A Finite Element Method Study of Stress Distribution in Dental Hard Tissues: Impact of Access Cavity Design and Restoration Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
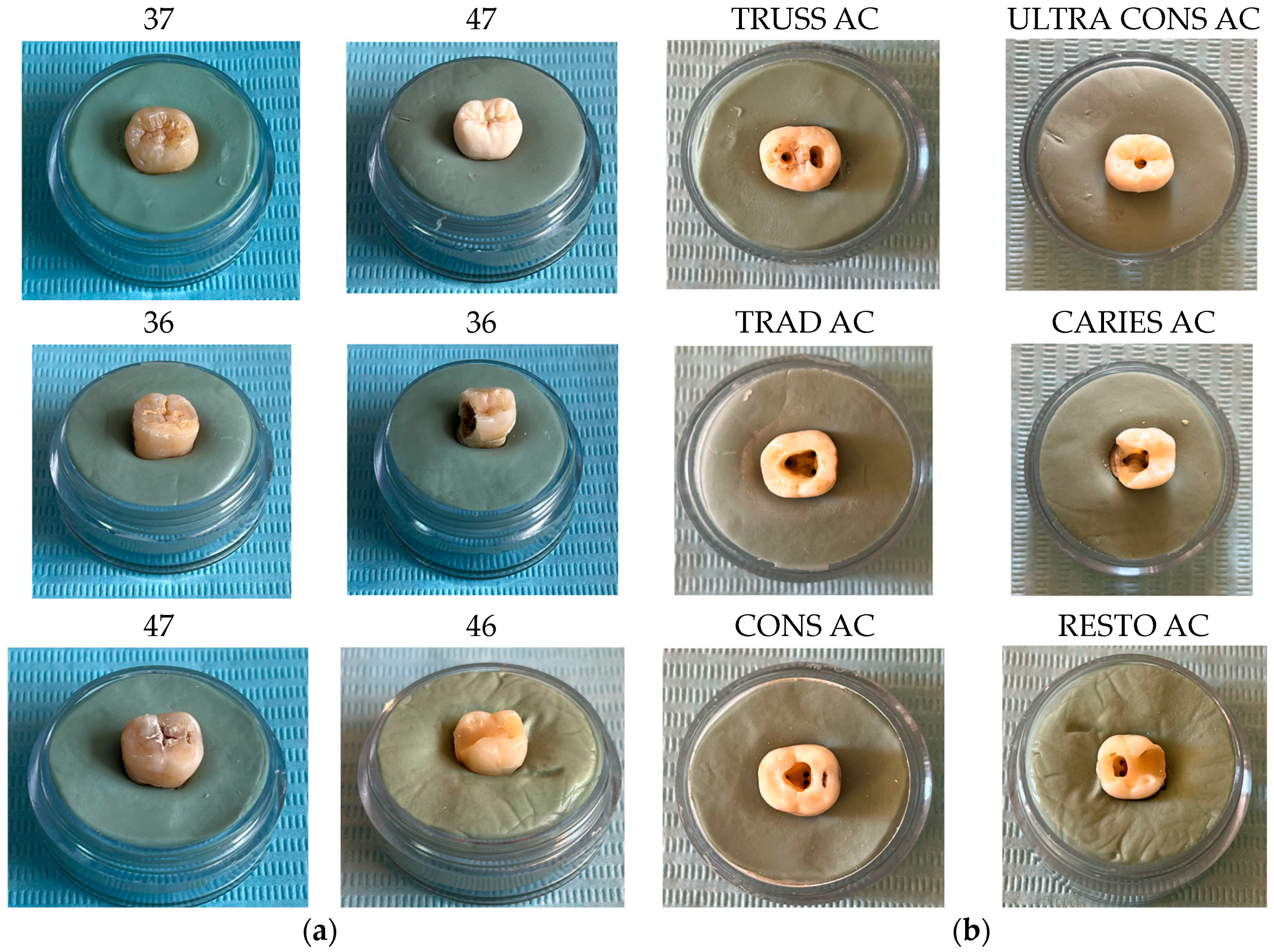
2.1.1. Selection and Preparation of Teeth
- -
- Molar 37 (without carious lesions)—TrussAC;
- -
- Molar 47 (without carious lesions)—UltraAC;
- -
- Molar 36 (tooth with wear on the occlusal surface)—TradAC;
- -
- Molar 36 (occluso-distal carious process)—CariesAC;
- -
- Molar 47 (occlusal carious process)—ConsAC;
- -
- Molar 46 (coronary obturation)—RestoAC.
2.1.2. Hardware and Appliances
- -
- several desktop computers, with 8 GB RAM memory, INTEL Core I3 processor with a frequency of 3.7 GHz;
- -
- a laptop type computer, with 16 GB RAM memory and INTEL Core I5 processor with a frequency of 2.6 GHz.
- Scale weight: approx. 1.25 kg
- Material: Weighing plate/SUS304 stainless steel
- Accuracy: 0.01 g
- Maximum admissible mass: 300 g
- Diameter of weighing plate: φ110 mm
- Power supply: 100 V AC adapter (included) or AA alkaline dry batteries × 6 (optional) Size: 188 × 216 × 58 mm
2.1.3. Software
2.2. Method
- −
- Methods and techniques of reverse engineering;
- −
- Methods and principles of CAD and direct engineering;
- −
- Techniques and methods specific to the FEM [26];
- −
- Methods and principles of the Mechanics of Continuous Media;
- −
- Techniques and methods specific to the Strength of Materials;
- −
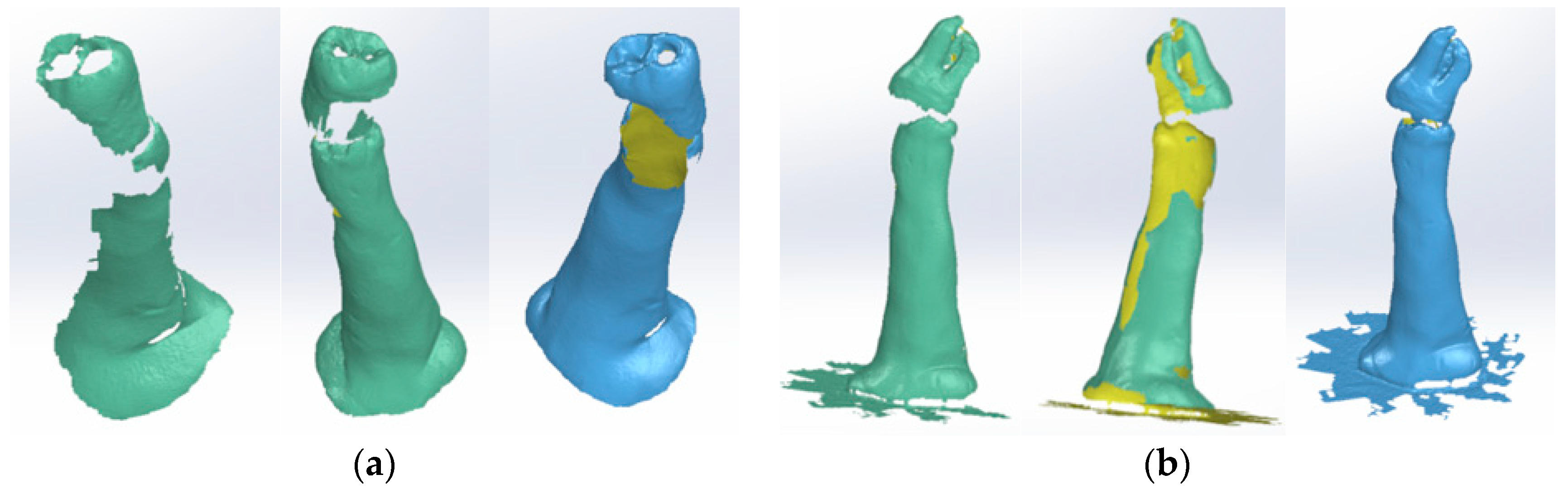
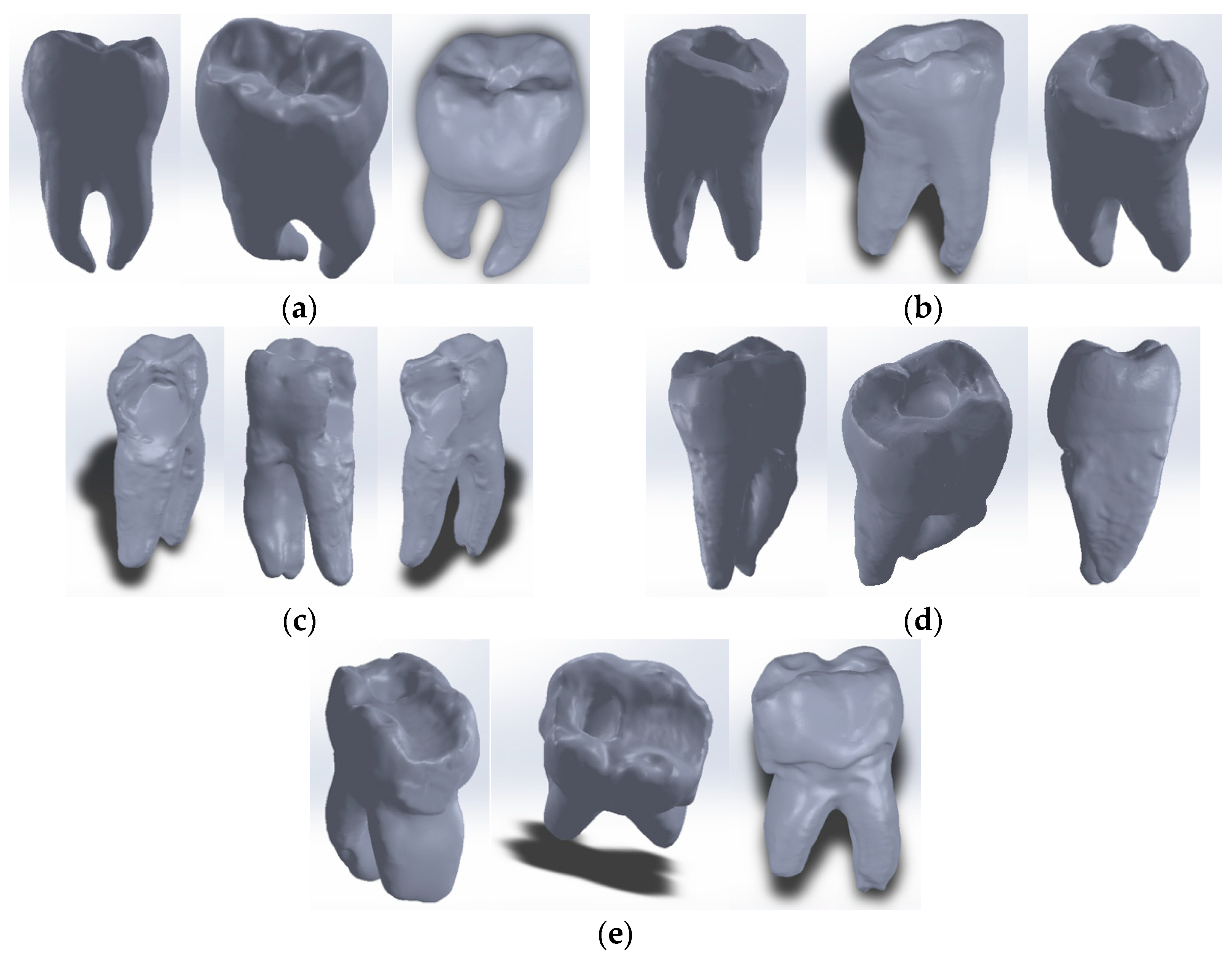
2.3. External Virtual Models of the Selected Molars
The Virtual Model of the Molar with TrussAC
2.4. The External Virtual Models of the Restored Molars
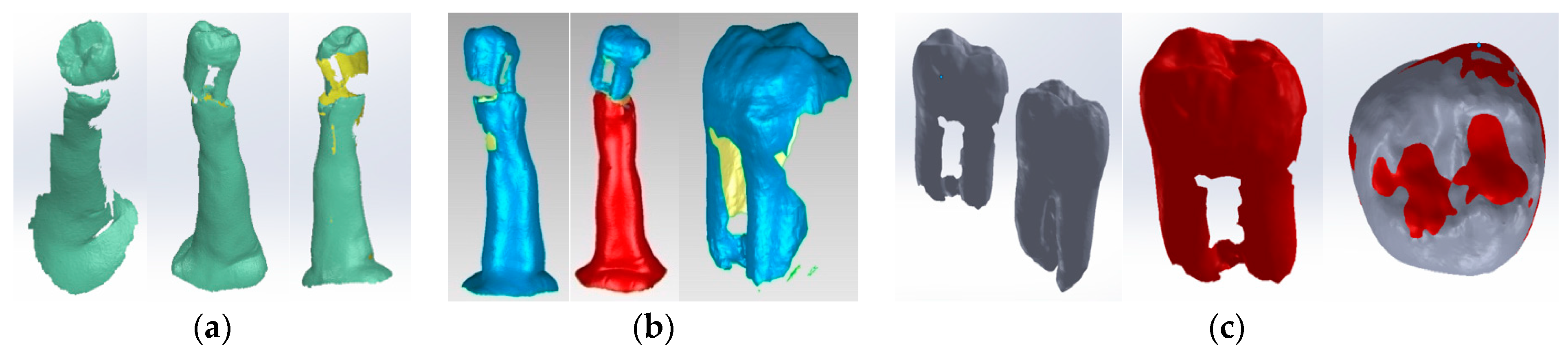
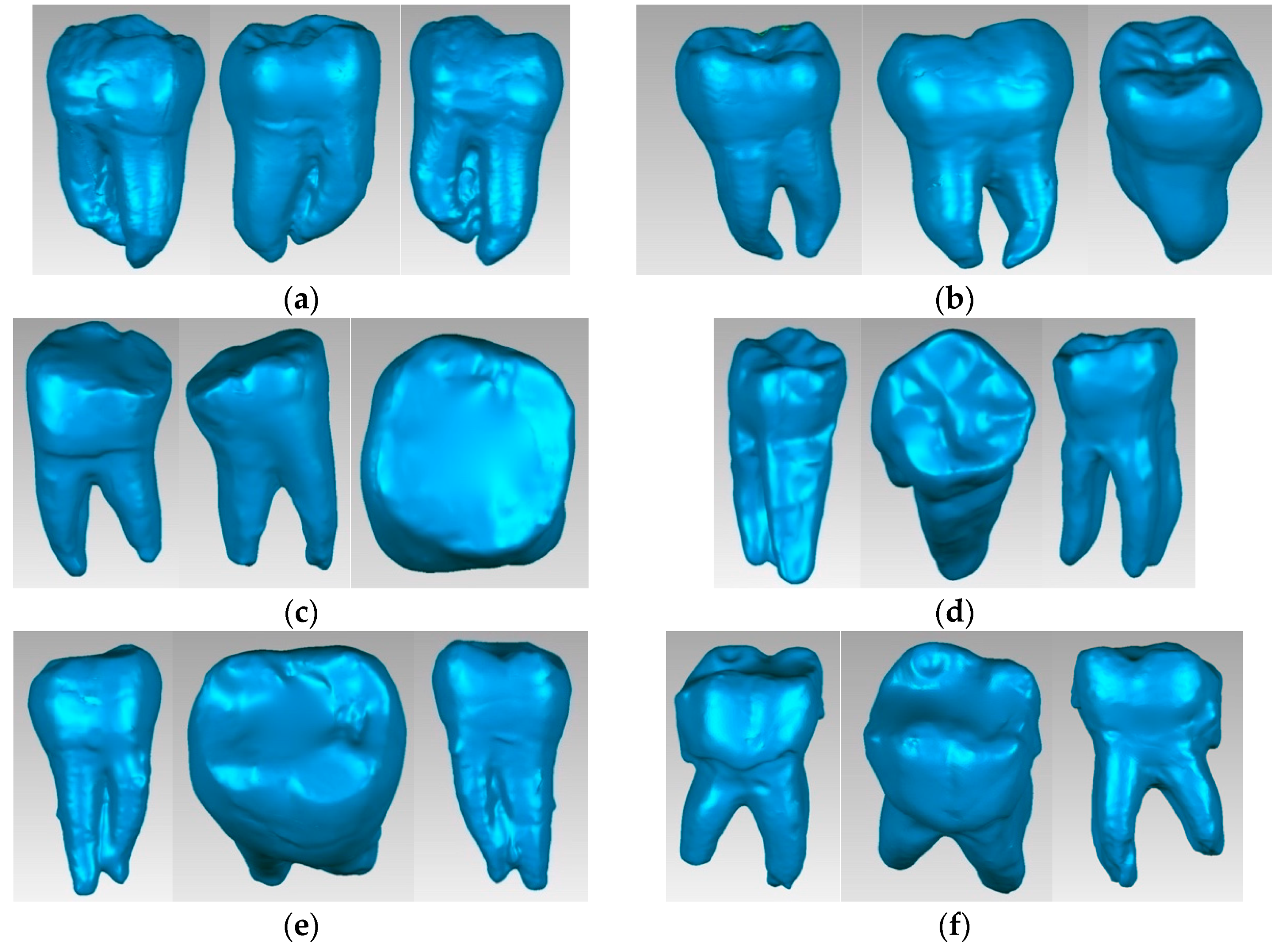
2.5. Virtual Models of Molars with Internal Anatomy
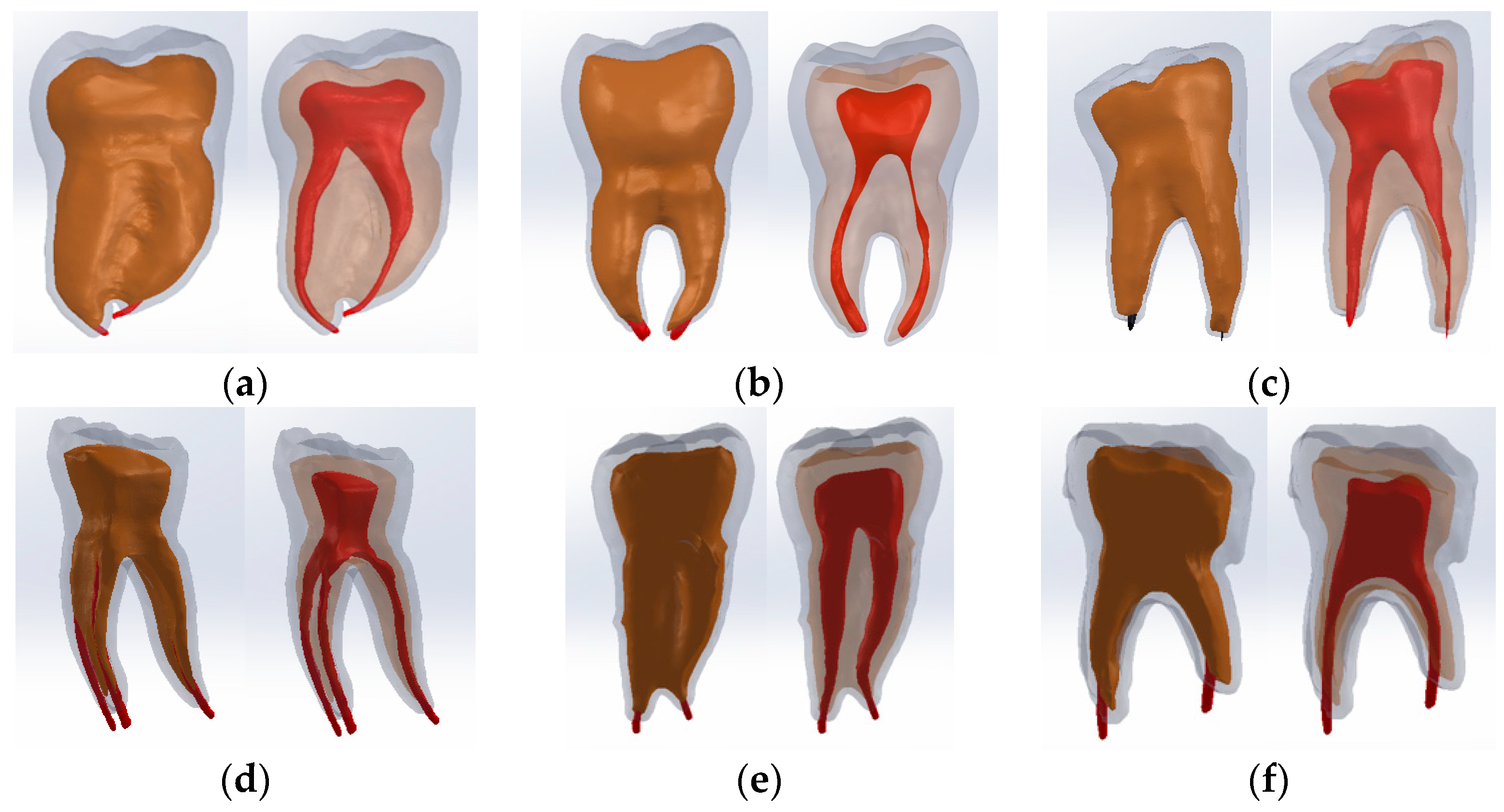
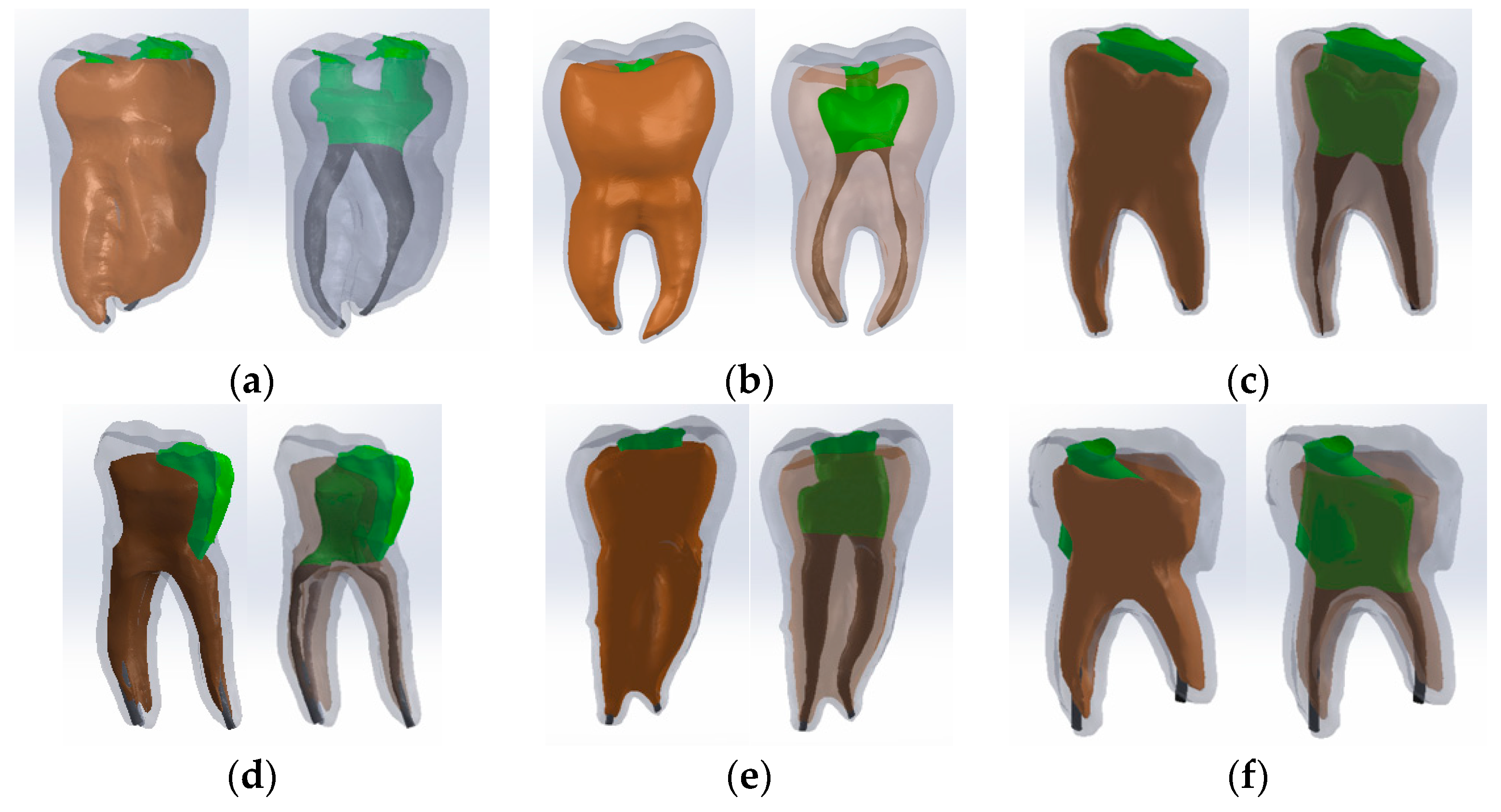
2.6. Virtual Models of Molars with Internal Anatomy Simulated, Root Canal Filling and Coronal Restoration
2.7. Simulation of the Mechanical Behavior of the Analyzed Molars
2.7.1. Establishing the Physico-Mechanical Properties of Materials Used in Finite Element Simulation
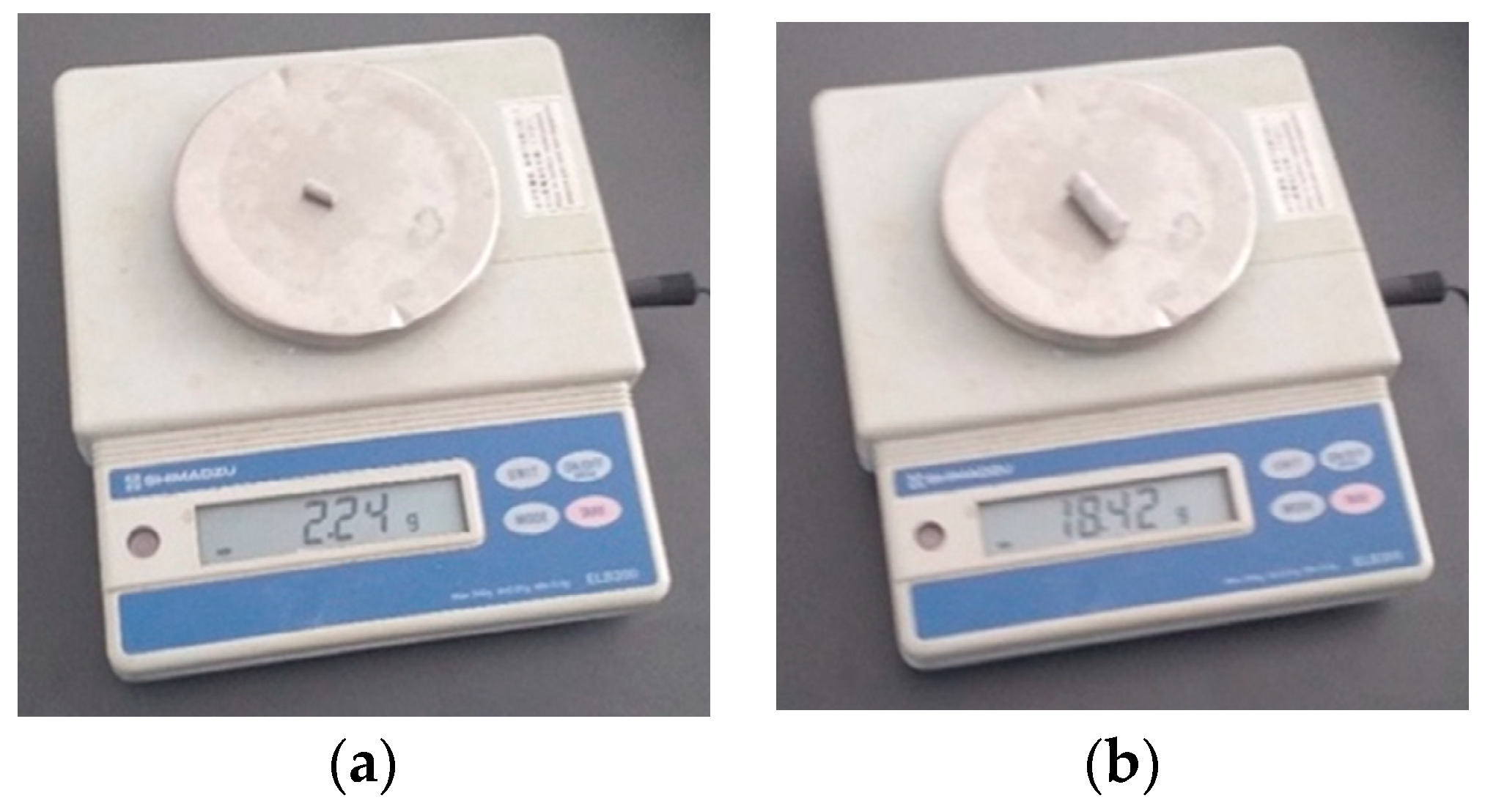
2.7.2. Determination of Densities for the Two Restorative Materials, Bulk Fill Composite Resin and Amalgam
- The density of the composite material is c = 1052.257 kg/;
- The density of the amalgam is a = 11,333.9 kg/.
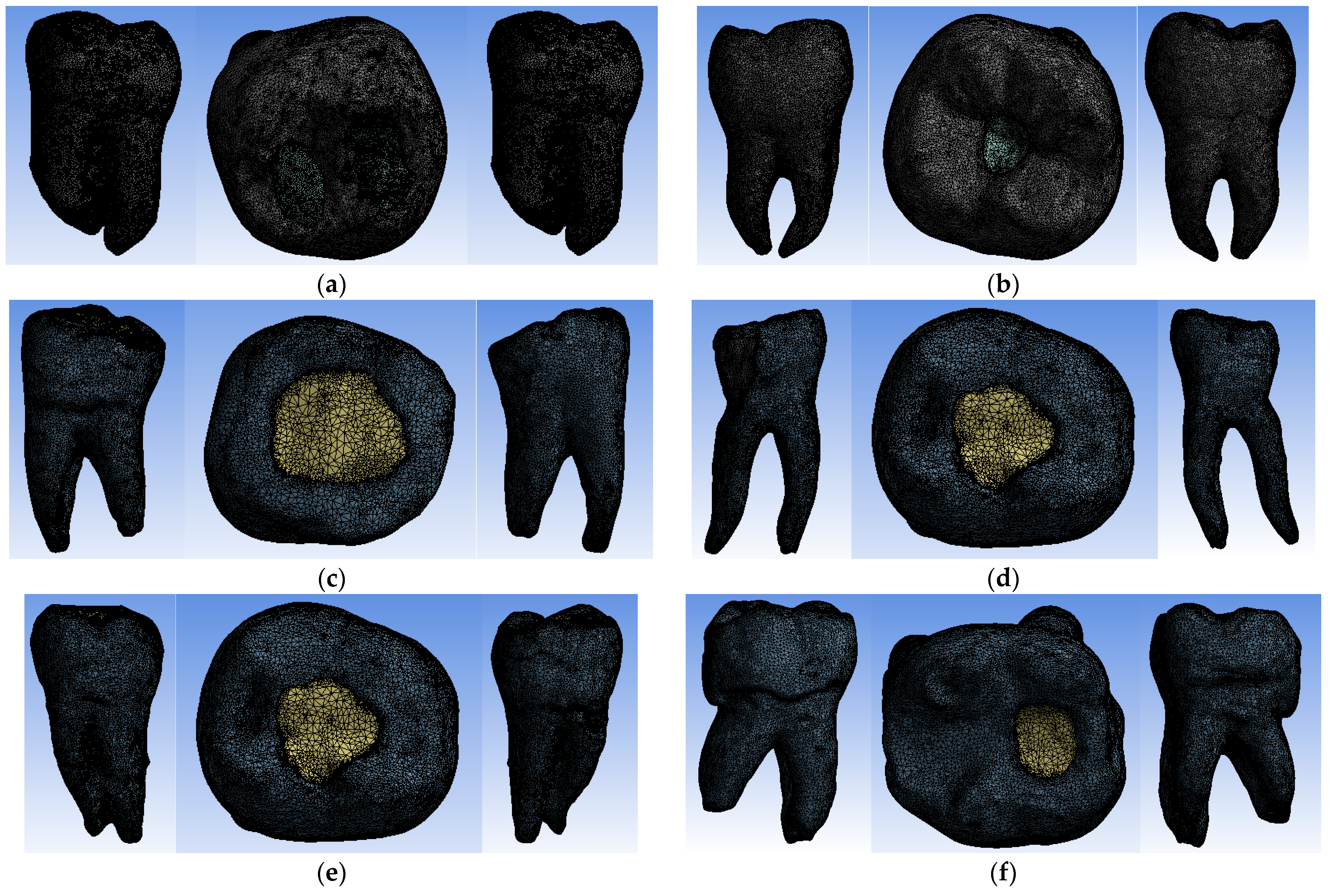
2.7.3. Dividing Virtual Models into Finite Elements
- −
- 1,637,959 nodes and 1,036,362 finite elements for the virtual model of the molar with Truss AC after the coronal obturation;
- −
- 1,076,862 nodes and 670,028 finite elements for the virtual model of the molar with UltraAC;
- −
- 737,746 nodes and 455,890 finite elements for the virtual molar model with TradAC;
- −
- 670,215 nodes and 421,462 finite elements for the virtual molar model with CariesAC;
- −
- 1,231,306 nodes and 783,696 finite elements for the virtual molar model with ConsAC;
- −
- 948,445 nodes and 595,301 elements finished for the virtual model of the molar with RestoAC.
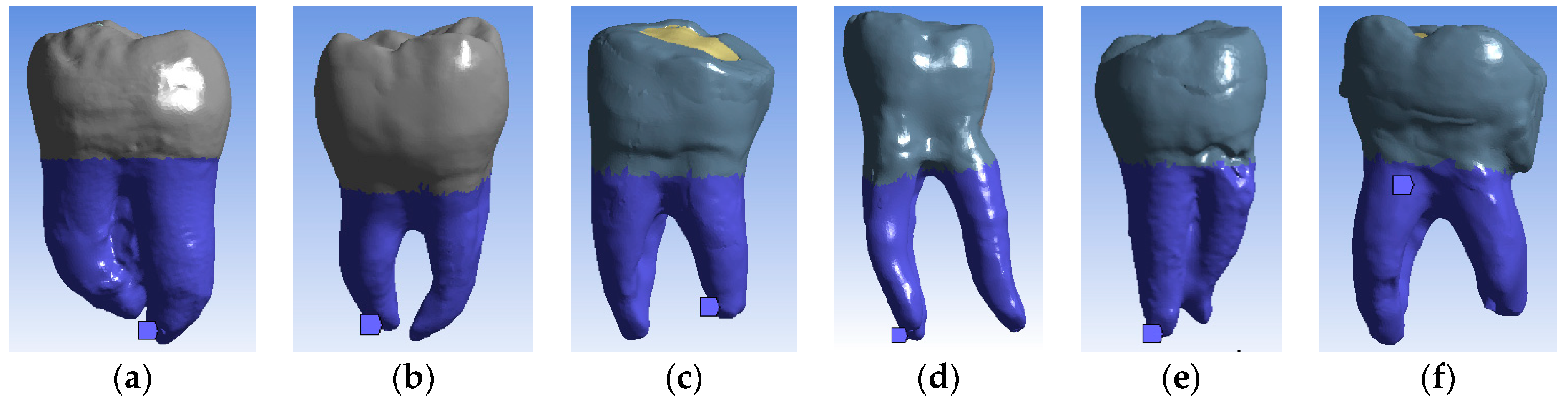
2.7.4. Imposing Mechanical Constraints in Finite Element Simulations
2.7.5. Imposition of Bruxism-Specific Mechanical Force Loading System to Simulate Mechanical Constraints in Finite Element Simulations
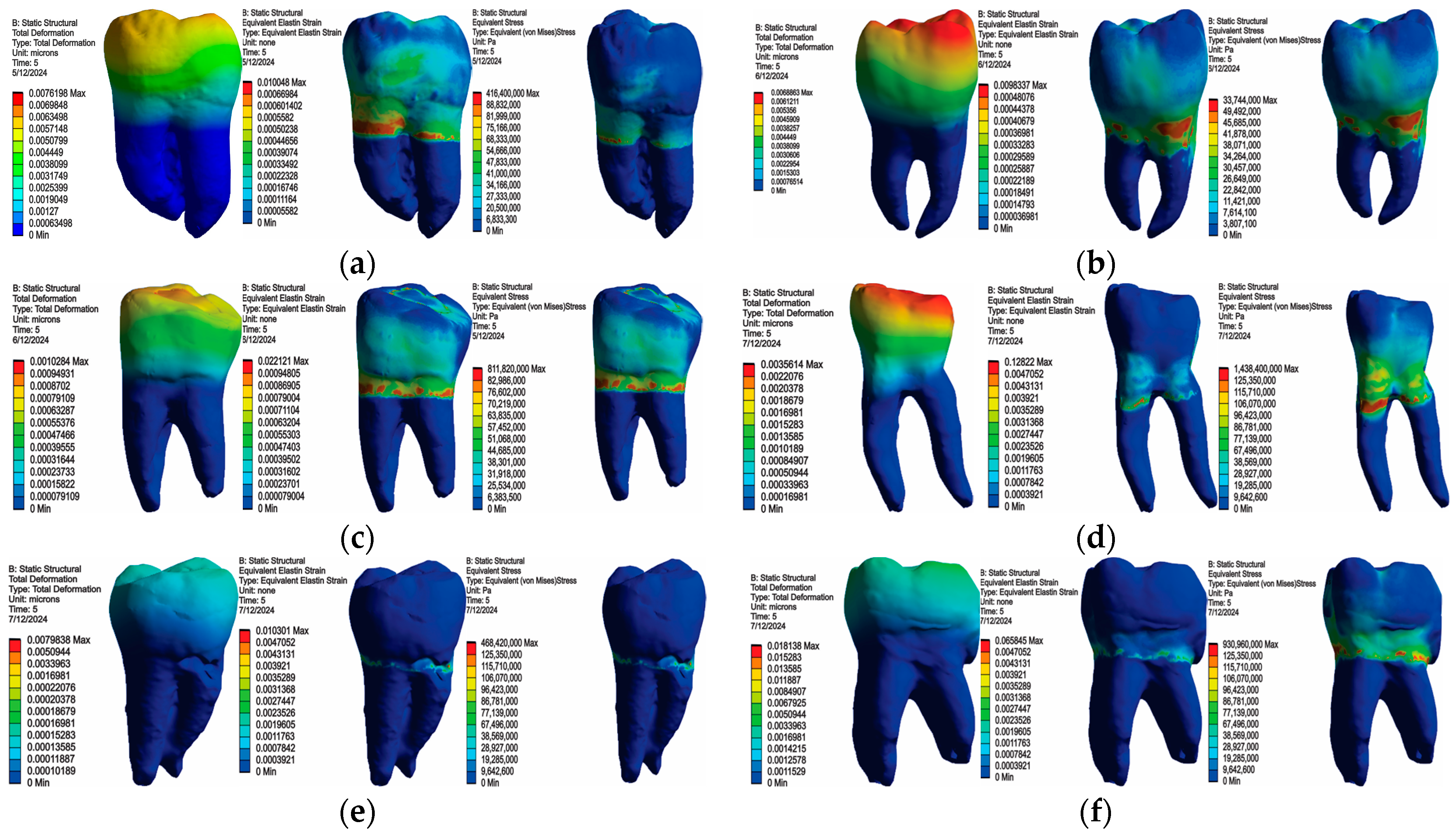
3. Results
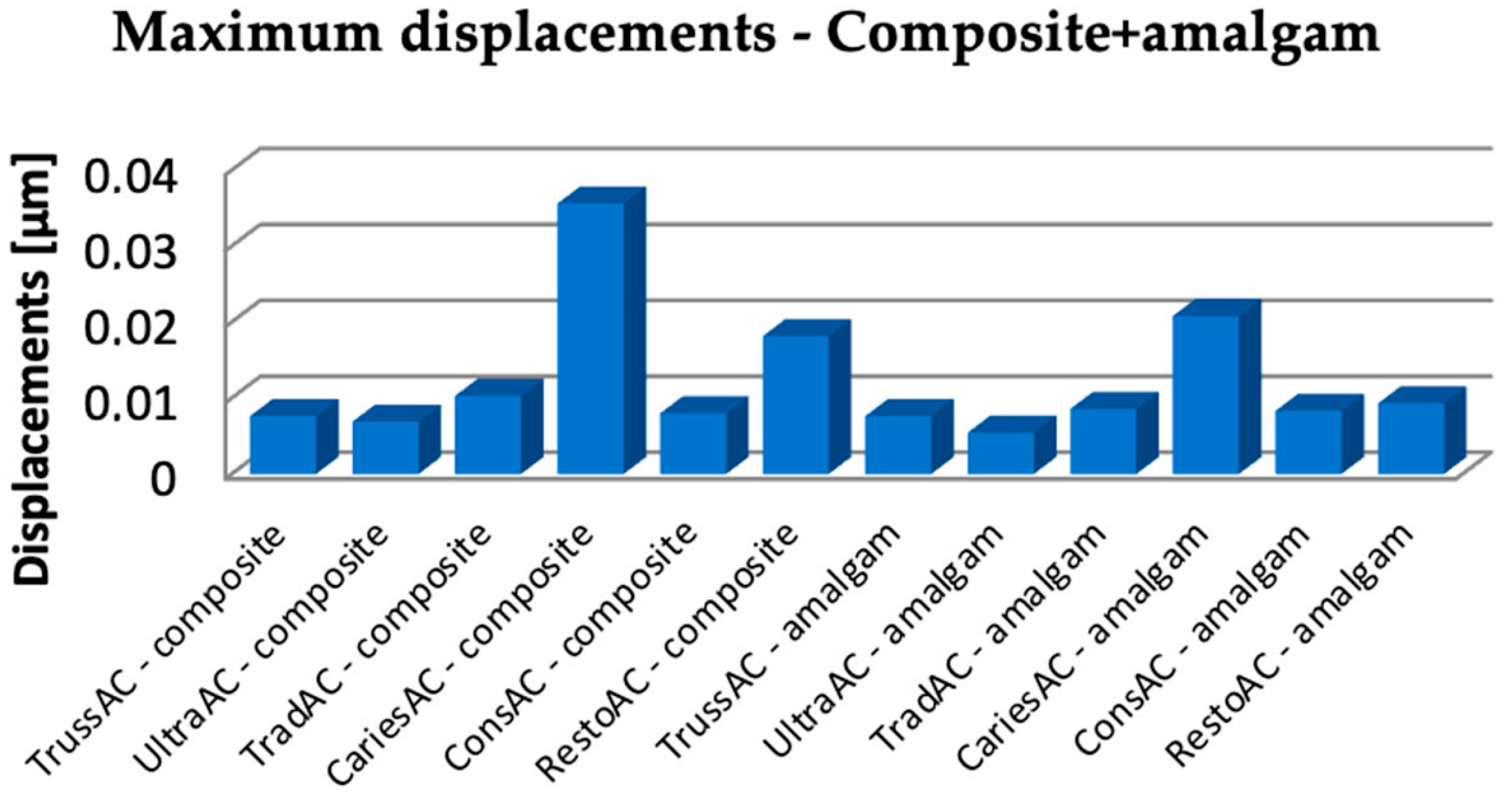
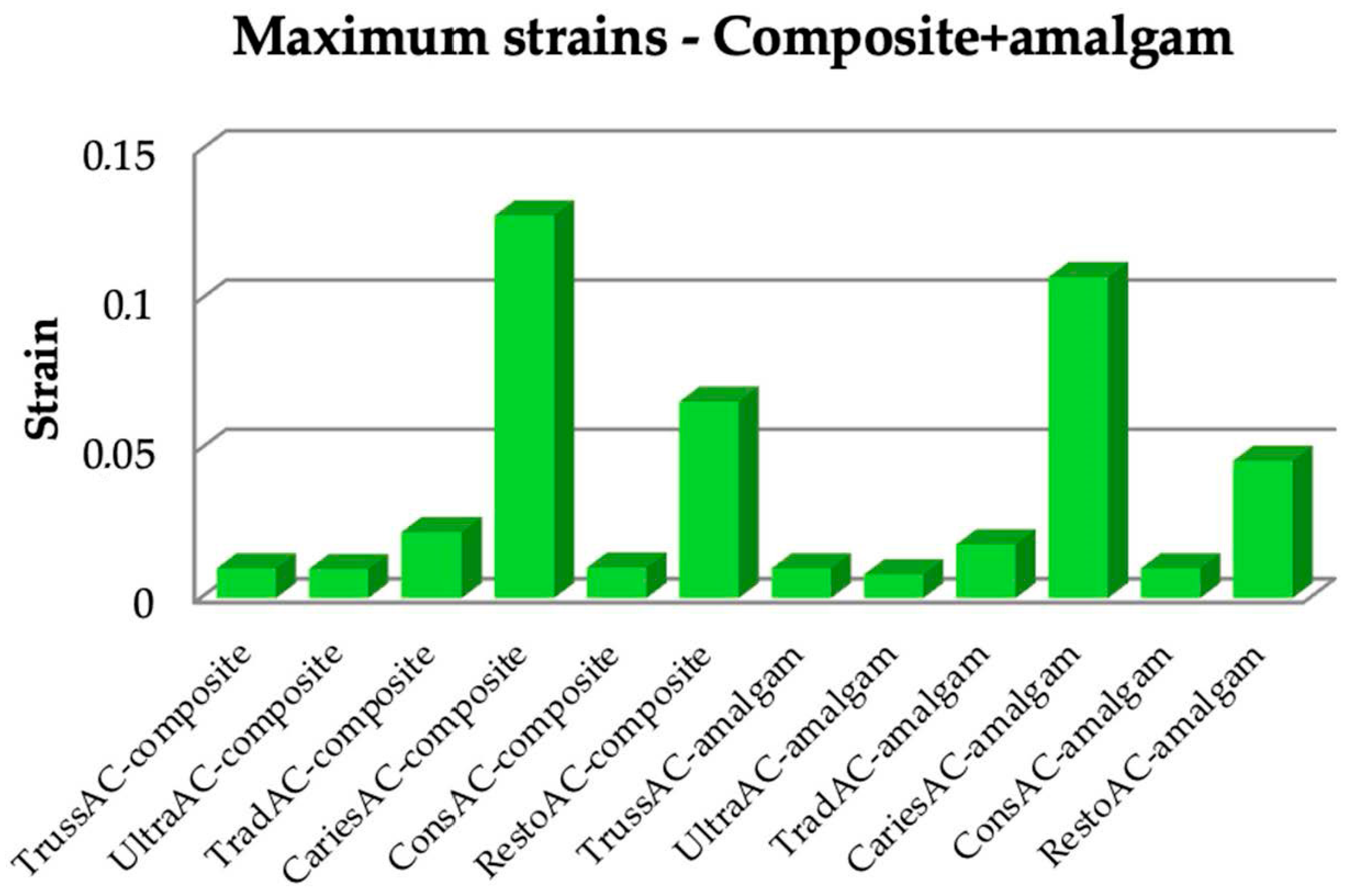
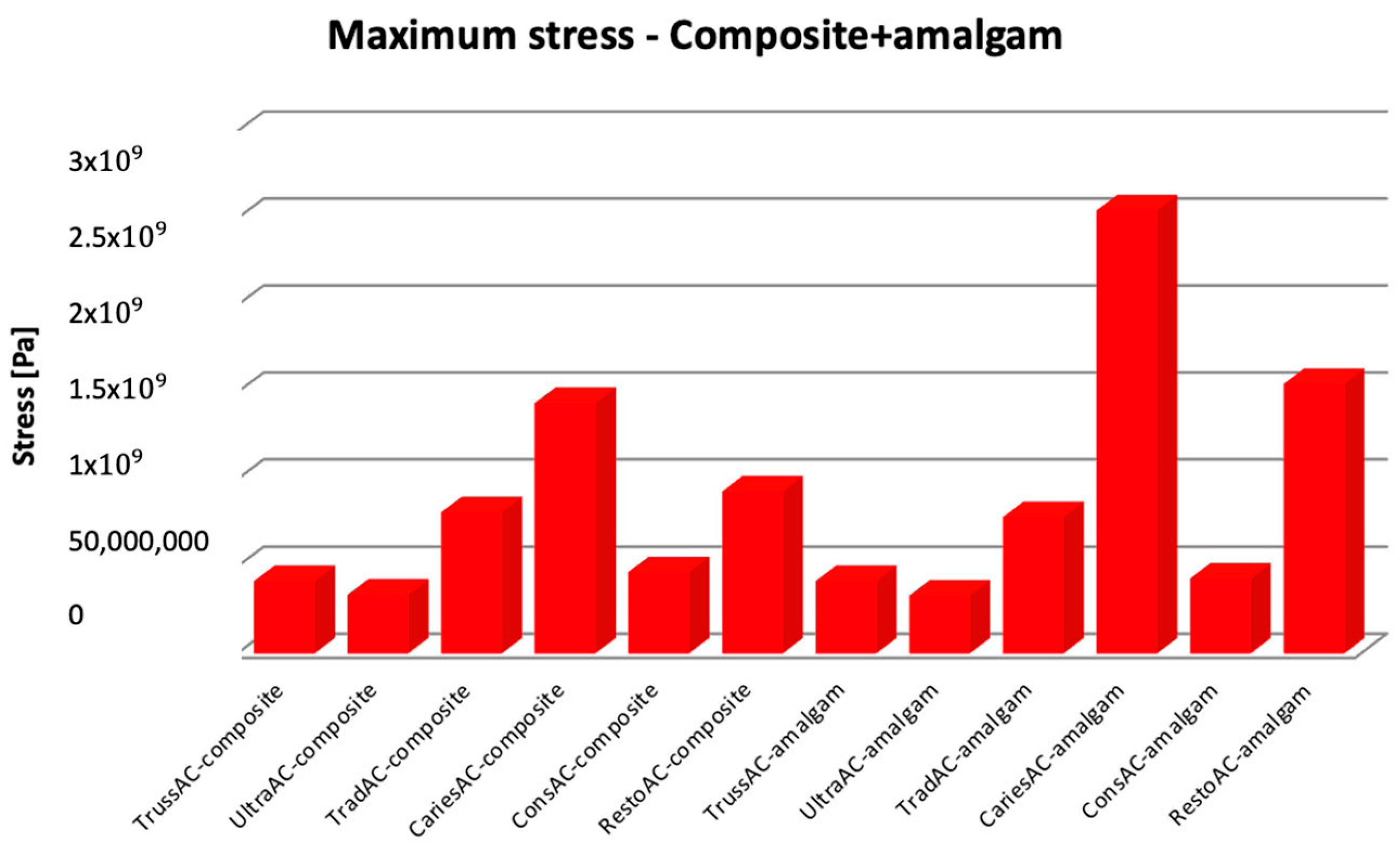
3.1. Numerical Results Obtained for Molars Restored with Bulk Fill Resin Composite and Bruxism-Specific Loading
3.2. Numerical Results Obtained for Amalgam and Bruxism-Specific Loading (Figure 18)
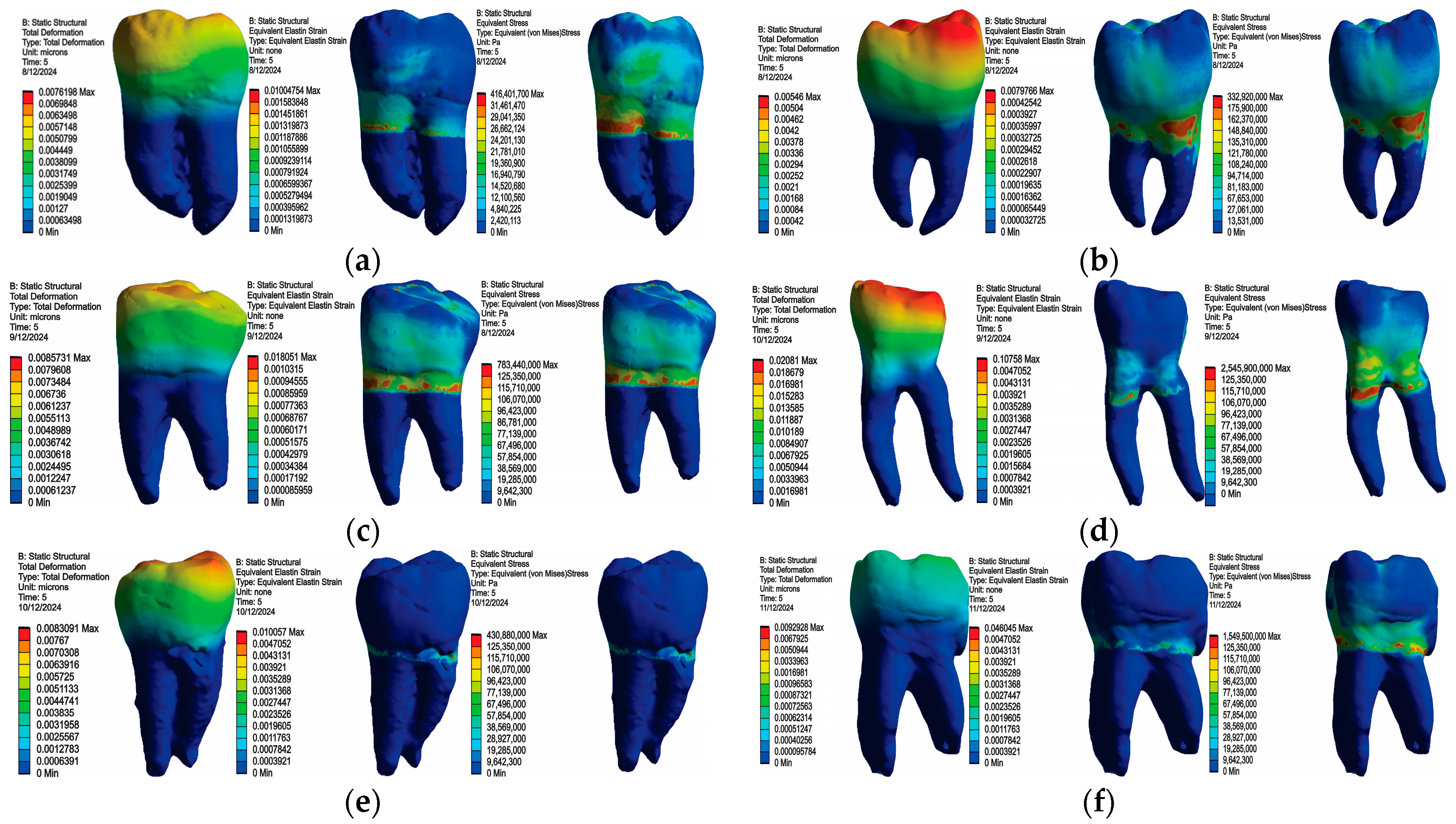
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clark, D.; Khademi, J. Modern molar endodontic access and directed dentin conservation. Dent. Clin. N. Am. 2010, 54, 249–273. [Google Scholar] [CrossRef] [PubMed]
- Abdulrazaq, L.; Ali, A.; Foschi, F. Minimally invasive access cavities in endodontics. J. Baghdad Coll. Dent. 2023, 35, 65–67. [Google Scholar] [CrossRef]
- Silva, E.J.N.L.; De-Deus, G.; Souza, E.M.; Belladonna, F.G.; Cavalcante, D.M.; Simões-Carvalho, M.; Versiani, M.A. Present status and future directions—Minimal endodontic access cavities. Int. Endod. J. 2022, 55 (Suppl. S3), 531–587. [Google Scholar] [CrossRef]
- Nunes, A.; Baroudi, K.; Barroso, L.; Silva, L.; Rosy, N.; Teixeira, A.; Lourenço, T.; Huguenin, J. Influence of different access cavity sizes on the direction of stress distribution in endodontically treated uniradicular maxillary premolar: A finite element analysis. Cadenos UniFOA 2023, 18, 1–10. [Google Scholar] [CrossRef]
- Maske, A.; Weschenfelder, V.M.; Soares Grecca Vilella, F.; Burnett Junior, L.H. Influence of access cavity design on fracture strength of endodontically treated lower molars. Aust. Endod. J. 2021, 47, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Wu, Y.; Smales, R.J. Identifying and reducing risks for potential fractures in endodontically treated teeth. J. Endod. 2010, 36, 60917. [Google Scholar] [CrossRef]
- Ree, M.; Schwartz, R.S. The endo-restorative interface: Current concepts. Dent. Clin. N. Am. 2010, 54, 345–374. [Google Scholar] [CrossRef]
- Silva, A.; Belladonna, F.; Rover, G.; Lopes, R.; Moreira, E.; De-Deus, G. Does ultraconservative access affect the efficacy of root canal treatment and the fracture resistance of two-rooted maxillary premolars? Int. Endod. J. 2020, 53, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Mandil, O.A.; Ghoulah, K.T.; Hazzam, B.M.; Alhijji, H.S.; Al Abbas, A.H.; Rehan, A.K.; Doumani, M.; Mandil, A.A. Modern versus Traditional Endodontic Access Cavity Designs. J. Pharm. Bioallied Sci. 2022, 14 (Suppl. S1), 24–27. [Google Scholar] [CrossRef]
- Shabbir, J.; Zehra, T.; Najmi, N.; Hasan, A.; Naz, M.; Piasecki, L.; Azim, A.A. Access Cavity Preparations: Classification and Literature Review of Traditional and Minimally Invasive Endodontic Access Cavity Designs. J. Endod. 2021, 47, 1229–1244. [Google Scholar] [CrossRef]
- Assif, D.; Nissan, J.; Gafni, Y.; Gordon, M. Assessment of the resistance to fracture of endodontically treated molars restored with amalgam. J. Prosthet. Dent. 2003, 89, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Mannocci, F.; Cowie, J. Restoration of endodontically treated teeth. Br. Dent. J. 2014, 216, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, R.G.; Lloyd, C.H. Dental amalgam: The history and legacy you perhaps never knew? Br. Dent. J. 2022, 232, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.J.M.C.; Rêgo, H.M.C.; Siddique, I.; Jessani, A. Five-Year Clinical Performance of Complex Class II Resin Composite and Amalgam Restorations—A Retrospective Study. Dent. J. 2023, 11, 88. [Google Scholar] [CrossRef]
- Alreshaid, L.; El-Badrawy, W.; Kulkarni, G.; Santos, M.J.; Prakki, A. Resin Composite Versus Amalgam Restorations Placed in United States Dental Schools. Oper. Dent. 2023, 48, 21–32. [Google Scholar] [CrossRef]
- Alreshaid, L.; El-Badrawy, W.; Lawrence, H.P.; Santos, M.J.; Prakki, A. Composite versus Amalgam Restorations Placed in Canadian Dental Schools. Oper. Dent. 2021, 46, 621–630. [Google Scholar] [CrossRef]
- Bailey, O.; Vernazza, C.R.; Stone, S.; Ternent, L.; Roche, A.G.; Lynch, C. Amalgam Phase-Down Part 2: UK-Based Knowledge Opinions, and Confidence in the Alternatives. JDR Clin. Trans. Res. 2022, 7, 50–60. [Google Scholar] [CrossRef]
- Opdam, N.J.M.; Bronkhorst, E.M.; Roeters, J.M.; Loomans, B.A.C. A retrospective clinical study on longevity of posterior composite and amalgam restorations. Dent. Mater. 2007, 23, 2–8. [Google Scholar] [CrossRef]
- Vidnes-Kopperud, S.; Tveit, A.B.; Gaarden, T.; Sandvik, L.; Espelid, I. Factors influencing dentists’ choice of amalgam and tooth-colored restorative materials for Class II preparations in younger patients. Acta Odontol. Scand. 2009, 67, 74–79. [Google Scholar] [CrossRef]
- Lukarcanin, J.; Sadıkoğlu, İ.S.; Yaşa, B.; Türkün, L.Ş.; Türkün, M. Comparison of Different Restoration Techniques for Endodontically Treated Teeth. Int. J. Biomater. 2022, 11, 6643825. [Google Scholar] [CrossRef]
- Kimble, P.; Stuhr, S.; McDonald, N.; Venugopalan, A.; Campos, M.S.; Cavalcanti, B. Decision Making in the Restoration of Endodontically Treated Teeth: Effect of Biomimetic Dentistry Training. Dent. J. 2023, 11, 159. [Google Scholar] [CrossRef] [PubMed]
- Larson, T.D. The effect of occlusal forces on restorations. Northwest Dent. 2012, 91, 29–35. [Google Scholar]
- Matos, J.D.M.d.; Queiroz, D.A.; Nakano, L.J.N.; Andrade, V.C.; Ribeiro, N.d.C.R.; Borges, A.L.S.; Bottino, M.A.; Lopes, G.d.R.S. Bioengineering Tools Applied to Dentistry: Validation Methods for In Vitro and In Silico Analysis. Dent. J. 2022, 10, 145. [Google Scholar] [CrossRef]
- Stănuşi, A.; Stănuşi, A.Ş.; Gîngu, O.; Mercuţ, V.; Osiac, E. Stereomicroscopic Aspects of Non-Carious Cervical Lesions. Diagnostics 2023, 13, 2590. [Google Scholar] [CrossRef]
- Ruddle, C. Endodontic access preparation an opening for success. Dent. Today 2007, 26, 116–119. [Google Scholar]
- Maksay, S.T.; Bistrian, D.A. Introducere în Metoda Elementelor Finite; Editura CERMI Iasi: Iași, Romania, 2008; ISBN 978-973-667-324-5. [Google Scholar]
- Belli, S.; Eraslan, O.; Eskitascioglu, G. Direct Restoration of Endodontically Treated Teeth: A Brief Summary of Materials and Techniques. Curr. Oral. Health Rep. 2015, 2, 182–189. [Google Scholar] [CrossRef]
- Rodrigues, M.P.; Soares, P.B.F.; Gomes, M.A.B.; Pereira, R.A.; Tantbirojn, D.; Versluis, A.; Soares, C.J. Direct resin composite restoration of endodontically-treated permanent molars in adolescents: Bite force and patient-specific finite element analysis. J. Appl. Oral. Sci. 2020, 28, e20190544. [Google Scholar] [CrossRef]
- Anand, M.; Karthikeyan, K.; Sekar, M. Current trend of restoration of endodontically treated teeth with extensive subgingival caries: A case series. J. Conserv. Dent. 2022, 25, 202–205. [Google Scholar]
- Vatu, M.; Vintilă, D.; Popa, D.L.; Mercuţ, R.; Popescu, S.M.; Vintila, G. Determining Mechanical Causes that Produce Dental Wear Using Finite Element Method. Appl. Mech. Mater. 2020, 896, 15–22. [Google Scholar] [CrossRef]
- Hsu, M.L.; Chang, C.L. Application of finite element analysis in dentistry. Finite Elem. Anal. 2010, 5, 43–66. [Google Scholar]
- Cicciù, M.; Cervino, G.; Bramanti, E.; Lauritano, F.; Lo Gudice, G.; Scappaticci, L.; Rapparini, A.; Guglielmino, E.; Risitano, G. FEM Analysis of Mandibular Prosthetic Overdenture Supported by Dental Implants: Evaluation of Different Retention Methods. Comput. Math. Methods Med. 2015, 2015, 943839. [Google Scholar] [CrossRef] [PubMed]
- Keulemans, F.; Shinya, A.; Lassila, L.V.; Vallittu, P.K.; Kleverlaan, C.J.; Feilzer, A.J.; De Moor, R.J. Three-dimensional finite element analysis of anterior two-unit cantilever resin-bonded fixed dental prostheses. Sci. World J. 2015, 2015, 864389. [Google Scholar] [CrossRef] [PubMed]
- Soliheen, M.A.M.; Kurniawan, D.; Nor, F.M. Stress Distribution between Bonding Surface of Dental Filling in Enamel and Dentine. Procedia Manuf. 2015, 2, 212–217. [Google Scholar] [CrossRef]
- Benazzi, S.; Nguyen, H.N.; Kullmer, O.; Kupczik, K. Dynamic Modelling of Tooth Deformation Using Occlusal Kinematics and Finite Element Analysis. PLoS ONE 2016, 11, e0152663. [Google Scholar] [CrossRef]
- Schliebe, L.R.S.O.; Braga, S.S.L.; da Silva Pereira, R.A.; Bicalho, A.A.; Verissimo, C.; Novais, V.R.; Versluis, A.; Soares, C.J. The new generation of conventional and bulk-fill composites do not reduce the shrinkage stress in endodontically-treated molars. Am. J. Dent. 2016, 29, 333–338. [Google Scholar]
- Yoon, H.G.; Oh, H.K.; Lee, D.Y.; Shin, J.H. 3-D finite element analysis of the effects of post location and loading location on stress distribution in root canals of the mandibular 1st molar. J. Appl. Oral. Sci. 2018, 26, e20160406. [Google Scholar] [CrossRef]
- Pereira, R.D.; Leoni, G.B.; Silva-Sousa, Y.T.; Gomes, E.A.; Dias, T.R.; Brito-Júnior, M.; Sousa-Neto, M.D. Impact of Conservative Endodontic Cavities on Root Canal Preparation and Biomechanical Behavior of Upper Premolars Restored with Different Materials. J. Endod. 2021, 47, 989–999. [Google Scholar] [CrossRef]
- Yoshimi, H.; Sasaguri, K.; Tamaki, K.; Sato, S. Identification of the occurrence and pattern of masseter muscle activities during sleep using EMG and accelerometer systems. Head. Face Med. 2009, 11, 5–7. [Google Scholar] [CrossRef]
- Sagl, B.; Schmid-Schwap, M.; Piehslinger, E.; Rausch-Fan, X.; Stavness, I. The effect of tooth cusp morphology and grinding direction on TMJ loading during bruxism. Front. Physiol. 2022, 13, 964930. [Google Scholar] [CrossRef]
- Sagl, B.; Schmid-Schwap, M.; Piehslinger, E.; Kundi, M.; Stavness, I. Effect of facet inclination and location on TMJ loading during bruxism: An in-silico study. J. Adv. Res. 2021, 35, 25–32. [Google Scholar] [CrossRef]
- Fransson, H.; Dawson, V. Tooth survival after endodontic treatment. Int. Endod. J. 2023, 56 (Suppl. S2), 140–153. [Google Scholar] [CrossRef] [PubMed]
- López-Valverde, I.; Vignoletti, F.; Vignoletti, G.; Martín, C.; Sanz, M. Long-term tooth survival and success following primary root canal treatment: A 5- to 37-year retrospective observation. Clin. Oral. Investig. 2023, 27, 3233–3244. [Google Scholar] [CrossRef] [PubMed]
- Tamse, A.; Fuss, Z.; Lustig, J.; Kaplavi, J. An evaluation of endodontically treated vertically fractured teeth. J. Endod. 1999, 25, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Popescu, S.M.; Diaconu, O.A.; Scrieciu, M.; Marinescu, I.R.; Drăghici, E.C.; Truşcă, A.G.; Bănică, A.C.; Vătu, M.; Mercuţ, V. Root fractures: Epidemiological, clinical and radiographic aspects. Rom. J. Morphol. Embryol. 2017, 58, 501–506. [Google Scholar] [PubMed]
- Tzimpoulas, N.E.; Alisafis, M.G.; Tzanetakis, G.N.; Kontakiotis, E.G. A prospective study of the extraction and retention incidence of endodontically treated teeth with uncertain prognosis after endodontic referral. J. Endod. 2012, 38, 1326–1329. [Google Scholar] [CrossRef]
- Singhal, Y.; Srivastava, N.; Rana, V.; Kaushik, N.; Reddy, V. Efficacy of Root Canal Instrumentation and Fracture Strength Assessment in Primary Molars after Preparing Two Different Shapes of Access Cavity: An Ex Vivo Histological Study. Int. J. Clin. Pediatr. Dent. 2021, 14, 518–524. [Google Scholar] [CrossRef]
- Dimitriu, B.; Vârlan, C.; Suciu, I.; Vârlan, V.; Bodnar, D. Current considerations concerning endodontically treated teeth: Alteration of hard dental tissues and biomechanical properties following endodontic therapy. J. Med. Life 2009, 2, 60–65. [Google Scholar]
- Taha, N.A.; Palamara, J.E.; Messer, H.H. Fracture strength and fracture patterns of root filled teeth restored with direct resin restorations. J. Dent. 2011, 39, 527–535. [Google Scholar] [CrossRef]
- Liao, W.C.; Chen, C.H.; Pan, Y.H.; Chang, M.C.; Jeng, J.H. Vertical Root Fracture in Non-Endodontically and Endodontically Treated Teeth: Current Understanding and Future Challenge. J. Pers. Med. 2021, 11, 1375. [Google Scholar] [CrossRef]
- Lavanya Priya, K.P.; Gill, S.; Banik, A.; Marvaniya, J.; Marella, K.; Anusha, Y.; Mustafa, M. A Retrospective Study on the Fracture Toughness of the Coronal Restorations in Endodontically Restored Teeth. An Original Research. J. Pharm. Bioallied Sci. 2023, 15 (Suppl. 1), 132–136. [Google Scholar] [CrossRef]
- Patil, P.; Newase, P.; Pawar, S.; Gosai, H.; Shah, D.; Parhad, S.M. Comparison of Fracture Resistance of Endodontically Treated Teeth With Traditional Endodontic Access Cavity, Conservative Endodontic Access Cavity, Truss Endodontic Access Cavity, and Ninja Endodontic Access Cavity Designs: An In Vitro Study. Cureus 2022, 14, e28090. [Google Scholar] [CrossRef] [PubMed]
- Daniel, A.; Saleh, A.R.; Al-Jadaa, A.; Kheder, W. Impact of Access Cavity Design on Fracture Resistance of Endodontically Treated Maxillary First Premolar: In Vitro. Braz. Dent. J. 2024, 35, e245676. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Chueh, L.H.; Hsiao, C.K.; Wu, H.P.; Chiang, C.P. First untoward events and reasons for tooth extraction after nonsurgical endodontic treatment in Taiwan. J. Endod. 2008, 34, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.L.; Mann, V.; Gulabivala, K. A prospective study of the factors affecting outcomes of non-surgical root canal treatment: Part 2: Tooth survival. Int. Endod. J. 2011, 44, 610–625. [Google Scholar] [CrossRef]
- Touré, B.; Faye, B.; Kane, A.W.; Lo, C.M.; Niang, B.; Boucher, Y. Analysis of reasons for extraction of endodontically treated teeth: A prospective study. J. Endod. 2011, 37, 1512–1515. [Google Scholar] [CrossRef]
- Krishan, R.; Paqué, F.; Ossareh, A.; Kishen, A.; Dao, T.; Friedman, S. Impacts of conservative endodontic cavity on root canal instrumentation efficacy and resistance to fracture assessed in incisors, premolars, and molars. J. Endod. 2014, 40, 1160–1166. [Google Scholar] [CrossRef]
- Tamse, A.; Kaffe, I.; Lustig, J.; Ganor, Y.; Fuss, Z. Radiographic features of vertically fractured endodontically treated mesial roots of mandibular molars. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2006, 101, 797–802. [Google Scholar] [CrossRef]
- Zadik, Y.; Sandler, V.; Bechor, R.; Salehrabi, R. Analysis of factors related to extraction of endodontically treated teeth. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2008, 106, e31–e35. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Shah, N.C.; Kumar, M.; Ishwar, S.; Purkayastha, D.D.; Mishra, D.; Shanbhag, A. Stress distribution pattern in two different no-post systems in endodontically treated maxillary central incisors: A three-dimensional finite element analysis. J. Conserv. Dent. Endod. 2024, 27, 572–576. [Google Scholar] [CrossRef]
- Kapetanaki, I.; Dimopoulos, F.; Gogos, C. Traditional and minimally invasive access cavities in endodontics: A literature review. Restor. Dent. Endod. 2021, 46, e46. [Google Scholar] [CrossRef]
- Silva, E.J.N.L.; Rover, G.; Belladonna, F.G.; De-Deus, G.; da Silveira Teixeira, C.; da Silva Fidalgo, T.K. Impact of contracted endodontic cavities on fracture resistance of endodontically treated teeth: A systematic review of in vitro studies. Clin. Oral. Investig. 2018, 22, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Plotino, G.; Grande, N.M.; Isufi, A.; Ioppolo, P.; Pedullà, E.; Bedini, R.; Gambarini, G.; Testarelli, L. Fracture Strength of Endodontically Treated Teeth with Different Access Cavity Designs. J. Endod. 2017, 43, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.; Meyer, C.A.; Yoo, E.; Vargas, J.A.; Liu, Y.; Jalali, P. Stress distribution in a tooth treated through minimally invasive access compared to one treated through traditional access: A finite element analysis study. J. Conserv. Dent. JCD 2018, 21, 505–509. [Google Scholar]
- Jiang, Q.; Huang, Y.; Tu, X.; Li, Z.; He, Y.; Yang, X. Biomechanical Properties of First Maxillary Molars with Different Endodontic Cavities: A Finite Element Analysis. J. Endod. 2018, 44, 1283–1288. [Google Scholar] [CrossRef]
- Lakshmisetty, H.; Raghu, R.; Shetty, A.; Rajasekhara, S.; Sharma, S.; Bharath, G. Biomechanical properties of mandibular first molar with truss and conventional access cavities: A finite element analysis. Endodontology 2022, 34, 265–269. [Google Scholar] [CrossRef]
- Saeed, M.; Al-Obadi, M.; Salim, A.; Alsawaf, A.Y.; Hadi, K. Impact of Access Cavity Design on Fracture Resistance of Endodontically Treated Molars: A Systematic Review. Clin. Cosmet. Investig. Dent. 2021, 13, 1–10. [Google Scholar] [CrossRef]
- Kataia, M.; Nawar, N.N.; Mohamed, S.H. Biomechanical behavior of two-rooted maxillary second premolar with different access cavities and apical preparations (A finite element analysis). Eur. Chem. Bull. 2023, 8, 1546–1558. [Google Scholar]
- Cobankara, F.K.; Unlu, N.; Cetin, A.R.; Ozkan, H.B. The effect of different restoration techniques on the fracture resistance of endodontically-treated molars. Oper. Dent. 2008, 33, 526–533. [Google Scholar] [CrossRef]
- Sengun, A.; Cobankara, F.K.; Orucoglu, H. Effect of a new restoration technique on fracture resistance of endodontically treated teeth. Dent. Traumatol. 2008, 24, 214–219. [Google Scholar] [CrossRef]
- Nezir, M.; Dinçtürk, B.A.; Sarı, C.; Alp, C.K.; Altınışık, H. Effect of fiber-reinforced direct restorative materials on the fracture resistance of endodontically treated mandibular molars restored with a conservative endodontic cavity design. Clin. Oral. Investig. 2024, 28, 316. [Google Scholar] [CrossRef]
- Plasmans, P.J.; Creugers, N.H.; Mulder, J. Long-term survival of extensive amalgam restorations. J. Dent. Res. 1998, 77, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Slutzky-Goldberg, I.; Slutzky, H.; Gorfil, C.; Smidt, A. Restoration of endodontically treated teeth review and treatment recommendations. Int. J. Dent. 2009, 150251. [Google Scholar] [CrossRef] [PubMed]
- Bianchi E Silva, A.A.; Ghiggi, P.C.; Mota, E.G.; Borges, G.A.; Burnett, L.H., Jr.; Spohr, A.M. Influence of restorative techniques on fracture load of endodontically treated premolars. Stomatologija 2013, 15, 123–128. [Google Scholar] [PubMed]
- Arya, A.; Grewal, M.S.; Arya, V.; Choudhary, E.; Duhan, J. Stress distribution of endodontically treated mandibular molars with varying amounts of tooth structure restored with direct composite resin with or without cuspal coverage: A 3D finite element analysis. J. Conserv. Dent. 2023, 26, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Geiger, S.; Paikin, L.; Gorfil, C.; Gordon, M. Fracture resistance of endodontically treated teeth restored with combined composite-amalgam restorations. Quintessence Int. 2008, 39, e58–e62. [Google Scholar]
- Gönder, H.Y.; Mohammadi, R.; Harmankaya, A.; Yüksel, İ.B.; Fidancıoğlu, Y.D.; Karabekiroğlu, S. Teeth Restored with Bulk–Fill Composites and Conventional Resin Composites; Investigation of Stress Distribution and Fracture Lifespan on Enamel, Dentin, and Restorative Materials via Three-Dimensional Finite Element Analysis. Polymers 2023, 15, 1637. [Google Scholar] [CrossRef]
- Ausiello, P.; Rengo, S.; Davidson, C.L.; Watts, D.C. Stress distributions in adhesively cemented ceramic and resin-composite Class II inlay restorations: A 3D-FEA study. Dent. Mater. 2004, 20, 862–872. [Google Scholar] [CrossRef]
- Roberson, T.M.; Heymann, H.O.; Swift, E.J. Sturdevant’s Art & Science of Operative Dentistry, 4th ed.; Mosby Inc.: Orlando, FL, USA, 2002; pp. 476–483. [Google Scholar]
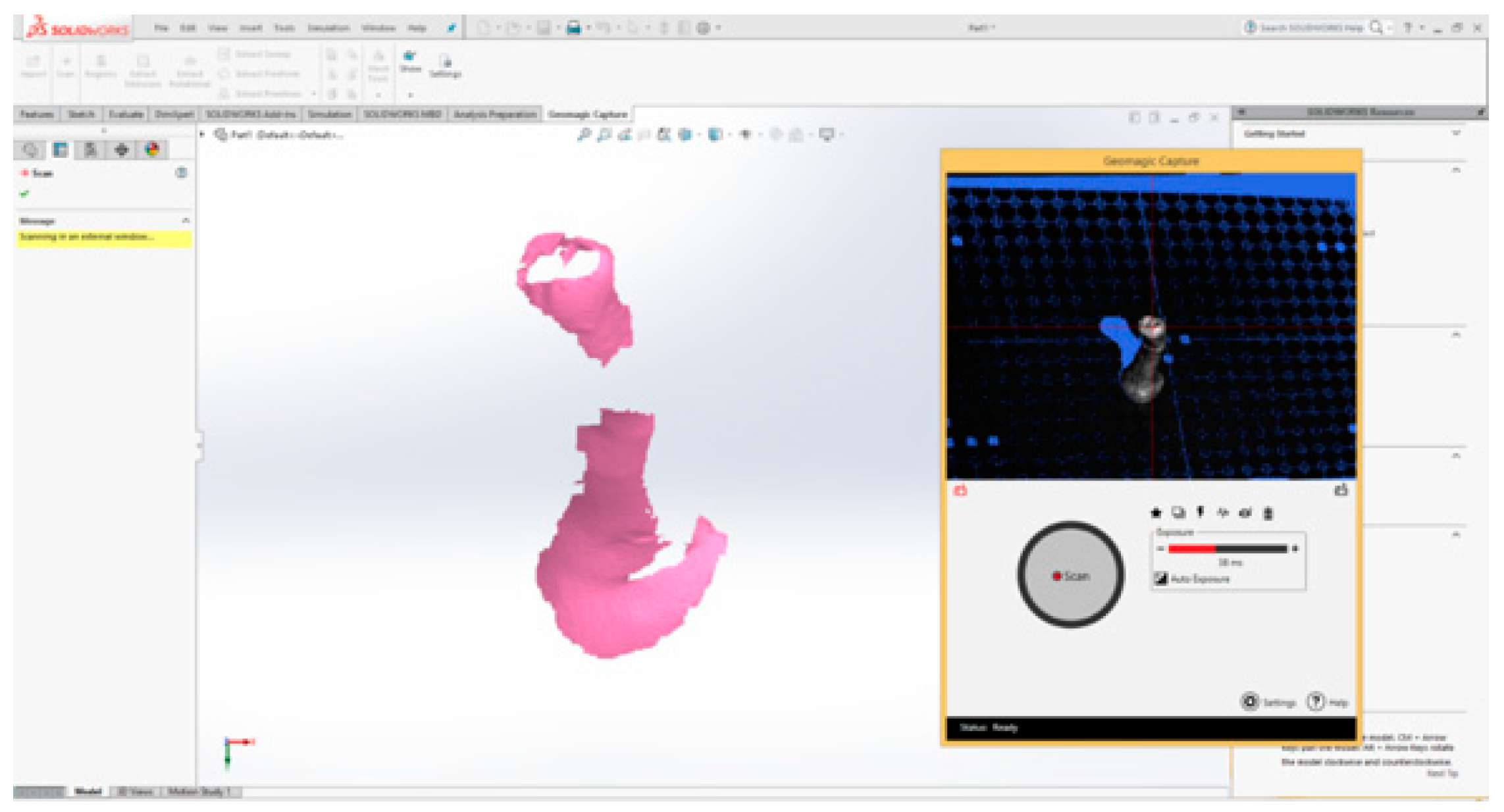





| Characteristics | 3D Systems Capture 3D |
|---|---|
| Net weight | 1.35 kg |
| Size (L × l × H) | 276 × 74 ×49 mm |
| Data capture rate | 98,500 points/scan |
| Resolution | 0.110 mm la 300 mm 0.180 mm la 480 mm |
| Accuracy | 0.060 mm |
| Standard distance | 300 mm |
| Scan depth | 180 mm |
| Field of view | 124 × 120 mm (zoom in) 190 × 175 mm (zoom out) |
| Material Name | Density [kg/m3] | Modulus of Elasticity E [Pa] | Poisson’s Ratio |
|---|---|---|---|
| Enamel | 2.958 | 7.79 × 1010 | 0.3 [33] |
| Dentine | 2.140 | 1.76 × 1010 | 0.25 [33] |
| Gutta-percha | 1.000 | 6.1 × 106 | 0.49 [37] |
| Amalgam | 11.334 | 5 × 1010 | 0.29 [34] |
| Bulk fill composite | 1.052 | 1.43 × 1010 | 0.24 [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boțilă, M.-R.; Popa, D.L.; Mercuț, R.; Iacov-Crăițoiu, M.M.; Scrieciu, M.; Popescu, S.M.; Mercuț, V. A Finite Element Method Study of Stress Distribution in Dental Hard Tissues: Impact of Access Cavity Design and Restoration Material. Bioengineering 2024, 11, 878. https://doi.org/10.3390/bioengineering11090878
Boțilă M-R, Popa DL, Mercuț R, Iacov-Crăițoiu MM, Scrieciu M, Popescu SM, Mercuț V. A Finite Element Method Study of Stress Distribution in Dental Hard Tissues: Impact of Access Cavity Design and Restoration Material. Bioengineering. 2024; 11(9):878. https://doi.org/10.3390/bioengineering11090878
Chicago/Turabian StyleBoțilă, Mihaela-Roxana, Dragos Laurențiu Popa, Răzvan Mercuț, Monica Mihaela Iacov-Crăițoiu, Monica Scrieciu, Sanda Mihaela Popescu, and Veronica Mercuț. 2024. "A Finite Element Method Study of Stress Distribution in Dental Hard Tissues: Impact of Access Cavity Design and Restoration Material" Bioengineering 11, no. 9: 878. https://doi.org/10.3390/bioengineering11090878
APA StyleBoțilă, M.-R., Popa, D. L., Mercuț, R., Iacov-Crăițoiu, M. M., Scrieciu, M., Popescu, S. M., & Mercuț, V. (2024). A Finite Element Method Study of Stress Distribution in Dental Hard Tissues: Impact of Access Cavity Design and Restoration Material. Bioengineering, 11(9), 878. https://doi.org/10.3390/bioengineering11090878






