1. Introduction
Computed tomography (CT) is a non-invasive, cross-sectional view of the body carried out by transmitting X-rays through the subject, which can provide information on the size, shape, and location of lesions, as well as tracking disease metastasis. However, compared to medical imaging techniques such as MRI and ultrasound, there are ongoing concerns about radiation exposure to subjects between imaging [
1]. Compared to humans, animals may be thought to require lower doses due to their smaller size, but higher doses are required to see their small anatomy at high resolution. In addition, animals are less able to communicate than humans, which can make radiation exposure more dangerous. Compared to standard-dose CT (SDCT), low-dose CT (LDCT) offers the advantage of reducing medical exposure by minimizing radiation dosage. However, this reduction comes at the cost of a lower signal-to-noise ratio (SNR), leading to diminished image quality. Consequently, there is a pressing need for alternative methods capable of producing high-quality images under low-dose conditions in animal CT imaging. Recently, super-resolution and denoising techniques using deep neural network learning have been proposed to overcome this quality degradation problem [
2,
3,
4].
One task that has benefited greatly from advances in deep learning technology is denoising. Previous deep learning-based image quality improvement research has used per-pixel loss functions based on mean square error (MSE) [
5]. While MSE has maintained high scores on measures such as peak signal-to-noise ratio (PSNR) by focusing on the similarity between pixels, it has limitations such as lack of high-frequency detail and poor quality in perceptual details [
6]. In addition, convolutional neural network (CNN)-based models require pairs of input data for training. Deep denoising networks are not a reliable solution for unpaired noisy images, but it is difficult to obtain such data for medical images such as CT scans. To address this limitation, many unsupervised deep learning models have been proposed.
Unsupervised learning, where the model identifies patterns or relationships in data without labeled training data, is particularly useful for tasks with scarce data. Cycle-consistent generative adversarial networks (CycleGAN) [
5] are a typical model in this category, using two GANs and cycle consistency loss. Various modifications to the generators, discriminators, and loss functions have been proposed to improve CycleGAN’s performance, including the application of AdaIN [
2] from StyleGAN and the addition of style reconstruction and perceptual losses [
5,
7].
Our method introduces several key innovations to address the limitations of traditional CT imaging. First, we enhance the generator network architecture by incorporating BlurPool [
8] layers to mitigate aliasing effects during down-sampling, thus preserving high-frequency details essential for accurate medical imaging. Aliasing occurs when high-frequency components of an image are misrepresented during down-sampling, leading to artifacts and loss of detail. This issue is particularly problematic in medical imaging, where precise details are crucial for accurate diagnosis [
9]. Second, we integrate the hierarchical feature synthesis (HFS) module [
10] into our residual network. HFS effectively aggregates residual features, preserving high-frequency details and improving feature representation. This module, combined with spatial and channel squeeze and excitation (scSE) blocks, enables the model to focus on relevant spatial information and enhance segmentation performance.
Additionally, our approach employs a multi-scale discriminator network that evaluates images at various resolutions. This multi-scale strategy improves the discriminator’s ability to capture fine details and patterns, resulting in superior image reconstructions compared to single-scale approaches.
Our contributions are as follows.
We propose an enhanced generator network architecture incorporating BlurPool layers to mitigate aliasing effects and improve image quality under low-dose conditions.
We apply the hierarchical feature synthesis (HFS) module to leverage spatial information effectively without loss, enhancing feature extraction and representation in medical imaging.
We utilize a multi-scale discriminator to improve the discrimination of fine details, such as edges and patterns, resulting in higher-quality image reconstructions.
2. Related Work
2.1. GAN-Based Solutions for CT Denoising
In the field of image enhancement or denoising, GAN-based solutions with adversarial training are better at constructing manifolds closer to the target data than MSE-based solutions and focus on perceptual quality rather than optimizing only for quantitative metrics [
11]. This has been shown to produce more cognitively compelling results [
3]. Thus, several studies have shown that GAN models are able to achieve high performance in CT denoising [
3,
4,
12].
GAN consists of two models: a generative model and a discriminative model. Researchers employ various CNN structures and explore different loss functions to enhance the performance of both the generator and discriminator models in denoising tasks. WGAN-VGG-GP [
3], which changes the objective to Wasserstein-1 distance to solve the problem of poor learning of Vanila GAN and introduces gradient penalty to the WGAN-GP model [
12] with VGG to measure perceptual differences, has shown great results in GAN-based CT Denoising research. It has been reported to achieve PSNR values of 23.39 and 22.16 with SSIM scores around 0.79, while also providing superior visual quality compared to both MSE-based and traditional GAN models [
3]. SAGAN [
4] employed a U-Net-based generator model and PatchGAN [
13]-based discriminator. Further, it tried to solve the problem of smoothing the edges of structures in the output of the GAN-based denoising model through sharpness loss. DU-GAN [
12] enables adversarial training in both the image domain and the gradient domain, which reduces noise while mitigating streak artifacts. DU-GAN achieved a PSNR of 22.31, RMSE of 0.0802, and the highest SSIM score of 0.7489 on the Mayo-10% dataset, outperforming both MSE-based and other GAN-based methods. This indicates that DU-GAN not only effectively reduces noise but also preserves important structural information, as reflected in its superior SSIM scores.
Many studies employing GAN models as above have significantly improved CT denoising performance. However, obtaining paired datasets in real-world medical settings remains challenging, necessitating denoising techniques applicable to unpaired datasets.
2.2. Techniques for Denoising with Unpaired Datasets
To solve the problem of denoising in unpaired datasets, researchers have attempted to utilize image domain translation. CycleGAN is a representative model of image domain translation [
14], which uses cycle consistency loss to learn translation between two different domains; i.e., it captures the unique features of one set of images and transfers them to another set.
Kang et al. [
15] introduced CycleGAN for unpaired multiphase computed tomography angiography (CTA) denoising. GAN generates high-quality samples quickly, but it has poor mode coverage and is prone to mode collapse. However, by introducing cycle consistency loss, the researchers demonstrated that CycleGAN works well without mode collapse on unpaired CT datasets. GAN-CIRCLE [
16] introduced Wasserstein distance into the framework based on CycleGAN and showed that by using unpaired datasets, the model can effectively learn more complex structured features. Cycle-free CycleGAN [
17] introduced AdaIN [
18], which is used in StyleGAN, and achieved efficient performance in unpaired CT denoising while reducing model complexity by using only a single invertible generator and discriminator. Yin et al. [
19]. introduced an attention-gate to CycleGAN to extract useful salient features and attempted to improve denoising performance.
There has also been recent work on LDCT denoising using contrastive learning, with NDCT as positive samples and LDCT as negative samples. In CCN-CL [
20], a contrastive regularization loss term was proposed and performed using a content–noise complementary learning strategy. ESAU-Net [
21], which utilizes a channel-wise self-attention mechanism in its ESAU-Net, improved global and local context preservation, leading to a 15% increase in image clarity. Yuanke Zhang et al. [
22] propose a bidirectional contrastive unsupervised denoising (BCUD) technique to solve the problem of difficulty in obtaining paired data between LDCT and NDCT; they use a bidirectional generator to generate synthetic images which are then used for training.
2.3. Recent Developments in Unsupervised Image-to-Image Translation
Translating a noisy image into a clean, high-quality image, image-to-image (I2I) translation provides new strategies for improving image quality, maintaining fine details, and handling the complexities of medical imaging data. The field of I2I translation has seen significant advances, which offer potential solutions to some of the challenges encountered in traditional convolutional neural networks (CNNs).
Recently, diffusion models have emerged as a powerful alternative to GANs for various image synthesis tasks, including medical imaging [
23]. Unlike GANs, which rely on adversarial training to generate images, diffusion models work by iteratively refining a noisy image towards the target image distribution. This approach has been shown to be highly effective in generating high-quality images with fewer artifacts [
24]. Arslan et al. [
25] introduced a self-consistent recursive diffusion bridge for medical image translation, demonstrating superior performance in tasks that require cross-modality synthesis, such as CT to MRI conversion. Incorporating diffusion models into CT denoising frameworks could potentially enhance the quality of the generated images by further reducing noise and artifacts.
Transformers [
26], known for their ability to capture long-range dependencies in data, have also been successfully applied to medical image synthesis. I2I-Mamba [
27] integrates selective state–space modeling (SSM) with a transformer architecture, enabling efficient context capture without the overhead typically associated with transformers. This model outperforms traditional CNNs in tasks like MRI-CT synthesis by maintaining both local precision and global contextual understanding, which is crucial for high-fidelity image reconstruction.
3. Methodology
3.1. Framework
The overall framework of the proposed network architecture is illustrated in
Figure 1. We proposed a low-dose CT denoising framework based on the CycleGAN model. We used unmatched pairs of LDCT and SDCT images. The network approaches employed in previous CycleGAN papers have certain drawbacks. One such disadvantage is the propensity to produce checkerboard artifacts attributed to deformations in the latent space. Additionally, the approach fails to adequately leverage the spatial information present in the input image. Consequently, we introduce an anti-aliasing generator and multi-scale discriminator as a remedy to address these aforementioned issues.
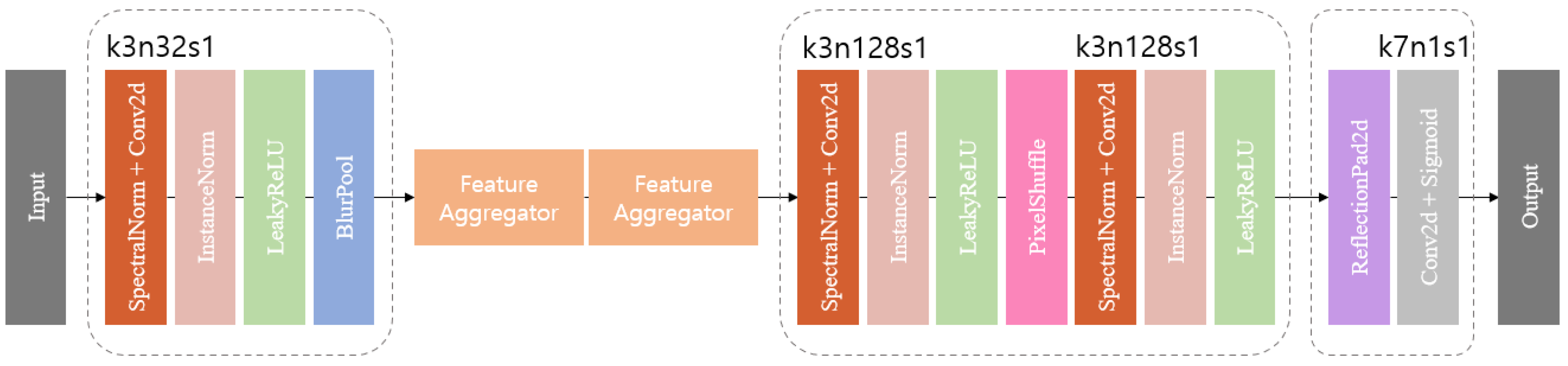
3.1.1. Anti-Aliasing Encoder and Decoder
The encoder incorporates spectral normalization [
28], instance normalization [
29], LeakyReLU [
30], and a BlurPool layer. Spectral normalization controls the Lipschitz constant, serving as a regularization term to prevent sensitivity to singular directions and promote adaptive regulation of the principal gradient direction, considering features in diverse directions. Instance normalization (IN) is used to calculate the mean and variance for each input sample to keep the style of each image independent. The two normalization operations bring the benefits of stability and style preservation. After two normalizations, the image is converted to a non-linear value by passing it through the LeakyReLU and then down-sampling it through the BlurPool layer. BlurPool is one of the techniques proposed to solve the problem of aliasing during pooling operations in deep learning. According to Richard Zhang et al. [
8], MaxPool [
31] is divided into two steps. The max kernel extracts the maximum value within each window, and sub-sampling uses the extracted value to reduce the resolution of the output. One thing to note is that aliasing may occur in the subsampling step, which breaks the moving homogeneity. BlurPool works by blurring high-frequency components by applying an anti-aliasing filter before the sub-sampling step.
The decoder consists of the same sequence as the encoder: spectral norm, instance norm, and LeakyReLU, with the last layer of up-sampling using PixelShuffle [
32]. PixelShuffle reduces the number of channels by 1/32 and adjusts the upscaling factor to 3. PixelShuffle works by taking pixels from each channel and arranging them in 3 × 3 groups, effectively scaling the height and width of the features by the square of scale to create a feature map in the channel axis and increasing scale by bringing the features created in the channel axis into one dimension. This process provides PixelShuffle with a significant advantage in terms of expressive power, as it possesses more network parameters compared to conventional deconvolution layers. The final stage (
Figure 2) consists of a reflection padding, a convolution layer, and a sigmoid, and uses the reflection padding earlier to use a kernel size of seven in the convolution layer.
3.1.2. Hierarchical Feature Aggregator
The feature extraction step in traditional CycleGAN is accomplished through U-Net [
33], which consists of an encoder to compress the image into a low-dimensional representation and a decoder to expand it back into a high-dimensional representation, utilizing skip-connections to integrate both low- and high-dimensional information. However, models like PixelShuffle face challenges related to learning time and gradient vanishing problems, limiting layer depth and struggling to reproduce high-frequency components. In contrast, ResNet [
31] effectively learns residuals and reproduces high-frequency components, making it frequently used in denoising tasks. According to Jie Liu et al. [
34], the conventional structure of ResNet does not fully exploit the capabilities of the residual block, leading to the proposal of a residual feature aggregation (RFA) structure to better utilize local residual learning [
34].
RFA was initially proposed for single-image super-resolution tasks. RFA structures aggregate residual features locally to improve the representation of fine details [
35]. This method enhances the learning of high-frequency components, resulting in sharper and more detailed image reconstructions. The RFA network combines multiple residual blocks and uses hierarchical feature synthesis to retain and propagate essential details throughout the network layers [
10].
In this study, the feature aggregator is based on the HFS network. The three residual blocks of the HFS are concatenated together with the output of the last residual block. Finally, a 1 × 1 convolution is applied to fuse these features with the identity features before adding them together. Unlike simply stacking multiple residual blocks, HFS leverages residual features non-locally. The hierarchical information contained in the residual blocks propagates to the end of the HFS module without loss or interference, leading to a more discriminative feature representation.
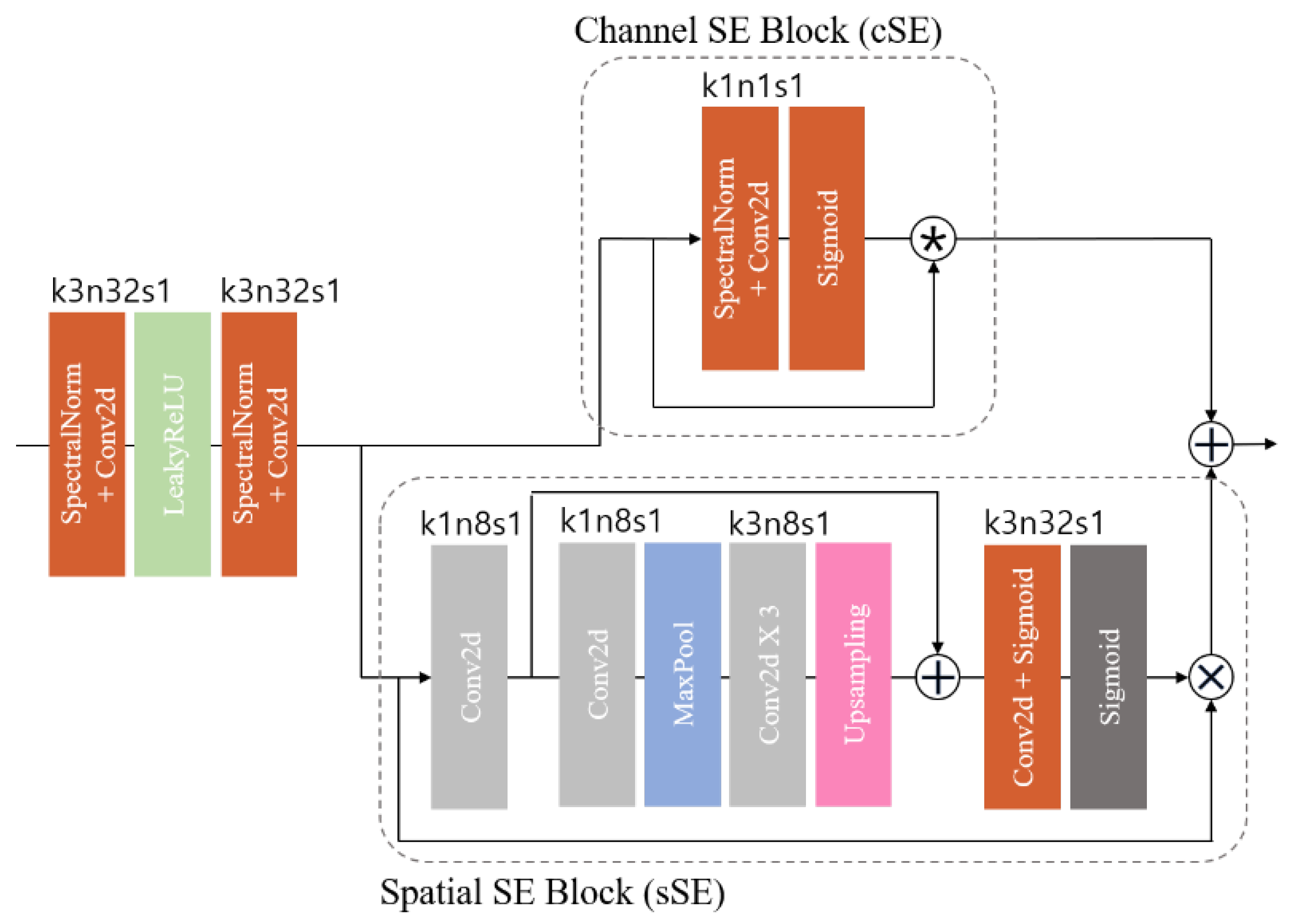
To enhance the effectiveness of the HFS framework, we employed spatial and channel squeeze and excitation (SE) blocks (scSE) (
Figure 3). The scSE blocks are an advancement of the squeeze and excitation (SE) block [
36], which learns channel-specific descriptors accounting for spatial dependence through global average pooling. This process resizes the input feature map to emphasize useful channels. The scSE combines the channel SE (cSE) block, which squeezes spatial regions and excites channels, with the spatial SE (sSE) block, which provides spatial attention without altering the receptive field.
In medical imaging, pixel-level spatial information is crucial for accurately segmenting anatomical structures [
10]. The spatial SE (sSE) block squeezes along channels and excites spatially, offering spatial attention while maintaining the receptive field. This enables the model to focus on specific regions of interest, enhancing segmentation performance. The scSE blocks, combining cSE and sSE blocks, recalibrate the feature map according to channel and space. These blocks are integrated into the residual block of the HFS module in this study.
3.2. Multi-Scale Discriminator Network Architecture
The discriminators in the model used the multi-scale discriminator method, which is a CycleGAN structure that performs image-to-image conversion and allows the discriminators to see the details of the image and understand and evaluate different information.
The multi-scale discriminator is a method that converts an input image to multiple resolutions, evaluates each version independently, and then aggregates them. By evaluating in multiple resolutions, it is possible to make more accurate evaluations of edges and patterns by utilizing information not only from high-resolution images but also from low-resolution images. Each image is extracted by random sampling to create a consistent image. In
Figure 4, the
is 512 × 512 and
is 256 × 256 and
is 128 × 128 in size.
Figure 4 shows the detail of the discriminator blocks. The first discriminator block consists of spectral normalization and convolution layers, followed by batch normalization and finally the LeakyReLU activation function and BlurPool layer. The second stage is the structure of the first discriminator block subtracted with the batch normalization. In the final stage, the ZeroPad2d layer is used to apply a convolution layer with a padding of 1 on top and bottom. The kernel size of the convolution layer is set to 4 and the stride to 1.
3.3. Loss Function
In this work, we use adversarial loss, cycle consistency loss, and identity loss to induce learning. Each loss is defined by Equations (1)–(3).
3.3.1. Adversarial Loss
Adversarial loss aims to minimize the cross-entropy between the distributions of generated and real data while maximizing the discriminator’s ability to differentiate between them. The loss encourages the generators to produce outputs closely resembling the target domain while ensuring that the discriminator can adeptly distinguish between generated and real samples. The adversarial loss
defined as follows:
where X and Y denote input data and target, respectively.
takes
as input and generates fake
.
is the i-th discriminator that judges the authenticity of the
data. We utilize an additional loss that is identical, employing
and
.
is the inverse of
, and
is also the inverse of
. For
,
are LDCT images and SDCT images, respectively. We set
.
With adversarial loss alone, the network cannot successfully learn the mapping relationship between the source and target domains, resulting in mode collapse. To deal with this issue, we applied cycle consistency loss.
3.3.2. Cycle Consistency Loss
Cycle consistency loss enables
, referred to as forward and backward cycle consistency, respectively. In fact, it imposes a shared latent spatial constraint to preserve the original content during the cycle reconstruction. The cycle consistency loss
is defined as follows:
3.3.3. Identity Loss
Adding loss of identity has several benefits. First, it preserves the content of the input images during translation, resulting in more visually consistent results. Second, it allows the model to better handle the task of translating between unmatched images by encouraging the model to maintain the structural integrity of the input images. The identity loss
is defined as follows:
3.3.4. Overall Joint Loss
Combining Equations (1)–(3), we obtain the overall joint loss function of the low-dose CT denoising framework, expressed as follows:
where λ is a weighting parameter to control the trade-off between adversarial loss, cycle consistency loss, and identity loss.
= 0.1,
= 10.0,
and = 2.0. The primary reason for this setup was to align the scales of each loss function. The
tends to have a larger value compared to the
and
, so we set
to a lower value to appropriately adjust the scale. Moreover, to minimize artifacts and distortions that could occur during the image reconstruction process, we selected hyperparameters that relatively increased the
and
. This ensured that the reconstructed images maintained structural consistency while providing visually realistic results.
4. Experiments
4.1. Datasets
In this study, we used unmatched pairs of LDCT and SDCT images of 10 clinically healthy dogs provided by the Animal Hospital of Konkuk University. We observed ethical considerations, and Institutional Review Board (IRB) approval was obtained for the animal experimentation. The training data consists of 3458 axial slices of LDCT and SDCT images of 9 dogs, respectively, and the test dataset consists of 941 axial slices of one dog.
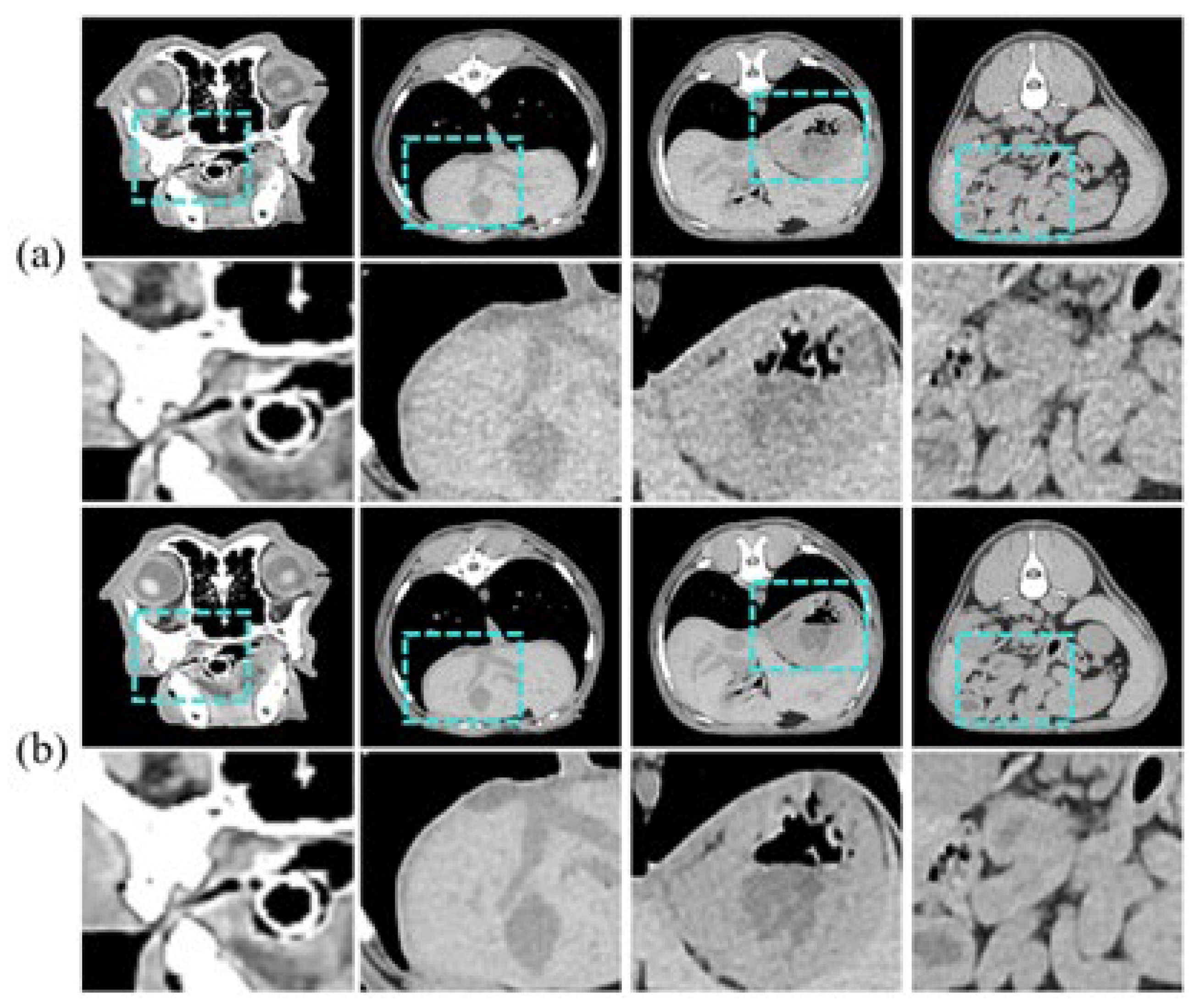
Figure 5 is an example of an input sample. The CT images are grayscale and have a pixel size of 512 × 512. Coronal currents were acquired using 30 mA and 150 mA for LDCT and SDCT, respectively.
4.2. Evaluation Framework
The evaluation involves two key aspects. First, precision measures the proportion of the generated image that aligns with the true image sample distribution. It essentially quantifies how accurately the generated image depicts the true image sample. Recall is the proportion of real-world footage that belongs to the distribution of generated footage samples, and recall is how much of the real-world footage is reproduced by the generated footage.
The current calculation method relies on relative density, which poses limitations in properly addressing phenomena such as densely packed generated images due to mode decay or truncation. Therefore, in this study, we introduce an improved precision and recall calculation method to address these shortcomings. The improved precision and recall [
37] uses the middle layer of the InceptionV3 model [
38] to extract the features of the generated images and the real images.
The regions of the real and generated images are then approximated by hyperspheres using the K-nearest neighbor method. Equation (5) gives the improved precision, which is the binary value of whether or not the generated data is present in the Kth closest region of the real image to the generated data and averaged. Equation (6) gives the improved recall, which is a binary value that averages over whether or not the actual data is present in the Kth closest region of the training data. Equation (7) is defined to compare the distance between feature vectors and is a functional expression that outputs 1 if a vector closer than the Kth feature vector exists and 0 otherwise.
In the following way, recall is quantified by querying for each real image whether the image is estimated manifold generated images.
where
are the set of features of the real image and the generated image, respectively, and
are the feature elements of the set
are the feature elements of
. The set
and outputs the feature vector that is the Kth closest to the feature vector
and outputs the Kth closest feature vector to the feature vector given as input to the function.
Additionally, there are two main approaches to validating model outcomes: k-fold cross-validation and single validation. While k-fold cross-validation is generally a useful technique for assessing model performance, it has certain drawbacks. In the case of sensitive medical imaging data, there is a risk of overestimating performance if slices from the same patient are mixed between the training and validation sets during the data splitting process. To address this, a patient-based k-fold cross-validation can be considered. However, if certain pathological features are unique to specific patients, this approach may introduce data imbalance across folds, leading to distorted performance evaluation. Moreover, dividing the data into multiple folds and training and evaluating the model on each fold is computationally intensive, significantly increasing training time. Therefore, in this study, we conducted an independent single validation. We aimed to enhance the reliability of the evaluation by using improved precision and recall metrics over traditional methods that rely on relative density. An appropriate validation split ratio of approximately 21% was used.
4.3. Implementation Details
In Equation (7), which is used to evaluate precision and recall for the generated images, K is 3. The data processing for precision and recall evaluation is as follows. When extracting image patches, the outside of the CT cylinder is fixed to a value such as −2048, so we set an ROI inside the cylinder and randomly sampled it with a size of 128 × 128. In the process, if the sampled patches contained many parts that were not the organs we wanted to see, we excluded them and resampled them.
We used min-max normalization to standardize the characteristics of the images to make consistent comparisons. In Equation (8), X is the value of each pixel in the original image and X′ is the normalized value. This ensures that the pixel values in the image are represented within a certain range.
When training the network, we used Adam [
39] as the optimizer and set
= 0.9,
= 0.999, and the learning rate was fixed at
. The key parameters used in training the CNN are provided in each figure. Weight initialization was performed using the Xavier uniform method. The training was performed on a single RTX GeForce 4090 with a batch size of 1 and input size of 512 for 5000 epochs.
In the selection of hyperparameters, consistent performance improvements were observed at specific values through experimentation. Although the chosen hyperparameters were not fully optimized due to computational constraints, they were found to be practical and effective configurations, as evidenced by the empirical performance observed in the experiments.
4.4. Ablation Study on Model Architecture
We adopted scSE blocks to maximize the performance of the HFS modules. In this section, we performed three ablation studies to validate the effectiveness of scSE. We compared cSE alone, sSE alone, and the scSE method, which combines cSE and sSE. Each method was evaluated based on whether it applied spatial squeeze and channel excitation or channel squeeze and spatial excitation, and we identified and described the features of each approach. First, cSE alone emphasized useful channels by squeezing them along spatial regions and excitations along channels. The results are shown in
Figure 6b and indicate reduced noise and improved perceptual quality compared to the conventional LDCT image. However, the contrast is still not sharp enough, and important structures are faintly visible. Second, sSE alone emphasizes pixel-wise information by spatially exciting them along the channel, as opposed to cSE. The results, shown in
Figure 6c, demonstrate improved contrast, making anatomical structures more distinct. However, the reconstructed high-resolution image appears uneven and less smooth. Finally, the scSE method combines the advantages of both cSE and sSE.
Figure 6d shows that scSE yields the most pronounced contrast, improving upon the limitations observed with sSE alone.
The quantitative evaluation of each item is shown in
Table 1. Precision and recall are evaluated by truncation 1.0 and 0.3. Truncation is a technique used to sample the best images from the trained distribution after training the GAN with the truncation trick. The farther away from the mean of the distribution, the more transformed images will be sampled; close to 0 means sampling near the mean, and close to 1 means sampling from the distribution as it is. Low and standard dose are scores for comparison between the different methods. The scores when applying cSE, sSE, and scSE to the base model show positive results, indicating that LDCT can be used as input data to produce images close to SDCT. The highest and next highest scores in each area are shown in bold and underscore, respectively. scSE yielded the highest values for precision and recall. Precision was next highest with cSE, and recall was next highest after that with sSE. The proposed scSE method was found to be the most effective in both quantitative and perceptual evaluation.
4.5. Comparison of Different Methods
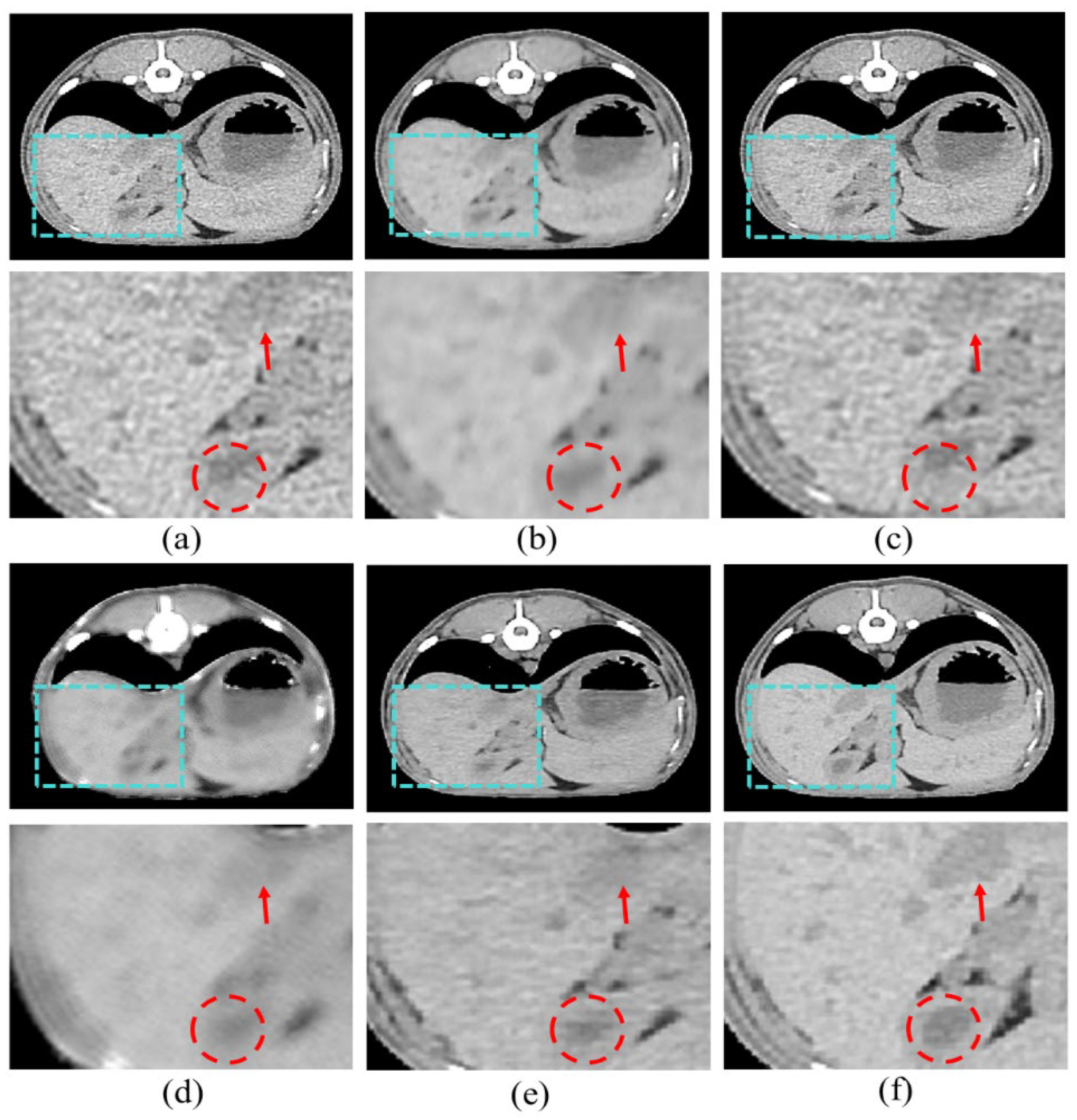
For the purpose of comparing noise reduction performance, we employed three distinct deep learning models. RED-CNN [
40], rooted in residual learning, enhances resolution by extracting features from high-resolution images at each layer and adding these features to the output of the previous layer. We utilized HFS to compare its effectiveness in feature extraction from images. WGAN-VGG [
3] is a model based on the Wasserstein GAN (WGAN) [
41], which improves the stability of GANs and the quality of generated images. We used it to compare with the anti-aliasing and stability effects of BlurPool and the regularization term that we used. Lastly, DU-GAN utilizes a gradient domain discriminator to enhance edge information and reduce streak artifacts caused by photon starvation. It focuses on increasing edge sharpness and reducing streak artifacts to generate high-resolution images. Therefore, we chose this model for comparison with the multi-scale discriminator.
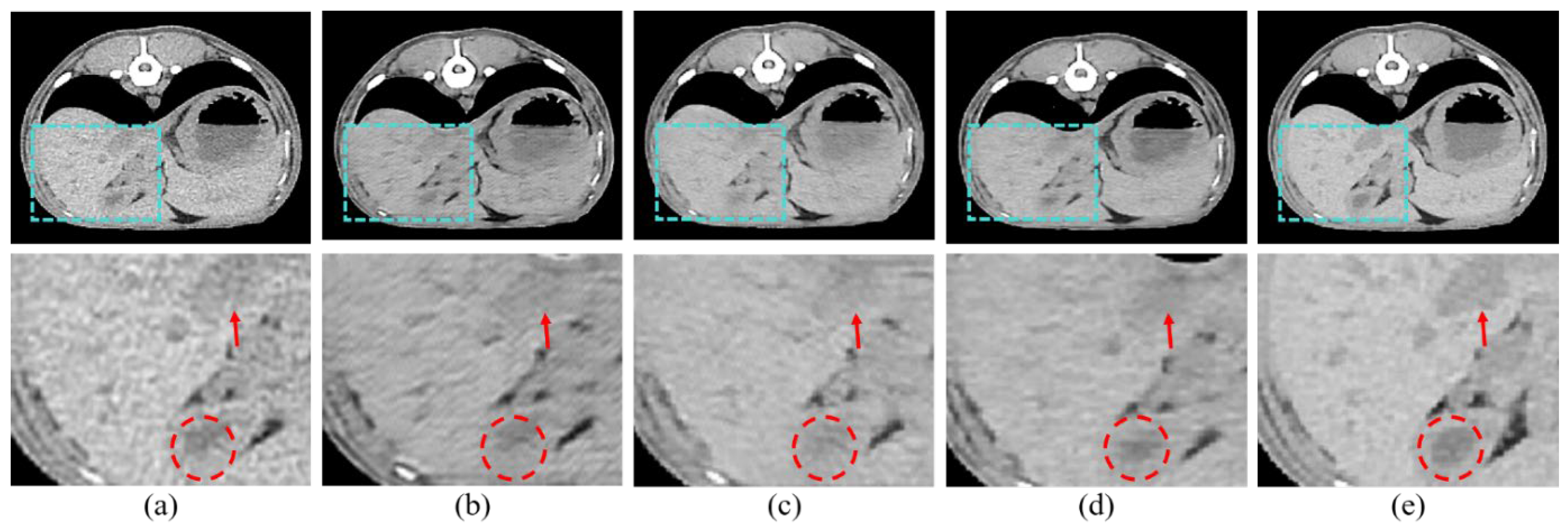
Figure 7 shows the resulting images of the proposed model and the comparison models.
Figure 7b is the result image for RED-CNN, indicating significant noise reduction compared to the LDCT images, though sharpness is still lacking.
Figure 7c shows the result for WGAN-VGG, which is sharper, but shows less noise reduction.
Figure 7d shows the results for DU-GAN, where the edges are cleaner and the denoising is effective, but sharpness is still an issue.
Figure 7e shows our proposed model, which addresses the shortcomings of the previous models and best represents the features of SDCT.
5. Discussion
In this study, we introduced a CycleGAN-based denoising method tailored for low-dose CT (LDCT) imaging, specifically targeting animal LDCT. This innovative approach surpasses conventional CT denoising methods, offering enhanced image quality while concurrently minimizing radiation exposure, thereby showcasing its potential in veterinary imaging. The key is the cohesive integration of several techniques adept at handling noise and deformation, critical aspects in medical image reconstruction. The utilization of BlurPool and regularization terms effectively mitigates distortion in high-resolution images, while PixelShuffle enhances expressiveness in the output. Additionally, the HFS structure and scSE block retain and emphasize features, facilitating the accurate reproduction of contrast and vital organs in the resulting image. The generator’s efficacy in producing realistic images heavily relies on the effectiveness of the discriminator, for which we introduce a multi-scale discriminator. This novel approach ensures accurate assessments of edges and patterns by incorporating information from various resolutions, thus effectively balancing the adversarial learning process and leading to improved overall performance.
Our results demonstrate that our proposed model outperforms comparative methods in both perceptual and quantitative evaluations. In our study, the results show that our model outperforms the comparative methods in both perceptual and quantitative evaluations in
Figure 7 and
Table 2. In addition, in
Figure 6 and
Table 1, the effectiveness of the proposed scSE method for denoising LDCT images in animal imaging was demonstrated. Through three ablation studies, we compared the performance of cSE alone, sSE alone, and the combined scSE method, revealing distinct advantages and limitations associated with each approach. The successful implementation of the scSE method underscores the importance of innovative approaches in addressing the challenges associated with LDCT imaging in veterinary medicine.
Our method offers a promising solution for improving diagnostic accuracy and patient care in veterinary imaging.
While our proposed method demonstrates greater performance in improving image quality compared to other methods, there is still room for improvement in terms of contrast and sharpness when compared to SDCT. In the future, there is potential for further enhancement of our denoising method and exploration of its applicability across diverse animal species and imaging modalities. This could involve refining existing components to improve the efficacy and efficiency of LDCT denoising in veterinary imaging, exploring the generalizability of our approach, and conducting real-world clinical validation to provide valuable insights into its practical utility and transformative impact on animal healthcare. Additionally, our target was the pre-contrast LDCT images. In this case, applying our model to LDCT images effectively reduced radiation exposure by producing images with noise levels comparable to SDCT images. However, when our model was applied to post-contrast LDCT images, it did not achieve the same level of quality as SDCT images. This is likely because post-contrast images exhibit greater contrast between areas with and without contrast agent, resulting in more distinct image features. This increased contrast can introduce complex and challenges in noise reduction, making it more difficult to achieve consistent results.
Deep learning tasks on medical images inherently face reliability challenges. While we have shown the reliability and perceptual superiority of the proposed low-dose CT denoising framework through precision and recall evaluation methods, there are still doubts about its practical clinical applicability, as it is not based on pixel-wise evaluations such as PSNR or SSIM. However, due to the unpaired nature of the dataset, employing this method remains challenging. As deep learning advances into real-world applications, it is clear that different evaluation methodologies must be considered.
6. Conclusions
In this paper, we propose a method to apply deep learning to LDCT to make it as good as SDCT. We call this method the low-dose CT denoising framework. The proposed framework is based on CycleGAN and proposes an anti-aliasing generator and multi-scale discriminator with various proven techniques. By overcoming the limitations of the existing CycleGAN for input images, the proposed framework outperforms existing models in both quantitative and perceptual evaluations in terms of preserving anatomical information and suppressing image noise. With a framework that can generate high-quality images under low-dose conditions, we expect to reduce radiation exposure to animals and make future animal CT imaging safer.
The CT dataset used in this study is sufficient to produce results, but we plan to accumulate more data and conduct various experiments on the proposed framework to extend it to various fields and tasks in the future.
Author Contributions
Y.S.: writing—original draft preparation, methodology; S.J.: writing—original draft preparation; J.L. writing—original draft preparation; H.C.: data curation, investigation; J.K.: data curation, investigation; H.S.: writing—review and editing; B.J.: writing—review and editing; Y.H.: conceptualization, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Konkuk University (Approval Code: KU24109) on 24 May 2024. All experimental procedures were conducted in accordance with the guidelines and regulations for the care and use of laboratory animals set forth by the IACUC.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brenner, D.J.; Hall, E.J. Computed tomography—An increasing source of radiation exposure. N. Engl. J. Med. 2007, 357, 2277–2284. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Ye, J.C. AdaIN-based tunable CycleGAN for efficient unsupervised low-dose CT denoising. IEEE Trans. Comput. Imaging 2021, 7, 73–85. [Google Scholar] [CrossRef]
- Yang, Q.; Yan, P.; Zhang, Y.; Yu, H.; Shi, Y.; Mou, X.; Kalra, M.K.; Zhang, Y.; Sun, L.; Wang, G. Low-dose CT image denoising using a generative adversarial network with Wasserstein distance and perceptual loss. IEEE Trans. Med. Imaging 2018, 37, 1348–1357. [Google Scholar] [CrossRef]
- Yi, X.; Babyn, P. Sharpness-aware low-dose CT denoising using conditional generative adversarial network. J. Digit. Imaging 2018, 31, 655–669. [Google Scholar] [CrossRef]
- Zhu, J.-Y.; Park, T.; Isola, P.; Efros, A. A Unpaired image-to-image translation using cycle-consistent adversarial networks. In Proceedings of the IEEE International Conference on Computer Vision, Venice, Italy, 22–29 October 2017. [Google Scholar]
- Hore, A.; Ziou, D. Image quality metrics: PSNR vs. SSIM. In Proceedings of the 2010 20th International Conference on Pattern Recognition, Istanbul, Turkey, 23–26 August 2010. [Google Scholar]
- Hu, W.; Li, M.; Ju, X. Improved cycleGAN for Image-to-Image Translation. 2021. Available online: https://weininghu1012.github.io/file/cpsc532L_report.pdf (accessed on 7 August 2024).
- Zhang, R. Making convolutional networks shift-invariant again. In Proceedings of the 36th International Conference on Machine Learning, Long Beach, CA, USA, 9–15 June 2019. [Google Scholar]
- Gan, Y.; Gong, J.; Ye, M.; Qian, Y.; Liu, K.; Zhang, S. GANs with multiple constraints for image translation. Complexity 2018, 2018, 4613935. [Google Scholar] [CrossRef]
- Jeong, D.; Hong, Y.; Lee, J.; Lee, S.B.; Cho, Y.J.; Shim, H.; Chang, H.-J. Improving the Reproducibility of Computed Tomography Radiomic Features Using an Enhanced Hierarchical Feature Synthesis Network. IEEE Access 2024, 12, 27648–27660. [Google Scholar] [CrossRef]
- Wang, Z.; Simoncelli, E.P.; Bovik, A.C. Multiscale structural similarity for image quality assessment. In Proceedings of the Thirty-Seventh Asilomar Conference on Signals, Systems & Computers, Pacific Grove, CA, USA, 9–12 November 2003. [Google Scholar]
- Huang, Z.; Zhang, J.; Zhang, Y.; Shan, H. DU-GAN: Generative adversarial networks with dual-domain U-Net-based discriminators for low-dose CT denoising. IEEE Trans. Instrum. Meas. 2021, 71, 4500512. [Google Scholar] [CrossRef]
- Isola, P.; Zhu, J.-Y.; Zhou, T.; Efros, A.A. Image-to-image translation with conditional adversarial networks. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Honolulu, HI, USA, 21–26 July 2017. [Google Scholar]
- Qu, X.; Wang, X.; Wang, Z.; Wang, L.; Zhang, L. Perceptual-DualGAN: Perceptual losses for image to image translation with generative adversarial nets. In Proceedings of the 2018 International Joint Conference on Neural Networks (IJCNN), Rio de Janeiro, Brazil, 8–13 July 2018. [Google Scholar]
- Kang, E.; Koo, H.J.; Yang, D.H.; Seo, J.B.; Ye, J.C. Cycle-consistent adversarial denoising network for multiphase coronary CT angiography. Med. Phys. 2019, 46, 550–562. [Google Scholar] [CrossRef]
- You, C.; Li, G.; Zhang, Y.; Zhang, X.; Shan, X.; Ju, S.; Zhao, Z.; Zhang, Z.; Cong, W.; Vannier, M.W.; et al. CT Super-resolution GAN Constrained by the Identical, Residual, and Cycle Learning Ensemble. arXiv 2018, arXiv:1808.04256v3. [Google Scholar] [CrossRef]
- Kwon, T.; Ye, J.C. Cycle-free CycleGAN using invertible generator for unsupervised low-dose CT denoising. IEEE Trans. Comput. Imaging 2021, 7, 1354–1368. [Google Scholar] [CrossRef]
- Huang, X.; Belongie, S. Arbitrary style transfer in real-time with adaptive instance normalization. In Proceedings of the IEEE International Conference on Computer Vision, Venice, Italy, 22–29 October 2017. [Google Scholar]
- Yin, Z.; Xia, K.; Wang, S.; He, Z.; Zhang, J.; Zu, B. Unpaired low-dose CT denoising via an improved cycle-consistent adversarial network with attention ensemble. Vis. Comput. 2023, 39, 4423–4444. [Google Scholar] [CrossRef]
- Tang, Y.; Du, Q.; Wang, J.; Wu, Z.; Li, Y.; Li, M.; Yang, X.; Zheng, J. CCN-CL: A content-noise complementary network with contrastive learning for low-dose computed tomography denoising. Comput. Biol. Med. 2022, 147, 105759. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Gao, Q.; Zhang, Y.; Shan, H. Ascon: Anatomy-aware supervised contrastive learning framework for low-dose ct denoising. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Vancouver, BC, Canada, 8–12 October 2023. [Google Scholar]
- Zhang, Y.; Zhang, R.; Cao, R.; Xu, F.; Jiang, F.; Meng, J.; Ma, F.; Guo, Y.; Liu, J. Unsupervised low-dose CT denoising using bidirectional contrastive network. Comput. Methods Programs Biomed. 2024, 251, 108206. [Google Scholar] [CrossRef]
- Sohl-Dickstein, J.; Weiss, E.; Maheswaranathan, N.; Ganguli, S. Deep unsupervised learning using nonequilibrium thermodynamics. In Proceedings of the International Conference on Machine Learning, Lille, France, 6–11 July 2015. [Google Scholar]
- Özbey, M.; Dalmaz, O.; Dar, S.U.; Bedel, H.A.; Özturk, Ş.; Güngör, A.; Çukur, T. Unsupervised medical image translation with adversarial diffusion models. IEEE Trans. Med. Imaging 2023, 42, 3524–3539. [Google Scholar] [CrossRef]
- Arslan, F.; Kabas, B.; Dalmaz, O.; Ozbey, M.; Çukur, T. Self-Consistent Recursive Diffusion Bridge for Medical Image Translation. arXiv 2024, arXiv:2405.06789. [Google Scholar]
- Vaswani, A. Attention is all you need. In Proceedings of the Advances in Neural Information Processing Systems, Long Beach, CA, USA, 4–9 December 2017. [Google Scholar]
- Atli, O.F.; Kabas, B.; Arslan, F.; Yurt, M.; Dalmaz, O.; Çukur, T. I2I-Mamba: Multi-modal medical image synthesis via selective state space modeling. arXiv 2024, arXiv:2405.14022. [Google Scholar]
- Miyato, T.; Kataoka, T.; Koyama, M.; Yoshida, Y. Spectral normalization for generative adversarial networks. arXiv 2018, arXiv:1802.05957. [Google Scholar]
- Ulyanov, D.; Vedaldi, A.; Lempitsky, V. Instance normalization: The missing ingredient for fast stylization. arXiv 2016, arXiv:1607.08022. [Google Scholar]
- Maas, A.L.; Hannun, A.Y.; Ng, A.Y. Rectifier nonlinearities improve neural network acoustic models. In Proceedings of the 30th International Conference on Machine Learning, Atlanta, GA, USA, 17–19 June 2013. [Google Scholar]
- Lecun, Y.; Bottou, L.; Bengio, Y.; Haffner, P. Gradient-based learning applied to document recognition. Proc. IEEE 1998, 86, 2278–2324. [Google Scholar] [CrossRef]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep residual learning for image recognition. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016. [Google Scholar]
- Ronneberger, O.; Fischer, P.; Brox, T. U-net: Convolutional networks for biomedical image segmentation. In Proceedings of the Medical Image Computing and Computer-Assisted Intervention–MICCAI 2015: 18th International Conference, Munich, Germany, 5–9 October 2015. [Google Scholar]
- Liu, J.; Zhang, W.; Tang, Y.; Tang, J.; Wu, G. Residual feature aggregation network for image super-resolution. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition, Seattle, WA, USA, 13–19 June 2020. [Google Scholar]
- Lee, S.B.; Cho, Y.J.; Hong, Y.; Jeong, D.; Lee, J.; Kim, S.H.; Lee, S.; Choi, Y.H. Deep learning-based image conversion improves the reproducibility of computed tomography radiomics features: A phantom study. Investig. Radiol. 2022, 57, 308–317. [Google Scholar] [CrossRef]
- Roy, A.G.; Navab, N.; Wachinger, C. Recalibrating fully convolutional networks with spatial and channel “squeeze and excitation” blocks. IEEE Trans. Med. Imaging 2018, 38, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Kynkäänniemi, T.; Karras, T.; Laine, S.; Lehtinen, J.; Aila, T. Improved precision and recall metric for assessing generative models. Adv. Neural Inf. Process. Syst. 2019, 32, 3927–3936. [Google Scholar]
- Szegedy, C.; Vanhoucke, V.; Ioffe, S.; Shlens, J.; Wojna, Z. Rethinking the inception architecture for computer vision. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016. [Google Scholar]
- Kingma, D.P.; Ba, J. Adam: A method for stochastic optimization. arXiv 2014, arXiv:1412.6980. [Google Scholar]
- Chen, H.; Zhang, Y.; Kalra, M.K.; Lin, F.; Chen, Y.; Liao, P.; Zhou, J.; Wang, G. Low-dose CT with a residual encoder-decoder convolutional neural network. IEEE Trans. Med. Imaging 2017, 36, 2524–2535. [Google Scholar] [CrossRef] [PubMed]
- Adler, J.; Lunz, S. Banach wasserstein gan. In Proceedings of the Advances in Neural Information Processing Systems 31, Montréal, QC, Canada, 3–8 December 2018. [Google Scholar]
Figure 1.
Low-dose CT denoising framework: X is LDCT; Y is SDCT. The network consists of two main components: the generator networks and the discriminator networks. The bounding boxes within the CT images are color-coded to represent different input sizes for the discriminators. The yellow boxes correspond to the discriminators, which process the image at a size of 512 × 512 pixels. The blue boxes correspond to at 256 × 256, and the green boxes represent at 128 × 128.
Figure 1.
Low-dose CT denoising framework: X is LDCT; Y is SDCT. The network consists of two main components: the generator networks and the discriminator networks. The bounding boxes within the CT images are color-coded to represent different input sizes for the discriminators. The yellow boxes correspond to the discriminators, which process the image at a size of 512 × 512 pixels. The blue boxes correspond to at 256 × 256, and the green boxes represent at 128 × 128.
Figure 2.
BlurPool generator structure, specifying the kernel size (k), number of feature maps (n), and stride (s) for each convolutional layer.
Figure 2.
BlurPool generator structure, specifying the kernel size (k), number of feature maps (n), and stride (s) for each convolutional layer.
Figure 3.
Feature attention block, specifying the kernel size (k), number of feature maps (n), and stride (s) for each convolutional layer.
Figure 3.
Feature attention block, specifying the kernel size (k), number of feature maps (n), and stride (s) for each convolutional layer.
Figure 4.
Multi-scale discriminator structure, specifying the kernel size (k), number of feature maps (n), and stride (s) for each convolutional layer.
Figure 4.
Multi-scale discriminator structure, specifying the kernel size (k), number of feature maps (n), and stride (s) for each convolutional layer.
Figure 5.
Image samples for training, showing diverse regions/tissues: (a) is LDCT and (b) is SDCT.
Figure 5.
Image samples for training, showing diverse regions/tissues: (a) is LDCT and (b) is SDCT.
Figure 6.
Ablation studies of the proposed method, (a) LDCT, (b) cSE alone, (c) sSE alone, (d) scSE, and (e) SDCT.
Figure 6.
Ablation studies of the proposed method, (a) LDCT, (b) cSE alone, (c) sSE alone, (d) scSE, and (e) SDCT.
Figure 7.
Results of the proposed model and other models: (a) LDCT, (b) RED-CNN, (c) WGAN-VGG, (d) DU-GAN, (e) Ours, and (f) SDCT.
Figure 7.
Results of the proposed model and other models: (a) LDCT, (b) RED-CNN, (c) WGAN-VGG, (d) DU-GAN, (e) Ours, and (f) SDCT.
Table 1.
Precision and recall evaluation of the proposed methods in ablation studies: The highest and next highest scores in each area are shown in bold and underline, respectively.
Table 1.
Precision and recall evaluation of the proposed methods in ablation studies: The highest and next highest scores in each area are shown in bold and underline, respectively.
| Ablation Term | Precision | | Recall | |
|---|
| Truncation | 1.0 | 0.3 | 1.0 | 0.3 |
| Low and standard dose | 0.45 | 0.45 | 0.66 | 0.37 |
| Base model + cSE | 0.88 | 0.91 | 0.90 | 0.88 |
| Base model + sSE | 0.91 | 0.91 | 0.9 | 0.9 |
| Base model + scSE | 0.93 | 0.94 | 0.92 | 0.91 |
Table 2.
Comparison model precision and recall evaluation: The highest and next highest scores in each area are shown in bold and underline, respectively.
Table 2.
Comparison model precision and recall evaluation: The highest and next highest scores in each area are shown in bold and underline, respectively.
| Ablation Term | Precision | | Recall | |
|---|
| Truncation | 1.0 | 0.3 | 1.0 | 0.3 |
| Low and standard dose | 0.45 | 0.45 | 0.66 | 0.37 |
| RED-CNN [40] | 0.86 | 0.83 | 0.91 | 0.93 |
| WGAN-VGG [3] | 0.71 | 0.70 | 0.82 | 0.85 |
| DU-GAN [12] | 0.94 | 0.93 | 0.87 | 0.84 |
| Proposed | 0.93 | 0.94 | 0.92 | 0.91 |
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).