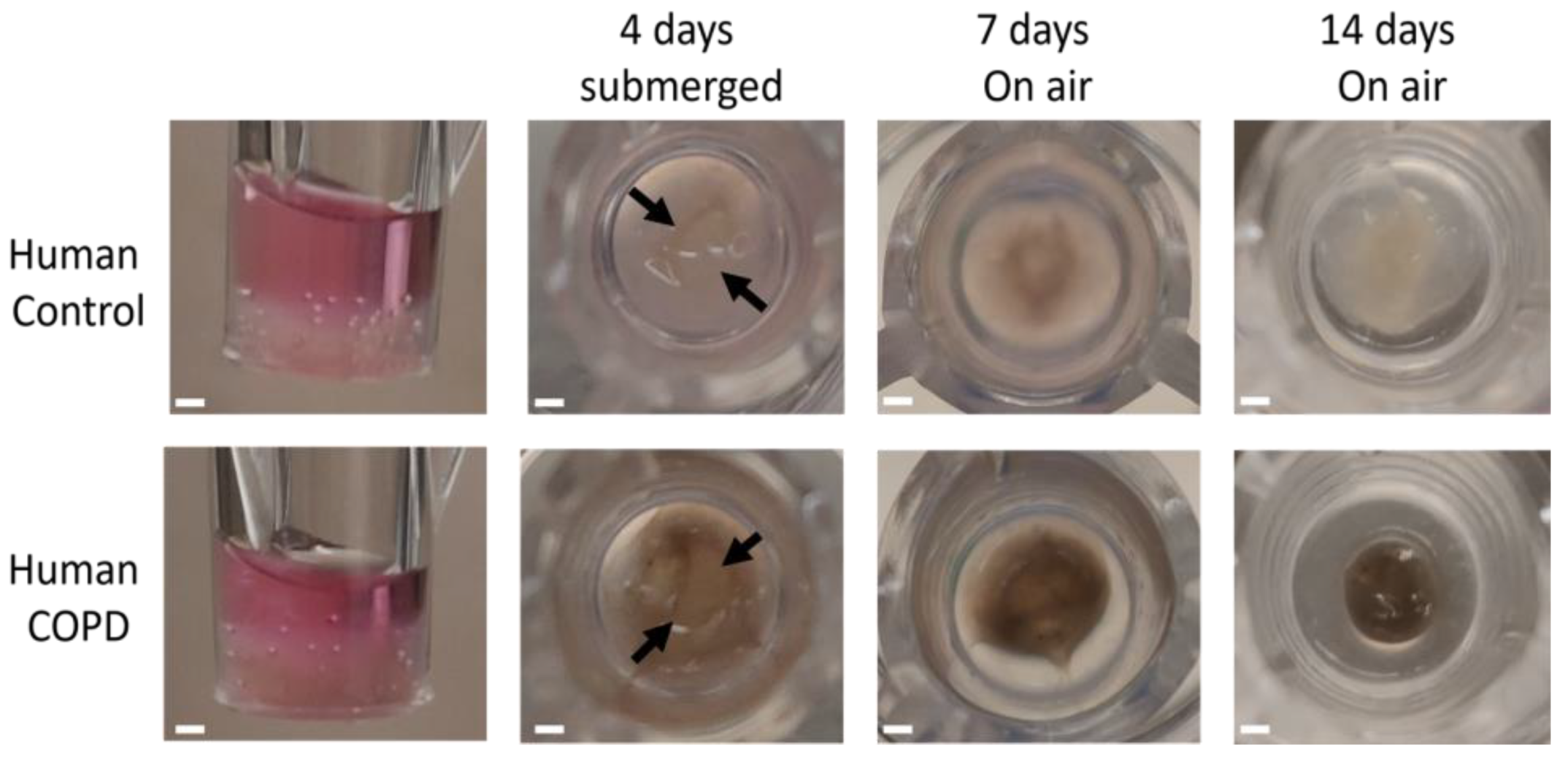
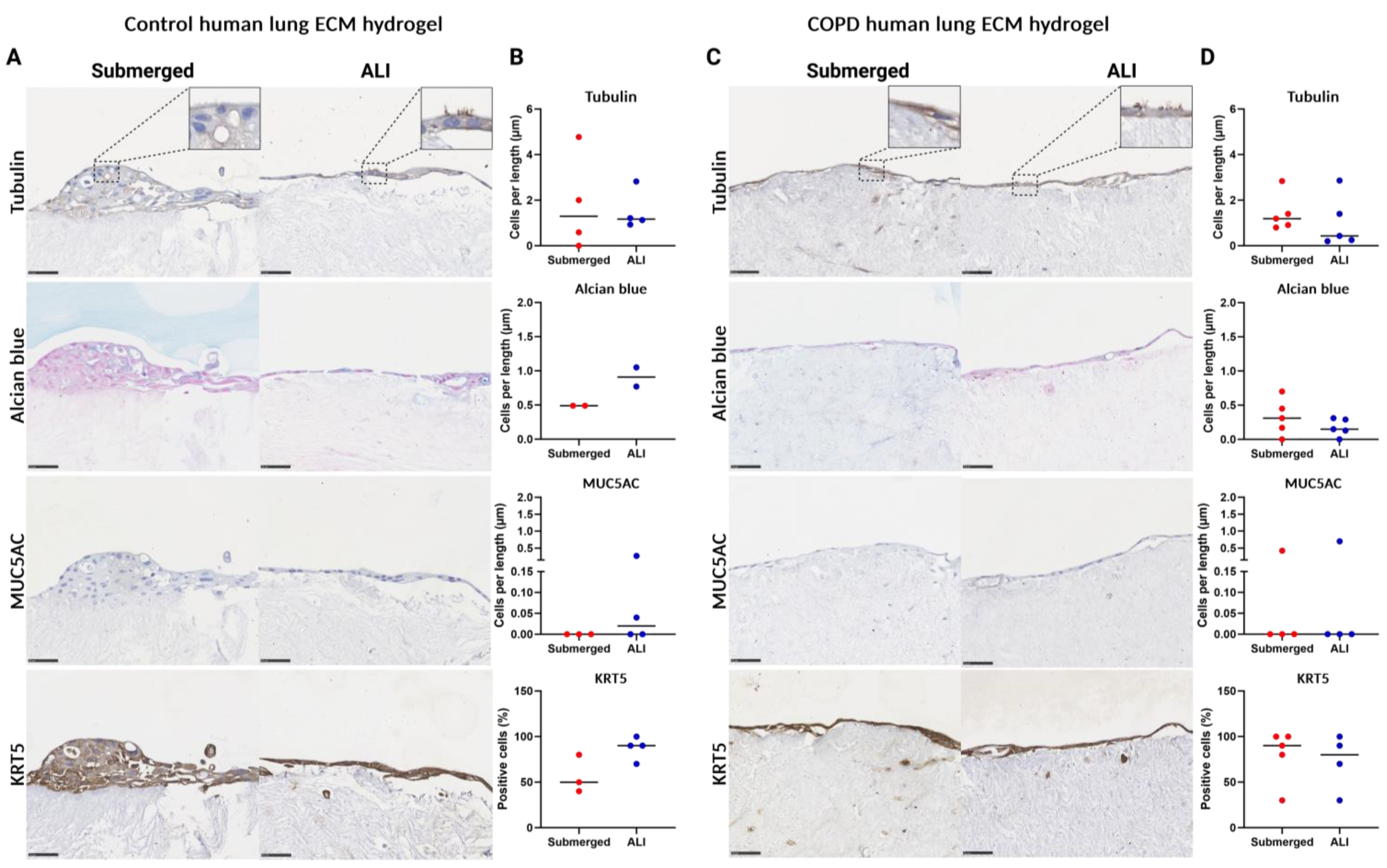
3.8. 3D ECM Hydrogel Co-Culture Is Possible with Both Control and COPD Lung ECM Hydrogels
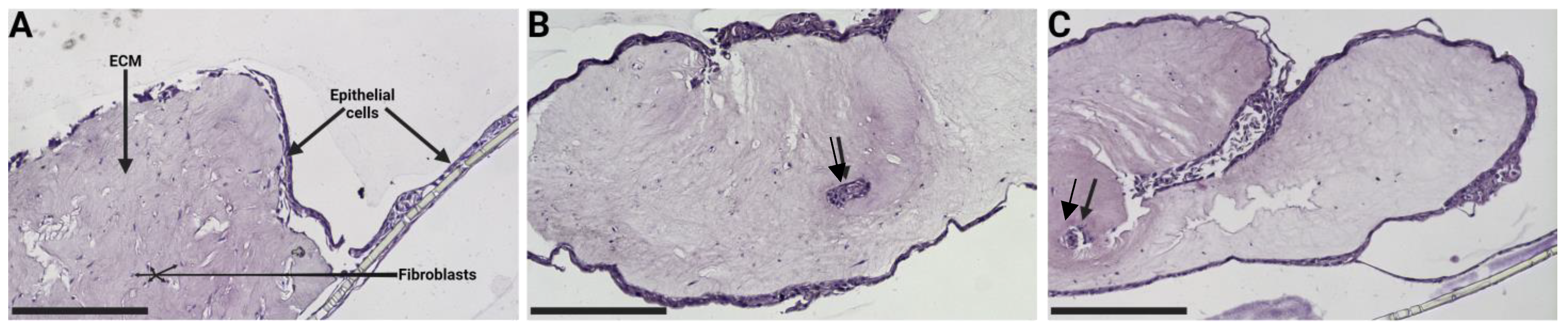
3.9. Changes in the Porcine ECM Organization after ALI Culture
3.10. Changes in the Human ECM Organization after ALI Culture
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quaderi, S.A.; Hurst, J.R. The unmet global burden of COPD. Glob Health Epidemiol Genom 2018, 3, e4. [Google Scholar] [CrossRef] [PubMed]
- Eisner, M.D.; Anthonisen, N.; Coultas, D.; Kuenzli, N.; Perez-Padilla, R.; Postma, D.; Romieu, I.; Silverman, E.K.; Balmes, J.R. An official American Thoracic Society public policy statement: Novel risk factors and the global burden of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010, 182, 693–718. [Google Scholar] [CrossRef] [PubMed]
- Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [CrossRef] [PubMed]
- Hogg, J.C.; Timens, W. The pathology of chronic obstructive pulmonary disease. Annu. Rev. Pathol. 2009, 4, 435–459. [Google Scholar] [CrossRef] [PubMed]
- Syamlal, G.; Doney, B.; Mazurek, J.M. Chronic Obstructive Pulmonary Disease Prevalence Among Adults Who Have Never Smoked, by Industry and Occupation—United States, 2013–2017. MMWR Morb. Mortal Wkly. Rep. 2019, 68, 303–307. [Google Scholar] [CrossRef]
- Agustí, A.; Celli, B.R.; Criner, G.J.; Halpin, D.; Anzueto, A.; Barnes, P.; Bourbeau, J.; Han, M.K.; Martinez, F.J.; Montes de Oca, M.; et al. Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD Executive Summary. Eur. Respir. J. 2023, 61, 819–837. [Google Scholar] [CrossRef]
- Hogg, J.C.; McDonough, J.E.; Gosselink, J.V.; Hayashi, S. What drives the peripheral lung-remodeling process in chronic obstructive pulmonary disease? Proc. Am. Thorac. Soc. 2009, 6, 668–672. [Google Scholar] [CrossRef]
- Rabe, K.F.; Watz, H. Chronic obstructive pulmonary disease. Lancet 2017, 389, 1931–1940. [Google Scholar] [CrossRef]
- Tam, A.; Wadsworth, S.; Dorscheid, D.; Man, S.F.; Sin, D.D. The airway epithelium: More than just a structural barrier. Ther. Adv. Respir. Dis. 2011, 5, 255–273. [Google Scholar] [CrossRef]
- Liu, C.; Li, P.; Zheng, J.; Wang, Y.; Wu, W.; Liu, X. Role of necroptosis in airflow limitation in chronic obstructive pulmonary disease: Focus on small-airway disease and emphysema. Cell Death Discov. 2022, 8, 363. [Google Scholar] [CrossRef]
- Pouwels, S.D.; Heijink, I.H.; Ten Hacken, N.H.; Vandenabeele, P.; Krysko, D.V.; Nawijn, M.C.; Van Oosterhout, A.J. DAMPs activating innate and adaptive immune responses in COPD. Mucosal Immunol. 2014, 7, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Crystal, R.G. Airway basal cells. The “smoking gun” of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2014, 190, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Osei, E.T.; Brandsma, C.-A.; Noordhoek, J.A.; Timens, W.; Postma, D.; Heijink, I. Crosstalk between epithelium and fibroblasts; implications for COPD. Eur. Respir. J. 2014, 44 (Suppl. 58), P3899. [Google Scholar]
- Fujita, Y.; Araya, J.; Ito, S.; Kobayashi, K.; Kosaka, N.; Yoshioka, Y.; Kadota, T.; Hara, H.; Kuwano, K.; Ochiya, T. Suppression of autophagy by extracellular vesicles promotes myofibroblast differentiation in COPD pathogenesis. J. Extracell. Vesicles 2015, 4, 28388. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Small airway fibrosis in COPD. Int. J. Biochem. Cell Biol. 2019, 116, 105598. [Google Scholar] [CrossRef]
- Hynes, R.O. The extracellular matrix: Not just pretty fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef]
- Hynes, R.O.; Naba, A. Overview of the Matrisome—An Inventory of Extracellular Matrix Constituents and Functions. Cold Spring Harb. Perspect. Biol. 2012, 4, a004903. [Google Scholar] [CrossRef]
- Jayadev, R.; Sherwood, D.R. Basement membranes. Curr. Biol. 2017, 27, R207–R211. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef]
- Sun, B. The mechanics of fibrillar collagen extracellular matrix. Cell Rep. Phys. Sci. 2021, 2, 100515. [Google Scholar] [CrossRef]
- Pomin, V.H.; Mulloy, B. Glycosaminoglycans and proteoglycans. Pharmaceuticals 2018, 11.1, 27. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.K.; Mauad, T.; Tjin, G.; Karlsson, J.C.; Westergren-Thorsson, G. The extracellular matrix—The under-recognized element in lung disease? J. Pathol. 2016, 240.4, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Karsdal, M.A.; Nielsen, M.J.; Sand, J.M.; Henriksen, K.; Genovese, F.; Bay-Jensen, A.C.; Smith, V.; Adamkewicz, J.I.; Christiansen, C.; Leeming, D.J. Extracellular matrix remodeling: The common denominator in connective tissue diseases. Possibilities for evaluation and current understanding of the matrix as more than a passive architecture, but a key player in tissue failure. Assay Drug Dev. Technol. 2013, 11, 70–92. [Google Scholar] [CrossRef] [PubMed]
- Muncie, J.M.; Weaver, V.M. The Physical and Biochemical Properties of the Extracellular Matrix Regulate Cell Fate. Curr. Top Dev. Biol. 2018, 130, 1–37. [Google Scholar] [CrossRef]
- Wrench, C.; Baker, J.; Fenwick, P.; Donnelly, L.; Barnes, P. Small airway fibroblasts from COPD patients are senescent and pro-fibrotic. Eur. Respir. J. 2018, 52, PA2172. [Google Scholar]
- Karakioulaki, M.; Papakonstantinou, E.; Stolz, D. Extracellular matrix remodelling in COPD. Eur. Respir. Rev. 2020, 29, 190124. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Schaible, N.; Hall, J.K.; Bartolák-Suki, E.; Deng, Y.; Herrmann, J.; Sonnenberg, A.; Behrsing, H.P.; Lutchen, K.R.; Krishnan, R.; et al. Multiscale stiffness of human emphysematous precision cut lung slices. Sci. Adv. 2023, 9, eadf2535. [Google Scholar] [CrossRef]
- Parameswaran, H.; Majumdar, A.; Suki, B. Linking microscopic spatial patterns of tissue destruction in emphysema to macroscopic decline in stiffness using a 3D computational model. PLoS Comput. Biol. 2011, 7, e1001125. [Google Scholar] [CrossRef]
- Annoni, R.; Lanças, T.; Yukimatsu Tanigawa, R.; de Medeiros Matsushita, M.; de Morais Fernezlian, S.; Bruno, A.; Fernando Ferraz da Silva, L.; Roughley, P.J.; Battaglia, S.; Dolhnikoff, M.; et al. Extracellular matrix composition in COPD. Eur. Respir. J. 2012, 40, 1362–1373. [Google Scholar] [CrossRef]
- Kutluk, H.; Bastounis, E.E.; Constantinou, I. Integration of Extracellular Matrices into Organ-on-Chip Systems. Adv. Healthc. Mater. 2023, 12, 2203256. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Raeber, G.P.; Zisch, A.H.; Tirelli, N.; Hubbell, J.A. Cell-Responsive Synthetic Hydrogels. Adv. Mater. 2003, 15, 888–892. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Yuan, T.; Zhang, L.; Li, K.; Fan, H.; Fan, Y.; Liang, J.; Zhang, X. Collagen hydrogel as an immunomodulatory scaffold in cartilage tissue engineering. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2014, 102, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Rowley, J.A.; Madlambayan, G.; Mooney, D.J. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials 1999, 20, 45–53. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Reis, L.A.; Chiu, L.L.Y.; Liang, Y.; Hyunh, K.; Momen, A.; Radisic, M. A peptide-modified chitosan–collagen hydrogel for cardiac cell culture and delivery. Acta Biomater. 2012, 8, 1022–1036. [Google Scholar] [CrossRef]
- Sackett, S.; Tremmel, D.; Ma, F.; Feeney, A.K.; Maguire, R.M.; Brown, M.E.; Zhou, Y.; Li, X.; O’Brien, C.; Li, L.; et al. Extracellular matrix scaffold and hydrogel derived from decellularized and delipidized human pancreas. Sci. Rep. 2018, 8, 10452. [Google Scholar] [CrossRef]
- Liguori, G.R.; Liguori, T.T.A.; de Moraes, S.R.; Sinkunas, V.; Terlizzi, V.; van Dongen, J.A.; Sharma, P.K.; Moreira, L.F.P.; Harmsen, M.C. Molecular and Biomechanical Clues From Cardiac Tissue Decellularized Extracellular Matrix Drive Stromal Cell Plasticity. Front. Bioeng. Biotechnol. 2020, 8, 520. [Google Scholar] [CrossRef]
- Hoffman, E.T.; Uhl, F.E.; Asarian, L.; Deng, B.; Becker, C.; Uriarte, J.J.; Downs, I.; Young, B.; Weiss, D.J. Regional and disease specific human lung extracellular matrix composition. Biomaterials 2023, 293, 121960. [Google Scholar] [CrossRef]
- de Hilster, R.H.J.; Sharma, P.K.; Jonker, M.R.; White, E.S.; Gercama, E.A.; Roobeek, M.; Timens, W.; Harmsen, M.C.; Hylkema, M.N.; Burgess, J.K. Human lung extracellular matrix hydrogels resemble the stiffness and viscoelasticity of native lung tissue. Am. J. Physiol Lung Cell Mol. Physiol. 2020, 318, L698–L704. [Google Scholar] [CrossRef]
- Pouliot, R.A.; Link, P.A.; Mikhaiel, N.S.; Schneck, M.B.; Valentine, M.S.; Kamga Gninzeko, F.J.; Herbert, J.A.; Sakagami, M.; Heise, R.L. Development and characterization of a naturally derived lung extracellular matrix hydrogel. J. Biomed. Mater. Res. A 2016, 104, 1922–1935. [Google Scholar] [CrossRef] [PubMed]
- Vieira Braga, F.A.; Kar, G.; Berg, M.; Carpaij, O.A.; Polanski, K.; Simon, L.M.; Brouwer, S.; Gomes, T.; Hesse, L.; Jiang, J. A cellular census of human lungs identifies novel cell states in health and in asthma. Nat. Med. 2019, 25, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Heijink, I.H.; Kies, P.; Kauffman, H.F.; Postma, D.S.; van Oosterhout, A.J.; Vellenga, E. Down-regulation of E-cadherin in human bronchial epithelial cells leads to epidermal growth factor receptor-dependent Th2 cell-promoting activity. J. Immunol. 2007, 178, 7678–7685. [Google Scholar] [CrossRef] [PubMed]
- Dinesh Kumar, N.; Ter Ellen, B.M.; Bouma, E.M.; Troost, B.; van de Pol, D.P.; van der Ende-Metselaar, H.H.; van Gosliga, D.; Apperloo, L.; Carpaij, O.A.; van den Berge, M. Moxidectin and ivermectin inhibit SARS-CoV-2 replication in Vero E6 cells but not in human primary bronchial epithelial cells. Antimicrob. Agents Chemother. 2022, 66, e01543-21. [Google Scholar] [CrossRef]
- Martinez-Garcia, F.D.; de Hilster, R.H.J.; Sharma, P.K.; Borghuis, T.; Hylkema, M.N.; Burgess, J.K.; Harmsen, M.C. Architecture and Composition Dictate Viscoelastic Properties of Organ-Derived Extracellular Matrix Hydrogels. Polymers 2021, 13, 3113. [Google Scholar] [CrossRef]
- Nizamoglu, M.; de Hilster, R.H.J.; Zhao, F.; Sharma, P.K.; Borghuis, T.; Harmsen, M.C.; Burgess, J.K. An in vitro model of fibrosis using crosslinked native extracellular matrix-derived hydrogels to modulate biomechanics without changing composition. Acta Biomater 2022, 147, 50–62. [Google Scholar] [CrossRef]
- Sharma, P.; Busscher, H.; Terwee, T.; Koopmans, S.; van Kooten, T. A comparative study on the viscoelastic properties of human and animal lenses. Exp. Eye Res. 2011, 93, 681–688. [Google Scholar] [CrossRef]
- Noordhoek, J.A.; Postma, D.S.; Chong, L.L.; Menkema, L.; Kauffman, H.F.; Timens, W.; van Straaten, J.F.; van der Geld, Y.M. Different modulation of decorin production by lung fibroblasts from patients with mild and severe emphysema. Copd 2005, 2, 17–25. [Google Scholar] [CrossRef]
- CORNING. Certificate of Analysis—CORNING® COLLAGEN I, RAT TAIL. Available online: https://ecatalog.corning.com/life-sciences/b2c/US/en/Surfaces/Extracellular-Matrices-ECMs/Corning%C2%AE-Collagen/p/354236 (accessed on 3 June 2021).
- Martinez-Garcia, F.D.; Valk, M.M.; Sharma, P.K.; Burgess, J.K.; Harmsen, M.C. Adipose Tissue-Derived Stromal Cells Alter the Mechanical Stability and Viscoelastic Properties of Gelatine Methacryloyl Hydrogels. Int. J. Mol. Sci. 2021, 22, 10153. [Google Scholar] [CrossRef]
- Lai, M.; Lü, B. Tissue preparation for microscopy and histology. In Comprehensive Sampling and Sample Preparation; Elsevier: Amsterdam, The Netherlands, 2012; pp. 53–93. ISBN 978-0-12-381374-9. [Google Scholar]
- Tasena, H.; Timens, W.; van den Berge, M.; van Broekhuizen, J.; Kennedy, B.K.; Hylkema, M.N.; Brandsma, C.A.; Heijink, I.H. MicroRNAs Associated with Chronic Mucus Hypersecretion in COPD Are Involved in Fibroblast-Epithelium Crosstalk. Cells 2022, 11, 526. [Google Scholar] [CrossRef]
- Spanjer, A.I.; Menzen, M.H.; Dijkstra, A.E.; van den Berge, M.; Boezen, H.M.; Nickle, D.C.; Sin, D.D.; Bossé, Y.; Brandsma, C.-A.; Timens, W. A pro-inflammatory role for the Frizzled-8 receptor in chronic bronchitis. Thorax 2016, 71, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008, 2008, pdb.prot4986. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Ngassie, M.L.K.; De Vries, M.; Borghuis, T.; Timens, W.; Sin, D.D.; Nickle, D.; Joubert, P.; Horvatovich, P.; Marko-Varga, G.; Teske, J.J. Age-associated differences in the human lung extracellular matrix. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2023. [Google Scholar]
- Wershof, E.; Park, D.; Barry, D.J.; Jenkins, R.P.; Rullan, A.; Wilkins, A.; Schlegelmilch, K.; Roxanis, I.; Anderson, K.I.; Bates, P.A.; et al. A FIJI macro for quantifying pattern in extracellular matrix. Life Sci. Alliance 2021, 4, L799–L814. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Schaefer, N.; Chu, H.W. Air-Liquid Interface Culture of Human and Mouse Airway Epithelial Cells. Methods Mol. Biol. 2018, 1809, 91–109. [Google Scholar] [CrossRef]
- Wu, R.; Sato, G.H.; Whitcutt, M.J. Developing differentiated epithelial cell cultures: Airway epithelial cells. Fundam. Appl. Toxicol. 1986, 6, 580–590. [Google Scholar] [CrossRef]
- Jain, P.; Rauer, S.B.; Möller, M.; Singh, S. Mimicking the Natural Basement Membrane for Advanced Tissue Engineering. Biomacromolecules 2022, 23, 3081–3103. [Google Scholar] [CrossRef]
- Heijink, I.H.; de Bruin, H.G.; Dennebos, R.; Jonker, M.R.; Noordhoek, J.A.; Brandsma, C.A.; van den Berge, M.; Postma, D.S. Cigarette smoke-induced epithelial expression of WNT-5B: Implications for COPD. Eur. Respir. J. 2016, 48, 504–515. [Google Scholar] [CrossRef]
- Hohenester, E.; Yurchenco, P.D. Laminins in basement membrane assembly. Cell Adh Migr. 2013, 7, 56–63. [Google Scholar] [CrossRef]
- Dekkers, B.G.J.; Saad, S.I.; van Spelde, L.J.; Burgess, J.K. Basement membranes in obstructive pulmonary diseases. Matrix Biol. Plus 2021, 12, 100092. [Google Scholar] [CrossRef] [PubMed]
- Liesker, J.J.; Hacken, N.H.T.; Zeinstra-Smith, M.; Rutgers, S.R.; Postma, D.S.; Timens, W. Reticular basement membrane in asthma and COPD: Similar thickness, yet different composition. Int. J. Chronic Obstr. Pulm. Dis. 2009, 4, 127–135. [Google Scholar]
- Kranenburg, A.R.; Willems-Widyastuti, A.; Mooi, W.J.; Sterk, P.J.; Alagappan, V.K.; de Boer, W.I.; Sharma, H.S. Enhanced bronchial expression of extracellular matrix proteins in chronic obstructive pulmonary disease. Am. J. Clin. Pathol. 2006, 126, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Prytherch, Z.; Job, C.; Marshall, H.; Oreffo, V.; Foster, M.; BéruBé, K. Tissue-Specific stem cell differentiation in an in vitro airway model. Macromol. Biosci. 2011, 11, 1467–1477. [Google Scholar] [CrossRef] [PubMed]
- Handorf, A.M.; Zhou, Y.; Halanski, M.A.; Li, W.J. Tissue stiffness dictates development, homeostasis, and disease progression. Organogenesis 2015, 11, 1–15. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Gu, L.; Klumpers, D.; Darnell, M.; Bencherif, S.A.; Weaver, J.C.; Huebsch, N.; Lee, H.P.; Lippens, E.; Duda, G.N.; et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2016, 15, 326–334. [Google Scholar] [CrossRef]
- Hinz, B.; McCulloch, C.A.; Coelho, N.M. Mechanical regulation of myofibroblast phenoconversion and collagen contraction. Exp Cell Res 2019, 379, 119–128. [Google Scholar] [CrossRef]
- Bourke, J.E.; Li, X.; Foster, S.R.; Wee, E.; Dagher, H.; Ziogas, J.; Harris, T.; Bonacci, J.V.; Stewart, A.G. Collagen remodelling by airway smooth muscle is resistant to steroids and β2-agonists. Eur. Respir. J. 2011, 37, 173–182. [Google Scholar] [CrossRef]
- Hameed, P.; Manivasagam, G. An overview of bio-actuation in collagen hydrogels: A mechanobiological phenomenon. Biophys. Rev. 2021, 13, 387–403. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, P.; Fang, X.; Lin, F.; Fang, J.; Xiong, C. Collagen gel contraction assays: From modelling wound healing to quantifying cellular interactions with three-dimensional extracellular matrices. Eur. J. Cell Biol. 2022, 101, 151253. [Google Scholar] [CrossRef]
- Sarrigiannidis, S.O.; Rey, J.M.; Dobre, O.; González-García, C.; Dalby, M.J.; Salmeron-Sanchez, M. A tough act to follow: Collagen hydrogel modifications to improve mechanical and growth factor loading capabilities. Mater Today Bio. 2021, 10, 100098. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kang, B.; Cui, X.; Lee, S.H.; Lee, K.; Cho, D.W.; Hwang, W.; Woodfield, T.B.; Lim, K.S.; Jang, J. Light-activated decellularized extracellular matrix-based bioinks for volumetric tissue analogs at the centimeter scale. Adv. Funct. Mater. 2021, 31, 2011252. [Google Scholar] [CrossRef]
- Hedström, U.; Öberg, L.; Vaarala, O.; Dellgren, G.; Silverborn, M.; Bjermer, L.; Westergren-Thorsson, G.; Hallgren, O.; Zhou, X. Impaired Differentiation of Chronic Obstructive Pulmonary Disease Bronchial Epithelial Cells Grown on Bronchial Scaffolds. Am. J. Respir. Cell Mol. Biol. 2021, 65, 201–213. [Google Scholar] [CrossRef]
- Jones, R.L.; Noble, P.B.; Elliot, J.G.; James, A.L. Airway remodelling in COPD: It’s not asthma! Respirology 2016, 21, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Toda, S.; Yokoi, F.; Yamada, S.; Yonemitsu, N.; Nishimura, T.; Watanabe, K.; Sugihara, H. Air exposure promotes fibroblast growth with increased expression of mitogen-activated protein kinase cascade. Biochem. Biophys. Res. Commun. 2000, 270, 961–966. [Google Scholar] [CrossRef]
- Nizamoglu, M.; Alleblas, F.; Koster, T.; Borghuis, T.; Vonk, J.M.; Thomas, M.J.; White, E.S.; Watson, C.K.; Timens, W.; Kasmi, K.C.E.; et al. Three dimensional fibrotic extracellular matrix directs microenvironment fiber remodeling by fibroblasts. Acta Biomater. 2024, 177, 118–131. [Google Scholar] [CrossRef]
- Wijsman, P.C.; Goorsenberg, A.W.; Keijzer, N.; d’Hooghe, J.N.; Ten Hacken, N.H.; Shah, P.L.; Weersink, E.J.; de Brito, J.M.; Costa, N.d.S.X.; Mauad, T. Airway wall extracellular matrix changes induced by bronchial thermoplasty in severe asthma. J. Allergy Clin. Immunol. 2024, 153, 435–446.e4. [Google Scholar] [CrossRef] [PubMed]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef]
- Mendoza-Novelo, B.; Avila, E.E.; Cauich-Rodríguez, J.V.; Jorge-Herrero, E.; Rojo, F.J.; Guinea, G.V.; Mata-Mata, J.L. Decellularization of pericardial tissue and its impact on tensile viscoelasticity and glycosaminoglycan content. Acta Biomater. 2011, 7, 1241–1248. [Google Scholar] [CrossRef]
- Higham, A.; Quinn, A.M.; Cançado, J.E.D.; Singh, D. The pathology of small airways disease in COPD: Historical aspects and future directions. Respir. Res. 2019, 20, 49. [Google Scholar] [CrossRef]
- Baker, B.M.; Trappmann, B.; Stapleton, S.C.; Toro, E.; Chen, C.S. Microfluidics embedded within extracellular matrix to define vascular architectures and pattern diffusive gradients. Lab A Chip 2013, 13, 3246–3252. [Google Scholar] [CrossRef] [PubMed]
- Vindin, H.; Mithieux, S.M.; Weiss, A.S. Elastin architecture. Matrix Biol. 2019, 84, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.D. A human breathing lung-on-a-chip. Ann. Am. Thorac. Soc. 2015, 12 (Suppl. 1), S42–S44. [Google Scholar] [CrossRef] [PubMed]
- Zamprogno, P.; Wüthrich, S.; Achenbach, S.; Thoma, G.; Stucki, J.D.; Hobi, N.; Schneider-Daum, N.; Lehr, C.M.; Huwer, H.; Geiser, T.; et al. Second-generation lung-on-a-chip with an array of stretchable alveoli made with a biological membrane. Commun. Biol. 2021, 4, 168. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Hilster, R.H.J.; Reinders-Luinge, M.A.; Schuil, A.; Borghuis, T.; Harmsen, M.C.; Burgess, J.K.; Hylkema, M.N. A 3D Epithelial–Mesenchymal Co-Culture Model of the Airway Wall Using Native Lung Extracellular Matrix. Bioengineering 2024, 11, 946. https://doi.org/10.3390/bioengineering11090946
de Hilster RHJ, Reinders-Luinge MA, Schuil A, Borghuis T, Harmsen MC, Burgess JK, Hylkema MN. A 3D Epithelial–Mesenchymal Co-Culture Model of the Airway Wall Using Native Lung Extracellular Matrix. Bioengineering. 2024; 11(9):946. https://doi.org/10.3390/bioengineering11090946
Chicago/Turabian Stylede Hilster, Roderick H. J., Marjan A. Reinders-Luinge, Annemarie Schuil, Theo Borghuis, Martin C. Harmsen, Janette K. Burgess, and Machteld N. Hylkema. 2024. "A 3D Epithelial–Mesenchymal Co-Culture Model of the Airway Wall Using Native Lung Extracellular Matrix" Bioengineering 11, no. 9: 946. https://doi.org/10.3390/bioengineering11090946
APA Stylede Hilster, R. H. J., Reinders-Luinge, M. A., Schuil, A., Borghuis, T., Harmsen, M. C., Burgess, J. K., & Hylkema, M. N. (2024). A 3D Epithelial–Mesenchymal Co-Culture Model of the Airway Wall Using Native Lung Extracellular Matrix. Bioengineering, 11(9), 946. https://doi.org/10.3390/bioengineering11090946







