The Synergic Effect of Tubal Endometriosis and Women’s Aging on Fallopian Tube Function: Insights from a 3D Mechanical Model
Abstract
1. Introduction
2. Methods and Materials
2.1. 3D Modeling of the Human Fallopian Tubes
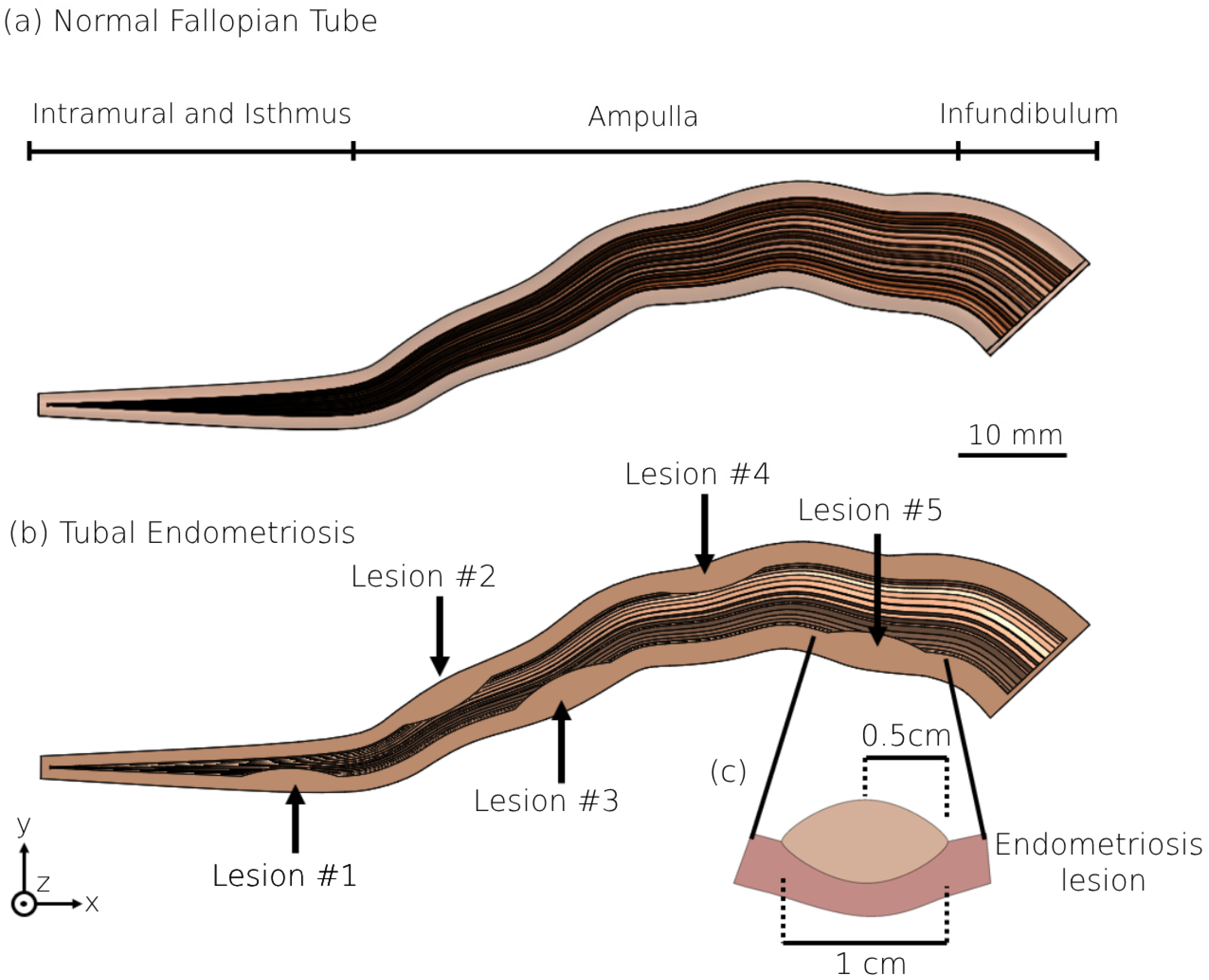
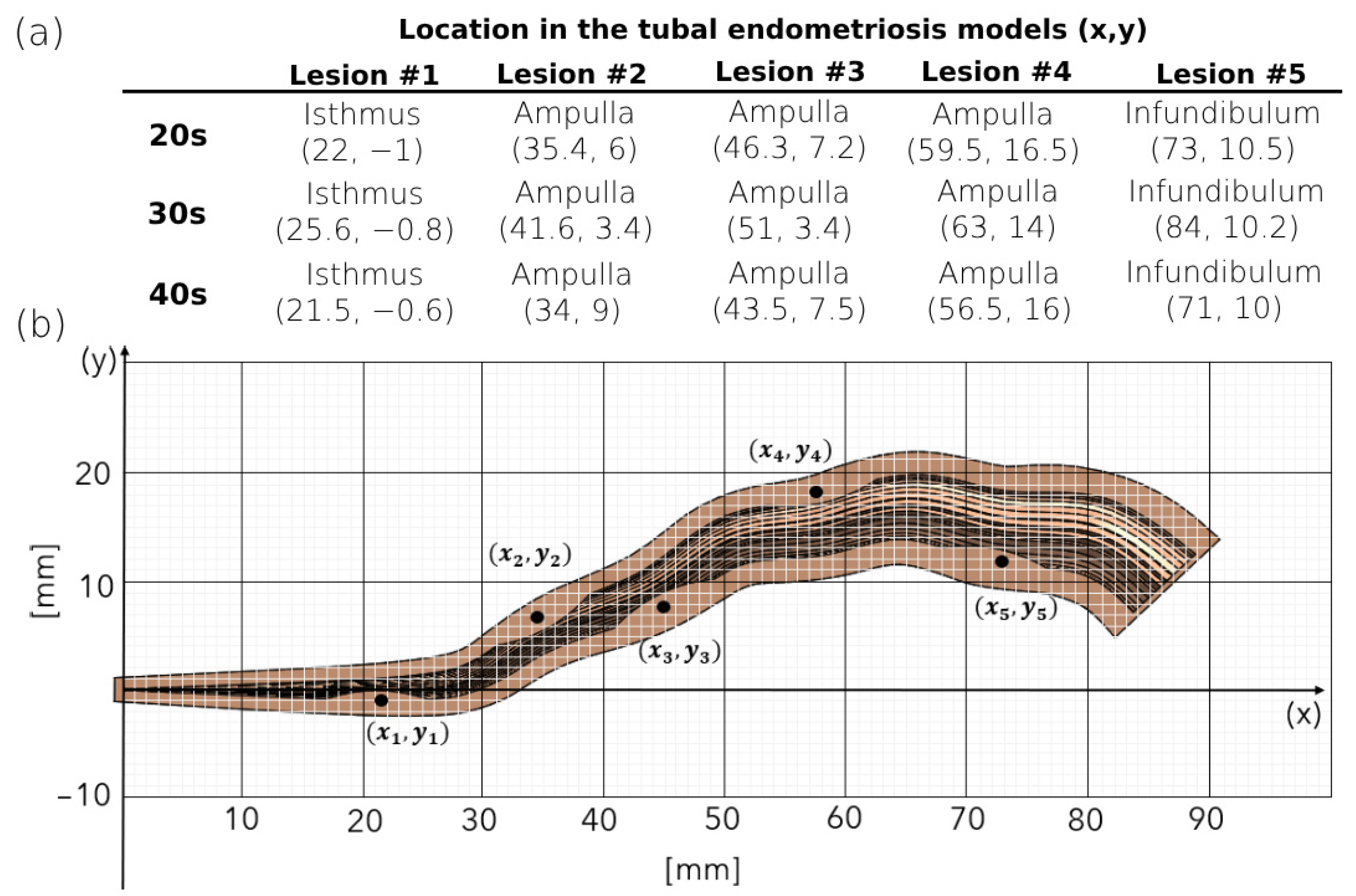
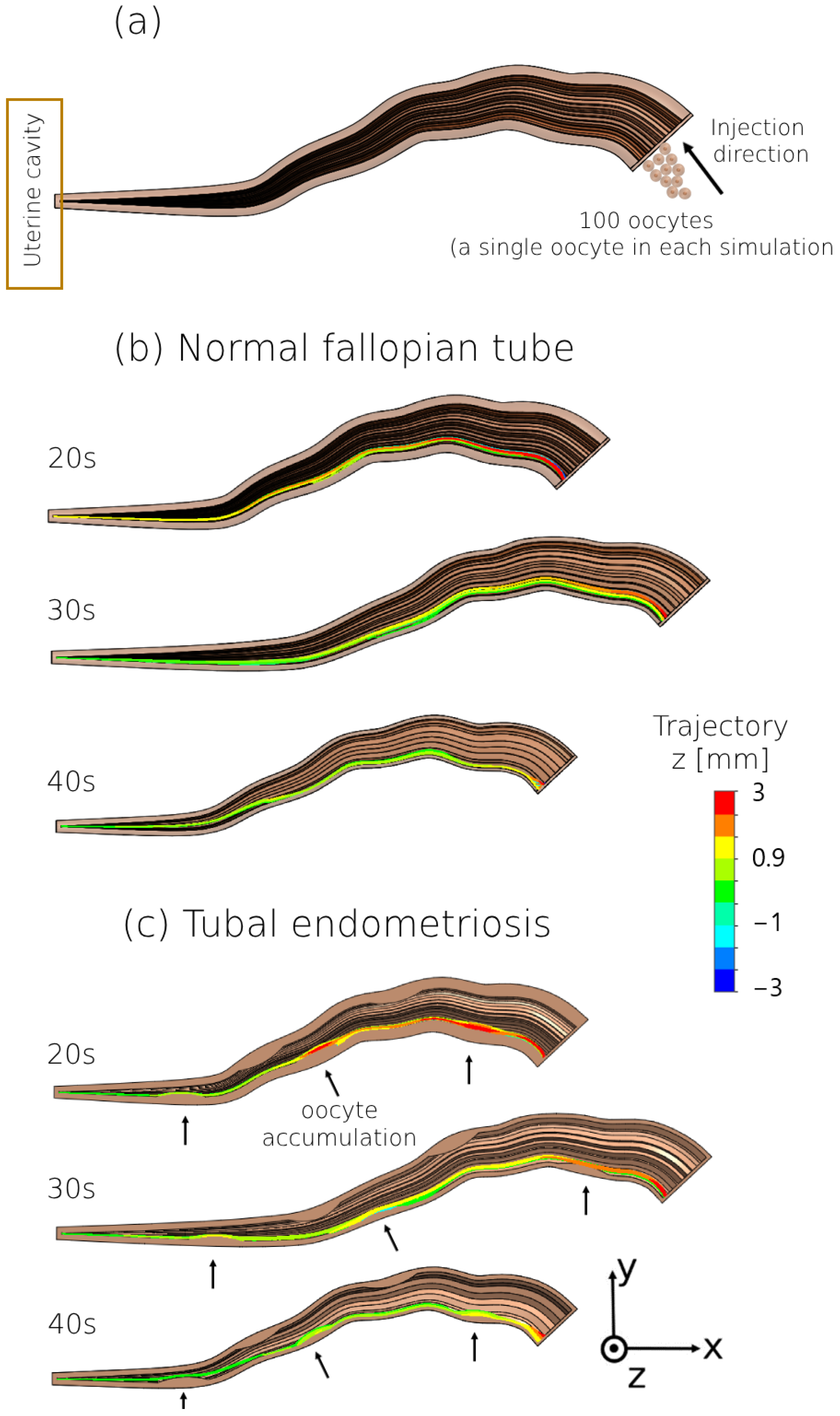
2.2. 3D Modeling of the Human Fallopian Tubes with Endometriosis
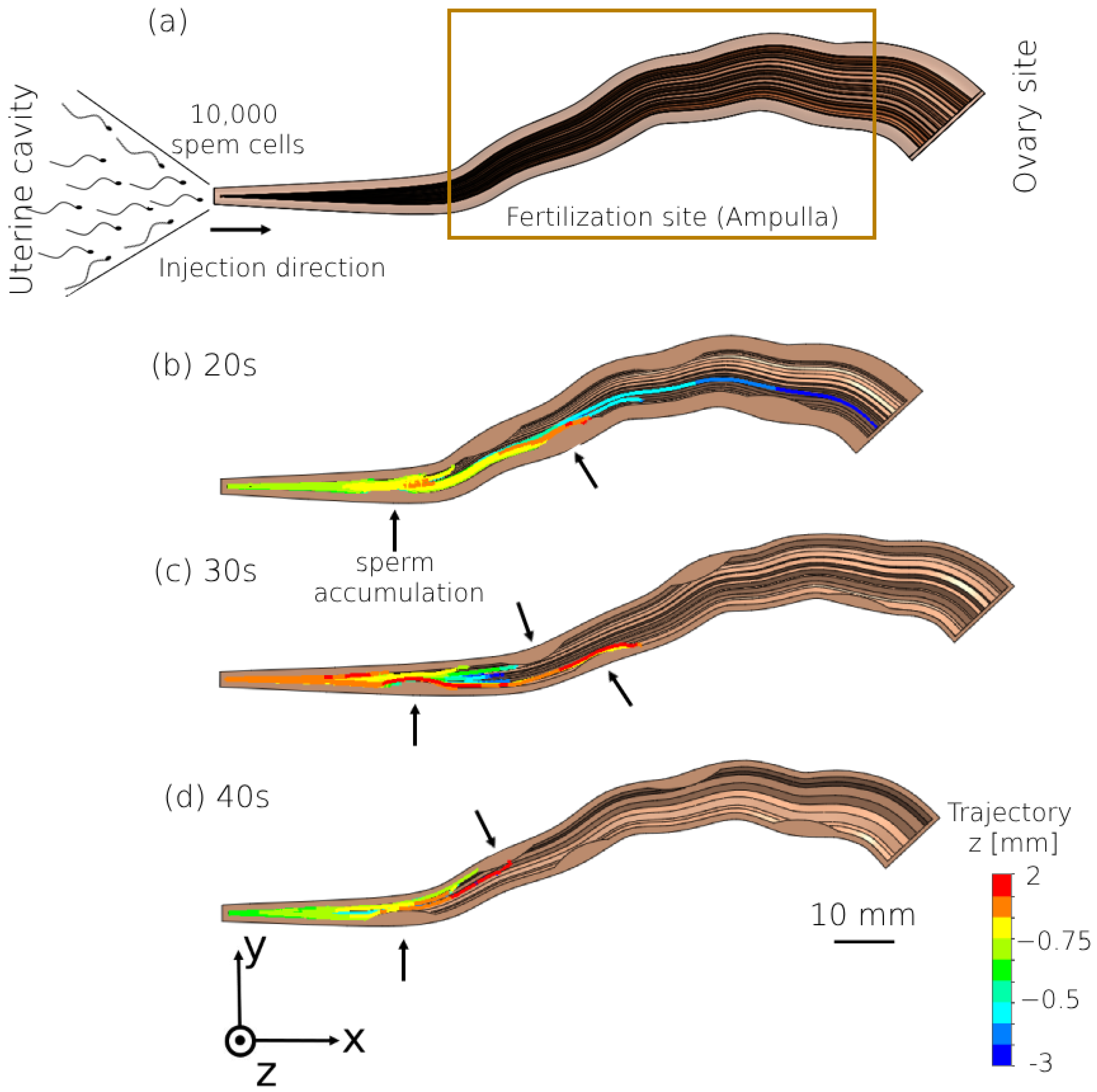
2.3. Peristalsis–Ciliary Flow
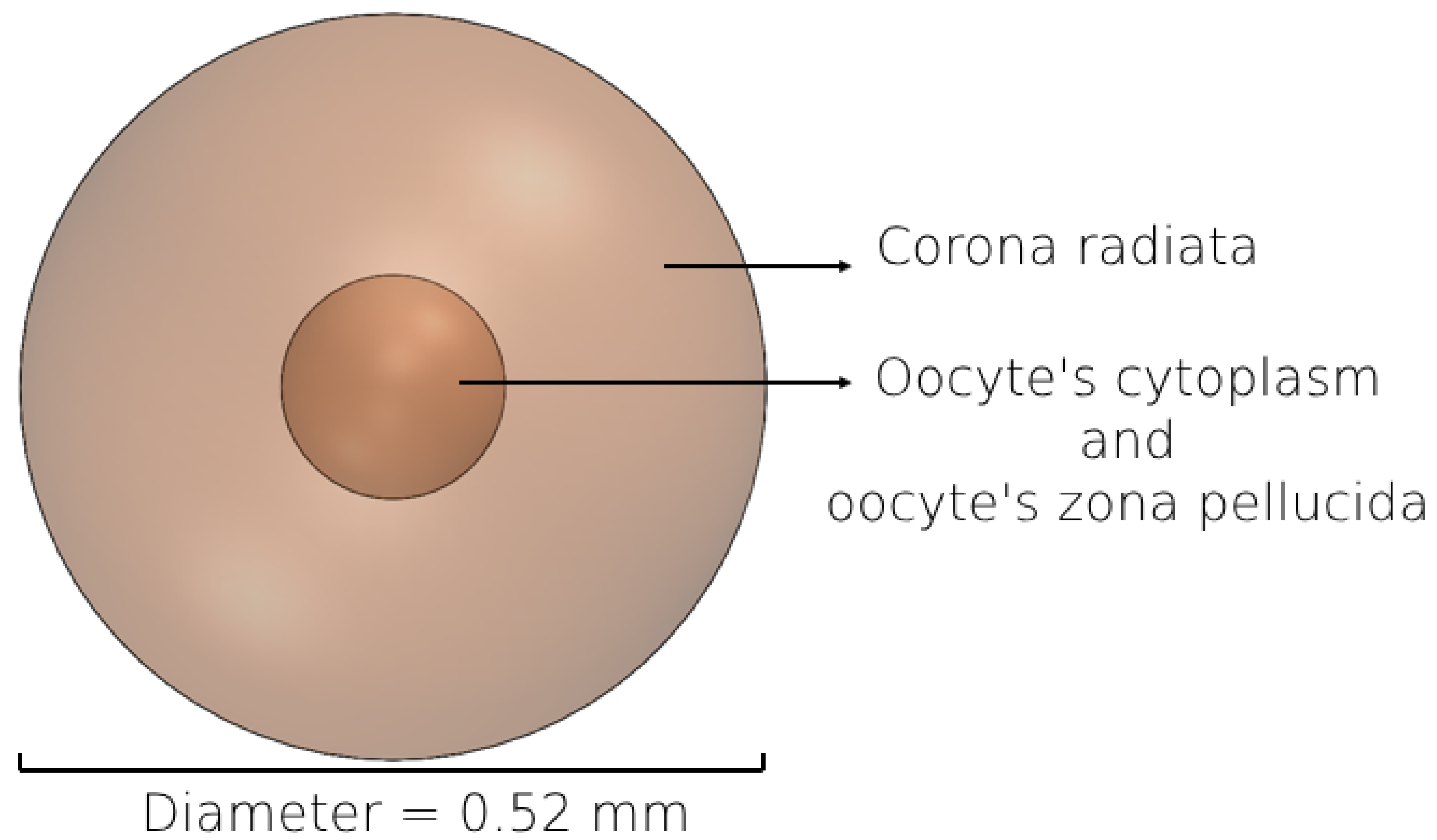
2.4. 3D Modeling of the Female Oocyte
2.5. 3D Human Sperm Model
2.6. Beat Pattern
2.7. Computerized Fluid Dynamic Simulation
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Szmelskyj, I.; Aquilina, L.; Szmelskyj, A.O. Anatomy and Physiology of the Reproductive System. In Acupuncture for IVF and Assisted Reproduction: An Integrated Approach to Treatment and Management, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2014; Chapter 2; pp. 23–58. [Google Scholar] [CrossRef]
- Lyons, R.A.; Saridogan, E.; Djahanbakhch, O. The Reproductive Significance of Human Fallopian Tube Cilia. Hum. Reprod. Update 2006, 12, 363–372. [Google Scholar] [CrossRef]
- Vitale, S.G.; Carugno, J.; D’alterio, M.N.; Mikuš, M.; Patrizio, P.; Angioni, S. A New Methodology to Assess Fallopian Tubes Microbiota and Its Impact on Female Fertility. Diagnostics 2022, 12, 1375. [Google Scholar] [CrossRef]
- Hunter, R.H.F. Have the Fallopian Tubes a Vital Rôle in Promoting Fertility? Acta Obstet. Gynecol. Scand. 1998, 77, 475–486. [Google Scholar] [CrossRef]
- Dulohery, K.; Trottmann, M.; Bour, S.; Liedl, B.; Alba-Alejandre, I.; Reese, S.; Hughes, B.; Stief, C.G.; Kölle, S. How Do Elevated Levels of Testosterone Affect the Function of the Human Fallopian Tube and Fertility?—New Insights. Mol. Reprod. Dev. 2020, 87, 30–44. [Google Scholar] [CrossRef]
- Briceag, I.; Costache, A.; Purcarea, V.L.; Cergan, R.; Dumitru, M.; Briceag, I.; Sajin, M.; Ispas, A.T. Fallopian Tubes-Literature Review of Anatomy and Etiology in Female Infertility. J. Med. Life 2015, 8, 129–131. [Google Scholar]
- Ambildhuke, K.; Pajai, S.; Chimegave, A.; Mundhada, R.; Kabra, P. A Review of Tubal Factors Affecting Fertility and Its Management. Cureus 2022, 14, e30990. [Google Scholar] [CrossRef]
- Posaci, C.; Camus, M.; Osmanagaoglu, K.; Devroey, P. Tubal Surgery in the Era of Assisted Reproductive Technology: Clinical Options. Hum. Reprod. 1999, 14, 120–136. [Google Scholar] [CrossRef]
- Gupta, N.; Sharma, J.B.; Mittal, S.; Singh, N.; Misra, R.; Kukreja, M. Genital Tuberculosis in Indian Infertility Patients. Int. J. Gynecol. Obstet. 2007, 97, 135–138. [Google Scholar] [CrossRef]
- Seiler, J.C. Factors Influencing the Outcome of Microsurgical Tubal Ligation Reversals. Am. J. Obstet. Gynecol. 1983, 146, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Mohd Nor, H.; Jayapragasam, K.J.; Abdullah, B.J.J. Diagnostic Image Quality of Hysterosalpingography: Ionic versus Non Ionic Water Soluble Iodinated Contrast Media. Biomed. Imaging Interv. J. 2009, 5, e29. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luciano, D.E.; Exacoustos, C.; Johns, D.A.; Luciano, A.A. Can Hysterosalpingo-Contrast Sonography Replace Hysterosalpingography in Confirming Tubal Blockage after Hysteroscopic Sterilization and in the Evaluation of the Uterus and Tubes in Infertile Patients? Am. J. Obstet. Gynecol. 2011, 204, 79.e1–79.e5. [Google Scholar] [CrossRef] [PubMed]
- Eddy, C.A.; Pauerstein, C.J. Anatomy and Physiology of the Fallopian Tube. Clin. Obstet. Gynecol. 1980, 23, 1177–1193. [Google Scholar] [CrossRef]
- Winuthayanon, W.; Li, S. Fallopian Tube/Oviduct: Structure and Cell Biology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 282–290. [Google Scholar]
- Han, X.; Wang, L.; Wang, S.; Chou, Y.; Liu, X.; Guo, C.; Chen, J. Radiographic Morphology of Fallopian Tubes in Women of Child-Bearing Potential: A Descriptive Study. J. Obstet. Gynaecol. Res. 2013, 39, 820–824. [Google Scholar] [CrossRef]
- Varga, I.; Kachlík, D.; Žišková, M.; Miko, M. Lymphatic Lacunae of the Mucosal Folds of Human Uterine Tubes—A Rediscovery of Forgotten Structures and Their Possible Role in Reproduction. Ann. Anat.—Anat. Anz. 2018, 219, 121–128. [Google Scholar] [CrossRef]
- Russo, A.; Burdette, J.E. Isolation of Fallopian Tube Epithelium for Assessment of Cilia Beating Frequency (CBF). Methods Mol. Biol. 2022, 2424, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Critoph, F.N.; Dennis, K.J. Ciliary Activity in the Human Oviduct. BJOG Int. J. Obstet. Gynaecol. 1977, 84, 216–218. [Google Scholar] [CrossRef]
- Wånggren, K.; Stavreus-Evers, A.; Olsson, C.; Andersson, E.; Gemzell-Danielsson, K. Regulation of Muscular Contractions in the Human Fallopian Tube through Prostaglandins and Progestagens. Hum. Reprod. 2008, 23, 2359–2368. [Google Scholar] [CrossRef] [PubMed]
- Eytan, O.; Jaffa, A.J.; Elad, D. Peristaltic Flow in a Tapered Channel: Application to Embryo Transport within the Uterine Cavity. Med. Eng. Phys. 2001, 23, 475–484. [Google Scholar] [CrossRef]
- Maia, H.S.; Coutinho, E.M. Peristalsis and Antiperistalsis of the Human Fallopian Tube during the Menstrual Cycle. Biol. Reprod. 1970, 2, 305–314. [Google Scholar] [CrossRef]
- Kunz, G.; Beil, D.; Deiniger, H.; Einspanier, A.; Mall, G.; Leyendecker, G. The uterine peristaltic pump: Normal and impeded sperm transport within the female genital tract. Fate Male Germ Cell 1997, 424, 267–277. [Google Scholar] [CrossRef]
- Kuijsters, N.P.M.; Methorst, W.G.; Kortenhorst, M.S.Q.; Rabotti, C.; Mischi, M.; Schoot, B.C. Uterine Peristalsis and Fertility: Current Knowledge and Future Perspectives: A Review and Meta-Analysis. Reprod. Biomed. Online 2017, 35, 50–71. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, J.; Zaltzhendler, O.; Bshara, M.; Jaffa, A.J.; Grisaru, D.; Duan, E.; Elad, D. Analysis of in Vivo Uterine Peristalsis in the Non-Pregnant Female Mouse. Interface Focus 2019, 9, 20180082. [Google Scholar] [CrossRef]
- Zhu, L.; Che, H.S.; Xiao, L.; Li, Y.P. Uterine Peristalsis before Embryo Transfer Affects the Chance of Clinical Pregnancy in Fresh and Frozen-Thawed Embryo Transfer Cycles. Hum. Reprod. 2014, 29, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Devi, J.; Medhi, T.; Hussain, F. Age Related Changes of Morphology, Length and Luminal Diameter of Human Fallopian Tube. IOSR J. Dent. Med. Sci. e-ISSN 2017, 16, 1–08. [Google Scholar] [CrossRef]
- Lisa, J.R.; Gioia, J.D.; Rubin, I.C. Observations on the Interstitial Portion of the Fallopian Tube. Surg. Gynecol. Obstet. 1954, 99, 159–169. [Google Scholar] [PubMed]
- Hwang, T.S.; Song, J. Morphometrical Changes of the Human Uterine Tubes According to Aging and Menstrual Cycle. Ann. Anat. 2004, 186, 263–269. [Google Scholar] [CrossRef]
- Kim-Bjorklund, T.; Landgren, B.M.; Hamberger, L.; Johannisson, E. Comparative Morphometric Study of the Endometrium, the Fallopian Tube, and the Corpus Luteum during the Postovulatory Phase in Normally Menstruating Women. Fertil. Steril. 1991, 56, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, H.; Sahu, S.K. A Morphological Study on Fallopian Tube. Int. J. Anat. Res. 2016, 4, 3066–3071. [Google Scholar] [CrossRef]
- Veerraju, T.S.N.V.V.; Sesi, D.A.V.S. A Study on Development and Morphometric Changes of Fallopian Tubes. Dent. Med. Sci. 2019, 18, 7–9. [Google Scholar] [CrossRef]
- Li, J.; Ning, Y.; Abushahin, N.; Yuan, Z.; Wang, Y.; Wang, Y.; Yuan, B.; Cragun, J.M.; Chambers, S.K.; Hatch, K.; et al. Secretory Cell Expansion with Aging: Risk for Pelvic Serous Carcinogenesis. Gynecol. Oncol. 2013, 131, 555–560. [Google Scholar] [CrossRef]
- Tao, T.; Lin, W.; Wang, Y.; Zhang, J.; Chambers, S.K.; Li, B.; Lea, J.; Wang, Y.; Wang, Y.; Zheng, W. Loss of Tubal Ciliated Cells as a Risk for “Ovarian” or Pelvic Serous Carcinoma. Am. J. Cancer Res. 2020, 10, 3815–3827. [Google Scholar] [CrossRef]
- Coan, M.; Vinciguerra, G.L.R.; Cesaratto, L.; Gardenal, E.; Bianchet, R.; Dassi, E.; Vecchione, A.; Baldassarre, G.; Spizzo, R.; Nicoloso, M.S. Exploring the Role of Fallopian Ciliated Cells in the Pathogenesis of High-Grade Serous Ovarian Cancer. Int. J. Mol. Sci. 2018, 19, 2512. [Google Scholar] [CrossRef] [PubMed]
- Şendur, H.N.; Cindil, E.; Cerit, M.N.; Kılıç, P.; Gültekin, I.İ.; Oktar, S.Ö. Evaluation of Effects of Aging on Skeletal Muscle Elasticity Using Shear Wave Elastography. Eur. J. Radiol. 2020, 128, 109038. [Google Scholar] [CrossRef] [PubMed]
- Shirasuna, K.; Iwata, H. Effect of Aging on the Female Reproductive Function. Contracept. Reprod. Med. 2017, 2, 23. [Google Scholar] [CrossRef] [PubMed]
- Barros, E.M.K.P.; Rodrigues, C.J.; Rodrigues, N.R.; Oliveira, R.P.; Barros, T.E.P.; Rodrigues, A.J. Aging of the Elastic and Collagen Fibers in the Human Cervical Interspinous Ligaments. Spine J. 2002, 2, 57–62. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, B.; Nezhat, F.; Ursillo, L.; Akerman, M.; Vintzileos, W.; White, M. Fallopian Tube Endometriosis in Women Undergoing Operative Video Laparoscopy and Its Clinical Implications. Fertil. Steril. 2020, 114, 1040–1048. [Google Scholar] [CrossRef]
- Hill, C.J.; Fakhreldin, M.; Maclean, A.; Dobson, L.; Nancarrow, L.; Bradfield, A.; Choi, F.; Daley, D.; Tempest, N.; Hapangama, D.K. Endometriosis and the Fallopian Tubes: Theories of Origin and Clinical Implications. J. Clin. Med. 2020, 9, 1905. [Google Scholar] [CrossRef]
- Jiao, H.N.; Song, W.; Feng, W.W.; Liu, H. Diagnosis and Treatment of Tubal Endometriosis in Women Undergoing Laparoscopy: A Case Series from a Single Hospital. World J. Clin. Cases 2022, 10, 12136–12145. [Google Scholar] [CrossRef]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis Is a Chronic Systemic Disease: Clinical Challenges and Novel Innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef]
- Donnez, J.; Nisolle, M.; Casanas-Roux, F. Three-Dimensional Architectures of Peritoneal Endometriosis. Fertil. Steril. 1992, 57, 980–983. [Google Scholar] [CrossRef]
- Salamanca, A.; Beltran, E. Subendometrial Contractility in Menstrual Phase Visualized by Transvaginal Sonography in Patients with Endometriosis. Fertil. Steril. 1995, 64, 193–195. [Google Scholar] [CrossRef]
- Richter, O.N.; Bartz, C.; Dowaji, J.; Kupka, M.; Reinsberg, J.; Ulrich, U.; Rath, W. Contractile Reactivity of Human Myometrium in Isolated Non-Pregnant Uteri. Hum. Reprod. 2006, 21, 36–45. [Google Scholar] [CrossRef][Green Version]
- He, Y.; Wu, H.; He, X.; Xing, Q.; Zhou, P.; Cao, Y.; Wei, Z. Administration of Atosiban in Patients with Endometriosis Undergoing Frozen–Thawed Embryo Transfer: A Prospective, Randomized Study. Fertil. Steril. 2016, 106, 416–422. [Google Scholar] [CrossRef]
- Li, X.; Kodithuwakku, S.P.; Chan, R.W.S.; Yeung, W.S.B.; Yao, Y.; Ng, E.H.Y.; Chiu, P.C.N.; Lee, C.L. Three-Dimensional Culture Models of Human Endometrium for Studying Trophoblast-Endometrium Interaction during Implantation. Reprod. Biol. Endocrinol. 2022, 20, 120. [Google Scholar] [CrossRef]
- Mbuguiro, W.; Gonzalez, A.N.; Mac Gabhann, F. Computational Models for Diagnosing and Treating Endometriosis. Front. Reprod. Health 2021, 3, 699133. [Google Scholar] [CrossRef]
- Borelli, V.; Martinelli, M.; Luppi, S.; Vita, F.; Romano, F.; Fanfani, F.; Trevisan, E.; Celsi, F.; Zabucchi, G.; Zanconati, F.; et al. Mast Cells in Peritoneal Fluid from Women with Endometriosis and Their Possible Role in Modulating Sperm Function. Front. Physiol. 2020, 10, 1543. [Google Scholar] [CrossRef]
- Oral, E.; Arici, A.; Olive, D.L.; Huszar, G. Peritoneal Fluid from Women with Moderate or Severe Endometriosis Inhibits Sperm Motility: The Role of Seminal Fluid Components. Fertil. Steril. 1996, 66, 787–792. [Google Scholar] [CrossRef]
- Garcia-Velasco, J.A.; Arici, A. Is the Endometrium or Oocyte/Embryo Affected in Endometriosis? Hum. Reprod. 1999, 14, 77–89. [Google Scholar] [CrossRef]
- Saetta, A.; Magro, M.; Oliver, R.; Odejinmi, F. Endometriosis and the Risk of Ectopic Pregnancy: 10-Year Retrospective Cohort Study. J. Endometr. Pelvic Pain Disord. 2020, 12, 10–15. [Google Scholar] [CrossRef]
- Mills, T.A.; Lavender, T. Advanced Maternal Age. Obstet. Gynaecol. Reprod. Med. 2014, 24, 85–90. [Google Scholar] [CrossRef]
- Zagorski, N. Journal Digest. Psychiatr. News 2019, 54, 2000. [Google Scholar] [CrossRef]
- Yong, P.J.; Matwani, S.; Brace, C.; Quaiattini, A.; Bedaiwy, M.A.; Albert, A.; Allaire, C. Endometriosis and Ectopic Pregnancy: A Meta-Analysis. J. Minim. Invasive Gynecol. 2020, 27, 352–361.e2. [Google Scholar] [CrossRef]
- Nassir, M.; Levi, M.; Dardikman-Yoffe, G.; Mirsky, S.K.; Shaked, N.T. Prediction of Sperm Progression in Three Dimensions Using Rapid Optical Imaging and Dynamic Mechanical Modeling. Cells 2022, 11, 1319. [Google Scholar] [CrossRef]
- Nassir, M.; Levi, M.; Wiser, A.; Shaked, N.T. Evaluation of Women’s Aging Influence on Sperm Passage inside the Fallopian Tube Using 3D Dynamic Mechanical Modeling. Front. Bioeng. Biotechnol. 2024, 12, 1324802. [Google Scholar] [CrossRef] [PubMed]
- Revzin, M.V.; Moshiri, M.; Katz, D.S.; Pellerito, J.S.; Gettle, L.M.; Menias, C.O. Imaging Evaluation of Fallopian Tubes and Related Disease: A Primer for Radiologists. Radiographics 2020, 40, 1473–1501. [Google Scholar] [CrossRef] [PubMed]
- Rimbach, S.; Wallwiener, D.; Barth, C.; Bekeredjian, R.; Hardt, S.; Bastert, G. Intraluminal Ultrasound Imaging of the Fallopian Tube Wall: Results of Standardized in Vitro Investigations of Pig and Human Tubal Specimens. Fertil. Steril. 1998, 70, 161–164. [Google Scholar] [CrossRef]
- Luo, H.; Li, S.; Kou, S.; Lin, Y.; Hagemann, I.S.; Zhu, Q. Enhanced 3D Visualization of Human Fallopian Tube Morphology Using a Miniature Optical Coherence Tomography Catheter. Biomed. Opt. Express 2023, 14, 3225. [Google Scholar] [CrossRef]
- De Geyter, C.; Steimann, S.; Fröhlich, J.M.; Wiesner, W.; Wight, E.; Steinbrich, W.; Pegios, W. Selective Visualization of the Fallopian Tube with Magnetic Resonance Imaging. Reprod. Biomed. Online 2007, 14, 593–597. [Google Scholar] [CrossRef] [PubMed]
- La Parra Casado, C.; Molina Fàbrega, R.; Forment Navarro, M.; Cano Gimeno, J. Fallopian Tube Disease on Magnetic Resonance Imaging. Radiol. (Engl. Ed.) 2013, 55, 385–397. [Google Scholar] [CrossRef]
- Wolman, I. Berek and Novak’s Gynecology 15th. J. Obstet. Gynaecol. India 2014, 64, 150–151. [Google Scholar] [CrossRef]
- Papathanasiou, A.; Djahanbakhch, O.; Saridogan, E.; Lyons, R.A. The Effect of Interleukin-6 on Ciliary Beat Frequency in the Human Fallopian Tube. Fertil. Steril. 2008, 90, 391–394. [Google Scholar] [CrossRef]
- Lyons, R.A.; Djahanbakhch, O.; Mahmood, T.; Saridogan, E.; Sattar, S.; Sheaff, M.T.; Naftalin, A.A.; Chenoy, R. Fallopian Tube Ciliary Beat Frequency in Relation to the Stage of Menstrual Cycle and Anatomical Site. Hum. Reprod. 2002, 17, 584–588. [Google Scholar] [CrossRef]
- van Gestel, I.; Ijland, M.M.; Hoogland, H.J.; Evers, J.L.H. Endometrial Wave-like Activity in the Non-Pregnant Uterus. Hum. Reprod. Update 2003, 9, 131–138. [Google Scholar] [CrossRef]
- De Ziegler, D.; Brioschi, P.A.; Fanchin, R.; Bulletti, C.; Ayoubi, J.M. Contractility of the Non-Pregnant Uterus. Infertil. Reprod. Med. Clin. N. Am. 2003, 14, 309–327. [Google Scholar]
- Singh, G.; Chanda, A. Mechanical Properties of Whole-Body Soft Human Tissues: A Review. Biomed. Mater. 2021, 16, 062004. [Google Scholar] [CrossRef] [PubMed]
- Baah-Dwomoh, A.; McGuire, J.; Tan, T.; De Vita, R. Mechanical Properties of Female Reproductive Organs and Supporting Connective Tissues: A Review of the Current State of Knowledge. Appl. Mech. Rev. 2016, 68, 060801. [Google Scholar] [CrossRef]
- Lasiene, K.; Lasys, V.; Glinskyte, S.; Valanciute, A.; Vitkus, A. Relevance and Methodology for the Morphological Analysis of Oocyte Quality in IVF and ICSI. J. Reprod. Stem Cell Biotechnol. 2011, 2, iv-79. [Google Scholar] [CrossRef][Green Version]
- Jurema, M.W.; Nogueira, D. In Vitro Maturation of Human Oocytes for Assisted Reproduction. Fertil. Steril. 2006, 86, 1277–1291. [Google Scholar] [CrossRef]
- Yang, D.; Yang, H.; Yang, B.; Wang, K.; Zhu, Q.; Wang, J.; Ding, F.; Rao, B.; Xue, R.; Peng, J.; et al. Embryological Characteristics of Human Oocytes with Agar-like Zona Pellucida and Its Clinical Treatment Strategy. Front. Endocrinol. 2022, 13, 859361. [Google Scholar] [CrossRef]
- Biology and Pathology of the Oocyte: Its Role in Fertility and Reproductive Medicine; Cambridge University Press: Cambridge, UK, 2003; ISBN 9780521799584.
- Tong, X.H.; Wu, L.M.; Jin, R.T.; Luo, L.H.; Luan, H.B.; Liu, Y.S. Fertilization Rates Are Improved after IVF If the Corona Radiata Is Left Intact in Vitrified-Warmed Human Oocytes. Hum. Reprod. 2012, 27, 3208–3214. [Google Scholar] [CrossRef] [PubMed]
- Goudet, G.; Leclercq, L.; Bézard, J.; Duchamp, G.; Guillaume, D.; Palmer, E. Chorionic Gonadotropin Secretion Is Associated with an Inhibition of Follicular Growth and an Improvement in Oocyte Competence for in Vitro Maturation in the Mare. Biol. Reprod. 1998, 58, 760–768. [Google Scholar] [CrossRef]
- Valeri, C.; Pappalardo, S.; De Felici, M.; Manna, C. Correlation of Oocyte Morphometry Parameters with Women’s Age. J. Assist. Reprod. Genet. 2011, 28, 545–552. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pors, S.E.; Nikiforov, D.; Cadenas, J.; Ghezelayagh, Z.; Wakimoto, Y.; Jara, L.A.Z.; Cheng, J.; Dueholm, M.; Macklon, K.T.; Flachs, E.M.; et al. Oocyte Diameter Predicts the Maturation Rate of Human Immature Oocytes Collected Ex Vivo. J. Assist. Reprod. Genet. 2022, 39, 2209–2214. [Google Scholar] [CrossRef]
- Khalilian, M.; Navidbakhsh, M.; Valojerdi, M.R.; Chizari, M.; Yazdi, P.E. Estimating Young’s Modulus of Zona Pellucida by Micropipette Aspiration in Combination with Theoretical Models of Ovum. J. R. Soc. Interface 2010, 7, 687–694. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, M.; Huang, J.; Sun, M.; Zhao, X.; Zhao, Q. Robotic Micropipette Aspiration for Multiple Cells. Micromachines 2019, 10, 348. [Google Scholar] [CrossRef]
- Murayama, Y.; Yoshida, M.; Mizuno, J.; Nakamura, H.; Inoue, S.; Watanabe, Y.; Akaishi, K.; Inui, H.; Constantinou, C.E.; Omata, S. Elasticity Measurement of Zona Pellucida Using a Micro Tactile Sensor to Evaluate Embryo Quality. J. Mamm. Ova Res. 2008, 25, 8–16. [Google Scholar] [CrossRef]
- Nassir, M.; Levi, M.; Shaked, N.T. Dynamic 3D Modeling for Humansperm Motility through the Female Cervical Canal and Uterine Cavity to Predict Sperm Chance of Reaching the Oocyte. Cells 2023, 12, 203. [Google Scholar] [CrossRef]
- Dardikman-Yoffe, G.; Mirsky, S.K.; Barnea, I.; Shaked, N.T. High-Resolution 4-D Acquisition of Freely Swimming Human Sperm Cells without Staining. Sci. Adv. 2020, 6, eaay7619. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen Sixth Edition; World Health Organization: Geneva, Switzerland, 2021; Volume V. [Google Scholar]
- World Health Organization. Examination and Processing of Human Semen; World Health Organization: Geneva, Switzerland, 2010; Volume V, ISBN 978-92-4-154778-9. [Google Scholar]
- Lindemann, C.B.; Lesich, K.A. Functional Anatomy of the Mammalian Sperm Flagellum. Cytoskeleton 2016, 73, 652–669. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.K.; O’Hanlon, D.E.; Harrold, S.; Man, S.T.; Wang, Y.Y.; Cone, R.; Hanes, J. Rapid Transport of Large Polymeric Nanoparticles in Fresh Undiluted Human Mucus. Proc. Natl. Acad. Sci. USA 2007, 104, 1482–1487. [Google Scholar] [CrossRef] [PubMed]
- Sobachkin, A.; Dumnov, G. Numerical Basis of CAD-Embedded CFD. NAFEMS World Congr. 2013, 2013, 1–20. [Google Scholar]
- Mediouni, M.; Madiouni, R.; Gardner, M.; Vaughan, N. Translational Medicine: Challenges and New Orthopaedic Vision (Mediouni-Model). Curr. Orthop. Pract. 2020, 31, 196–200. [Google Scholar] [CrossRef]
- Zeeshan, A.; Ijaz, N.; Bhatti, M.M.; Mann, A.B. Mathematical Study of Peristaltic Propulsion of Solid–Liquid Multiphase Flow with a Biorheological Fluid as the Base Fluid in a Duct. Chin. J. Phys. 2017, 55, 1596–1604. [Google Scholar] [CrossRef]
- Akbar, N.S.; Tripathi, D.; Khan, Z.H.; Bég, O.A. Mathematical Model for Ciliary-Induced Transport in MHD Flow of Cu-H2O Nanofluids with Magnetic Induction. Chin. J. Phys. 2017, 55, 947–962. [Google Scholar] [CrossRef]
- Ashraf, H.; Siddiqui, A.M.; Rana, M.A. Fallopian Tube Analysis of the Peristaltic-Ciliary Flow of Third Grade Fluid in a Finite Narrow Tube. Chin. J. Phys. 2018, 56, 605–621. [Google Scholar] [CrossRef]
- Hayat, T.; Nazar, H.; Imtiaz, M.; Alsaedi, A.; Ayub, M. Axisymmetric Squeezing Flow of Third Grade Fluid in Presence of Convective Conditions. Chin. J. Phys. 2017, 55, 738–754. [Google Scholar] [CrossRef]
- Ashraf, M.M.; Hasan, N.; Lewis, L.; Hasan, M.R.; Ray, P. A Systematic Literature Review of the Application of Information Communication Technology for Visually Impaired People. Int. J. Disabil. Manag. 2016, 11, e6. [Google Scholar] [CrossRef]
- Kissler, S.; Zangos, S.; Wiegratz, I.; Kohl, J.; Rody, A.; Gaetje, R.; Doebert, N.; Wildt, L.; Kunz, G.; Leyendecker, G.; et al. Utero-Tubal Sperm Transport and Its Impairment in Endometriosis and Adenomyosis. Ann. N. Y. Acad. Sci. 2007, 1101, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Reeve, L.; Lashen, H.; Pacey, A.A. Endometriosis Affects Sperm-Endosalpingeal Interactions. Hum. Reprod. 2005, 20, 448–451. [Google Scholar] [CrossRef]


| Density | |
| Specific heat | |
| Thermal conductivity | |
| Dynamic viscosity |
| Numbers of Simulations for Women’s Age Group | Total Number of Sperm/Oocyte Models in a Single Simulation | With/Without Endometriosis |
|---|---|---|
| 1 | 10,000 sperm models | Without |
| 1 | 10,000 sperm models | With |
| 100 | 1 single oocyte model | Without |
| 100 | 1 single oocyte model | With |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nassir, M.; Levi, M.; Shaked, N.T. The Synergic Effect of Tubal Endometriosis and Women’s Aging on Fallopian Tube Function: Insights from a 3D Mechanical Model. Bioengineering 2024, 11, 852. https://doi.org/10.3390/bioengineering11080852
Nassir M, Levi M, Shaked NT. The Synergic Effect of Tubal Endometriosis and Women’s Aging on Fallopian Tube Function: Insights from a 3D Mechanical Model. Bioengineering. 2024; 11(8):852. https://doi.org/10.3390/bioengineering11080852
Chicago/Turabian StyleNassir, Mayssam, Mattan Levi, and Natan T. Shaked. 2024. "The Synergic Effect of Tubal Endometriosis and Women’s Aging on Fallopian Tube Function: Insights from a 3D Mechanical Model" Bioengineering 11, no. 8: 852. https://doi.org/10.3390/bioengineering11080852
APA StyleNassir, M., Levi, M., & Shaked, N. T. (2024). The Synergic Effect of Tubal Endometriosis and Women’s Aging on Fallopian Tube Function: Insights from a 3D Mechanical Model. Bioengineering, 11(8), 852. https://doi.org/10.3390/bioengineering11080852









