Encapsulation of Inositol Hexakisphosphate with Chitosan via Gelation to Facilitate Cellular Delivery and Programmed Cell Death in Human Breast Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
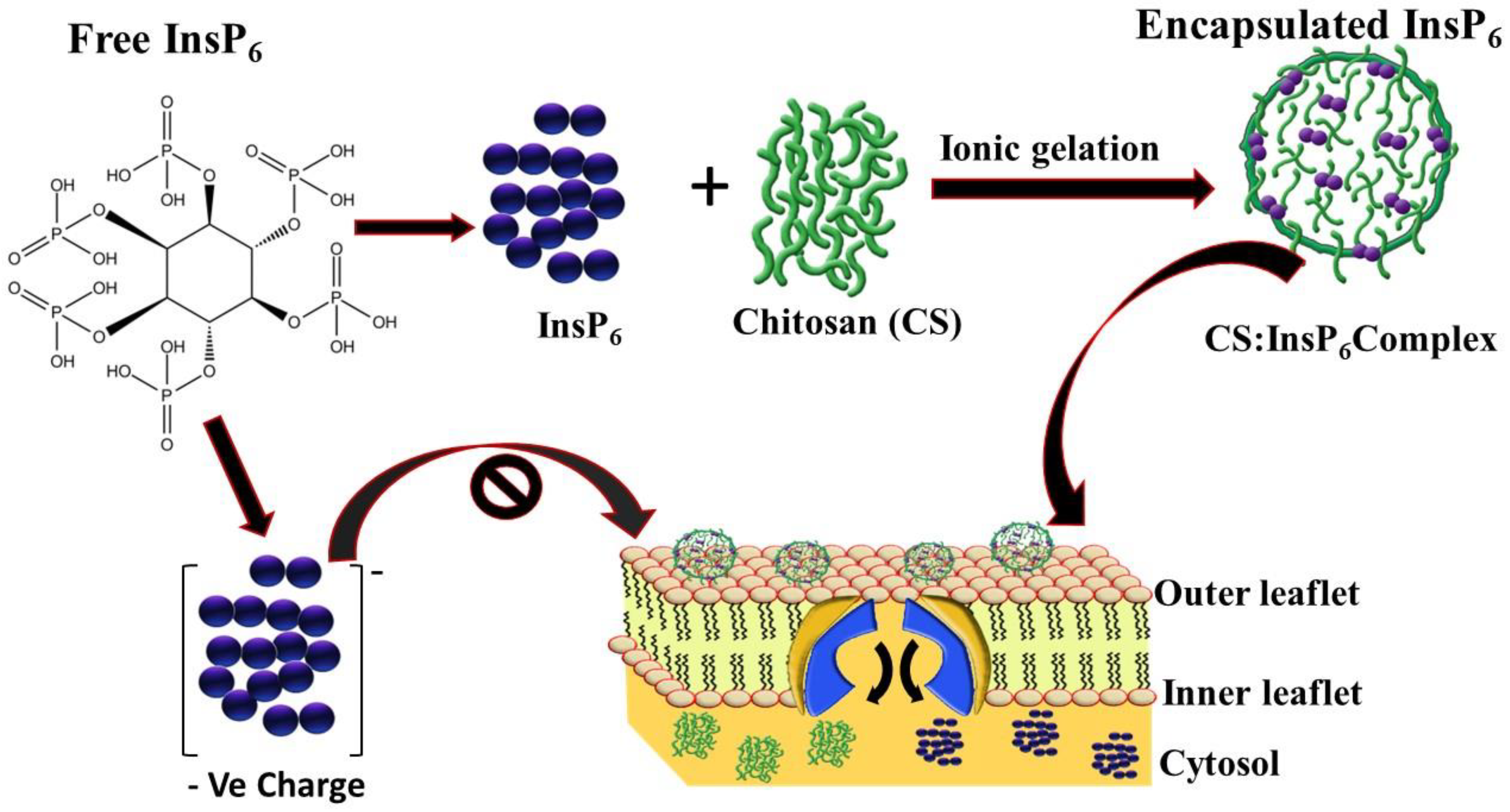
2.2. Encapsulation of InsP6 with Chitosan
2.3. Scanning Electron Microscopy (SEM)
2.4. Fourier Transform Infrared (FTIR) Spectra Studies
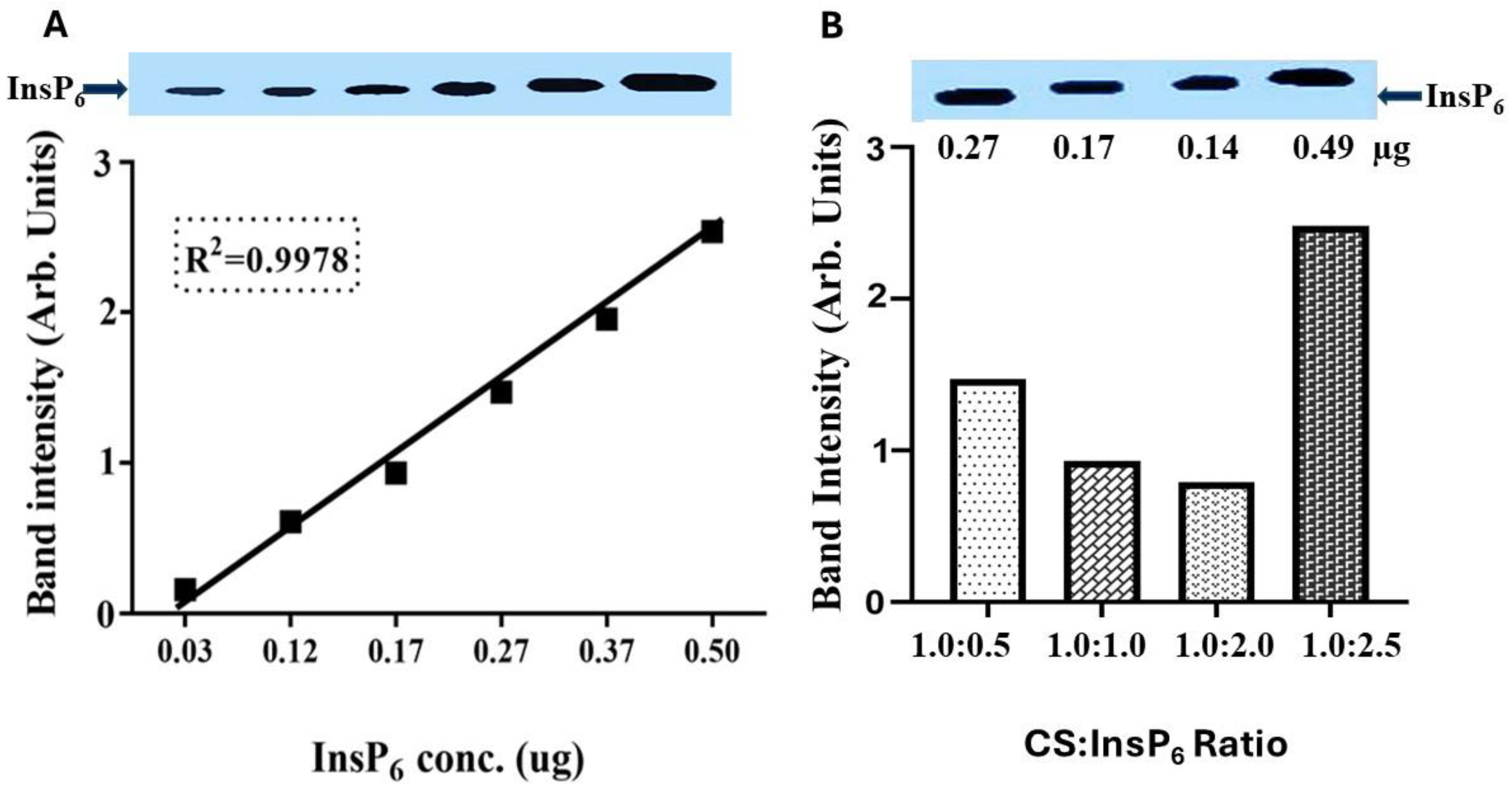
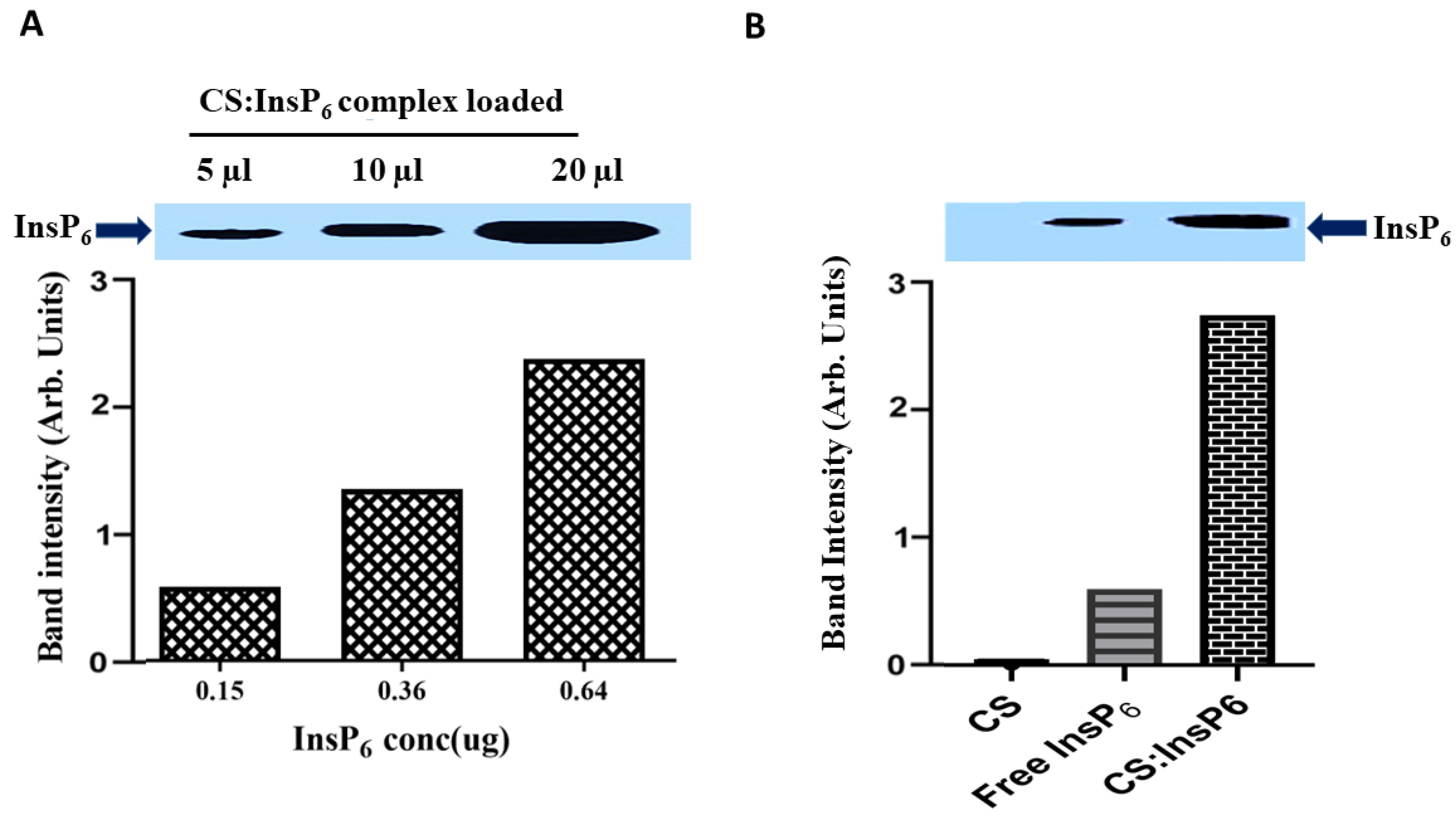
2.5. Detection of InsP6 by Polyacrylamide Gel Electrophoresis (PAGE)
2.6. Detection of InsP6 by Atomic Absorption Spectrophotometry
2.7. Cell Culture
2.8. MTT Assay
2.9. Acridine Orange/Ethidium Bromide Staining
2.10. Determination of Reactive Oxygen Species (ROS) Production
2.11. Flow Cytometry
2.12. Statistical Calculations
3. Results
3.1. Encapsulation of InsP6 with Chitosan and Its Physicochemical Characterization
3.2. Cellular Uptake of Encapsulated InsP6 by MCF-7 Cells
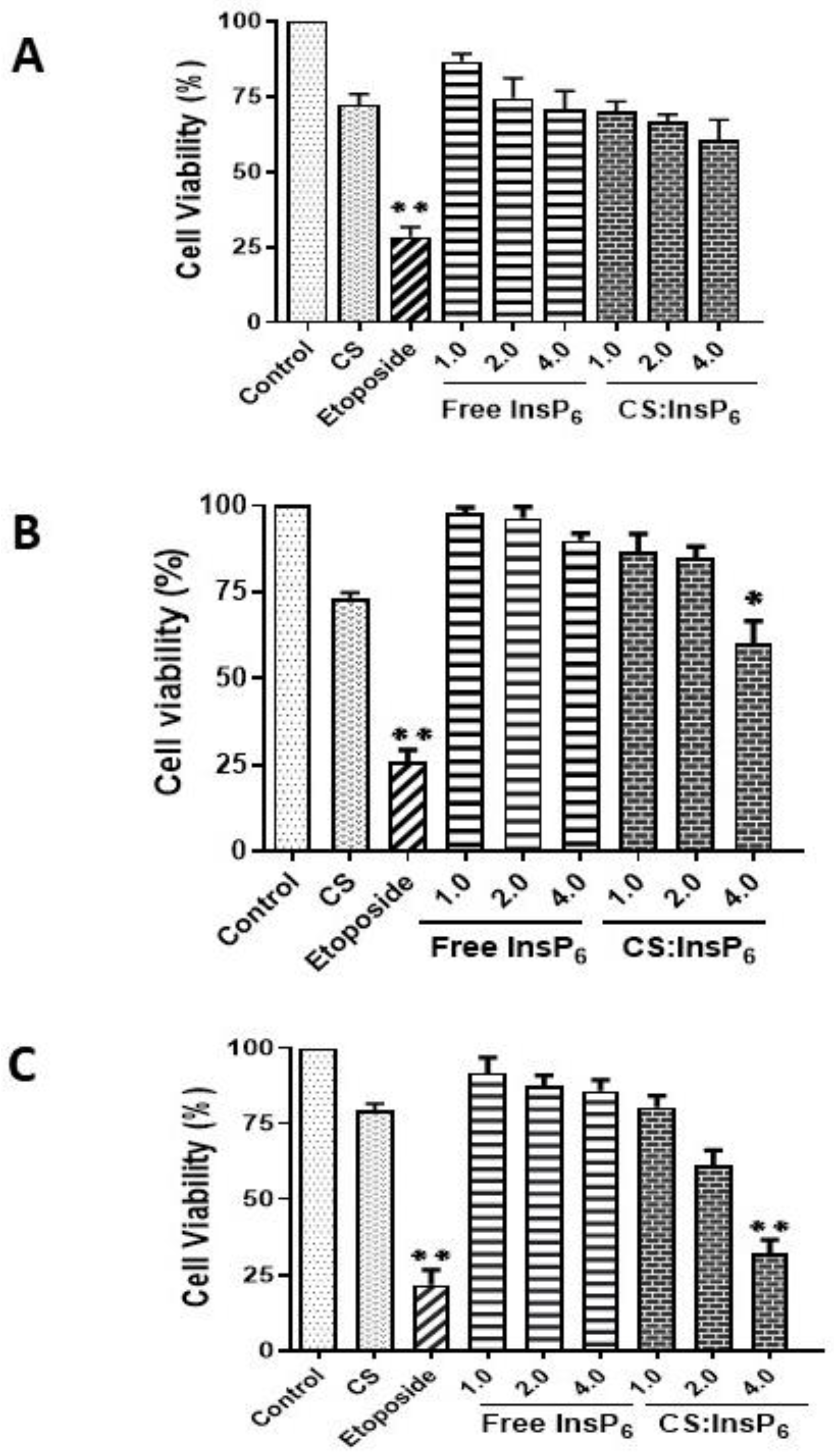
3.3. Effect of Encapsulated InsP6 on Cell Viability in MCF-7 Cells
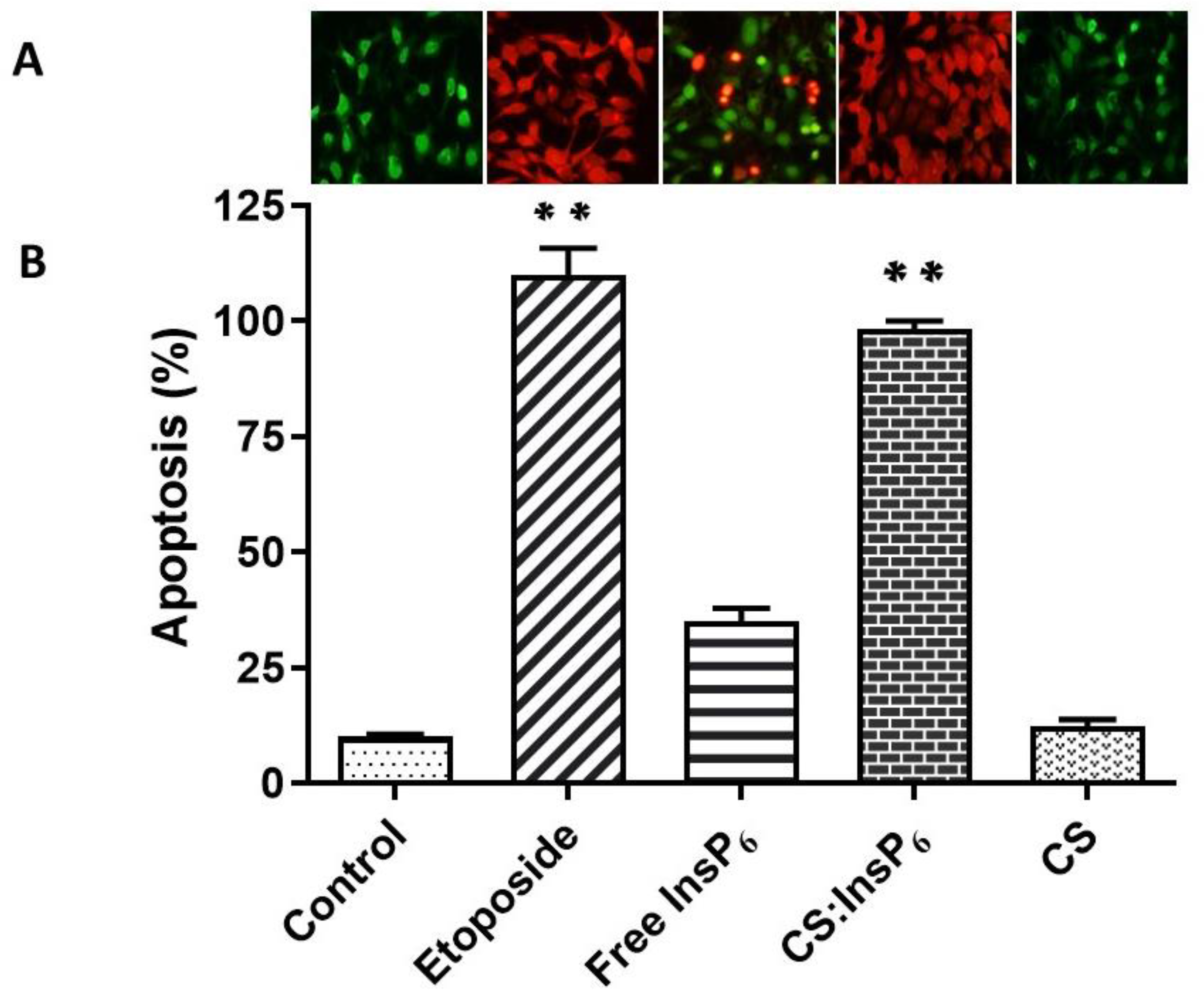
3.4. Effect of Encapsulated InsP6 on Apoptosis in MCF-7 Cells
3.5. Encapsulated InsP6 Induced Cell Death via the Generation Reactive Oxygen Species
3.6. Encapsulated InsP6 Induces Apoptosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berridge, M.J.; Irvine, R.F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature 1984, 312, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Abel, K.; Anderson, R.A.; Shears, S.B. Phosphatidylinositol and inositol phosphate metabolism. J. Cell Sci. 2001, 114, 2207–2208. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Mumtaz, H.; Ali, N. Role of inositol polyphosphates in programmed cell death. Mol. Cell. Biochem. 2009, 328, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, M.; Dinicola, S.; Bevilacqua, A.; Cucina, A. Broad spectrum anticancer activity of myo-inositol and inositol hexakisphosphate. Int. J. Endocrinol. 2016, 2016, 5616807. [Google Scholar] [CrossRef] [PubMed]
- Vucenik, I. Bioactivity of inositol phosphates. Molecules 2021, 26, 5042. [Google Scholar] [CrossRef] [PubMed]
- Shears, S.B.; Wang, H. Inositol phosphate kinases: Expanding the biological significance of the universal core of the protein kinase fold. Adv. Biol. Regul. 2019, 71, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Irvine, R.F.; Schell, M.J. Back in the water: The return of the inositol phosphates. Nat. Rev. Mol. Cell Biol. 2001, 2, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.R.; Sathe, S.K.; Salunkhe, D.K. Phytates in legumes and cereals. Adv. Food Res. 1982, 28, 1–92. [Google Scholar] [CrossRef]
- Holub, B.J. Metabolism and function of myo-inositol and inositol phospholipids. Annu. Rev. Nutr. 1986, 6, 563–597. [Google Scholar] [CrossRef]
- Kalam Shamsuddin, A.; Bose, S. IP6 (Inositol Hexaphosphate) as a signaling molecule. Curr. Signal Transduct. Ther. 2012, 7, 289–304. [Google Scholar] [CrossRef]
- Grases, F.; Costa-Bauza, A. Key aspects of myo-inositol hexaphosphate (phytate) and pathological calcifications. Molecules 2019, 24, 4434. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.; Wilson, M.S.; Eisenbeis, V.B.; Harmel, R.K.; Riemer, E.; Haas, T.M.; Wittwer, C.; Jork, N.; Gu, C.; Shears, S.B.; et al. Analysis of inositol phosphate metabolism by capillary electrophoresis electrospray ionization mass spectrometry. Nat. Commun. 2020, 11, 6035. [Google Scholar] [CrossRef] [PubMed]
- Ferry, S.; Matsuda, M.; Yoshida, H.; Hirata, M. Inositol hexakisphosphate blocks tumor cell growth by activating apoptotic machinery as well as by inhibiting the Akt/NFκB-mediated cell survival pathway. Carcinogenesis 2002, 23, 2031–2041. [Google Scholar] [CrossRef] [PubMed]
- Vucenik, I.; Shamsuddin, A.M. Protection against cancer by dietary IP6 and inositol. Nutr. Cancer 2006, 55, 109–125. [Google Scholar] [CrossRef]
- Paplomata, E.; O’Regan, R. The PI3K/AKT/mTOR pathway in breast cancer: Targets, trials and biomarkers. Ther. Adv. Med. Oncol. 2014, 6, 154–166. [Google Scholar] [CrossRef]
- Joh, E.H.; Hollenbaugh, J.A.; Kim, B.; Kim, D.H. Pleckstrin homology domain of Akt kinase: A proof of principle for highly specific and effective non-enzymatic anti-cancer target. PLoS ONE 2012, 7, e50424. [Google Scholar] [CrossRef]
- Singh, R.P.; Agarwal, C.; Agarwal, R. Inositol hexaphosphate inhibits growth, and induces G1 arrest and apoptotic death of prostate carcinoma DU145 cells: Modulation of CDKI-CDK-cyclin and pRb-related protein-E2F complexes. Carcinogenesis 2003, 24, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Kapral, M.; Wawszczyk, J.; Jesse, K.; Paul-Samojedny, M.; Kuśmierz, D.; Węglarz, L. Inositol hexaphosphate inhibits proliferation and induces apoptosis of colon cancer cells by suppressing the AKT/mTOR signaling pathway. Molecules 2017, 22, 1657. [Google Scholar] [CrossRef] [PubMed]
- Vucenik, I.; Shamsuddin, A.M. Cancer inhibition by inositol hexaphosphate (IP6) and inositol: From laboratory to clinic. J. Nutr. 2003, 133, 3778S–3784S. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Welch, D.R.; Hurst, D.R. Defining the hallmarks of metastasis. Cancer Res. 2019, 79, 3011–3027. [Google Scholar] [CrossRef] [PubMed]
- Lawson, D.A.; Kessenbrock, K.; Davis, R.T.; Pervolarakis, N.; Werb, Z. Tumour heterogeneity and metastasis at single-cell resolution. Nat. Cell Biol. 2018, 20, 1349–1360. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Thapa, S.; Rather, R.A.; Singh, S.K.; Bhagat, M. Insights into the Role of Defective Apoptosis in Cancer Pathogenesis and Therapy. In Regulation and Dysfunction of Apoptosis; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Neophytou, C.M.; Trougakos, I.P.; Erin, N.; Papageorgis, P. Apoptosis Deregulation and the Development of Cancer Multi-Drug Resistance. Cancers 2021, 13, 4363. [Google Scholar] [CrossRef]
- Lopez, J.; Tait, S.W. Mitochondrial apoptosis: Killing cancer using the enemy within. Br. J. Cancer 2015, 112, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Kalkan, H. The Program Cell Death (Apoptosis) and the Therapy of Cancer. In Regulation and Dysfunction of Apoptosis; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Yu, L.; Zhu, G.; Zhang, Z.; Yu, Y.; Zeng, L.; Xu, Z.; Weng, J.; Xia, J.; Li, J.; Pathak, J.L. Apoptotic bodies: Bioactive treasure left behind by the dying cells with robust diagnostic and therapeutic application potentials. J. Nanobiotechnol. 2023, 21, 218. [Google Scholar] [CrossRef]
- Yanumula, A.; Cusick, J.K. Biochemistry, Extrinsic Pathway of Apoptosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Al-Anbaky, Q.; Al-Karakooly, Z.; Kilaparty, S.P.; Agrawal, M.; Albkuri, Y.M.; RanguMagar, A.B.; Ghosh, A.; Ali, N. Cytotoxicity of manganese (III) complex in human breast adenocarcinoma cell line is mediated by the generation of reactive oxygen species followed by mitochondrial damage. Int. J. Toxicol. 2016, 35, 672–682. [Google Scholar] [CrossRef]
- Shears, S.B. Inositol pyrophosphates: Why so many phosphates? Adv. Biol. Regul. 2015, 57, 203–216. [Google Scholar] [CrossRef]
- Green, D.R.; Kroemer, G. The pathophysiology of mitochondrial cell death. Science 2004, 305, 626–629. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H. Extrinsic and Intrinsic Apoptosis Signal Pathway Review [Internet]. In Apoptosis and Medicine; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Wei, S.; Nguyen, T.H.; Jo, Y.; Zhang, Y.; Park, W.; Gariani, K.; Oh, C.M.; Kim, H.H.; Ha, K.T.; et al. Mitochondria-associated programmed cell death as a therapeutic target for age-related disease. Exp. Mol. Med. 2023, 55, 1595–1619. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Cunha, G.M.; McKinlay, C.J.; Vargas, J.R.; Jessen, H.J.; Waymouth, R.M.; Wender, P.A. Delivery of inorganic polyphosphate into cells using amphipathic oligocarbonate transporters. ACS Cent. Sci. 2018, 4, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Scheideler, M.; Vidakovic, I.; Prassl, R. Lipid nanocarriers for microRNA delivery. Chem. Phys. Lipids 2020, 226, 104837. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, A.; Grabowska, A.; Stolnik, S. Pathways of cellular internalisation of liposomes delivered siRNA and effects on siRNA engagement with target mRNA and silencing in cancer cells. Sci. Rep. 2018, 8, 3748. [Google Scholar] [CrossRef]
- Li, X.; Gu, C.; Hostachy, S.; Sahu, S.; Wittwer, C.; Jessen, H.J.; Fiedler, D.; Wang, H.; Shears, S.B. Control of XPR1-dependent cellular phosphate efflux by InsP8 is an exemplar for functionally-exclusive inositol pyrophosphate signaling. Proc. Natl. Acad. Sci. USA 2020, 117, 3568–3574. [Google Scholar] [CrossRef] [PubMed]
- Austin, S.; Mayer, A. Phosphate homeostasis—A vital metabolic equilibrium maintained through the INPHORS signaling pathway. Front. Microbiol. 2020, 11, 1367. [Google Scholar] [CrossRef]
- Li, X.; Kirkpatrick, R.B.; Wang, X.; Tucker, C.J.; Shukla, A.; Jessen, H.J.; Wang, H.; Shears, S.B.; Gu, C. Homeostatic coordination of cellular phosphate uptake and efflux requires an organelle-based receptor for the inositol pyrophosphate IP8. Cell Rep. 2024, 25, 6. [Google Scholar] [CrossRef]
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesàro, A. “The good, the bad and the ugly” of chitosans. Mar. Drugs 2016, 14, 99. [Google Scholar] [CrossRef]
- Manna, S.; Seth, A.; Gupta, P.; Nandi, G.; Dutta, R.; Jana, S.; Jana, S. Chitosan derivatives as carriers for drug delivery and biomedical applications. ACS Biomater. Sci. Eng. 2023, 9, 2181–2202. [Google Scholar] [CrossRef]
- Lee, H.; Jeong, C.; Ghafoor, K.; Cho, S.; Park, J. Oral delivery of insulin using chitosan capsules cross-linked with phytic acid. Bio-Med. Mater. Eng. 2011, 21, 25–36. [Google Scholar] [CrossRef]
- Sachdeva, B.; Sachdeva, P.; Negi, A.; Ghosh, S.; Han, S.; Dewanjee, S.; Jha, S.K.; Bhaska, R.; Sinha, J.K.; Paiva-Santos, A.C.; et al. Chitosan nanoparticles-based cancer drug delivery: Application and challenges. Mar. Drugs 2023, 21, 211. [Google Scholar] [CrossRef] [PubMed]
- Tığlı Aydın, R.S.; Pulat, M. 5-Fluorouracil encapsulated chitosan nanoparticles for pH-stimulated drug delivery: Evaluation of controlled release kinetics. J. Nanomater. 2012, 2012, 313961. [Google Scholar] [CrossRef]
- Sangnim, T.; Dheer, D.; Jangra, N.; Huanbutta, K.; Puri, V.; Sharma, A. Chitosan in oral drug delivery formulations: A review. Pharmaceutics 2023, 15, 2361. [Google Scholar] [CrossRef] [PubMed]
- Motiei, M.; Kashanian, S.; Taherpour, A. Hydrophobic amino acids grafted onto chitosan: A novel amphiphilic chitosan nanocarrier for hydrophobic drugs. Drug Dev. Ind. Pharm. 2017, 43, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Jadaun, A.; Arora, V.; Sinha, R.K.; Biyani, N.; Jain, V.K. In vitro toxicity assessment of chitosan oligosaccharide coated iron oxide nanoparticles. Toxicol. Rep. 2015, 2, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Munshi, P.; Padmanabhan, S.; Sullivan, S.Z.; Mustafa, T.A.; Brezden, A.M.; Ghosh, A. An Economically Viable Process for the Synthesis of a Chiral Oxazolidinone (S)-4-Benzyl-2-oxazolidinone) from Amino Acid. Part. Sci. Technol. 2011, 29, 466–474. [Google Scholar] [CrossRef]
- Majeed, W.; Bourdo, S.; Petibone, D.M.; Saini, V.; Vang, K.B.; Nima, Z.A.; Alghazali, K.M.; Darrigues, E.; Ghosh, A.; Watanabe, F.; et al. The role of surface chemistry in the cytotoxicity profile of graphene. J. Appl. Toxicol. 2017, 37, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Losito, O.; Szijgyarto, Z.; Resnick, A.C.; Saiardi, A. Inositol pyrophosphates and their unique metabolic complexity: Analysis by gel electrophoresis. PLoS ONE 2009, 4, e5580. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.S.; Bulley, S.J.; Pisani, F.; Irvine, R.F.; Saiardi, A. A novel method for the purification of inositol phosphates from biological samples reveals that no phytate is present in human plasma or urine. Open Biol. 2015, 5, 150014. [Google Scholar] [CrossRef]
- Alkarakooly, Z.; Al-Anbaky, Q.A.; Kannan, K.; Ali, N. Metabolic reprogramming by Dichloroacetic acid potentiates photodynamic therapy of human breast adenocarcinoma MCF-7 cells. PLoS ONE 2018, 13, e0206182. [Google Scholar] [CrossRef]
- Kilaparty, S.P.; Agarwal, R.; Singh, P.; Kannan, K.; Ali, N. Endoplasmic reticulum stress-induced apoptosis accompanies enhanced expression of multiple inositol polyphosphate phosphatase 1 (Minpp1): A possible role for Minpp1 in cellular stress response. Cell Stress Chaperones 2016, 21, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Hassen, S.; Ali, N. Changes in cellular levels of inositol polyphosphates during apoptosis. Mol. Cell. Biochem. 2010, 345, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Shamsuddin, A.M.; Vucenik, I.; Cole, K.E. IP6: A novel anti-cancer agent. Life Sci. 1997, 61, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Song, Y.; Cui, L.; Wen, Z.; Lu, X. Inositol hexaphosphate suppresses growth and induces apoptosis in HT-29 colorectal cancer cells in culture: PI3K/Akt pathway as a potential target. Int. J. Clin. Exp. Pathol. 2015, 1402–1410. [Google Scholar]
- Wundenberg, T.; Mayr, G.W. Synthesis and biological actions of diphosphoinositol phosphates (inositol pyrophosphates), regulators of cell homeostasis. Biol. Chem. 2012, 393, 979–998. [Google Scholar] [CrossRef] [PubMed]
- Tu-Sekine, B.; Kim, S.F. The Inositol Phosphate System—A coordinator of metabolic adaptability. Int. J. Mol. Sci. 2022, 23, 6747. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Shi, L.; Zhu, L.; Chen, Y.; Zhu, H.; Cheng, W.; Chen, A.F.; Fu, C. Functions, mechanisms, and therapeutic applications of the inositol pyrophosphates 5PP-InsP5 and InsP8 in mammalian cells. J. Cardiovasc. Trans. Res. 2024, 17, 197–215. [Google Scholar] [CrossRef] [PubMed]
- Letcher, A.J.; Schell, M.J.; Irvine, R.F. Do mammals make all their own inositol hexakisphosphate? Biochem. J. 2008, 416, 263–270. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Dünnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef]
- Wei, S.; Ching, Y.C.; Chuah, C.H. Synthesis of chitosan aerogels as promising carriers for drug delivery: A review. Carbohydr. Polym. 2020, 231, 115744. [Google Scholar] [CrossRef]
- Kadhim, I.H. Encapsulation of Inositol Hexakisphosphate to Facilitate Cellular Entry and Programmed Cell Death in Breast Cancer Cells. Masters’ Thesis, University of Arkansas, Little Rock, AR, USA, 2017. [Google Scholar]
- Wrońska, N.; Katir, N.; Nowak-Lange, M.; El Kadib, A.; Lisowska, K. Biodegradable Chitosan-Based Films as an Alternative to Plastic Packaging. Foods 2023, 12, 3519. [Google Scholar] [CrossRef] [PubMed]
- Martău, G.A.; Mihai, M.; Vodnar, D.C. The Use of Chitosan, Alginate, and Pectin in the Biomedical and Food Sector-Biocompatibility, Bioadhesiveness, and Biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Weiskirchen, R. What Does the “AKT” Stand for in the Name “AKT Kinase”? Some Historical Comments. Front. Oncol. 2020, 10, 1329. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadhim, I.H.; Oluremi, A.S.; Chhetri, B.P.; Ghosh, A.; Ali, N. Encapsulation of Inositol Hexakisphosphate with Chitosan via Gelation to Facilitate Cellular Delivery and Programmed Cell Death in Human Breast Cancer Cells. Bioengineering 2024, 11, 931. https://doi.org/10.3390/bioengineering11090931
Kadhim IH, Oluremi AS, Chhetri BP, Ghosh A, Ali N. Encapsulation of Inositol Hexakisphosphate with Chitosan via Gelation to Facilitate Cellular Delivery and Programmed Cell Death in Human Breast Cancer Cells. Bioengineering. 2024; 11(9):931. https://doi.org/10.3390/bioengineering11090931
Chicago/Turabian StyleKadhim, Ilham H., Adeolu S. Oluremi, Bijay P. Chhetri, Anindya Ghosh, and Nawab Ali. 2024. "Encapsulation of Inositol Hexakisphosphate with Chitosan via Gelation to Facilitate Cellular Delivery and Programmed Cell Death in Human Breast Cancer Cells" Bioengineering 11, no. 9: 931. https://doi.org/10.3390/bioengineering11090931
APA StyleKadhim, I. H., Oluremi, A. S., Chhetri, B. P., Ghosh, A., & Ali, N. (2024). Encapsulation of Inositol Hexakisphosphate with Chitosan via Gelation to Facilitate Cellular Delivery and Programmed Cell Death in Human Breast Cancer Cells. Bioengineering, 11(9), 931. https://doi.org/10.3390/bioengineering11090931










